Abstract
Ongoing activity in nociceptors, a driver of spontaneous pain, can be generated in dorsal root ganglion neurons in the absence of sensory generator potentials if one or more of three neurophysiological alterations occur - prolonged depolarization of resting membrane potential (RMP), hyperpolarization of action potential (AP) threshold, and/or increased amplitude of depolarizing spontaneous fluctuations of membrane potential (DSFs) to bridge the gap between RMP and AP threshold. Previous work showed that acute, sustained exposure to serotonin (5-HT) hyperpolarized AP threshold and potentiated DSFs, leading to ongoing activity if a separate source of maintained depolarization was present. Cellular signaling pathways that increase DSF amplitude and promote ongoing activity acutely in nociceptors are not known for any neuromodulator. Here, isolated DRG neurons from male rats were used to define the pathway by which low concentrations of 5-HT enhance DSFs, hyperpolarize AP threshold, and promote ongoing activity. A selective 5-HT4 receptor antagonist blocked these 5-HT-induced hyperexcitable effects, while a selective 5-HT4 agonist mimicked the effects of 5-HT. Inhibition of cAMP effectors, protein kinase A (PKA) and exchange protein activated by cAMP (EPAC), attenuated 5-HT’s hyperexcitable effects, but a blocker of hyperpolarization-activated cyclic nucleotide-gated (HCN) channels had no significant effect. 5-HT4-dependent PKA activation was specific to DRG neurons that bind isolectin B4 (a nonpeptidergic nociceptor marker). 5-HT’s effects on AP threshold, DSFs, and ongoing activity were mimicked by a cAMP analog. Sustained exposure to 5-HT promotes ongoing activity in nonpeptidergic nociceptors through the Gs-coupled 5-HT4 receptor and downstream cAMP signaling involving both PKA and EPAC.
Keywords: Nociceptor, ongoing pain, serotonin, 5-HT4 receptor, cAMP, hyperexcitability
Graphical Abstract
1. Introduction
Ongoing (apparently spontaneous) pain at rest is a major complaint of patients suffering from many forms of acute and chronic pain. The finding that activation of even a few human nociceptors in vivo evokes immediate pain (Nagi et al., 2019; Ochoa and Torebjörk, 1989) and the high incidence of ongoing activity (OA, often referred to as spontaneous activity) in C-fiber nociceptors in microneurographic recordings from patients with ongoing neuropathic pain (Kleggetveit et al., 2012; Serra et al., 2012) indicate that a major driver of ongoing human pain is OA in nociceptors. Electrophysiological recordings from single dorsal root ganglion (DRG) neurons dissociated from human patients (North et al., 2019) and recordings from rodent nociceptors in preclinical neuropathic, inflammatory, and incisional pain models (Bedi et al., 2010; Djouhri et al., 2006; Xu and Brennan, 2010) also demonstrate an increased incidence of OA in DRG neurons linked to ongoing pain. The occurrence of pain-related OA in dissociated nociceptors in vitro that is similar in pattern and incidence to that occurring in vivo during persistent neuropathic pain (Bedi et al., 2010) provides a special opportunity to define the cellular mechanisms in nociceptors that enable their ongoing and spontaneous activity, thereby promoting ongoing pain.
As illustrated by our findings in a model of neuropathic pain (Odem et al., 2018), three basic neurophysiological alterations can generate OA in nociceptors in the absence of sensory generator potentials - prolonged depolarization of the resting membrane potential (RMP), hyperpolarization of the action potential (AP) threshold, and an increase in the incidence of large depolarizing spontaneous fluctuations (DSFs) of membrane potential that can intermittently bridge the gap between RMP and AP threshold. Large DSFs are prominent in neurons with OA, and they play a major role in controlling the low firing rate and irregular pattern of OA observed in DRG neurons dissociated in animal models of persistent pain, including chronic constriction injury of the sciatic nerve (Study and Kral, 1996), spinal cord injury (Berkey et al., 2020; Odem et al., 2018), and treatment with the chemotherapeutic cisplatin (Laumet et al., 2020), as well as neurons dissociated from humans with neuropathic pain (R.Y. North, M.A. Odem, P.M. Dougherty, E.T. Walters, unpublished observations). While cell signaling contributions to persistent OA after spinal cord injury (SCI) have been described (Bavencoffe et al., 2016; Berkey et al., 2020; Garza Carbajal et al., 2020), nothing is known about cell signaling mechanisms that may enhance DSFs and promote OA acutely.
OA can be enhanced acutely (5–60 min) in the absence of prior neuropathy by the inflammatory mediator and neuromodulator serotonin (5-hydroxytryptamine, 5-HT) via decreased AP threshold and increased DSF amplitude under experimentally depolarized conditions (Odem et al., 2018). This study found no effect of 5-HT on RMP. Thus, 5-HT application combined with a source of depolarization (to −45 mV, within the physiological range) for 30–60 s provides a useful model for defining cell signaling mechanisms by which OA in nociceptors is enhanced acutely. While 5-HT-stimulated cell signaling that may lower AP threshold in nociceptors has been explored (Cardenas et al., 2001; Gold et al., 1996), cell signaling pathways that enhance DSFs and OA acutely after any kind of stimulation have not been described.
Serotonin was linked to pain and nociceptor hyperexcitability in a number of previous studies. Intradermal application of 5-HT produces hyperalgesia in mice and rats (Lin et al., 2011; Taiwo and Levine, 1992) and pain in humans (Schmelz et al., 2003). Serotonin application to dissociated DRG neurons can decrease the current needed to elicit an AP, increase the AP amplitude (Cardenas et al., 2001), and increase AP firing frequency during depolarizing current steps (Salzer et al., 2016). Several studies of 5-HT effects on nociceptor excitability have examined the effects of micromolar concentrations, often using relatively fast perfusion. Because exposure of nociceptor somata to inflammatory mediators such as 5-HT is likely to occur through prolonged exposure to low concentrations in the blood and/or cerebrospinal fluid (CSF), it is important to also examine the effects of relatively low concentrations over longer time periods.
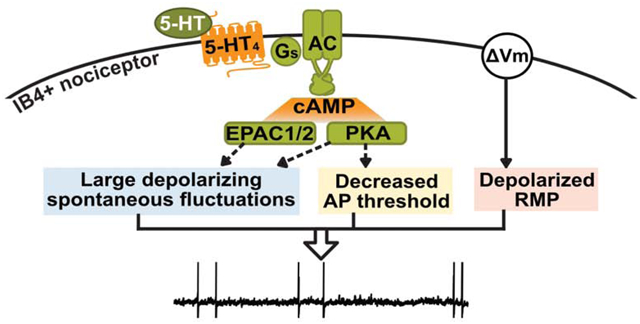
Multiple signaling pathways have been implicated in 5-HT effects on nociceptor excitability and pain, potentially linked to numerous 5-HT receptors expressed in the DRG, including those coupled to Gs, Gi, and Gq, as well as 5-HT-gated cation channels (Chen et al., 1998; Lin et al., 2011; Nicholson et al., 2003; Ohta et al., 2006). Our objective was to define the predominant cell signaling mechanism by which 5-HT enhances DSFs and associated OA. Because 5-HT is known to strongly activate protein kinase A (PKA) in nociceptors (Isensee et al., 2017) and our previous studies linked activity of PKA and exchange protein activated by cAMP (EPAC) to chronic pain-related OA (Bavencoffe et al., 2016; Berkey et al., 2020), we hypothesized that Gs-coupled, 5-HT receptor (5-HTR)-induced cAMP signaling mediates the enhancement of DSFs, reduction of AP threshold, and consequent potentiation of OA by 5-HT. Combining electrophysiology, automated DSF analysis, high content microscopy, and Ca2+ imaging, we tested this hypothesis and defined cAMP signaling via PKA and EPAC downstream of the 5-HT4 receptor as a critical signaling pathway mediating acute enhancement of OA by 5-HT.
2. Materials and Methods
2.1. Animals
All procedures were in accordance with the guidelines of the International Association for the Study of Pain and were approved by the Institutional Animal Care and Use Committee. Male Sprague-Dawley rats (200–300 g, 2 per cage) were maintained in the McGovern Medical School animal research facility under a 12:12 h reversed light/dark cycle, and experiments were performed during the dark phase. Rats had access to food and water ad libitum.
2.2. Dissociation and culture of DRG neurons
Rats were euthanized by intraperitoneal injection of pentobarbital/phenytoin (0.9 mL; Euthasol, Virbac AH, Inc) followed by transcardial perfusion of ice-cold phosphate-buffered saline (PBS, Sigma-Aldrich). DRG from spinal levels T8 to L6 were excised and digested with trypsin (0.3 mg/mL, Worthington) and collagenase D (1.4 mg/mL, Sigma-Aldrich) in Dulbecco’s Modified Eagle Medium (DMEM; Sigma-Aldrich) for 40 min at 34°C. Digested DRG were washed twice with warm (37°C) DMEM followed by mechanical trituration in DMEM with fire-polished Pasteur pipettes. Dissociated cells were seeded on glass coverslips (for electrophysiological recordings or Ca2+ imaging) or a 96-well cell culture microplate (for high content microscopy) coated with poly-L-ornithine solution (0.01%). Cell cultures were incubated overnight (5% CO2, 95% humidity, 37°C) in DMEM without serum, growth factors, or other supplements, and experiments were performed 18–28 h post-dissociation.
2.3. Whole-cell recordings from dissociated DRG neurons
Small- to medium-sized DRG neurons (soma diameter ≤30 μm and input capacitance ≤45 pF) that were not in visible contact with other neurons or debris were recorded in whole-cell configuration at room temperature using either a Zeiss Axiovert 200M with 40X magnification and a HEKA EPC10 amplifier or an Olympus IX-71 with 40X magnification and a MultiClamp 700B amplifier (Molecular Devices). Patch pipettes were pulled from borosilicate glass capillaries with a 1.5 mm outer diameter and 0.86 mm inner diameter (Sutter Instrument Co) using a Sutter P-97 Flaming/Brown Micropipette Puller followed by polishing. Patch pipettes were filled with an intracellular-like solution (ICS; in mM: 134 KCl, 1.6 MgCl2, 13.2 NaCl, 3 EGTA, 9 HEPES, 4 Mg-ATP, and 0.3 Na-GTP, adjusted to pH 7.2 with KOH and osmolarity 300 with sucrose) and had an electrode resistance of 2–10 MΩ. The extracellular solution (ECS) contained (in mM): 140 NaCl, 3 KCl, 1.8 CaCl2, 2 MgCl2, 10 HEPES, and 10 glucose and was adjusted to pH 7.4 with NaOH and osmolarity 320 with sucrose. After forming a tight seal, the membrane was ruptured to establish whole-cell configuration under voltage clamp, and membrane resistance and input capacitance were measured. The liquid junction potential (~4.3 mV) was not corrected.
To measure OA at rest, neurons were recorded in current clamp with no current injection for a minimum of 1 min. Next, current was injected to maintain the membrane potential at approximately −60 mV while a series of 2-ms depolarizing current injections (200 ms sweep interval, +10 pA or +20 pA increments) were used to measure AP voltage threshold and other AP properties. This was followed by a series of 2-s depolarizing current injections (4-s sweep intervals, increments of +5 pA, +10 pA, or +20 pA) to measure rheobase and to provide an additional measure of AP voltage threshold. Each of these procedures yields consistent estimates of AP voltage threshold (Odem et al., 2018); in this study our primary measure was the AP threshold determined with 2-s pulses. To measure OA at −45 mV, current was injected to maintain a membrane potential of approximately −45 mV for at least 30 s. DSFs were quantified using our algorithm SFA.py coded in Python v3.5.2 (Python Software Foundation), as described previously (Odem et al., 2018), from the 30-s traces recorded at −45 mV. In addition to subthreshold DSFs identified by the program, suprathreshold DSFs were included, and the amplitude was calculated as the difference between the start membrane potential of the event and the AP threshold. DSFs were not quantified from cells exhibiting firing frequencies above ~1.5 Hz.
For neurons treated with 5-HT (serotonin hydrochloride, Abcam and Sigma-Aldrich) or prucalopride (Sigma-Aldrich), recordings were made from cells that had been pre-exposed to the drug (at least 5 min), and the drug was continuously present during the recordings (up to 60 min). There was no apparent change in the 5-HT effects on the properties measured over this period. To test the effects of 5-HT receptor antagonists, PKA inhibitors, EPAC inhibitors, or a hyperpolarization-activated cyclic-nucleotide-gated channel (HCN) inhibitor, coverslips were incubated at 37°C in ECS containing GR113808, ketanserin-tartrate salt, myristoylated PKI 14–22 amide (all from Sigma-Aldrich), RS127445, granisetron, SB269970, CE3F4, H-89, ZD7288 (all from Cayman Chemical), or ESI-05 (synthesized as described by Chen et al., 2013) for a minimum of 5 min before being transferred to the recording chamber containing the inhibitor plus 5-HT in ECS. With the exception of 8-Br-cAMP, all drugs were added to the ECS. The chambers were not continuously perfused. 8-Br-cAMP (Sigma-Aldrich) was diluted in ICS for intracellular delivery via the patch pipette (resistance of 2–6 MΩ). For intracellular dialysis of 8-Br-cAMP, after obtaining whole-cell configuration, the cell was maintained at −60 mV under voltage clamp for at least 3 min before switching to current clamp and proceeding with data collection. All data shown are from non-accommodating (NA) type neurons, excluding the rapidly accommodating (RA) type neurons (Odem et al., 2018).
2.4. High content microscopy and subpopulation analysis
The following materials were used. Primary antibodies: chicken anti-PGP9.5 (1:4000, Novus Biologicals, # NB110–58872), rabbit monoclonal anti-phospho RII (S99) (1:1000, clone 151, Abcam, # ab32390), and mouse anti-CGRP (1:1000, Santacruz #SC-57053). Secondary antibodies (all 1:1000): goat anti-chicken-DyLight 755, goat anti-rabbit 568, donkey anti-mouse AF 647. Isolectin B4-FITC (1:1500, MilliporeSigma, #L2895) and DAPI.
Following pharmacological treatments at 37°C, cells were fixed with 4% paraformaldehyde for 10 min then washed with PBS. Cells were then blocked for one h at room temperature with blocking solution containing 1% bovine serum albumin and 0.075% Triton X-100 in PBS followed by incubation with primary antibodies in blocking solution at 4°C overnight. After washing, cells were incubated with secondary antibodies and DAPI in blocking solution or with isolectin B4 (IB4) in IB4 buffer (100 μM each MgCl2, CaCl2, and MnCl2 in PBS) at room temperature in the dark for one h followed by three final PBS washes.
Plates were imaged using a Cellomics CX5 microscope (Thermo Scientific) with a 10x objective following modified protocols described by Isensee et al., 2018, 2017, 2014. Cellomics software package (Thermo Scientific) was used to analyze 1104×1104 pixel images. Neurons were identified by PGP 9.5 staining intensity. When appropriate, bleed through between channels was compensated with raw fluorescence data from fluorescence controls using the slope determined by linear regression (Prism, GraphPad) as described by Roederer, 2002.
One- and two-dimensional density plots were generated using FlowJo (Becton Dickinson). Gating of subpopulations, based on neuronal soma area, CGRP expression, and IB4 binding as established previously by Garza Carbajal et al., 2020, was performed by setting thresholds at local minima of probability in 2D plots and corroborated by 3D cluster analysis. Individual cells used to perform the cluster analysis were normalized between the minimal (0.001%) and maximal (0.999%) fluorescence levels per channel. Cluster analysis (k-medians) was performed using Cluster 3.0 software (de Hoon et al., 2004). Three-dimensional (3D) plots were constructed using Plotly (Chart Studio). Data analysis and graph plots were performed using Prism.
2.5. Calcium imaging
Overnight cultured DRG neurons were loaded with 2 μM Fura-2AM (TEFLabs.com) in DMEM in the cell culture incubator for 1 h. Neurons on coverslips were placed in a diamond-shaped RC-40 low profile bath chamber on a QE-1 quick exchange platform (both from Warner Instruments) and were constantly perfused with the same ECS used for electrophysiological recording. Images were acquired on a live cell fluorescence system, including Nikon Eclipse TE2000-U microscope with 40X oil objective lens and a Sutter Instrument Lambda DG-4 ultra-high-speed wavelength switching system controlled by SlideBook software v5.0. Fura-2 fluorescence signals were excited alternately at 340 nm and 380 nm every 3 s and detected at 510 nm emission. 5-HT (300 nM or 10 μM) or capsaicin (500 nM, Sigma-Aldrich) diluted in ECS or KCl (60 mM, made by substituting 57 mM NaCl from ECS with equimolar KCl) was applied to neurons through whole chamber perfusion by a peristaltic pump at a flow rate of approximately 2 ml/min. 5-HT was applied 90–120 s following 2 min baseline recording. Capsaicin was applied following 5-HT stimulation to identify capsaicin-sensitive nociceptors. KCl was applied at the end of each recording to confirm responses from live neurons. Fura-2 fluorescence ratios (F340/F380) were analyzed using SlideBook to estimate intracellular Ca2+ concentration ([Ca2+]i) changes.
2.6. Statistical analysis
All data sets were tested for normality with the Shapiro-Wilk and D’Agostino and Pearson tests. Normally distributed data were analyzed by parametric tests: t-test or 1- or 2-way ANOVA followed by Dunnett’s or Tukey’s multiple comparisons tests using Prism v8 (GraphPad Software, Inc). Non-normally distributed data were analyzed by non-parametric tests: Mann-Whitney or Kruskal-Wallis (GraphPad Software, Inc). If any data set within an analysis failed to pass either normality test, non-parametric statistics were performed. Comparisons of incidences were made with Fisher’s exact test. All tests were two-tailed, with one exception. The dose-response relationship for 5-HT and OA was tested with a one-tailed Fisher’s exact test on the basis of prior observations indicating that the incidence of OA, which is low under our control conditions, would be increased but not decreased by 5-HT treatment (Odem et al., 2019 and unpublished observations). All t-tests were unpaired unless otherwise stated. Dose-response curves were fitted using Prism v8.
3. Results
3.1. Dose-response relationship of the effects of 5-HT on hyperexcitability properties
We found previously that prolonged exposure (~5–60 min) of dissociated DRG neurons to 100 nM 5-HT promoted OA by increasing DSF amplitude and decreasing AP voltage threshold. The OA occurred under artificially depolarized conditions (−45 mV) in small- and medium-sized (< 30 μm) NA neurons (Odem et al., 2018). We have now examined the dose-response relationships of 5-HT’s effects on functionally important excitability properties under the same conditions. Consistent with earlier observations that low and high doses of 5-HT enhance a voltage-gated Na+ current (Cardenas et al., 1997a, 2001; Gold et al., 1996) and with our earlier observations (Odem et al., 2018), prolonged 5-HT treatment hyperpolarized the AP voltage threshold (Fig. 1A; 1-way ANOVA, F(6,76) = 2.28, p = 0.045) at 10 nM and 100 nM compared to the vehicle control. Also consistent with our preceding study (Odem et al., 2018), 5-HT increased the incidence of medium-(3–5 mV) and large-amplitude (>5 mV) DSFs recorded while the membrane potential was held at approximately −45 mV under current clamp (Fig. 1B; Kruskal-Wallis, p = 0.0024 and p = 0.018, respectively) at concentrations of 10 nM and 100 nM. The greatest increase in the incidence of OA at −45 mV occurred at 100 nM 5-HT (Fig. 1C; 1-sided Fisher’s exact test, p = 0.005) with a mean AP firing frequency of 0.28 Hz under these conditions (data not shown). There were trends toward increased OA incidence with concentrations as low as 10 nM (p = 0.065) and as high as 10 μM 5-HT (p = 0.11). Furthermore, there was an overall effect of 5-HT across all doses in reducing the rheobase during 2-s depolarizing test pulses, although individual doses showed no significant effects (Fig. 1D; Kruskal-Wallis, p = 0.048). None of the tested 5-HT concentrations produced significant effects on either RMP (Fig. 1A; 1-way ANOVA, F(6,76) = 1.61, p = 0.16) or OA recorded at RMP (Fig. 1C). These results show that OA and two of its major electrophysiological mechanisms (increased DSF amplitude and decreased AP voltage threshold) in probable nociceptors are sensitive to relatively low concentrations of 5-HT. In addition, the results confirm that, while these 5-HT concentrations enhance AP generation by at least two general electrophysiological alterations, this enhancement is only expressed when the membrane potential is maintained at a relatively depolarized level within the physiological range.
Fig. 1. Dose-response effects of 5-HT on RMP, AP threshold, DSFs, and OA.
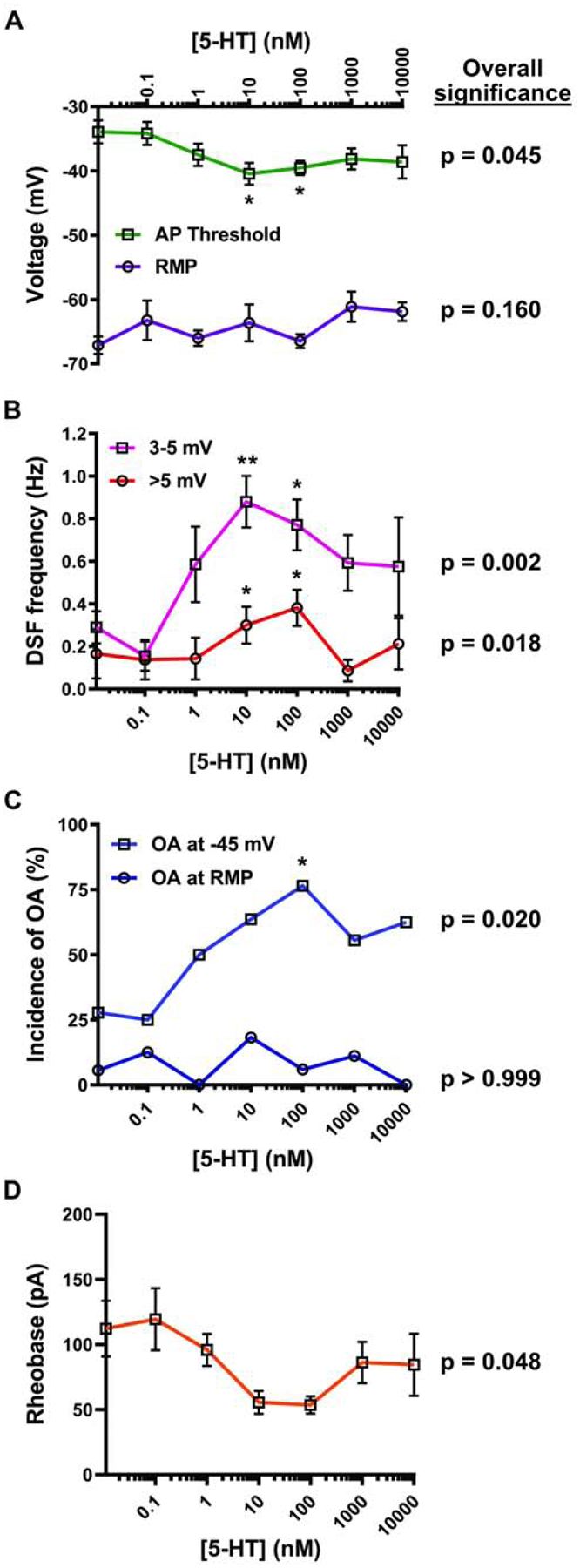
DRG neurons were treated with 5-HT or vehicle before and during recording. Each concentration is represented by a minimum of 8 cells from at least 2 rats. P values listed under overall significance indicate the result of ANOVA, Kruskal-Wallis, or Fisher’s exact tests assessing the presence of an overall effect across all doses. (A) 5-HT effects on RMP and AP threshold. Mean ± SEM. * Dunnett’s adjusted p value < 0.05. (B) 5-HT effects on the frequency of medium-amplitude (3–5 mV) and large-amplitude (>5 mV) DSFs during 30-s depolarization to −45 mV. Mean ± SEM. * Dunn’s adjusted p value < 0.05, ** p < 0.01. (C) 5-HT effects on the incidence of OA measured during a ≥ 60-s recording at RMP and then during a 30-s depolarization to −45 mV. P values indicate the result of Fisher’s exact tests comparing no 5-HT to all 5-HT concentrations combined. * p < 0.0083 (after Bonferroni correction for 6 comparisons). (D) 5-HT effects on rheobase. Mean ± SEM. Kruskal-Wallis test revealed a significant overall difference, but Dunn’s test did not reveal differences at individual doses.
3.2. 5-HT4 receptors are required for potentiation of DSFs and OA induced by 5-HT in probable nociceptors
To begin to elucidate the cell signaling pathways by which 5-HT modulates DSFs and AP threshold, and their effects on OA, we asked which 5-HT receptors are required. Selective antagonists of 5-HT receptors known to be expressed in the DRG were added to the 5-HT solution. The incidence of OA recorded at −45 mV in the presence of 100–300 nM 5-HT was blocked by the selective 5-HT4 receptor antagonist GR113808 (Fig. 2A, B; Fisher’s exact test, p = 0.0002) but not by the other 5-HT receptor antagonists tested. GR113808 reduced the frequencies of medium-amplitude (3–5 mV; Kruskal-Wallis, p = 0.01) and large-amplitude (>5 mV; Kruskal-Wallis, p = 0.0007) DSFs at −45 mV during exposure to 5-HT (Fig. 2C). Additionally, treatment with GR113808 resulted in a more depolarized AP threshold in the presence of 5-HT compared with vehicle/5-HT (Fig. 2D; Kruskal-Wallis, p = 0.024). Moreover, GR113808 prevented reduction of the rheobase by 5-HT (Fig. 2E; Kruskal-Wallis, p = 0.0099). Selective antagonists of 5-HT2A/2C (ketanserin), 5-HT2B (RS127445) or 5-HT3 (granisetron) had no effect on any of these measures (Fig. 2A, C, D), and there was no significant effect on RMP by any of the agents tested (data not shown).
Fig. 2. 5-HT4 receptor is required for potentiation of DSFs and OA by 5-HT.
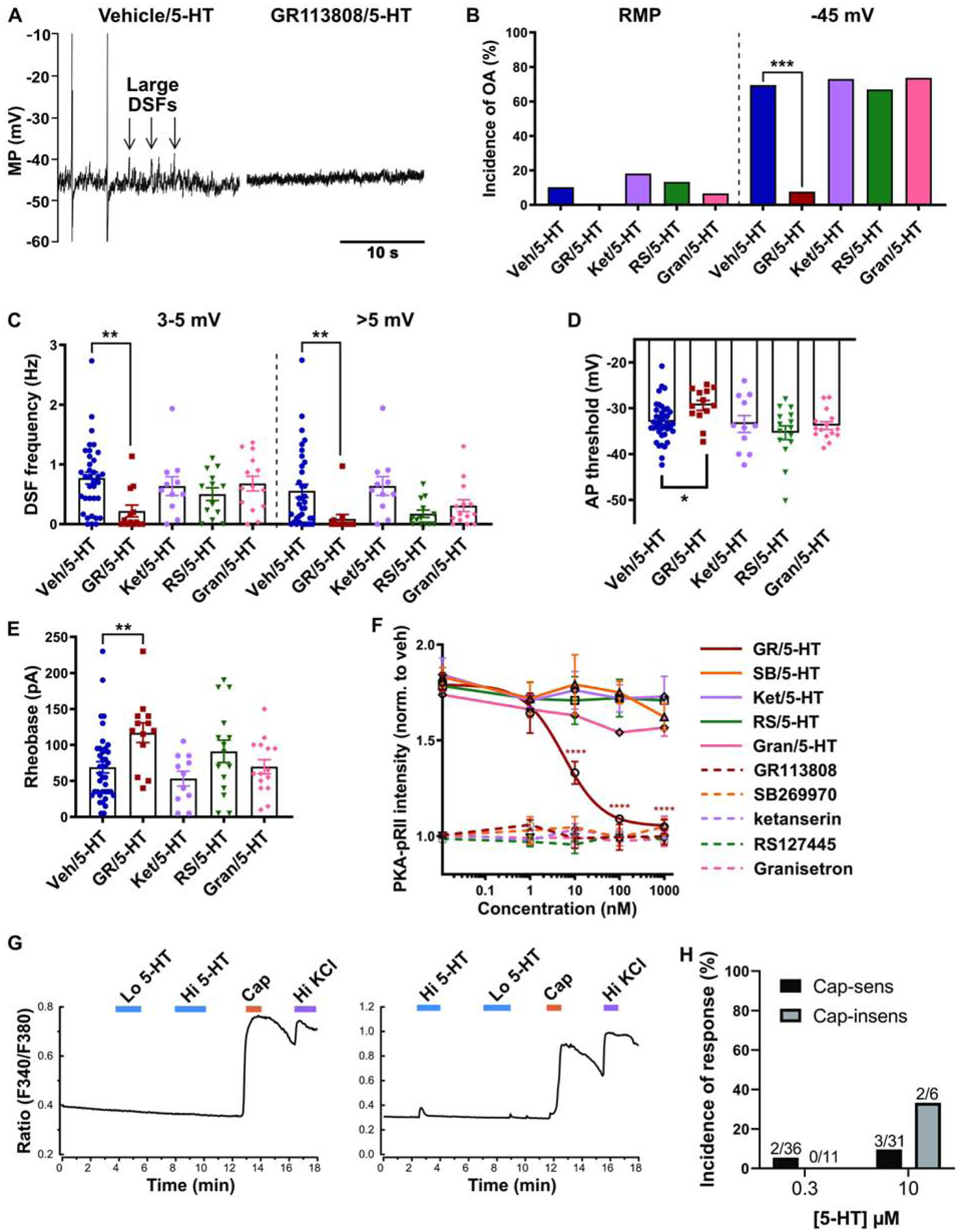
For electrophysiological recordings, cells were pre-treated for 5–15 min with 1 μM of the 5-HTR antagonist or vehicle control before cells were patched in the presence of the antagonist plus 100–300 nM 5-HT. (A) Representative recordings at −45 mV in different neurons exposed to vehicle/5-HT or GR113808/5-HT. Note the two APs (OA) and large DSFs in the left panel. MP = membrane potential. (B) 5-HTR antagonist effects on the incidence of OA during exposure to 5-HT. OA was measured at RMP for ≥60 s and then during depolarization to −45 mV for 30 s. *** p < 0.00025 (after Bonferroni correction for 4 comparisons). (C-E) 5-HTR antagonist effects on the frequency of medium- (3–5 mV) and large-amplitude (> 5 mV) DSFs during 30-s depolarization to −45 mV (C), AP threshold (D), and rheobase (E) in the presence of 5-HT. Mean ± SEM. * Dunn’s adjusted p value < 0.05. ** p < 0.01. (F) PKA activation measured by PKA-pRII staining in rat DRG neurons. Different concentrations of antagonists were applied 30 min prior to 5-min co-application of the antagonist and 100 nM 5-HT. Each data point represents the mean ± SEM of 3–5 separate experiments. **** Dunnett’s adjusted p value < 0.0001. (G) Representative traces of Fura-2 ratio changes used to indicate increases in [Ca2+]i during superfusion of 300 nM or 10 μM 5-HT in single capsaicin-sensitive neurons. (H) Incidence of 5-HT-associated increases in [Ca2+]i as measured with Fura-2. Cap-sens = capsaicin sensitive. Cap-insens = capsaicin-insensitive. Lo 5-HT = 300 nM 5-HT. Hi 5-HT = 10 μM 5-HT. Cap = 500 nM capsaicin. Hi KCl = 60 mM KCl.
In high-content imaging studies using PKA-pRII as a surrogate readout of PKA activation (Isensee et al., 2014), the selective 5-HT4 antagonist GR113808 blocked PKA activation in response to 100 nM 5-HT in a dose-dependent manner (Fig. 2F; IC50 = 5.55 nM; 2-way ANOVA, F(16,63) = 3.18, p = 0.0005), confirming a previous report (Isensee et al., 2017). Surprisingly, although there is evidence for expression of the Gs-coupled 5-HT7 receptor in rodent DRG neurons (Chen et al., 1998; Lin et al., 2011; Ohta et al., 2006), a selective antagonist of 5-HT7 (SB269970) had no appreciable effect on PKA activation by 5-HT (Fig. 2F). As expected, 5-HT2A/2C (ketanserin), 5-HT2B (RS127445) and 5-HT3 (granisetron) antagonists had no significant effect on PKA activation (Fig. 2F). These results suggest that, at these 5-HT concentrations, the Gs-coupled 5-HT7 receptor does not play a role in PKA activation in primary DRG neurons, and the Gq-coupled receptors (5-HT2) and 5-HT-gated cation channels (5-HT3) do not alter PKA activation produced by activation of the 5-HT4 receptor.
To further address whether Gq-coupled 5-HT2 receptors might contribute to nociceptor responses to 5-HT under our potentially desensitizing electrophysiological testing conditions (relatively slow delivery and continuing exposure to 5-HT for at least several minutes), we measured Ca2+ responses to 5-HT in probable nociceptors using Fura-2. Example recordings from a neuron that failed to respond to 90–120 s superfusion with either 300 nM or 10 μM 5-HT and another neuron that showed a weak response to the higher concentration are shown in Fig. 2G. Only 5.6% (2/36) and 9.7% (3/31) of neurons responsive to capsaicin (i.e. probable nociceptors) showed detectable increases in [Ca2+]i during superfusion with 300 nM or 10 μM 5-HT, respectively (Fig. 2H). Among the capsaicin-insensitive neurons, 0% (0/11) and 33.3% (2/6) showed an increase in [Ca2+]i in response to 300 nM or 10 μM 5-HT, respectively. This suggests that increasing 5-HT concentration relatively slowly (over tens of seconds under our superfusion conditions) elicits Ca2+ responses in only a small subset of DRG neurons (4.3 and 13% of all neurons tested in 0.3 and 10 μM 5-HT, respectively). Taken together, these data indicate that DSFs and associated OA are enhanced in a sustained manner by relatively low concentrations of 5-HT that fail to evoke Ca2+ responses in most of the neurons sampled, and these effects are mediated primarily by the Gs-coupled 5-HT4 receptor.
3.3. 5-HT4 activation can induce hyperexcitability in probable nociceptors
Is selective activation of the 5-HT4 receptor sufficient to enhance DSFs and promote OA in small DRG neurons? To answer this question, we used a selective 5-HT4 agonist, prucalopride, and compared the dose dependence of its activation of PKA to the activation of PKA by 5-HT, along with the inhibition of these effects by an effective dose of the 5-HT4 receptor antagonist GR113808 (Fig. 3A). Activation of PKA by prucalopride and 5-HT exhibited a similar dose dependence, with an EC50 of 4.53 (95% confidence interval (CI) = 1.78 – 11.45, R2 = 0.89) for 5-HT and 10.23 nM (95% CI = 4.06 – 25.61, R2 = 0.89) for prucalopride. A 2-way ANOVA revealed significant overall effects of dose (F(5,48) = 38.83, p < 0.0001) and drug (F(3,48) = 56.32, p < 0.0001). Importantly, GR113808 (1 μM) significantly reduced PKA activation by both 5-HT and prucalopride at all doses ≥ 10 nM and ≥ 100 nM, respectively (Fig. 3A).
Figure 3. Selective 5-HT4 receptor activation induces hyperexcitability in probable nociceptors.
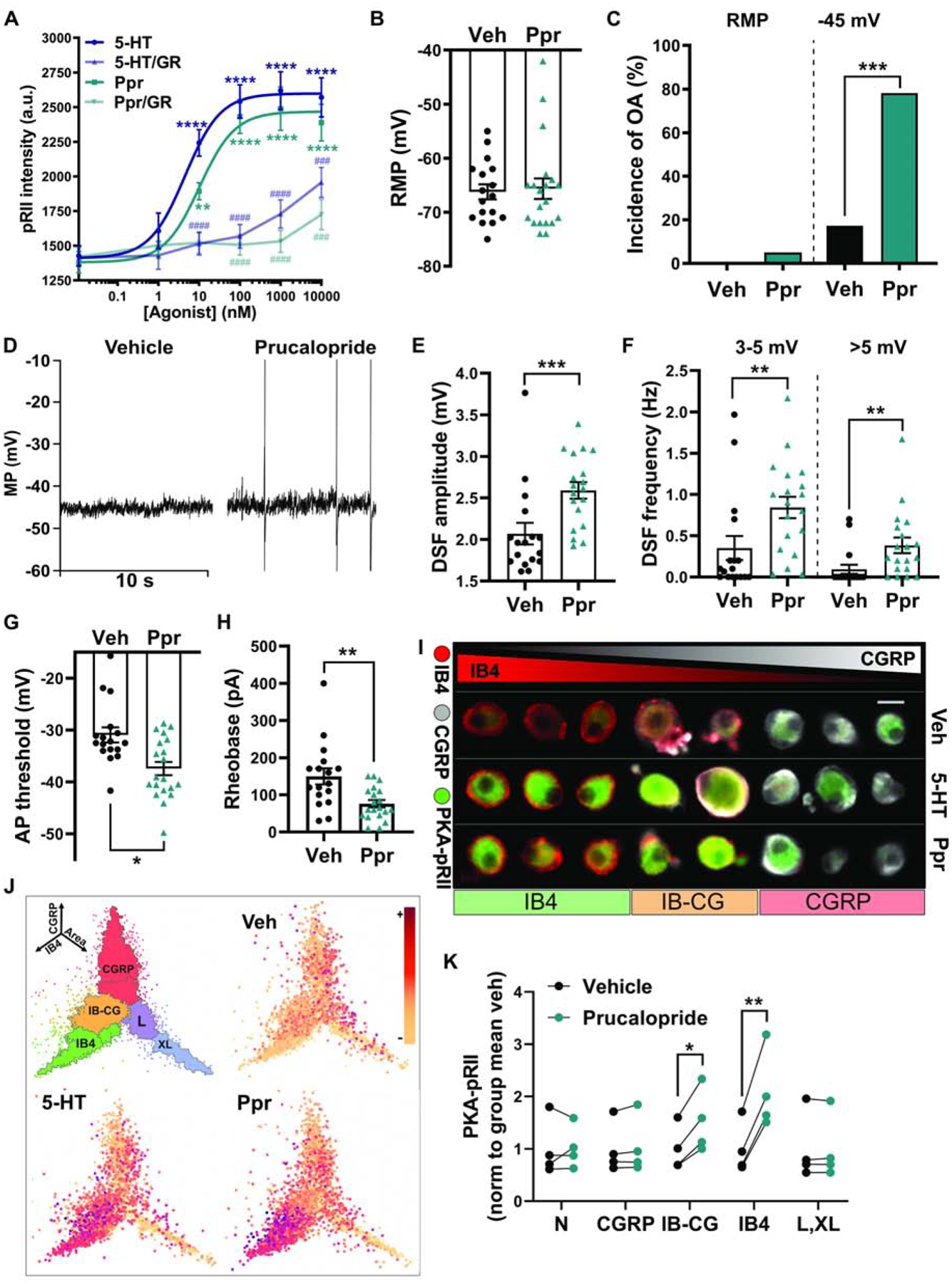
(A) Dose-dependent PKA activation by 5-HT and the 5-HT4 agonist prucalopride (Ppr), and inhibition of this effect by the 5-HT4 receptor antagonist GR113808 (GR). Vehicle (DMSO) or 1 μM GR113808 was co-applied with 5-HT or prucalopride for 5 min. Each data point represents the mean ± SEM of 3 separate experiments. Asterisks indicate significant difference of the agonist dose compared with no agonist. Hash marks indicate significant difference in the presence of antagonist compared with no antagonist. ** Dunnett’s adjusted p value < 0.01, ### Tukey’s adjusted p value < 0.001, ****/#### Dunnett’s/Tukey’s adjusted p value < 0.0001. (B) Lack of effect of prucalopride on RMP measured during electrophysiological recordings from cells continuously exposed to 300 nM prucalopride before and during recording. Mean ± SEM. (C) Effect of prucalopride on the incidence of OA determined from ≥60-s recordings at RMP then 30-s recordings at ~−45 mV. *** p < 0.001. (D) Representative traces of neurons treated with vehicle (DMSO) or prucalopride and depolarized to −45 mV. (E, F) Effects of prucalopride on the mean DSF amplitude and the frequency of medium- (3–5 mV) and large-amplitude (> 5 mV) DSFs during 30-s depolarization to −45 mV. Mean ± SEM. ** p < 0.01 *** p < 0.001. (G) Effect of prucalopride on the AP voltage threshold. Mean ± SEM. * p < 0.05. (H) Effect of prucalopride on the rheobase. Mean ± SEM. ** p < 0.01. (I) Examples of cell staining for PKA-pRII. Scale bar = 25 μm. (J) PKA-RII phosphorylation in controls, 300 nM 5-HT and 300 nM prucalopride, shown as coordinates of soma area (X-axis), CGRP staining intensity (Y-axis), and IB4 staining intensity (Z-axis). n > 15,000 neurons. (K) Prucalopride responses measured by PKA-pRII levels in specific neuronal subpopulations: N (negative for IB4 and CGRP and small somata), CGRP, IB-CG (weak staining for both IB4 and CGRP), L,XL (large and extra-large somata), and IB4. Each point represents the mean from one experiment normalized to the subpopulation baseline from the same experiment (n = 4 rats). * p < 0.05 ** p < 0.01.
Exposure to prucalopride (300 nM, ~5–60 min), like 5-HT, had no effect on RMP (Fig. 3B; Mann-Whitney, p = 0.79) and did not induce OA at rest (Fig. 3C, D). Prucalopride strongly increased the incidence of OA upon depolarization to −45 mV (Fig. 3C, D; Fisher’s exact test, p = 0.0002). The modest experimental depolarization also revealed increases in mean DSF amplitude (Fig. 3D, E; Mann-Whitney, p = 0.0006) and increased frequencies of medium- and large-amplitude DSFs (Fig. 3F; Mann-Whitney, p = 0.0022 and p = 0.0014, respectively), similar to that observed with comparable concentrations of 5-HT (Fig. 1; see also Odem et al., 2018). Prucalopride significantly hyperpolarized AP voltage threshold (Fig. 3G; Mann-Whitney, p = 0.0036) and markedly reduced rheobase (Fig. 3H; Mann-Whitney, p = 0.0024).
DRG neurons comprise several distinct subpopulations, even when excluding large cells as done in our patch clamp experiments. Therefore, we used a high-content microscopy approach to examine 5-HT4-induced PKA responses in DRG neuron subpopulations, as distinguished by the markers CGRP (for peptidergic nociceptors) and IB4-binding (for non-peptidergic nociceptors), as well as soma size (Garza Carbajal et al., 2020). The strongest PKA activation by prucalopride occurred in the nonpeptidergic nociceptors (IB4, 2.2-fold increase, paired t test, p = 0.0042), followed by an apparently weaker but still significant response in neurons positive for both IB4 binding and CGRP (IB-CG, 1.5-fold increase, paired t test, p = 0.011); no response was observed in the peptidergic nociceptors (CGRP), small- to medium-sized neurons negative for both markers (N), or large neurons (L, XL) (Fig. 3I–K). These results indicate that activation of 5-HT4 receptors is both necessary and sufficient to activate PKA and to promote larger DSFs, reduce AP threshold, and increase the incidence of OA under depolarized conditions in response to 5-HT, and that this modulation is specific to IB4+ and IB4+/CGRP+ nociceptors.
3.4. PKA activity is required for potentiation of DSFs and OA by 5-HT
Downstream of 5-HT4 activation, cAMP production is increased, leading to activation of its effectors, among which PKA is best known (Isensee et al., 2017) (Fig. 3A). PKA activity has previously been implicated in 5-HT effects on voltage-gated Na+ current that should reduce AP voltage threshold and rheobase (Cardenas et al., 2001; Gold et al., 1996). To test the necessity of PKA activity for 5-HT’s effects on OA, DSFs, AP voltage threshold, and rheobase, we utilized two different types of PKA inhibitor: H-89, a small-molecule competitive inhibitor, and myr-PKI 14–22, a peptide that mimics endogenous PKA inhibitors. Both H-89 and PKI blocked 5-HT potentiation of OA at −45 mV (Fig. 4A; Fisher’s exact tests, p = 0.01 and p = 0.0051, respectively). Similarly, H-89 and PKI both attenuated the frequency of medium-amplitude DSFs (3–5 mV; Kruskal-Wallis, p = 0.0008) and large-amplitude DSFs (>5 mV; Kruskal-Wallis, p = 0.0018) at −45 mV (Fig. 4B). PKI effectively blocked hyperpolarization of the AP voltage threshold by 5-HT (Fig. 4C; Kruskal-Wallis, p = 0.0036). However, inhibition of PKA activity was not sufficient to significantly block the reduction of rheobase by 5-HT (Fig. 4D; Kruskal-Wallis, p = 0.11). As anticipated, none of these treatments affected RMP (Fig. 4C) or incidence of OA at RMP (Fig. 4A). These results demonstrate that PKA plays a critical role in enhancement of DSFs, reduction of AP threshold, and associated potentiation of OA by 5-HT, but plays a lesser role in decreasing rheobase.
Figure 4. PKA activity is required for potentiation of DSFs and OA by 5-HT.
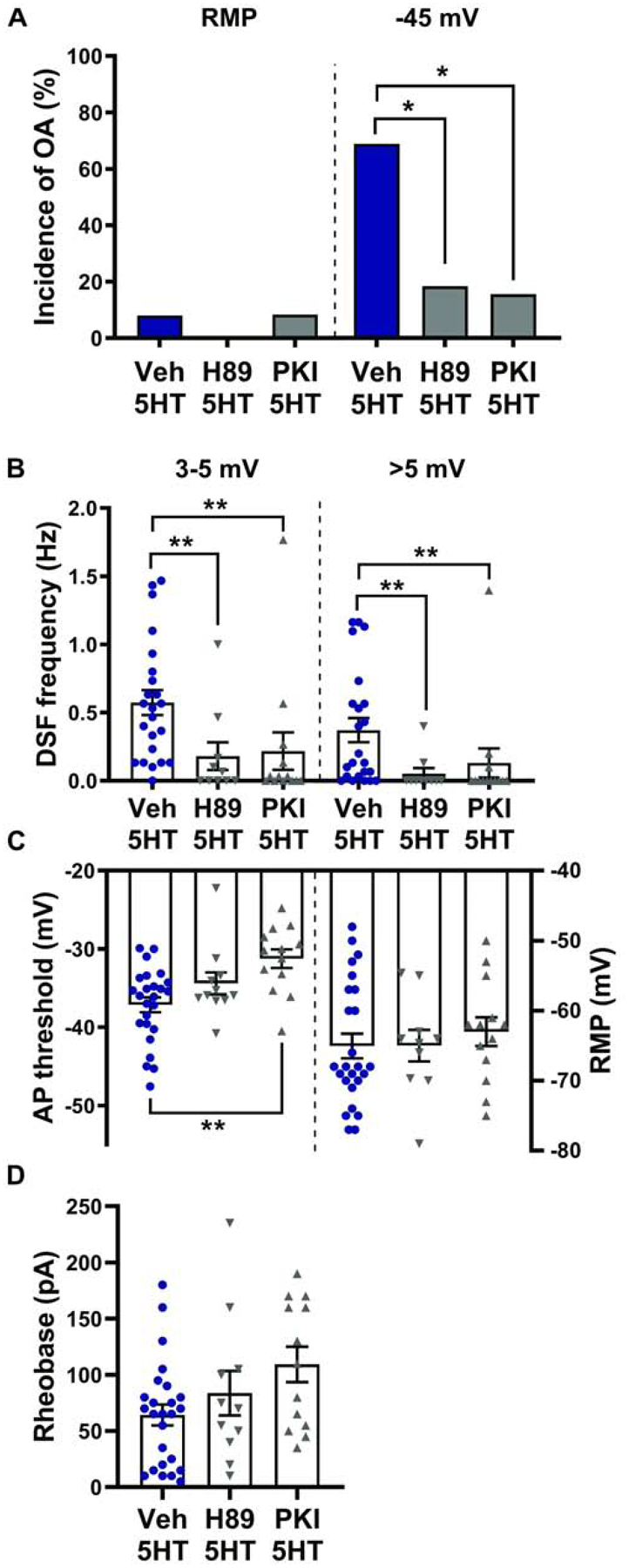
Rat DRG neurons were treated with 10 μM H-89, 1 μM myr-PKI 14–22, or vehicle before and during exposure to 100–300 nM 5-HT. (A) Effects of H-89 and PKI on the incidence of OA during 5-HT exposure. The occurrence of OA was determined from ≥ 60-s recordings at RMP then 30-s recordings at ~−45 mV. * p < 0.025 (after Bonferroni correction for 2 comparisons). (B-D) Effects of H-89 and PKI on the frequency of medium- (3–5 mV) and large-amplitude (>5 mV) DSFs during 30-s depolarization to −45 mV (B), AP voltage threshold (C), and rheobase (D) in the presence of 5-HT. Mean ± SEM. ** Dunn’s adjusted p value < 0.01.
3.5. EPAC activity contributes to induction of hyperexcitability by 5-HT
While PKA is the cAMP effector best known for enhancing nociceptor activity and pain, important roles are also played by EPAC (Berkey et al., 2020; Fu et al., 2019; Huang and Gu, 2017; Pan et al., 2019; Singhmar et al., 2018). The relationship between 5-HT signaling and EPAC activity has begun to be elucidated in the brain and spinal cord (Cochet et al., 2013; Fields et al., 2015; Lin et al., 2003), but whether EPAC activity is involved in 5-HT effects on DRG neurons is unknown. To test this possibility, we employed an EPAC1 inhibitor, CE3F4, and EPAC2 inhibitor, ESI05, alone and in combination. The concentrations used (10 μM CE3F4 and 5 μM ESI-05) were previously found to strongly inhibit both spontaneous activity (OA at RMP) and enhancement of DSFs at ~−45 mV months after spinal cord injury when the inhibitors were applied individually (to rat DRG neurons) or in combination (to mouse DRG neurons) (Berkey et al., 2020). Here, significant attenuation of OA generated acutely at −45 mV in the presence of 5-HT in rat DRG neurons was only observed when CE3F4 and ESI05 were combined (Fig. 5A; Fisher’s exact test, p = 0.002). There was a trend toward reduced OA incidence with ESI05 alone (p = 0.032), which was not significant after Bonferroni correction for multiple comparisons. Similarly, the frequency of large-amplitude (>5 mV) DSFs at −45 mV in the presence of 5-HT was significantly attenuated by the combination of CE3F4 and ESI05 (Fig. 5B, right panel; Kruskal-Wallis, p = 0.017). No significant attenuation of medium-amplitude DSFs (Fig. 5B, left; Kruskal-Wallis, p = 0.24) or AP threshold (Fig. 5C) was observed, but possible trends toward more depolarized AP thresholds in the presence of 5-HT were observed with CE3F4 or ESI05 alone or in combination (1-way ANOVA, F(3,87) = 2.23, p = 0.091). Weak individual effects of EPAC1 and EPAC2 on AP threshold and DSFs might combine to produce significant effects on OA. No effect of EPAC inhibitors was found on 5-HT-induced reduction of rheobase (Fig.5D; Kruskal-Wallis, p = 0.92), and none of these treatments impacted RMP (Fig. 5E; Kruskal-Wallis, p = 0.89). Taken together, these findings suggest that EPAC activity contributes to the increased incidence of OA caused by 5-HT under depolarized conditions primarily by increasing DSF amplitude.
Figure 5. EPAC activity contributes to hyperexcitability induced by 5-HT.
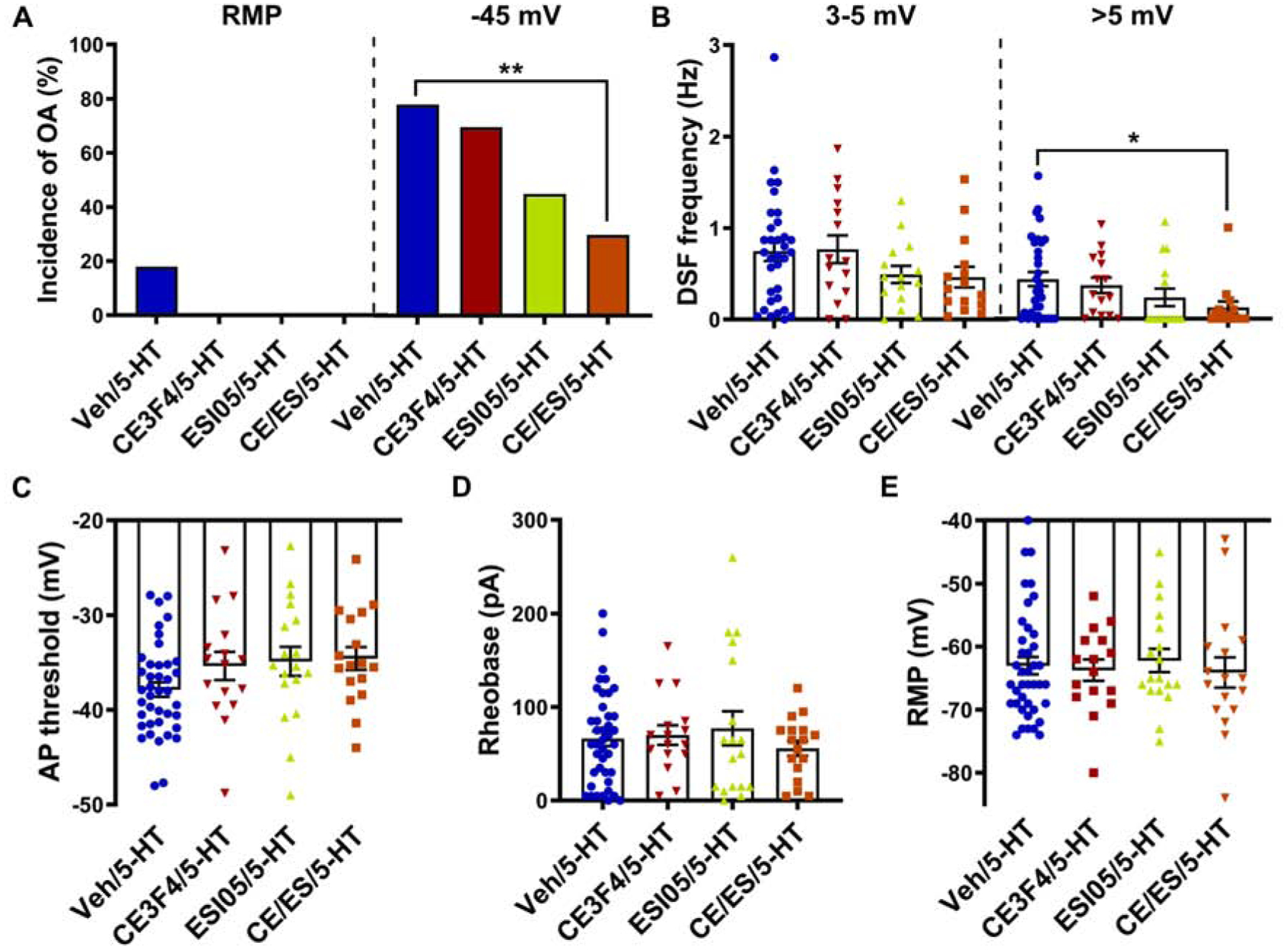
Cells were treated with CE3F4 (10 μM) or ESI-05 (5 μM) alone or in combination or vehicle control before and during exposure to 100–300 nM 5-HT. (A) Effects of EPAC inhibitors on the incidence of OA was measured during ≥60-s recordings at RMP then during 30-s depolarization to −45 mV in the presence of 5-HT. ** p < 0.0033 (Bonferroni correction for 3 comparisons). (B) Effects of EPAC inhibitors on the frequency of medium- (3–5 mV) and large-amplitude (>5 mV) DSFs during 30-s depolarization to −45 mV in the presence of 5-HT. Mean ± SEM. * Dunn’s adjusted p value < 0.05. (C) Effects of EPAC inhibitors on the AP voltage threshold during exposure to 5-HT. Mean ± SEM. (D, E) Effects of EPAC inhibitors on rheobase (D) and RMP (E) during exposure to 5-HT.
3.6. Nociceptor hyperexcitability induced by 5-HT does not require HCN activity
The other major cAMP effector, HCN channels, may contribute modestly to RMP in nociceptors (Du et al., 2014), and these channels are known to be important for some forms of pain (e.g., Emery et al., 2011; Weng et al., 2012). To examine whether HCN channels play a substantial role in the enhancement of DSFs caused by 5-HT at −45 mV, we tested the effects of blocking the channels with a widely used HCN inhibitor, ZD7288 (e.g., Du et al., 2014), before and during exposure to 5-HT. ZD7288 had no significant effect on the incidence of OA at −45 mV (Fig. 6A; Fisher’s exact test, p = 0.26), frequency of medium- and large-amplitude DSFs at −45 mV (Fig. 6B; Mann-Whitney, p = 0.28 and p = 0.32, respectively), firing frequency at −45 mV (Fig. 6C; Mann-Whitney, p = 0.13), AP threshold (Fig. 6D; t test, p = 0.64), rheobase (Fig. 6E; t test, p = 0.39), or RMP (data not shown) during exposure to 5-HT. These results suggest that HCN activity is not a major contributor to the effects of 5-HT on OA and DSFs at −45 mV, nor to AP threshold or rheobase in DRG neurons.
Figure 6. Nociceptor hyperexcitability induced by 5-HT does not require HCN activity.
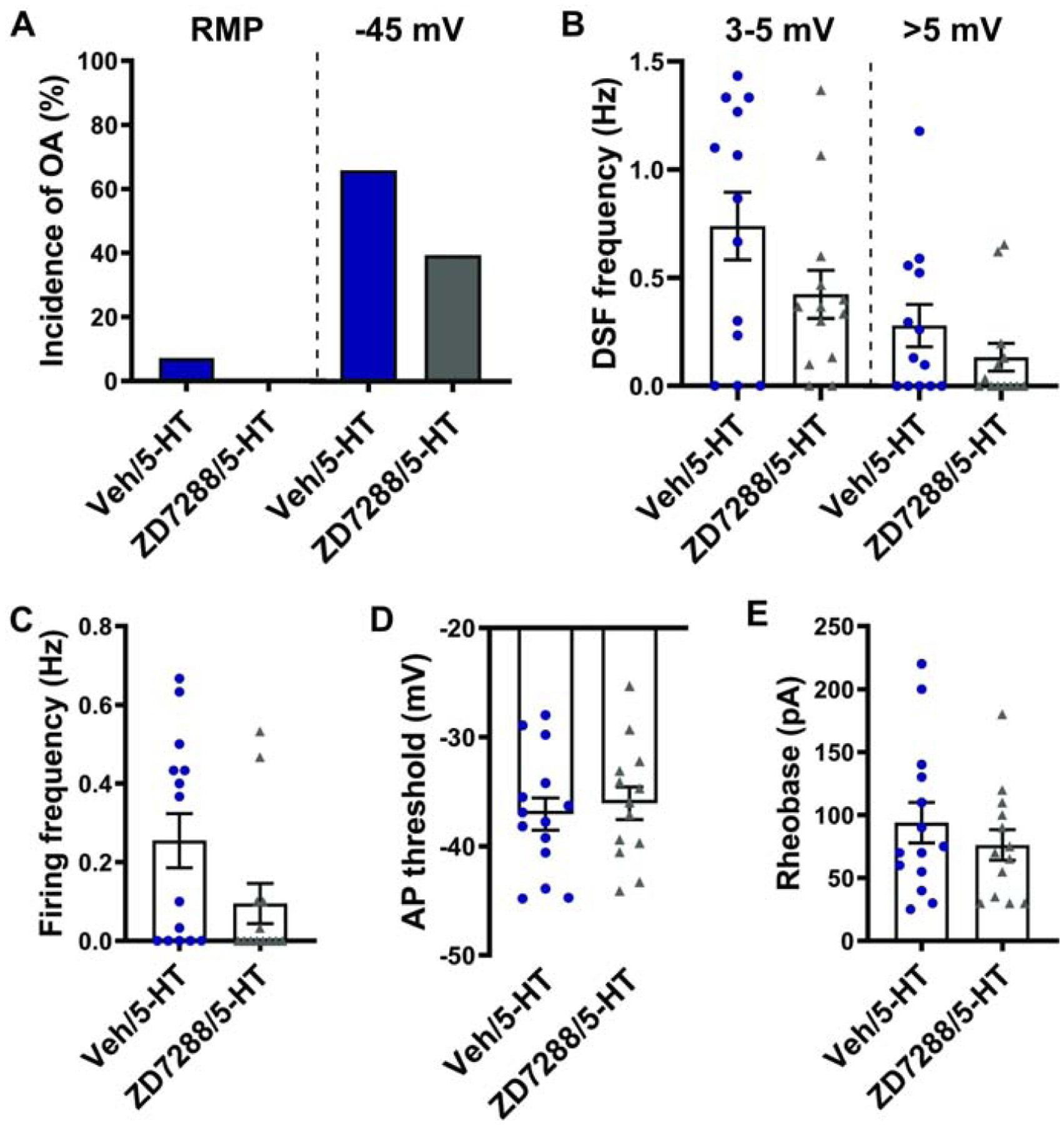
Rat DRG neurons were treated 100 μM ZD7288 before and during exposure to 100 nM 5-HT. (A) Effect of ZD7288 on the incidence of OA during exposure to 5-HT. The occurrence of OA was measured during ≥60-s recordings at RMP then during 30-s depolarization to −45 mV. (B-E) Effects of ZD7288 on the frequency of medium- (3–5 mV) and large-amplitude (>5 mV) DSFs during 30-s depolarization to −45 mV (B), frequency of AP firing during 30-s recordings at −45 mV (C), AP voltage threshold (D), and rheobase (E) in the presence of 5-HT. Mean ± SEM.
3.7. Activation of cAMP signaling is sufficient to induce hyperexcitability in probable nociceptors
While we know that cAMP signaling is important for maintaining chronic nociceptor hyperactivity in vitro after SCI (Bavencoffe 2016; Berkey 2020), and that 5-HT stimulates cAMP signaling in nociceptors (e.g., Fig. 3A), it is not known whether an acute increase in cAMP is sufficient to enhance DSFs and promote OA. To answer this question, we applied the cAMP analog 8-Br-cAMP intracellularly via the patch pipette. Similar to extracellular application of 5-HT, intracellular dialysis of 8-Br-cAMP did not induce OA at rest, but it increased the incidence of OA when the neuron was experimentally depolarized to a holding potential of ~−45 mV (Fig. 7A; Fisher’s exact test, p = 0.028) and reduced the rheobase (Fig. 7D; t test, p = 0.013). Also similar to 5-HT, 8-Br-cAMP increased the frequency of medium- (3–5 mV) and large-amplitude (>5 mV) DSFs at −45 mV (Fig. 7B; Mann-Whitney, p = 0.0001 and p = 0.0024, respectively) and hyperpolarized the AP voltage threshold (Fig. 7C; Mann-Whitney, p = 0.016). Unlike 5-HT or the selective 5-HT4 agonist prucalopride, 8-Br-cAMP significantly depolarized the RMP (Fig. 7E; t test, p = 0.0085), indicating that activation of the cAMP signaling pathway can affect RMP (see also Momin and McNaughton, 2009), albeit not when cAMP signaling is stimulated by the 5-HT4 receptor. These results demonstrate that cAMP signaling is sufficient to induce a hyperactive state that promotes OA in probable nociceptors.
Figure 7. Activation of cAMP signaling by the cAMP analog 8-Br-cAMP is sufficient to potentiate DSFs and OA in isolated DRG neurons.
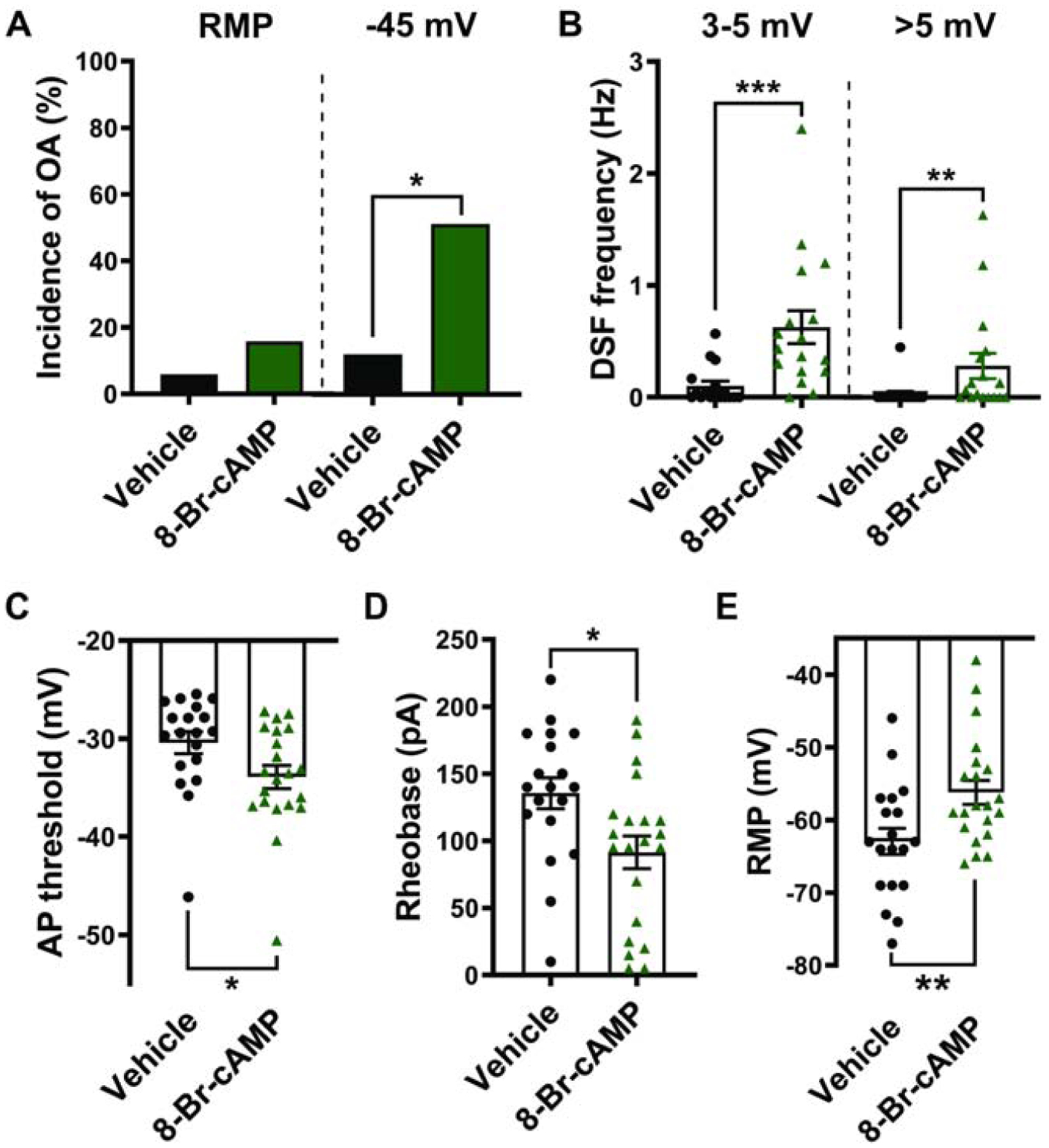
Patch pipettes contained 100 μM 8-Br-cAMP or vehicle in the ICS. (A) Effect of 8-Br-cAMP on the incidence of OA. The occurrence of OA was measured during ≥60-s recording at RMP then during 30-s depolarization to −45 mV. * p < 0.05. (B-E) Effect of 8-Br-cAMP on the frequency of medium- (3–5 mV) and large-amplitude (>5 mV) DSFs during 30-s depolarization to −45 mV (B), AP threshold (C), rheobase (D), and RMP (E). Mean ± SEM. * p < 0.05, ** p < 0.01, *** p < 0.001.
4. Discussion
We showed previously that 5-HT promotes OA in DRG neurons by enhancing DSFs and reducing AP threshold (Odem et al., 2018). Here we show that acute, sustained exposure to low concentrations of 5-HT promotes OA through the Gs-coupled 5-HT4 receptor and downstream cAMP signaling, predominantly via activation of PKA, with some contributions from EPAC. The similar effects produced by a cAMP analog suggest that any signal that activates cAMP signaling in nociceptors has the potential to promote OA by increasing DSF amplitude and decreasing AP threshold.
4.1. Nociceptor hyperexcitability can be driven acutely by stimulation of 5-HT4 receptors
Our results show that 5-HT4 receptors play a crucial role in hyperexcitability induced by prolonged exposure to 5-HT, which extends early evidence that 5-HT4 receptors increase tetrodotoxin-insensitive sodium currents in DRG neurons (Cardenas et al., 1997a). However, numerous 5-HT receptors are expressed in primary somatosensory neurons and several have been implicated in peripheral sensitization (reviewed by Loyd et al., 2013). For example, the 5-HT2A antagonist ketanserin can attenuate 5-HT-induced thermal hyperalgesia (Tokunaga et al., 1998), and a 5-HT2B/C antagonist can inhibit 5-HT-induced mechanical allodynia but not 5-HT-induced thermal hyperalgesia in rodents (Lin et al., 2011). Overexpression studies in HEK293T cells showed that 5-HT can evoke robust Ca2+ transients via 5-HT2 receptors (Lin et al., 2011). Fast superfusion of 10 μM 5-HT produced rapidly activating and inactivating [Ca2+]i responses in 67% of capsaicin-sensitive neurons tested in rats (Linhart et al., 2003), in contrast to the lack of effect we found with a 5-HT2 antagonist during 5–60 min bath application of 100–300 nM 5-HT. Similarly, only 3.4% of mouse DRG neurons responded to 1 μM 5-HT (Lin et al., 2011). However, significant [Ca2+]i responses to 5-HT were reported in capsaicin-sensitive rat trigeminal ganglion neurons, which were attenuated by antagonists of 5-HT2A receptors and 5-HT3 receptors (Loyd et al., 2011). Ionotropic 5-HT3 receptors typically activate and desensitize rapidly. In rat DRG neurons, a 5-HT2 antagonist abolished the increase in AP discharge during depolarizing steps produced by rapid perfusion of 10 μM 5-HT (Salzer et al., 2016). Different results among these and other studies might be explained by procedural, species, and sex differences, but it seems likely that a major factor contributing to the lack of action potentials or calcium responses evoked by 5-HT at RMP in our experiments arose from our attempt to mimic sustained exposure to 5-HT during in vivo inflammation. Slow, prolonged administration of 5-HT is likely to substantially desensitize many 5-HT receptors in nociceptors. In addition, 5-HT2 and 5-HT4 receptors may function in partially separate nociceptor subpopulations, as 5-HT2A receptors are mainly expressed in CGRP-synthesizing (peptidergic) small DRG neurons (Okamoto et al., 2002), whereas we found 5-HT4-induced PKA-RII phosphorylation (used here as an indirect measurement of cAMP generation) to be largely restricted to IB4+ (nonpeptidergic) and IB4+/CGRP+ nociceptors. In mice, 5-HT4 receptor expression was also shown by single-cell RNA-seq profiling to be restricted to nonpeptidergic DRG neurons (Mousebrain.org, November 7, 2020; Usoskin et al., 2015).
In addition to the 5-HT4 family of Gs-coupled receptors, there is evidence for DRG neuron expression of Gs-coupled 5-HT7 and 5-HT6 receptors. 5-HT7 activity can potentiate T-type Ca2+ current via PKA-dependent mechanisms in Xenopus oocytes co-expressing 5-HT7 and Cav3.2 (Kim et al., 2006), and a 5-HT7 agonist mimics potentiating effects of 5-HT on increases of [Ca2+]i evoked by capsaicin in DRG neurons (Ohta et al., 2006). However, we were unable to block 5-HT activation of PKA in DRG neurons with a selective 5-HT7 antagonist (SB269970), suggesting 5-HT7 activity in these neurons is not a major contributor to cAMP signaling evoked by low concentrations of 5-HT. The evidence for expression of 5-HT6 receptors in rat lumbar DRGs is mixed. For example, 5-HT6 receptor mRNA was reported to be increased after plantar injection of bee venom (Liu et al., 2005), but 5-HT6 receptor mRNA was not detected in rat DRG neurons by in situ hybridization (Nicholson et al., 2003) or in cultured DRG neurons by RT-PCR (Chen et al., 1998). Because of the close similarity of the effects of the 5-HT4 receptor agonist prucalopride to those produced by 5-HT, and the complete blockade of 5-HT-induced OA, DSF enhancement, and PKA activation by the 5-HT4 receptor antagonist GR113808, we conclude that other Gs-coupled 5-HT receptors are less important under our conditions, although potential contributions of 5-HT6 receptors have not been tested directly. Besides Gs- and Gq-coupled receptors, DRG neurons express Gi-coupled 5-HT1/5 receptors. Any 5-HT1/5-mediated effects on OA remain to be elucidated and they may be complex because Gi-coupled receptors usually decrease cAMP signaling, and they have been linked to inhibitory effects of 5-HT on nociceptors (Cardenas et al., 1997b).
4.2. Acute hyperexcitability induced by 5-HT depends upon cAMP signaling involving PKA and EPAC
The promotion of OA by the cAMP analog 8-Br-cAMP showed that activation of cAMP signaling is sufficient to reduce AP threshold and enhance DSFs to increase nociceptor excitability. This suggests that other mediators, such as PGE2, that activate Gs-coupled receptors may enhance OA by similar mechanisms. While 8-Br-cAMP depolarized RMP (to ~−57 mV), further depolarization (experimentally, to ~−45 mV) was required to reveal effects on OA, as was also observed with 5-HT. Direct activation of adenylyl cyclase with forskolin produces effects similar to those of 8-Br-cAMP delivery (A. Bavencoffe, E.T. Walters, and C.W. Dessauer, unpublished observations). The lack of a significant increase in OA at RMP induced by 8-Br-cAMP despite the cAMP analog depolarizing RMP, reducing AP threshold, and enhancing DSFs can be explained by its insufficient alteration of some or all of the electrophysiological components that drive OA. Specifically, the hyperpolarization of AP threshold and increased incidence of large DSFs induced by 8-Br-cAMP were modest compared to what was observed with 5-HT, and the depolarization of RMP by 8-Br-cAMP to ~−57 mV is not close to the −45 mV holding potential we used to enable OA during 5-HT treatment. The weaker effects of a relatively high concentration of 8-Br-cAMP compared to those of low doses of 5-HT are consistent with findings that OA generation in nociceptors involves interaction of cAMP signaling with at least one other signaling pathway. Interestingly, depolarization to −45 mV, where we see the marked effects on OA and DSFs with cAMP activation, also activates the extracellular signal-regulated kinase (ERK) pathway in IB4+ nociceptors (Garza Carbajal et al., 2020).
The antagonists of effectors downstream of cAMP that produced the largest attenuation of 5-HT-induced hyperexcitability were inhibitors of PKA, which blocked enhancement of OA, DSFs, and excitability by 5-HT. We note that, while our measure of cAMP production in response to 5-HT and its analogs was type II PKA activity, this does not rule out the interesting possibility of significant contributions of type I PKAs to at least some of the 5-HT effects we observed. PKA activity was previously linked to potentiation by 5-HT of transient receptor potential V1 (TRPV1) function (Ohta et al., 2006) and tetrodotoxin-resistant (TTX-R) Na+ current (Scroggs, 2011) in isolated DRG neurons, as well as to 5-HT-induced hyperalgesia in vivo (Aley and Levine, 1999). Whether enhanced TRPV1 or TTX-R Na+ currents are involved in 5-HT-induced OA, and the extent to which the mechanisms described in this study contribute to hyperalgesia in vivo, are not yet known. While a number of studies demonstrated a role for EPAC in neuropathic and inflammatory pain (reviewed by Huang and Gu, 2017; Li et al., 2019), the link between 5-HT signaling and EPAC activity in nociceptors had not been explored. We found that EPAC1 and 2 activity (primarily in combination) contributed significantly to the hyperexcitable state induced by 5-HT. Lastly, we found statistically insignificant trends for reduced excitability when inhibiting HCN channels during 5-HT treatment, suggesting that (at most) HCN channels are a minor contributor to the 5-HT-induced effects we measured. This is consistent with the enhancement of DSFs and OA occurring primarily at relatively depolarized membrane potentials where few HCN channels should be active. We failed to prevent 5-HT’s reduction of rheobase (measured from a holding potential of −60 mV) by inhibiting any one of the cAMP effectors tested (PKA, EPAC1/2, and HCN), suggesting that additive effects of these components of cAMP signaling, and perhaps other cell signaling pathways, drive this manifestation of 5-HT-induced hyperexcitability.
4.3. Functional implications of 5-HT4-dependent cAMP signaling in nociceptors
Nociceptor OA induced by injury or inflammation may be generated in peripheral terminals, axonal neuromas, and/or the soma, with each site potentially exposed persistently to inflammatory mediators, including 5-HT (discussed by Walters, 2019). For example, 5-HT is elevated in the CSF and plasma acutely following SCI (Brodner et al., 1980; Sharma et al., 1993). Because DRG are perfused by blood in addition to CSF (Abram et al., 2006; Godel et al., 2016), circulating and intrathecal 5-HT might contribute to the early C-fiber OA generated within the DRG in vivo after SCI (Bedi et al., 2010). An important question for future investigation is whether the effects of 5-HT on somal excitability observed in DRG neurons dissociated from normal rats in this study can be altered by prior inflammation or nerve injury.
The 5-HT4 receptor-dependent effects we found in nociceptor somata may also contribute within peripheral branches to pain caused by peripheral tissue injury, such as incision. The reduced rheobase may be particularly relevant in the periphery, where increased 5-HT4 activity in nociceptors may render their peripheral terminals more sensitive to sensory generator potentials and thereby contribute to hyperalgesia. Our findings set the stage for multidisciplinary approaches to define potentially important contributions of 5-HT-stimulated cAMP signaling in nociceptors -- within the DRG and at sites of injury -- to behaviorally expressed ongoing pain. They also suggest that peripheral 5-HT4 receptors might be a useful target for treating some forms of ongoing pain clinically.
Highlights.
Sustained exposure to 5-HT induces nociceptor hyperexcitability via 5-HT4 receptors
The hyperexcitable effects promote ongoing activity during modest depolarization
A major effect is enhancement of irregular, depolarizing spontaneous fluctuations
cAMP, PKA, and EPAC mediate hyperexcitable effects that promote ongoing activity
Acknowledgements
The authors thank Drs. Max Odem and Ryan Cassidy for instruction and consultation on the use of SFA.py and thank Dr. Xiaodong Cheng for the generous gift of ESI-05.
Funding: This work was supported by The National Institute of Neurological Diseases and Stroke Grants NS091759 to C.W.D. and E.T.W. and NS111521 to E.T.W. and M.X.Z., The National Institute of General Medical Sciences Awards F31FM133203 to E.R.L. and T32GM120011 to C.W.D, and a Charlene Kopchick Fellowship to E.R.L. Other than providing financial support, the funding sources had no direct involvement in the research.
Abbreviations:
- 5-HT
serotonin
- 5-HTR
serotonin receptor
- 8-Br-cAMP
8-bromo-cyclic adenosine monophosphate
- ANOVA
analysis of variance
- AP
action potential
- cAMP
cyclic adenosine monophosphate
- [Ca2+]i
intracellular Ca2+ concentration
- Cav3.2
voltage-gated calcium channel 3.2
- CGRP
calcitonin gene-related peptide
- CI
confidence interval
- CSF
cerebrospinal fluid
- DMEM
Dulbecco’s Modified Eagle Medium
- DMSO
dimethyl sulfoxide
- DRG
dorsal root ganglion
- DSF
depolarizing spontaneous fluctuation
- EC50
concentration that produces the half maximal stimulatory effect
- ECS
extracellular solution
- EPAC
exchange protein activated by cAMP
- GPCR
G protein-coupled receptor
- HCN
hyperpolarization-activated cyclic nucleotide-gated channel
- IB4
isolectin B4
- IC50
concentration that produces the half maximal inhibitory effect
- ICS
intracellular solution
- myr-PKI 14–22
myristoylated protein kinase inhibitor 14–22
- NA
nonaccommodating
- OA
ongoing activity
- PGE2
prostaglandin E2
- PGP9.5
protein gene product 9.5
- PKA
protein kinase A
- RA
rapidly accommodating
- RMP
resting membrane potential
- SCI
spinal cord injury
- TRPV1
transient receptor potential vanilloid 1
- TTX-R
tetrodotoxin-resistant
- Veh
vehicle
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest: None
References
- Abram SE, Yi J, Fuchs A, Hogan QH, 2006. Permeability of injured and intact peripheral nerves and dorsal root ganglia. Anesthesiology 105, 146–153. 10.1097/00000542-200607000-00024 [DOI] [PubMed] [Google Scholar]
- Aley KO, Levine JD, 1999. Role of Protein Kinase A in the Maintenance of Inflammatory Pain. J Neurosci 19, 2181–2186. 10.1523/JNEUROSCI.19-06-02181.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavencoffe A, Li Y, Wu Z, Yang Q, Herrera J, Kennedy EJ, Walters ET, Dessauer CW, 2016. Persistent Electrical Activity in Primary Nociceptors after Spinal Cord Injury Is Maintained by Scaffolded Adenylyl Cyclase and Protein Kinase A and Is Associated with Altered Adenylyl Cyclase Regulation. J Neurosci 36, 1660–1668. 10.1523/JNEUROSCI.0895-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedi SS, Yang Q, Crook RJ, Du J, Wu Z, Fishman HM, Grill RJ, Carlton SM, Walters ET, 2010. Chronic spontaneous activity generated in the somata of primary nociceptors is associated with pain-related behavior after spinal cord injury. J Neurosci 30, 14870–14882. 10.1523/JNEUROSCI.2428-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkey SC, Herrera JJ, Odem MA, Rahman S, Cheruvu SS, Cheng X, Walters ET, Dessauer CW, Bavencoffe AG, 2020. EPAC1 and EPAC2 promote nociceptor hyperactivity associated with chronic pain after spinal cord injury. Neurobiol Pain 7, 100040 10.1016/j.ynpai.2019.100040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodner RA, Dohrmann GJ, Roth RH, Rubin RA, 1980. Correlation of cerebrospinal fluid serotonin and altered spinal cord blood flow in experimental trauma. Surg Neurol 13, 337–343. [PubMed] [Google Scholar]
- Cardenas CG, Del Mar LP, Cooper BY, and Scroggs RS, 1997a. 5HT4 receptors couple positively to tetrodotoxin-insensitive sodium channels in a subpopulation of capsaicin-sensitive rat sensory neurons. J Neurosci 17, 7181–7189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas CG, Del Mar LP, Scroggs RS, 1997b. Two Parallel Signaling Pathways Couple 5HT 1A Receptors to N- and L-Type Calcium Channels in C-Like Rat Dorsal Root Ganglion Cells. J Neurophysiol 77, 3284–3296. 10.1152/jn.1997.77.6.3284 [DOI] [PubMed] [Google Scholar]
- Cardenas LM, Cardenas CG, Scroggs RS, 2001. 5HT Increases Excitability of Nociceptor-Like Rat Dorsal Root Ganglion Neurons Via cAMP-Coupled TTX-Resistant Na + Channels. J Neurophysiol 86, 241–248. 10.1152/jn.2001.86.1.241 [DOI] [PubMed] [Google Scholar]
- Chen H, Tsalkova T, Chepurny OG, Mei FC, Holz GG, Cheng X, Zhou J, 2013. Identification and characterization of small molecules as potent and specific EPAC2 antagonists. J Med Chem 56, 952–962. 10.1021/jm3014162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JJ, Vasko MR, Wu X, Staeva TP, Baez M, Zgombick JM, Nelson DL, 1998. Multiple subtypes of serotonin receptors are expressed in rat sensory neurons in culture. J Pharmacol Exp Ther 287, 1119–1127. [PubMed] [Google Scholar]
- Cochet M, Donneger R, Cassier E, Gaven F, Lichtenthaler SF, Marin P, Bockaert J, Dumuis A, Claeysen S, 2013. 5-HT4 receptors constitutively promote the non-amyloidogenic pathway of APP cleavage and interact with ADAM10. ACS Chem Neurosci 4, 130–140. 10.1021/cn300095t [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Hoon MJL, Imoto S, Nolan J, Miyano S, 2004. Open source clustering software. Bioinformatics 20, 1453–1454. 10.1093/bioinformatics/bth078 [DOI] [PubMed] [Google Scholar]
- Djouhri L, Koutsikou S, Fang X, McMullan S, Lawson SN, 2006. Spontaneous pain, both neuropathic and inflammatory, is related to frequency of spontaneous firing in intact C-fiber nociceptors. J Neurosci 26, 1281–1292. 10.1523/JNEUROSCI.3388-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du X, Hao H, Gigout S, Huang D, Yang Y, Li L, Wang C, Sundt D, Jaffe DB, Zhang H, Gamper N, 2014. Control of somatic membrane potential in nociceptive neurons and its implications for peripheral nociceptive transmission. Pain 155, 2306–2322. 10.1016/j.pain.2014.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery EC, Young GT, Berrocoso EM, Chen L, McNaughton PA, 2011. HCN2 ion channels play a central role in inflammatory and neuropathic pain. Science 333, 1462–1466. 10.1126/science.1206243 [DOI] [PubMed] [Google Scholar]
- Fields DP, Springborn SR, Mitchell GS, 2015. Spinal 5-HT7 receptors induce phrenic motor facilitation via EPAC-mTORC1 signaling. J Neurophysiol 114, 2015–2022. 10.1152/jn.00374.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu W, Nelson TS, Santos DF, Doolen S, Gutierrez JJP, Ye N, Zhou JK Taylor B, 2019. An NPY Y1 receptor antagonist unmasks latent sensitization and reveals the contribution of protein kinase A and Epac to chronic inflammatory pain. Pain 160, 1754–1765. 10.1097/j.pain.0000000000001557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garza Carbajal A, Bavencoffe A, Walters ET, Dessauer CW, 2020. Depolarization-dependent C-Raf signaling promotes hyperexcitability and reduces opioid sensitivity of isolated nociceptors after spinal cord injury. J Neurosci 10.1523/JNEUROSCI.0810-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godel T, Pham M, Heiland S, Bendszus M, Bäumer P, 2016. Human dorsal-root-ganglion perfusion measured in-vivo by MRI. Neuroimage 141, 81–87. 10.1016/j.neuroimage.2016.07.030 [DOI] [PubMed] [Google Scholar]
- Gold MS, Reichling DB, Shuster MJ, Levine JD, 1996. Hyperalgesic agents increase a tetrodotoxin-resistant Na+ current in nociceptors. Proc Natl Acad Sci USA 93, 1108–1112. 10.1073/pnas.93.3.1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L-Y, Gu Y, 2017. Epac and Nociceptor Sensitization. Mol Pain 13, 1744806917716234. 10.1177/1744806917716234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isensee J, Diskar M, Waldherr S, Buschow R, Hasenauer J, Prinz A, Allgower F, Herberg FW, Hucho T, 2014. Pain modulators regulate the dynamics of PKA-RII phosphorylation in subgroups of sensory neurons. J Cell Sci 127, 216–229. 10.1242/jcs.136580 [DOI] [PubMed] [Google Scholar]
- Isensee J, Kaufholz M, Knape MJ, Hasenauer J, Hammerich H, Gonczarowska-Jorge H, Zahedi RP, Schwede F, Herberg FW, Hucho T, 2018. PKA-RII subunit phosphorylation precedes activation by cAMP and regulates activity termination. J Cell Biol 217, 2167–2184. 10.1083/jcb.201708053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isensee J, Krahé L, Moeller K, Pereira V, Sexton JE, Sun X, Emery E, Wood JN, Hucho T, 2017. Synergistic regulation of serotonin and opioid signaling contributes to pain insensitivity in Nav1.7 knockout mice. Sci Signal 10, eaah4874 10.1126/scisignal.aah4874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J-A, Park J-Y, Kang H-W, Huh S-U, Jeong S-W, Lee J-H, 2006. Augmentation of Cav3.2 T-type calcium channel activity by cAMP-dependent protein kinase A. J Pharmacol Exp Ther 318, 230–237. 10.1124/jpet.106.101402 [DOI] [PubMed] [Google Scholar]
- Kleggetveit IP, Namer B, Schmidt R, Helås T, Rückel M, Ørstavik K, Schmelz M, Jørum E, 2012. High spontaneous activity of C-nociceptors in painful polyneuropathy: Pain 153, 2040–2047. 10.1016/j.pain.2012.05.017 [DOI] [PubMed] [Google Scholar]
- Laumet G, Bavencoffe A, Edralin JD, Huo X-J, Walters ET, Dantzer R, Heijnen CJ, Kavelaars A, 2020. Interleukin-10 resolves pain hypersensitivity induced by cisplatin by reversing sensory neuron hyperexcitability. Pain. 10.1097/j.pain.0000000000001921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z-H, Cui D, Qiu C-J, Song X-J, 2019. Cyclic nucleotide signaling in sensory neuron hyperexcitability and chronic pain after nerve injury. Neurobiol Pain 6, 100028 10.1016/j.ynpai.2019.100028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SL, Johnson-Farley NN, Lubinsky DR, Cowen DS, 2003. Coupling of neuronal 5-HT7 receptors to activation of extracellular-regulated kinase through a protein kinase A-independent pathway that can utilize Epac J Neurochem 87, 1076–1085. 10.1046/j.1471-4159.2003.02076.x [DOI] [PubMed] [Google Scholar]
- Lin S-Y, Chang W-J, Lin C-S, Huang C-Y, Wang H-F, Sun W-H, 2011. Serotonin Receptor 5-HT2B Mediates Serotonin-Induced Mechanical Hyperalgesia. J Neurosci 31, 1410–1418. 10.1523/JNEUROSCI.4682-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linhart O, Obreja O, and Kress M, 2003. The inflammatory mediators serotonin, prostaglandin E2 and bradykinin evoke calcium influx in rat sensory neurons. Neuroscience 118, 69–74. 10.1016/s0306-4522(02)00960-0 [DOI] [PubMed] [Google Scholar]
- Linnarsson Lab. Mousebrain.org. http://mousebrain.org/genesearch.html (accessed 9.10.20)
- Liu XY, Wu SX, Wang YY, Wang W, Zhou L, Li YQ, 2005. Changes of 5-HT receptor subtype mRNAs in rat dorsal root ganglion by bee venom-induced inflammatory pain. Neurosci Lett 375, 42–46. 10.1016/j.neulet.2004.10.06 [DOI] [PubMed] [Google Scholar]
- Loyd DR, Henry MA, Hargreaves KM, 2013. Serotonergic neuromodulation of peripheral nociceptors. Semin Cell Dev Biol 24, 51–57. 10.1016/j.semcdb.2012.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loyd DR, Weiss G, Henry MA, Hargreaves KM, 2011. Serotonin increases the functional activity of capsaicin-sensitive rat trigeminal nociceptors via peripheral serotonin receptors. Pain 152, 2267–2276. 10.1016/j.pain.2011.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momin A, McNaughton PA, 2009. Regulation of firing frequency in nociceptive neurons by pro-inflammatory mediators. Exp Brain Res 196, 45–52. 10.1007/s00221-009-1744-2 [DOI] [PubMed] [Google Scholar]
- Nagi SS, Marshall AG, Makdani A, Jarocka E, Liljencrantz J, Ridderström M, Shaikh S, O’Neill F, Saade D, Donkervoort S, Foley AR, Minde J, Trulsson M, Cole J, Bönnemann CG, Chesler AT, Bushnell MC, McGlone F, Olausson H, 2019. An ultrafast system for signaling mechanical pain in human skin. Sci Adv 5, 1297 10.1126/sciadv.aaw1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson R, Small J, Dixon AK, Spanswick D, Lee K, 2003. Serotonin receptor mRNA expression in rat dorsal root ganglion neurons. Neurosci Lett 337, 119–122. 10.1016/s0304-3940(02)01256-9 [DOI] [PubMed] [Google Scholar]
- North RY, Li Y, Ray P, Rhines LD, Tatsui CE, Rao G, Johansson CA, Zhang H, Kim YH, Zhang B, Dussor G, Kim TH, Price TJ, Dougherty PM, 2019. Electrophysiological and transcriptomic correlates of neuropathic pain in human dorsal root ganglion neurons. Brain 142, 1215–1226. 10.1093/brain/awz063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa J, Torebjörk E, 1989. Sensations evoked by intraneural microstimulation of C nociceptor fibres in human skin nerves. J Physiol 415, 583–599. 10.1113/jphysiol.1989.sp017737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odem MA, Bavencoffe AG, Cassidy RM, Lopez ER, Tian J, Dessauer CW, Walters ET, 2018. Isolated nociceptors reveal multiple specializations for generating irregular ongoing activity associated with ongoing pain. Pain 159, 2347–2362. 10.1097/j.pain.0000000000001341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta T, Ikemi Y, Murakami M, Imagawa T, Otsuguro K-I, Ito S, 2006. Potentiation of transient receptor potential V1 functions by the activation of metabotropic 5-HT receptors in rat primary sensory neurons. J Physiol 576, 809–822. 10.1113/jphysiol.2006.112250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto K, Imbe H, Morikawa Y, Itoh M, Sekimoto M, Nemoto K, Senba E 2002. 5-HT2A receptor subtype in the peripheral branch of sensory fibers is involved in the potentiation of inflammatory pain in rats. Pain 99, 133–143. 10.1016/S0304-3959(02)00070-2 [DOI] [PubMed] [Google Scholar]
- Pan P, Huang S-S, Shen S-R, Lu C-E, Qin Y-B, Zhang J-L, Cao S, 2019. Role of p120 Catenin in Epac1-Induced Chronic Postsurgical Pain in Rats. Pain Res Manag. 10.1155/2019/9017931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roederer M, 2002. Compensation in flow cytometry Curr Protoc Cytom Chapter 1, Unit 1.14. 10.1002/0471142956.cy0114s22 [DOI] [PubMed] [Google Scholar]
- Salzer I, Gantumur E, Yousuf A, Boehm S, 2016. Control of sensory neuron excitability by serotonin involves 5HT2C receptors and Ca(2+)-activated chloride channels. Neuropharmacology 110, 277–286. 10.1016/j.neuropharm.2016.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelz M, Schmidt R, Weidner C, Hilliges M, Torebjörk HE, Handwerker HO, 2003. Chemical Response Pattern of Different Classes of C-Nociceptors to Pruritogens and Algogens. J Neurophysiol 89, 2441–2448. 10.1152/jn.01139.2002 [DOI] [PubMed] [Google Scholar]
- Scroggs RS, 2011. Up-regulation of low-threshold tetrodotoxin-resistant Na+ current via activation of a cyclic AMP/protein kinase A pathway in nociceptor-like rat dorsal root ganglion cells. Neuroscience 186, 13–20. 10.1016/j.neuroscience.2011.04.046 [DOI] [PubMed] [Google Scholar]
- Serra J, Bostock H, Solà R, Aleu J, García E, Cokic B, Navarro X, Quiles C, 2012. Microneurographic identification of spontaneous activity in C-nociceptors in neuropathic pain states in humans and rats. Pain 153, 42–55. 10.1016/j.pain.2011.08.015 [DOI] [PubMed] [Google Scholar]
- Sharma HS, Olsson Y, Nyberg F, Dey PK, 1993. Prostaglandins modulate alterations of microvascular permeability, blood flow, edema and serotonin levels following spinal cord injury: an experimental study in the rat. Neuroscience 57, 443–449. 10.1016/0306-4522(93)90076-r [DOI] [PubMed] [Google Scholar]
- Singhmar P, Huo X, Li Y, Dougherty PM, Mei F, Cheng X, Heijnen CJ, Kavelaars A, 2018. Orally active Epac inhibitor reverses mechanical allodynia and loss of intraepidermal nerve fibers in a mouse model of chemotherapy-induced peripheral neuropathy. Pain 159, 884–893. 10.1097/j.pain.0000000000001160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Study RE, Kral MG, 1996. Spontaneous action potential activity in isolated dorsal root ganglion neurons from rats with a painful neuropathy. Pain 65, 235–242. 10.1016/0304-3959(95)00216-2 [DOI] [PubMed] [Google Scholar]
- Taiwo YO, Levine JD, 1992. Serotonin is a directly-acting hyperalgesic agent in the rat. Neuroscience 48, 485–490. 10.1016/0306-4522(92)90508-Y [DOI] [PubMed] [Google Scholar]
- Tokunaga A, Saika M, Senba E, 1998. 5-HT2A receptor subtype is involved in the thermal hyperalgesic mechanism of serotonin in the periphery. Pain 76, 349–355. 10.1016/s0304-3959(98)00066-9 [DOI] [PubMed] [Google Scholar]
- Usoskin D, Furlan A, Islam S, Abdo H, Lönnerberg P, Lou D, Hjerling-Leffler J, Haeggström J, Kharchenko O, Kharchenko PV, Linnarsson S, Ernfors P, 2015. Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat Neurosci 18, 145–153. 10.1038/nn.3881 [DOI] [PubMed] [Google Scholar]
- Walters ET, 2019. Adaptive mechanisms driving maladaptive pain: how chronic ongoing activity in primary nociceptors can enhance evolutionary fitness after severe injury. Philos Trans R Soc Lond, B, Biol Sci 374, 20190277 10.1098/rstb.2019.0277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng X, Smith T, Sathish J, Djouhri L, 2012. Chronic inflammatory pain is associated with increased excitability and hyperpolarization-activated current (Ih) in C- but not Aδ-nociceptors. Pain 153, 900–914. 10.1016/j.pain.2012.01.019 [DOI] [PubMed] [Google Scholar]
- Xu J, Brennan TJ, 2010. Guarding pain and spontaneous activity of nociceptors after skin versus skin plus deep tissue incision. Anesthesiology 112, 153–164. 10.1097/ALN.0b013e3181c2952e [DOI] [PMC free article] [PubMed] [Google Scholar]


