Abstract
Advancing age is the major risk factor for cardiovascular diseases, driven largely by vascular endothelial dysfunction (impaired endothelium-dependent dilation, EDD) and aortic stiffening (increased aortic pulse wave velocity, aPWV). In humans, vascular aging occurs in the presence of differences in diet and physical activity, but the interactive effects of these factors are unknown. We assessed carotid artery EDD and aPWV across the lifespan in mice consuming standard (normal) low-fat chow (NC) or a high-fat/high-sucrose Western diet (WD) in the absence (sedentary, SED) or presence (voluntary wheel running, VWR) of aerobic exercise. Aging impaired nitric oxide-mediated EDD (peak EDD 88±12% 6 mo. P = 0.003 vs. 59±9% 27 mo. NC-SED), which was accelerated by WD (60±18% 6 mo. WD-SED). In NC mice, aPWV increased 32% with age (423±13 cm/sec 24 mo. P < 0.001 vs. 321±12 cm/sec 6 mo.) and absolute values were an additional ~10 % higher at any age in WD mice (P = 0.042 vs. NC-SED). Increases in aPWV with age in NC and WD mice were associated with 30-65% increases in aortic intrinsic wall stiffness (6 vs. 19-27 mo., P = 0.007). Lifelong aerobic exercise prevented age- and WD- related vascular dysfunction across the lifespan, and this protection appeared to be mediated by mitigation of vascular mitochondrial oxidative stress and inflammation. Our results depict the temporal impairment of vascular function over the lifespan in mice, acceleration and exacerbation of that dysfunction with WD consumption, the remarkable protective effects of voluntary aerobic exercise, and the underlying mechanisms.
Keywords: arterial stiffness, endothelium-dependent vasodilation, vascular mitochondria, superoxide, nitric oxide, intrinsic stiffness, wheel running
INTRODUCTION
Advancing age is the major risk factor for cardiovascular diseases (CVD), which remain the leading cause of death in developed societies (Timmis, Townsend et al. 2020, Virani, Alonso et al. 2020). The age-related increase in CVD risk is largely attributable to the development of vascular dysfunction, specifically vascular endothelial dysfunction and stiffening of the large elastic arteries (Lakatta and Levy 2003, Seals 2014). Endothelial function, commonly assessed as the vasodilatory response to a physical or chemical stimulus, declines with age and is an independent risk factor for CVD (Yeboah, Crouse et al. 2007, Yeboah, Folsom et al. 2009, Lind, Berglund et al. 2011, Seals, Jablonski et al. 2011, Seals, Kaplon et al. 2014). Stiffening of the large elastic arteries, particularly the aorta, can be assessed in humans via the gold standard measure pulse wave velocity, which increases with age and independently predicts CVD and other common disorders of aging, including cognitive impairment (Scuteri, Brancati et al. 2005, Waldstein, Giggey et al. 2005, Elias, Robbins et al. 2009, Mitchell, Hwang et al. 2010, Ben-Shlomo, Spears et al. 2014).
The key upstream mechanisms underlying age-related arterial dysfunction are vascular oxidative stress and inflammation, which interact in feed-forward fashion to impair endothelial function in large part by decreasing the bioavailability of key vasodilatory molecule nitric oxide (NO), which, in turn, promotes changes in the endothelial milieu toward a vasoconstrictive and pro-inflammatory state (van der Loo, Labugger et al. 2000, Lakatta 2003, Seals, Jablonski et al. 2011, Seals, Kaplon et al. 2014, Donato, Machin et al. 2018, Ungvari, Tarantini et al. 2018). Oxidative stress- and inflammation-driven signaling are important mediators of aortic stiffening via adverse remodeling of the arterial wall, though endothelial dysfunction and age-related increases in vasomotor tone also likely contribute (Lakatta and Levy 2003, Zieman, Melenovsky et al. 2005, Soucy, Ryoo et al. 2006, Fleenor, Seals et al. 2012). A growing body of literature suggests that dysregulated vascular mitochondria are an important source of oxidative stress in the setting of age-related vascular dysfunction (Davidson and Duchen 2007, Widlansky and Gutterman 2011, Kluge, Fetterman et al. 2013, Gioscia-Ryan, Battson et al. 2016, Rossman, Gioscia-Ryan et al. 2020).
Vascular aging in humans occurs in the presence of, and is influenced by, lifestyle factors such as diet and physical activity, but how these factors interact with primary vascular aging processes is incompletely understood. Consumption of a “Western-style” dietary pattern, characterized by high levels of saturated fat and sugar, and low levels of fiber and nutrients, is prevalent in developed and developing societies and has been linked to increased CVD risk (Hu, Rimm et al. 2000, Fung, Rimm et al. 2001, Yusuf, Reddy et al. 2001, Iqbal, Anand et al. 2008). Although dietary patterns are frequently maintained over the course of adult life, there is little information about how consumption of a Western diet (WD) interacts with the changes that occur to arteries with primary aging. In contrast to WD, regular aerobic exercise has numerous established health benefits, including decreasing CVD risk; attributable in part to salutary effects on vascular function (Seals 2014, Seals, Kaplon et al. 2014). Aerobic exercise has protective effects on the vasculature in the context of short-term high-fat or WD consumption in young animals (Park, Booth et al. 2012, Langbein, Hofmann et al. 2015) and one prior investigation in our laboratory showed that late-life voluntary wheel running prevented the deleterious effects of short-term WD consumption on vascular function in old mice ( Lesniewski, Zigler et al. 2013). However, to our knowledge, whether exercise performed over the course of the lifespan can protect arteries from the combined stressors of aging and chronic WD consumption has never been investigated.
A better understanding of the interactive effects of aging, diet and exercise on vascular function across the lifespan would have important biomedical and societal implications for informing interventions designed to preserve vascular health in humans, thereby decreasing CVD risk. However, these interactions remain incompletely characterized, possibly due to the difficult and time-consuming nature of longitudinal studies in people and even, though to a lesser degree, experimental animal models. Accordingly, here we performed a series of longitudinal and cross-sectional studies in C57BL/6 mice, an established model of human vascular aging (Sindler, Fleenor et al. 2011, Fleenor, Sindler et al. 2013, LaRocca, Gioscia-Ryan et al. 2013). We assessed the key expressions of vascular function – endothelial function and aortic stiffness – and markers of oxidative stress, mitochondrial oxidative stress, and inflammation at multiple timepoints across the lifespan in mice consuming either conventional (“normal”) low-fat rodent chow or a 41% fat/18% sucrose WD while remaining sedentary or performing aerobic exercise in the form of voluntary wheel running.
EXPERIMENTAL PROCEDURES
Ethical Approval.
All experiments were approved by the University of Colorado Boulder Institutional Animal Care and Use Committee (protocol # 2539) and adhered to all guidelines as set forth in the Guide for Care and Use of Laboratory Animals (National Research Council, 2011; Grundy 2015).
Overall study design and experimental animals.
To examine the interactive effects of aging, WD consumption, and exercise across the lifespan, we studied separate cohorts of male, C57BL/6 mice. Male, but not female, mice of this strain consistently exhibit the key features of age-related vascular dysfunction in humans (Sindler, Fleenor et al. 2011, Fleenor, Sindler et al. 2013, LaRocca, Gioscia-Ryan et al. 2013); in addition, this work was funded and initiated in 2012, i.e., prior to the NIH mandate of studying (and providing the budget to study) both sexes in preclinical experiments. Each cohort was randomly divided into the 4 treatment groups described below and aged for a predetermined period of time. All experimental animals were obtained from Charles River at 8-10 weeks of age and allowed to acclimate to our facilities for at least 2 weeks prior to the beginning of study procedures. Mice were singly housed in standard cages in our vivarium throughout the study, maintained on a 12:12 hour light/dark cycle and permitted ad libitum access to food and water. Daily animal health checks were performed by laboratory or institutional laboratory animal staff, under the supervision of the institutional veterinarian.
Treatment groups.
At 3 months of age, baseline measurement of aortic stiffness (described below) was obtained. Mice were then randomly assigned to a diet (normal chow, NC, or Western diet, WD) and exercise (sedentary, SED, or voluntary wheel running, VWR) condition, yielding 4 treatment groups as follows: normal chow sedentary (NC-SED), Western diet sedentary (WD-SED), normal chow voluntary wheel running (NC-VWR) and Western diet voluntary wheel running (WD-VWR).
Diets.
Normal chow was provided as Envigo 7917 (14% kcal from fat, 62% carbohydrate, 24% protein) whereas the Western diet (Harlan Teklad Diet 96132)—composed of 40.6% kcal from fat (beef tallow and vegetable shortening), 40.7% carbohydrate (including 18.2% of total caloric content as sucrose), and 18.7% protein—was chosen to reflect a typical high-fat, high-sugar, low fiber dietary pattern typical of Western societies (Lesniewski, Zigler et al. 2013). Mice were permitted ad libitum access to food throughout the study.
Voluntary exercise.
Mice assigned to voluntary wheel running were provided with ad libitum access to a running wheel in their home cage (Ware Pet Products small flying saucer, product number 03281); a subset of mice from each group (n=5–6 per cohort) were placed in specialized cages (Lafayette Instruments, Lafayette, IN, USA) equipped with running wheels attached to electronic monitoring software (Activity Wheel Monitoring, Lafayette, IN) to enable quantification of running volume (Durrant, Seals et al. 2009, Fleenor, Marshall et al. 2010, Lesniewski, Zigler et al. 2013, Gioscia-Ryan, Clayton et al. 2020). Running distance was recorded from each exercising group for a continuous 72-hour period approximately once per month. Data are presented as the mean running volume per day.
Experimental timeline.
As illustrated in Figure 1, we performed repeated non-terminal measures of physiological function at baseline and approximately every 3 months. To obtain data and samples from terminal experiments at various ages across the lifespan, separate cohorts of mice were aged to 6 mo, 13 mo, 19 mo, and 27 mo, the latter of which represents the median survival age for C57/BL6 mice consuming normal rodent chow (Mercken, Mitchell et al. 2014, Mitchell, Martin-Montalvo et al. 2014). We selected these ages to determine the temporal pattern of changes in vascular function across the lifespan.
Figure 1. Study overview.
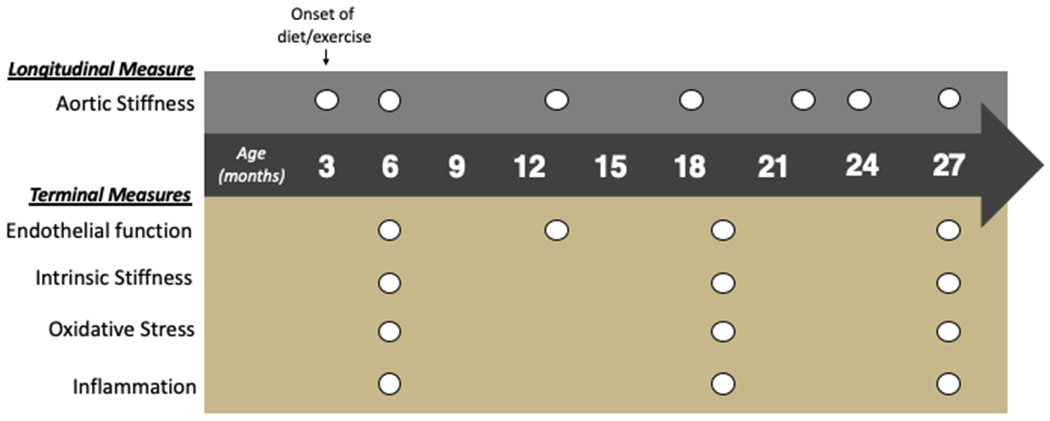
At 3 months of age, mice were randomly assigned to diet (normal rodent chow, NC, or Western diet, WD) and exercise (sedentary, SED, or voluntary wheel running, VWR) conditions, which were continued for the duration of the study. Non-invasive measurements (top) of aortic stiffness (pulse wave velocity) were obtained at intervals of approximately 3-6 months. Terminal measurements (bottom) of endothelial function in isolated carotid arteries were performed at 4 pre-determined ages (6 mo, 13 mo, 19 mo, and 27 mo), at which times aortic tissue was collected for determination of intrinsic mechanical stiffness, oxidative stress, and inflammation.
Longitudinal assessments
Aortic pulse wave velocity (aPWV).
Aortic stiffness was assessed as aortic pulse wave velocity using the Doppler ultrasound procedure previously described by our laboratory (Sindler, Fleenor et al. 2011, Fleenor, Seals et al. 2012, Gioscia-Ryan, Clayton et al. 2020). While maintained under light anesthesia with inhaled isoflurane (1.5-2%), mice were positioned supine on a warmed platform with paws secured to ECG leads. Doppler probes were placed at the transverse aortic arch and abdominal aorta to detect pulse waves. Three consecutive 2-second recordings were used to determine time delay between the ECG R-wave and the foot of the Doppler signal at each site (timeabdominal and timetransverse). Aortic PWV was calculated as (physical distance between the two probes) / (timeabdominal-timetransverse) and reported in cm/sec.
Terminal assessments and sample collection
Running wheels were removed from cages at least 24 hr prior to terminal measurements to prevent any influence of recent exercise. Mice were euthanized via exsanguination under inhaled isoflurane anesthesia.
Aorta were harvested and dissected free of surrounding connective tissue in ice-cold physiological saline. Segments of thoracic aorta were collected for determination of intrinsic stiffness and superoxide bioactivity (described below). The remainder of the aorta were snap-frozen in liquid nitrogen and stored at −80 C until use for determination of aortic cytokines (as described previously (Lesniewski, Durrant et al. 2011, Gioscia-Ryan, Battson et al. 2018, Ballak, Brunt et al. 2019), see below).
Vascular endothelial function.
Carotid artery endothelial function was assessed via pressure myography as we have previously described (Sindler, Fleenor et al. 2011, LaRocca, Gioscia-Ryan et al. 2013, Lesniewski, Zigler et al. 2013, Gioscia-Ryan, LaRocca et al. 2014). Carotid arteries were harvested, dissected free of connective tissue, and cannulated onto glass pipet tips in a myograph chamber (DMT, Inc. Arhaus Denmark) while submerged in warm physiological saline solution. Arteries were gravity pressurized and allowed to equilibrate for at least 40 minutes prior to beginning experiments. Vessels were visualized on a computer screen via an inverted microscope and diameter was measured manually via Myoview 1.2p software. Pre-constriction of at least 15% was achieved with addition of 2 μM phenylephrine, after which dilation in response to increasing doses of acetylcholine (1 x 10−9 to 1 x 10−4 mol/L). Arteries were washed with multiple changes of warm physiological saline prior to subsequent assessments of EDD in the presence of pharmacological modulators (below).
Nitric oxide component of EDD.
To determine the contribution of nitric oxide to the vasodilatory response to ACh, a subset of carotid arteries was pre-incubated with the endothelial nitric oxide synthase inhibitor L-NAME (0.1 mmol/L; Sigma-Aldrich Corp.) for 40 minutes prior to assessment of the dose-response to ACh as described above.
Tonic suppression of EDD by mitochondrial oxidative stress.
To determine the contribution of mitochondrial oxidative stress to suppression of vasodilation, a subset of arteries was pre-incubated with the mitochondria-specific antioxidant, MitoQ (100 μM) for 40 minutes prior to assessment of the dose-response to ACh as described above (Gioscia-Ryan, LaRocca et al. 2014).
Endothelium independent dilation and maximal vessel diameter.
Following all ACh dose-response experiments, endothelium-independent dilation (EID) was assessed as the dose-response to increasing doses of the direct NO donor sodium nitroprusside (SNP). Arteries were then incubated in calcium-free physiological saline to determine maximal vessel diameter in the absence of smooth muscle tone.
Calculations.
To account for differences in vessel diameter, all dilation responses are expressed on a percentage basis. Pre-constriction was calculated as percentage of maximal diameter according to the following formula:
Dm is the maximal lumen diameter achieved throughout the experimental day, Dp is the steady-state lumen diameter after the addition of a 2 μM dose of phenylephrine.
Vasodilator responses were recorded as actual diameters and expressed as a percentage of maximal possible vasodilator response according to the following formula:
Dm is maximal diameter achieved throughout the experimental day, Ds is the steady-state diameter recorded after the addition of either acetylcholine or sodium nitroprusside, and Dp is the steady-state diameter following pre-constriction with phenylephrine before the first addition of drug.
Peak EDD and EID were considered the highest value of dilation achieved in a given dose-response. The nitric oxide component of dilation was calculated as the difference between peak EDD in the absence vs. presence of L-NAME, expressed as a percent. In addition, carotid artery sensitivity (EC50) to acetylcholine was also assessed.
Aortic Intrinsic Stiffness and Biochemistry
Given that there were no differences in our key functional outcomes (EDD and aPWV) between 6 and 13 mo. of age in any of the treatment groups, the following measures using aortic tissue are presented only for samples obtained at 6, 19, and 27 mo.
Intrinsic Mechanical Stiffness.
Two ~1-mm segments of the thoracic aorta were used for determination of intrinsic mechanical stiffness by incremental stress-strain testing via wire myography, as described previously by our laboratory (Fleenor, Eng et al. 2014, LaRocca, Hearon et al. 2014, Gioscia-Ryan, Battson et al. 2018). Aortic segments were loaded into a warmed (37 C°) wire myograph chamber (DMT, Arhaus, Denmark) containing calcium-free phosphate-buffered saline. After three cycles of pre-stretching, ring diameter was increased to achieve 1mN force and then incrementally stretched by ~10% every 3 minutes until failure. The force corresponding to each stretching interval was used to calculate stress and strain, as follows:
Strain (λ) = Δ d/d(i); d= diameter; d(i)= initial diameter; Stress (t) = λL/2HD; L= one-dimensional load; H= wall thickness determined by histology; D= vessel length
The collagen-dominant elastic modulus was determined as the slope of the linear regression fit to the final four points of the stress-strain curve (Fleenor, Sindler et al. 2012, LaRocca, Hearon et al. 2014, Gioscia-Ryan, Battson et al. 2018).
Whole cell and mitochondrial superoxide bioactivity.
Two 1-mm segments of thoracic aorta were used for determination of aortic whole-cell and mitochondrial superoxide bioactivity (1, 1-mm segment for each) via electron paramagnetic resonance spectroscopy using the spin probes CMH (whole-cell superoxide) and MitoTempo-H (mitochondria-specific superoxide), as reported previously (Dikalova, Bikineyeva et al. 2010, Fleenor, Seals et al. 2012, Gioscia-Ryan, LaRocca et al. 2014, LaRocca, Hearon et al. 2014).
Inflammatory cytokines.
Aortic inflammatory cytokine protein levels were determined as previously described by our laboratory (Lesniewski, Durrant et al. 2011, Gioscia-Ryan, Battson et al. 2018, Ballak, Brunt et al. 2019) using a commercially available multiplex ELISA kit (Ciraplex, Aushon Biosystems, Billerica, MA). Aorta were lysed in RIPA buffer with phosphatase and protease inhibitors. 15 μg micrograms of protein were used for the assay, which was performed according to the manufacturer’s instructions for detection of Interleukin (IL)1β, IL6, Interferon (IFN)-γ, Tumor Necrosis Factor (TNF)-α, and IL2. Images were captured using Cirascan imager (Aushon), and results were analyzed with Cirasoft software (Aushon). If levels of a given cytokine were undetectable (e.g. fell below the limits of detection of the assay), samples were excluded from the analysis.
Statistical Analysis.
Data were assessed for outliers using the Grubb’s test (GraphPad online calculator) prior to statistical analysis with an exclusion threshold of 0.05. After confirming normality, data for all cross-sectional outcomes were analyzed by two-way analysis of variance (ANOVA) with main effects of group and age, whereas the longitudinal measure (aPWV) were analyzed using a linear mixed model accounting for group and age comparisons. Sidak’s multiple comparisons post hoc test was performed when significant main effect (group and age) differences were detected using a p value of < 0.05. All data are presented as means ± SD. Statistics were calculated using Graph Pad Prism version 8 (Graph Pad Software, La Jolla, CA).
RESULTS
The overall study design and timing of primary outcome measurements is illustrated in Figure 1. Diet (NC or WD) and exercise (SED or VWR) conditions were initiated at 3 months of age and continued for the duration of the study. Non-invasive measurements were obtained at intervals of approximately 3-6 months, whereas terminal measurements and harvesting of arterial tissue for biochemical assessments were performed at pre-determined timepoints (6 mo, 13 mo, 19 mo, and 27 mo.). Due to the anticipated early mortality in the sedentary mice consuming WD (Baur, Pearson et al. 2006, Pearson, Baur et al. 2008), no terminal measurements were obtained from this group at 27 mo. Average running distance was similar (main effect of group, P = 0.87) between mice consuming NC and WD at all time points assessed, and decreased with age in both groups (average distance covered in 24 hours 7.62 ± 5.16 km at 6 mo. of age vs 1.92 ± 2.04 km at 27 mo. of age, P < 0.0001), consistent with previous studies (Lesniewski, Zigler et al. 2013, Goh and Ladiges 2015, Gioscia-Ryan, Clayton et al. 2020). As expected, body weight was 30-60% higher in the WD-SED mice than in the NC groups and the WD-VWR group at all ages (all P < 0.0001). There were no other body weight differences detected between groups.
Western diet accelerates, and voluntary aerobic exercise prevents, age-associated, nitric oxide-mediated impairments in endothelial function throughout the lifespan.
Age-related endothelial dysfunction developed later in life, evidenced by a 26% decline in peak isolated carotid artery dilation to ACh at 27 mo. of age in NC-SED mice (P = 0.001 vs. 6 mo. NC-SED) (Figure 2A). Endothelial dysfunction was substantially accelerated by WD consumption, such that by 6 mo. of age peak EDD in WD-SED mice was 36% lower than that of age-matched NC-SED mice (P < 0.0001) and comparable in magnitude to that of 27 mo. NC-SED mice, suggesting substantial impairment of endothelial function already in early adulthood. Lifelong aerobic exercise on both diet backgrounds preserved endothelial function throughout the lifespan, with peak EDD in NC-VWR and WD-VWR groups remaining at young adult control levels (e.g., not different from 6 mo. NC-SED) throughout the lifespan. There were no differences in carotid artery sensitivity to ACh (Table 1). Furthermore, there were no group differences across treatment nor time in the vasodilatory response to the direct nitric oxide donor sodium nitroprusside, indicating that the age, WD, and exercise effects on vasodilatory response to ACh were specific to the endothelium and not due to reduced vascular smooth muscle sensitivity to NO (Figure 2B).
Figure 2. Western diet accelerates, and voluntary aerobic exercise prevents, age-associated, impairments in nitric oxide-mediated endothelium-dependent dilation throughout the lifespan.
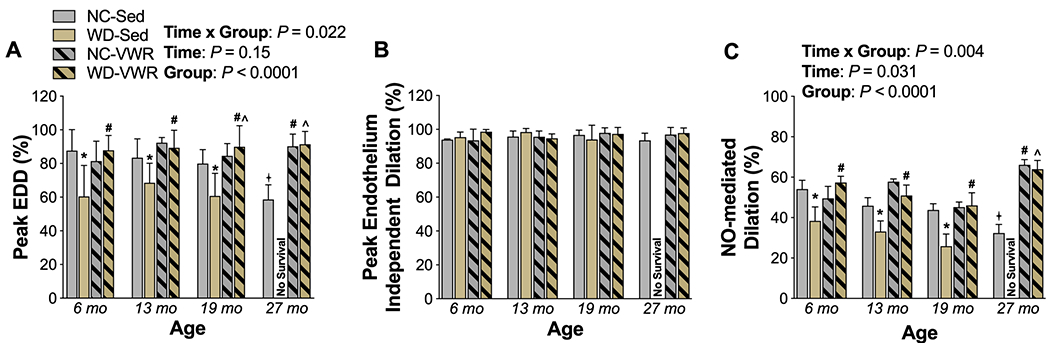
A) Peak carotid artery endothelium-dependent dilation (EDD) to acetylcholine (ACh) in normal chow sedentary (NC-SED), Western diet sedentary (WD-SED), NC voluntary wheel running (NC-VWR), and WD voluntary wheel running (WD-VWR) mice at ages indicated on the x-axis. B) Peak carotid artery endothelium-independent dilation in response to the direct nitric oxide (NO) donor sodium nitroprusside (SNP). C) Proportion of EDD mediated by NO, assessed as the difference between peak EDD in the absence vs. presence of L-NAME.
n = 7-13 (NC-SED; all time-points); n = 6-10 (WD-SED; 6, 13 and 19 mo); n = 7-27 (NC-VWR; all time-points); n = 5-8 (WD-VWR; all time-points). Data are the mean ± SD. *P < 0.05 Two-way ANOVA, main effect of group with post-hoc test demonstrating a significant difference between NC-SED vs. WD-SED; #P < 0.05 main effect of group with post-hoc test demonstrating a significant difference between NC-VWR vs. NC-SED and WD-VWR vs. WD-SED; ^P < 0.05 main effect of group with post-hoc testing demonstrating a significant difference between NC-SED vs. WD-VWR; +P < 0.05 significant difference over time within group.
Table 1.
Carotid artery sensitivity (EC50) to acetylcholine
| EC50 (logM) | |||||
|---|---|---|---|---|---|
| Age | NC-Sed | WD-Sed | NC-VWR | WD-VWR | |
| 6 mo | −7.4 ± 0.5 | −7.1 ± 1.0 | −7.7 ± 0.8 | −7.5 ± 0.5 | |
| 13 mo | −7.4 ± 0.8 | −7.7 ± 0.8 | −7.5 ± 0.5 | −7.5 ± 0.5 | |
| 19 mo | −7.6 ± 0.7 | −7.0 ± 0.9 | −7.4 ± 0.8 | −6.8 ± 0.4 | |
| 27 mo | −7.6 ± 0.8 | No Survival | −7.7 ± 0.6 | −7.5 ± 1.0 | |
NC-Sed: Normal Chow-Sedentary; WD-Sed: Western Diet-Sedentary; NC-VWR: Normal Chow- Voluntary Wheel Running; WD-VWR: Western Diet-Voluntary Wheel Running. Data are presented as mean ± SD. There were no statistically significant differences among groups (main effect of timepoint, p = 0.34; main effect of group, p = 0.16).
Both the age and WD-related impairments in EDD appeared to be largely mediated by a decrease in bioavailability of NO. For example, the NO-mediated component of EDD (determined as the difference in EDD in the absence vs. presence of L-NAME) was ~35% lower in 27 mo. vs 6 mo. NC-SED mice (Figure 2C). The age-related decline in NO-mediated dilation was accelerated by WD, such that decrements (as compared to 6 mo. NC-SED) were observed beginning at 6 mo. and persisted across all timepoints. NO-mediated dilation was completely preserved by voluntary wheel running on both diet backgrounds, with the NO component of dilation remaining comparable to 6 mo. NC-SED in NC-VWR and WD-VWR at all ages.
Western diet exacerbates, and voluntary aerobic exercise prevents, age-associated aortic stiffening.
Aortic pulse-wave velocity, the most well-established in vivo measure of aortic stiffness, was significantly increased by 18 months of age in NC-SED mice and continued to increase through 24 mo. of age to ~40% above young adult baseline levels (Figure 3A). This age-related aortic stiffening was exacerbated in sedentary mice consuming Western diet, such that aPWV values in WD-SED were higher vs. age-matched NC-SED beginning at 22 mo. of age. Lifelong aerobic exercise prevented the age- and Western diet- associated increases in aortic stiffness, with aPWV levels in voluntary wheel running mice from both diet groups remaining unchanged compared to baseline across the entire lifespan. The group differences in aPWV did not appear to be related to changes in arterial blood pressure, as there were no consistent changes nor group differences in tail-cuff blood pressure over the lifespan (data not shown).
Figure 3. WD exacerbates, and voluntary aerobic exercise prevents, age-associated aortic stiffening.
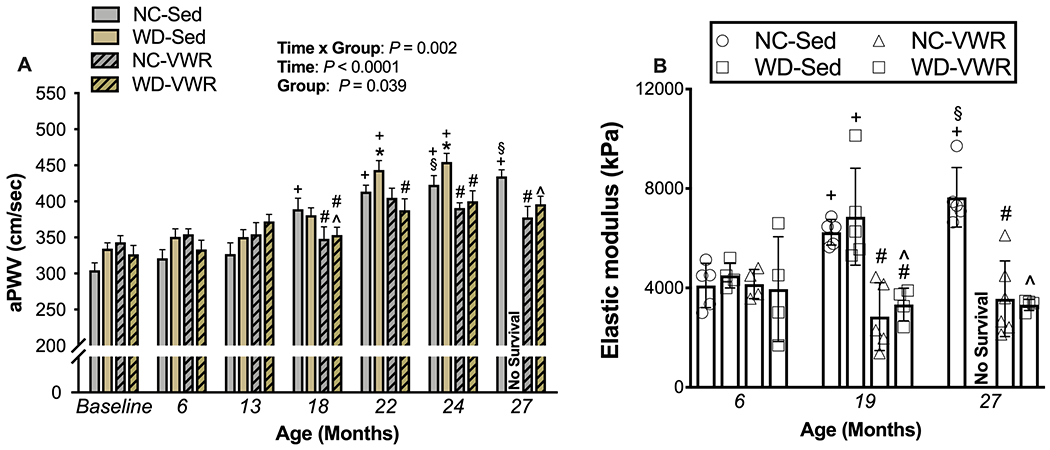
A) Aortic stiffening as measured by aortic pulse wave velocity across the lifespan (ages indicated on x-axis) in normal chow (NC-SED; n = 17-29), Western diet (WD-SED; n = 12-27), NC voluntary wheel running (NC-VWR; n = 4-15) and WD-VWR (WD-VWR; 5-31).
B) Intrinsic mechanical stiffness (elastic modulus) in isolated aorta rings from NC-SED (n = 5), WD-SED (n = 4–5), NC-VWR (n = 4–6) and WD-VWR (n = 4).
Data are the mean ± SD. *P < 0.05 main effect of group with post-hoc test demonstrating a significant difference between NC-SED vs. WD-SED; #P < 0.05 main effect of group with post-hoc test demonstrating a significant difference between NC-VWR vs. NC-SED and WD-VWR vs. WD; ^P < 0.05 main effect of group with post-hoc testing demonstrating a significant difference between NC-SED vs. WD-VWR; +P < 0.05 significant difference over time within group.
In concert with the age- and WD-related increases in aPWV, intrinsic mechanical stiffness of the aorta—determined via ex vivo stress-strain testing of aortic rings and expressed as elastic modulus—increased 38-42% from 6 to 19-27 months of age in the NC-SED and WD-SED groups (Figure 3B). Increases in intrinsic aortic stiffness were completely prevented by lifelong aerobic exercise in both NC-VWR and WD-VWR mice, consistent with the protective effects of lifelong aerobic exercise against age-related increases in aPWV.
Age-related vascular dysfunction and its acceleration by Western diet are linked to aortic whole cell and mitochondria-derived oxidative stress, which are prevented by lifelong aerobic exercise.
Aortic whole-cell (Figure 4A) superoxide was elevated ~2-fold in NC-SED mice by 27 mo. of age (P = 0.035 vs. 6 mo.), consistent with age-related vascular oxidative stress. Given the growing body of literature supporting an important role for mitochondria as a source of vascular oxidative stress, we also assessed mitochondria-specific superoxide, which was increased ~ 2-fold as well at 27 mo. of age (Figure 4B). WD consumption accelerated and exacerbated the age-associated increases in whole-cell and mitochondrial superoxide, such that ~2.5-3-fold elevations (whole cell: P = 0.032; mitochondrial: P = 0.005 vs. 6 mo. NC-SED) in aortic whole-cell and mitochondrial superoxide were evident by 6 mo. of age in WD-SED mice and persisted across the shortened lifespan of those animals (19 mo. timepoint). Lifelong voluntary aerobic exercise prevented the increases in aortic whole-cell and mitochondrial superoxide observed with aging in both NC-VWR and WD-VWR groups.
Figure 4. Age-related vascular dysfunction and its acceleration by Western diet consumption are linked to aortic whole cell and mitochondria-derived oxidative stress, which are prevented by lifelong exercise.
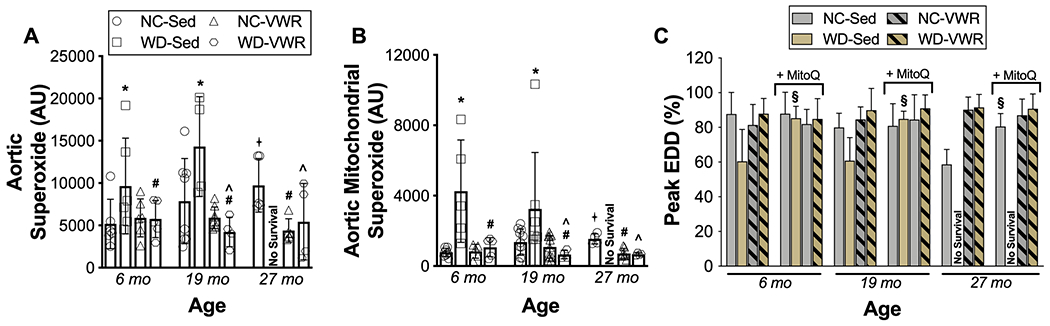
Whole-cell (A) and mitochondria-specific (B) superoxide production in aortic segments from normal chow (NC-SED; n = 4-8/group), Western diet (WD-SED; n = 4-7/group), NC voluntary wheel running (NC-VWR; n = 5-13/group), and WD voluntary wheel running (WD-VWR; n = 4-5/group) mice at 6, 19 and 27 mo. of age. C) Peak endothelium-dependent dilation (EDD) to ACh in the absence versus presence of acute ex vivo treatment with the mitochondria-specific antioxidant MitoQ (NC-Sed, n = 4-6/group; WD-Sed, n = 4-5/group; NC-VWR, n = 5-8/group; WD-VWR, n= 4-5/group). Data are the mean ± SD.
*P < 0.05 main effect of group with post-hoc test demonstrating a significant difference between NC-SED vs. WD-SED; #P < 0.05 main effect of group with post-hoc test demonstrating a significant difference between NC-VWR vs. NC-SED and WD-VWR vs. WD-SED; ^P < 0.05 main effect of group with post-hoc testing demonstrating a significant difference between NC-SED vs. WD-VWR; +P < 0.05 significant difference over time within group. § p<0.05 MitoQ treated vs. untreated within group and timepoint.
To determine the functional contribution of elevated vascular mitochondrial oxidative stress to age- and WD-related vascular dysfunction, we assessed EDD in isolated carotid arteries following acute ex vivo application of the mitochondria-targeted antioxidant MitoQ (Gioscia-Ryan, LaRocca et al. 2014) (Figure 4C). The higher aortic mitochondrial superoxide levels with aging and WD consumption were associated with greater tonic suppression of endothelial function by mitochondrial oxidative stress, as indicated by significant acute improvements in EDD in the presence vs. absence of MitoQ in 27 mo. NC-SED and all ages of WD-SED mice (i.e., all groups displaying baseline impairments in EDD). Consistent with the suppression of age- and WD- related elevations in whole-cell and mitochondrial superoxide, lifelong voluntary aerobic exercise prevented the mitochondrial oxidative-stress mediated suppression of EDD, evidenced by no further improvement in EDD with acute MitoQ in NC-VWR and WD-VWR groups at all ages.
Age-related vascular dysfunction and its acceleration by WD consumption are associated with aortic inflammation, which is attenuated by lifelong exercise.
In sedentary mice consuming NC, aging was associated with ~4-fold elevations in the aortic pro-inflammatory cytokines IL1β, IL6, IFN-γ, TNF-α and IL-2 (Figure 5 A–E) by 27 mo. of age (P = 0.001 vs. 6 mo). Consumption of WD in sedentary mice accelerated age-related aortic inflammation, such that ~ 2-fold elevations in cytokine levels (P = 0.042 vs. 6 mo. NC-SED) were observed already by 6 mo. of age in WD-SED, with further increases (to levels at least 4-6 times greater than 6 mo. NC-SED) observed by 19 mo. of age. VWR completely prevented (in NC-fed mice) or markedly attenuated (in the setting of WD consumption) age- and diet- associated elevations in pro-inflammatory cytokines.
Figure 5. Age-related vascular dysfunction and its acceleration by Western diet consumption are accompanied by aortic inflammation, which is attenuated by lifelong exercise.
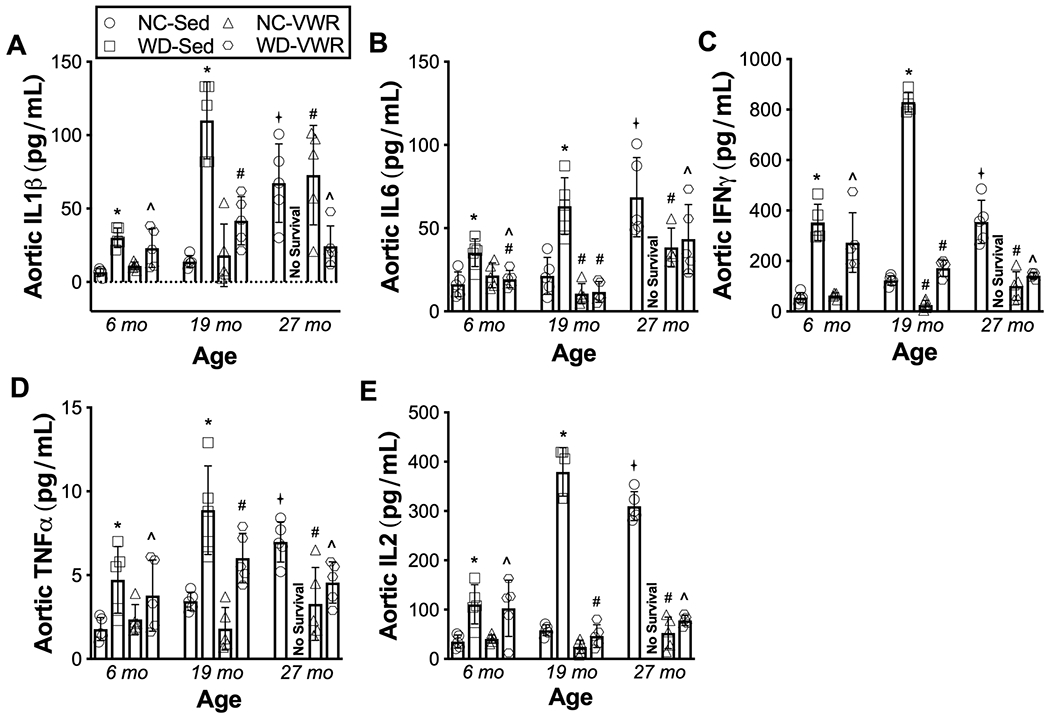
Aortic protein abundance of inflammatory cytokines A) interleukin (IL)-1β, B) IL-6, C) interferon (IFN)-γ, D) tumor necrosis factor (TNF)-α, and E) IL-2 in aortic lysates from normal chow sedentary (NC-SED), Western diet sedentary (WD-SED), NC voluntary wheel running (NC-VWR), and WD voluntary wheel running (WD-VWR) mice at 6, 19 and 27mo of age. n = 5 (NC-SED; all time-points); n = 5 (WD-SED; 6, 13 and 19 mo); n = 5 (NC-VWR; all time-points); n = 5 (WD-VWR; all time-points).
Data are the mean ± SD. *P < 0.05 main effect of group with post-hoc test demonstrating a significant difference between NC-SED vs. WD-SED; #P < 0.05 main effect of group with post-hoc test demonstrating a significant difference between NC-VWR vs. NC-SED and WD-VWR vs. WD-SED; ^P < 0.05 main effect of group with post-hoc testing demonstrating a significant difference between NC-SED vs. WD-VWR; +P < 0.05 significant difference over time within group.
DISCUSSION
Overall, the results of this study provide the first insight into vascular aging across the lifespan as modulated by two common lifestyle behaviors observed in human populations—consumption of a “Western” vs. non-Western dietary pattern and the presence vs. absence of regular aerobic exercise. We show that age-associated impairments in the two key expressions of vascular function, nitric oxide-mediated endothelium-dependent dilation and aortic stiffening, were accelerated and/or exacerbated by lifelong Western diet consumption, accompanied by corresponding worsening of age-related vascular oxidative stress—including excessive mitochondria-derived reactive oxygen species production—and inflammation. Lifelong aerobic exercise completely protected arteries from both the separate and combined effects of aging and consuming a Western diet, preserving vascular function across the lifespan with attendant prevention or marked attenuation of vascular oxidative stress and inflammation.
By assessing vascular function at several points over the lifespan in sedentary mice consuming standard rodent chow, we were able to establish the temporal pattern by which vascular function becomes impaired with adult aging in the absence of adverse dietary or beneficial physical activity exposure. In this setting, “primary aging” in mice was characterized by the development of vascular endothelial dysfunction and aortic stiffening, extending previous findings based on cross-sectional observations in rodents and humans (Vaitkevicius, Fleg et al. 1993, Celermajer, Sorensen et al. 1994, Lakatta and Levy 2003, Mitchell, Parise et al. 2004, Seals, Jablonski et al. 2011, Donato, Walker et al. 2013, Seals, Kaplon et al. 2014). Our results indicate that aortic stiffening increased progressively with advancing age; aPWV was significantly increased compared to baseline by later middle-age (~18 months) and there was a further, significant increase in older adulthood (24 months). This pattern is consistent with longitudinal carotid-femoral pulse-wave data from adult humans in the Baltimore Longitudinal Study of Aging (AlGhatrif, Strait et al. 2013). The age-related increase in aortic stiffening appeared to be at least partially attributable to arterial structural remodeling, as evidenced by greater intrinsic mechanical stiffness of isolated aortic rings at later ages (19 and 27 mo.).
We also observed significant impairments in NO-mediated endothelial function in later life (27 months) which were not apparent at the middle-age timepoint (19 months), suggesting that the onset of endothelial dysfunction occurred sometime between 19 and 27 months. Based on human data demonstrating reduced endothelial function by the 5th to 6th decade of life (Celermajer, Sorensen et al. 1994, Taddei, Virdis et al. 1995, Brandes, Fleming et al. 2005, Seals, Jablonski et al. 2011)—equivalent to ~20-24 months in C57BL/6 mice (Dutta and Sengupta 2016, Hagan and Catherine 2017)—it is plausible that decrements in peak EDD may be present as early as 20-21 months; however, because of the terminal nature of our endothelial function measurements performed at 4 pre-determined ages, we cannot provide additional temporal resolution regarding the onset of endothelial dysfunction. Nevertheless, our data demonstrating the development of vascular dysfunction with primary aging provide an important framework for investigating its modification by Western diet and aerobic exercise.
A key objective of this study was to determine how lifelong consumption of a Western diet, a common occurrence in human populations of both developed and developing nations, interacts with the changes that occur to vascular function with primary aging. Our results demonstrate that lifelong Western diet consumption markedly accelerates vascular endothelial dysfunction; by 6 months of age, peak EDD in the Western diet-fed mice was already impaired similarly to that of the oldest (27 mo.) normal chow-fed mice. We also found that Western diet exacerbated aortic stiffening, with aPWV in WD-SED mice significantly higher than that of age-matched mice consuming normal chow, due at least in part to adverse structural remodeling, as indicated by higher intrinsic mechanical stiffness (vs. 6 mo. NC-SED) at 19 mo. in WD-SED. Previous work indicates that high-fat or Western diet consumption can induce endothelial dysfunction and promote arterial stiffening and remodeling in young animals (Lesniewski, Zigler et al. 2013, García-Prieto and Fernández-Alfonso 2016, Battson, Lee et al. 2018, Kramer, França et al. 2018, Elrashidy, Zhang et al. 2019) and a prior study from our laboratory demonstrated that short-term (10-14 weeks) Western diet feeding in older mice further worsened already existing age-related endothelial dysfunction (Lesniewski, Zigler et al. 2013). Our findings here reinforce and extend this previous work by showing that the deleterious effects of lifelong WD consumption extend throughout the lifespan and compound the declines in vascular function that occur with age. Our findings may also have translational implications. Epidemiological data link Western dietary patterns to increased CVD risk (Hu, Rimm et al. 2000, Fung, Rimm et al. 2001, Yusuf, Reddy et al. 2001, Iqbal, Anand et al. 2008), and our results suggest that some of this risk likely is attributable to exacerbation of the already significant vascular dysfunction that occurs with natural adult aging.
The most remarkable finding of the present study was that we show for the first time that lifelong voluntary wheel running completely prevented the impairments in vascular function induced by primary aging and, even more impressively, the additive dysfunction induced by lifelong Western diet consumption. Interestingly, the preservation of vascular function by aerobic exercise persisted to the oldest ages despite the well-documented substantially lower volume of voluntary running exercise observed in older compared to younger mice (Durrant, Seals et al. 2009, Fleenor, Marshall et al. 2010, Lesniewski, Zigler et al. 2013, Goh and Ladiges 2015, Gioscia-Ryan, Clayton et al. 2020). The present results demonstrating the effects of lifelong voluntary wheel running extend previous findings demonstrating that short-term exercise interventions initiated in late life improve vascular function in mice in settings of aging alone and aging compounded by short-term Western diet (Durrant, Seals et al. 2009, Fleenor, Marshall et al. 2010, Lesniewski, Zigler et al. 2013, Gu, Wang et al. 2014). In humans, later-life aerobic exercise training interventions improve vascular endothelial function (DeSouza, Shapiro et al. 2000, Seals, Desouza et al. 2008, Pierce, Donato et al. 2011, Nowak, Rossman et al. 2018) and some studies suggest favorable effects on arterial stiffness, though these results are less consistent, possibly related to the relatively short duration of the intervention period (Tanaka, Dinenno et al. 2000, Moreau, Donato et al. 2003, Nowak, Rossman et al. 2018). There are limited data on the effects of regular aerobic exercise over longer periods of adulthood in humans. Cross-sectional analyses suggest that habitually aerobic exercise trained older adults have preserved endothelial function and that the age-associated increase in arterial stiffness is attenuated by 50% or more compared with sedentary individuals (Vaitkevicius, Fleg et al. 1993, Seals, Desouza et al. 2008, Gando, Kawano et al. 2010, Laurent, Marenco et al. 2011, Seals 2014, Pierce 2017, Nowak, Rossman et al. 2018, Shibata, Fujimoto et al. 2018, Tanaka, Palta et al. 2018). This, combined with our results, suggest that maintenance of physical activity in general, and regular aerobic exercise in particular, throughout life may be critical for optimizing vascular health. Additionally, our results indicate that the vascular protective effects of regular, lifelong aerobic exercise may persist even in the face of adverse effects of prolonged exposure to consumption of a Western diet. Future work is warranted to determine the translational potential of these observations—particularly with respect to the optimal timing and duration of aerobic exercise required to confer vascular-protective effects in human as compared to rodent aging (Demetrius 2005) - and to establish whether the salutary effects of voluntary running extend to other forms of aerobic exercise (e.g., cycling, swimming) commonly performed by older adults.
As expected, the development of age-related vascular dysfunction was accompanied by aortic oxidative stress and inflammation, consistent with previous work establishing these mechanisms as key drivers of vascular aging (Lakatta 2003, Seals, Kaplon et al. 2014, Donato, Machin et al. 2018, Ungvari, Tarantini et al. 2018). Emerging evidence supports an important role for vascular mitochondria dysregulation as an important factor contributing to age-related vascular oxidative stress and inflammation (Davidson and Duchen 2007, Widlansky and Gutterman 2011, Kluge, Fetterman et al. 2013, Rossman, Gioscia-Ryan et al. 2020). Consistent with this idea, we observed elevations in aortic mitochondria-specific superoxide across the lifespan in sedentary mice consuming normal chow, and associated tonic suppression of EDD, evidenced by restoration of EDD with the ex vivo application of a mitochondrial antioxidant (MitoQ) to isolated arteries (Gioscia-Ryan, LaRocca et al. 2014). Together, these observations support the growing body of literature indicating that vascular mitochondria are an important source of reactive oxygen species bioactivity and oxidative stress in aging arteries (Rossman, Gioscia-Ryan et al. 2020).
The exacerbation of vascular dysfunction by Western diet consumption was mediated, at least in part, by exaggeration of the same mechanisms observed to mediate primary vascular aging, as age-related elevations in aortic superoxide, mitochondria-specific superoxide, and inflammation were more pronounced in mice consuming Western diet throughout the lifespan. This is in line with evidence that Western diet intake promotes oxidative stress, inflammation, and impairs mitochondrial function in many tissues, including in the vasculature (Lesniewski, Zigler et al. 2013, Gioscia-Ryan, Battson et al. 2016, Kramer, França et al. 2018). The results of the present study provide the first evidence that lifelong aerobic exercise decreases total arterial superoxide production, mediated at least in part by decreased mitochondrial superoxide production, and suppresses inflammation with aging, even in animals consuming a pro-inflammatory WD. These findings extend our previous findings that improvements in vascular function following late-life exercise interventions in mice are mediated by decreases in oxidative stress and inflammation (Durrant, Seals et al. 2009, Fleenor, Marshall et al. 2010, Lesniewski, Durrant et al. 2011, Lesniewski, Zigler et al. 2013) and have also been associated with improvements in vascular mitochondrial health markers in some models (Knaub, McCune et al. 2013, Gu, Wang et al. 2014, Keller, Knaub et al. 2015, Gioscia-Ryan, Battson et al. 2016). Taken together, the present results along with our earlier findings demonstrate that regular aerobic exercise preserves vascular function with aging and consumption of a WD by inhibiting vascular oxidative stress, inflammation, and adverse mitochondrial alterations.
Conclusion
In summary, this study provides novel insight into the interactive influences of primary aging, Western diet consumption, and aerobic exercise on vascular function across the lifespan in mice. Our data indicate that lifelong aerobic exercise is a powerful intervention which largely prevents the development of age-related vascular endothelial dysfunction and arterial stiffening, even when compounded by Western diet, by mitigating vascular oxidative stress and inflammation (Figure 6). Our results contribute to the understanding of the interplay among aging and these real-world lifestyle factors on vascular function and could inform efforts aimed at identifying effective strategies to preserve cardiovascular health across the lifespan in humans.
Figure 6. Working hypothesis.
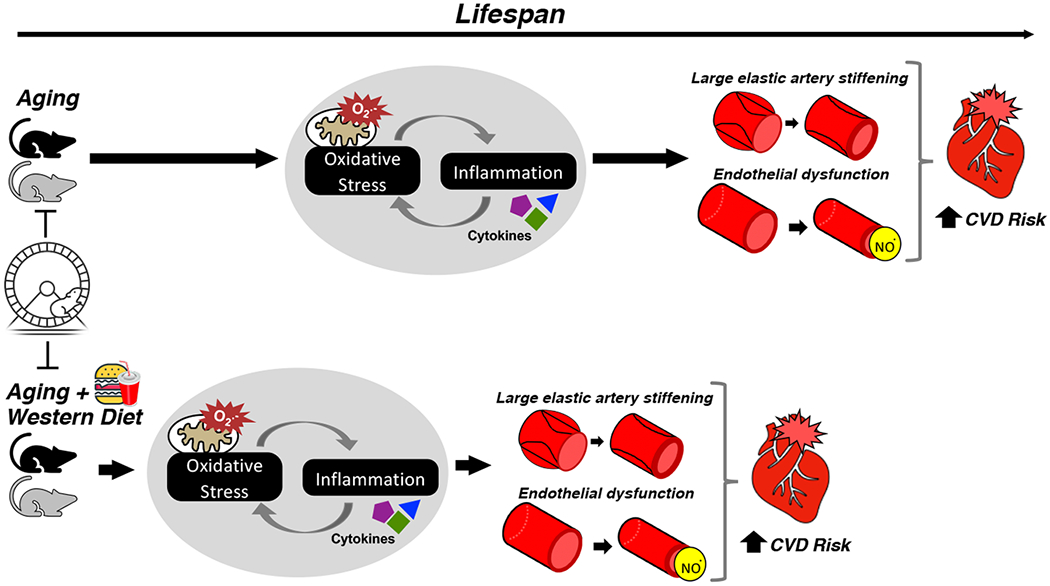
We studied interactions of common lifestyle factors and vascular aging across the lifespan in mice. Age-related nitric oxide-mediated endothelial dysfunction and aortic stiffening were accelerated and exacerbated by lifelong Western diet, with corresponding worsening of age-related vascular mitochondria-derived oxidative stress and inflammation. Lifelong exercise preserved vascular function throughout life regardless of diet, with attendant amelioration of oxidative stress and inflammation.
Supplementary Material
Key Points.
Overall, the results of the present study:
Establish the temporal pattern of age-related vascular dysfunction across the adult lifespan in sedentary mice consuming a non-Western diet, and the underlying mechanisms;
Demonstrate that consuming a Western diet accelerates and exacerbates vascular aging across the lifespan in sedentary mice;
Show that lifelong voluntary aerobic exercise has remarkable protective effects on vascular function throughout the lifespan, in the setting of aging alone, as well as aging compounded by Western diet consumption;
Indicate that amelioration of mitochondrial oxidative stress and inflammation are key mechanisms underlying the voluntary aerobic exercise-associated preservation of vascular function across the lifespan in both the presence and absence of a Western dietary pattern.
Acknowledgements:
The authors would like to thank Tom LaRocca, Alyssa Evans, and the University of Colorado Boulder laboratory animal veterinarian and staff for their study assistance.
Funding: this study was supported by grants from the National Institutes of Health (HL107120, AG000279, AG047784, DK007135, HL151022)
Footnotes
Conflict of Interest: All authors declare that they have no conflicts of interest
Data Availability: the data from this study are not publicly available but are available from the corresponding author on reasonable request.
References
- AlGhatrif M, Strait JB, Morrell CH, Canepa M, Wright J, Elango P, Scuteri A, Najjar SS, Ferrucci L and Lakatta EG (2013). Longitudinal trajectories of arterial stiffness and the role of blood pressure: the Baltimore Longitudinal Study of Aging. Hypertension 62(5): 934–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballak DB, Brunt VE, Sapinsley ZJ, Ziemba BP, Richey JJ, Zigler MC, Johnson LC, Gioscia-Ryan RA, Culp-Hill R, Eisenmesser EZ, D'Alessandro A, Dinarello CA and Seals DR (2019). Short-term interleukin-37 treatment improves vascular endothelial function, endurance exercise capacity, and whole-body glucose metabolism in old mice. Aging Cell: e13074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battson ML, Lee DM, Jarrell DK, Hou S, Ecton KE, Weir TL and Gentile CL (2018). Suppression of gut dysbiosis reverses Western diet-induced vascular dysfunction. Am J Physiol Endocrinol Metab 314(5): E468–E477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R and Sinclair DA (2006). Resveratrol improves health and survival of mice on a high-calorie diet. Nature 444(7117): 337–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shlomo Y, Spears M, Boustred C, May M, Anderson SG, Benjamin EJ, Boutouyrie P, Cameron J, Chen CH, Cruickshank JK, Hwang SJ, Lakatta EG, Laurent S, Maldonado J, Mitchell GF, Najjar SS, Newman AB, Ohishi M, Pannier B, Pereira T, Vasan RS, Shokawa T, Sutton-Tyrell K, Verbeke F, Wang KL, Webb DJ, Willum Hansen T, Zoungas S, McEniery CM, Cockcroft JR and Wilkinson IB (2014). Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta-analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol 63(7): 636–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandes RP, Fleming I and Busse R (2005). Endothelial aging."Cardiovasc Res 66(2): 286–294. [DOI] [PubMed] [Google Scholar]
- Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J and Deanfield JE (1994). Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol 24(2): 471–476. [DOI] [PubMed] [Google Scholar]
- Davidson SM and Duchen MR (2007). Endothelial mitochondria: contributing to vascular function and disease. Circ Res 100(8): 1128–1141. [DOI] [PubMed] [Google Scholar]
- Demetrius L (2005). Of mice and men. When it comes to studying ageing and the means to slow it down, mice are not just small humans. EMBO Rep 6 Spec No: S39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSouza CA, Shapiro LF, Clevenger CM, Dinenno FA, Monahan KD, Tanaka H and Seals DR (2000). Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation 102(12): 1351–1357. [DOI] [PubMed] [Google Scholar]
- Dikalova AE, Bikineyeva AT, Budzyn K, Nazarewicz RR, McCann L, Lewis W, Harrison DG and Dikalov SI (2010). Therapeutic targeting of mitochondrial superoxide in hypertension. Circ Res 107(1): 106–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato AJ, Machin DR and Lesniewski LA (2018). Mechanisms of Dysfunction in the Aging Vasculature and Role in Age-Related Disease. Circ Res 123(7): 825–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato AJ, Walker AE, Magerko KA, Bramwell RC, Black AD, Henson GD, Lawson BR, Lesniewski LA and Seals DR (2013). Life-long caloric restriction reduces oxidative stress and preserves nitric oxide bioavailability and function in arteries of old mice. Aging Cell 12(5): 772–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durrant JR, Seals DR, Connell ML, Russell MJ, Lawson BR, Folian BJ, Donato AJ and Lesniewski LA (2009). Voluntary wheel running restores endothelial function in conduit arteries of old mice: direct evidence for reduced oxidative stress, increased superoxide dismutase activity and down-regulation of NADPH oxidase. J Physiol 587(Pt 13): 3271–3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta S and Sengupta P (2016). Men and mice: Relating their ages. Life Sci 152: 244–248. [DOI] [PubMed] [Google Scholar]
- Elias MF, Robbins MA, Budge MM, Abhayaratna WP, Dore GA and Elias PK (2009). Arterial pulse wave velocity and cognition with advancing age. Hypertension 53(4): 668–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elrashidy RA, Zhang J and Liu G (2019). Long-term consumption of Western diet contributes to endothelial dysfunction and aortic remodeling in rats: Implication of Rho-kinase signaling. Clin Exp Hypertens 41(2): 174–180. [DOI] [PubMed] [Google Scholar]
- Fleenor BS, Eng JS, Sindler AL, Pham BT, Kloor JD and Seals DR (2014). Superoxide signaling in perivascular adipose tissue promotes age-related artery stiffness. Aging Cell 13(3): 576–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleenor BS, Marshall KD, Durrant JR, Lesniewski LA and Seals DR (2010). Arterial stiffening with ageing is associated with transforming growth factor-β1-related changes in adventitial collagen: reversal by aerobic exercise. J Physiol 588(Pt 20): 3971–3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleenor BS, Seals DR, Zigler ML and Sindler AL (2012). Superoxide-lowering therapy with TEMPOL reverses arterial dysfunction with aging in mice. Aging Cell 11(2): 269–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleenor BS, Sindler AL, Eng JS, Nair DP, Dodson RB and Seals DR (2012). Sodium nitrite de-stiffening of large elastic arteries with aging: role of normalization of advanced glycation end-products. Exp Gerontol 47(8): 588–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleenor BS, Sindler AL, Marvi NK, Howell KL, Zigler ML, Yoshizawa M and Seals DR (2013). Curcumin ameliorates arterial dysfunction and oxidative stress with aging. Exp Gerontol 48(2): 269–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung TT, Rimm EB, Spiegelman D, Rifai N, Tofler GH, Willett WC and Hu FB (2001). Association between dietary patterns and plasma biomarkers of obesity and cardiovascular disease risk. Am J Clin Nutr 73(1): 61–67. [DOI] [PubMed] [Google Scholar]
- Gando Y, Kawano H, Yamamoto K, Sanada K, Tanimoto M, Oh T, Ohmori Y, Miyatani M, Usui C, Takahashi E, Tabata I, Higuchi M and Miyachi M (2010). Age and cardiorespiratory fitness are associated with arterial stiffening and left ventricular remodelling. J Hum Hypertens 24(3): 197–206. [DOI] [PubMed] [Google Scholar]
- García-Prieto CF and Fernández-Alfonso MS (2016). Caloric Restriction as a Strategy to Improve Vascular Dysfunction in Metabolic Disorders. Nutrients 8(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gioscia-Ryan RA, Battson ML, Cuevas LM, Eng JS, Murphy MP and Seals DR (2018). Mitochondria-targeted antioxidant therapy with MitoQ ameliorates aortic stiffening in old mice. J Appl Physiol (1985) 124(5): 1194–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gioscia-Ryan RA, Battson ML, Cuevas LM, Zigler MC, Sindler AL and Seals DR (2016). Voluntary aerobic exercise increases arterial resilience and mitochondrial health with aging in mice. Aging (Albany NY) 8(11): 2897–2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gioscia-Ryan RA, Clayton ZS, Fleenor BS, Eng JS, Johnson LC, Rossman MJ, Zigler MC, Evans TD and Seals DR (2020). Late-life voluntary wheel running reverses age-related aortic stiffness in mice: a translational model for studying mechanisms of exercise-mediated arterial de-stiffening. Geroscience. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gioscia-Ryan RA, LaRocca TJ, Sindler AL, Zigler MC, Murphy MP and Seals DR (2014). Mitochondria-targeted antioxidant (MitoQ) ameliorates age-related arterial endothelial dysfunction in mice. J Physiol 592(12): 2549–2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh J and Ladiges W (2015). Voluntary Wheel Running in Mice. Curr Protoc Mouse Biol 5(4): 283–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy D (2015). Principles and standards for reporting animal experiments in The Journal of Physiology and Experimental Physiology. J Physiol 593(12): 2547–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Q, Wang B, Zhang XF, Ma YP, Liu JD and Wang XZ (2014). Chronic aerobic exercise training attenuates aortic stiffening and endothelial dysfunction through preserving aortic mitochondrial function in aged rats. Exp Gerontol 56: 37–44. [DOI] [PubMed] [Google Scholar]
- Hagan C (2017). When are mice considered old? Jackson Laboratories; 2020. [Google Scholar]
- Hu FB, Rimm EB, Stampfer MJ, Ascherio A, Spiegelman D and Willett WC (2000). Prospective study of major dietary patterns and risk of coronary heart disease in men. Am J Clin Nutr 72(4): 912–921. [DOI] [PubMed] [Google Scholar]
- Iqbal R, Anand S, Ounpuu S, Islam S, Zhang X, Rangarajan S, Chifamba J, Al-Hinai A, Keltai M, Yusuf S and Investigators IS (2008). Dietary patterns and the risk of acute myocardial infarction in 52 countries: results of the INTERHEART study. Circulation 118(19): 1929–1937. [DOI] [PubMed] [Google Scholar]
- Keller AC, Knaub LA, Miller MW, Birdsey N, Klemm DJ and Reusch JE (2015). Saxagliptin restores vascular mitochondrial exercise response in the Goto-Kakizaki rat. J Cardiovasc Pharmacol 65(2): 137–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluge MA, Fetterman JL and Vita JA (2013). Mitochondria and endothelial function. Circ Res 112(8): 1171–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knaub LA, McCune S, Chicco AJ, Miller M, Moore RL, Birdsey N, Lloyd MI, Villarreal J, Keller AC, Watson PA and Reusch JE (2013). Impaired response to exercise intervention in the vasculature in metabolic syndrome. Diab Vasc Dis Res 10(3): 222–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn JC, Azar J, Seta F and Reinhart-King CA (2018). High-Fat, High-Sugar Diet-Induced Subendothelial Matrix Stiffening is Mitigated by Exercise. Cardiovasc Eng Technol 9(1): 84–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer B, França LM, Zhang Y, Paes AMA, Gerdes AM and Carrillo-Sepulveda MA (2018). Western diet triggers Toll-like receptor 4 signaling-induced endothelial dysfunction in female Wistar rats. Am J Physiol Heart Circ Physiol 315(6): H1735–H1747. [DOI] [PubMed] [Google Scholar]
- La Favor JD, Anderson EJ, Dawkins JT, Hickner RC and Wingard CJ (2013). Exercise prevents Western diet-associated erectile dysfunction and coronary artery endothelial dysfunction: response to acute apocynin and sepiapterin treatment. Am J Physiol Regul Integr Comp Physiol 305(4): R423–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakatta EG (2003). Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part III: cellular and molecular clues to heart and arterial aging. Circulation 107(3): 490–497. [DOI] [PubMed] [Google Scholar]
- Lakatta EG and Levy D (2003). Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a “set up” for vascular disease. Circulation 107(1): 139–146. [DOI] [PubMed] [Google Scholar]
- Langbein H, Hofmann A, Brunssen C, Goettsch W and Morawietz H (2015). Impact of high-fat diet and voluntary running on body weight and endothelial function in LDL receptor knockout mice. Atheroscler Suppl 18: 59–66. [DOI] [PubMed] [Google Scholar]
- LaRocca TJ, Gioscia-Ryan RA, Hearon CM and Seals DR (2013). The autophagy enhancer spermidine reverses arterial aging. Mech Ageing Dev 134(7–8): 314–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRocca TJ, Hearon CM, Henson GD and Seals DR (2014). Mitochondrial quality control and age-associated arterial stiffening. Exp Gerontol 58: 78–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent P, Marenco P, Castagna O, Smulyan H, Blacher J and Safar ME (2011). Differences in central systolic blood pressure and aortic stiffness between aerobically trained and sedentary individuals. J Am Soc Hypertens 5(2): 85–93. [DOI] [PubMed] [Google Scholar]
- Lesniewski LA, Durrant JR, Connell ML, Henson GD, Black AD, Donato AJ and Seals DR (2011). Aerobic exercise reverses arterial inflammation with aging in mice. Am J Physiol Heart Circ Physiol 301(3): H1025–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesniewski LA, Zigler ML, Durrant JR, Nowlan MJ, Folian BJ, Donato AJ and Seals DR (2013). Aging compounds western diet-associated large artery endothelial dysfunction in mice: prevention by voluntary aerobic exercise. Exp Gerontol 48(11): 1218–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind L, Berglund L, Larsson A and Sundström J (2011). Endothelial function in resistance and conduit arteries and 5-year risk of cardiovascular disease. Circulation 123(14): 1545–1551. [DOI] [PubMed] [Google Scholar]
- Mercken EM, Mitchell SJ, Martin-Montalvo A, Minor RK, Almeida M, Gomes AP, Scheibye-Knudsen M, Palacios HH, Licata JJ, Zhang Y, Becker KG, Khraiwesh H, González-Reyes JA, Villalba JM, Baur JA, Elliott P, Westphal C, Vlasuk GP, Ellis JL, Sinclair DA, Bernier M and de Cabo R (2014). SRT2104 extends survival of male mice on a standard diet and preserves bone and muscle mass. Aging Cell 13(5): 787–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meziat C, Boulghobra D, Strock E, Battault S, Bornard I, Walther G and Reboul C (2019). Exercise training restores eNOS activation in the perivascular adipose tissue of obese rats: Impact on vascular function. Nitric Oxide 86: 63–67. [DOI] [PubMed] [Google Scholar]
- Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, Vita JA, Levy D and Benjamin EJ (2010). Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation 121(4): 505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell GF, Parise H, Benjamin EJ, Larson MG, Keyes MJ, Vita JA, Vasan RS and Levy D (2004). Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: the Framingham Heart Study. Hypertension 43(6): 1239–1245. [DOI] [PubMed] [Google Scholar]
- Mitchell SJ, Martin-Montalvo A, Mercken EM, Palacios HH, Ward TM, Abulwerdi G, Minor RK, Vlasuk GP, Ellis JL, Sinclair DA, Dawson J, Allison DB, Zhang Y, Becker KG, Bernier M and de Cabo R (2014). The SIRT1 activator SRT1720 extends lifespan and improves health of mice fed a standard diet. Cell Rep 6(5): 836–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau KL, Donato AJ, Seals DR, DeSouza CA and Tanaka H (2003). Regular exercise, hormone replacement therapy and the age-related decline in carotid arterial compliance in healthy women. Cardiovasc Res 57(3): 861–868. [DOI] [PubMed] [Google Scholar]
- Nowak KL, Rossman MJ, Chonchol M and Seals DR (2018). Strategies for Achieving Healthy Vascular Aging. Hypertension 71(3): 389–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Y, Booth FW, Lee S, Laye MJ and Zhang C (2012). Physical activity opposes coronary vascular dysfunction induced during high fat feeding in mice. J Physiol 590(17): 4255–4268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, Swindell WR, Kamara D, Minor RK, Perez E, Jamieson HA, Zhang Y, Dunn SR, Sharma K, Pleshko N, Woollett LA, Csiszar A, Ikeno Y, Le Couteur D, Elliott PJ, Becker KG, Navas P, Ingram DK, Wolf NS, Ungvari Z, Sinclair DA and de Cabo R (2008). Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab 8(2): 157–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce GL (2017). Aortic Stiffness in Aging and Hypertension: Prevention and Treatment with Habitual Aerobic Exercise. Curr Hypertens Rep 19(11): 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce GL, Donato AJ, LaRocca TJ, Eskurza I, Silver AE and Seals DR (2011). Habitually exercising older men do not demonstrate age-associated vascular endothelial oxidative stress. Aging Cell 10(6): 1032–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossman MJ, Gioscia-Ryan RA, Clayton ZS, Murphy MP and Seals DR (2020). Targeting mitochondrial fitness as a strategy for healthy vascular aging. Clin Sci (Lond) 134(12): 1491–1519. [DOI] [PubMed] [Google Scholar]
- Scuteri A, Brancati AM, Gianni W, Assisi A and Volpe M (2005). Arterial stiffness is an independent risk factor for cognitive impairment in the elderly: a pilot study. J Hypertens 23(6): 1211–1216. [DOI] [PubMed] [Google Scholar]
- Seals DR (2014). Edward F. Adolph Distinguished Lecture: The remarkable anti-aging effects of aerobic exercise on systemic arteries. J Appl Physiol (1985) 117(5): 425–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seals DR, Desouza CA, Donato AJ and Tanaka H (2008). Habitual exercise and arterial aging. J Appl Physiol (1985) 105(4): 1323–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seals DR, Jablonski KL and Donato AJ (2011). Aging and vascular endothelial function in humans. Clin Sci (Lond) 120(9): 357–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seals DR, Kaplon RE, Gioscia-Ryan RA and LaRocca TJ (2014). You're only as old as your arteries: translational strategies for preserving vascular endothelial function with aging. Physiology (Bethesda) 29(4): 250–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata S, Fujimoto N, Hastings JL, Carrick-Ranson G, Bhella PS, Hearon CM and Levine BD (2018). The effect of lifelong exercise frequency on arterial stiffness. J Physiol 596(14): 2783–2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sindler AL, Fleenor BS, Calvert JW, Marshall KD, Zigler ML, Lefer DJ and Seals DR (2011). Nitrite supplementation reverses vascular endothelial dysfunction and large elastic artery stiffness with aging. Aging Cell 10(3): 429–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soucy KG, Ryoo S, Benjo A, Lim HK, Gupta G, Sohi JS, Elser J, Aon MA, Nyhan D, Shoukas AA and Berkowitz DE (2006). Impaired shear stress-induced nitric oxide production through decreased NOS phosphorylation contributes to age-related vascular stiffness. J Appl Physiol (1985) 101(6): 1751–1759. [DOI] [PubMed] [Google Scholar]
- Taddei S, Virdis A, Mattei P, Ghiadoni L, Gennari A, Fasolo CB, Sudano I and Salvetti A (1995). Aging and endothelial function in normotensive subjects and patients with essential hypertension. Circulation 91(7): 1981–1987. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Dinenno FA, Monahan KD, Clevenger CM, DeSouza CA and Seals DR (2000). Aging, habitual exercise, and dynamic arterial compliance. Circulation 102(11): 1270–1275. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Palta P, Folsom AR, Meyer ML, Matsushita K, Evenson KR, Aguilar D and Heiss G (2018). Habitual physical activity and central artery stiffening in older adults: the Atherosclerosis Risk in Communities study. J Hypertens 36(9): 1889–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmis A, Townsend N, Gale CP, Torbica A, Lettino M, Petersen SE, Mossialos EA, Maggioni AP, Kazakiewicz D, May HT, De Smedt D, Flather M, Zuhlke L, Beltrame JF, Huculeci R, Tavazzi L, Hindricks G, Bax J, Casadei B, Achenbach S, Wright L, Vardas P and E. S. o. Cardiology (2020). European Society of Cardiology: Cardiovascular Disease Statistics 2019. Eur Heart J 41(1): 12–85. [DOI] [PubMed] [Google Scholar]
- Ungvari Z, Tarantini S, Donato AJ, Galvan V and Csiszar A (2018). Mechanisms of Vascular Aging. Circ Res 123(7): 849–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaitkevicius PV, Fleg JL, Engel JH, O'Connor FC, Wright JG, Lakatta LE, Yin FC and Lakatta EG (1993). “Effects of age and aerobic capacity on arterial stiffness in healthy adults.” Circulation 88(4 Pt 1): 1456–1462. [DOI] [PubMed] [Google Scholar]
- van der Loo B, Labugger R, Skepper JN, Bachschmid M, Kilo J, Powell JM, Palacios-Callender M, Erusalimsky JD, Quaschning T, Malinski T, Gygi D, Ullrich V and Lüscher TF (2000). Enhanced peroxynitrite formation is associated with vascular aging. J Exp Med 192(12): 1731–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Shay CM, Spartano NL, Stokes A, Tirschwell DL, VanWagner LB, Tsao CW and A. H. A. C. o. E. a. P. S. C. a. S. S. Subcommittee (2020). Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation 141(9): e139–e596. [DOI] [PubMed] [Google Scholar]
- Waldstein SR, Giggey PP, Thayer JF and Zonderman AB (2005). Nonlinear relations of blood pressure to cognitive function: the Baltimore Longitudinal Study of Aging. Hypertension 45(3): 374–379. [DOI] [PubMed] [Google Scholar]
- Widlansky ME and Gutterman DD (2011). Regulation of endothelial function by mitochondrial reactive oxygen species. Antioxid Redox Signal 15(6): 1517–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodman CR, Thompson MA, Turk JR and Laughlin MH (2005). Endurance exercise training improves endothelium-dependent relaxation in brachial arteries from hypercholesterolemic male pigs. J Appl Physiol (1985) 99(4): 1412–1421. [DOI] [PubMed] [Google Scholar]
- Yeboah J, Crouse JR, Hsu FC, Burke GL and Herrington DM (2007). Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: the Cardiovascular Health Study. Circulation 115(18): 2390–2397. [DOI] [PubMed] [Google Scholar]
- Yeboah J, Folsom AR, Burke GL, Johnson C, Polak JF, Post W, Lima JA, Crouse JR and Herrington DM (2009). Predictive value of brachial flow-mediated dilation for incident cardiovascular events in a population-based study: the multi-ethnic study of atherosclerosis. Circulation 120(6): 502–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusuf S, Reddy S, Ounpuu S and Anand S (2001). Global burden of cardiovascular diseases: part I: general considerations, the epidemiologic transition, risk factors, and impact of urbanization. Circulation 104(22): 2746–2753. [DOI] [PubMed] [Google Scholar]
- Zieman SJ, Melenovsky V and Kass DA (2005). Mechanisms, pathophysiology, and therapy of arterial stiffness.” Arterioscler Thromb Vasc Biol 25(5): 932–943. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


