Abstract
Primary brain tumors are a heterogeneous group of malignancies that originate in cells of the central nervous system. A variety of models tractable for preclinical studies have been developed to recapitulate human brain tumors, allowing us to understand the underlying pathobiology and explore potential treatments. However, many promising therapeutic strategies identified using preclinical models have shown limited efficacy or failed at the clinical trial stage. The inability to develop therapeutic strategies that significantly improve survival rates in patients highlight the compelling need to revisit the design of currently available animal models and explore the use of new models that allow us to bridge the gap between promising preclinical findings and clinical translation. In this review, we will discuss current strategies used to model glioblastoma, the most malignant brain tumor in adults and highlight the shortcomings of specific models that must be circumvented for the development of innovative therapeutic strategies.
Keywords: Glioma, Glioblastoma, Brain tumor model, Therapeutic development, neurooncology
1. Introduction
Primary brain tumors are a group of heterogeneous tumors of the central nervous system (CNS), associated with significant morbidity and mortality. The most common primary malignant brain tumors are diffusely infiltrating gliomas of glial cell origin such as astrocytomas (1) of which, grade IV astroctyoma, also known as glioblastoma (GBM) is the most common (2). Preclinical brain tumor models have played a fundamental role in understanding tumor biology and developing anti-tumor strategies. An ideal experimental model must meet a number of requirements; I) genetic background that resembles human tumors; II) tumor microenvironment that resembles human tumors; III) intratumoral heterogeneity; IV) reproducibility and V) cost-effectiveness (3). From a therapeutic development point of view, a critical goal of studies utilizing preclinical brain tumor models is the ability to predict response in patients and provide insight into predictive biomarkers.
Preclinical brain tumor studies include syngeneic models, genetically engineered models (GEMs) and xenografts (cell line-based and patient derived). Preclinical studies have been predominantly performed on rodents, however the use of canines, vertebrates and arthropods to model brain tumors has provided great insight into the pathobiology of the disease. Current available models however remain imperfect due to the difficulty in recapitulating the genetic heterogeneity and tumor immune microenvironment of human tumors, at a reasonable cost and technical feasibility. In this review, we highlight the key in vitro and in vivo models used to study malignant brain tumors in adults.
Malignant brain tumors
Glioblastoma (GBM) is the most common malignant brain tumor in adults (4). The disease has a dismal prognosis with a median 5-year survival rate of 5.8% (5). GBM can be classified as isocitrate dehydrogenase (IDH)- wildtype (WT) also known as primary GBM or IDH-mutant, classically termed secondary GBM (6). IDH-WT GBMs are more common and aggressive with a worse prognosis than the mutant type. IDH-WT GBMs are characterized by over-expression and amplification of the epidermal growth factor receptor (EGFR) gene, mutations of the Telomerase Reverse Transcriptase (TERT) promoter, deletion of the cyclin-dependent kinase Inhibitor 2A (CDKN2A) gene, mutations of tumor protein p53 (TP53) and Phosphatase and tensin homolog (PTEN) gene. IDH-mutant GBMs, which develop from pre-existent lower grade astrocytoma, are characterized by mutations in TP53, IDH1 and α thalassemia/mental retardation syndrome X-linked (ATRX) (7, 8)
GBMs are troublesome to treat due to their diffuse growth and invasive properties rendering them difficult to remove surgically. There is a migration-proliferation dichotomy in GBM with an inverse correlation between migration and proliferation, which further complicates the treatment process (9, 10). An understanding of the molecular events underlying gliomagenesis is crucial for the development of targeted therapy. GBM was originally classified into 4 transcriptomic subgroups according to their differential gene expression patterns: Classical, Proneural, Neural and Mesenchymal (11). However, subsequent studies found that non-neoplastic cells contaminated in tumor tissues reflected the neural subtype (12).
Classical GBM is characterized by amplification of EGFR resulting in dysregulation of the phosphoinositide 3-kinase (PI3K)/AKT pathway, which can also be disrupted if there is loss of PTEN (13). The proneural GBM subtype is characterized by over-expression of platelet-derived growth factor receptor (PDGFR)(14), mutations in IDH1 (15), loss-of-function in CDKN2A/B (16) and TP53 (17). The mesenchymal GBM subgroup is associated with increased expression of genes in the tumor necrosis factor (TNF) super family pathway and nuclear factor (NF)-κB pathway (18). This subtype presents with frequent mutations in the neurofibromatosis tumor suppressor NF1, PTEN and TP53 genes (19)
2. Syngeneic implantation models
Syngeneic implantation models have been widely used to investigate GBM. Tumorigenesis is induced using carcinogens or via genetic modification. Syngeneic models allow the study of GBM biology and therapeutics in the presence of a functional immune system and are therefore pertinent for immunotherapy studies. Syngeneic models are also highly reproducible and cost-effective (20).
Carcinogen induced glioma cell lines:
Gliomas in rodents can be induced with injection of N-nitroso compounds and were first generated by the administration of carcinogen ethyl nitrosourea (ENU) in the 1970s (Figure 2A). ENU is an alkylating agent administered through the placenta at the 15-18th day of pregnancy resulting in brain tumors with various mutations in the litters. The B-Raf proto-oncogene (Braf), which encodes a serine/threonine protein kinase that activates the mitogen activated protein kinase effector arm of receptor tyrosine kinase signaling, is a key mutation in ENU-induced glioma formation in rats. Other induced mutations include Tp53, Pdgfrα, deletion of Cdkn2a, and amplification of Egfr (21). Commonly used cell lines for use in mice include GL261 and CT-2A. These cell lines were first generated using injection of the carcinogen 3-methylcholantrene (3) resulting in tumors that harbor key morphological characteristics of GBM. Commonly used lines for use in rats include 9L/Lac-Z, F98, RG2, and C6. Tumors grown from the C6 cell line are diffusively invasive and those derived from 9L/LacZ are aggressive, infiltrative and angiogenic (22), typical of that seen in human GBM.
Figure 2: Alternative models of glioblastoma.
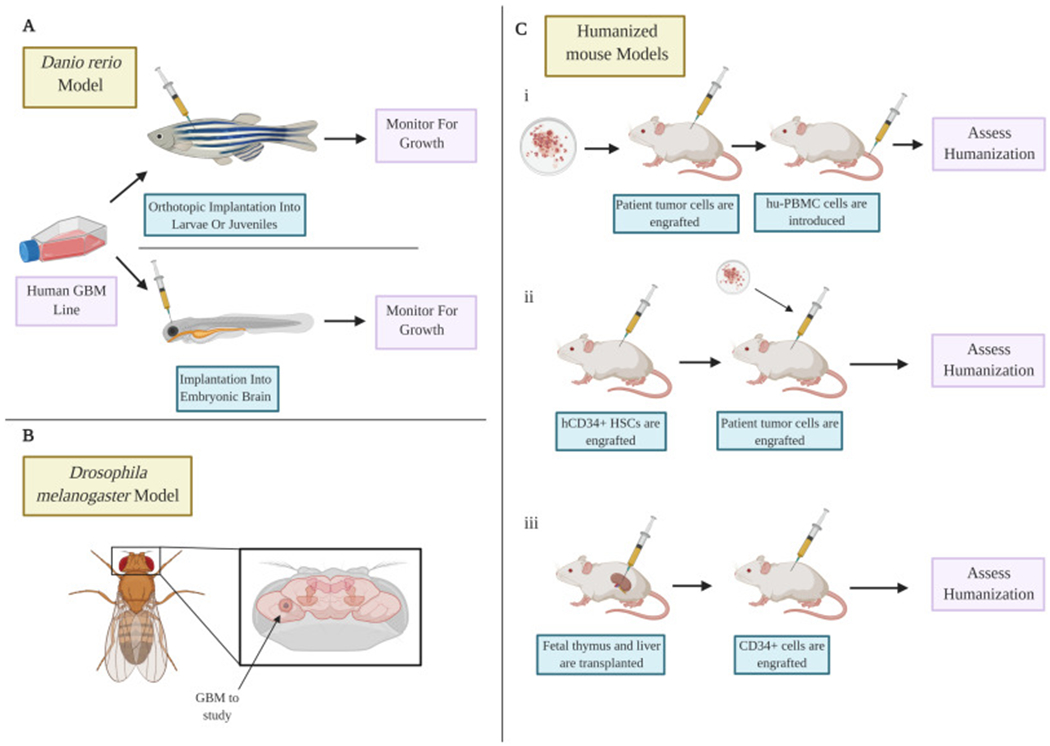
A) The zebrafish model using both embryos and adult species allow modeling of the disease to be performed quickly and can allow tumors to be imaged in real time. B) The drosophila model allows the study of glioblastoma using various genetic manipulations. C) Humanized mouse models allow modeling of tumors with partially humanized immune systems using three methods. (i) hu-PBMC cells are introduced after patient tumor cell engraftment. (ii) hCD34+ stem cells are engrafted before the patient tumor cells are added. (iii) Humanized mouse models can be created through the transplantation of fetal liver and thymus under the kidney capsule. CD34+ cells are engrafted afterwards.
An advantage of syngeneic immunocompetent animals is that all arms of the immune system are present and therefore able to interact with the developing tumor. Lack of rejection of the cell lines by the hosts immune system is of particular benefit in immunotherapy studies (23). In a recent study, we generated distinct intact and resected syngeneic mouse GBM-tumor models and utilized RNA-sequencing and time of flight cytometry (CyTOF) to identify immunologically-inert and -active GBM types. Given the efficacy of immunotherapy in highly malignant brain-tumors, glioblastomas (GBM) is currently limited, the findings of our study will significantly help in making informed choices of GBM models for immunotherapeutic interventions and therefore offer a potential to facilitate immune-therapies in GBM patients (24)
However, limitations of these models include genetic drift due to the extensive number of passages the lines undergo prior to use, thereby limiting our understanding of their genetic profile and phenotype (25). Engrafted syngeneic rodent models also lack the stepwise genetic changes seen in tumor progression. Furthermore, the tumors grow as circumscribed tumors without infiltrating the parenchyma and therefore do not fully recapitulate the original tumor phenotype (26).
3. Genetically engineered mouse models (GEM)
GEMs are an important method for delineating underlying genetic alterations responsible for tumor progression. The emergence of these models has led to a better understanding of the effect of particular genes and their mutated counterparts on tumorigenesis. GEMs involve the delivery of cancer initiating genes using viral vectors to initiate tumor formation. GEMs are advantageous in allowing perturbations of key signaling pathways such as EGFR and PDGFR (27–29) (Table 1). Gene expression can also be controlled using various strategies such as tet-regulation to control the expression or inactivation of genes (30). There are a number of limitations of using GEMs for inducing glioma formation. First, GEMs can be time-consuming to develop and may not lead to sufficient tumor formation (20). Secondly GEMs lead to formation of tumors with homogenous genetic changes whereas human GBM cells are heterogeneous. Furthermore, the genetic background of rodent strains can affect the tumor biology, gene function and tumor susceptibility (31). Mice heterozygous for particular mutations develop tumors of higher grade in certain strains such as C57Bl/6J mice compared to 129S4/SvJae mice. Biological differences between mice and human cells may significantly affect tumor development. For example, longer telomeres in mice compared to humans may be responsible for the variation in tumor formation spectrums (32).
Table 1:
Genetically engineered mouse models of human gliomas
| Tumor type | Transgene | Knock out/Knock in | Incidences | Grade |
|---|---|---|---|---|
| High-grade astrocytoma | Src transgene | 10–20% at later | III | |
| HRAS V12 and AKT | 40% by 16–20 weeks | III–IV | ||
| GFAP-Cre | NF1 + Trp53 cis | 30–75% by 15–55 weeks | II–IV | |
| GFAP-T121 transgene | PTEN+/− | 100% by 4–32 weeks | II-III | |
| GFAP-HRAS V12 | Floxed NF1 + Trp53 knockout | 100% by 2–16 weeks | III–IV | |
| GFAP-Cre | Floxed NF1 + Trp53 knockout | 30–75% by 15–55 weeks | II–IV | |
| Glioblastoma | ||||
| Kras and AKT (RCAS virus) | Cdkn2a knockout | 42–49% by 12 weeks | IV | |
| EGFRvIII(Ad-Cre virus) | Cdkn2a, PTEN F/F | 100% by 5–13 weeks | IV | |
| NES-CreER | Floxed NF1, Floxed PTEN, Floxed Trp53 | 100% by 24–56 weeks | III–IV | |
| PDGFB (RCAS virus) | Cdkn2a knockout, Trp53 knockout | 100% by 4–7 weeks | IV | |
| EGFRvIII(Ad-Cre virus) | PTEN F/F | 93% by 6–15 weeks | II–IV | |
| HRAS V12 and AKT | Trp53 knockout | 100% by 10–13 weeks | IV |
GEMs using RCAS-tVA:
The replication competent avian-like sarcoma (RCAS) virus and its avian tumor virus A (tVA) cell surface receptor is a popular GEM that has been developed to induce glioma formation. Using this system, desired oncogenes can be somatically transferred into target cells that have been engineered to express the tVA receptor under the control of a cell-type specific promoter such as Nestin. The resultant tumors develop into distinct tumor subtypes according to the oncogene of interest (33). The effects of the specific oncogenes in different intracranial locations such as the subventricular zone (svz), cortex and cerebellum (14, 34) can then be assessed. This is important due to the key differences in mice and human anatomy. In adult mouse brain, the svz contains neural stem cells tightly associated with ependymal cells, which generate neuroblasts that migrate to the olfactory bulb (35). These stem cells are morphologically different in the svz of the lateral ventricle compared to the svz of the third ventricle (36). The human svz, in contrast, possesses astrocytes with stem cell properties and limited evidence of neuroblast migration (33). The svz in children is also different to that of adults. Human third ventricle svz in children contains cells positive for nestin, glial fibrillary acidic protein (GFAP), brain fatty acid binding protein, and SRY-Box Transcription Factor 2 (SOX2). In young mice, third ventricle svz contains Sox2-positive cells but lacks GFAP and nestin positive cells (37).
The RVAS-tVA model can also be used to generate gliomas in rats. Transgenic tVA rats co-infected with Pdgf-a and Tp53 Short hairpin RNA (shRNA) RCAS viruses lead to the formation of tumors with evidence of pseudopalisading necrosis, microvascular proliferation and invasion into healthy tissue (38). In one unique study, gene expression of tVA rat gliomas was compared to human tumors, mice and canine tumors. Tumors in rats had reduced expression of glioma-related genes Insulin-like growth factor binding protein 2 (Igfbp2) and bone morphogenetic protein 7 (Bmp7) compared to the other tumors. These tumors had a small percentage of unique differentially expressed genes (DEGs) and the largest overlap with common DEGs, suggesting that rat gliomas closely resemble human gliomas (39)
The RCAS-tVA model of glioma has several advantages compared to transgenic and knockout models. Firstly, it is a cost effective method of assessing multiple genes and their effect on tumor growth using a single tVA mouse strain. Secondly, RCAS viruses do not replicate in mammalian cells allowing the preservation of signaling between tumor cells and neighboring healthy cells, which is often lost in other model systems that affect whole tissues and are less specific. This system also allows both spatial and temporal regulation of gene expression (40, 41). However, shortcomings of the RCAS-tVA system include the limited vector insert capacity of RCAS, which excludes the study of important oncogenes such as complementary DNA (cDNA) for EGFR. This system is also limited in the number of cells that become infected and therefore weaker oncogenes may not result in tumor formation in vivo (41)
GEM using Cre-LoxP:
Another powerful gene editing technology is the Cre-LoxP system that can be used to induce site-specific recombination using the Cre recombinase enzyme between two loxP recognition sites. It has been used to create transgenic strains of mice capable of expressing WT and/or vIII human EGFR by inserting minigenes consisting of a floxed transcriptional/translational stop cassette inserted between a ubiquitous promoter and the EGFR cDNAs (WT or vIII). Spatiotemporal control over EGFR expression can take place by injecting Cre recombinase to remove the floxed stop cassette. The EgfrvIII/EgfrWT expressing mice develop GBMs with high penetrance in 6 to 8 weeks. Somatic expression of mutant EgfrvIII in the CNS leads to the formation of aggressive tumors with migration of GBM cells along distinct structures such as the white matter tracts, blood vessel basement membrane and subdural sheets (42). However, this system is limited due to it being time-consuming and expensive (43).
GEM using Sleeping Beauty transposon:
Sleeping Beauty (SB) is a system that can be used to identify genetic drivers of cancers in rodent models. It is a transposon/transposase system that can be used to overexpress or inactivate genes of interest. Mice that carry different transposon and transposase transgene combinations can lead to the development of infiltrating gliomas and this system can be used to help identify genes that play an important role in gliomagenesis (44, 45).
The use of GEMs in immunotherapy: Immunological studies are predominantly performed in syngeneic models; however, GEMs are an alternative strategy that can be considered. GEM-derived tumor cells retain their immunogenicity as evidenced by their failure to grow when transplanted in WT mice but ability to grow in immunodeficient mice. GEM-derived tumors are limited partly due to our limited knowledge of the expressed tumor antigens that may be recognized by T cells. Introduction of tumor antigens to allow tracking of tumor-specific T responses may be able to overcome this limitation. In low immunogenic tumors, this method has demonstrated increased tumor immunogenicity with a potent anti-tumor T-cell response (46, 47) followed by a regulatory T-cell-mediated immunosuppression (48). The use of GEMs for GBM research however has been hampered due to the concerns regarding reproducibility, latency of tumor formation and lack of a consensus regarding tumor immunogenicity.
4. Traditional xenograft mouse models
Xenograft models involve transplanting human cancer cells into an immunocompromised rodent. Biopsies from GBM patients can be processed in tissue culture flasks and passaged to yield monolayer cell lines in serum-containing medium. Intracerebral implantation of these cell lines in immunodeficient animals leads to the formation of tumors with typical characteristics of GBM (49). Several established GBM cell lines have been widely used and cited in the literature, including U87, U251 and T98G, which have all provided useful information on the nature of GBM tumors. The two most widely studied are the U87 and U251 cell lines, which were generated from GBM patients and subsequently cultured in vitro and xenografted into immunodeficient nonobese diabetic/severe combined Immunodeficiency (NOD/SCID) mice, or NOD/SCID IL-2Rγ-null (NSG) mice (50, 51). These cell lines retain genetic mutations and can be used to study various signaling pathways. They represent a rapid and reproducible method of investigating GBM, manifest reliable disease progression and can be expanded to provide a large yield of tumor cells (3). Cell line derived xenografts display angiogenesis and evidence of tissue invasion to a limited extent, however they do not possess single cell infiltration in the brain and do not fully recapitulate the heterogeneity and phenotypes of human GBM. Additionally, established cell lines passaged in monolayers often possess abnormal expression of collagens and integrins and an up-regulation of immunological markers such as major histocompatibility complex (MHC) and cytokines (52). Profiles from array-comparative genomic hybridization (aCGH) of GBM cell lines are significantly different from those typically found in primary GBM (53). Genomic alterations in adherent serum-growing cultures often do not correspond well to the genotype of the original tumors (54). Whole-genome sequencing has revealed several copy number variations and translocations, possibly acquired during extensive cell passaging with fetal bovine serum, altering genomes, transcriptomes and genetic stability (49). The traditional human GBM cell lines are therefore imperfect models for GBM and if used must be fully authenticated.
5. Patient derived xenograft (PDX) and xenografts generated from patient-derived cancer stem cells
Patient-derived xenografts (PDX) involve direct implantation of freshly biopsied tumor tissue or cultured tumor spheres into immunodeficient animals (49). Transplanting biopsied specimens into flank subcutaneous space has been widely used due to practicality reasons (e.g., technical feasibility and easy visual follow-up of tumor formation). Flank tumors grown in immunodeficient mice are useful for maintaining genetic driver alterations in patients and testing direct drug activity on the tumors. A large collection of extensively characterized GBM PDXs has been established and these are available to the wide research community (55). However, one major disadvantage of subcutaneous models is that the tumor microenvironment does not reproduce the environment in which the tumor grows (29). Intracranial tumors can be established using a heterotopic-to-orthotopic approach. In the orthotopic model, biopsy tissue is grown on agar coated flasks supplemented with medium to form spheroids. The spheroids possess a similar architecture to the original tumor tissue and the molecular profile is stable over time. The spheroids can be implanted into the brains of immunodeficient mice using stereotactic devices (Figure 1) or by a freehand procedure. Direct transplantation of acutely dissociated cells from GBM patients is an alternative approach to generating orthotopic GBM PDX as this has been shown to engraft at a high take rate (75.7%), recapitulating histopathological properties and maintaining the genomic characteristics of parental tumors (56). PDX cells benefit from not being subjected to stresses that can arise in cell cultures as they are propagated in successive generations of mice (57) however the success rate and length of engraftment is affected by tumor origin and aggressiveness (58).
Figure 1: In vivo models of glioblastoma in rodents.
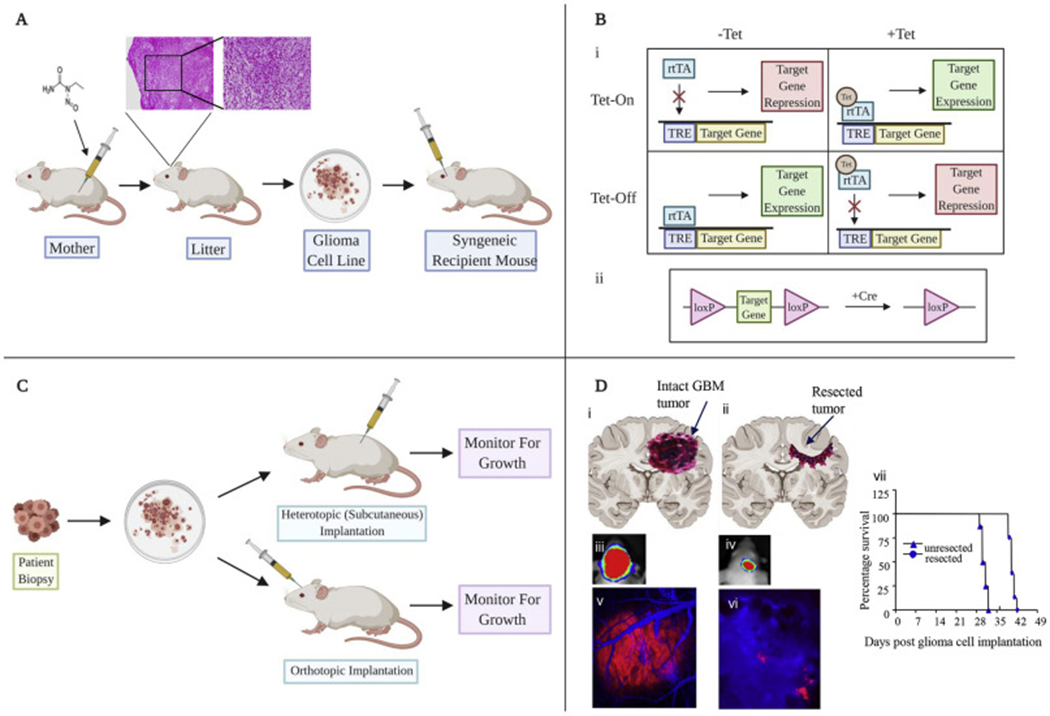
A) Traditional methods include ENU administration in pregnant rodents leading to tumor formation, which can be harvested and processed into cell lines in vitro. B) Genetically engineered systems include reversible systems using Tet regulation (i) or Cre recombinase (ii). C) Patient derived xenografts can be injected subcutaneously or directly into cerebral cortex. D) Resection models designed to recapitulate the tumor environment following primary resection of tumors. (i,ii) Cartoons showing GBM tumors before and after tumor resection in the brain mice. (iii-vii). Mice with established GBM-Fluc-mCherry GBMs were imaged by bioluminescence imaging (iii,iv) and intravital microscopy (v,vi) before and after tumor resection. Kaplan-Meier survival curves of mice with and without resected U87-Fluc-mCherry tumors (vii) (adapted from Kauer et al 2012) (82).
Glioma stem cell-based xenografts
GBM growth is driven by a sub-population of cancer stem cells (GSCs) that is capable of contributing to tumor initiation and therapeutic resistance (59). These cells have the ability to self-renew in culture and form neurospheres in the presence of appropriate growth factors. Patient biopsies are dissociated enzymatically and propagated in neurobasal serum free growth media, with the addition of supplements and growth factors (60). GSC cultures obtained from patients contain stem-like cells and express astroglial and neuronal markers in culture and in vivo (61, 62). The tumor stem cells form neurospheres(63) that are capable of proliferation, differentiation and self-renewal (63, 64). The tumors formed are similar in phenotype and genotype to human tumors (61, 65, 66). This suggests that GSC are useful for authentically replicating human GBM tumors in mice. However, there is a discrepancy in success rates of tumor formation. This could be due to lack of uniformity in culture methods (67). There is also controversy regarding identification of GSCs and the reliability of the CD133 antigen, commonly used as a GSC marker (68). It has been widely perceived that brain tumors do not arise from cancer stem cells that are CD133 negative (61, 69). However, studies have shown that human gliomas do not express CD133 consistently or abundantly and some cells may have no detectable CD133+ cells (70) yet have similar properties to CD133+ cells (71, 72). Tumors may also initially express little CD133+, but with serial passaging in vivo, there may be upregulation of CD133 expression associated with the onset of angiogenesis, suggesting that CD133 expression is not required for tumor initiation but may be important for tumor progression (70). Cells are often a mix of both CD133-positive and negative cells (56) and the cell ratios may determine the type of tumors formed. Tumors with low CD133− cell ratios are typically characteristic of the mesenchymal type whereas those with high CD133− cell ratios lead to tumors typical of the proneural subtype (56).
IDH1/2 mutant glioma models
IDH1/2 mutant glioma is a major and distinct subset of human glioma with longer survival times, higher concentration of 2-hydroxyglutarate (2-HG), increased cytosine methylation and reduced immune infiltrates compared to the WT counterpart (73). Over 90% of these tumors have the IDH1R13H variant, which plays a major role in driving glioma formation. Knock-in of Idh1R132H in the mouse brain SVZ leads to proliferation of neural stem/progenitor cells and the formation of nodules (74). Idh1R132H in co-operation with Pdgfra, and loss of Cdkn2a, Atrx, and Pten can promote glioma development, resembling proneural human IDH1 mutant GBM (75). Growing IDH mutant PDX is very difficult both in culture and in vivo and the lack of cell lines with endogenous mutations pose difficulties. A limited number of studies have described the isolation and expansion of glioma brain tumor stem cell lines such as BT142, that retains the endogenous IDH1R13H mutation in culture and can be propagated in NOD SCID mice (76). Generation of orthotopic IDH1-mutant glioma xenografts by direct implantation of biopsy specimens or briefly cultured cells requires the presence of tertiary genetic alterations such as amplification of Pdgfra in astrocytic gliomas and activating mutations in the PI3K-mTOR signaling pathway in oligodendroglial tumors (77, 78). Mass spectroscopy analysis of patient-derived IDH-mutant vs wild-type glioma xenografts identified differences in phospholipid and glucose metabolism (79).
6. Models recapitulating clinical GBM resection
Although the clinical standard of care for patients with GBM includes surgical debulking (80), most pre-clinical GBM models focus on treating solid intact intracranial tumors. Typically, standard orthotopic xenografts involve implanting tumor cells 2-3mm lateral to the bregma and at a depth of 2-3mm (Figure 1). Several resection models have been created in rodents to mimic tumor debulking in patients. The first intracranial resection model was created in a rats where a fluorescent dissecting microscope was used to guide microsurgical resection and aspiration of the tumor (81) however this did not lead to a survival benefit compared to controls. We integrated fluorescent and bioluminescent markers and optical imaging to simultaneously confirm the presence of established tumors, visualize the extent of tumor resection and serially monitor tumor regrowth after resection. The inclusion of real-time fluorescence microscopy permitted visualization of residual tumor cells and associated blood vessels in resected tumor. Post-resection bioluminescence imaging permitted gross assessment of the extent of tumor removal. This model allows exploration of anti-GBM therapeutics using mouse models with greater clinical relevance than models focused on treating unperturbed tumor mass (82). The major advantage of the resection model is its ability to reflect the debulking of tumors which takes place clinically. However, the model is limited by the time needed to perform the experiments due to the additional need for craniectomy.
Damage of healthy, non-tumorous tissue results in an anti-inflammatory response characterized by T cell infiltration into the lesion (83). Based on the hypothesis that a first-line treatment of maximal surgical tumor resection would invoke an acute immune reaction, possibly enough to break the immune tolerance within the tumor microenvironment, we developed syngeneic mouse tumor models of GBM resection and characterized the immune response of intact and resected tumors. Our results indicate that tumor resection decreases the number of tumor-associated myeloid-derived suppressor cells (MDSC) and simultaneously increases the number of effector T lymphocytes recruited into the remaining tumor area (84). Modulating the nonspecific immune reaction after tumor debulking toward a tumor-specific immune response may be an alternative immunotherapy strategy in GBM treatment.
7. Emerging preclinical models
Danio Rerio model (Zebrafish):
The danio rerio model is a new tool available for deciphering the pathology of brain tumors. Comparison of fish and human cancer gene signatures reveals a great resemblance of genes involved in regulating cell cycle, apoptosis and DNA repair (85). Implantation of human glioma cells in zebrafish can result in xenografts with similar morphology to those obtained from mice such as intact vessels and invasiveness (3). Zebrafish have transparent bodies and lack an adaptive immune system until 6 weeks with a dense microenvironment similar to the human brain. Therefore, early tumorigenesis can be studied effectively without interference by the adaptive immune system (Figure 2A) (86).
Human GBM cells have been successfully implanted into the embryonic brain 3 days post fertilization (dpf) (87) and in adults. Juvenile and adult zebrafish are thought to have fully functioning CNS similar to human brains and are able to accommodate more cells than the larvae model. However, at older stages, the brain is more difficult to image as optical transparency is lost. The danio rerio model has shown advantages including its relative low cost, easy visualization of internal structures, and rapid embryonic development. Limitations of the model include differences in the microenvironment between humans and fish and in the optimal temperature for human (37°C) and fish cells (28°C) (86). Recent advances include the development of optically clear adult zebrafish, which can engraft human tumors at 37°C (88).
Drosophila melanogaster (Fruit fly):
The Drosophila melanogaster model has emerged as an alternative to rodent models of glioma (Figure 2). Approximately 75% of human genes share functional orthologs in Drosophila (89). The fruit fly has many salient features which make it attractive for the study of various diseases. The fruit fly brain is capable of numerous complex tasks including regulation of circadian rhythms, memory and sleep. The CNS elicits neurological responses to drugs that resemble mammalian systems (90). The Drosophila model has been proven to be a powerful system to study tumor initiation and identify numerous signaling pathways affected in cancers and has recently been used as a model for investigating brain tumors. Glioma can be induced using the GAL4/upstream activating sequence (Gal4-UAS) system, by overexpressing homologs of human tyrosine kinase receptors under the control of the glia-specific promoter reverse polarity (repo). Glial overexpression of Egfr, Pi3k, activated Pdgfr/vascular endothelial growth factor receptor (Vegf) homolog, activated fibroblast growth factor receptor 1 homolog or insulin receptor leads to proliferation, migration and invasion of glial cells (91). Constitutive coactivation of Egfr-Ras proteins and PI3K pathways initiate inappropriate cellular growth with the fly orthologs CyclinE, Cdc25, and Myc being rate-limiting genes required for glial neoplasia (92). The formation of these tumors requires the activation of known downstream pathways such as Akt signaling (92) which may activate and overexpress right open reading frame kinases (RIOK) leading to transformation of GBM cells (93). The drosophila model can also be used to gain insight into mechanisms underlying disrupted asymmetric cellular division in cancer stem cells, such as centrosome dysfunction leading to tumors by perturbing stem cell division (94). Drosophila larval neuroblasts generate differentiating cells by segregating the growth inhibitor Brat and the transcription factor Prospero into one daughter cell. Inhibiting Brat or Prospero leads to neoplastic proliferation of neuroblasts (95), which is associated with the upregulation of Notch signaling. In human GBM, tripartite motif-containing protein 3 (TRIM3), the human ortholog of Drosophila Brat, suppresses NOTCH1 signaling and markedly attenuates the glioma stem cell component (96).
The Drosophila model has several advantages for studying brain tumors. These include a short life span, easy handling, rapid generation of offspring in large numbers, and availability of many tissue specific promoters. The resultant tumors also invade into nearby structures and can be easily quantified (92). The model is a versatile genetic model system and therefore allows several genetic aberrations to be tested. However, notable differences between the Drosophila model and humans such as anatomical variation and differences in the immune system limit its applicability and use (97).
Organoid models of GBM:
Three-dimensional (3D) culture of organoids has been emerging as an ex vivo experimental system for glioma research. Organoid models of GBM allow investigations of the biology of GBM in the context of the tumor environment, as cell-cell and cell-extracellular matrix interactions present in 3D organoids are considered to model tumors in vivo. Organoid models of GBM can be classified into two types. The first model type involves 3D culture of GBM cells directly derived from biopsies to generate organoids (98, 99). GBM organoids grown in defined serum and matrigel-free conditions recapitulate inter and intra-tumoral heterogeneity of primary tumors and can be used for xenografting, and in vitro testing of drugs and tumor response to Chimeric antigen receptor (CAR)-T cells (99). The second model is based on the induction of GBM oncogenesis in embryonic stem cell (ESC) or induced pluripotent stem cell (iPSCs)-derived cerebral organoids (100, 101). Oncogene transduction and clustered regularly interspaced short palindromic repeats (CRISPR) editing of tumor suppressor genes in human cerebral organoids results in the formation of invasive GBM (100, 101). Cerebral organoids can be also used to transplant patient-derived GBM stem cells to initiate the growth of invasive GBM within the 3D brain environment (101, 102).
Organotypic brain slice cultures:
Organotypic brain slice cultures are useful in investigating cellular and molecular processes of the brain in vitro as they maintain the normal architecture (103). Using this model, glioma cells have been shown to infiltrate the brain directed by interactions with the host vasculature (104). Slice cultures can be incubated with fluorescent antibodies to allow real time imaging of tumor cell invasion (103) and measure movement in the microenvironment (105).
Humanized mice tumor models:
Humanized mice models are a robust platform in which a functional human immune system is engrafted into immunodeficient mice (106) These mice display a chimeric immune system, with only a proportion of the total immune cells in the peripheral blood being of human origin. Engraftment, development and functionality of the human immune system in the host depend partially on the immunodeficient mouse strain used for the development of the model, as well as on the method and protocol chosen to generate the humanized mice. There are three main ways of developing mice with a functional human system (107, 108). Human peripheral blood mononuclear cell-engrafted NSG™ mice (hu-PBMC)-exhibit high functional T-cells reconstitution but will inevitably develop Graft versus host disease (GvHD) between 4-6 weeks post-engraftment (109). In human CD34+ hematopoietic stem cell-engrafted NSG™ mice (hCD34+ HSC), the immune reconstitution includes all human hematopoietic lineages, as in the hu-PBMC model, however some immune cell types are not fully functional due to the lack of human cytokines and growth factors in the murine environment (107, 108). Moreover, T-cells are murine MHC restricted, and cannot recognize antigens presented on human leukocyte antigen (HLA) efficiently (110). Finally, Bone Marrow Liver Thymic (BLT) mice are developed by co-transplantation of fetal thymus and fetal liver under the kidney capsule, coupled with engraftment of CD34+ cells derived from the same fetal liver (111) This model has the most functional immune system out of the three however, these mice eventually develop GvHD (onset >20 weeks post-engraftment) (112). In recent years, an increasing number of transgenic mice have been designed to express human cytokines, with the goal of supporting a better immune reconstitution, delaying the onset of GvHD and T-cell recognition of human (107, 113).
Humanized mice have been employed in cancer research, using cell lines or PDX to establish tumor formation with infiltration of the human immune cells in the tumor microenvironment (107, 114, 115). To generate humanized mice bearing solid PDX, the graft is generally implanted subcutaneously into a previously humanized mouse (116, 117). Alternatively, tumor cell lines can be injected directly in the desired region or systemically for a model of metastases (116, 118). Humanized mice have been employed to study the effects of different immunotherapeutic approaches for human GBM. Patient-derived PBMCs have been used to successfully develop humanized mice which mimic the patient T-cell immune response (119, 120). In both cases, MHC-gene double knockout mice (deficient in both murine MHC class I and II) is used to delay the onset of GvHD in subcutaneous flank human GBM models. Orthotopic brain tumor models have also been established in BLT mice by intracranial engraftment of different GBM cells or patient-derived GBM xenografts (121). In this case, humanized mice are first established followed by injection of GBM cells once there is reconstitution of the human immune system (122).
Canine brain tumor models:
Intracranial tumors that spontaneously arise in dogs are drawing attention as a large animal disease model in neuro-oncology (123) The canine gene families associated with cancer are closer to humans, than the relationship between a mouse and a human and gliomas in dogs share similar morphological (124) and immunological characteristics with human gliomas (124).
A recent comprehensive characterization of the molecular landscape of canine gliomas revealed somatic alterations that converge with human glioma drivers such as the receptor tyrosine kinases, Tp53 and cell-cycle pathways, and IDH1R132 (125). The size of the dog brain also allows opportunities to test a variety of drug delivery approaches, such as convection-enhanced delivery, which are difficult in rodents (126)
8. Conclusions and perspectives
GBM remains the focus of interest for many researchers due to its poor outcomes and lack of curative therapy. The vast array of GBM models include autochthonous models such as syngeneic implantation of cell lines, xenograft models (subcutaneous, orthotopic) and resection models, and now cover small to large model organisms (Table 2). GBM cell-line xenografts generally have the advantages of high engraftment and growth rates. However, they do not possess the stepwise genetic alterations that occur during human gliomagenesis (29). Patient-derived xenografts may retain the genetic and histological features of the primary tumor but cannot adequately reflect the host’s antitumor immunity seen in human GBM. GEMs allow us to pinpoint genetic alterations involved in tumor initiation and progression, however tumors are usually composed of cells with homogeneous genetic changes, and therefore GEMs cannot completely reflect the intra-tumoral genomic and phenotypic heterogeneity of GBM (29).
Table 2:
Summary of the characteristics of a variety of brain tumor models
| Recapitulation of patient tumor genetics | Heterogeneity | Tumor microenvironment (TME) | Immunotherapy research | Technical difficulty | Costs | Note | |
|---|---|---|---|---|---|---|---|
| Syngeneic implantation model | Poor | No | Yes | Yes | Low | Low | Robust in vivo model |
| Genetically engineered mouse model (GEMM) | Yes, specific gene alterations | No | Yes | Yes | Moderate | Relatively high for generation | In vivo functional genomics |
| Traditional xenografts | Limited | No | Yes, but limited due to human-mouse interaction | No | Low | Moderate | Robust in vivo model |
| PDX or stem cell based xenografts | Yes | Yes | Yes, but limited due to human-mouse interaction | No | Moderate | Moderate | Non-immunotherapy targeted approaches |
| Mouse resection models | Varies | Varies | Resection induces changes in TME | Yes, if syngeneic model used | Moderate-high | Moderate | Mimicking clinical surgery |
| Danio Rerio model (Zebrafish) | Yes, if patient-derived cells used | Yes, if patient-derived cells used | TME in zebrafish models not well understood. | No | Low | Low | Robust screening possible |
| Drosophila melanogaster (Fruit fly) | Yes, specific gene alterations can be made | No | Yes, but relevance to human biology unclear | Possible | Low | Low | Robust screening possible |
| Organoid models of GBM | Yes | Yes | Yes | No (except short-term T cell testing) | Moderate | Relatively low | Relatively short-term studies |
| Organotypic brain slice cultures | Yes | Yes | Yes | Unknown | Low | low | Relatively short-term studies |
| Humanized mice tumor model | Yes, if PDX or glioma stem cells used | Yes, if patient-derived cells used | Yes, but partially human cell-mouse brain interaction | Yes | Moderate (humanized mice commercially available) | High | Expensive |
| Canine brain tumor model | Yes | Yes | Yes | Yes | Low | High, Veterinary skill and facility needed | Large animal model |
Advantages of rodent models include their availability and familiarity of use amongst researchers, the presence of a blood brain barrier (BBB) and ability to test therapeutic agents. However, rodents have their congenital shortcomings. Only 85% of human genes have the homologous orthologues in mouse, and 20% of orthologues have significantly different functions (127). Furthermore, human cancer cells transplanted in rodent xenograft models locate in a different microenvironment from human GBM and the use of immunocompromised mice also reduces normal immune responses involved in tumor formation in patients (128). Other problems of rodent models include cost of breeding and maintenance, time associated with acquiring skills and ethical considerations (129). Zebrafish (Danio rerio) is a promising xenograft tumor model system for studies of tumor invasion. However, as it is a newer model, its translational value toward clinical trials is currently unclear. Parallel studies with mice models may be used to validate data and if successful eventually reduce the need to use mouse models (130).
Despite extensive search for therapies for GBM, none has been developed that culminates into true benefits as a sole agent. The immune system has garnered the most attention and several therapeutic agents modulating the immune system have been developed, which have since been tested in clinical trials. However, despite many pre-clinical studies being conducted in GBM, a small proportion proceed to clinical trials or have meaningful benefits in patients. Nevertheless, clinical trials are still guided by preclinical studies, and to select and use models that are most appropriate to individual research needs will continue to be important (131). Limitations of available technologies and intrinsic differences between species make it a challenge to create a model representing human GBM morphologically, phenotypically and genetically, and balance clinical representation and costs and feasibility. Further research into the effect of age, sex and species or strain on the outcome of GBM is necessary. Given an expanding array of available models, coordinated efforts by clinicians and researchers in the field may be warranted to generate guidelines of appropriate use of pre-clinical brain tumor models.
Acknowledgments
This work was supported by NIH grants R01-CA201148 (K.S.) and by DoD grant LC180495. We would like to thank Maria Sanchez for helping with the literature review.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interests
K.S. owns equity in and is a member of the Board of Directors of AMASA Therapeutics, a company developing stem cell-based therapies for cancer. K.S.’s interests were reviewed and are managed by Brigham and Women’s Hospital and Partners Healthcare in accordance with their conflict of interest policies. The other authors declare that they have no competing interests.
References
- 1.Perkins A & Liu G (2016) Primary Brain Tumors in Adults: Diagnosis and Treatment. Am Fam Physician 93(3):211–217. [PubMed] [Google Scholar]
- 2.Ostrom QT, et al. (2019) CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2012-2016. Neuro Oncol 21(Suppl 5):v1–v100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lenting K, Verhaak R, Ter Laan M, Wesseling P, & Leenders W (2017) Glioma: experimental models and reality. Acta neuropathologica 133(2):263–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thakkar JP, et al. (2014) Epidemiologic and molecular prognostic review of glioblastoma. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 23(10):1985–1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arvold ND & Reardon DA (2014) Treatment options and outcomes for glioblastoma in the elderly patient. Clin Interv Aging 9:357–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen AL, Holmen SL, & Colman H (2013) IDH1 and IDH2 mutations in gliomas. Current neurology and neuroscience reports 13(5):345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yan H, et al. (2009) IDH1 and IDH2 mutations in gliomas. N Engl J Med 360(8):765–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Appin CL, et al. (2013) Glioblastoma with oligodendroglioma component (GBM-O): molecular genetic and clinical characteristics. Brain Pathol 23(4):454–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giese A & Westphal M (1996) Glioma invasion in the central nervous system. Neurosurgery 39(2):235–250; discussion 250-232. [DOI] [PubMed] [Google Scholar]
- 10.Wang SD, et al. (2012) EphB2 receptor controls proliferation/migration dichotomy of glioblastoma by interacting with focal adhesion kinase. Oncogene 31(50):5132–5143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verhaak RG, et al. (2010) Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 17(1):98–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Q, et al. (2017) Tumor Evolution of Glioma-Intrinsic Gene Expression Subtypes Associates with Immunological Changes in the Microenvironment. Cancer Cell 32(1):42–56 e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Georgescu MM (2010) PTEN Tumor Suppressor Network in PI3K-Akt Pathway Control. Genes Cancer 1(12):1170–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Westermark B (2014) Platelet-derived growth factor in glioblastoma-driver or biomarker? Ups J Med Sci 119(4):298–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Waitkus MS, Diplas BH, & Yan H (2016) Isocitrate dehydrogenase mutations in gliomas. Neuro Oncol 18(1):16–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brennan CW, et al. (2013) The somatic genomic landscape of glioblastoma. Cell 155(2):462–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ham SW, et al. (2019) TP53 gain-of-function mutation promotes inflammation in glioblastoma. Cell death and differentiation 26(3):409–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soubannier V & Stifani S (2017) NF-kappaB Signalling in Glioblastoma. Biomedicines 5(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Behnan J, Finocchiaro G, & Hanna G (2019) The landscape of the mesenchymal signature in brain tumours. Brain 142(4):847–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Day CP, Merlino G, & Van Dyke T (2015) Preclinical mouse cancer models: a maze of opportunities and challenges. Cell 163(1):39–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Q, et al. (2016) Braf Mutations Initiate the Development of Rat Gliomas Induced by Postnatal Exposure to N-Ethyl-N-Nitrosourea. The American journal of pathology 186(10):2569–2576. [DOI] [PubMed] [Google Scholar]
- 22.Plate KH, Breier G, Millauer B, Ullrich A, & Risau W (1993) Up-regulation of vascular endothelial growth factor and its cognate receptors in a rat glioma model of tumor angiogenesis. Cancer Res 53(23):5822–5827. [PubMed] [Google Scholar]
- 23.Oh T, et al. (2014) Immunocompetent murine models for the study of glioblastoma immunotherapy. J Transl Med 12:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khalsa JK, et al. (2020) Immune phenotyping of diverse syngeneic murine brain tumors identifies immunologically distinct types. Nature communications 11(1):3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sibenaller ZA, et al. (2005) Genetic characterization of commonly used glioma cell lines in the rat animal model system. Neurosurgical focus 19(4):E1. [DOI] [PubMed] [Google Scholar]
- 26.Yeo AT & Charest A (2017) Immune Checkpoint Blockade Biology in Mouse Models of Glioblastoma. Journal of cellular biochemistry 118(9):2516–2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hara T & Verma IM (2019) Modeling Gliomas Using Two Recombinases. Cancer Res 79(15):3983–3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huse JT & Holland EC (Targeting brain cancer: advances in the molecular pathology of malignant glioma and medulloblastoma. Nat Rev Cancer 10(5):319–331. [DOI] [PubMed] [Google Scholar]
- 29.Huszthy PC, et al. (2012) In vivo models of primary brain tumors: pitfalls and perspectives. Neuro Oncol 14(8):979–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baron U & Bujard H (2000) Tet repressor-based system for regulated gene expression in eukaryotic cells: principles and advances. Methods in enzymology 327:401–421. [DOI] [PubMed] [Google Scholar]
- 31.Reilly KM (2009) Brain tumor susceptibility: the role of genetic factors and uses of mouse models to unravel risk. Brain Pathol 19(1):121–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bernal A & Tusell L (2018) Telomeres: Implications for Cancer Development. Int J Mol Sci 19(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ahronian LG & Lewis BC (2014) Using the RCAS-TVA system to model human cancer in mice. Cold Spring Harb Protoc 2014(11):1128–1135. [DOI] [PubMed] [Google Scholar]
- 34.Dai C, et al. (2001) PDGF autocrine stimulation dedifferentiates cultured astrocytes and induces oligodendrogliomas and oligoastrocytomas from neural progenitors and astrocytes in vivo. Genes Dev 15(15):1913–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Semple BD, Blomgren K, Gimlin K, Ferriero DM, & Noble-Haeusslein LJ (2013) Brain development in rodents and humans: Identifying benchmarks of maturation and vulnerability to injury across species. Prog Neurobiol 106-107:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee DY, Gianino SM, & Gutmann DH (2012) Innate neural stem cell heterogeneity determines the patterning of glioma formation in children. Cancer Cell 22(1):131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dahiya S, Lee DY, & Gutmann DH (2011) Comparative characterization of the human and mouse third ventricle germinal zones. J Neuropathol Exp Neurol 70(7):622–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Connolly NP, et al. (2017) Genetically engineered rat gliomas: PDGF-driven tumor initiation and progression in tv-a transgenic rats recreate key features of human brain cancer. PloS one 12(3):e0174557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Connolly NP, et al. (2018) Cross-species transcriptional analysis reveals conserved and host-specific neoplastic processes in mammalian glioma. Scientific reports 8(1):1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Federspiel MJ, Bates P, Young JA, Varmus HE, & Hughes SH (1994) A system for tissue-specific gene targeting: transgenic mice susceptible to subgroup A avian leukosis virus-based retroviral vectors. Proc Natl Acad Sci U S A 91(23):11241–11245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.von Werder A, Seidler B, Schmid RM, Schneider G, & Saur D (2012) Production of avian retroviruses and tissue-specific somatic retroviral gene transfer in vivo using the RCAS/TVA system. Nature protocols 7(6):1167–1183. [DOI] [PubMed] [Google Scholar]
- 42.Zhu H, et al. (2009) Oncogenic EGFR signaling cooperates with loss of tumor suppressor gene functions in gliomagenesis. Proc Natl Acad Sci U S A 106(8):2712–2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Frese KK & Tuveson DA (2007) Maximizing mouse cancer models. Nat Rev Cancer 7(9):645–658. [DOI] [PubMed] [Google Scholar]
- 44.Bender AM, et al. (2010) Sleeping beauty-mediated somatic mutagenesis implicates CSF1 in the formation of high-grade astrocytomas. Cancer Res 70(9):3557–3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Collier LS, et al. (2009) Whole-body sleeping beauty mutagenesis can cause penetrant leukemia/lymphoma and rare high-grade glioma without associated embryonic lethality. Cancer Res 69(21):8429–8437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.DuPage M, et al. (2011) Endogenous T cell responses to antigens expressed in lung adenocarcinomas delay malignant tumor progression. Cancer Cell 19(1):72–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.DuPage M, Mazumdar C, Schmidt LM, Cheung AF, & Jacks T (2012) Expression of tumour-specific antigens underlies cancer immunoediting. Nature 482(7385):405–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Joshi NS, et al. (2015) Regulatory T Cells in Tumor-Associated Tertiary Lymphoid Structures Suppress Anti-tumor T Cell Responses. Immunity 43(3):579–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kijima N & Kanemura Y (2017) Mouse Models of Glioblastoma Glioblastoma, ed De Vleeschouwer SBrisbane (AU)). [PubMed] [Google Scholar]
- 50.Ponten J & Macintyre EH (1968) Long term culture of normal and neoplastic human glia. Acta Pathol Microbiol Scand 74(4):465–486. [DOI] [PubMed] [Google Scholar]
- 51.Westermark B, Ponten J, & Hugosson R (1973) Determinants for the establishment of permanent tissue culture lines from human gliomas. Acta Pathol Microbiol Scand A 81(6):791–805. [DOI] [PubMed] [Google Scholar]
- 52.Anderson RC, et al. (2002) Changes in the immunologic phenotype of human malignant glioma cells after passaging in vitro. Clin Immunol 102(1):84–95. [DOI] [PubMed] [Google Scholar]
- 53.Nigro JM, et al. (2005) Integrated array-comparative genomic hybridization and expression array profiles identify clinically relevant molecular subtypes of glioblastoma. Cancer Res 65(5):1678–1686. [DOI] [PubMed] [Google Scholar]
- 54.Lee J, et al. (2006) Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell 9(5):391–403. [DOI] [PubMed] [Google Scholar]
- 55.Vaubel RA, et al. (2020) Genomic and Phenotypic Characterization of a Broad Panel of Patient-Derived Xenografts Reflects the Diversity of Glioblastoma. Clin Cancer Res 26(5):1094–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Joo KM, et al. (2013) Patient-specific orthotopic glioblastoma xenograft models recapitulate the histopathology and biology of human glioblastomas in situ. Cell reports 3(1):260–273. [DOI] [PubMed] [Google Scholar]
- 57.Daniel VC, et al. (2009) A primary xenograft model of small-cell lung cancer reveals irreversible changes in gene expression imposed by culture in vitro. Cancer Res 69(8):3364–3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cho SY (2020) Patient-derived xenografts as compatible models for precision oncology. Lab Anim Res 36:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dalerba P, Cho RW, & Clarke MF (2007) Cancer stem cells: models and concepts. Annu Rev Med 58:267–284. [DOI] [PubMed] [Google Scholar]
- 60.Zhang L, et al. (2020) The necessity for standardization of glioma stem cell culture: a systematic review. Stem cell research & therapy 11(1):84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Galli R, et al. (2004) Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res 64(19):7011–7021. [DOI] [PubMed] [Google Scholar]
- 62.Ignatova TN, et al. (2002) Human cortical glial tumors contain neural stem-like cells expressing astroglial and neuronal markers in vitro. Glia 39(3):193–206. [DOI] [PubMed] [Google Scholar]
- 63.Yuan X, et al. (2004) Isolation of cancer stem cells from adult glioblastoma multiforme. Oncogene 23(58):9392–9400. [DOI] [PubMed] [Google Scholar]
- 64.Singh SK, et al. (2003) Identification of a cancer stem cell in human brain tumors. Cancer Res 63(18):5821–5828. [PubMed] [Google Scholar]
- 65.Wakimoto H, et al. (2009) Human glioblastoma-derived cancer stem cells: establishment of invasive glioma models and treatment with oncolytic herpes simplex virus vectors. Cancer Res 69(8):3472–3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wakimoto H, et al. (2012) Maintenance of primary tumor phenotype and genotype in glioblastoma stem cells. Neuro Oncol 14(2):132–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kelly PN, Dakic A, Adams JM, Nutt SL, & Strasser A (2007) Tumor growth need not be driven by rare cancer stem cells. Science 317(5836):337. [DOI] [PubMed] [Google Scholar]
- 68.Clement V, Dutoit V, Marino D, Dietrich PY, & Radovanovic I (2009) Limits of CD133 as a marker of glioma self-renewing cells. Int J Cancer 125(1):244–248. [DOI] [PubMed] [Google Scholar]
- 69.Singh SK, et al. (2004) Identification of human brain tumour initiating cells. Nature 432(7015):396–401. [DOI] [PubMed] [Google Scholar]
- 70.Wang J, et al. (2008) CD133 negative glioma cells form tumors in nude rats and give rise to CD133 positive cells. Int J Cancer 122(4):761–768. [DOI] [PubMed] [Google Scholar]
- 71.Chen R, et al. (2010) A hierarchy of self-renewing tumor-initiating cell types in glioblastoma. Cancer Cell 17(4):362–375. [DOI] [PubMed] [Google Scholar]
- 72.Ogden AT, et al. (2008) Identification of A2B5+CD133− tumor-initiating cells in adult human gliomas. Neurosurgery 62(2):505–514; discussion 514-505. [DOI] [PubMed] [Google Scholar]
- 73.Amankulor NM, et al. (2017) Mutant IDH1 regulates the tumor-associated immune system in gliomas. Genes Dev 31(8):774–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bardella C, et al. (2016) Expression of Idh1(R132H) in the Murine Subventricular Zone Stem Cell Niche Recapitulates Features of Early Gliomagenesis. Cancer Cell 30(4):578–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Philip B, et al. (2018) Mutant IDH1 Promotes Glioma Formation In Vivo. Cell reports 23(5):1553–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Luchman HA, Chesnelong C, Cairncross JG, & Weiss S (2013) Spontaneous loss of heterozygosity leading to homozygous R132H in a patient-derived IDH1 mutant cell line. Neuro Oncol 15(8):979–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tateishi K, et al. (2019) PI3K/AKT/mTOR Pathway Alterations Promote Malignant Progression and Xenograft Formation in Oligodendroglial Tumors. Clin Cancer Res 25(14):4375–4387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wakimoto H, et al. (2014) Targetable signaling pathway mutations are associated with malignant phenotype in IDH-mutant gliomas. Clin Cancer Res 20(11):2898–2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fack F, et al. (2017) Altered metabolic landscape in IDH-mutant gliomas affects phospholipid, energy, and oxidative stress pathways. EMBO Mol Med 9(12):1681–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Minniti G, et al. (2008) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma in elderly patients. J Neurooncol 88(1):97–103. [DOI] [PubMed] [Google Scholar]
- 81.Akbar U, et al. (2009) Delivery of temozolomide to the tumor bed via biodegradable gel matrices in a novel model of intracranial glioma with resection. J Neurooncol 94(2):203–212. [DOI] [PubMed] [Google Scholar]
- 82.Kauer TM, Figueiredo JL, Hingtgen S, & Shah K (2012) Encapsulated therapeutic stem cells implanted in the tumor resection cavity induce cell death in gliomas. Nat Neurosci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Valparaiso AP, Vicente DA, Bograd BA, Elster EA, & Davis TA (2015) Modeling acute traumatic injury. The Journal of surgical research 194(1):220–232. [DOI] [PubMed] [Google Scholar]
- 84.Choi SH, et al. (2017) Tumor resection boosts therapeutic efficacy of encapsulated stem cells expressing a highly secretable variant of interferon-beta in glioblastomas. Clin Cancer Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Etchin J, Kanki JP, & Look AT (2011) Zebrafish as a model for the study of human cancer. Methods Cell Biol 105:309–337. [DOI] [PubMed] [Google Scholar]
- 86.Vittori M, Motaln H, & Turnsek TL (2015) The study of glioma by xenotransplantation in zebrafish early life stages. J Histochem Cytochem 63(10):749–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zeng A, et al. (2017) Identify a Blood-Brain Barrier Penetrating Drug-TNB using Zebrafish Orthotopic Glioblastoma Xenograft Model. Scientific reports 7(1):14372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yan C, et al. (2019) Visualizing Engrafted Human Cancer and Therapy Responses in Immunodeficient Zebrafish. Cell 177(7):1903–1914 e1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Reiter LT, Potocki L, Chien S, Gribskov M, & Bier E (2001) A systematic analysis of human disease-associated gene sequences in Drosophila melanogaster. Genome Res 11(6):1114–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.McClung C & Hirsh J (1998) Stereotypic behavioral responses to free-base cocaine and the development of behavioral sensitization in Drosophila. Current biology : CB 8(2):109–112. [DOI] [PubMed] [Google Scholar]
- 91.Witte HT, Jeibmann A, Klambt C, & Paulus W (2009) Modeling glioma growth and invasion in Drosophila melanogaster. Neoplasia 11(9):882–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Read RD, Cavenee WK, Furnari FB, & Thomas JB (2009) A drosophila model for EGFR-Ras and PI3K-dependent human glioma. PLoS genetics 5(2):e1000374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Read RD, et al. (2013) A kinome-wide RNAi screen in Drosophila Glia reveals that the RIO kinases mediate cell proliferation and survival through TORC2-Akt signaling in glioblastoma. PLoS genetics 9(2):e1003253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Castellanos E, Dominguez P, & Gonzalez C (2008) Centrosome dysfunction in Drosophila neural stem cells causes tumors that are not due to genome instability. Current biology : CB 18(16):1209–1214. [DOI] [PubMed] [Google Scholar]
- 95.Betschinger J, Mechtler K, & Knoblich JA (2006) Asymmetric segregation of the tumor suppressor brat regulates self-renewal in Drosophila neural stem cells. Cell 124(6):1241–1253. [DOI] [PubMed] [Google Scholar]
- 96.Mukherjee S, et al. (2016) Drosophila Brat and Human Ortholog TRIM3 Maintain Stem Cell Equilibrium and Suppress Brain Tumorigenesis by Attenuating Notch Nuclear Transport. Cancer Res 76(8):2443–2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Awasaki T & Lee T (2011) New tools for the analysis of glial cell biology in Drosophila. Glia 59(9):1377–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hubert CG, et al. (2016) A Three-Dimensional Organoid Culture System Derived from Human Glioblastomas Recapitulates the Hypoxic Gradients and Cancer Stem Cell Heterogeneity of Tumors Found In Vivo. Cancer Res 76(8):2465–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jacob F, et al. (2020) A Patient-Derived Glioblastoma Organoid Model and Biobank Recapitulates Inter- and Intra-tumoral Heterogeneity. Cell 180(1):188–204 e122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bian S, et al. (2018) Genetically engineered cerebral organoids model brain tumor formation. Nat Methods 15(8):631–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ogawa J, Pao GM, Shokhirev MN, & Verma IM (2018) Glioblastoma Model Using Human Cerebral Organoids. Cell reports 23(4):1220–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Linkous A, et al. (2019) Modeling Patient-Derived Glioblastoma with Cerebral Organoids. Cell reports 26(12):3203–3211 e3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hailer NP, Jarhult JD, & Nitsch R (1996) Resting microglial cells in vitro: analysis of morphology and adhesion molecule expression in organotypic hippocampal slice cultures. Glia 18(4):319–331. [DOI] [PubMed] [Google Scholar]
- 104.Farin A, et al. (2006) Transplanted glioma cells migrate and proliferate on host brain vasculature: a dynamic analysis. Glia 53(8):799–808. [DOI] [PubMed] [Google Scholar]
- 105.Eisemann T, et al. (2018) An advanced glioma cell invasion assay based on organotypic brain slice cultures. BMC Cancer 18(1):103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Traggiai E, et al. (2004) Development of a human adaptive immune system in cord blood cell-transplanted mice. Science 304(5667):104–107. [DOI] [PubMed] [Google Scholar]
- 107.De La Rochere P, et al. (2018) Humanized Mice for the Study of Immuno-Oncology. Trends Immunol 39(9):748–763. [DOI] [PubMed] [Google Scholar]
- 108.Pearson T, Greiner DL, & Shultz LD (2008) Creation of “humanized” mice to study human immunity. Curr Protoc Immunol Chapter 15:Unit 15 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.van Rijn RS, et al. (2003) A new xenograft model for graft-versus-host disease by intravenous transfer of human peripheral blood mononuclear cells in RAG2−/− gammac−/− double-mutant mice. Blood 102(7):2522–2531. [DOI] [PubMed] [Google Scholar]
- 110.Allen TM, et al. (2019) Humanized immune system mouse models: progress, challenges and opportunities. Nature immunology 20(7):770–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lan P, Tonomura N, Shimizu A, Wang S, & Yang YG (2006) Reconstitution of a functional human immune system in immunodeficient mice through combined human fetal thymus/liver and CD34+ cell transplantation. Blood 108(2):487–492. [DOI] [PubMed] [Google Scholar]
- 112.Greenblatt MB, et al. (2012) Graft versus host disease in the bone marrow, liver and thymus humanized mouse model. PloS one 7(9):e44664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ito R, Takahashi T, & Ito M (2018) Humanized mouse models: Application to human diseases. Journal of cellular physiology 233(5):3723–3728. [DOI] [PubMed] [Google Scholar]
- 114.Walsh NC, et al. (2017) Humanized Mouse Models of Clinical Disease. Annual review of pathology 12:187–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wege AK (2018) Humanized Mouse Models for the Preclinical Assessment of Cancer Immunotherapy. BioDrugs : clinical immunotherapeutics, biopharmaceuticals and gene therapy 32(3):245–266. [DOI] [PubMed] [Google Scholar]
- 116.Wang M, et al. (2018) Humanized mice in studying efficacy and mechanisms of PD-1-targeted cancer immunotherapy. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 32(3): 1537–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yao LC, et al. (2019) Creation of PDX-Bearing Humanized Mice to Study Immuno-oncology. Methods Mol Biol 1953:241–252. [DOI] [PubMed] [Google Scholar]
- 118.Liu D, et al. (2012) IL-15 protects NKT cells from inhibition by tumor-associated macrophages and enhances antimetastatic activity. The Journal of clinical investigation 122(6):2221–2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Akiyama Y, et al. (2017) The anti-tumor activity of the STAT3 inhibitor STX-0119 occurs via promotion of tumor-infiltrating lymphocyte accumulation in temozolomide-resistant glioblastoma cell line. Immunol Lett 190:20–25. [DOI] [PubMed] [Google Scholar]
- 120.Ashizawa T, et al. (2017) Antitumor Effect of Programmed Death-1 (PD-1) Blockade in Humanized the NOG-MHC Double Knockout Mouse. Clin Cancer Res 23(1): 149–158. [DOI] [PubMed] [Google Scholar]
- 121.Zhai L, et al. (2017) Infiltrating T Cells Increase IDO1 Expression in Glioblastoma and Contribute to Decreased Patient Survival. Clin Cancer Res 23(21):6650–6660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wainwright DA, et al. (2012) IDO expression in brain tumors increases the recruitment of regulatory T cells and negatively impacts survival. Clin Cancer Res 18(22):6110–6121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.LeBlanc AK, et al. (2016) Creation of an NCI comparative brain tumor consortium: informing the translation of new knowledge from canine to human brain tumor patients. Neuro Oncol 18(9): 1209–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hicks J, Platt S, Kent M, & Haley A (2017) Canine brain tumours: a model for the human disease? Vet Comp Oncol 15(1):252–272. [DOI] [PubMed] [Google Scholar]
- 125.Amin SB, et al. (2020) Comparative Molecular Life History of Spontaneous Canine and Human Gliomas. Cancer Cell 37(2):243–257 e247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Young JS, et al. (2018) Convection-Enhanced Delivery of Polymeric Nanoparticles Encapsulating Chemotherapy in Canines with Spontaneous Supratentorial Tumors. World Neurosurg 117:e698–e704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Strand AD, et al. (2007) Conservation of regional gene expression in mouse and human brain. PLoS genetics 3(4):e59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Xiao W, Sohrabi A, & Seidlits SK (2017) Integrating the glioblastoma microenvironment into engineered experimental models. Future Sci OA 3(3):FS0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Vandamme TF (2014) Use of rodents as models of human diseases. J Pharm Bioallied Sci 6(1):2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yang XJ, et al. (2013) A novel zebrafish xenotransplantation model for study of glioma stem cell invasion. PloS one 8(4):e61801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Vanderbeek AM, et al. (2018) The clinical trials landscape for glioblastoma: is it adequate to develop new treatments? Neuro Oncol 20(8): 1034–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]


