Abstract
Background:
Guidelines recommend extended chemoprophylaxis for venous thromboembolism in high-risk patients having operations for inflammatory bowel disease. Quantifying patients’ risk of venous thromboembolism, however, remains challenging. We sought (1) to identify factors associated with postdischarge venous thromboembolism in patients undergoing colorectal resection for inflammatory bowel disease and (2) to develop a postdischarge venous thromboembolism risk calculator to guide prescribing of extended chemoprophylaxis.
Methods:
Patients who underwent an operation for inflammatory bowel disease from 2012 to 2018 were identified from the American College of Surgeons National Surgical Quality Improvement Program for colectomy and proctectomy procedure targeted modules. Postdischarge venous thromboembolism included pulmonary embolism or deep vein thrombosis diagnosed after discharge from the index hospitalization. Multivariable logistic regression estimated the association of patient/operative factors with postdischarge venous thromboembolism. A postdischarge venous thromboembolism risk calculator was subsequently constructed.
Results:
Of 18,990 patients, 199 (1.1%) developed a postdischarge venous thromboembolism within the first 30 postoperative days. Preoperative factors associated with postdischarge venous thromboembolism included body mass index (1.9% with body mass index ≥35 vs 0.8% with body mass index 18.5–24.9; odds ratio 2.34 [95% confidence interval 1.49–3.67]), steroid use (1.3% vs 0.7%; odds ratio 1.91 [95% confidence interval 1.37–2.66]), and ulcerative colitis (1.5% vs 0.8% with Crohn’s disease; odds ratio 1.76 [95% confidence interval 1.32–2.34]). Minimally invasive surgery was associated with postdischarge venous thromboembolism (1.2% vs 0.9% with open; odds ratio 1.42 [95% confidence interval 1.05–1.92]), as was anastomotic leak (2.8% vs 1.0%; odds ratio 2.24 [95% confidence interval 1.31–3.83]) and ileus (2.1% vs 0.9%; odds ratio 2.60 [95% confidence interval 1.91–3.54]). The predicted probability of postdischarge venous thromboembolism ranged from 0.2% to 14.3% based on individual risk factors.
Conclusion:
Preoperative, intraoperative, and postoperative factors are associated with postdischarge venous thromboembolism after an operation for inflammatory bowel disease. A postdischarge venous thromboembolism risk calculator was developed which can be used to tailor extended venous thromboembolism chemoprophylaxis by individual risk.
Introduction
Despite the preventable nature of venous thromboembolism (VTE), pulmonary embolism (PE), and deep vein thrombosis (DVT), VTE remains a leading cause of morbidity and mortality.1,2 A number of risk factors for VTE have been described, which are rooted in Virchow’s triad of venous stasis, vascular injury, and immobility that can disrupt the balance of procoagulant and anticoagulant serum properties in favor of thrombosis.3 Based on these principles, both postoperative patients and those with comorbid inflammatory conditions are at particularly increased risk of VTE.4–6 To combat this risk, the Centers for Medicare and Medicaid Services have advocated for comprehensive VTE prophylaxis for hospitalized, postoperative patients.7 But VTE risk extends beyond hospital discharge, with approximately one-third of VTEs diagnosed in the postdischarge setting.8,9 Enhanced recovery pathways and decreasing length of stay have the potential to increase the diagnoses of postdischarge VTE. Thus, the guidelines of the American College of Chest Physicians for the prevention of VTE have advocated for extended chemoprophylaxis in select patients at increased risk of postdischarge VTE.10
Inflammatory bowel disease (IBD), inclusive of Crohn’s disease and ulcerative colitis, is an inflammatory condition associated with a 3-fold increased risk of VTE in comparison to the general population, with further increased risk in hospitalized medical patients and patients requiring operative therapy.11–14 For postoperative IBD patients specifically, VTE risk exceeds that of patients undergoing colorectal resection for other benign indications and even exceeds VTE risk after resection for malignancy in some analyses.15–18 Thus, postoperative IBD patients are a relatively high-risk group and should be considered for extended VTE chemoprophylaxis based on their risk profile.19–21
Identifying which postoperative IBD patients are at the greatest risk of VTE remains a challenge. Currently available instruments to evaluate postdischarge VTE risk are limited in that they are not specific to postoperative IBD patients or rely heavily on preoperative patient-specific risk factors.22–24 Studies of postdischarge VTEs in other patient populations have demonstrated that, in addition to preoperative patient specific factors, both operative and postoperative factors are associated with the development of postdischarge VTE.25,26 Thus, we sought (1) to identify preoperative, intraoperative, and postoperative risk factors associated with postdischarge VTE in a national cohort of patients undergoing colectomy or proctectomy for IBD and (2) to develop a postdischarge VTE risk calculator to identify high-risk patients for extended chemoprophylaxis.
Methods
Data source and patient population
Patients who underwent colon or rectal resection between January 1, 2012 and December 31, 2018 were identified from the colectomy and proctectomy procedure targeted participant use data files of the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP). The ACS NSQIP is a validated, prospective registry of a sample of patients undergoing select operations at participating hospitals.27 Briefly, trained nurse abstractors collect over 150 variables, including preoperative, intraoperative, and 30-day postoperative outcome data.28,29 The integrity of the registry data is audited to ensure excellent inter-rater reliability.30 Beginning in 2012, procedure-specific modules were developed for various organ systems, including colectomy and proctectomy. These modules allow for abstraction of additional procedure specific variables, inclusive of preoperative workup and additional postoperative complications.
Patients with an International Classification of Diseases versions 9 or 10 code corresponding to a diagnosis of IBD and a Current Procedural Terminology code corresponding to partial or total colectomy or proctectomy were included for analysis. Exclusions included patients with a duration of stay exceeding 30 days, those diagnosed with an inpatient VTE, or who suffered an inpatient death.
Primary outcome and predictors
Postdischarge VTE included either PE or DVT diagnosed after the date of index hospitalization discharge but within 30 days of operative resection. Per ACS NSQIP definitions, the diagnosis of a PE required a new diagnosis of a blood clot in a pulmonary artery identified on imaging, and the diagnosis of a DVT required a new diagnosis of a blood clot or thrombus in the venous system identified on imaging and for which therapeutic anticoagulation was either recommended or administered. Accordingly, mesenteric and portal venous thrombosis are included within the definition of DVT.
A review of the literature was undertaken to identify potential preoperative, intraoperative, and postoperative factors associated with postdischarge VTE. Key preoperative factors included age, sex, race, body mass index (BMI; calculated as weight in kilograms divided by height in meters squared [kg/m2] and categorized as underweight <18.5, normal weight 18.5–24.9, overweight 25.0–29.9, obese 30.0–34.9, and morbidly obese ≥35.0), select preoperative comorbidities, functional status as determined by the American Society of Anesthesiologists classification, preoperative labs inclusive of albumin and platelet count, and indication for operative indication (Crohn’s disease or ulcerative colitis); there were no available variables to determine the severity or duration of IBD in this database. Intraoperative factors evaluated for association with postdischarge VTE included the classification as an emergency operation, operative time, procedure type categorized as (1) ileocecectomy, (2) partial or total colectomy, or (3) proctectomy with or without colectomy, and operative approach (open or minimally invasive). Patients who underwent minimally invasive operations that were converted to open were classified as open. Postoperative factors evaluated for association with postdischarge VTE included anastomotic leak, ileus, surgical site infection (including superficial, deep, and organ space infections), pneumonia, reintubation, renal failure, transfusion, reoperation, and duration of hospital stay. Only those complications that were diagnosed during the index hospitalization were included for analysis. Undated complications derived from the colectomy and proctectomy procedure targeted data were reviewed for readmission International Classification of Diseases versions 9 and 10 codes potentially related to these conditions. Patients who were readmitted with a corresponding diagnosis were not considered to have had that diagnosis at hospital discharge, and therefore, those complications were not included in analyses.
Statistical analysis
The overall rate of VTE was reported as inpatient and postdischarge VTEs. The frequency of postdischarge VTE based on preoperative, intraoperative, and postoperative factors was evaluated on bivariate analysis using separate χ2 tests for categorical variables and t tests for continuous variables. Factors with statistically significant associations on bivariate analyses based on a predetermined P < .05 were entered into a multivariable logistic regression model, with collinear predictors removed progressively via forward selection until a final model was estimated. Model diagnostics evaluating discrimination and calibration included the C-statistic and Hosmer and Lemeshow (HL) χ2, respectively. A Hosmer and Lemeshow chi-squared value with a P < .05 indicates poor calibration.31,32 Internal validation was performed using 20 iterations of 10-fold cross validation, with model diagnostics averaged over each iteration.33
The final regression model was estimated on the logit scale and used to generate a risk calculator. Beta coefficients corresponding to the individual risk factors for each patient were summed with the model intercept, equaling the log probability (LP) of the outcome (postdischarge VTE) for each patient. Next, the LP was exponentiated to generate the predicted probability of postdischarge VTE for each patient using the following equation: probability of event = exp(LP)/(1+exp[LP]).34–37 Predicted probabilities were plotted for the entire cohort to demonstrate variation in postdischarge VTE risk.
All statistical analyses were completed in Stata version 14.2 (Stata Corp, College Station, TX). This study was determined to be exempt from review by the Institutional Review Board at Northwestern University based on use of deidentified data.
Results
Cohort description
Of 19,695 patients who underwent colectomy or proctectomy for IBD, 538 (2.7%) developed a VTE, 339 (63.0%) of whom were diagnosed during inpatient recovery and 199 (37.0%) after hospital discharge. After excluding 295 patients with a length of stay greater than 30 days, 294 patients who developed an inpatient VTE, and 116 who suffered an inpatient death, a total of 18,990 patients at risk for postdischarge VTE were included for final analyses. The postdischarge VTE rate was 1.1%. The mean patient age was 42.7 years, 50.4% of patients were female, and 80.0% were White. Of the analyzed patients, 43.4% had a normal BMI, 63.5% were on preoperative steroids, and 66.0% had a diagnosis of Crohn’s disease. The most common procedure was an ileocecectomy (40.0%), and the majority of cases were performed via a minimally invasive approach (55.5%). The most common postoperative complication was ileus, which occurred in 15.5% of patients. Among patients who developed a postdischarge VTE, 155 were readmitted within 30 days of the index operation (77.9% vs 13.6% of patients who did not develop a postdischarge VTE; P < .001). Additional demographic information is listed in Table I.
Table I.
General cohort characteristics of patients undergoing colorectal resection for inflammatory bowel disease
| Patient characteristic (N = 18,990) | n (%) |
|---|---|
| Preoperative factors | |
| Age, y, mean (SD) | 42.7 (16.2) |
| Sex | |
| Male | 9,413 (49.6) |
| Female | 9,577 (50.4) |
| Race | |
| White | 15,197 (80.0) |
| Black | 1,315 (6.9) |
| Asian | 243 (1.3) |
| Other/not reported | 2,235 (11.8) |
| BMI | |
| <18.5 | 1,514 (8.1) |
| 18.5–24.9 | 8,130 (43.4) |
| 25.0–29.9 | 5,116 (27.3) |
| 30.0–34.9 | 2,469 (13.2) |
| ≥35.0 | 1,505 (8.0) |
| Comorbidities | |
| Bleeding disorder | 542 (2.9) |
| Dyspnea (moderate exertion/rest) | 480 (2.5) |
| Hypertension | 3,226 (17.0) |
| Diabetes | 924 (4.9) |
| Steroid use | 12,049 (63.5) |
| Weight loss >10% in past 6 months | 1,954 (10.3) |
| Functional status | |
| Independent | 18,804 (99.1) |
| Dependent | 163 (0.9) |
| ASA Class | |
| I/II | 10,686 (56.3) |
| III/IV/V | 8,284 (43.7) |
| Preoperative albumin <3 g/dL | 3,282 (22.8) |
| Preoperative platelet count | |
| <150,000 | 546 (3.1) |
| 150,000–400,000 | 13,357 (74.8) |
| >400,000 | 3,951 (22.1) |
| Operative indication | |
| Crohn’s disease | 12,540 (66.0) |
| Ulcerative colitis | 6,450 (34.0) |
| Intraoperative factors | |
| Emergency case classification | 1,351 (7.1) |
| Operative time, min, mean (SD) | 191.1 (93.4) |
| Procedure type | |
| Ileocecectomy | 7,586 (40.0) |
| Colectomy | 7,435 (39.2) |
| Proctectomy +/− colectomy | 3,969 (20.9) |
| Operative approach | |
| Open | 8,445 (44.5) |
| Minimally invasive | 10,545 (55.5) |
| Postoperative factors | |
| Inpatient postoperative complications | |
| Anastomotic leak | 568 (3.0) |
| Ileus | 2,940 (15.5) |
| SSI | 1,008 (5.3) |
| Pneumonia | 175 (0.9) |
| Reintubation | 94 (0.5) |
| Renal failure | 57 (0.3) |
| Transfusion | 1,756 (9.3) |
| Reoperation | 519 (2.7) |
| Length of stay, d, mean, (SD) | 6.3 (4.3) |
| Outcomes | |
| Postdischarge DVT | 173 (0.9) |
| Postdischarge PE | 36 (0.2) |
| Any postdischarge VTE | 199 (1.1) |
ASA, American Society of Anesthesiologists; COPD, chronic obstructive pulmonary disease; SSI, surgical site infection.
Factors associated with postdischarge VTE
Several preoperative factors were associated with postdischarge VTE. IBD patients with BMI in the obese and morbidly obese categories more frequently developed postdischarge VTEs after colectomy or proctectomy than patients with normal BMIs (1.4% if BMI 30.0–34.9; odds ratio [OR] 1.74 [95% confidence interval (CI) 1.15–2.63; P = .009], 1.9% if BMI ≥35.0; OR 2.34 [95% C11.49–3.67]; P < .001 vs 0.8% if BMI 18.5–24.9). Preoperative steroid use was also associated with postdischarge VTE (1.3% vs 0.7%; OR 1.91 [95% CI 1.37–2.66]; P < .001), as was a diagnosis of ulcerative colitis as compared with Crohn’s disease (1.5% vs 0.8%, OR 1.76 [95% CI 1.32–2.34]; P < .001). There were no differences in the frequency of postdischarge VTE diagnosis based on other preoperative patient factors (Table II).
Table II.
Characteristics of patients who develop postdischarge VTE after IBD surgery
| Total patients N = 18,990 | No pdVTE |
pdVTE |
P value |
|---|---|---|---|
|
n (%) |
|||
| 18,791 (99.0) | 199 (1.1) | ||
| Preoperative factors | |||
| Age, y | |||
| <30 | 4,963 (99.1) | 47 (0.9) | .549 |
| 30–40 | 4,226 (98.8) | 50 (1.2) | |
| 40–55 | 4,695 (99.1) | 45 (1.0) | |
| ≥55 | 4,907 (98.9) | 57 (1.2) | |
| Sex | |||
| Male | 9,315 (99.0) | 98 (1.0) | .927 |
| Female | 9,476 (99.0) | 101 (1.1) | |
| Race | |||
| White | 15,030 (98.9) | 167 (1.1) | .562 |
| Black | 1,305 (99.2) | 10 (0.8) | |
| Asian | 241 (99.2) | 2 (0.8) | |
| Other/not reported | 2,215 (99.1) | 20 (0.9) | |
| BMI | |||
| <18.5 | 1,504 (99.3) | 10 (0.7) | <.001 |
| 18.5–24.9 | 8,065 (99.2) | 65 (0.8) | |
| 25.0–29.9 | 5,057 (98.9) | 59 (1.2) | |
| 30.0–34.9 | 2,434 (98.6) | 35 (1.4) | |
| ≥35.0 | 1,477 (98.1) | 28 (1.9) | |
| Comorbidities | |||
| Bleeding disorder | 535 (98.7) | 7 (1.3) | .572 |
| Dyspnea (moderate exertion/rest) | 476 (99.2) | 4 (0.8) | .640 |
| Hypertension | 3,194 (99.0) | 32 (1.0) | .732 |
| Diabetes | 916 (99.1) | 8 (0.9) | .577 |
| Steroid use | 11,897 (98.7) | 152 (1.3) | <.001 |
| Weight loss >10% in past 6 months | 1,935 (99.0) | 19 (1.0) | .729 |
| Functional status | |||
| Independent | 18,607 (99.0) | 197 (1.1) | .823 |
| Dependent | 161 (98.8) | 2 (1.2) | |
| ASA Class | |||
| I/II | 10,585 (99.1) | 101 (1.0) | .129 |
| III/IV/V | 8,187 (98.8) | 97 (1.2) | |
| Preoperative albumin <3 g/dL | 3,241 (98.8) | 41 (1.3) | .252 |
| Preoperative platelet count | |||
| <150,000 | 540 (98.9) | 6 (1.1) | .978 |
| 150,000–400,000 | 13,218 (99.0) | 139 (1.0) | |
| >400,000 | 3,911 (99.0) | 40 (1.0) | |
| Operative indication | |||
| Crohn’s disease | 12,438 (99.2) | 102 (0.8) | <.001 |
| Ulcerative colitis | 6,353 (98.5) | 97 (1.5) | |
| Intraoperative factors | |||
| Emergency case classification | 1,334 (98.7) | 17 (1.3) | .431 |
| Operative time, h | |||
| <2 | 4,089 (99.3) | 28 (0.7) | .049 |
| 2–3 | 5,938 (99.0) | 63 (1.1) | |
| 3–4 | 4,250 (98.8) | 52 (1.2) | |
| >4 | 4,511 (98.8) | 56 (1.2) | |
| Procedure type | |||
| Ileocectomy | 7,529 (99.3) | 57 (0.8) | .005 |
| Colectomy | 7,343 (98.8) | 92 (1.2) | |
| Proctectomy | 3,919 (98.7) | 50 (1.3) | |
| Operative approach | |||
| Open | 8,374 (99.2) | 72 (0.9) | .018 |
| Minimally invasive | 10,417 (98.8) | 127 (1.2) | |
| Postoperative factors | |||
| Inpatient postoperative complications | |||
| Anastomotic leak | 552 (97.2) | 16 (2.8) | <.001 |
| Ileus | 2,879 (97.9) | 61 (2.1) | <.001 |
| SSI | 1,000 (99.2) | 8 (0.8) | .415 |
| Pneumonia | 172 (98.3) | 3 (1.7) | .384 |
| Reintubation | 92 (97.9) | 2 (2.1) | .303 |
| Renal failure | 56 (98.3) | 1 (1.8) | .600 |
| Transfusion | 1,730 (98.5) | 26 (1.5) | .062 |
| Reoperation | 511 (98.5) | 8 (1.5) | .263 |
| Length of stay, d | |||
| <4 | 4,335 (99.0) | 43 (1.0) | .926 |
| 4–5 | 3,580 (99.0) | 37 (1.0) | |
| 5–7 | 4,844 (98.9) | 55 (1.1) | |
| ≥7 | 6,032 (99.0) | 64 (1.1) | |
ASA, American Society of Anesthesiologists; COPD, chronic obstructive pulmonary disease; pdVTE, post-discharge venous thromboembolism; SSI, skin/soft tissue infection.
Intraoperative factors with increased postdischarge VTE rates included greater operative times (eg, 1.2% of patients with operative time >4 hours vs 0.7% <2 hours; P =.049), type of operations (1.3% of patients undergoing proctectomy, 1.2% colectomy vs 0.8% ileocecectomy; P =.005), and operative approach (1.2% of patients after minimally invasive surgery vs 0.9% open; P =.018). These factors were collinear, and operative approach remained statistically significant in the final adjusted model (OR 1.42 [95% CI 1.05–1.92], P =.022 Table III). With regard to postoperative complications, both anastomotic leak (2.8% vs 1.0%; OR 2.24 [95% CI 1.31–3.83], P = .003) and postoperative ileus (2.1% vs 0.9%; OR 2.60 [95% CI 1.91–3.54], P < .001) were associated with postdischarge VTE. Other postoperative complications were not associated with postdischarge VTE nor was postoperative hospital length of stay (Table II).
Table III.
Association between patient characteristics and postdischarge VTE after IBD surgery
| Adjusted OR (95% CI) | P value | |
|---|---|---|
| BMI | ||
| <18.5 | 0.80 (0.41–1.57) | .520 |
| 18.5–24.9 | 1.00 | REF |
| 25.0–29.9 | 1.42 (1.00–2.03) | .051 |
| 30.0–34.9 | 1.74 (1.15–2.63) | .009 |
| ≥35 | 2.34 (1.49–3.67) | <.001 |
| Preoperative steroids | ||
| No | 1.00 | REF |
| Yes | 1.91 (1.37–2.66) | <.001 |
| IBD type | ||
| Crohn’s disease | 1.00 | REF |
| Ulcerative colitis | 1.76 (1.32–2.34) | <.001 |
| Operative approach | ||
| Open | 1.00 | REF |
| MIS | 1.42 (1.05–1.92) | .022 |
| Anastomotic leak | ||
| No | 1.00 | REF |
| Yes | 2.24 (1.31–3.83) | .003 |
| Postoperative ileus | ||
| No | 1.00 | REF |
| Yes | 2.60 (1.91–3.54) | <.001 |
MIS, minimally invasive surgery; REF, reference.
Postdischarge VTE risk calculator
Beta coefficients for preoperative, intraoperative, and postoperative factors associated with postdischarge VTE are listed in Supplemental Table I along with the model constant for use as a postdischarge VTE risk calculator. Using the calculator, the predicted probability of postdischarge VTE ranged from 0.2% to 14.3% (Fig 1); 40.6% of patients had a postdischarge VTE risk of 1.0% or greater, and 10.2% of patients had a risk of 2.0% or greater. The model’s C-statistic was 0.691 and HL χ2 P = .336 demonstrating good discrimination and calibration. Internal validation via 20 iterations of 10-fold cross validation yielded a mean C-statistic of 0.665.
Fig 1.
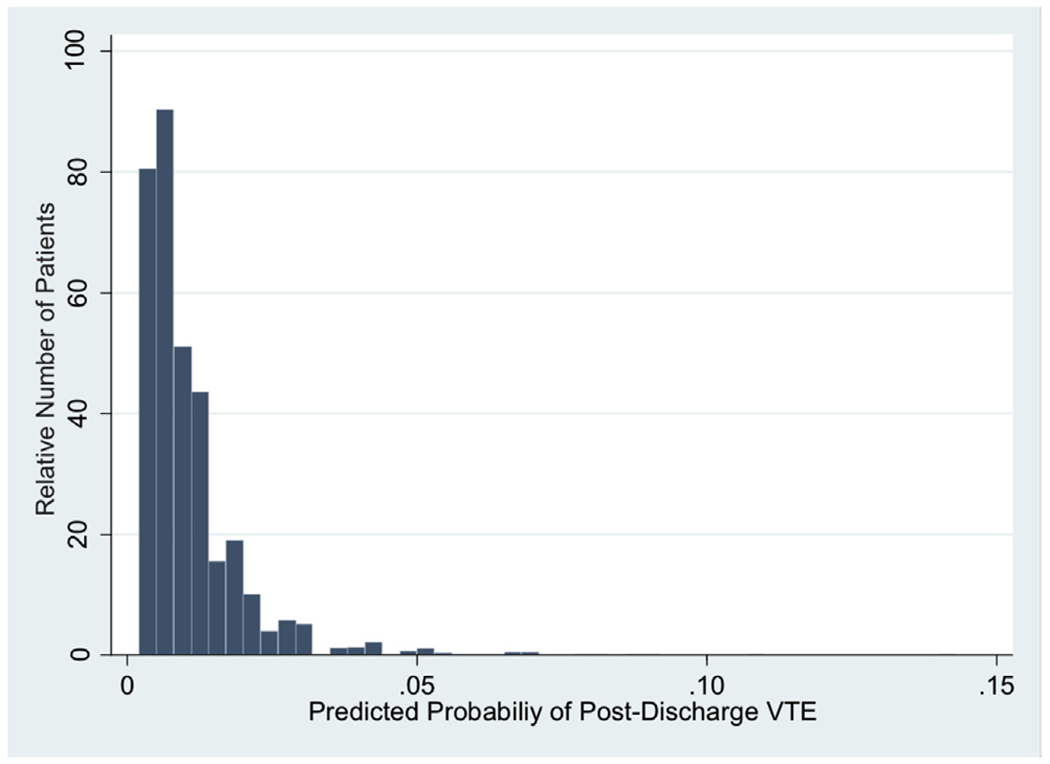
Predicted probability of postdischarge VTE after IBD surgery.
Discussion
VTE risk is increased after many types of operations and extends beyond hospital discharge. In particular, patients undergoing specific types of operations, such as major orthopedic, bariatric, and abdominal or pelvic operations, or IBD resections have a notable risk of postdischarge VTE.9,16,38–40 Although clinical practice guidelines recommend extended chemoprophylaxis for high-risk patients postoperatively, identifying those high-risk patients remains difficult. Using only information available at hospital discharge, we identified preoperative, intraoperative, and postoperative factors associated with postdischarge VTE in patients undergoing colorectal resection for IBD. BMI, preoperative steroid use, IBD type, operative approach, and inpatient postoperative diagnoses of anastomotic leak and ileus were associated with postdischarge VTE. A postdischarge risk calculator was constructed to quantify relative risk based upon these factors and individual patient’s predicted risk ranged from 0.2% to 14.3%.
Overall VTE Risk
Overall, 2.7% of patients developed a VTE after a colon or rectal resection for IBD, of whom 37.0% were diagnosed after hospital discharge for a postdischarge VTE rate of 1.1%. These findings are consistent with previous evaluations of VTE after operations for IBD, with overall VTE rates of approximately 2.5% and with 40% of the events occurring after hospital discharge.24,41,42 Furthermore, these rates are notably greater than VTE rates in patients undergoing colorectal resection for benign indications.16,42
Factors Associated with Post-Discharge VTE
Preoperative, intraoperative, and postoperative factors were associated with postdischarge VTE in this study of patients undergoing colon or rectal resection for IBD. Patients with an obese BMI had a 74% relative increased odds of postdischarge VTE, while those with morbidly obese BMIs had a greater than 2-fold relative increased odds of postdischarge VTE. These findings are consistent with previous reports of the association between BMI and VTE in postoperative IBD patient43 However, our study is somewhat unique, because no prior study has identified an association between BMI and postdischarge VTE in postoperative IBD patients, despite evidence that BMI is associated with postdischarge VTE in abdominal malignancies and patients undergoing colon or rectal resection for other indications.16,26,44,45
Preoperative steroid use was also associated with postdischarge VTE, which has been previously demonstrated.24,46 This association could be a surrogate for severe forms of IBD or acute inflammation, which are known to increase VTE risk47,48; exogenous steroid use, however, is associated with VTEs, regardless of indication, which may be related to increased production of clotting factors.49 Patients with ulcerative colitis in our study had a 76% relative increased odds of postdischarge VTE compared with patients with Crohn’s disease, as has been reported in other studies.41,42,50,51 Similar to steroid use, it has been hypothesized that this association could be related to inflammation severity and resultant alterations to the coagulation cascade in IBD.52–54 Because ulcerative colitis is a contiguous inflammatory condition, a greater inflammatory response may be seen in these patients compared with the isolated and/or sporadic inflammation seen in Crohn’s disease. Patients undergoing restorative proctocolectomy for ulcerative colitis can be particularly susceptible to portal venous thrombosis, which may arise, in part, from mesenteric stretching and tension during ileoanal pouch creation.55 Furthermore, patients with Crohn’s disease often require resection for fibrostenotic disease, which is thought to be a noninflamed sequela of quiescent inflammation.56,57
Several intraoperative factors were associated with postdischarge VTE on bivariate analyses, including operative time, type of procedure, and operative approach. In constructing a multivariable adjusted model, these factors were collinear, with operative approach generating the best model fit. Our finding that minimally invasive operative approaches are associated with postdischarge VTE is contrary to prior work indicating that minimally invasive surgery is associated with inpatient VTE in postoperative IBD patients but not postdischarge VTE.24 A prior NSQIP analysis of patients undergoing colorectal resection for IBD, however, did identify findings consistent with our study that minimally invasive surgery is associated with a decreased odds of inpatient VTE, but an increased odds of postdischarge VTE.16 This finding could be related to a time bias, because patients who undergo minimally invasive colon operations are discharged typically sooner, which may shift the distribution of VTE diagnoses from the inpatient setting to outpatient.58 Alternatively, providers may be less likely to prescribe postdischarge chemoprophylaxis to patients undergoing minimally invasive surgery because of a perceived decreased risk of VTE; however, VTE rates may be as high as 10% in ultrasonographically screened patients after laparoscopic colon resections.59
Finally, inpatient diagnoses of postoperative ileus and anastomotic leak were associated with postdischarge VTE. Previous studies have identified that postoperative complications typically compound after colorectal surgery, with complications being the greatest risk factors for postdischarge VTE.16,60 Specifically, postoperative ileus has been identified as a high-risk complication associated with VTE after laparoscopic colon resection.61 Furthermore, both anastomotic leak and ileus have been identified as risk factors for postdischarge VTE in patients undergoing resection for colorectal cancer.26 Thus, despite no prior study to the best of our knowledge that has identified an association between anastomotic leak or postoperative ileus and postdischarge VTE in patients undergoing colon or rectal surgery for IBD, our findings are not surprising.
Postdischarge VTE risk calculator
Although randomized controlled trials have shown the benefit of extended chemoprophylaxis in high-risk patients, such as those undergoing abdominal or pelvic resection for cancer, no such trials have been performed after IBD surgery.62–64 Clinical practice guidelines including those put forward by the American Society of Colon and Rectal Surgeons recommend extended chemoprophylaxis in high-risk patients as defined as a 6% risk of overall VTE by the American College of Chest Physicians guidelines.10,19 The overall rate of VTE in our study is well below 6%, thus identification of risk factors known at the time of discharge that are associated with postdischarge VTE in patients undergoing IBD surgery is warranted to best select patients for extended chemoprophylaxis.
Prior tools to risk stratifying these patients have been limited. The Caprini score was developed to estimate overall VTE risk in postoperative patients after major operations,22,65 but the Caprini score cannot discriminate between inpatient and postdischarge VTEs, does not include postoperative complications, and identifies the same risk for patients with ulcerative colitis and Crohn’s disease. A VTE risk calculator for IBD patients has also been developed, but this calculator is not specific to postoperative patients, who have a different risk profile than patients admitted for IBD who do not require operative treatment.23 While Benlice et al developed a nomogram to indicate risk for postdischarge VTE in IBD patients, their nomogram relies on just 3 statistically significant risk factors, none of which are from the postoperative period.24 The risk calculator described in our study was developed using the same methodology that has been used in other ACS NSQIP calculators, includes variables from all phases of care, and has good internal validity. Thus, the calculator we offer can help providers to indicate high-risk patients who may derive the greatest benefit from extended VTE chemoprophylaxis after colon or rectal resection for IBD.
An example application of the postdischarge VTE risk calculator from the study population is as follows: a 50-year-old woman with a BMI of 28.4 on preoperative steroids for ulcerative colitis underwent an open protocolectomy with ileopouch anal anastomosis and diverting loop ileostomy. Her postoperative course was complicated by anastomotic leak and postoperative ileus, which were managed conservatively. She was discharged from the hospital on postoperative day 9. Thus, her risk factors for postdischarge VTE as identified in this study include an overweight BMI (beta coefficient 0.35), preoperative steroids (beta coefficient 0.65), ulcerative colitis (beta coefficient 0.57), an open operation (beta coefficient 0.00), anastomotic leak (beta coefficient 0.81), and postoperative ileus (beta coefficient 0.95). By summing the listed beta coefficients (3.33) with the model intercept (−5.97), the LP of postdischarge VTE is calculated as −2.64. The event probability is then calculated as exp(LP)/(1+exp[LP]) = 6.7%, we would suggest that this risk would warrant extended postdischarge prophylaxis for VTE.
Although prospective studies have not identified a specific postdischarge VTE risk threshold above which postoperative IBD patients should receive postdischarge VTE prophylaxis, the postdischarge VTE risk generated by this calculator can be interpreted within the context of current guidelines and cost-effectiveness analyses. For example, the American College of Chest Physician guidelines define high-risk patients as those with an overall VTE risk >6%.10 Considering that approximately 40% of postoperative VTEs are diagnosed in the postdischarge setting, high-risk could be defined as >2.4%.24,42 Similarly, a cost-effectiveness analysis of postdischarge chemoprophylaxis in patients after abdominal oncologic resections identified a threshold of 2.4%, above which postdischarge chemoprophylaxis was the dominant strategy.66 The cost effectiveness of postdischarge chemoprophylaxis after operative resection of Crohn’s disease, however, favored selective prescribing at risk thresholds >4.9%.67 Specific decisions regarding postdischarge prophylaxis should be made within the context of the provider and patient’s risk aversion, and a calculated relative risk of postdischarge VTE will aide in that decision making process.
Limitations
First, ACS NSQIP contains observational data from which association but not causation can be derived. Second, some individual factors, such as prothrombotic gene mutations, severity of disease, and duration of IBD symptoms, may be associated with VTE risk but are not available in ACS NSQIP, and thus were not included in this study. Third, there is no information available in ACS NSQIP regarding prophylaxis compliance, including chemoprophylaxis during the inpatient setting, which has been demonstrated to decrease VTE rates. Furthermore, there are no data available regarding prescribing or compliance of extended chemoprophylaxis. Thus, the calculated VTE rates are not adjusted for the use of inpatient or postdischarge prophylaxis, and the true VTE rates in the absence of prophylaxis are unknown; however, prescription rates of postdischarge chemoprophylaxis are approximately 10% after IBD surgery, thus the majority of patients in this study likely did not receive postdischarge chemoprophylaxis.68 Fourth, the postoperative risk of VTE extends beyond 30 days, but we were only able to evaluate 30-day outcome data in this study based on data collection techniques used by ACS NSQIP. Fifth, surveillance strategies are not included in the ACS NSQIP data. We are unable to evaluate for asymptomatic VTEs diagnosed via screening, which would be documented VTEs within the ACS NSQIP data but are of unclear clinical significance. Additionally, given the increased baseline risk of VTE in IBD patients, it is unclear how many, if any, of the described VTEs were present preoperatively. Finally, a time bias may exist in this study in that patients with greater inpatient durations of stay have a shorter period at risk of postdischarge VTE within the 30-day collection period of ACS NSQIP. However, these data limitations likely will not change the risk factors associated with postdischarge VTE identified in this study but may underestimate the true postdischarge VTE risk.
In conclusion, overall, 2.7% of patients developed a VTE after colon or rectal resection for IBD, with 1.1% of patients diagnosed after hospital discharge. Patient-specific risk factors associated with postdischarge VTE include BMI, steroid use, IBD type, operative approach, anastomotic leak, and ileus. A postdischarge VTE risk calculator was constructed and can be used to identify high-risk patients at the time of discharge who merit extended chemoprophylaxis after colon or rectal resection for IBD.
Supplementary Material
Acknowledgments
Funding/Support
TKY is supported by the Agency for Healthcare Research and Quality (5T32HS000078); ADY by the National Heart, Lung and Blood Institute (K08NIHL145139); DJB by a Veteran’s Administration Merit Award (I01HX002290); KYB by the Agency for Healthcare Research and Quality (R01HS024516); and RPM by the Agency for Healthcare Research and Quality (K12HS026385) and an Institutional Research Grant from the American Cancer Society (IRG-18-163-24).
Footnotes
These findings were presented as an oral presentation at the 15th Annual Academic Surgical Congress, Orlando, FL on February 5, 2020.
Conflict of interest/Disclosures
None declared.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at https://doi.org/10.1016/j.surg.2020.09.006.
References
- 1.Tooher R, Middleton P, Pham C, et al. A systematic review of strategies to improve prophylaxis for venous thromboembolism in hospitals. Ann Surg. 2005;241:397–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cardoso LF, Krokoscz DV, de Paiva EF, et al. Results of a venous thromboembolism prophylaxis program for hospitalized patients. Vasc Health Risk Manag. 2016;121:491–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stone J, Hangge P, Albadawi H, et al. Deep vein thrombosis: pathogenesis, diagnosis, and medical management. Cardiovasc Diagn Ther. 2017;7(Suppl 3): S276–S284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gangireddy C, Rectenwald JR, Upchurch GR, et al. Risk factors and clinical impact of postoperative symptomatic venous thromboembolism. J Vasc Surg. 2007;45:335–341. [DOI] [PubMed] [Google Scholar]
- 5.Agnelli G Prevention of venous thromboembolism in surgical patients. Circulation. 2004;110(24 Suppl 1):IV4–IV12. [DOI] [PubMed] [Google Scholar]
- 6.Saghazadeh A, Rezaei N. Inflammation as a cause of venous thromboembolism. Crit Rev Oncol Hematol. 2016;99:272–285. [DOI] [PubMed] [Google Scholar]
- 7.Agency for Healthcare Research and Quality (AHRQ). Preventing hospital-associated venous thromboembolism; 2016. https://www.ahrq.gov/patient-safety/resources/vtguide/index.html. Accessed November 22, 2019.
- 8.Merkow RP, Bilimoria KY, McCarter MD, et al. Post-discharge venous thromboembolism after cancer surgery: extending the case for etended prohylaxis. Ann Surg. 2011;2554:131–137. [DOI] [PubMed] [Google Scholar]
- 9.Shah DR, Wang H, Bold RJ, et al. Nomograms to predict risk of in-hospital and post-discharge venous thromboembolism after abdominal and thoracic surgery: an American College of Surgeons National Surgical Quality Improvement Program analysis. J Surg Res. 2013;183:462–471. [DOI] [PubMed] [Google Scholar]
- 10.Gould MK, Garcia DA, Wren SM, et al. Prevention of VTE in nonorthopedic surgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e227S–e277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCurdy JD, Kuenzig ME, Smith G, et al. Risk of venous thromboembolism after hospital discharge in patients with inflammatory bowel disease: a population-based study [e-pub ahead of print]. Inflammatory Bowel Dis. 2020. 10.1093/ibd/izaa002. Accessed March 1, 2020. [DOI] [PubMed] [Google Scholar]
- 12.Kaddourah O, Numan L, Jeepalyam S, Abughanimeh O, Ghanimeh MA, Abuamr K. Venous thromboembolism prophylaxis in inflammatory bowel disease flare-ups. Ann Gastroenterol. 2019;32:578–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Collaborative Writing Group for Surgical Care and Outcomes Assessment Program—Comparative Effectivness Research Translation Network (SCOAP-CERTAIN), Nelson DW, Simianu VV, et al. Thromboembolic complications and prophylaxis patterns in colorectal surgery. JAMA Surg. 2015;150:712–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bernstein CN, Blanchard JF, Houston DS, Wajda A. The incidence of deep venous thrombosis and pulmonary embolism among patients with inflammatory bowel disease: A population-based cohort study. Thromb Haemost. 2001;85:430–434. [PubMed] [Google Scholar]
- 15.Murthy SK, Nguyen GC. Venous thromboembolism in inflammatory bowel disease: an epidemiological review. Am J Gastroenterol. 2011;106:713–718. [DOI] [PubMed] [Google Scholar]
- 16.Alhassan N, Trepanier M, Sabapathy C, et al. Risk factors for post-discharge venous thromboembolism in patients undergoing colorectal resection: a NSQIP analysis. Tech Coloproctol. 2018;22:955–964. [DOI] [PubMed] [Google Scholar]
- 17.Gross ME, Vogler SA, Mone MC, Sheng X, Sklow B. The importance of extended postoperative venous thromboembolism prophylaxis in IBD: a National Surgical Quality Improvement Program analysis. Dis Colon Rectum. 2014;57:482–489. [DOI] [PubMed] [Google Scholar]
- 18.Ali F, Al-Kindi SG, Blank JJ, Peterson CY, Ludwig KA, Ridolfi TJ. Elevated venous thromboembolism risk following colectomy for IBD is equal to those for colorectal cancer for ninety days after surgery. Dis Colon Rectum. 2018;61:375–381. [DOI] [PubMed] [Google Scholar]
- 19.Fleming F, Gaertner W, Ternent CA, et al. The American Society of Colon and Rectal Surgeons Clinical Practice Guideline for the Prevention of Venous Thromboembolic Disease in Colorectal Surgery. Dis Colon Rectum. 2018;61:14–20. [DOI] [PubMed] [Google Scholar]
- 20.McKechnie T, Wang J, Springer JE, Gross PL, Forbes S, Eskicioglu C. Extended thromboprophylaxis following colorectal surgery in patients with inflammatory bowel disease: a comprehensive systematic clinical review. Colorectal Dis. 2020;22:663–678. [DOI] [PubMed] [Google Scholar]
- 21.Merkow RP. Is there a role for extended venous thromboembolism chemoprophylaxis in IBD? Dis Colon Rectum. 2014;57:413–414. [DOI] [PubMed] [Google Scholar]
- 22.Bahl V, Hu HM, Henke PK, Wakefield TW, Campbell DA Jr, Caprini JA. A validation study of a retrospective venous thromboembolism risk scoring method. Ann Surg. 2010;251:344–350. [DOI] [PubMed] [Google Scholar]
- 23.McCurdy JD, Israel A, Hasan M, et al. A clinical predictive model for posthospitalisation venous thromboembolism in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2019;49:1493–1501. [DOI] [PubMed] [Google Scholar]
- 24.Benlice C, Holubar SD, Gorgun E, et al. Extended venous thromboembolism prophylaxis after elective surgery for IBD patients: nomogram-based risk assessment and prediction from nationwide cohort. Dis Colon Rectum. 2018;61:1170–1179. [DOI] [PubMed] [Google Scholar]
- 25.Aminian A, Andalib A, Khorgami Z, et al. Who should get extended thromboprophylaxis after bariatric surgery?: a risk assessment tool to guide indications for post-discharge pharmacoprophylaxis. Ann Surg. 2017;265:143–150. [DOI] [PubMed] [Google Scholar]
- 26.Schlick CJR, Liu JY, Yang AD, Bentrem DJ, Bilimoria KY, Merkow RP. Pre-operative, intra-operative, and post-operative factors associated with postdischarge venous thromboembolism following colorectal cancer resection. J Gastrointest Surg. 2020;24:144–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ingraham AM, Richards KE, Hall BL, Ko CY. Quality improvement in surgery: the American College of Surgeons National Surgical Quality Improvement Program approach. Adv Surg. 2010;44:251–267. [DOI] [PubMed] [Google Scholar]
- 28.Cohen ME, Ko CY, Bilimoria KY, et al. Optimizing ACS NSQIP modeling for evaluation of surgical quality and risk: patient risk adjustment, procedure mix adjustment, shrinkage adjustment, and surgical factors. J Am Coll Surg. 2013;217:336–346. [DOI] [PubMed] [Google Scholar]
- 29.Henderson WG, Daley J. Design and statistical methodology of the National Surgical Quality Improvement Program: why is it what it is? Am J Surg. 2009;198(5 Suppl):S19–S27. [DOI] [PubMed] [Google Scholar]
- 30.Shiloach M, Frencher SK Jr, Steeger JE, et al. Toward robust information: data quality and inter-rater reliability in the American College of Surgeons National Surgical Quality Improvement Program. J Am Coll Surg. 2010;210:6–16. [DOI] [PubMed] [Google Scholar]
- 31.Merkow RP, Hall BL, Cohen ME, et al. Relevance of the c-statistic when evaluating risk-adjustment models in surgery. J Am Coll Surg. 2012;214:822–830. [DOI] [PubMed] [Google Scholar]
- 32.Steyerberg EW, Vickers AJ, Cook NR, et al. Assessing the performance of prediction models: a framework for traditional and novel measures. Epidemiology. 2010;21:128–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jung Y, Hu J. A K-fold averaging cross-vaidation procedure. J Nonparametr Stat. 2015;27:167–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bilimoria KY, Liu Y, Paruch JL, et al. Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J Am Coll Surg. 2013;217:833–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cohen ME, Bilimoria KY, Ko CY, Hall BL. Development of an American College of Surgeons National Surgery Quality Improvement Program: morbidity and mortality risk calculator for colorectal surgery. J Am Coll Surg. 2009;208: 1009–1016. [DOI] [PubMed] [Google Scholar]
- 36.Mansmann U, Rieger A, Strahwald B, Crispin A. Risk calculators-methods, development, implementtion, and validation. Int J Colorectal Dis. 2016;31:1111–1116. [DOI] [PubMed] [Google Scholar]
- 37.Parikh P, Shiloach M, Cohen ME, et al. Pancreatectomy risk calculator: an ACS-NSQIP resource. HPB (Oxford). 2010;12:488–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clark LN, Helm MC, Gould JC. Practice patterns regarding post-discharge chemoprophylaxis for venous thromboembolism following bariatric surgery in the United States. Surg Obes Relat Dis. 2019;15:703–709. [DOI] [PubMed] [Google Scholar]
- 39.Flevas DA, Megaloikonomos PD, Dimopoulos L, Mitsiokapa E, Koulouvaris P, Mavrogenis AF. Thromboembolism prophylaxis in orthopaedics: an update. EFORT Open Rev. 2018;3:136–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schlick CJR, Merkow RP, Yang AD, Bentrem DJ. Post-discharge venous thromboembolism after pancreatectomy for malignancy: predicting risk based on preoperative, intraoperative, and postoperative factors [e-pub ahead of print]. J Surg Oncol. 2020. 10.1002/jso.26046. Accessed July 15, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wallaert JB, De Martino RR, Marsicovetere PS, et al. Venous thromboembolism after surgery for inflammatory bowel disease: are there modifiable risk factors? Data from ACS NSQIP. Dis Colon Rectum. 2012;55:1138–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wilson MZ, Connelly TM, Tinsley A, Hollenbeak CS, Koltun WA, Messaris E. Ulcerative colitis is associated with an increased risk of venous thromboembolism in the postoperative period: the results of a matched cohort analysis. Ann Surg. 2015;261:1160–1166. [DOI] [PubMed] [Google Scholar]
- 43.Merrill A, Millham F. Increased risk of postoperative deep vein thrombosis and pulmonary embolism in patients with inflammatory bowel disease: a study of National Surgical Quality Improvement Program patients. Arch Surg. 2012;147:120–124. [DOI] [PubMed] [Google Scholar]
- 44.Beal EW, Tumin D, Chakedis J, et al. Which patients require extended thromboprophylaxis after colectomy? Modeling risk and assessing indications for post-discharge pharmacoprophylaxis. World J Surg. 2018;42:2242–2251. [DOI] [PubMed] [Google Scholar]
- 45.Parkin L, Sweetland S, Balkwill A, et al. Body mass index, surgery, and risk of venous thromboembolism in middle-aged women: a cohort study. Circulation. 2012;125:1897–1904. [DOI] [PubMed] [Google Scholar]
- 46.McKenna NP, Behm KT, Ubl DS, et al. Analysis of postoperative venous thromboembolism in patients with chronic ulcerative colitis: is it the disease or the operation? Dis Colon Rectum. 2017;60:714–722. [DOI] [PubMed] [Google Scholar]
- 47.Andrade AR, Barros LL, Azevedo MFC, et al. Risk of thrombosis and mortality in inflammatory bowel disease. Clin Transl Gastroenterol. 2018;9:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alkim H, Koksal AR, Boga S, Sen I, Alkim C. Etiopathogenesis, prevention, and treatment of thromboembolism in inflammatory bowel disease. Clin Appl Thromb Hemost. 2017;23:501–510. [DOI] [PubMed] [Google Scholar]
- 49.Johannesdottir SA, Horvath-Puho E, Dekkers OM, et al. Use of glucocorticoids and risk of venous thromboembolism: a nationwide population-based case-control study. JAMA Intern Med. 2013;173:743–752. [DOI] [PubMed] [Google Scholar]
- 50.Moghadamyeghaneh Z, Hanna MH, Carmichael JC, Nguyen NT, Stamos MJ. A nationwide analysis of postoperative deep vein thrombosis and pulmonary embolism in colon and rectal surgery. J Gastrointest Surg. 2014;18:2169–2177. [DOI] [PubMed] [Google Scholar]
- 51.Scarpa M, Pilon F, Pengo V, et al. Deep venous thrombosis after surgery for inflammatory bowel disease: is standard dose low molecular weight heparin prophylaxis enough? World J Surg. 2010;34:1629–1636. [DOI] [PubMed] [Google Scholar]
- 52.Jackson LM, O’Gorman PJ, O’Connell J, Cronin CC, Cotter KP, Shanahan F. Thrombosis in inflammatory bowel disease: clinical setting, procoagulant profile and factor V Leiden. QJM. 1997;90:183–188. [DOI] [PubMed] [Google Scholar]
- 53.Saibeni S, Bottasso B, Spina L, et al. Assessment of thrombin-activatable fibrinolysis inhibitor (TAFI) plasma levels in inflammatory bowel diseases. Am J Gastroenterol. 2004;99:1966–1970. [DOI] [PubMed] [Google Scholar]
- 54.Saibeni S, Cattaneo M, Vecchi M, et al. Low vitamin B(6) plasma levels, a risk factor for thrombosis, in inflammatory bowel disease: role of inflammation and correlation with acute phase reactants. Am J Gastroenterol. 2003;98:112–117. [DOI] [PubMed] [Google Scholar]
- 55.Remzi FH, Fazio VW, Oncel M, et al. Portal vein thrombi after restorative proctocolectomy. Surgery. 2002;132:655–661:discussion 661-662. [DOI] [PubMed] [Google Scholar]
- 56.Chang CW, Wong JM, Tung CC, Shih IL, Wang HY, Wei SC. Intestinal stricture in Crohn’s disease. Intest Res. 2015;13:19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li J, Mao R, Kurada S, et al. Pathogenesis of fibrostenosing Crohn’s disease. Transl Res. 2019;209:39–54. [DOI] [PubMed] [Google Scholar]
- 58.Alizadeh RF, Chaudhury HH, Li S, et al. Ileocolic resection for Crohn’s disease: a minimally invasive approach claims its place. Am Surg. 2018;84:1639–1644. [PubMed] [Google Scholar]
- 59.Vedovati MC, Becattini C, Rondelli F, et al. A randomized study on 1-week versus 4-week prophylaxis for venous thromboembolism after laparoscopic surgery for colorectal cancer. Ann Surg. 2014;259:665–669. [DOI] [PubMed] [Google Scholar]
- 60.Tevis SE, Kennedy GD. Postoperative complications: looking forward to a safer future. Clin Colon Rectal Surg. 2016;29:246–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kronberg U, Kiran RP, Soliman MS, et al. A characterization of factors determining postoperative ileus after laparoscopic colectomy enables the generation of a novel predictive score. Ann Surg. 2011;253:78–81. [DOI] [PubMed] [Google Scholar]
- 62.Bergqvist D, Agnelli G, Cohen AT, et al. , and the ENOXACAN II Investigators. Duration of prophylaxis against venous thromboembolism with enoxaparin after surgery for cancer. N Engl J Med. 2002;346:975–980. [DOI] [PubMed] [Google Scholar]
- 63.Kakkar VV, Balibrea JL, Martinez-Gonzalez J, Prandoni P, CANBESURE Study Group. Extended prophylaxis with bemiparin for the prevention of venous thromboembolism after abdominal or pelvic surgery for cancer: the CANBESURE randomized study. J Thromb Haemost. 2010;8:1223–1229. [DOI] [PubMed] [Google Scholar]
- 64.Rasmussen MS, Jorgensen LN, Wille-Jorgensen P. Prolonged thromboprophylaxis with low molecular weight heparin for abdominal or pelvic surgery. Cochrane Database Syst Rev. 2009;21:CD004318. [DOI] [PubMed] [Google Scholar]
- 65.Caprini JA. Thrombosis risk assessment as a guide to quality patient care. Dis Mon. 2005;51:70–78. [DOI] [PubMed] [Google Scholar]
- 66.Iannuzzi JC, Rickles AS, Kelly KN, et al. Defining high risk: cost-effectiveness of extended-duration thromboprophylaxis following major oncologic abdominal surgery. J Gastrointest Surg. 2014;18:60–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Leeds IL, DiBrito SR, Canner JK, Haut ER, Safar B. Cost-benefit limitations of extended, outpatient venous thromboembolism prophylaxis following surgery for Crohn’s disease. Dis Colon Rectum. 2019;62:1371–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mukkamala A, Montgomery JR, De Roo AC, Ogilvie JW Jr, Regenbogen SE. Population-based analysis of adherence to post-discharge extended VTE prophylaxis after colorectal resection [e-pub ahead of print]. Dis Colon Rectum. 2020. 10.1097/DCR.0000000000001650. Accessed July 15, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


