Abstract
Pseudomonas aeruginosa (P. aeruginosa) is an opportunistic bacterium commonly found in wound infections and airways of cystic fibrosis patients. P. aeruginosa readily forms biofilms which can reduce the efficacy of antibiotics used to eradicate the pathogen. We have previously shown that a Specialized Pro-resolving Mediator (SPM), Lipoxin A4 (LxA4) is a quorum sensing inhibitor which can reduce P. aeruginosa virulence. In this study, we examined the direct actions of LxA4 and RvD2 on P. aeruginosa biofilm formation and virulence gene expression. The influence of LxA4 on antibiotic efficacy and the combined effects on biofilm formation were also investigated. LxA4 and RvD2 reduced P. aeruginosa biofilm formation and virulence gene expression. LxA4 increased ciprofloxacin inhibition on biofilm formation but did not affect ciprofloxacin’s action on non-adherent bacteria. On the other hand, LxA4 increased bacterial killing action of imipenem but did not affect imipenem’s action on biofilm. We also found that Journal LxA4 can increase ciprofloxacin’s bacterial killing ability in established biofilm. Together these results suggest that LxA4 has direct effects on P. aeruginosa biofilm formation and can increase antibiotic efficacy directly.
Keywords: Lipoxin A4, Resolvin, Pseudomonas aeruginosa, biofilm, antibiotics, Pro-resolving mediators
1. Introduction
Pseudomonas aeruginosa (P. aeruginosa) is a gram-negative bacterium which is a major cause of nosocomial infections and secondary infections in immunocompromised patients (1). Biofilms are found to be present in burns and chronic open wounds, in infections involving indwelling catheters, and surgical implants (2). The ability of P. aeruginosa to form cooperative biofilm communities adds to the difficulty in treating and eradicating these infections. P. aeruginosa virulence is controlled by a bacterial population density-mediated signaling network called quorum sensing (QS) (3). QS signaling in P. aeruginosa involves four major interconnected signaling pathways (3). The signaling molecules for these pathways are: N-(3-oxododeconoyl)-L-homoserine lactone for the las pathway, N-butanoyl-L-homoserine lactone (BHL) for the rhl pathway, 2-heptyl-3-hydroxy-4-quinolone (Pseudomonas Quinolone Signal, PQS) pathway and 2-(2-hydroxyphenyl)-thiazole-4-carbaldehyde (Integrated Quorum Sensing, IQS) (3–5). When a critical population density is reached, P. aeruginosa secretes these molecules which then act on their respective receptors to trigger coordinated expression of virulence genes (5). With respect to the signaling hierarchy, the las signaling system appears to sit upstream of the PQS, IQS and rhl pathways, with PQS having influence over rhl as well (5). Biofilm production is initiated, exotoxins such as pyocyanin and elastase are produced, and antibiotic resistance genes are activated (6–8).
Biofilms are bacterial extracellular matrices containing populations which adhere to each other and often to solid surfaces. These biofilms encapsulate bacterial cells with proteins and nuclear material using exopolysaccharides (9). This increases bacterial virulence by reducing the ability of antibiotics or immune cells to penetrate the biofilm and clear the infection. Intrinsic and acquired antibiotic resistance causes P. aeruginosa to be increasingly challenging to eradicate.
Specialized Pro-Resolving Mediators (SPMs) are lipids, endogenously produced by the host as part of inflammation resolution (10, 11). During inflammation or infection, there is transcellular biosynthesis of lipoxins from arachidonic acid and resolvins from omega-3 fatty acids (11). The transcellular biosynthesis may occur between neutrophils, monocyte/macrophages, and epithelial cells (12). Lipoxin A4 (LxA4) has been reported to decrease neutrophil activation, increase monocyte/macrophage recruitment, and attenuate proinflammatory cytokine production in models of infection and sepsis (12–14). Furthermore, previous studies have shown that LxA4 can directly increase the phagocytic ability of neutrophils isolated from septic mice (14) and promote macrophage phagocytosis of apoptotic neutrophils (15, 16). Similarly, resolvins of the D-series derived from docosahexaenoic acid (22:6) and E-series derived from eicosapentaenoic acid (20:5) reduced inflammation and decreased bacterial load in different models of inflammation and infection (17–21). Furthermore, RvD2 decreased antibiotic requirements in an in vivo model of infection (20). Although there have been many studies which have focused on SPM action on host response in inflammation and infection, little work has been conducted to examine if SPMs have direct effects on bacteria growth or virulence.
Many studies have targeted synthetic inhibitory molecules to antagonize QS signaling (22). We have previously shown that LxA4 is a potent inhibitor of QS signaling by acting as a strong antagonist of the LasR receptor (23). Furthermore, P. aeruginosa secretes an arachidonate 15-lipoxygenase enzyme (LoxA) which may alter the local environment by synthesizing SPMs such as LxA4 (24). On the other hand, there is evidence that P. aeruginosa inhibits production of 15-epi-LxA4, suggesting that the bacteria possesses a mechanism to reduce resolution in the local environment (25). Therefore, we reasoned that LxA4 may directly reduce P. aeruginosa biofilm formation.
Two common antibiotics used in the treatment of P. aeruginosa infections are ciprofloxacin and imipenem (2). P. aeruginosa has been shown to develop resistance to ciprofloxacin and imipenem (26–28). Ciprofloxacin (a topoisomerase inhibitor) (29) at sub-inhibitory concentrations may cause increased formation of biofilm (30). Ciprofloxacin has been shown to increase biofilm-associated bacterial cells’ antibiotic resistance above that of non-adherent cells, only recovering susceptibility after 5–10 passages (31). Ciprofloxacin and imipenem (a cell wall inhibitor) (29) have both shown QS-inhibitory action on P. aeruginosa (32, 33). Ciprofloxacin decreases expression for a range of QS-regulated virulence factors, suggesting the mechanism of QS inhibition may be mediated by alterations to membrane permeability and the flux of the QS autoinducer signaling molecule (33, 34). It is therefore plausible that a known QS inhibitor such as LxA4 may augment the actions of these antibiotics.
In this study, we used an established in vitro system to examine the effects of LxA4 and other SPMs on P. aeruginosa biofilm formation. We also examined the effects of SPMs on virulence gene expression. We investigated the potential synergistic action of LxA4 with antibiotics to reduce bacterial biofilm formation and/or increase antibiotic bactericidal activity.
2. Materials and methods
2.1. Culture preparation and biofilm growth:
P. aeruginosa ATCC 27853™ (American Type Culture Collection, Manassas, VA, USA) was streaked on a Tryptic soy agar plate (TSA; Ward’s Scientific, Rochester, NY, USA) and incubated overnight at 37°C. From the streaked plate, liquid cultures were made by swabbing P. aeruginosa with a wooden applicator stick and depositing into glass culture tubes with 3 mL LB broth (Luria-Bertani broth; Gibco: Gaithersburg, MD, USA). The cultures were incubated for 5 hr at 37°C with shaking (180 rpm) and pelleted by centrifuging for 6 min at 9100 g. LB broth was removed, and pellets were washed 3 times with M63 minimal media (Amresco, Cleveland, OH, USA) supplemented with 1 mM MgSO4, 0.2% glucose, and 0.5% casamino acids (Fisher BioReagents, Pittsburgh, PA, USA). Cultures were diluted in M63 to an OD600 between 0.05 – 0.10. 125 μL of the cultures were plated into each well of U-bottom 96-well microplates. 0.1 – 10 nM of SPM (obtained by organic synthesis, see section 2.9), FPR2 peptide agonist WKYMVm (Tocris, Minneapolis, MN, USA) or saline (Molecular Biologicals International, Irvine, CA, USA) was added to the wells. The OD600 was measured to verify consistent plating of the culture (Biotek Synergy H1 plate reader; Biotek, Winooski, VT, USA). The plates were incubated overnight (19 – 20 hr) at 37°C. As lowered pH is found in airways of cystic fibrosis patients (35), these studies were conducted at lowered pH to better simulate the clinically relevant conditions, which are environments P. aeruginosa may be prevalent.
2.2. Biofilm assay:
To assay the biofilm biomass, plates were gently submerged in ddH2O three times to rinse off the non-adherent bacteria. 155 μL of 0.1% crystal violet in ddH2O (Sigma, St. Louis, MO, USA.) was added to each well for 15 min. The plates were washed in ddH2O three times to remove excess crystal violet stain and dried upside down overnight. 175 μL of modified biofilm dissolving solution (36) (10% SDS dissolved in 80% ethanol) was added to each well and incubated for 15 min. The solution was transferred to flat-bottom microtiter plates and OD600 was measured to quantify the crystal violet staining.
2.3. Bacteria colonies:
To estimate non-adherent colony forming units (CFUs), cultures from 2–3 wells of each treatment group were recovered prior to washing the plate in ddH2O. These cultures were diluted in saline, 100 μL were spread on TSA plates, incubated overnight at 37°C, and colonies counted.
2.4. Antibiotic experiments:
For experiments using antibiotics, dilutions were made such that 0.1 – 10 μg/mL of antibiotic was added to treatment wells. LxA4 (1, 10 nM) or vehicle saline was added to the 96-well microplates first, followed by ciprofloxacin (Enzo, Farmingdale, NY, USA), imipenem (Alfa Aesar, Tewksbury, MA, USA) or saline with gentle swirling. In these studies, culture was plated at densities at which LxA4 alone did not affect biofilm formation. The plates were incubated at 37°C for 20 hr. Biofilm assays or CFU counts of non-adherent bacteria were performed as described in 2.2 and 2.3.
2.5. Pre-formed biofilm assay:
Untreated biofilm was grown for 20 hr before addition of antibiotics and LxA4 for 6 hr. At the end of treatment incubation time, biofilm biomass was quantitated as described in 2.2.
2.6. MTT assay:
Based on methods previously published (37–39) with modifications as follows. In brief, 100 μL untreated biofilm was grown for 20 hr. The plates were gently submerged in 1X PBS (Molecular Biologicals International, Irvine, CA, USA) three times to rinse off the culture and non-adherent bacteria. Ciprofloxacin with or without LxA4 in M63 media was added (125 μL) and incubated with pre-formed biofilm for 6 hr, then rinsed in 1X PBS three times. Thiazolyl Blue tetrazolium bromide (MTT) (Alfa Aesar, Tewksbury, MA, USA) stock solution was made to a concentration of 5 mg/mL in 1X PBS, and a working solution was made in M63 to a final concentration of 0.45 mg/mL. 150 μL of this MTT working solution was plated and incubated for 4 hr at 37°C. The solution was pipetted from the plate, and 175 μL of solubilization solution (alkaline DMSO: 0.5mM NaOH in DMSO) was incubated for 30m at 37°C. The solubilized formazan was transferred to flat-bottom microtiter plates and OD570 was measured to quantify the formazan, reflecting the relative amount of metabolically active bacterial cells in the biofilm.
2.7. Growth curves:
Cultures were prepared as in 2.1. 200 μL of the cultures were plated into flat bottom 96-well microtiter plates. 0.01 – 100 nM of LxA4 or RvD2 or saline solution was added to control wells. Empty wells were filled with 200 μL ddH2O to serve as a moisture reservoir. The plates were covered and shaken at 37°C, and OD600 was measured every 10 min for 15 hr in a microplate reader.
2.8. Gene expression via RT-PCR and qRT-PCR:
Cultures were prepared as in section 2.1 and incubated for 8 hr at 37°C with shaking (180 rpm). 1 mL of the cultures were aliquoted, and 1 nM of each treatment or saline was added to the tubes. Treatments were incubated for designated time points before RNA extraction. RNA was extracted using Trizol Max Bacterial RNA isolation Kit (Thermo Fisher Scientific, Waltham, MA). RNA purity (Absorbance ratio A260/A280, A260/A230) and RNA concentration were measured using a nanophotometer (IMPLEN P330; Implen, Los Angeles, CA, USA). Extracted RNA was stored at −70°C until further use.
Approximately 1 μg of RNA was reverse transcribed using High Capacity cDNA reverse transcription kit (Thermo Fisher Scientific, Waltham, MA, USA). RNase Inhibitor (Thermo Fisher Scientific Waltham, MA, USA) was used in all reactions to prevent RNA degradation. Reverse Transcription was performed using kit protocol and recommended conditions. cDNA purity (Absorbance ratio A260/A280, A260/A230) and concentration was measured using a nanophotometer. Samples were stored at −20°C until further use.
Quantitative real-time PCR (qRT-PCR) was performed by SYBR green method using Fast SYBR green master mix (Thermo Fisher Scientific, Waltham, MA, USA) and primers (Table 1). Primer sequences for genes pqsA, rhlA, and 16S rRNA were obtained from published work (40). 16S rRNA was used as the endogenous control. The program used for qRT-PCR was adapted from recommended thermal cycler conditions with modifications according to primers used (Table 2) and carried out in an Eppendorf Realplex Mastercycler (Eppendorf, Westbury, NY, USA).
Table 1.
Primer sequences used for virulence gene expression qRT-PCR.
| GENE NAME | PRIMER SEQUENCES | AMPLICON LENGTH |
|---|---|---|
| 16S rRNA | Forward: 5’-GGAGAAAGTGGGGGATCTTC-3’ Reverse: 5’-CCGGTGCTTATTCTGTTGGT-3’ |
316 |
| rhlA | Forward: 5’-GCGCGAAAGTCTGTTGGTAT-3’ Reverse: 5’-ATTTCCACCTCGTCGTCCTT-3’ |
249 |
| pqsA | Forward: 5’-ACCGCGAAGGACACACTATC-3’ Reverse: 5’-GGCAGGTAGGAACCAGAACC-3’ |
297 |
Table 2.
Thermal cycler program optimized for virulence gene expression qRT-PCR.
| STEP | NAME | Temp (°C) | Duration (sec) | Number of Cycles |
|---|---|---|---|---|
| 1 | Initiation | 95 | 120 | 1 |
| 2 | DNA polymerase activation | 95 | 20 | 1 |
| 3 | Denaturation | 95 | 3 | 50 |
| 4 | Anneal and Extend | 60 | 30 | 50 |
| 5 | Endpoint | 60 | 120 | 1 |
2.9. SPM preparation:
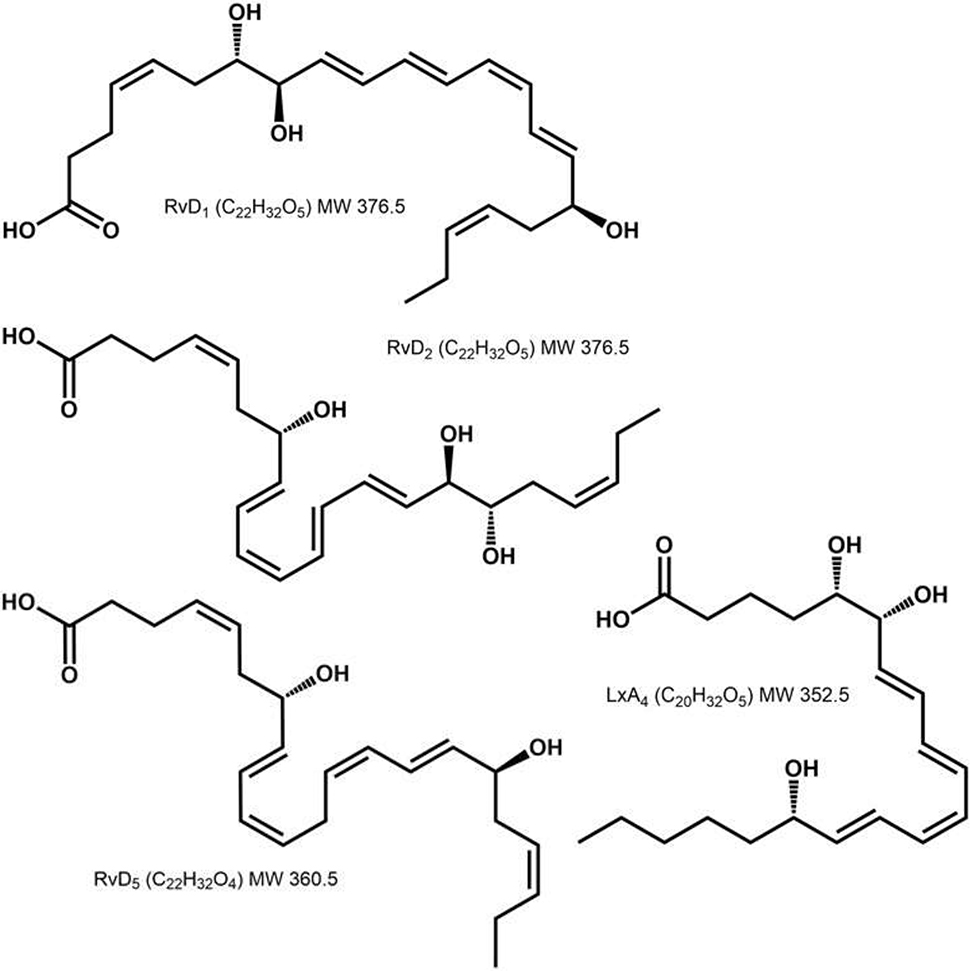
All SPMs (LxA4, RvD1, RvD2, RvD5) were prepared by total organic synthesis (41–44). Purity of the compounds was measured by HPLC-Mass Spectrometry and was determined to be > 98%. The SPMs were diluted in saline and bubbled with argon to displace oxygen. Structures of SPMs used are shown in Figure 1.
Figure 1.
Chemical structures of specialized pro-resolving mediators RvD1, RvD2, RvD5, and LxA4. All three resolvins are 22C chains; RvD1 has hydroxyl groups at C7, 8 and 17; RvD2 has hydroxyl groups at C7, 16 and 17; and RvD5 has hydroxyl groups at C7 and 17 (21, 42–44, 64). LxA4 is a 20C chain with hydroxyl groups on C5, 6 and 15 (41).
2.10. Statistical analyses:
All analyses ware performed using GraphPad Prism (San Diego, CA, USA). Biofilm data were subjected to one-way ANOVA. Dunnett’s test for multiple groups was then used to test for significance compared to controls. For growth curves, areas under the curve were calculated and then subjected to one-way ANOVA. Student’s t-test was used to test significance between antibiotic and antibiotic + LxA4 at each concentration of antibiotic. IC50 was measured after drawing individual cubic spline plots for each treatment. The average IC50 for each treatment was then calculated and compared using t-test. In all analyses, P < 0.05 was taken as significant. All data is expressed as mean ± s.e.m. For gene expression, relative gene quantification was done by ΔCT method (45). Relative quantification was performed by calculating average of triplicate CT values. 1/ΔCT values were used to measure statistical significance between treated and untreated controls of the same time point.
3. Results
3.1. Biofilm formation:
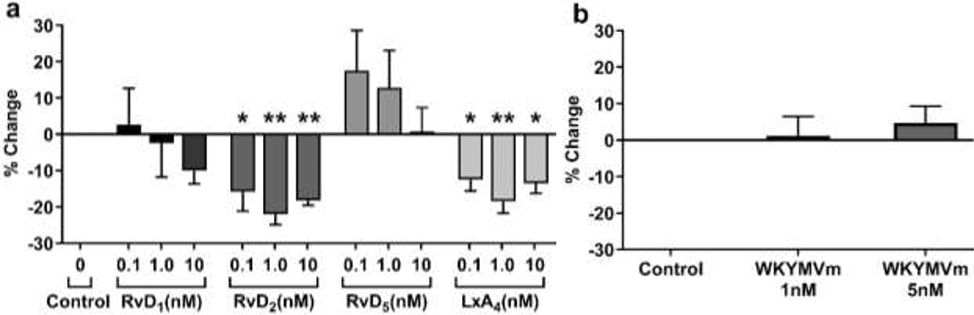
P. aeruginosa cultures were incubated with saline vehicle or SPM (LxA4, RvD1, RvD2, RvD5) in 96 well plates for 20 hr. At the end of the incubation period, wells were washed and stained with crystal violet. After drying, the stained biofilm mass was removed with detergent solution containing SDS and ethanol. LxA4 and RvD2 (0.1 – 10 nM) significantly reduced biofilm formation, but RvD1 and RvD5 did not (Figure 2a). The results suggest that there is SPM selectivity in biofilm reduction. To further examine other receptor-mediated effects, we performed studies with a FPR2 receptor peptide agonist, WKYMVm. The FPR2 peptide had no significant effect on biofilm formation (Figure 2b).
Figure 2.
RvD2 and LxA4 reduce P. aeruginosa biofilm formation. (a) In a 96-well plate, cultures were treated with multiple concentrations of each SPM and incubated at 37°C overnight. The cultures and apical biofilm were washed away, and the remaining apical biofilm rings were stained with 0.1% crystal violet. Absorbance was measured at 600 nm. RvD2 significantly reduced biofilm formation at all three concentrations, with treatment at 0.1 nM less effective than at 1 nM or 10 nM. LxA4 significantly reduced biofilm formation, though 0.1 nM and 10 nM were less effective than 1 nM. (b) Effects of FPR2 receptor peptide agonist on P. aeruginosa biofilm formation. Cultures were treated with multiple concentrations of the peptide agonist WKYMVm. Peptide agonist WKYMVm did not significantly affect the formation of biofilm at either concentration tested. Data are mean ± s.e.m. of percent change from control adjusted to zero. * = p < 0.05; ** = p < 0.01; n = 3 independent experiments for all treatments.
3.2. Growth curves:
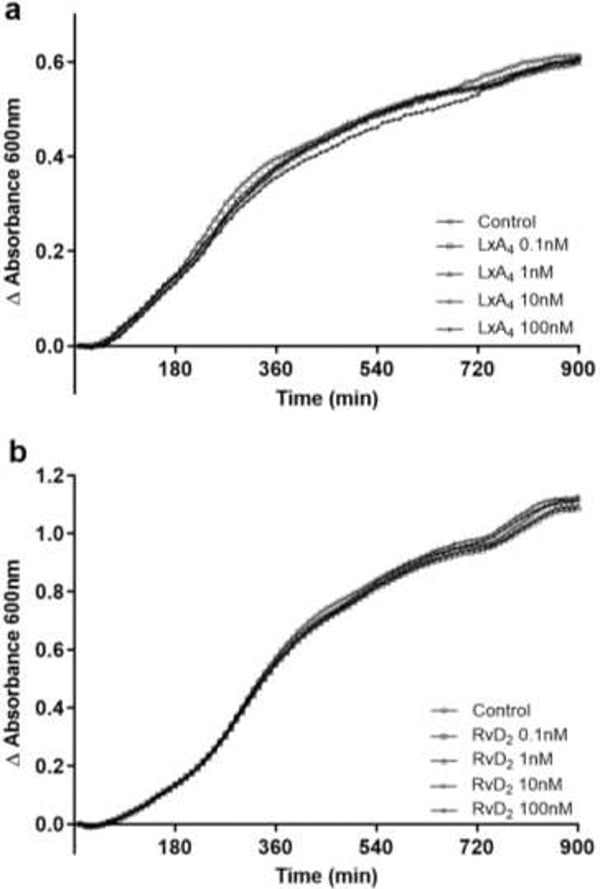
In these studies, we examined if LxA4 and RvD2 had any effect on non-adherent P. aeruginosa growth rate. Neither LxA4 nor RvD2 had a significant effect on non-adherent P. aeruginosa growth, suggesting that the reduction in biofilm biomass was not a result of an inhibitory effect on cell growth (Figure 3).
Figure 3.
Non-adherent cell growth of P. aeruginosa was unaffected by LxA4 or RvD2. SPMs were added to P. aeruginosa cultures in 96-well plates and incubated with orbital shaking at 37°C in a microplate reader for 15 hr. Absorbance was measured every 10 min. Neither LxA4 (a) nor RvD2 (b) showed any significant effect on non-adherent cell growth in minimal media. n = 3 (LxA4) and n = 4 (RvD2) independent experiments.
3.3. Effects of SPMs on quorum-sensing gene expression:
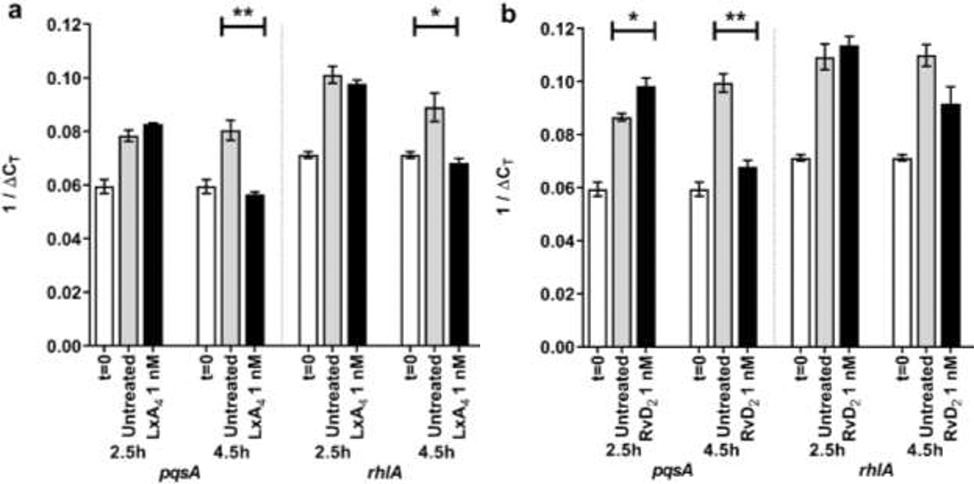
P. aeruginosa was incubated with either saline or 1 nM SPMs (LxA4 or RvD2). At 2.5 hr, Pseudomonas aeruginosa untreated controls increased expression of the two virulence genes investigated (pqsA, rhlA) compared to expression at t=0 (Figure 4). LxA4 did not significantly affect gene expression at 2.5 hr (Figure 4a). At 4.5 hr, LxA4 reduced expression of pqsA and rhlA compared to saline controls at the same time point (Figure 4a). On the other hand, RvD2 increased expression of pqsA at 2.5 hr, but at 4.5 hr, RvD2 significantly reduced expression of pqsA (Figure 4b). There was a strong tendency for RvD2 to inhibit rhlA expression at 4.5 hr, but the inhibition did not reach significance (Figure 4b). These results suggest that both LxA4 and RvD2 can inhibit expression of virulence genes at 4.5 hr after treatment. The results also suggest that at these initial time points of biofilm formation, LxA4 has a greater effect on downregulation of virulence genes compared to RvD2. Therefore, only LxA4 was used in further experiments.
Figure 4.
Effects of SPMs on Pseudomonas aeruginosa quorum-sensing virulence gene expression. (a) 1/ΔCT of two virulence genes’ expression when treated with 1 nM LxA4. LxA4 significantly reduced both genes’ expression at 4.5 hr. (b) 1/ΔCT of two virulence genes’ expression when treated with 1 nM RvD2. RvD2 significantly reduced pqsA gene expression at 4.5 hr. Data are mean ± s.e.m., with p-values determined by unpaired t-test comparing untreated control to treatment group of same timepoint. * p < 0.05, ** p < 0.01. n = 3 independent experiments.
3.4. Interactions of LxA4 with ciprofloxacin:
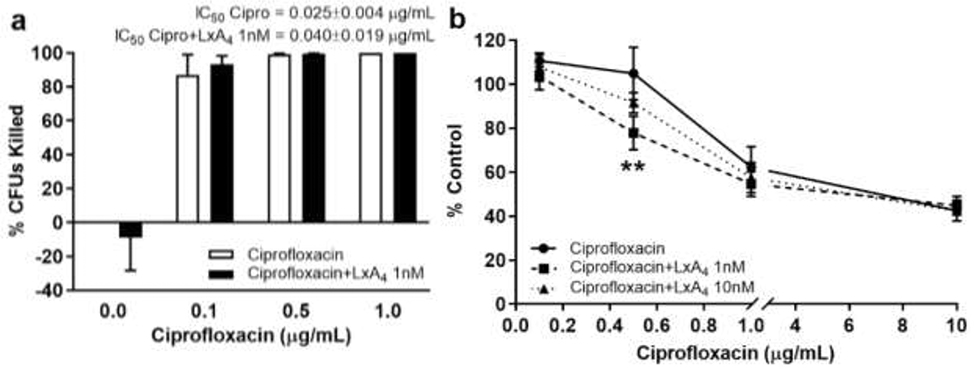
LxA4 (1, 10 nM) or saline was incubated with ciprofloxacin (0.1 – 50 μg/mL) for 20h. Biofilm was then measured. The number of non-adherent bacteria (CFUs) was measured in parallel. At 0.1 and 0.5 μg/mL concentrations, ciprofloxacin did not affect P. aeruginosa biofilm formation (Figure 5b). It should be noted that 0.5 μg/mL ciprofloxacin was bactericidal i.e. causing 99% inhibition of non-adherent P. aeruginosa growth (Figure 5a). Interestingly, LxA4 (1 nM) significantly reduced biofilm formation when incubated with ciprofloxacin (0.5 μg/mL) (Figure 5b), providing evidence that any effects of LxA4 on antibiotic efficacy were synergistic. LxA4 (1 nM), however, did not have any effect on ciprofloxacin-induced inhibition of bacterial growth (Figure 5a). These results suggest that a bactericidal concentration of ciprofloxacin did not affect biofilm formation without concomitant addition of LxA4.
Figure 5.
LxA4 treatment can aid the efficacy of the antibiotic ciprofloxacin in reducing biofilm formation. Cultures were treated in a 96-well plate overnight with multiple concentrations of ciprofloxacin and LxA4. The cultures and apical biofilm were washed away, and the remaining apical biofilm rings were stained with 0.1% crystal violet. Absorbance was measured at 600 nm. Some cultures were recovered from the 96-well plate, diluted and spread on tryptic soy agar plates to incubate overnight at 37°C. Colonies were counted the next day. (a) LxA4 does not have an effect when combined with ciprofloxacin on non-adherent cell growth, determined by colony forming units (CFUs). (b) When combined with LxA4, biofilm formation is significantly reduced in ciprofloxacin treatments at and above bactericidal doses (0.5 μg/mL). CFU data are mean ± s.e.m. of percent change from control adjusted to zero. Biofilm data are mean ± s.e.m. percent of control. ** = p < 0.01; CFUs n = 4 independent experiments; biofilm n = 6 (1 nM) and n = 3 (10 nM) independent experiments.
3.5. Interactions of LxA4 with imipenem:
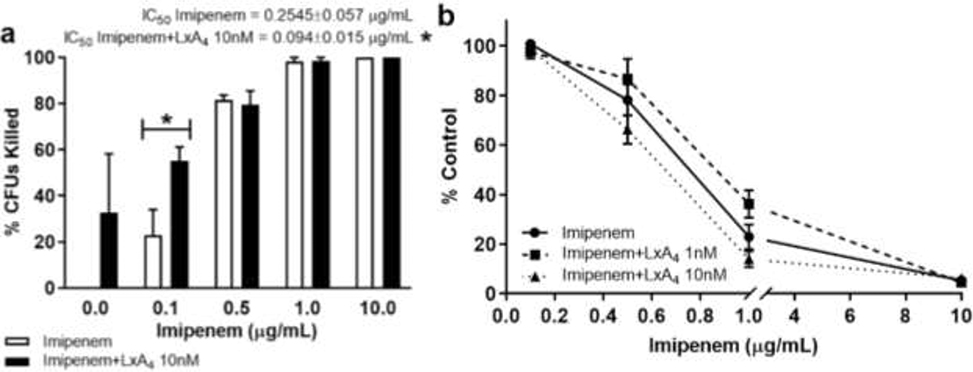
LxA4 (1, 10 nM) or saline was incubated with imipenem (0.1 – 10 μg/mL) for 20 hr before biofilm mass measurement. As in studies with ciprofloxacin, the number of non-adherent bacteria (CFUs) was measured in parallel. Imipenem dose-dependently reduced biofilm formation (Figure 6b). LxA4 did not affect imipenem induced biofilm reduction. Imipenem dose-dependently reduced non-adherent bacterial growth (Figure 6a). Unexpectedly, LxA4 (10 nM) enhanced the bacterial killing action of imipenem at 1 μg/mL (Figure 6a). Overall, IC50 of imipenem was significantly lowered by LxA4 (Figure 6a). These findings suggest that LxA4 increased the efficacy of imipenem’s bactericidal activity in vitro.
Figure 6.
LxA4 in combination with imipenem significantly decreased non-adherent cell growth but not biofilm formation. Cultures were treated in a 96-well plate overnight with multiple concentrations of imipenem and LxA4. The cultures and apical biofilm were washed away, and the remaining apical biofilm rings were stained with 0.1% crystal violet. Absorbance was measured at 600 nm. Some cultures were recovered from a 96-well plate, diluted and spread on tryptic soy agar plates to incubate overnight at 37°C. Colonies were counted the next day. (a) LxA4 (10 nM) in combination with imipenem (0.1 μg/mL) significantly decreased non-adherent cell growth. (b) LxA4 does not significantly affect the efficacy of imipenem against biofilm formation. These data combined with the ciprofloxacin data (Fig. 4) suggests the synergistic action of LxA4 with antibiotics is dependent on the class of antibiotics and their mechanism of action. CFU data are mean ± s.e.m. of percent change from control adjusted to zero. Biofilm data are mean ± s.e.m. percent of control. * = p < 0.05; CFUs n = 4 independent experiments; biofilm n = 3 (LxA4 1 nM) and n = 4 (LxA4 10 nM) independent experiments.
3.6. Interaction of LxA4 with ciprofloxacin in pre-formed biofilm:
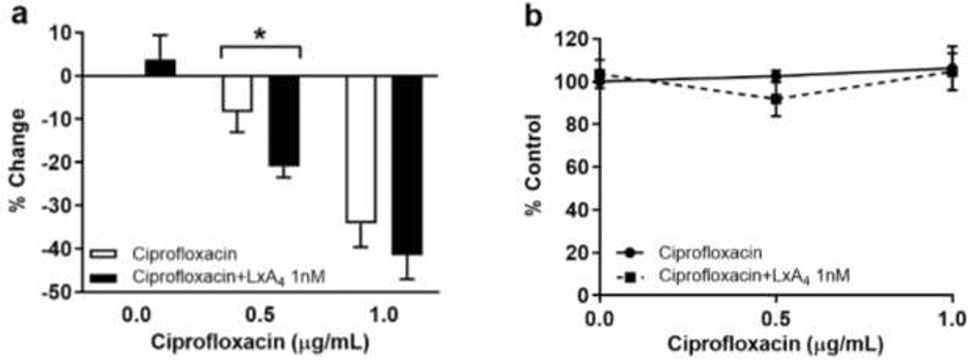
In these studies, P. aeruginosa biofilm was grown for 20 hr before addition of ciprofloxacin (0.5, 1 μg/mL) with or without LxA4. Bacterial cell viability within the biofilm was then assessed by the MTT assay. Our results show that ciprofloxacin alone at 0.5 μg/mL had no effect on P. aeruginosa viability within the biofilm, but addition of LxA4 increased the bactericidal activity of ciprofloxacin (Figure 7a). LxA4 had no effect on the action of ciprofloxacin on pre-formed P. aeruginosa biofilm biomass (Figure 7b).
Figure 7.
Effects of LxA4 on ciprofloxacin action in pre-formed biofilms. (a) LxA4 (1 nM) significantly increased the efficacy of ciprofloxacin (0.5 μg/mL) to reduce the amount of metabolically active bacterial cells associated with the biofilm, determined by MTT assay. (b) LxA4 does not significantly alter the efficacy of ciprofloxacin to reduce biofilm biomass, determined by crystal violet staining. This suggests that LxA4 treatment can aid the ciprofloxacin in accessing the bacteria associated with a pre-formed biofilm. Viability data are mean ± s.e.m. of percent change from control adjusted to zero. Biofilm data are mean ± s.e.m. percent of control. * = p < 0.05; viability n = 5 independent experiments; biomass n = 5 independent experiments.
4. Discussion
This study provides evidence that LxA4 and RvD2 have beneficial effects to reduce P. aeruginosa biofilm formation directly and reduce virulence gene expression. LxA4 improved the efficacy of antibiotics (ciprofloxacin and imipenem). With respect to its effects in concert with antibiotics, LxA4 increased efficacy of ciprofloxacin (0.5 μg/mL) to reduce biofilm formation and increased killing of biofilm-associated P. aeruginosa in pre-formed biofilm. This action did not affect ciprofloxacin’s bacterial killing of non-adherent cells. On the other hand, LxA4 did not affect imipenem action on biofilm formation, but unexpectedly increased imipenem’s bacterial killing of non-adherent cells. LxA4 alone had no effect on P. aeruginosa growth.
The inflammation resolution actions of SPMs have been documented in various models of in vivo inflammation and infection (12, 13, 17, 18, 21, 46). With respect to infection, SPMs have been shown to reduce systemic inflammation as well as bacterial load (13, 18, 21). In mammalian cells, these actions are mediated by specific G-protein receptors. Specifically, LxA4 binds to the formyl peptide receptor 2 (FPR2) while RvD1 binds both FPR2 and GPR 32 (47, 48). RvD5 binds only GPR 32 while RvD2 binds only GPR 18 (18, 20). The direct actions of these SPMs on bacterial cells, however, have not been examined extensively.
LxA4 and RvD2, but not RvD1 or RvD5, reduced P. aeruginosa biofilm formation. As LxA4 shares a similar receptor with RvD1, while RvD2 does not share a similar receptor with either SPM, the mechanism of biofilm inhibition is likely not related to structural binding to a common receptor, such as FPR2. In our experiment, using an FPR2 peptide agonist did not have any significant effect on biofilm formation. This is consistent with other studies showing that the formyl peptide receptors are mammalian receptors and thus P. aeruginosa is unlikely to be affected by FPR agonists (49). We have shown that LxA4 binds to the lasR receptor and inhibits the quorum sensing pathway (23). Taken together, our results suggest that LxA4 reduces biofilm formation through a QS inhibitory mechanism. This conclusion is supported by our results showing that neither LxA4 nor RvD2 given alone, altered non-adherent cell growth in any way. Additionally, there was no significant dose-dependent effect of LxA4 or RvD2 at the concentrations tested. This would suggest that the biofilm inhibitory mechanism is easily saturated. We have previously shown similar results when looking at the effects of LxA4 in reducing pyocyanin release from P. aeruginosa (23). These data have significant clinical relevance because diminishing biofilm formation should augment immune cell clearance of the pathogen. This possibility is a subject of ongoing studies in our lab.
Virulence gene pqsA is indispensable for biofilm formation in Pseudomonas aeruginosa (50). PqsA is required to produce metabolites such as 2,4-dihydroxyquinoline (DHQ) and 4-hydroxy-2-alkylquinolines (HAQs) which act as signals for cell-cell communication (51). As both LxA4 and RvD2 downregulated pqsA expression, it suggests that the mechanism by which LxA4 and RvD2 inhibit biofilm formation is at least partially through down regulation of pqsA expression. pqsA is downstream of lasR and therefore from other data published by our lab (23), the effect seen on pqsA could be a result of LxA4 affecting lasR. rhlA is required for biosynthesis of rhamnolipids, which are dimers of 3-hydroxy fatty acids linked to a mono- or di-rhamnose moiety by an O-glycosidic link (52). Importantly, rhamnolipids are vital to maintaining the architecture of biofilms and protect the biofilms against host neutrophils (52–54). Our results showing that LxA4 significantly reduced both pqsA and rhlA expression further supports the notion that LxA4 can directly suppress P. aeruginosa virulence. As RvD2 increased pqsA at 2.5 hr and had a smaller reducing effect at 4.5 hr than LxA4, it suggests that RvD2 is less potent than LxA4 in decreasing virulence at these time points. Interestingly, neither LxA4 nor RvD2 had any effect on reducing virulence gene expression at 2.5 hr. This result suggests that early virulence (including early biofilm formation) is not inhibited by either SPM and would therefore account for the modest decreases in biofilm formation with both SPMs. This result also suggests that SPM downregulation of virulence genes is not through early inhibition of transcription, but a later suppression of these genes.
P. aeruginosa biofilm is an exopolysaccharide matrix that encapsulates the bacterial cells and provides a protection against antibiotics (26, 28). The mechanism for this resistance is now believed to be dependent on the type of antibiotic used. Fluoroquinolone antibiotics such as ciprofloxacin can penetrate P. aeruginosa biofilm, while aminoglycoside penetration is limited (26). However, penetration of ciprofloxacin into the biofilm does not affect the biofilm-associated P. aeruginosa viability. It is thought that one mechanism of ciprofloxacin resistance in biofilm is due to a subpopulation switch to the persister phenotype, which contributes to a failure in biofilm eradication (55).
It is important to note that we performed counts of non-adherent bacteria in wells side by side to wells used for biofilm biomass determination. By employing this method, we were able to ascertain the bacteria killing activity of the antibiotic in a physiologically relevant setting where P. aeruginosa actively forms biofilm. The results strongly suggest that a ciprofloxacin concentration (0.5 μg/mL) that is bactericidal to non-adherent bacteria does not reduce biofilm formation to any significant degree. This concentration is consistent with published work showing that it is indeed bactericidal for P. aeruginosa (56), supporting our methodology. We show that LxA4 can increase efficacy of ciprofloxacin to reduce biofilm formation. As there is evidence that LxA4 can inhibit quorum sensing (23), it is possible that LxA4 can reduce the ciprofloxacin-mediated switch of P. aeruginosa to the persister phenotype and reduce P. aeruginosa biofilm. It is also possible that by reducing rhamnolipid production, ciprofloxacin can more easily destroy biofilm architecture.
With respect to pre-formed or existing biofilm, our results suggest that ciprofloxacin at 0.5 μg/mL did not significantly kill biofilm associated P. aeruginosa. We show for the first time that LxA4 promoted the bactericidal actions of ciprofloxacin in pre-formed biofilm matrix, which supports the plausibility of LxA4 as adjunctive treatment for P. aeruginosa infections. There was no effect of ciprofloxacin on biofilm mass nor did LxA4 have any effect on this. The results suggest that neither ciprofloxacin at bactericidal concentrations nor LxA4 have any effect on destroying pre-formed biofilm structure. If combined treatments of SPMs can help make classically used antibiotics effective against multi-drug resistant bacterial infections, we have a method to combat the growing public health crisis of multi-drug resistant pathogens.
Imipenem is an antibiotic which works by inhibiting cell wall synthesis of gram negative and gram positive bacteria, where it inactivates specific proteins involved in the last stages of cell wall synthesis (29). Imipenem alone dose-dependently inhibited biofilm formation and LxA4 did not affect this action. Others have shown that imipenem works on the las QS system, just as LxA4 (57). It is unlikely that LxA4 affected imipenem’s action through the las QS system because there was no effect on biofilm formation. On the other hand, LxA4 increased bactericidal efficacy of imipenem on non-adherent bacteria at the low dose of 0.1 μg/mL imipenem but not at higher doses. At higher doses (≥ 0.5 μg/mL), imipenem caused ≥ 80% bacterial death, making it difficult to increase the bactericidal action of imipenem any further. The effect of LxA4 on the action of low dose imipenem was unexpected because LxA4 does not have any effect on P. aeruginosa growth. We are currently investigating if this effect with imipenem is specific to P. aeruginosa or whether it extends to other bacterial pathogens.
The beneficial effects of combining an SPM such as LxA4 with an antibiotic may be of great significance in the topical treatment of wound infections. This is because wounds infected with P. aeruginosa have decreased healing due to excessive neutrophil infiltration and increased inflammatory cytokine release (58, 59). It is plausible that adjunctive or concomitant use of antibiotic and LxA4 may have beneficial actions to increase efficacy of the antibiotic but also to increase wound healing through inflammation resolution. However, the therapeutic window for LxA4’s effects on antibiotic efficacy is small, between 1 – 10 nM LxA4, and is similar to LxA4’s effect on biofilm formation (Figure 2). The small therapeutic window may be due to the rapid saturation of its actions.
P. aeruginosa lung infections are common in cystic fibrosis patients. The bacteria use mechanisms such as biofilm formation to evade antibiotic killing or host defense mechanisms. These persistent infections cause chronic inflammation. Studies have shown that SPM production is reduced in cystic fibrosis (60, 61). Importantly, use of an SPM such as LxA4 has been reported to decrease both bacterial burden and disease severity in vivo (61). In vitro studies showing that LxA4 delayed P. aeruginosa colonization of airway epithelial cells (62) and augmented airway tissue repair (63) provide possible mechanisms for the beneficial actions of LxA4 in cystic fibrosis. Our study adds to current mechanistic knowledge by showing that LxA4 can directly reduce P. aeruginosa biofilm formation and importantly increases the efficacy of two antibiotics commonly used against P. aeruginosa infections.
4.1. Conclusions
In summary, our results show that LxA4 and RvD2 can directly reduce P. aeruginosa biofilm formation. LxA4 and RvD2 can also downregulate virulence gene expression. LxA4 enhances the efficacy of antibiotics directly against P. aeruginosa biofilm formation and bacterial cells within existing biofilm. These studies also provide evidence that further investigation into the antimicrobial mechanisms of RvD2 is warranted. The results suggest that there is relative selectivity in SPM inhibition of biofilm formation.
HIGHLIGHTS.
Lipoxin A4 (LxA4) and Resolvin D2 (RvD2) directly reduce Pseudomonas aeruginosa biofilm formation.
LxA4 and RvD2 reduce expression of Pseudomonas aeruginosa virulence genes.
LxA4 augments ciprofloxacin’s effects on biofilm reduction but does not affect ciprofloxacin’s bactericidal action on non-adherent cells.
LxA4 augments imipenem’s bactericidal action on non-adherent cells but does not affect imipenem’s action on Pseudomonas biofilm formation.
LxA4 augments ciprofloxacin’s antimicrobial action within pre-formed Pseudomonas biofilm
ACKNOWLEDGEMENTS
The work was supported by NIH RO1 AI128202 and a grant from the New Jersey Health Foundation. We thank Rachael Wilson for helping with completion of this work.
Source of support: This work was supported by the NIH (RO1 AI128202) and the New Jersey Health Foundation
Abbreviations:
- SPMs
Specialized Pro-resolving Mediators
- LxA4
Lipoxin A4
- Rv
Resolvin
- CFUs
Colony forming units
- PQS
Pseudomonas Quinolone Signal
- IQS
Integrated Quorum Sensing Signal
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gellatly SL, and Hancock RE (2013) Pseudomonas aeruginosa: new insights into pathogenesis and host defenses. Pathog Dis 67, 159–173 [DOI] [PubMed] [Google Scholar]
- 2.Munguia J, and Nizet V (2017) Pharmacological Targeting of the Host-Pathogen Interaction: Alternatives to Classical Antibiotics to Combat Drug-Resistant Superbugs. Trends Pharmacol Sci 38, 473–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jimenez PN, Koch G, Thompson JA, Xavier KB, Cool RH, and Quax WJ (2012) The multiple signaling systems regulating virulence in Pseudomonas aeruginosa. Microbiol Mol Biol Rev 76, 46–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Loughlin CT, Miller LC, Siryaporn A, Drescher K, Semmelhack MF, and Bassler BL (2013) A quorum-sensing inhibitor blocks Pseudomonas aeruginosa virulence and biofilm formation. Proc Natl Acad Sci U S A 110, 17981–17986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee J, and Zhang L (2015) The hierarchy quorum sensing network in Pseudomonas aeruginosa. Protein Cell 6, 26–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith RS, Harris SG, Phipps R, and Iglewski B (2002) The Pseudomonas aeruginosa quorum-sensing molecule N-(3-oxododecanoyl)homoserine lactone contributes to virulence and induces inflammation in vivo. J Bacteriol 184, 1132–1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dumas JL, van Delden C, Perron K, and Kohler T (2006) Analysis of antibiotic resistance gene expression in Pseudomonas aeruginosa by quantitative real-time-PCR. FEMS Microbiol Lett 254, 217–225 [DOI] [PubMed] [Google Scholar]
- 8.Kalia M, Yadav VK, Singh PK, Sharma D, Pandey H, Narvi SS, and Agarwal V (2015) Effect of Cinnamon Oil on Quorum Sensing-Controlled Virulence Factors and Biofilm Formation in Pseudomonas aeruginosa. PLoS One 10, e0135495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davies DG, Parsek MR, Pearson JP, Iglewski BH, Costerton JW, and Greenberg EP (1998) The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science 280, 295–298 [DOI] [PubMed] [Google Scholar]
- 10.Serhan CN (1994) Lipoxin biosynthesis and its impact in inflammatory and vascular events. Biochim Biophys Acta 1212, 1–25 [DOI] [PubMed] [Google Scholar]
- 11.Serhan CN (2014) Pro-resolving lipid mediators are leads for resolution physiology. Nature 510, 92–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bannenberg G, Moussignac RL, Gronert K, Devchand PR, Schmidt BA, Guilford WJ, Bauman JG, Subramanyam B, Perez HD, Parkinson JF, and Serhan CN (2004) Lipoxins and novel 15-epi-lipoxin analogs display potent anti-inflammatory actions after oral administration. Br J Pharmacol 143, 43–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walker J, Dichter E, Lacorte G, Kerner D, Spur B, Rodriguez A, and Yin K (2011) Lipoxin a4 increases survival by decreasing systemic inflammation and bacterial load in sepsis. Shock 36, 410–416 [DOI] [PubMed] [Google Scholar]
- 14.Wu B, Walker J, Spur B, Rodriguez A, and Yin K (2015) Effects of Lipoxin A4 on antimicrobial actions of neutrophils in sepsis. Prostaglandins Leukot Essent Fatty Acids 94, 55–64 [DOI] [PubMed] [Google Scholar]
- 15.Godson C, Mitchell S, Harvey K, Petasis NA, Hogg N, and Brady HR (2000) Cutting edge: lipoxins rapidly stimulate nonphlogistic phagocytosis of apoptotic neutrophils by monocyte-derived macrophages. J Immunol 164, 1663–1667 [DOI] [PubMed] [Google Scholar]
- 16.Reville K, Crean JK, Vivers S, Dransfield I, and Godson C (2006) Lipoxin A4 redistributes myosin IIA and Cdc42 in macrophages: implications for phagocytosis of apoptotic leukocytes. J Immunol 176, 1878–1888 [DOI] [PubMed] [Google Scholar]
- 17.Chen F, Fan XH, Wu YP, Zhu JL, Wang F, Bo LL, Li JB, Bao R, and Deng XM (2014) Resolvin D1 improves survival in experimental sepsis through reducing bacterial load and preventing excessive activation of inflammatory response. Eur J Clin Microbiol Infect Dis 33, 457–464 [DOI] [PubMed] [Google Scholar]
- 18.Chiang N, Dalli J, Colas RA, and Serhan CN (2015) Identification of resolvin D2 receptor mediating resolution of infections and organ protection. J Exp Med 212, 1203–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chiang N, de la Rosa X, Libreros S, and Serhan CN (2017) Novel Resolvin D2 Receptor Axis in Infectious Inflammation. J Immunol 198, 842–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chiang N, Fredman G, Backhed F, Oh SF, Vickery T, Schmidt BA, and Serhan CN (2012) Infection regulates pro-resolving mediators that lower antibiotic requirements. Nature 484, 524–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spite M, Norling LV, Summers L, Yang R, Cooper D, Petasis NA, Flower RJ, Perretti M, and Serhan CN (2009) Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature 461, 1287–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kalia VC (2013) Quorum sensing inhibitors: an overview. Biotechnol Adv 31, 224–245 [DOI] [PubMed] [Google Scholar]
- 23.Wu B, Capilato J, Pham MP, Walker J, Spur B, Rodriguez A, Perez LJ, and Yin K (2016) Lipoxin A4 augments host defense in sepsis and reduces Pseudomonas aeruginosa virulence through quorum sensing inhibition. FASEB J 30, 2400–2410 [DOI] [PubMed] [Google Scholar]
- 24.Vance RE, Hong S, Gronert K, Serhan CN, and Mekalanos JJ (2004) The opportunistic pathogen Pseudomonas aeruginosa carries a secretable arachidonate 15-lipoxygenase. Proc Natl Acad Sci U S A 101, 2135–2139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flitter BA, Hvorecny KL, Ono E, Eddens T, Yang J, Kwak DH, Bahl CD, Hampton TH, Morisseau C, Hammock BD, Liu X, Lee JS, Kolls JK, Levy BD, Madden DR, and Bomberger JM (2017) Pseudomonas aeruginosa sabotages the generation of host proresolving lipid mediators. Proc Natl Acad Sci U S A 114, 136–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walters MC 3rd, Roe F, Bugnicourt A, Franklin MJ, and Stewart PS (2003) Contributions of antibiotic penetration, oxygen limitation, and low metabolic activity to tolerance of Pseudomonas aeruginosa biofilms to ciprofloxacin and tobramycin. Antimicrob Agents Chemother 47, 317–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lynch MJ, Drusano GL, and Mobley HL (1987) Emergence of resistance to imipenem in Pseudomonas aeruginosa. Antimicrob Agents Chemother 31, 1892–1896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Musafer HK, Kuchma SL, Naimie AA, Schwartzman JD, Al-Mathkhury HJ, and O’Toole GA (2014) Investigating the link between imipenem resistance and biofilm formation by Pseudomonas aeruginosa. Microb Ecol 68, 111–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rang HP, Dale MM, Ritter JM, Flower RJ, and Henderson G (2014) Antibacterial Drugs In Rang and Dale’s Pharmacology pp. 622–637, Elsevier Churchill Livingstone Publishers [Google Scholar]
- 30.Gotoh H, Zhang Y, Dallo SF, Hong S, Kasaraneni N, and Weitao T (2008) Pseudomonas aeruginosa, under DNA replication inhibition, tends to form biofilms via Arr. Res Microbiol 159, 294–302 [DOI] [PubMed] [Google Scholar]
- 31.Oldak E, and Trafny EA (2005) Secretion of proteases by Pseudomonas aeruginosa biofilms exposed to ciprofloxacin. Antimicrob Agents Chemother 49, 3281–3288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.El-Mowafy SA, Abd El Galil KH, Habib EE, and Shaaban MI (2017) Quorum sensing inhibitory activity of sub-inhibitory concentrations of beta-lactams. Afr Health Sci 17, 199–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Skindersoe ME, Alhede M, Phipps R, Yang L, Jensen PO, Rasmussen TB, Bjarnsholt T, Tolker-Nielsen T, Hoiby N, and Givskov M (2008) Effects of antibiotics on quorum sensing in Pseudomonas aeruginosa. Antimicrob Agents Chemother 52, 3648–3663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bortolotti D, Trapella C, Bragonzi A, Marchetti P, Zanirato V, Alogna A, Gentili V, Cervellati C, Valacchi G, Sicolo M, Turrin G, Fantinati A, Di Luca D, and Rizzo R (2019) Conjugation of LasR Quorum-Sensing Inhibitors with Ciprofloxacin Decreases the Antibiotic Tolerance of P. aeruginosa Clinical Strains. Journal of Chemistry 2019 [Google Scholar]
- 35.McShane D, Davies JC, Davies MG, Bush A, Geddes DM, and Alton EW (2003) Airway surface pH in subjects with cystic fibrosis. Eur Respir J 21, 37–42 [DOI] [PubMed] [Google Scholar]
- 36.Tram G, Korolik V, and Day CJ (2013) MBDS Solvent: An improved method for assessment of biofilms. Advances in Microbiology 3, 200–204 [Google Scholar]
- 37.Cady NC, McKean KA, Behnke J, Kubec R, Mosier AP, Kasper SH, Burz DS, and Musah RA (2012) Inhibition of biofilm formation, quorum sensing and infection in Pseudomonas aeruginosa by natural products-inspired organosulfur compounds. PLoS One 7, e38492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65, 55–63 [DOI] [PubMed] [Google Scholar]
- 39.Stiefel P, Rosenberg U, Schneider J, Mauerhofer S, Maniura-Weber K, and Ren Q (2016) Is biofilm removal properly assessed? Comparison of different quantification methods in a 96-well plate system. Appl Microbiol Biotechnol 100, 4135–4145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Magalhaes AP, Franca A, Pereira MO, and Cerca N (2019) RNA-based qPCR as a tool to quantify and to characterize dual-species biofilms. Sci Rep 9, 13639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rodríguez AR, Nomen M, Spur BW, Godfroid J-J, and Lee T-H (2000) Total synthesis of lipoxin A4 and lipoxin B4 from butadiene. Tetrahedron Letters 41, 823–826 [Google Scholar]
- 42.Rodriguez AR, and Spur BW (2012) Total synthesis of Resolvin D1, a potent anti-inflammatory lipid mediator. Tetrahedron Letters 53, 6990–6994 [Google Scholar]
- 43.Rodriguez AR, and Spur BW (2005) First total synthesis of 7(S), 17(S)-Resolvin D5, a potent anti-inflammatory docosanoid. Tetrahedron Letters 46, 3623–3627 [Google Scholar]
- 44.Rodriguez AR, and Spur BW (2004) First total synthesis of 7(S), 16(R), 17(S)-Resolvin D2, a potent anti-inflammatory lipid mediator. Tetrahedron Letters 45, 8717–8720 [Google Scholar]
- 45.Livak KJ, and Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25, 402–408 [DOI] [PubMed] [Google Scholar]
- 46.Seki H, Fukunaga K, Arita M, Arai H, Nakanishi H, Taguchi R, Miyasho T, Takamiya R, Asano K, Ishizaka A, Takeda J, and Levy BD (2010) The anti-inflammatory and proresolving mediator resolvin E1 protects mice from bacterial pneumonia and acute lung injury. J Immunol 184, 836–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fiore S, Maddox JF, Perez HD, and Serhan CN (1994) Identification of a human cDNA encoding a functional high affinity lipoxin A4 receptor. J Exp Med 180, 253–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Krishnamoorthy S, Recchiuti A, Chiang N, Yacoubian S, Lee CH, Yang R, Petasis NA, and Serhan CN (2010) Resolvin D1 binds human phagocytes with evidence for proresolving receptors. Proc Natl Acad Sci U S A 107, 1660–1665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bufe B, Schumann T, Kappl R, Bogeski I, Kummerow C, Podgorska M, Smola S, Hoth M, and Zufall F (2015) Recognition of bacterial signal peptides by mammalian formyl peptide receptors: a new mechanism for sensing pathogens. J Biol Chem 290, 7369–7387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kang D, Turner KE, and Kirienko NV (2017) PqsA Promotes Pyoverdine Production via Biofilm Formation. Pathogens 7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lepine F, Dekimpe V, Lesic B, Milot S, Lesimple A, Mamer OA, Rahme LG, and Deziel E (2007) PqsA is required for the biosynthesis of 2,4-dihydroxyquinoline (DHQ), a newly identified metabolite produced by Pseudomonas aeruginosa and Burkholderia thailandensis. Biol Chem 388, 839–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nickzad A, and Deziel E (2014) The involvement of rhamnolipids in microbial cell adhesion and biofilm development - an approach for control? Lett Appl Microbiol 58, 447–453 [DOI] [PubMed] [Google Scholar]
- 53.Van Gennip M, Christensen LD, Alhede M, Phipps R, Jensen PO, Christophersen L, Pamp SJ, Moser C, Mikkelsen PJ, Koh AY, Tolker-Nielsen T, Pier GB, Hoiby N, Givskov M, and Bjarnsholt T (2009) Inactivation of the rhlA gene in Pseudomonas aeruginosa prevents rhamnolipid production, disabling the protection against polymorphonuclear leukocytes. APMIS 117, 537–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Davey ME, Caiazza NC, and O’Toole GA (2003) Rhamnolipid surfactant production affects biofilm architecture in Pseudomonas aeruginosa PAO1. J Bacteriol 185, 1027–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Soares A, Roussel V, Pestel-Caron M, Barreau M, Caron F, Bouffartigues E, Chevalier S, and Etienne M (2019) Understanding Ciprofloxacin Failure in Pseudomonas aeruginosa Biofilm: Persister Cells Survive Matrix Disruption. Front Microbiol 10, 2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Preston CA, Khoury AE, Reid G, Bruce AW, and Costerton JW (1996) Pseudomonas aeruginosa biofilms are more susceptible to ciprofloxacin than to tobramycin. Int J Antimicrob Agents 7, 251–256 [DOI] [PubMed] [Google Scholar]
- 57.Zhao J, Jiang H, Cheng W, Wu J, Zhao J, Wang J, and Dong L (2015) The role of quorum sensing system in antimicrobial induced ampC expression in Pseudomonas aeruginosa biofilm. J Basic Microbiol 55, 671–678 [DOI] [PubMed] [Google Scholar]
- 58.Fazli M, Bjarnsholt T, Kirketerp-Moller K, Jorgensen A, Andersen CB, Givskov M, and Tolker-Nielsen T (2011) Quantitative analysis of the cellular inflammatory response against biofilm bacteria in chronic wounds. Wound Repair Regen 19, 387–391 [DOI] [PubMed] [Google Scholar]
- 59.Pastar I, Nusbaum AG, Gil J, Patel SB, Chen J, Valdes J, Stojadinovic O, Plano LR, Tomic-Canic M, and Davis SC (2013) Interactions of methicillin resistant Staphylococcus aureus USA300 and Pseudomonas aeruginosa in polymicrobial wound infection. PLoS One 8, e56846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ringholz FC, Buchanan PJ, Clarke DT, Millar RG, McDermott M, Linnane B, Harvey BJ, McNally P, and Urbach V (2014) Reduced 15-lipoxygenase 2 and lipoxin A4/leukotriene B4 ratio in children with cystic fibrosis. Eur Respir J 44, 394–404 [DOI] [PubMed] [Google Scholar]
- 61.Karp CL, Flick LM, Park KW, Softic S, Greer TM, Keledjian R, Yang R, Uddin J, Guggino WB, Atabani SF, Belkaid Y, Xu Y, Whitsett JA, Accurso FJ, Wills-Karp M, and Petasis NA (2004) Defective lipoxin-mediated anti-inflammatory activity in the cystic fibrosis airway. Nat Immunol 5, 388–392 [DOI] [PubMed] [Google Scholar]
- 62.Higgins G, Fustero Torre C, Tyrrell J, McNally P, Harvey BJ, and Urbach V (2016) Lipoxin A4 prevents tight junction disruption and delays the colonization of cystic fibrosis bronchial epithelial cells by Pseudomonas aeruginosa. Am J Physiol Lung Cell Mol Physiol 310, L1053–1061 [DOI] [PubMed] [Google Scholar]
- 63.Buchanan PJ, McNally P, Harvey BJ, and Urbach V (2013) Lipoxin A(4)-mediated KATP potassium channel activation results in cystic fibrosis airway epithelial repair. Am J Physiol Lung Cell Mol Physiol 305, L193–201 [DOI] [PubMed] [Google Scholar]
- 64.Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, and Moussignac RL (2002) Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med 196, 1025–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]