Abstract
Objective
To categorise the variants of uncertain significance found with prenatal chromosomal microarray and determine the proportion of such variants that are associated with a well-known phenotype in order to establish how often they remain truly of uncertain significance.
Design
Retrospective cohort study.
Setting
The University of California, San Francisco.
Population
All patients with a variant of uncertain significance on prenatal microarray between 2014 and 2018.
Methods
Each variant was classified as a copy number variant that (a) contains Online Mendelian Inheritance in Man (OMIM)-annotated disease-causing genes (‘OMIM morbid genes’); (b) confers autosomal recessive carrier status; (c) is associated with incomplete penetrance; (d) is >1 Mb in size without OMIM morbid genes; (e) demonstrates mosaicism; or (f) contains significant regions of homozygosity. For each variant of uncertain significance, we examined the existing literature to determine whether the predicted phenotype(s) was known.
Main outcome measure
Prevalence and classification of variants and how much information is available regarding the likelihood of an affected phenotype.
Results
Of 970 prenatal microarrays, 55 (5.8%) had at least one variant of uncertain significance. The most common were copy number variants containing OMIM morbid genes (36.8%). In all, 48 (84.2%) were associated with a known phenotype; 55 (96.5%) had data available regarding the likelihood of an affected phenotype.
Conclusions
The prevalence of variants of uncertain significance with prenatal microarray was 5.8%. In the large majority of cases, data were available regarding the predicted phenotype.
Keywords: Microarray, prenatal, variants of uncertain significance
Tweetable abstract
Variants of uncertain significance occur in 5.8% of prenatal microarrays. In the overwhelming majority of cases, outcome information is available.
Introduction
In a 2012 study by Wapner et al., chromosomal microarray (CMA) revealed clinically relevant deletions or duplications in 6.0% of fetuses with structural anomalies identified on ultrasound who had a normal karyotype.1 As a result, the American College of Obstetricians and Gynecologists (ACOG) and the Society for Maternal-Fetal Medicine (SMFM) have recommended CMA as the initial genetic test of choice for fetuses with both ultrasound anomalies and stillbirth.2
Many providers and patients worry about potential variants of uncertain significance (VUSs), especially in the prenatal setting, when sending a CMA. These are copy number variants (CNVs) that cannot reliably be characterised as pathogenic or benign based on the current level of evidence and can cause considerable anxiety for parents.3 Women sometimes decline CMA for prenatal diagnosis due to fear of identifying a VUS. Given the limited phenotypic information provided by ultrasound, a prenatal VUS can also be considerably more difficult to interpret than a postnatal VUS. Currently, guidelines for how to categorise or report these cases only exist for postnatal microarrays.4 In the aforementioned study by Wapner et al., the incidence of VUSs was 2.5%.1 However, likely pathogenic VUSs were grouped with pathogenic results, which may underestimate the true prevalence of VUSs in the population. Other studies have described a wide variation in rates of VUSs, ranging from 0 to 6.7%, with different reporting criteria used.5-14
Furthermore, there is a considerable degree of variation among different types of VUSs, ranging from a likely carrier status of an autosomal recessive condition, to a condition that is autosomal dominant but has incomplete penetrance. Some CNVs are associated with genes known to cause genetic disorders, whereas others do not contain genes known to cause disease. For some types of VUSs, clear data exist on the potential outcomes and the probability that the fetus will be affected, whereas in others there is a paucity of information available for counselling. VUSs are therefore associated with variable degrees of uncertainty.
Our objective was to categorise different types of VUSs and determine the prevalence of each type in prenatal CMAs performed at our institution. In addition, we sought to determine the proportion of VUSs that were associated with a well-known phenotype, allowing reasonable prediction of the likelihood of an affected phenotype, with the ultimate goal to establish the proportion of VUSs that remain truly uncertain.
Methods
All patients who had prenatal CMA with a VUS reported at our centre since our clinical laboratory began offering this test in 2014 were included. The primary outcome was the proportion of VUSs that were associated with a well-known phenotype (the specific disease that manifests from an underlying genetic change) allowing reasonable prediction of the likelihood of an affected outcome (whether or not the disease will be expressed). After categorising the different types of VUSs, the secondary outcome was the prevalence of each VUS category in prenatal CMAs.
Single nucleotide polymorphism (SNP)-based CMAs have the ability to detect deletions and duplications (broadly termed copy number variants) in the genome. They may also detect regions where variants are the same on both alleles (known as regions of homozygosity or loss of heterozygosity). Of note, CMA-based genetic testing does not detect missense, nonsense or frameshift mutations; these may only be detected with sequencing-based technologies. Prenatal whole genome SNP-based cytogenomic CMAs were performed using the Illumina CytoSNP-850K Platform with genome build hg19/GRCh37 to detect genome-wide genomic CNVs and regions of homozygosity (ROH). There was no modification to the array platform or specifications during the period of the study. This array platform contains over 850 000 SNPs in 15x redundancy throughout the genome with enriched coverage for 3262 genes of known relevance in both constitutional and cancer applications. The average probe spacing across the whole array was approximately 1.8 kb. CNVs in the backbone of the genome involving less than 16 contiguous probes were not reported. Therefore, the overall effective resolution across the whole array was approximately 29.0 kb. Benign and likely benign CNVs were not reported for prenatal testing. Classification of clinical significance of CNVs was based on the ACMGG standards and guidelines; other factors may also affect these interpretations that have not been routinely considered in common laboratory guidelines.4
Data were analysed using BlueFuse software (Illumina, San Diego, CA, USA) and compared with available data in UC-Santa Cruz Genome Browser, Online Mendelian Inheritance in Man (OMIM), Database of Genomic Variants (DGV), the Database of Chromosomal Imbalance and Phenotype in Humans Using Ensemble Resources (DECIPHER), Clinical Genome Resource (ClinGen) and ClinGen Dosage Sensitivity Map.
We categorised variants as: (a) CNV that includes one or more OMIM-annotated disease-causing genes (‘OMIM morbid genes’); (b) CNV conferring autosomal recessive carrier status (‘Likely carrier’); (c) pathogenic CNV with incomplete penetrance; (d) region(s) of homozygosity (ROH) >10 Mb in interstitial regions or >3 Mb in terminal regions in chromosomes with imprinted genes or ROHs constituting >3.5% of the genome (‘Large ROH’); (e) CNV size >1 Mb without OMIM morbid genes (‘Large size’); (f) mosaic. The rationales for the VUS categories chosen are summarised in Table 1.
Table 1.
Categories of prenatal variants of uncertain significance
| Category and rationale | Specific phenotype |
Data available regarding likelihood of an affected fetus |
|---|---|---|
| CNVs with OMIM morbid genes | Variable | Variable |
| CNVs that are found in known disease-causing genes, but the particular variant has never been reported before | ||
| Likely carrier | Yes | Yes |
| In cases of autosomal recessive disorders, a deletion or duplication may be found on one allele. It is unknown whether there is a sequencing error on the other allele until sequencing testing is performed | ||
| Incomplete penetrance | Yes | Yes |
| Patients with a pathogenic variant may not express the phenotype at all, or may do so only after a certain age | ||
| Large ROH | Variable | Yes |
| ROHs can occur in the setting of ancestral relatedness, consanguinity or UPD. If there are two copies of pathogenic variants with sequencing errors in a disease associated with autosomal recessive inheritance, or if UPD occurs in a chromosome with imprinted genes, there may be an affected fetus. Our institution defines large ROHs as >10 Mb in interstitial regions or >3 Mb in terminal regions in chromosomes with imprinted genes, or ROHs constituting >3.5% of the genome | ||
| Large size | No | Yes |
| Deletions or duplications >1 Mb without any OMIM morbid genes | ||
| Mosaicism | Yes | Yes |
| Patients with mosaic CNVs tend to have a less severe phenotype than those without mosaicism |
CNV, copy number variant; ROH, region of homozygosity; UPD, uniparental disomy.
For each CNV, the specific outcome and likelihood of an affected phenotype was evaluated using OMIM, PubMed, the UCSC genome browser, ClinGen, DECIPHER and the Database of Genomic Variants.
CNVs with OMIM morbid genes
Copy number variants in this category are those that include known OMIM morbid genes but in which the particular variant has not been previously reported to be associated with the disease. One common example is when a patient has a duplication but pathogenicity has only been shown when a deletion is present. Another common scenario occurs when there is a deletion but pathogenicity has only been shown in a missense variant. In some cases, these are associated with a low risk of phenotypic abnormality (for example, full duplications are less likely to cause an affected phenotype compared with deletions, whereas partial duplications are thought to carry an increased probability of haploinsufficiency),15 but the data available regarding the likelihood of an affected phenotype are limited. Each CNV in this category, as well as the available data for the CNV, was evaluated to determine whether the likelihood of an affected phenotype could be predicted.
Likely carrier
In cases of autosomal recessive disorders, two pathogenic variants in the same gene (or different genes in cases of digenic inheritance) on two alleles are required. While CMA can detect potentially larger pathogenic deletions or duplications, it cannot resolve single nucleotide variants or deletions that are <20 kb. Therefore, prior to separate sequencing-based testing that would be recommended to rule out alterations on the second genomic copy, these variants are reported as VUSs. However, if a pathogenic variant is not present on the other allele, the carrier state is not associated with phenotypic abnormality in the vast majority of cases. We therefore categorised these as having a known phenotype, with adequate data regarding the potential phenotype and prognosis.
Incomplete penetrance
Some CNVs, or individual genes within CNVs, are known to exhibit incomplete penetrance, where not all patients who have a pathogenic variant will express the phenotype. Penetrance can also be age-dependent and some disorders may present at any point from the prenatal period to later adulthood. Even within families, penetrance can differ between individuals. For many variants with incomplete penetrance, the penetrance in the population is relatively well defined, and this can be used to counsel patients in pregnancy.
Large regions of homozygosity
A SNP microarray can detect ROHs in which the two genomic copies are identical. ROHs can occur in the setting of ancestral relatedness, consanguinity or uniparental disomy (UPD). A single large ROH or multiple ROHs of one chromosome are more likely to represent UPD and can be confirmed with methylation, microsatellite or parental microarray studies. Long, uninterrupted ROHs on multiple chromosomes are more likely due to consanguinity. Small ROHs (<4 Mb) are frequent in the population and likely represent ancestral relatedness.16 Areas of ROHs are at risk of unmasking of autosomal recessive conditions and when these are present, follow-up sequencing of particular genes may be recommended to determine whether homozygous pathogenic variants in OMIM morbid genes exist. In a study of 53 postnatal cases of patients with developmental delays and ROHs on CMA, whole exome sequencing confirmed homozygous pathogenic variants in 11.3% of cases.17
Reporting of ROHs is not standardised and reporting thresholds vary greatly between laboratories. In one study surveying 18 labs in the USA, seven reported ROH ≥10 Mb, two reported ≥5 MB and one reported ≥8 Mb in terms of threshold for reporting ROH. For multiple ROHs over the genome, three labs used a reporting threshold of >2-3% of the genome. A total of four labs used a combination of percentage and size of ROH. Our lab reports ROH ≥10 Mb on chromosomes with known imprinting-related disorders (chromosomes 6, 7, 11, 14, 15, 20) or constituting >3.5% of the genome as a VUS in prenatal cases.
We categorised ROHs as having variably known phenotypes. ROHs on chromosomes with known imprinting-related disorders often have a well-defined phenotype, whereas those constituting a large proportion of the genome do not have a well-defined phenotype. However, there was usually a large amount of data available to counsel patients about the likelihood of an affected fetus in these cases.
Large size
At our institution, CNVs without OMIM morbid genes that are <1Mb are not reported in prenatal cases; those >1 Mb are reported as VUSs. CNVs over this size threshold are generally thought to carry an increased risk of clinical significance, as CNVs >1 Mb are found in <2% of the normal population but are found in nearly 15% of patients with developmental delay, dysmorphic features or congenital anomalies.18 A population-based study has indicated that individuals with a CNV >1 Mb tend to have somewhat decreased educational attainment compared with the general population, even if they do not have a neuropsychiatric diagnosis.19 Losses of large amounts of chromosomal material can disrupt transcription of many genes and potentially more general genomic architecture, which may lead to abnormal phenotypes. CNVs that were >1Mb without OMIM morbid genes were categorised as not having a well-known phenotype; however, there data were still available to predict the likelihood of having an affected fetus.
Mosaicism
CMA can detect mosaicism above a level of approximately 10–20%, depending on the specific assay and laboratory-validated reporting standards. Patients with mosaic CNVs tend to have a less severe phenotype than those without mosaicism and the severity often increases proportionally with the level of mosaicism. However, if CMA is performed on cultured cells, there may be preferential growth of the normal cell line, and the level of mosaicism may appear to be artificially low. Follow-up testing with fluorescence in situ hybridisation (FISH) may provide a more accurate assessment of the degree of mosaicism. Given the uncertain degree of severity of the phenotype, these variants are classified as uncertain. Depending on the precise CNV, in many cases there are data available about the potential phenotype and how likely a fetus would be affected, yet some uncertainty often remains about the severity and degree of abnormality.
Known phenotype and data available in counseling
For each category, we considered whether the potential phenotypes associated with the VUS were known or uncertain. VUSs with incomplete penetrance, AR carrier status and mosaicism were considered to be associated with a well-described phenotype, whereas the others were not. We also determined the availability of data regarding the likelihood of an affected phenotype. VUSs with incomplete penetrance, likely carrier status, mosaicism and large size, had a known likelihood of having the phenotype associated with a disease. For example, in most cases of incomplete penetrance, the percentage of those affected is known and therefore the likelihood of a fetus having the phenotype was considered to be known. VUSs due to ROH are known to have a higher risk of disease, the larger the size or proportion of the genome, with available data to estimate risk. CNVs with OMIM morbid genes were individually examined to determine the data available regarding the likelihood of an affected phenotype. Appendix S1 provides specific information on each VUS: testing indication; CNV location; size and inheritance; gene and disease; category of VUS; pregnancy outcome; and categorization of the phenotype and confidence in counselling.
Trends in the number of microarrays and percentage of VUSs over time were calculated using logistic regression. Data were analysed using STATA 15.1 (Stata Statistical Software, College Station, TX, USA).
No funding was provided for this study. A core outcome set was not utilised for this study. Patients were not involved in this study.
Results
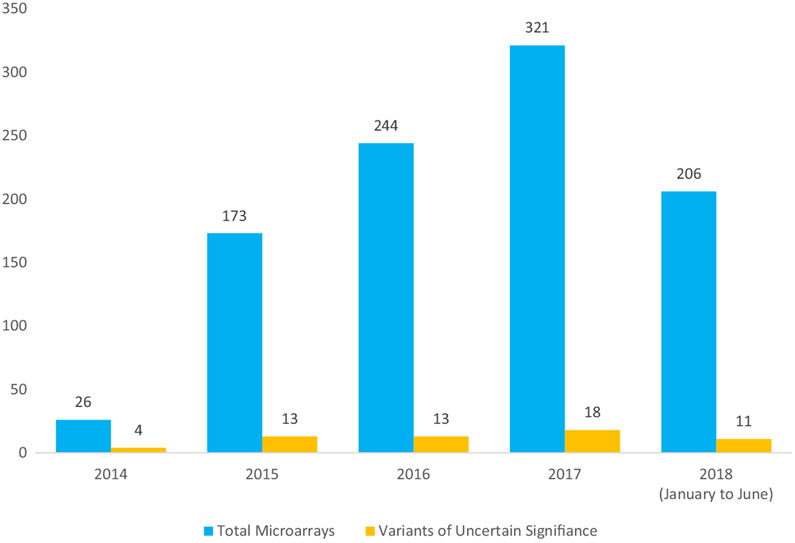
There were 970 prenatal CMAs performed at our institution in the study period. The number of CMAs and VUSs is shown in Figure 1. The total number of CMAs increased over time (26 in 2014, 173 in 2015, 244 in 2016, 321 in 2017, and 206 in the first half of 2018 (P < 0.05). Of all prenatal CMAs, 55 had a VUS for an overall rate of 5.8%. There were two patients with two VUSs reported (see Appendix S1 for a complete description of VUSs). The rate of VUSs among CMAs showed a decreasing trend over time (15.4% in 2014, 5.8% in 2015, 5.9% in 2016, 5.0% in 2017, and 4.4% in 2018, P = 0.14). This trend is likely due to ever-increasing publicly available genomic data, as well as expansion of laboratory-internal historical case data, both of which assist in interpretation.
Figure 1.
Variants of uncertain significance in prenatal chromosomal microarrays over time.
Demographic information for the results reported as a VUS are shown in Table 2. The median maternal age was 34 years, and the population was predominantly white, Asian and Hispanic. The majority (87.3%) of pregnancies were singletons. Most CMAs were performed from amniotic fluid samples (63.6%). The most common indications for CMA testing were advanced maternal age (45.5%), ultrasound abnormalities (40.0%) and abnormal serum analytes (25.5%). Of note, some patients had more than one indication for CMA. Most ultrasounds had at least one anomaly (61.8%), the most common being face/neck (18.2%) and cardiac anomalies (16.4%). Most pregnancies resulted in a full-term birth (58.2%) but 16.4% ended in a termination of pregnancy, 12.7% resulted in a preterm birth and 12.7% had an unknown outcome.
Table 2.
Patient demographics among those with a variant of uncertain significance result on prenatal microarray
| Category | Results |
|---|---|
| Maternal age, median (range) | 34 (17–45) |
| Race/ethnicity, n (%) | |
| White | 19 (34.5) |
| Asian | 13 (23.6) |
| Hispanic | 12 (21.8) |
| Black | 3 (5.5) |
| Other/unknown | 8 (14.5) |
| Number of fetuses, n (%) | |
| Singleton | 48 (87.3) |
| Twins | 7 (12.7) |
| Method of invasive testing, n (%) | |
| CVS | 20 (36.4) |
| Amniocentesis | 35 (63.6) |
| Indication for testing, n (%) | |
| AMA | 25 (45.5) |
| Ultrasound abnormalities | 22 (40.0) |
| Abnormal serum analytes | 14 (25.5) |
| Family history of genetic disorder | 12 (21.8) |
| Abnormal NIPT | 3 (5.5) |
| Other | 7 (12.7) |
| Ultrasound anomalies | |
| Normal | 21 (38.2) |
| Face/neck* | 10 (18.2) |
| Cardiac | 9 (16.4) |
| Fluid** | 8 (14.5) |
| Placental/cord*** | 5 (9.1) |
| Central nervous system | 4 (7.3) |
| Skeletal | 4 (7.3) |
| Growth restriction | 3 (5.5) |
| Pulmonary/thoracic | 2 (3.6) |
| Gastrointestinal/abdominal | 2 (3.6) |
| Genitourinary | 2 (3.6) |
| Hydrops | 1 (1.8) |
| Unknown | 3 (5.5) |
| Outcome, n (%) | |
| Full-term birth | 32 (58.2) |
| Termination of pregnancy | 9 (16.4) |
| Preterm birth | 7 (12.7) |
| Unknown | 7 (12.7) |
AMA, advanced maternal age; CVS, chorionic villous sampling; NIPT, noninvasive prenatal testing.
Includes enlarged nuchal translucency.
Includes polyhydramnios and oligohydramnios.
Includes single umbilical artery.
Each VUS was categorised as described above. The most common type of VUS was a CNV with known OMIM morbid genes (22, 38.6%), followed by carrier status (19, 33.3%) (Table 3). Each CNV with OMIM morbid genes was evaluated to identify the level of evidence available in the literature regarding outcomes; data were available regarding the likelihood of adverse outcome in 20 of 22 of these cases. Other types of VUSs were also categorised as to whether a phenotype is known and the certainty regarding prognosis, based on the category of VUS (Table 1). Ultimately, 48 (84.2%) of all VUSs were associated with a known phenotype and 55 (96.5%) had data available regarding the likelihood of an affected phenotype.
Table 3.
Categories of variants of uncertain significance in prenatal microarray performed at UCSF
| Category | Number (%) |
|---|---|
| CNV with OMIM morbid genes | 22 (38.6) |
| Likely carrier | 19 (33.3) |
| Incomplete penetrance | 7 (12.3) |
| Large ROH | 5 (8.8) |
| Large size | 3 (5.3) |
| Mosaicism | 1 (1.8) |
CNV, copy number variant; ROH, region of homozygosity.
Parental samples were available for 24 VUSs (Appendix S1): 17 CNVs with OMIM morbid genes, four that were likely carriers, two based on large size and one with incomplete penetrance. CNVs were confirmed to be inherited in 22 and de novo in two cases. In cases of a VUS with OMIM morbid genes or based on large size, those that were inherited from an unaffected parent were considered less likely to be pathogenic. In one case of a CNV with OMIM morbid genes that was considered suspicious for pathogenicity, the mother terminated the pregnancy after it was confirmed to be de novo (Case #38). Inheritance was considered not helpful in cases of a VUS due to carrier status and was more commonly already known prior to testing. In addition, inheritance was found to be paternal in one case of a VUS due to incomplete penetrance but this information was less helpful for interpretation, as a normal parent does not necessarily predict a normal offspring (Case #11). In the remaining cases, parental studies were offered but the parents did not pursue testing.
Upon chart review, three of the 19 VUSs classified as likely carriers had sequencing that confirmed carrier status. Among the CNVs categorised as likely carriers, five were likely carriers of alpha thalassaemia. Of these, four likely had the cis deletion, with at least one parent with either a normal MCV or no alpha thalassaemia variant on testing. The fifth had a homozygous deletion of HBA2, indicating the trans deletion. One VUS classified as a large ROH was later confirmed as pathogenic for Prader–-Willi syndrome after methylation studies were performed.
Discussion
Main findings
In our study population of 970 prenatal CMAs, 5.8% were categorised as a VUS, consistent with what is reported in the literature. The most common type was a CNV with OMIM morbid genes (36.8%). Among the VUSs, 84.2%% were associated with a known phenotype and the large majority (96.5%) had data available regarding the potential type and chance of an affected phenotype. In other words, although technically classified as being ‘of uncertain significance,’ in most cases a risk and prognosis could be provided to the pregnant woman about the type and chance of an abnormal outcome in her fetus.
Strengths and limitations
The strengths of our study include the large sample size and detailed assessment of each VUS. Each VUS was evaluated at the same institution using a standardised approach and a single CMA platform, which increased internal validity and the ability to more readily compare rates across time. While our study population was relatively diverse, with 34.5% white, 23.6% Asian and 21.8% Hispanic patients, the percentage of black patients (5.5%) and patients of other ethnic backgrounds was low, which may limit generalisability. All cases were analysed from one academic medical centre in one city in the USA, which also limits generalisability. The strength of one site and the use of one CMA platform make the results more readily comparable across time.
Interpretation
The aggregate prevalence of VUS results in our prenatal microarray cohort was 5.8% but this rate may decrease over time as more information regarding variants is collected and entered into publicly available databases. It is important for providers to recognise that there are different types of VUSs and that some may be resolved or clarified with further testing.
Further testing in individual cases includes parental testing, which can provide more certainty in counselling some cases of CNVs that include OMIM morbid genes or are large VUSs, but that have not been previously reported. Cases considered a VUS due to carrier status can be clarified with testing of the parents or the other allele, to assess for a second variant associated with an autosomal recessive condition. Finally, in some cases with large regions of homozygosity, testing for carrier status of genes within the region can clarify risk.
Several publicly available databases exist for CNVs, including the Database of Genomic Variants (DGV) and the Database of Chromosomal Imbalance and Phenotype in Humans Using Ensemble Resources (DECIPHER). Testing laboratories should be encouraged to deposit CNVs into these databases to increase the information that is available for accurate classification. In addition, non-European patients have historically been under-represented in genomic databases, which can affect variant interpretation. CNVs with OMIM morbid genes and large CNVs in particular will improve over time with more entries from diverse populations into these databases.
Conclusion
Whereas 5.8% of prenatal CMA tests yield a VUS, in most cases information regarding the degree of risk and the potential phenotype(s) can be provided. For patients considering CMA who are concerned about detection of a VUS, reassurance can be given that findings with truly uncertain significance are uncommon.
Supplementary Material
Appendix S1. Description of the prenatal variants of uncertain significance in the cohort.
Video S1. Author insights.
Acknowledgments
Disclosure of interests
TNS is supported by grant 5K12HD001262-18 from the National Institutes of Health (NIH). The contents of the publication are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. TNS is also supported by grants from the Fetal Health Foundation and Brianna Marie Foundation. Ultragenyx has provided financial support for studies conducted through the UCSF Center for Maternal–Fetal Precision Medicine. MEN is a consultant to Invitae and has received research funding from Natera, but this funding was not applied to this study. The other authors declare no conflicts of interest. Completed disclosure of interest forms are available to view online as supporting information.
Footnotes
Details of ethics approval
This study was approved by the Institutional Review Board at the University of California, San Francisco (No. 18-24687) on 8 May 2018.
Supporting Information
Additional supporting information may be found online in the Supporting Information section at the end of the article.
The abstract for this paper was presented in poster format (abstract #880) at the 39th Society for Maternal-Fetal Medicine Meeting, which took place from 11 to 16 February 2019 in Las Vegas, Nevada.
References
- 1.Wapner RJ, Martin CL, Levy B, Ballif BC, Eng CM, Zachary JM, et al. Chromosomal microarray versus karyotyping for prenatal diagnosis. N Engl J Med 2012;367:2175–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Committee Opinion No. 682: Microarrays and next-generation sequencing technology: the use of advanced genetic diagnostic tools in obstetrics and gynecology. Obs Gynecol 2016;128(6).e262–e268. [DOI] [PubMed] [Google Scholar]
- 3.Bernhardt BA, Soucier D, Hanson K, Savage MS, Jackson L, Wapner RJ. Women’s experiences receiving abnormal prenatal chromosomal microarray testing results. Genet Med 2013;15:139–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kearney HM, Thorland EC, Brown KK, Quintero-Rivera F, South ST. American College of Medical Genetics standards and guidelines for interpretation and reporting of postnatal constitutional copy number variants. Genet Med 2011;13:680–5. [DOI] [PubMed] [Google Scholar]
- 5.Fiorentino F, Caiazzo F, Napolitano S, Spizzichino L, Bono S, Sessa M, et al. Introducing array comparative genomic hybridization into routine prenatal diagnosis practice: a prospective study on over 1000 consecutive clinical cases. Prenat Diagn 2011;31:1270–82. [DOI] [PubMed] [Google Scholar]
- 6.Armengol L, Nevado J, Serra-Juhé C, Plaja A, Mediano C, García-Santiago FA, et al. Clinical utility of chromosomal microarray analysis in invasive prenatal diagnosis. Hum Genet 2012;131:513–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shaffer LG, Dabell MP, Fisher AJ, Coppinger J, Bandholz AM, Ellison JW, et al. Experience with microarray-based comparative genomic hybridization for prenatal diagnosis in over 5000 pregnancies. Prenat Diagn 2012;32:976–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ganesamoorthy D, Bruno DL, McGillivray G, Norris F, White SM, Adroub S, et al. Meeting the challenge of interpreting high-resolution single nucleotide polymorphism array data in prenatal diagnosis: Does increased diagnostic power outweigh the dilemma of rare variants? BJOG 2013;120:594–606. [DOI] [PubMed] [Google Scholar]
- 9.Mademont-Soler I, Morales C, Soler A, Martínez-Crespo JM, Shen Y, Margarit E, et al. Prenatal diagnosis of chromosomal abnormalities in fetuses with abnormal cardiac ultrasound findings: Evaluation of chromosomal microarray-based analysis. Ultrasound Obstet Gynecol 2013;41:375–82. [DOI] [PubMed] [Google Scholar]
- 10.Oneda B, Baldinger R, Reissmann R, Reshetnikova I, Krejci P, Masood R, et al. High-resolution chromosomal microarrays in prenatal diagnosis significantly increase diagnostic power. Prenat Diagn 2014;34:525–33. [DOI] [PubMed] [Google Scholar]
- 11.Brady PD, Delle Chiaie B, Christenhusz G, Dierickx K, Van Den Bogaert K, Menten B, et al. A prospective study of the clinical utility of prenatal chromosomal microarray analysis in fetuses with ultrasound abnormalities and an exploration of a framework for reporting unclassified variants and risk factors. Genet Med 2014;16:469–76. [DOI] [PubMed] [Google Scholar]
- 12.Klugman S, Suskin B, Spencer BL, Dar P, Bajaj K, Powers J, et al. Clinical utility of chromosomal microarray analysis in prenatal diagnosis: report of first 6 months in clinical practice. J Matern Fetal Neonatal Med 2014;27:1333–8. [DOI] [PubMed] [Google Scholar]
- 13.Coppinger J, Alliman S, Lamb AN, Torchia BS, Bejjani BA, Shaffer LG. Whole-genome microarray analysis in prenatal specimens identifies clinically significant chromosome alterations without increase in results of unclear significance compared to targeted microarray. Prenat Diagn 2009;29:1156–66. [DOI] [PubMed] [Google Scholar]
- 14.Van den Veyver IB, Patel A, Shaw CA, Pursley AN, Kang S-HL, Simovich MJ, et al. Clinical use of array comparative genomic hybridization (aCGH) for prenatal diagnosis in 300 cases. Prenat Diagn 2009;29:29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Riggs ER, Andersen EF, Cherry AM, Kantarci S, Kearney H, Patel A, et al. Technical standards for the interpretation and reporting of constitutional copy-number variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics (ACMG) and the Clinical Genome Resource (ClinGen). Genet Med 2020;22:245–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grote L, Myers M, Lovell A, Saal H, Lipscomb SK. Variability in laboratory reporting practices for regions of homozygosity indicating parental relatedness as identified by SNP microarray testing. Genet Med 2012;14:971–6. [DOI] [PubMed] [Google Scholar]
- 17.Prasad A, Sdano MA, Vanzo RJ, Mowery-Rushton PA, Serrano MA, Hensel CH, et al. Clinical utility of exome sequencing in individuals with large homozygous regions detected by chromosomal microarray analysis. BMC Med Genet 2018;19:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cooper GM, Mefford HC. Detection of copy number variation using SNP genotyping. Methods Mol Biol 2011;767:243–52. [DOI] [PubMed] [Google Scholar]
- 19.Männik K, Mägi R, Macé A, Cole B, Guyatt AL, Shihab HA, et al. Copy number variations and cognitive phenotypes in unselected populations. JAMA 2015;313:2044–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Description of the prenatal variants of uncertain significance in the cohort.
Video S1. Author insights.