Abstract
Objective:
To evaluate safety and pharmacokinetics (PK) of maraviroc administered with standard antiretroviral prophylaxis to HIV-1 exposed infants and to determine the appropriate dose of maraviroc during the first 6 weeks of life.
Design:
Phase I, multi-center, open label study enrolling two sequential cohorts.
Methods:
IMPAACT 2007 participants enrolled by day 3 of life and were stratified by exposure to maternal efavirenz. Cohort 1 participants received two single 8 mg/kg maraviroc doses one week apart with PK sampling after each dose. Cohort 2 participants received 8 mg/kg maraviroc twice daily through 6 weeks of life with PK sampling at weeks 1 and 4. Maraviroc exposure target was Cavg ≥ 75ng/mL. Laboratory and clinical evaluations assessed safety.
Results:
Fifteen Cohort 1 and 32 Cohort 2 HIV-exposed neonates were enrolled (median gestational age 39 weeks, 51% male). All 13 evaluable Cohort 1 infants met the PK target. Median exposure for the 25 evaluable Cohort 2 infants met the PK target but variability was high, with 17–33% of infants below target at Weeks 1 and 4. PK target achievement was similar between efavirenz exposure strata. No Grade 3+ toxicities, early study, or treatment discontinuations due to maraviroc occurred.
Conclusions:
Median maraviroc exposure met the Cavg target in neonates receiving 8 mg/kg twice daily, although exposures were variable. Maternal efavirenz use did not impact maraviroc exposure and no discontinuations were due to maraviroc toxicity/intolerance. No infants acquired HIV-1 infection during follow-up. Maraviroc 8 mg/kg twice daily appears safe and well-tolerated during the first 6 weeks of life.
Keywords: Maraviroc, HIV, pharmacokinetics, neonate, antiretroviral
INTRODUCTION
Despite the significant decline in perinatal transmission of HIV associated with the scale-up of antiretroviral therapy (ART) for pregnant women living with HIV, there were still ~160,000 new pediatric infections worldwide in 2017 and only half of children diagnosed with HIV (HIV+) were receiving ART[1]. Early treatment, within the first few weeks of life, confers lifesaving immunologic and virologic benefits to infants with perinatally-acquired HIV. Thus, there remains a critical need for antiretrovirals (ARVs) for use in HIV prophylaxis and treatment of this population. Due to a lack of ARVs with appropriate formulations and sufficient safety and pharmacokinetic (PK) data for neonates, treatment options remain significantly limited. In the last fourteen years, only two ARVs have been licensed for use in neonates, emtricitabine in 2006 and raltegravir in 2017. It is well-established that drug disposition in neonates and young infants is different from that in older individuals due to the effects of development and maturation of the physiologic processes responsible for drug absorption, distribution, metabolism and excretion[2]. Dosing of ARVs in this population must strike the fine balance between providing sufficient drug to achieve virologic suppression and avoiding excessive concentrations with the potential for toxicity[3]. Because many infants living with HIV have a purely CCR5-tropic virus, maraviroc, a CCR5 receptor antagonist approved for treatment of HIV-1 infection in adults, is attractive as a potential component of neonatal prophylaxis and treatment regimens[4]. It may also be valuable for infants born to mothers whose HIV strains are resistant to commonly used ARV classes such as nonnucleoside reverse transcriptase inhibitors (NNRTIs).
Data from a study of maraviroc in combination with optimized background therapy in ARV-experienced children 2 to <18 years of age demonstrated the tolerability, effectiveness, and safety of maraviroc in this age group and led to its approval in 2016 to treat children two years of age and older living with HIV-1 infection[5]. Normal growth and maturation during the first two years of life may have a large impact on maraviroc absorption, distribution, metabolism, and elimination, so it cannot be administered to younger infants until its PK and safety are studied in this population. Maraviroc is metabolized by CYP3A and in adults, a dose increase is required when it is administered with the CYP3A agonist efavirenz[6]. Efavirenz is readily transferred from mother to infant across the placenta during pregnancy and via breast milk after delivery[7, 8]. Efavirenz use during pregnancy and lactation may result in accumulation of biologically active plasma efavirenz concentrations in neonates with the potential to induce neonatal CYP3A-mediated maraviroc clearance. Thus, the impact of in utero and postnatal exposure from maternal efavirenz on infant maraviroc exposure must also be determined.
The overarching goals of this study were to evaluate the safety, tolerability, and PK of maraviroc solution when administered with routine ARV prophylaxis to HIV-1 exposed neonates in addition to determining appropriate dose options for maraviroc solution over the first six weeks of life to achieve exposure within the range observed in large Phase IIb/III adult studies and the Phase II pediatric study[5, 9–11].
METHODS
Study Design
International Maternal Pediatric Adolescent AIDS Clinical Trials Network (IMPAACT) 2007 was a Phase I, multi-center, open-label study of maraviroc safety and PK in full-term, HIV-1-exposed neonates receiving standard ARV prophylaxis for prevention of perinatal HIV transmission. Mother-infant pairs were enrolled within three days of infant birth and infants received their first dose of maraviroc solution at Entry. Study design included two sequential dosing cohorts. Due to the significant drug-drug PK interaction between maraviroc and efavirenz in adults, infants were stratified by exposure to maternal efavirenz. Cohort 1 was stratified by in utero exposure to maternal efavirenz and Cohort 2 was stratified by exposure to maternal efavirenz through breastfeeding after birth. Protocol approval was obtained from all required institutional review boards, ethics committees, and applicable regulatory entities at all participating sites and signed informed consent was obtained from the mothers of all infants before participation in the trial.
Cohort 1 infants received approximately 8 mg/kg maraviroc oral solution as single doses at Entry and Week 1 (7–14 days of life), with intensive PK sampling after each dose (Stratum 1A: infants without in utero exposure to maternal efavirenz, Stratum 1B: infants with in utero exposure to maternal efavirenz). The protocol was designed to achieve a target of 12 evaluable infants (6 in each stratum) receiving the dose of maraviroc which passed the safety and PK guidelines for each stratum and could be recommended for the corresponding stratum in Cohort 2. Based on these data, Cohort 2 infants received chronic dosing with 8 mg/kg maraviroc oral solution twice daily from entry through Week 6 (35–42 days of life), with intensive PK sampling at Weeks 1 and 4. (Stratum 2A: infants without exposure to maternal efavirenz either in utero and if breastfeeding, while breastfeeding, Stratum 2B: infants with exposure to maternal efavirenz both in utero and during breastfeeding). The protocol was designed to achieve a target of 24 evaluable infants (12 in each stratum) receiving the final recommended dose of maraviroc which passed safety and PK criteria for each stratum.
Infants were followed for 16 weeks (112–140 days of life). Laboratory and clinical evaluations assessed safety at Entry and Weeks 1, 2, 6, and 16 in Cohort 1 and at Weeks 1, 4, 6, 12, and 16 in Cohort 2. Study evaluations included physical exam, medical history, diagnoses, signs and symptoms, chemistries, and hematologies. Adverse events (AE) were graded according to the Division of AIDS Table for Grading the Severity of Adult and Pediatric Adverse Events, Corrected Version 2.1, dated July 2017 [12]. All toxicities were monitored by the Core Team monthly and Grade 3 or greater AEs were monitored until resolution. Safety endpoints included any life-threatening AE, including death, assessed by the Core Team as at least possibly related to the study drug; AEs of Grade 3 or greater judged by the Core Team to be probably or definitely related to the study drug, or that result in permanent discontinuation of study drug due to an AE, judged by the Core Team to be at least possibly related to study drug. Dose-finding safety assessments were done for each stratum within a cohort through Day 7-post dose (Cohort 1), and through Week 6 (Cohort 2). Infants were deemed evaluable for dose-finding safety assessments if they received the required amount of maraviroc per protocol or had met any of the safety endpoints. The primary and secondary safety analyses were carried out for both cohorts through Week 6 and Week 16, respectively, using the safety endpoints described above, and included all infants who received at least one dose of maraviroc.
Mothers had evaluations performed at screening and Entry and exited the study after the Entry visit. Inclusion criteria for infants were an estimated gestational age of at least 37 weeks, birth weight of at least 2 kg, an age of ≤ 3 days old, absence of a potent CYP3A inhibitor or inducer as part of the background ARV prophylaxis, Grade 0 (normal) alanine aminotransferase (ALT), ≤ Grade 1 aspartate aminotransferase (AST) and total bilirubin, ≤ Grade 2 hemoglobin, white blood cell counts, and platelet counts, and born after singleton delivery. Exclusion criteria included maternal use of maraviroc during pregnancy, infant weight below 2 kg, infants with a positive HIV-1 nucleic acid test at Entry, and infant or breastfeeding mother receipt of any protocol-defined disallowed medications.
Pharmacokinetic Methods
Plasma samples were analyzed for maraviroc by the IMPAACT Pharmacology Specialty Laboratory at the University of Alabama Birmingham using a validated analytical method based on protein precipitation, followed by HPLC/MS/MS analysis. The lower limit of quantification (LLQ) was 5 ng/mL using a 20 μL aliquot of Lithium Heparin plasma. The higher limit of quantification (HLQ) was 2,000 ng/mL.
In Cohort 1, samples were collected pre-dose, and at 1–2, 4–8, 11–13, 20–24, and 48–72 hours post dose at Entry. At Week 1, samples were collected pre-dose, and at 1–2 and 22–26 hours post dose. In Cohort 2, at both study visits at Weeks 1 and 4, samples were collected at pre-dose, and at 1–2, 3–5, 6–8, and 11–13 hours post dose. Standard non-compartmental methods for PK parameter derivation were performed using Phoenix WinNonlin (Certara USA, Inc). Maraviroc exposure target was Cavg of ≥ 75 ng/mL, which is the exposure associated with near-maximal efficacy in the treatment-naïve adult study (MERIT) of maraviroc given with zidovudine and lamivudine[13]. Maraviroc concentration values below the level of quantification (BLQ), <5 ng/mL were set to zero. Elimination rate constants were calculated from two or three declining concentrations after the peak concentration. AUC was calculated by the linear trapezoidal rule. For Cohort 1, AUC(0−∞) was used. For Cohort 2, AUC(0-τ) was used. Because the trough values were drawn over a range of times (11–13 hours post dose), the predicted AUC(0-τ) was reported and used to calculate Cavg. Infants were deemed evaluable for the PK analyses if protocol-specified maraviroc dosing was maintained through completed PK visits.
RESULTS
Forty-seven maraviroc-naïve, HIV-exposed neonates and their mothers enrolled from the USA (42%), Thailand (6%), Kenya (4%), and South Africa (47%). The majority identified as Black/African American and all infants received at least one dose of maraviroc (Table 1).
Table 1.
Infant Baseline Characteristics and Background Antiretrovirals (All Treated Infants)a
| Cohort 1 | Cohort 2 | |||||
|---|---|---|---|---|---|---|
| Stratum 1A N=8 (%) | Stratum 1B N=7 (%) | Total N=15 (%) | Stratum 2A N=16 (%) | Stratum 2B N=16 (%) | Total N=32 (%) | |
| Sex | ||||||
| Female | 5 (63) | 4 (57) | 9 (60) | 7 (44) | 7 (44) | 14 (44) |
| Male | 3 (38) | 3 (43) | 6 (40) | 9 (56) | 9 (56) | 18 (56) |
| Race | ||||||
| Asian | 0 (0) | 0 (0) | 0 (0) | 3 (19) | 0 (0) | 3 (9) |
| Black or African American | 5 (63) | 7 (100) | 12 (80) | 10 (63) | 16 (100) | 26 (81) |
| White | 3 (38) | 0 (0) | 3 (20) | 3 (19) | 0 (0) | 3 (9) |
| Gestational age (weeks) | ||||||
| Median | 38.5 | 39.0 | 39.0 | 39.0 | 40.0 | 39.0 |
| Q1,Q3b | 38.0,39.5 | 38.0,39.0 | 38.0,39.0 | 38.0,39.5 | 39.0,40.5 | 38.5,40.0 |
| Birth Weight (kg) | ||||||
| Median | 3.2 | 3.4 | 3.3 | 3.0 | 3.0 | 3.0 |
| Q1,Q3b | 2.8,3.5 | 2.9,3.6 | 2.9,3.5 | 2.9,3.2 | 2.8,3.2 | 2.8,3.2 |
| Background ARVs NRTI Only | ||||||
| 3TC, ZDV | 0 (0) | 0 (0) | 0 (0) | 1 (6) | 0 (0) | 1 (3) |
| ZDV | 7 (88) | 0 (0) | 7 (47) | 11 (69) | 0 (0) | 11 (34) |
| NNRTI(+/− NRTI) | ||||||
| 3TC, NVP, ZDV | 0 (0) | 0 (0) | 0 (0) | 3 (19) | 0 (0) | 3 (9) |
| NVP | 0 (0) | 7 (100) | 7 (47) | 0 (0) | 12 (75) | 12 (38) |
| NVP, ZDV | 1 (13) | 0 (0) | 1 (7) | 1 (6) | 4 (25) | 5 (16) |
ARV=antiretrovirals, NRTI=nucleoside reverse transcriptase inhibitor, NNRTI=non-nucleoside reverse transcriptase inhibitor, NVP=nevirapine, ZDV=zidovudine
Q1, Q3=25th and 75th percentiles, respectively
Pharmacokinetics
Cohort 1
Fifteen participants were enrolled into Cohort 1, eight in Stratum 1A and seven in Stratum 1B. Two of the participants in Stratum 1A did not receive the second dose of maraviroc, therefore did not complete the Week 1 PK sampling and were not evaluable for dose-finding purposes. Six participants in Stratum 1A and seven in Stratum 1B were evaluable for dose-finding purposes.
Maraviroc average concentration (Cavg) was determined for all infants at the Entry visit after the initial dose. At the Week 1 visit, the pre-dose samples were all below the LLQ and the vast majority of the 22–26 hour post-dose samples were below the LLQ, leaving only the 1–2 hour post-dose sample with a measurable concentration (the maximum concentration observed at that visit for all infants). This precluded an AUC calculation using standard non-compartmental methods for the Week 1 visit in Cohort 1. Pharmacokinetic parameters for all infants in Cohort 1 are shown in Table 2 and median concentration versus time curves are shown in Figure 1A.
Table 2.
Summary PK Parameters by Study Cohorta
| PK Parameters | Cohort 1 (Entry) N=13 | Cohort 1 (Week 1) N=13 | Cohort 2 (Week 1) N=25 | Cohort 2 (Week 4) N=25 |
|---|---|---|---|---|
| Cmax (ng/mL) | 510 [30–1618] | 137 [9–609] | 284 [34–1468] | 304 [77–793] |
| Tmax (h) | 1.8 [1.1–12.8] | 1.2 [1.1–1.3] | 1.6 [0.8–6.2] | 1.5 [0.0–11.4] |
| AUC (ng*h/mL) | 3734 [1123–15343] | ND | 1616 [205–6788] | 1123 [395–5859] |
| Cl/F (L/hr) | 6.7 [2.0–30.2] | ND | 15.9 [3.0–122.0] | 22.6 [4.3–63.3] |
| Cavg (ng/mL) | 311 [94–1279] | ND | 135 [17–566] | 93.6 [33–488] |
Median [minimum-maximum] except for N; AUC=Area under the concentration time curve to infinity for Cohort 1 (single dose), and to 12 hours for Cohort 2 (steady-state); Cavg= average concentration (AUC divided by tau set to 12 hours); Cl/F = oral clearance; Cmax = maximum observed concentration; Tmax = time of Cmax; ND = not determined.
Figure 1A:
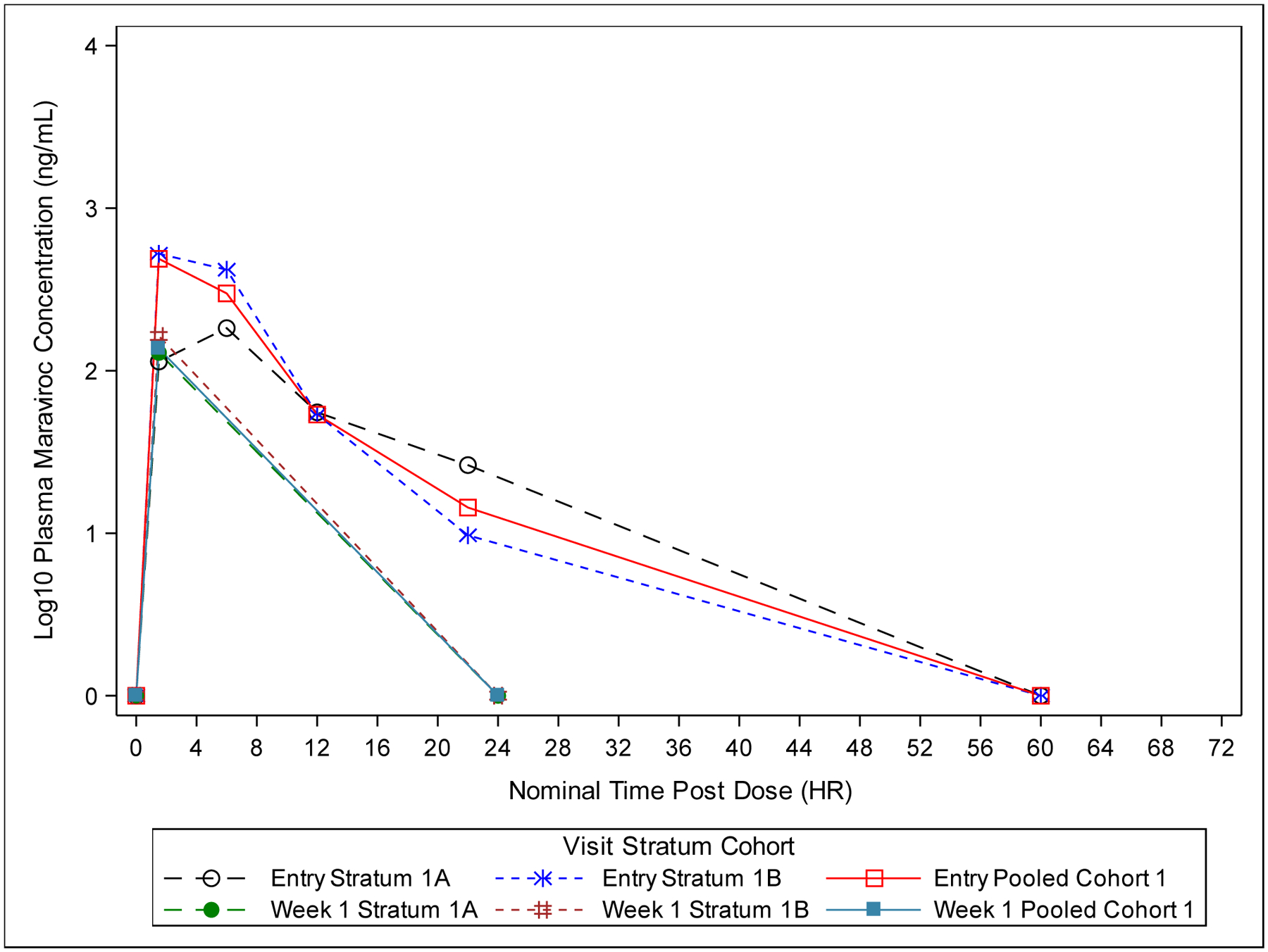
Plot of Median Plasma Maraviroc Concentration-Time (Semi-Log) for Cohort 1 (All Dose-Finding Evaluable Infants). Values below the Lower Limit of Quantification (<5.00 ng/mL) are set to 0.
In Strata 1A and 1B, the Cavg exceeded the target value of 75 ng/mL in all infants when using a tau (dosing interval) of 12 hours (Figure 2A). For Cohort 1, infants received maraviroc doses ranging from 20–40 mg (7.82–10.08 mg/kg) at Entry (within 3 days of birth) and 25–40 mg (7.89–10.13 mg/kg) at Week 1 (sparse PK sampling). Using a tau of 24 hours, 4 of 6 participants in Stratum 1A and 0 participants in Stratum 1B had a Cavg above 75 ng/mL with the 8 mg/kg dose used in Cohort 1. The median Cmax values for the Entry and Week 1 visits for Stratum 1A were 227 ng/mL and 129 ng/mL, respectively. PK parameters showed high intra- and inter-participant variability, with Cmax concentrations ranging from 30 ng/mL to 1618 ng/mL at Entry and 23 ng/mL to 296 ng/mL at Week 1. For Stratum 1B, median Cmax at Entry and Week 1 were 551 ng/mL and 163 ng/mL. Similar to Stratum 1A, variability was high, with Cmax concentrations ranging from 204 ng/mL to 1153 ng/mL at Entry and 9 ng/mL to 609 ng/mL at Week 1. Maraviroc exposures were comparable between the strata in Cohort 1. Based on the evaluation of Cohort 1 PK results, particularly Cavg, a dose of 8 mg/kg every 12 hours was selected for administration in Strata 2A and 2B of Cohort 2.
Figure 2A:
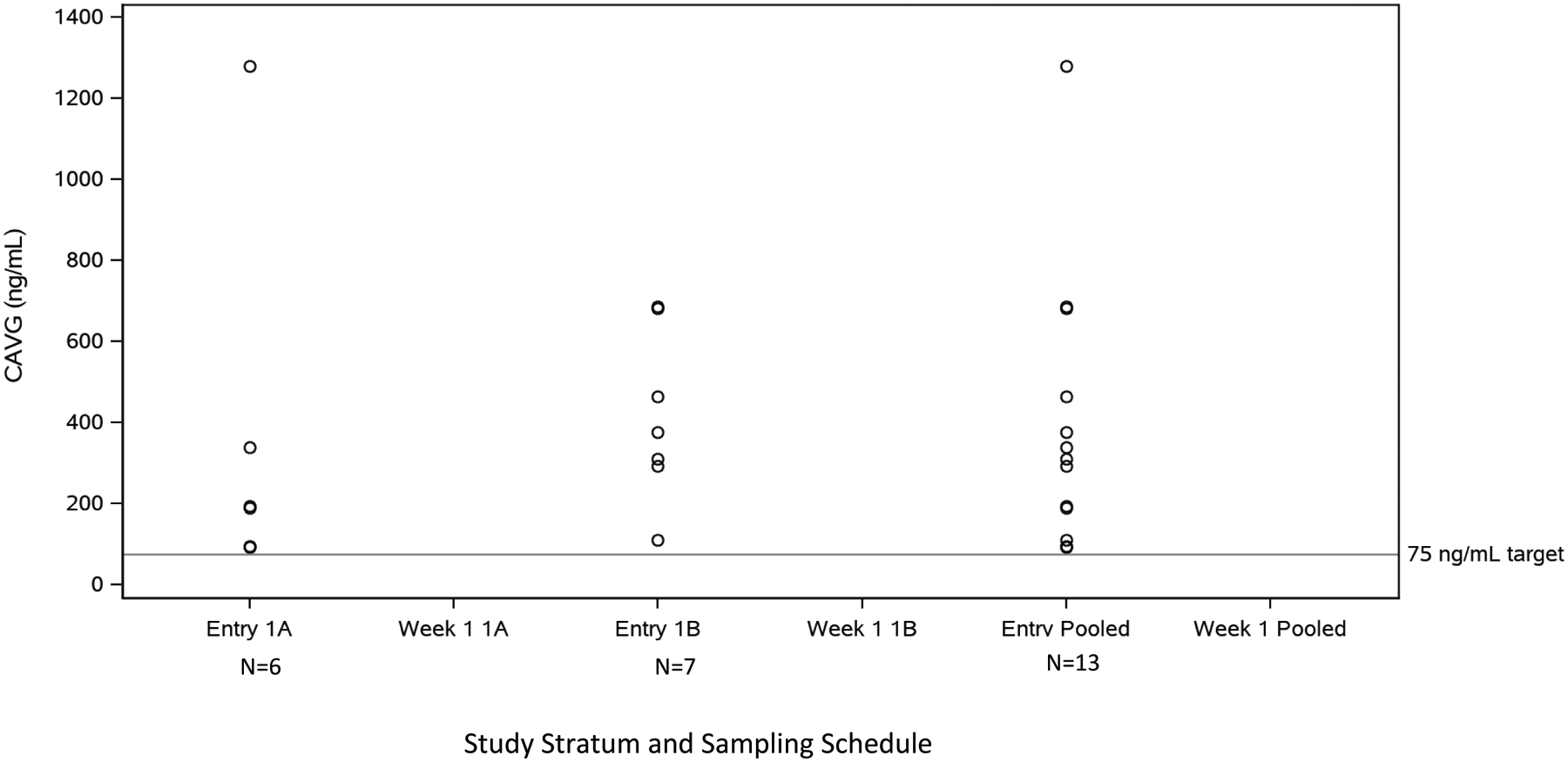
CAVG Summary of Cohort 1 PK Dose-Finding Evaluable Infants. CAVG calculated for Q12hr dosing.
Cohort 2
Thirty-two participants were enrolled into Cohort 2, sixteen in Stratum 2A and sixteen in Stratum 2B. For Cohort 2, infants received maraviroc doses ranging from 20–30 mg (6.76–9.47 mg/kg) BID at Week 1 and 20–40 mg (6.13–9.86 mg/kg) BID at Week 4. Three participants in Stratum 2A and 4 participants in Stratum 2B were not evaluable for PK. One Stratum 2A participant was unevaluable owing to a missed PK sampling visit and the other two due to caregiver withdrawal from participation prior to completing intensive PK sampling at Weeks 1 and 4. In Stratum 2B, four participants were unevaluable due to exclusionary total bilirubin inadvertently identified after entry (1), caregiver decision to withdraw participation prior to completing PK samplings (2), and nonadherence to study drug (1). Therefore, thirteen participants in Stratum 2A and twelve participants in Stratum 2B were PK evaluable for dose-finding purposes (Table 2).
In Stratum 2A, Cavg achieved the target of 75 ng/mL in ten of thirteen infants at Week 1 and in four of thirteen infants at Week 4. Two infants were below the Cavg target at both Week 1 and Week 4. Median (range) Cavg values were 152 (20–566) and 94 (33–488) ng/mL at Weeks 1 and 4, respectively. Median (range) Cmax values were 257 (52–1468) ng/mL at Week 1 and 417 (125–793) ng/mL at Week 4. Median concentration versus time curves for Cohort 2 visits are shown in Figure 1B.
Figure 1B:
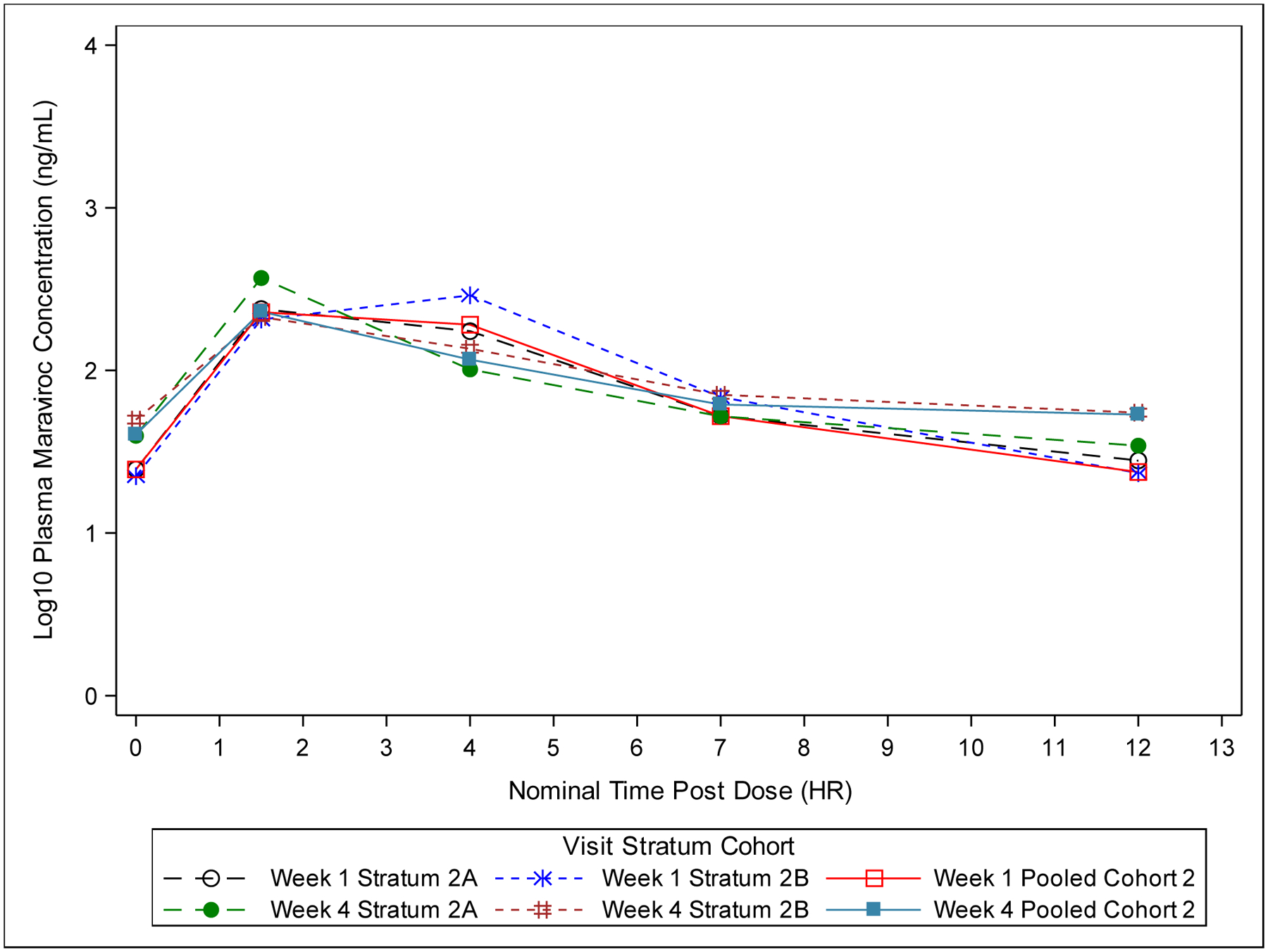
Plot of Median Plasma Maraviroc Concentration-Time (Semi-Log) for Cohort 2 (All Dose-Finding Evaluable Infants). Values below the Lower Limit of Quantification (<5.00 ng/mL) are set to 0.
In Stratum 2B, Cavg achieved the target of 75 ng/mL in eight of twelve infants at Week 1 and in five of twelve infants at Week 4. Four infants were below the Cavg target at both the Week 1 and Week 4 visits. Median (range) Cavg values were 125 (17–551) and 101 (43–351) ng/mL at Weeks 1 and 4, respectively. Median (range) Cmax values were 309 (34–1274) ng/mL at Week 1 and 222 (77–739) ng/mL at Week 4 (Figure 2B). Similar to Cohort 1, Cohort 2 infants displayed high PK variability and maraviroc exposures were comparable between strata.
Figure 2B:
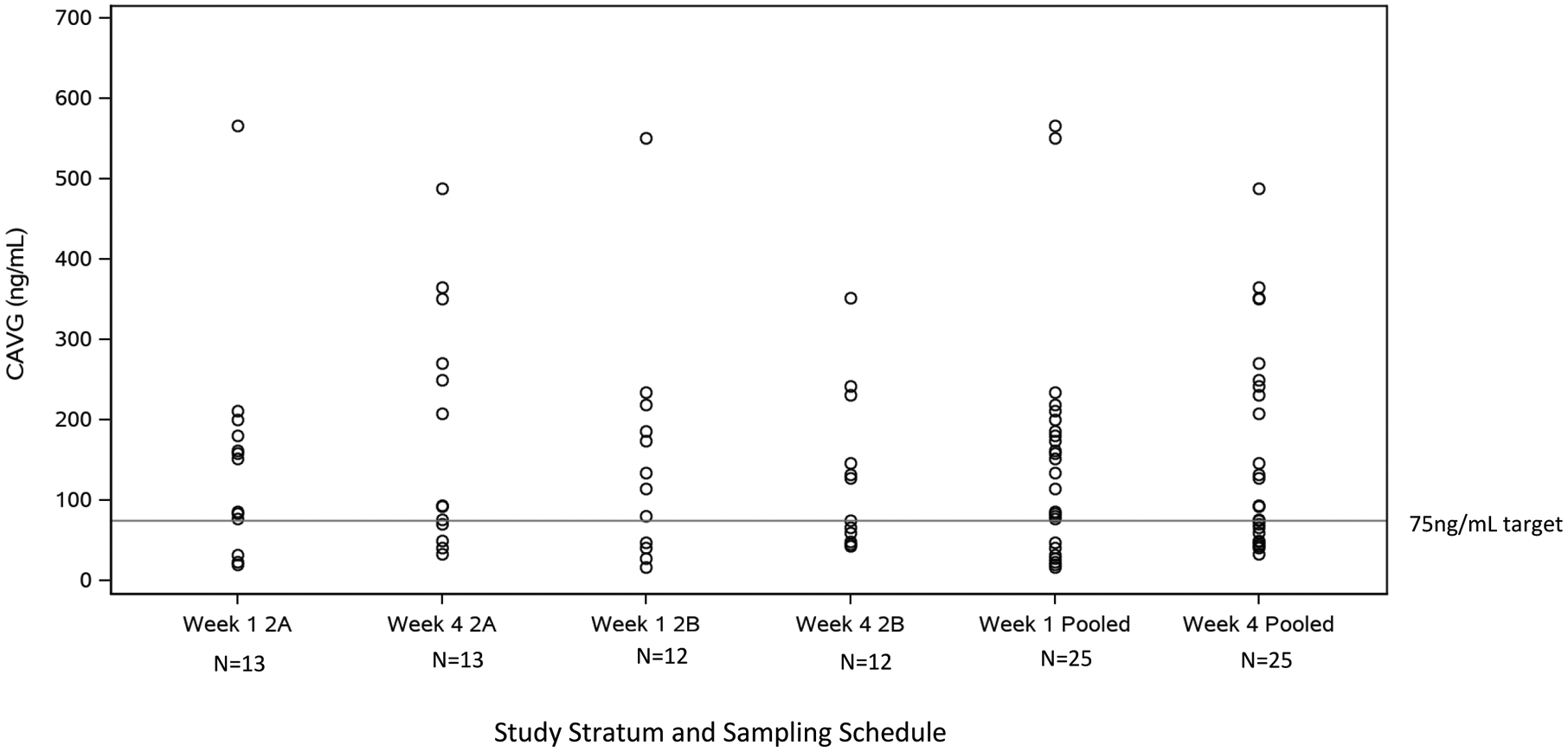
CAVG Summary of Cohort 2 PK Dose-Finding Evaluable Infants. CAVG calculated for Q12hr dosing.
Safety
There were no participants who received at least 1 dose of maraviroc that met any safety endpoints in the primary and secondary safety analyses through Week 6 and through Week 16 of follow-up, respectively, as determined by the Core Team (Stratum 1A (n=8): 0%, 95% CI [0.0, 36.9]; 1B (n=7): (0%), [0.0, 41.0]; 2A (n=16): (0%), [0.0, 20.6]; 2B (n=16): (0%), [0.0, 20.6]).
The only Grade 3 or greater AE in Cohort 1 through Week 16 included 1 of 8 infants in Stratum 1A with ophthalmia neonatorum and staphylococcal sepsis. No Grade 3 or greater AEs were noted in Stratum 1B. Through Week 16, Stratum 2A had a total of 7 of 16 infants with Grade 3 or greater AEs: (3) elevated bilirubin levels; (1) infant with elevated bilirubin and Grade 4 neutropenia; (1) infant with failure to thrive; (1) infant with anemia; and (1) infant with hypoxia and bacterial pneumonia. Stratum 2B had 5 of 16 infants with Grade 3 or greater AEs: (3) infants with neutropenia, (1) infant with failure to thrive and weight decrease, and (1) infant with anemia and jaundice. All Grade 3 or greater AEs were deemed unrelated to maraviroc by the Core Team. No early study or early treatment discontinuations were noted due to maraviroc and no enrolled infants acquired HIV-1 infection during follow-up.
DISCUSSION
This Phase I study evaluated the safety, tolerability, and PK of maraviroc solution when administered with routine ARV prophylaxis to perinatally HIV-1 exposed infants through the first 6 weeks of life. Maraviroc was determined to be safe and well-tolerated in this cohort in whom it was initiated within three days of life and administered at a dose of approximately 8mg/kg twice daily (range: 6.13 to 10.13 mg/kg; 20–40 mg BID) through Week 6. High intra- and inter-participant variability was seen with maraviroc exposures, though no safety concerns were identified through 16 weeks of follow-up. Historically, new ARVs in neonates have been studied largely in those at high risk of perinatal HIV-1 acquisition. Our study, however, enrolled participants regardless of risk status, making the findings applicable to a broader population. Additionally, the rigorous evaluation of the use of maraviroc in neonates adds an additional antiretroviral drug class for use for HIV prevention in neonates.
Though high intra- and inter-participant variability was demonstrated with respect to achieving the target exposure at a dose of 8mg/kg twice daily, this finding in neonates is not unique to maraviroc. Studies evaluating the PK of the protease inhibitor, nelfinavir, in neonates showed high variability and failure to meet exposure targets in almost half of infants receiving doses ranging from 10 mg three times daily to 60 mg/kg twice daily[14–16]. These data suggest that an increased dose to nearly 120mg/kg/day did not significantly impact achievement of target exposures for nelfinavir. Difficulty with achieving optimal exposure, given the variability with dosing and rapid physiologic development, has been seen with other ARVs in young infants. A PK analysis of the protease inhibitor lopinavir/ritonavir in nine infants 3.6–5.9 weeks of age in the IMPAACT P1030 study demonstrated high variability and a median lopinavir exposure that was roughly half that in infants six weeks to six months of age[17]. However, 4 of 9 infants had exposures in the range of older infants and a higher dose might have put these infants at risk of possible toxicity. The one participant in that trial who had a 50% lopinavir/ritonavir dosage increase based on PK results subsequently required dosage reduction for excessive drug concentrations and possible drug-related toxicity. A second study evaluating lopinavir/ritonavir population PK from 2 weeks to 6 months of age found high apparent clearance in the youngest infants which decreased with age[18].
Raltegravir, the only other antiretroviral recently studied in neonates, requires an 8-fold increase in the total daily dose over the first four weeks of life to maintain therapeutic drug concentrations, consistent with metabolism of raltegravir by UGT1A1 and the known rapid increase in activity of this enzyme over the first few weeks of life. The final neonatal raltegravir dosing recommendations include an increase from 1.5 mg/kg once daily to 6 mg/kg twice daily over the first four weeks of life in order to maintain adequate plasma exposures in the face of rapid maturation of UGT1A1 activity[19, 20]. In contrast, our data indicate that maraviroc, whose main route of elimination is CYP3A metabolism, can be dosed at 8 mg/kg twice daily throughout the first six weeks of life. As compared to UGT1A1 metabolism, which rapidly matures over the first few weeks of life, CYP3A metabolism is slower to mature and occurs over the first few years[21–23]. As a result, maraviroc does not require a dosing change in the first weeks of life, making it easier to administer.
In this study, enrolled infants were stratified by exposure to maternal efavirenz. Maraviroc clearance in adults is induced when co-administered with efavirenz, a CYP3A inducer, and the recommended maraviroc dose is doubled in adults receiving both agents[6]. In areas with high HIV-1 seroprevalence, efavirenz is commonly used during pregnancy and is known to be transferred from mother-to-fetus across the placenta and after birth from mother-to-infant via breast milk[7, 8]. Theoretically, the transfer of efavirenz may result in the accumulation of biologically active plasma efavirenz concentrations in neonates with the potential to induce neonatal clearance of maraviroc. However, maternal efavirenz use had no clinically relevant effect on neonatal maraviroc exposure in our study participants, likely due to the relative immaturity of CYP3A metabolism at birth and its very gradual increase early in life. Therefore, in settings where efavirenz is routinely used in pregnancy, suboptimal neonatal maraviroc exposures related to maternal use of efavirenz should not be an issue.
It is notable that the tolerability and safety of maraviroc, despite PK variability, was found to be favorable in this study wherein no participants met any safety endpoints in the primary and secondary analyses at Week 6 and through Week 16 of follow-up, respectively. The lack of early treatment discontinuations related to maraviroc or reported HIV-1 transmission events, supports the notion that maraviroc is safe in this population, diversifying the options for prophylaxis and presumptive treatment of term infants at risk for perinatally-acquired HIV-1 infection.
CONCLUSION
Maraviroc solution appears safe and well-tolerated at the dose of approximately 8 mg/kg given twice daily over the first six weeks of life in HIV-1-exposed neonates at risk of acquiring infection. There were no safety concerns identified through 16 weeks of follow-up, as none of the participants who received at least one dose of the study drug met any of the safety endpoints. As with other ARVs in young infants, maraviroc PK parameters showed high intra- and inter-participant variability, but approximately two-thirds of the infants in Cohort 2 achieved the PK target of Cavg ≥ 75 ng/mL at Weeks 1 and 4. Maternal efavirenz use prenatally or during breastfeeding appeared to have no effect on maraviroc exposure so infant dose adjustment should not be required. Maraviroc is a promising agent in combination with standard ARVs for prophylaxis and presumptive treatment of HIV-1-exposed term neonates.
ACKNOWLEDGEMENTS
Overall support for the International Maternal Pediatric Adolescent AIDS Clinical Trials Network (IMPAACT) was provided by the National Institute of Allergy and Infectious Diseases (NIAID) with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Institute of Mental Health (NIMH), all components of the National Institutes of Health (NIH), under Award Numbers UM1AI068632 (IMPAACT LOC), UM1AI068616 (IMPAACT SDMC) and UM1AI106716 (IMPAACT LC), and by NICHD contract number HHSN275201800001I. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Additional funding support and study product was provided by ViiV Healthcare.
We gratefully acknowledge the participants and their families for their participation in addition to the contributions of the site investigators and staff who conducted the IMPAACT 2007 study:
SOUTH AFRICA. Perinatal HIV Research Unit, University of the Witwatersrand: Ntatule Hilda Ndiweni, Bcur; Zaakirah Essack, BPharm; Mandisa Nyati, MBChB. CAPRISA Umlazi Clinical Research Site: Lorna Pillay; Rosemary Gazu, Dip; Natasha Pillay, Diploma IT; Damien Sookoo. THAILAND. Siriraj Hospital, Mahidol University: Kulkanya Chokephaibulkit, MD; Supattra Rungmaitree, MD, MSc; Keswadee Lapphra, MD; Orasri Wittawatmongkol, MD. KENYA. Kenya Medical Research Institute - Walter Reed Project Clinical Research Center: Isaac Tsikhutsu, MMED; Edner Openda, BSc; Priscillah Bii, HND; David Wekulo, BSc. UNITED STATES. Ann & Robert H. Lurie Children’s Hospital of Chicago: Ellen Chadwick, MD; Jessica D’Angelo, APN; Margaret Ann Sanders, MPH. University of Southern California: Alice Stek, MD; Mikhaela Cielo, MD; LaShonda Spencer, MD; Yvonne Morales, LVN. University of Colorado, Children’s Hospital Colorado: Christiana Smith-Anderson MD, MSc; Kacey Navarro, MSN, FNP-C; Carrie Glenny, MS, RN; Elizabeth McFarland, MD. Rush University - Cook County Hospital Chicago: Maureen McNichols RN, MSN; Julie Schmidt, MD; Helen Cejtin, MD; Ixchell Ortiz-Estes, MSN, CPNP. St. Jude Children’s Research Hospital: Katherine Knapp, MD; Nehali Patel, MD; Patricia M. Flynn, MD; Jill Utech, RN, MSN. IMPAACT Operations Center: Kathleen George, MPH; Shane Reynolds, MSHS. IMPAACT Laboratory Center: William Murtaugh, MPH. IMPAACT Statistical and Data Analysis Center: Terence Fenton, EdD; Michelle Hsu, MS; Jamie Branco-Ricard, MPH; Victoria Wong, BS. IMPAACT Data Management Center: Barbara Heckman, BS; Kyle Whitson, MA; Shawn Ward, MS. ViiV Healthcare: Navdeep K. Thoofer.
REFERENCES
- 1.UNAIDS. Global HIV & AIDS statistics — 2019 fact sheet. Available from: https://www.unaids.org/en/resources/fact-sheet.
- 2.Smits A, Annaert P, Allegaert K. Drug disposition and clinical practice in neonates: cross talk between developmental physiology and pharmacology. Int J Pharm 2013; 452(1–2):8–13. [DOI] [PubMed] [Google Scholar]
- 3.Clarke DF, Penazzato M, Capparelli E, Cressey TR, Siberry G, Sugandhi N, et al. Prevention and treatment of HIV infection in neonates: evidence base for existing WHO dosing recommendations and implementation considerations. Expert Rev Clin Pharmacol 2018; 11(1):83–93. [DOI] [PubMed] [Google Scholar]
- 4.Church JD, Huang W, Mwatha A, Musoke P, Jackson JB, Bagenda D, et al. Analysis of HIV tropism in Ugandan infants. Curr HIV Res 2010; 8(7):498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giaquinto C, Mawela MP, Chokephaibulkit K, Negra MD, Mitha IH, Fourie J, et al. Pharmacokinetics, Safety and Efficacy of Maraviroc in Treatment-experienced Pediatric Patients Infected With CCR5-Tropic HIV-1. Pediatr Infect Dis J 2018; 37(5):459–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.ViiV Healthcare. Maraviroc Package Insert. 2015.
- 7.McCormack SA, Best BM. Protecting the fetus against HIV infection: a systematic review of placental transfer of antiretrovirals. Clin Pharmacokinet 2014; 53(11):989–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olagunju A, Bolaji OO, Amara A, Waitt C, Else L, Soyinka J, et al. Development, validation and clinical application of a novel method for the quantification of efavirenz in dried breast milk spots using LC-MS/MS. J Antimicrob Chemother 2015; 70(2):555–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cooper DA, Heera J, Goodrich J, Tawadrous M, Saag M, Dejesus E, et al. Maraviroc versus efavirenz, both in combination with zidovudine-lamivudine, for the treatment of antiretroviral-naive subjects with CCR5-tropic HIV-1 infection. J Infect Dis 2010; 201(6):803–813. [DOI] [PubMed] [Google Scholar]
- 10.Gulick RM, Lalezari J, Goodrich J, Clumeck N, DeJesus E, Horban A, et al. Maraviroc for previously treated patients with R5 HIV-1 infection. N Engl J Med 2008; 359(14):1429–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saag M, Goodrich J, Fatkenheuer G, Clotet B, Clumeck N, Sullivan J, et al. A double-blind, placebo-controlled trial of maraviroc in treatment-experienced patients infected with non-R5 HIV-1. J Infect Dis 2009; 199(11):1638–1647. [DOI] [PubMed] [Google Scholar]
- 12.U.S. Department of Health and Human Services NIoH, National Institute of Allergy and Infectious Diseases, Division of AIDS. Division of AIDS (DAIDS) Table for Grading the Severity of Adult and Pediatric Adverse Events, Corrected Version 2.1. July 2017.
- 13.Sierra-Madero J, Di Perri G, Wood R, Saag M, Frank I, Craig C, et al. Efficacy and safety of maraviroc versus efavirenz, both with zidovudine/lamivudine: 96-week results from the MERIT study. HIV Clin Trials 2010; 11(3):125–132. [DOI] [PubMed] [Google Scholar]
- 14.Rongkavilit C, van Heeswijk RP, Limpongsanurak S, Thaithumyanon P, Boonrod C, Hassink EA, et al. Dose-escalating study of the safety and pharmacokinetics of nelfinavir in HIV-exposed neonates. J Acquir Immune Defic Syndr 2002; 29(5):455–463. [DOI] [PubMed] [Google Scholar]
- 15.Mirochnick M, Stek A, Acevedo M, Keller M, Holland D, Capparelli E, et al. Safety and pharmacokinetics of nelfinavir coadministered with zidovudine and lamivudine in infants during the first 6 weeks of life. J Acquir Immune Defic Syndr 2005; 39(2):189–194. [PubMed] [Google Scholar]
- 16.Mirochnick M, Nielsen-Saines K, Pilotto JH, Pinto J, Veloso VG, Rossi S, et al. Nelfinavir and Lamivudine pharmacokinetics during the first two weeks of life. Pediatr Infect Dis J 2011; 30(9):769–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chadwick EG, Pinto J, Yogev R, Alvero CG, Hughes MD, Palumbo P, et al. Early initiation of lopinavir/ritonavir in infants less than 6 weeks of age: pharmacokinetics and 24-week safety and efficacy. Pediatr Infect Dis J 2009; 28(3):215–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nikanjam M, Chadwick EG, Robbins B, Alvero C, Palumbo P, Yogev R, et al. Assessment of lopinavir pharmacokinetics with respect to developmental changes in infants and the impact on weight band-based dosing. Clin Pharmacol Ther 2012; 91(2):243–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clarke DF, Mirochnick M, Acosta EP, Capparelli E, Chain A, Teppler H, et al. Use of Modeling and Simulations to Determine Raltegravir Dosing in Neonates: A Model for Safely and Efficiently Determining Appropriate Neonatal Dosing Regimens: IMPAACT P1110. J Acquir Immune Defic Syndr 2019; 82(4):392–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clarke DF, Acosta EP, Cababasay M, Wang J, Chain A, Teppler H, et al. Raltegravir (RAL) in Neonates: Dosing, Pharmacokinetics (PK), and Safety in HIV-1-Exposed Neonates at Risk of Infection (IMPAACT P1110). J Acquir Immune Defic Syndr 2020; 84(1):70–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu H, Rosenbaum S. Developmental pharmacokinetics in pediatric populations. J Pediatr Pharmacol Ther 2014; 19(4):262–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krekels EH, Danhof M, Tibboel D, Knibbe CA. Ontogeny of hepatic glucuronidation; methods and results. Curr Drug Metab 2012; 13(6):728–743. [DOI] [PubMed] [Google Scholar]
- 23.Kawade N, Onishi S. The prenatal and postnatal development of UDP-glucuronyltransferase activity towards bilirubin and the effect of premature birth on this activity in the human liver. Biochem J 1981; 196(1):257–260. [DOI] [PMC free article] [PubMed] [Google Scholar]


