Abstract
IL-23 is an inflammatory cytokine that plays an essential role in Th17 immunity by enhancing Th17 cell proliferation and survival, and Th17 cytokine production. IL-23 has pathogenic roles in the development of Th17-mediated inflammatory diseases including psoriasis. Despite successful treatment of psoriasis by blocking IL-23, the regulation of IL-23 expression in psoriasis patients is largely unknown. Dendritic cells are generally considered to be the primary source of IL-23 in psoriasis. While high levels of IL-23 are found in psoriatic epidermis, IL-23 expression in psoriatic keratinoctyes remains a controversial issue. In this study, we demonstrated that IL-23 production is induced by a combination of TNFα and IL-17A in human keratinocytes. Additionally, this IL-23 induction by TNFα and IL-17A is further increased in psoriatic keratinocytes and is enhanced by EGFR signaling. Although IL-23 is also robustly induced by toll-like receptor agonists in dendritic cells and macrophages, IL-23 expression in these cell types is not regulated by TNFα, IL-17A, and EGFR signaling. Given that IL-23 is essential for maintaining Th17 activation, IL-23 induction by TNFα, IL-17A, and EGF in keratinocytes could play an important pathological role in psoriasis pathogenesis as well as the cutaneous rash associated with EGFR inhibition therapy.
Keywords: Tumor necrosis factor alpha (TNFα), Interleukin 17A (IL-17A), epidermal growth factor (EGF), psoriasis, keratinocytes
Introduction
IL-23 is a heterodimeric cytokine composed of a unique p19 subunit and a p40 subunit that is shared with IL-12[1]. It is an inflammatory cytokine essential for maintaining Th17 cell activity by enhancing Th17 cell proliferation, survival, and Th17 cytokine production[2]. IL-23 plays many critical roles in psoriasis pathogenesis, as demonstrated by its overexpression in psoriatic skin[3], the induction of psoriasis-like phenotypes in mice by intradermal IL-23 injection[4], the association of genetic variants in genes of IL-23 signaling with susceptibility or resistance to psoriasis[5,6], and the alleviation of psoriatic conditions by anti–IL-23 antibodies in mouse models[7]. More importantly, antibodies against either IL23p40 or IL23p19 have demonstrated clinical efficacy in human psoriasis therapy[8,9].
Despite the importance of IL-23 in psoriasis pathogenesis, the regulation of IL-23 expression in psoriasis remains largely elusive. IL-23 is primarily expressed in macrophages and dendritic cells in response to bacterial and viral infections and pathogen-associated molecular patterns[10–12], and dendritic cells are generally considered to be the primary source of IL-23 in psoriasis[3]. Despite high levels of IL-23 found in the epidermis of psoriatic skin[13–17], IL-23 expression in keratinoctyes remains a controversial issue. The expression of IL-23 mRNA has been detected in keratinocytes under different conditions[14,18,19]. However, only low levels of IL-23 protein were detected in keratinocytes under culture conditions that are not relevant to psoriasis[19]. To date, whether or not IL-23 protein is produced from keratinocytes remains a debatable issue. In this study, we aimed to determine whether IL-23 cytokine is expressed in keratinocytes and to identify the signals that trigger IL-23 expression in keratinocytes in an attempt to define the role of keratinocyte IL-23 expression in psoriasis pathogenesis.
Materials and Methods
Primary keratinocyte culture and reagents
Skin biopsy specimens (6 mm) were obtained from both the lesional skin of patients with psoriasis and the skin of individuals without psoriasis under an Oregon Health & Science University Institutional Review Board-approved protocol. The epidermis was trimmed off and minced into 1×1 mm2 skin islets. The skin islets were planted on collagen-coated plates as described[20]. Keratinocytes from the outgrowth were trypsinized and plated in 12-well plates for the assay. The keratinocyte cultures were switched to CnT-07 keratinocyte medium (Zen Bio) that contains 10 ng/ml EGF or Epilife medium with or without human keratinocyte growth supplement (Invitrogen) that contains 0.2 ng/ml EGF for 3 days before cytokine treatments, which included 10 ng/ml TNFα (Prospec), 20 ng/ml IL-17A (Cell Signaling), 20 ng/ml IL-22 (Cell Signaling), 10 ng/ml interferonγ (Prospec), 20 ng/ml IL-17F (Cell Signaling), and 10 ng/ml growth factors EGF (Millipore), 10 ng/ml transforming growth factor alpha (TGFα; Biolegend), amphiregulin (AREG; R&D Systems), and 10 ng/ml HB-EGF (R&D Systems) as indicated. The EGFR inhibitors erolotinib, gefitinib, and lapatinib were purchased from SelleckChem. The toll like receptor (TLR) agonist peptidoglycan was purchased from Sigma.
Monocyte derived dendritic cells and THP1 macrophage
Monocytes from peripheral blood of healthy donors were isolated by EasySep CD14 positive selection kit (Stemcell). Monocyte-derived dendritic cells (MoDC) were differentiated from CD14+ monocytes in StemXVivo DC Medium (R&D) with 50 ng/ml of GM-CSF and 20 ng/ml of IL-4 for 7 days. THP1 macrophages were induced from the human monocytic leukemia cell line THP1 (ATCC) by treating cells with 100 nM phorbol ester 12-O-tetradecanoylphorbol-13-acetate (TPA) for 3 days in RPMI1640 medium.
Quantitative RT-PCR
Total RNA was extracted from cultured keratinocytes with RNeasy Kit (Qiagen). RNA was reverse transcribed with High-Capacity RNA-to-cDNA™ Kit (ThermoFisher). All experiments were done in triplicate using SYBR Green PCR Master Mix (ThermoFisher). Human IL23p19 mRNA was measured by qRT-PCR using human IL23p19 primers (Fwd: GAGCCTTCTCTGCTCCCTGAT, Rev: AGTTGGCTGAGGCCCAGTAG) and IL23p40 primers (Fwd: AGGGACATCATCAAACCTGACC, Rev: GCTGAGGTCTTGTCCGTGAA). Gene expression data were collected using a 7900HT thermocycler (Applied Biosystems). The levels of human IL23p19 and IL23p40 mRNA were normalized to GAPDH (Fwd: ATCAAGAAGGTGGTGAAGCA; Rev: GTCGCTGTTGAAGTCAGAGGA).
IL-23 ELISA
IL-23 protein expression in primary keratinocyte culture supernatant was quantified by human IL-23 Ready-Set-Go ELISA kit (eBioscience). Measurements were done in duplicate. The optical density was measured at 450 nM with a SpectraMax 384 microplate reader (Molecular Devices).
Statistics
Statistical analyses were carried out using GraphPAD Prism version 4. One-way ANOVA was used to test for statistical significance.
Results and Discussion
IL-23 is induced by TNFα and IL-17A in human keratinocytes
To investigate the possibility of keratinocytes acting as a source of IL-23 production in psoriasis, we first analyzed the production of IL-23 by primary human keratinocytes in response to potential psoriatic cytokines including TNFα, IL-17A, IL-22, IL-17F, and interferonγ (IFN-γ).
We found that maximal IL23p19 mRNA was induced by combined treatment with TNFα and IL-17A (Fig 1A). Marginal IL23p19 expression was induced by either TNFα or IL-17A alone. IL23p19 expression was moderately induced by TNFα and IL-17F. IL-23 induction by TNFα and IL-17A was confirmed at the protein level by ELISA (Fig 1B). Approximately 60 pg/ml of IL-23 was detected in the culture medium of keratinocytes treated with TNFα and IL-17A. IL-23 protein at the level of less than 10 pg/ml has been previously detected from keratinocytes under co-culturing condition with J558-CD40L myeloma cells in the presence of peptidoglycan, poly(I:C), IFN-γ, and IL-1β, an artificial condition not relevant to psoriasis[19]. Considering the linear range of conventional IL-23 ELISA is between 16–2,000 pg/mL (eBioscience), our data showing IL-23 induction by TNFα and IL-17A at 60 pg/ml level provides the first evidence that IL-23 protein is expressed in keratinocytes under conditions that are relevant to psoriasis.
Figure 1.
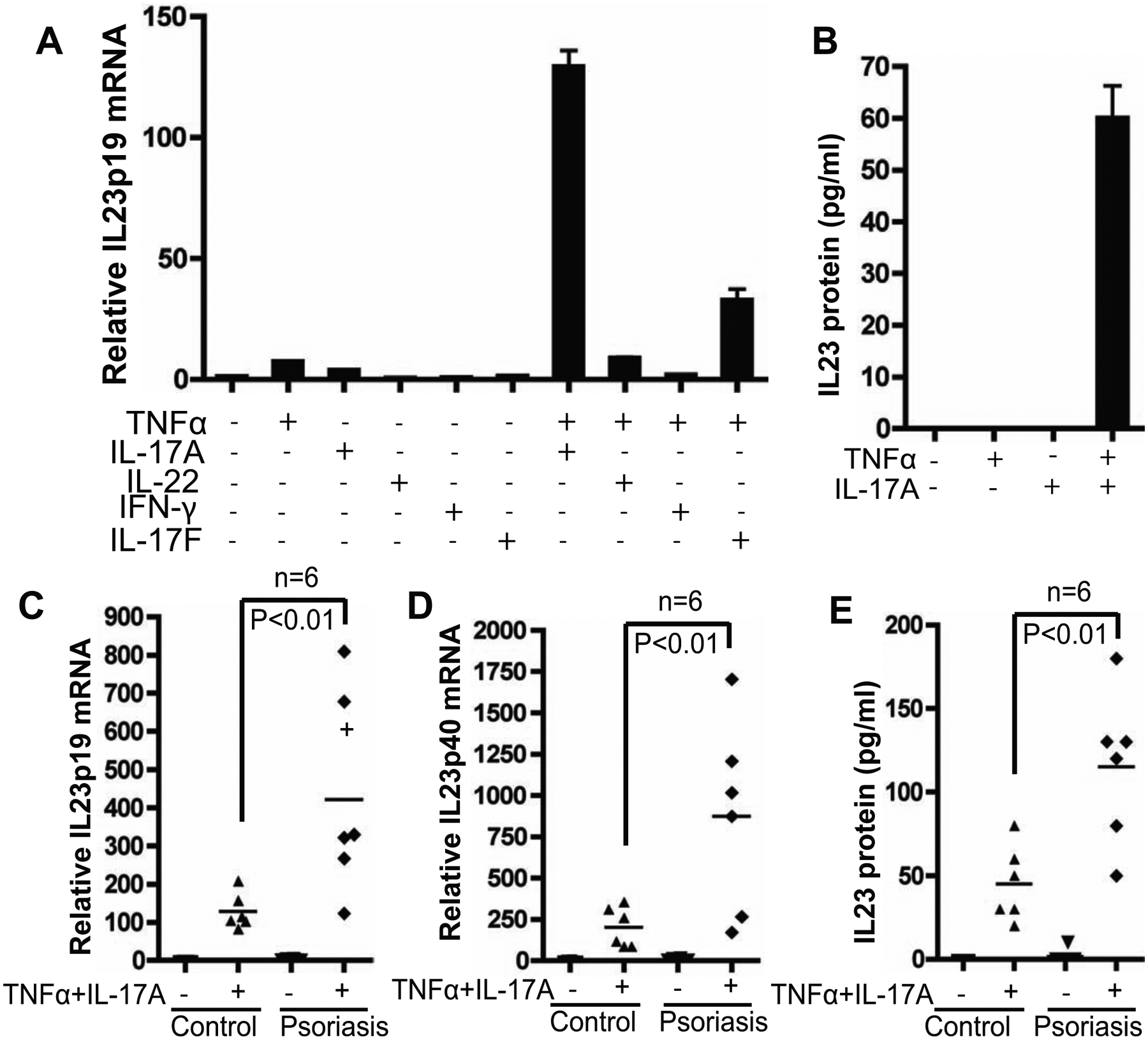
IL-23 is induced by TNFα and IL-17A in human primary keratinocytes and enhanced in psoriatic keratinocytes. A) IL23p19 induction by TNFα and IL-17A in human primary keratinocytes. Human primary keratinocytes were isolated from non-psoriatic individuals and cultured in CnT-07 medium. After treating the keratinocytes with cytokines as indicated for 24 hours, the total RNA was extracted for qRT-PCR analysis of IL-23p19 mRNA. B) ELISA quantification of IL-23 protein in the supernatant of human primary keratinocytes culture treated with indicated cytokines. C) IL-23 induction is enhanced in psoriatic keratinocytes. Keratinocytes were isolated from the lesional skin of patients with psoriasis (n=6) and from non-psoriatic individuals (n=6). After treating the keratinocytes with TNFα and IL-17A for 24 hours, the total RNA was extracted for qRT-PCR analysis of IL-23p19 mRNA, and D) IL23p40 mRNA. E) ELISA quantification of IL-23 protein in the culture supernatants of primary keratinocytes from patients with or without psoriasis.
IL-23 induction by TNFα/IL-17A is enhanced in psoriatic keratinocytes
Compared with the keratinocytes from non-psoriatic individuals, keratinocytes from lesional skin of psoriasis patients showed significantly higher expression of both IL23p19 and IL23p40 mRNA in response to TNFα and IL-17A (Fig 1C&D). ELISA analysis confirmed that IL-23 protein induction was consistently enhanced in psoriatic keratinocytes over levels seen in non-psoriatic individuals (Fig 1E). Enhanced IL-23 induction by TNFα and IL-17A in psoriatic keratinocytes suggests an intrinsic aberration in psoriatic keratinocytes that might contribute to enhanced IL-23 expression in psoriasis. A number of psoriasis-associated SNPs have been found in genes involved in NF-κB and IL-23 signaling pathways[5], yet the functional relationships between these two signaling pathways are largely unknown. Because both TNFα and IL-17A signals are transduced by NF-κB[21], aberrant NF-κB activation may contribute to enhanced IL-23 induction by TNFα and IL-17A in psoriatic keratinocytes, thus providing a functional connection between NF-κB and IL-23 signaling pathways in psoriasis.
Overexpression of IL-23 in psoriatic epidermis has been demonstrated by multiple groups[13–16]. Furthermore, IL-23 in psoriatic epidermis is significantly diminished upon UV irradiation therapy[15]. Since UV irradiation preferentially depletes intraepidermal T cells[22], this suggests that IL-23 in psoriatic epidermis is possibly regulated by IL-17A from intraepidermal T cells. The link between IL-23 and TNFα is supported by the reduction of IL-23 expression in skin from psoriasis patients treated with anti-TNFα antibodies[23,24]. While the mechanism of TNFα and IL-17A synergy remains to be determined, the mechanism of IL-23 induction by TNFα in keratinocytes has been demonstrated to be regulated by epigenetic control through H3K9 dimethylation[14]. Given that IL-23 is essential for Th17 cell activation[25], IL-23 induction by TNFα and IL-17A thus constitutes a positive feedback loop of Th17 activation involving Th17 cell activation by IL-23 from keratinocytes and keratinocyte activation by TNFα and IL-17A from Th17 cells. This positive feedback loop may explain how chronic inflammation is maintained in psoriasis.
IL-23 is not induced by TNFα and IL-17A in macrophages and dendritic cells
IL-23 is primarily expressed in macrophages and dendritic cells in response to bacterial or viral infection[10–12], and macrophages and dendritic cells have been considered to be the major cell types responsible for IL-23 overexpression in psoriasis[3]. Despite the detection of IL-23 overexpression in psoriatic epidermis in the previous studies[13–16], psoriatic keratinocytes have not been considered as the primary source of IL-23 overproduction in psoriasis. This conclusion is mainly based upon the results of experiments that showed monocyte-derived dendritic cells produced nearly 1,000-fold more IL-23 than keratinocytes in response to combined treatment with peptidoglycan (PGN), poly(I:C), IFN-γ, and IL-1β, and co-culturing with J558-CD40L myeloma cells, an artificial condition not relevant to psoriasis[19]. Therefore, we evaluated IL-23 induction by TNFα and IL-17A in different cell types.
Through analysis of different cell types from non-psoriatic individuals including keratinocytes, fibroblasts, MoDC, and THP1 macrophages, we found that IL-23 induction by the combination of TNFα and IL-17A was only specific to keratinocytes (Fig 2A). In contrast, IL23p19 was induced by the toll-like receptor (TLR) agonist PGN in the macrophages derived from THP1 monocytes but not in keratinocytes (Fig 2B). IL23p19 was gradually induced by TNFα and IL-17A in keratinocytes but not in macrophages (Fig 2C). Although the IL-23 protein level from PGN-treated macrophages was almost 10-fold higher than that induced by TNFα and IL-17A in keratinocytes, IL-23 protein induction by TNFα/IL-17A was not detected in macrophages (Fig 2D). The receptors for TNFα and IL-17A are expressed in both macrophages and dendritic cells [26,27]. This was further confirmed by analysis of TNFR1 and IL-17RA expression in the cells used in this study including THP1 macrophages and MoDC (Supplementary Figure S1). Inability to induce IL-23 by TNFα and IL-17A in macrophages and MoDC suggests that IL-23 expression is mediated through different signaling pathways in macrophages and dendritic cells.
Figure 2.
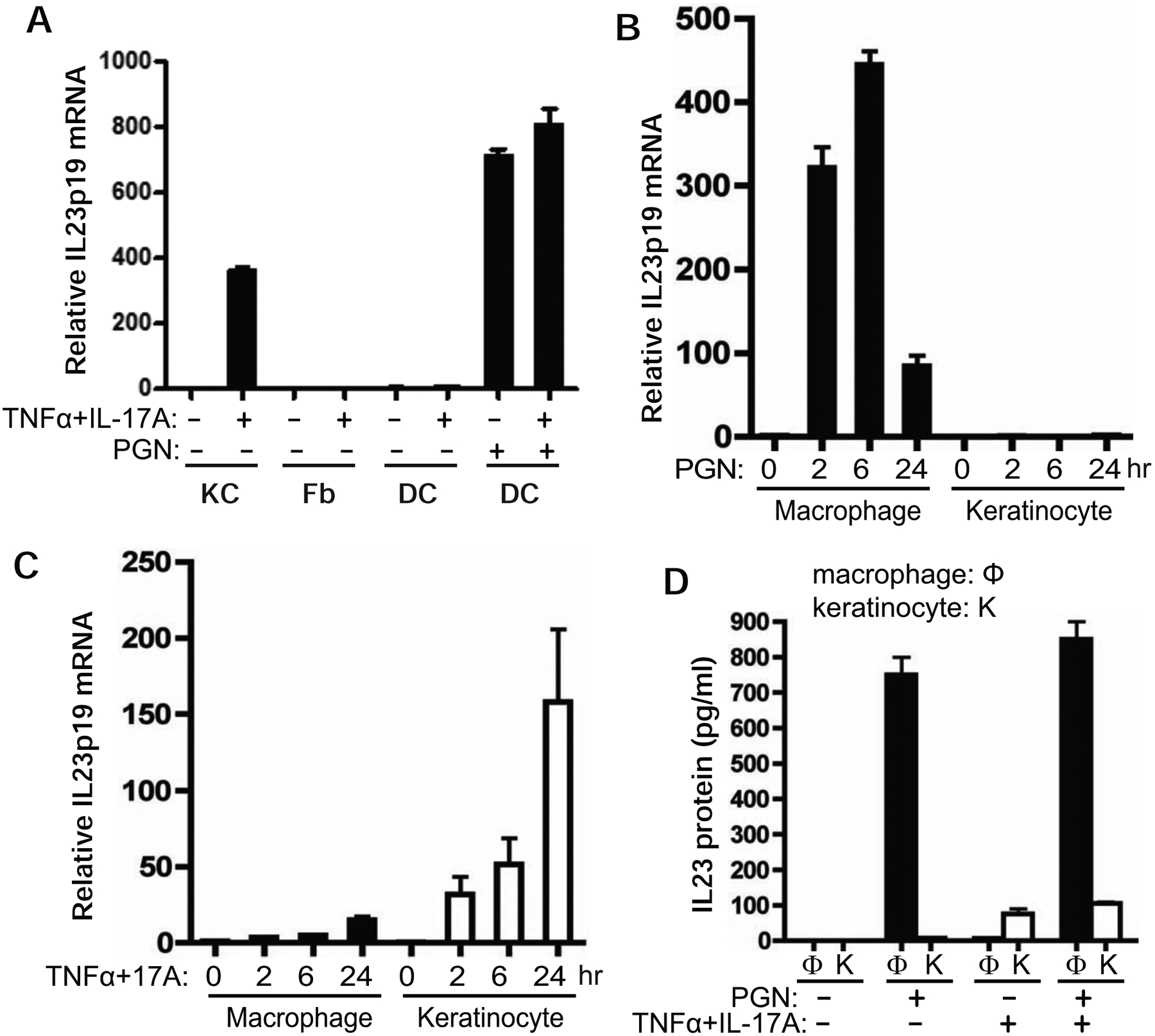
IL-23 induction by TNFα/IL-17A is specific to keratinocytes but not to macrophages and dendritic cells. A) IL23p19 mRNA is induced by TNFα/IL-17A in keratinocytes but not in fibroblast cells (Fb) derived from skin of healthy individuals or dendritic cells derived from blood (DC). The dendritic cells were treated with 10 μg/ml peptidoglycan (PGN) for 3 hours as control. B) THP1 macrophages and non-psoriatic keratinocytes were treated with 10 μg/ml of PGN for 2, 6, and 24 hours. C) THP1 macrophages and non-psoriatic keratinocytes were treated with TNFα/IL-17A for 2, 6, and 24 hours. The total RNA was collected for qRT-PCR analysis of IL23p19 mRNA. D) Comparison of IL-23 protein induction by TNFα/IL-17A versus PNG in macrophages (Φ) versus keratinocytes (K). Both macrophages and keratinocytes were treated with PGN and/or TNFα/IL-17A for 24 hours. The culture supernatants were collected for IL-23 ELISA measurement.
There is some clinical evidence showing that IL-23 expression in psoriatic skin is reduced by TNFα blockers [23,24]. It remains to be determined whether the reduction of IL-23 by TNFα blockade results from its action on psoriatic keratinocytes and/or dendritic cells. There is evidence to show reduced dermal IL-23/CD11c double positive cells in patients treated with TNFα blockers [24]. This appears to support the notion that TNFα blockers inhibit IL-23 expression in CD11c+ dendritic cells in psoriasis. However, there is no convincing data to determine whether the reduction of IL-23/CD11c+ cells in psoriatic skin is a result of direct inhibition of IL-23 expression in the CD11c+ cells by a TNFα blocker.
On the contrary, TNFα appears to be inhibitory on IL-23 expression in macrophages and dendritic cells. In vitro studies showed that LPS-induced IL-23 expression is inhibited in macrophages and dendritic cells pretreated with TNFα for more than 12 hours [28]. No inhibition was observed when LPS was added at the same time with TNFα. Consistent with these data, we found the induction of IL-23p40 mRNA and IL-23 protein by PGN was suppressed in dendritic cells pretreated with TNFα (Supplementary data Figure S2) but not in the cells co-treated with PGN and TNFα (Supplementary data Figure S3). These results suggest IL-23 expression in dendritic cells is not positively regulated by TNFα. The paradoxical action of TNFα on IL-23 expression in dendritic cells calls for further studies on IL-23 regulation by TNFα in dendritic cells, particularly in CD11c+ dendritic cells that are more relevant to psoriasis.
IL-23 induction by TNFα and IL-17A in keratinocytes is enhanced by EGFR signaling
Synergistic induction of psoriatic genes by TNFα and IL-17A in human keratinocytes has been reported by several groups[18,29,30]. However, IL-23 induction by TNFα and IL-17A was not investigated. Even when IL23p19 mRNA was listed as one of the upregulated genes in keratinocytes treated with TNFα and IL-17 by microarray analysis[18], it was not validated by qRT-PCR or ELISA. Analysis of the experimental conditions revealed that IL23p19 induction by TNFα and IL-17A in the CnT-07 medium used in our study was much higher than that in the Epilife medium used in these previous studies (Fig 3A). In analysis of the active components in CnT-07 medium, epidermal growth factor (EGF) was identified as the key factor contributing to IL23p19 induction in response to TNFα and IL-17A (Fig 3B). EGF concentration in the CnT-07 medium (10 ng/ml) is 50-fold more than that in Epilife medium (0.2 ng/ml); supplementing EGF to Epilife medium was sufficient to increase IL23p19 to a level similar to that in CnT-07 medium (Fig 3B). The addition of EGF significantly increased IL-23 protein induction by TNFα and IL-17A from undetectable to the level equivalent to that in CnT medium (Fig 3C). This may explain why IL-23 induction by TNFα and IL-17A was not identified in the previous studies.
Figure 3.
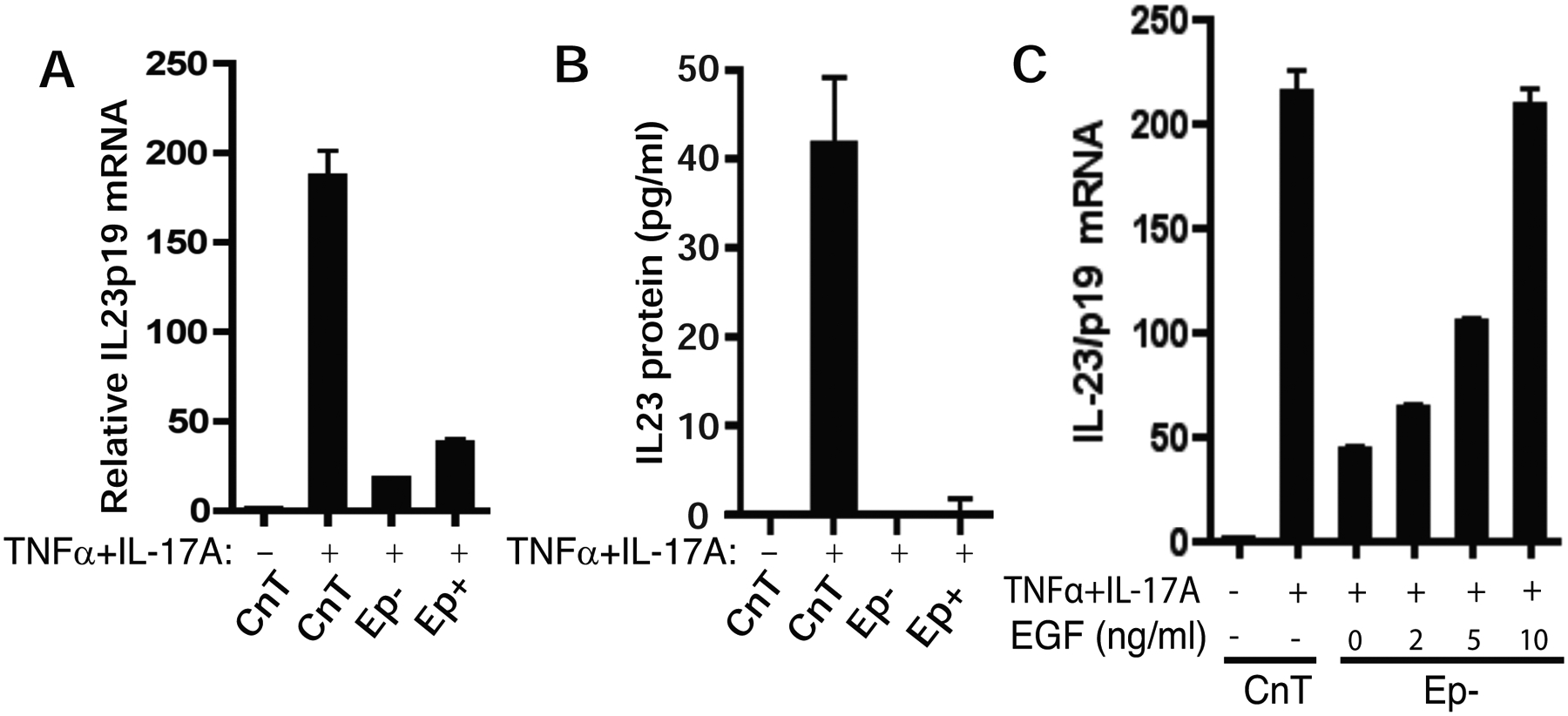
EGF is required for IL-23 induction by TNFα and IL-17A in keratinocytes. A) IL23p19 induction by TNFα and IL-17A is compromised in Epilife medium which contains 0.2 ng/ml EGF in the growth supplement. Non-psoriatic keratinocytes were cultured in CnT-07 medium (which contains 10 ng/ml EGF), Epilife medium with (Ep+) or without (Ep−) growth supplement containing 0.2 mg/ml EGF and treated with TNFα and IL-17A for 24 hours. Total RNA was collected for qRT-PCR analysis of IL23p19 mRNA. B) ELISA quantification of IL-23 protein in keratinocyte culture with CnT-07 or Epilife medium. C) IL23p19 induction by TNFα/IL-17A is enhanced by supplementing EGF in Epilife medium. Keratinocytes in the Epilife medium without growth supplement (Ep−) were supplemented with EGF at indicated concentration and treated with TNFα/IL-17A.
The activation of EGFR signaling has been implicated in psoriasis[31]. A number of EGF family members are overexpressed in psoriatic skin including, TGF-α[32], amphiregulin (AREG)[33], and heparin-binding EGF-like growth factor (HB-EGF)[34]. The signaling of these growth factors are mediated through EGFR[35]. Moreover, transgenic mice with overexpression of AREG in the basal epidermal layer (K14-AREG) develop psoriasis-like features[36] . Similar to EGF, these EGF-like molecules enhanced TNFα and IL-17A-mediated IL-23 induction (Fig. 4A).
Figure 4.
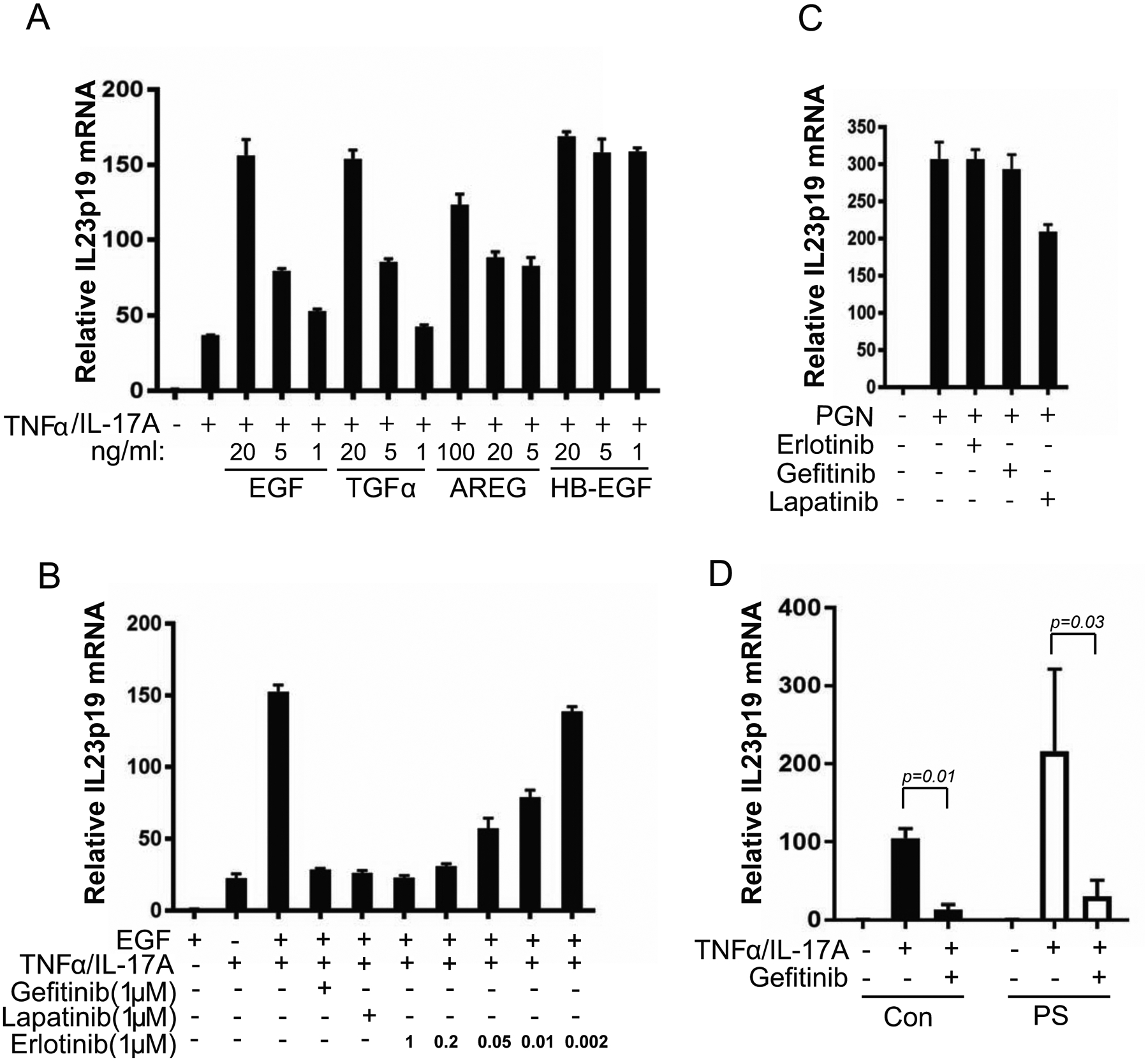
EGFR inhibition blocks IL-23 induction by TNFα/IL-17A in keratinocytes but not IL-23 induction by PGN in THP1 macrophages. A) IL23p19 induction by TNFα/IL-17A was enhanced by EGF-like molecules, TGFα (10 ng/ml), amphiregulin (AREG, 10 ng/ml), and heparin-binding EGF-like growth factor (HB-EGF, 10 ng/ml). B) Suppression of TNFα/IL-17A mediated IL23p19 induction by EGFR inhibitors. Keratinocytes in Epilife medium without growth supplement were supplemented with 10ng/ml EGF and treated with EGFR inhibitors at indicated concentration immediately followed by TNFα/IL-17A treatment for 24 hours. The total RNA was extracted for qRT-PCR analysis of IL-23/p19 mRNA. C) THP1 macrophages were treated with EGFR inhibitors (1μM) followed by 10 μg/ml peptidoglycan for 3 hours. Total RNA was extracted for qRT-PCR analysis of IL-23/p19 mRNA. D) Suppression of TNFα/IL-17A-mediated IL23p19 induction in psoriatic keratinocytes by EGFR inhibition. Both control keratinocytes (Con, n=4) and psoriatic keratinocytes (PS, n=4) were cultured in CnT-07 medium with or without Gefitinib (1μM) followed by followed by TNFα/IL-17A treatment for 24 hours. The total RNA was extracted for qRT-PCR analysis of IL-23p19 mRNA.
To test whether EGFR signaling is necessary for TNFα and IL-17A induced IL-23 expression, IL-23 induction by TNFα and IL-17A was measured in keratinocytes treated with pharmacologic EGFR inhibitors. TNFα and IL-17A induced IL23p19 expression was suppressed by all the EGFR inhibitors tested including erlotinib, gefitinib, and lapatinib (Fig 4B). Erlotinib showed dosage dependent inhibition of IL23p19 expression (Fig 4B). On the contrary, IL-23 induction by PGN in THP1 macrophages was not affected by EGFR inhibitors (Fig 4C). Similar to control keratinocytes, IL23p19 induction by TNFα and IL-17A in psoriatic keratinocytes was suppressed by EGFR inhibition under CnT-07 culture condition (Fig 4D). The data suggest that EGFR signaling contributes to IL-23 induction by TNFα and IL-17A; therefore, blocking EGFR signaling could be an effective approach for psoriasis therapy by blocking IL-23 expression from keratinocytes.
Indeed, since epidermal hyperproliferation is the most prominent feature of psoriasis, EGFR inhibition has been proposed for psoriasis therapy[37]. So far, however, there have been no reports of a clinical trial for psoriasis therapy with EGFR inhibitors. Fortuitous improvement of psoriasis has been noted during treatment with EGFR inhibitors in cancer patients who also have psoriasis[38,39]. However, worsening of psoriasis by an EGFR inhibitor was also reported in a breast cancer patient with combined chemotherapy[40]. An acneiform papulopustular skin eruption with secondary bacterial colonization is a major side effect of EGFR inhibition therapy[41], and topical or systemic antibiotic applications are effective for treatment or prevention of these side effects[42]. This might imply that EGFR inhibition compromises antibacterial activity in skin. Given that Th17 immunity is a primary defense system protecting surface tissues from bacterial infection, the essential role of EGFR signaling in IL-23 expression in keratinocytes suggests a molecular basis for the development of the skin rash and bacterial colonization associated with EGFR inhibition therapy.
In conclusion, this study provides the first evidence that IL-23 cytokine is induced by a synergistic action of TNFα, IL-17A, and EGF signaling in keratinocytes. Given that IL-23 is essential for maintaining Th17 activation in psoriasis, IL-23 induction by TNFα, IL-17A, and EGF in keratinocytes could play important pathological roles in psoriasis pathogenesis, and its inhibition in the skin rash associated with EGFR inhibition therapy.
Supplementary Material
Highlights:
IL-23 is induced by TNFα and IL-17A in keratinocytes.
IL-23 induction by TNFα and IL-17A is enhanced in psoriatic keratinocytes.
EGFR signaling is required for effective IL-23 induction by TNFα and IL-17A in keratinocytes.
IL-23 induction by TNFα and IL-17A is specific to keratinocytes but not monocytes.
Acknowledgments
We thank Melanie Swinson for procuring clinical samples and Clara Stemwedel for editing the manuscript. This study was partially supported by NIAMS R03 AR066736, and R01 AR070645.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Oppmann B, Lesley R, Blom B, Timans JC, Xu Y, Hunte B, Vega F, Yu N, Wang J, Singh K, Zonin F, Vaisberg E, Churakova T, Liu M, Gorman D, Wagner J, Zurawski S, Liu Y, Abrams JS, Moore KW, Rennick D, de Waal-Malefyt R, Hannum C, Bazan JF, Kastelein RA, Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12, Immunity. 13 (2000) 715–725. [DOI] [PubMed] [Google Scholar]
- [2].Hawkes JE, Yan BY, Chan TC, Krueger JG, Discovery of the IL-23/IL-17 Signaling Pathway and the Treatment of Psoriasis, J. Immunol 201 (2018) 1605–1613. 10.4049/jimmunol.1800013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Lee E, Trepicchio WL, Oestreicher JL, Pittman D, Wang F, Chamian F, Dhodapkar M, Krueger JG, Increased expression of interleukin 23 p19 and p40 in lesional skin of patients with psoriasis vulgaris, J. Exp. Med 199 (2004) 125–130. 10.1084/jem.20030451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Chan JR, Blumenschein W, Murphy E, Diveu C, Wiekowski M, Abbondanzo S, Lucian L, Geissler R, Brodie S, Kimball AB, Gorman DM, Smith K, de Waal Malefyt R, Kastelein RA, McClanahan TK, Bowman EP, IL-23 stimulates epidermal hyperplasia via TNF and IL-20R2-dependent mechanisms with implications for psoriasis pathogenesis, J. Exp. Med 203 (2006) 2577–2587. 10.1084/jem.20060244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Nair RP, Duffin KC, Helms C, Ding J, Stuart PE, Goldgar D, Gudjonsson JE, Li Y, Tejasvi T, Feng B-J, Ruether A, Schreiber S, Weichenthal M, Gladman D, Rahman P, Schrodi SJ, Prahalad S, Guthery SL, Fischer J, Liao W, Kwok P-Y, Menter A, Lathrop GM, Wise CA, Begovich AB, Voorhees JJ, Elder JT, Krueger GG, Bowcock AM, Abecasis GR, Collaborative Association Study of Psoriasis, Genome-wide scan reveals association of psoriasis with IL-23 and NF-kappaB pathways, Nat. Genet 41 (2009) 199–204. 10.1038/ng.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Di Meglio P, Villanova F, Napolitano L, Tosi I, Terranova Barberio M, Mak RK, Nutland S, Smith CH, Barker JNWN, Todd JA, Nestle FO, The IL23R A/Gln381 allele promotes IL-23 unresponsiveness in human memory T-helper 17 cells and impairs Th17 responses in psoriasis patients, J. Invest. Dermatol 133 (2013) 2381–2389. 10.1038/jid.2013.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Tonel G, Conrad C, Laggner U, Di Meglio P, Grys K, McClanahan TK, Blumenschein WM, Qin J-Z, Xin H, Oldham E, Kastelein R, Nickoloff BJ, Nestle FO, Cutting edge: A critical functional role for IL-23 in psoriasis, J. Immunol 185 (2010) 5688–5691. 10.4049/jimmunol.1001538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Krueger GG, Langley RG, Leonardi C, Yeilding N, Guzzo C, Wang Y, Dooley LT, Lebwohl M, CNTO 1275 Psoriasis Study Group, A human interleukin-12/23 monoclonal antibody for the treatment of psoriasis, N. Engl. J. Med 356 (2007) 580–592. 10.1056/NEJMoa062382. [DOI] [PubMed] [Google Scholar]
- [9].Gordon KB, Duffin KC, Bissonnette R, Prinz JC, Wasfi Y, Li S, Shen Y-K, Szapary P, Randazzo B, Reich K, A Phase 2 Trial of Guselkumab versus Adalimumab for Plaque Psoriasis, N. Engl. J. Med 373 (2015) 136–144. 10.1056/NEJMoa1501646. [DOI] [PubMed] [Google Scholar]
- [10].Pirhonen J, Matikainen S, Julkunen I, Regulation of virus-induced IL-12 and IL-23 expression in human macrophages, J. Immunol 169 (2002) 5673–5678. [DOI] [PubMed] [Google Scholar]
- [11].Smits HH, van Beelen AJ, Hessle C, Westland R, de Jong E, Soeteman E, Wold A, Wierenga EA, Kapsenberg ML, Commensal Gram-negative bacteria prime human dendritic cells for enhanced IL-23 and IL-27 expression and enhanced Th1 development, Eur. J. Immunol 34 (2004) 1371–1380. 10.1002/eji.200324815. [DOI] [PubMed] [Google Scholar]
- [12].Gerosa F, Baldani-Guerra B, Lyakh LA, Batoni G, Esin S, Winkler-Pickett RT, Consolaro MR, De Marchi M, Giachino D, Robbiano A, Astegiano M, Sambataro A, Kastelein RA, Carra G, Trinchieri G, Differential regulation of interleukin 12 and interleukin 23 production in human dendritic cells, J. Exp. Med 205 (2008) 1447–1461. 10.1084/jem.20071450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Guttman-Yassky E, Lowes MA, Fuentes-Duculan J, Zaba LC, Cardinale I, Nograles KE, Khatcherian A, Novitskaya I, Carucci JA, Bergman R, Krueger JG, Low expression of the IL-23/Th17 pathway in atopic dermatitis compared to psoriasis, J. Immunol 181 (2008) 7420–7427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Li H, Yao Q, Mariscal AG, Wu X, Hülse J, Pedersen E, Helin K, Waisman A, Vinkel C, Thomsen SF, Avgustinova A, Benitah SA, Lovato P, Norsgaard H, Mortensen MS, Veng L, Rozell B, Brakebusch C, Epigenetic control of IL-23 expression in keratinocytes is important for chronic skin inflammation, Nat Commun. 9 (2018) 1420 10.1038/s41467-018-03704-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Piskin G, Tursen U, Sylva-Steenland RMR, Bos JD, Teunissen MBM, Clinical improvement in chronic plaque-type psoriasis lesions after narrow-band UVB therapy is accompanied by a decrease in the expression of IFN-gamma inducers -- IL-12, IL-18 and IL-23, Exp. Dermatol 13 (2004) 764–772. 10.1111/j.0906-6705.2004.00246.x. [DOI] [PubMed] [Google Scholar]
- [16].Winge MCG, Ohyama B, Dey CN, Boxer LM, Li W, Ehsani-Chimeh N, Truong AK, Wu D, Armstrong AW, Makino T, Davidson M, Starcevic D, Kislat A, Nguyen NT, Hashimoto T, Homey B, Khavari PA, Bradley M, Waterman EA, Marinkovich MP, RAC1 activation drives pathologic interactions between the epidermis and immune cells, J. Clin. Invest 126 (2016) 2661–2677. 10.1172/JCI85738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Tu Z, Zhang S, Zhou G, Zhou L, Xiang Q, Chen Q, Zhao P, Zhan H, Zhou H, Sun L, LMO4 Is a Disease-Provocative Transcription Coregulator Activated by IL-23 in Psoriatic Keratinocytes, J. Invest. Dermatol 138 (2018) 1078–1087. 10.1016/j.jid.2017.12.010. [DOI] [PubMed] [Google Scholar]
- [18].Chiricozzi A, Guttman-Yassky E, Suárez-Fariñas M, Nograles KE, Tian S, Cardinale I, Chimenti S, Krueger JG, Integrative responses to IL-17 and TNF-α in human keratinocytes account for key inflammatory pathogenic circuits in psoriasis, J. Invest. Dermatol 131 (2011) 677–687. 10.1038/jid.2010.340. [DOI] [PubMed] [Google Scholar]
- [19].Piskin G, Sylva-Steenland RMR, Bos JD, Teunissen MBM, In vitro and in situ expression of IL-23 by keratinocytes in healthy skin and psoriasis lesions: enhanced expression in psoriatic skin, J. Immunol 176 (2006) 1908–1915. [DOI] [PubMed] [Google Scholar]
- [20].Aasen T, Izpisúa Belmonte JC, Isolation and cultivation of human keratinocytes from skin or plucked hair for the generation of induced pluripotent stem cells, Nat Protoc. 5 (2010) 371–382. 10.1038/nprot.2009.241. [DOI] [PubMed] [Google Scholar]
- [21].Amatya N, Garg AV, Gaffen SL, IL-17 Signaling: The Yin and the Yang, Trends Immunol. 38 (2017) 310–322. 10.1016/j.it.2017.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Krueger JG, Wolfe JT, Nabeya RT, Vallat VP, Gilleaudeau P, Heftler NS, Austin LM, Gottlieb AB, Successful ultraviolet B treatment of psoriasis is accompanied by a reversal of keratinocyte pathology and by selective depletion of intraepidermal T cells, J. Exp. Med 182 (1995) 2057–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Gottlieb AB, Chamian F, Masud S, Cardinale I, Abello MV, Lowes MA, Chen F, Magliocco M, Krueger JG, TNF inhibition rapidly down-regulates multiple proinflammatory pathways in psoriasis plaques, J. Immunol 175 (2005) 2721–2729. [DOI] [PubMed] [Google Scholar]
- [24].Zaba LC, Cardinale I, Gilleaudeau P, Sullivan-Whalen M, Suárez-Fariñas M, Suárez Fariñas M, Fuentes-Duculan J, Novitskaya I, Khatcherian A, Bluth MJ, Lowes MA, Krueger JG, Amelioration of epidermal hyperplasia by TNF inhibition is associated with reduced Th17 responses, J. Exp. Med 204 (2007) 3183–3194. 10.1084/jem.20071094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].McGeachy MJ, Chen Y, Tato CM, Laurence A, Joyce-Shaikh B, Blumenschein WM, McClanahan TK, O’Shea JJ, Cua DJ, The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo, Nat. Immunol 10 (2009) 314–324. 10.1038/ni.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Schling P, Rudolph C, Heimerl S, Fruth S, Schmitz G, Expression of tumor necrosis factor alpha and its receptors during cellular differentiation, Cytokine. 33 (2006) 239–245. 10.1016/j.cyto.2006.02.007. [DOI] [PubMed] [Google Scholar]
- [27].Ge S, Hertel B, Susnik N, Rong S, Dittrich AM, Schmitt R, Haller H, von Vietinghoff S, Interleukin 17 receptor A modulates monocyte subsets and macrophage generation in vivo, PLoS ONE. 9 (2014) e85461 10.1371/journal.pone.0085461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Zakharova M, Ziegler HK, Paradoxical anti-inflammatory actions of TNF-alpha: inhibition of IL-12 and IL-23 via TNF receptor 1 in macrophages and dendritic cells, J. Immunol 175 (2005) 5024–5033. 10.4049/jimmunol.175.8.5024. [DOI] [PubMed] [Google Scholar]
- [29].Guilloteau K, Paris I, Pedretti N, Boniface K, Juchaux F, Huguier V, Guillet G, Bernard F-X, Lecron J-C, Morel F, Skin Inflammation Induced by the Synergistic Action of IL-17A, IL-22, Oncostatin M, IL-1{alpha}, and TNF-{alpha} Recapitulates Some Features of Psoriasis, J. Immunol 184 (2010) 5263–5270. 10.4049/jimmunol.0902464. [DOI] [PubMed] [Google Scholar]
- [30].Johnston A, Fritz Y, Dawes SM, Diaconu D, Al-Attar PM, Guzman AM, Chen CS, Fu W, Gudjonsson JE, McCormick TS, Ward NL, Keratinocyte overexpression of IL-17C promotes psoriasiform skin inflammation, J. Immunol 190 (2013) 2252–2262. 10.4049/jimmunol.1201505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Nanney LB, Stoscheck CM, Magid M, King LE, Altered [125I]epidermal growth factor binding and receptor distribution in psoriasis, J. Invest. Dermatol 86 (1986) 260–265. 10.1111/1523-1747.ep12285389. [DOI] [PubMed] [Google Scholar]
- [32].Elder JT, Fisher GJ, Lindquist PB, Bennett GL, Pittelkow MR, Coffey RJ, Ellingsworth L, Derynck R, Voorhees JJ, Overexpression of transforming growth factor alpha in psoriatic epidermis, Science. 243 (1989) 811–814. [DOI] [PubMed] [Google Scholar]
- [33].Cook PW, Pittelkow MR, Keeble WW, Graves-Deal R, Coffey RJ, Shipley GD, Amphiregulin messenger RNA is elevated in psoriatic epidermis and gastrointestinal carcinomas, Cancer Res. 52 (1992) 3224–3227. [PubMed] [Google Scholar]
- [34].Li B, Tsoi LC, Swindell WR, Gudjonsson JE, Tejasvi T, Johnston A, Ding J, Stuart PE, Xing X, Kochkodan JJ, Voorhees JJ, Kang HM, Nair RP, Abecasis GR, Elder JT, Transcriptome analysis of psoriasis in a large case-control sample: RNA-seq provides insights into disease mechanisms, J. Invest. Dermatol 134 (2014) 1828–1838. 10.1038/jid.2014.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Pastore S, Mascia F, Mariani V, Girolomoni G, The epidermal growth factor receptor system in skin repair and inflammation, J. Invest. Dermatol 128 (2008) 1365–1374. 10.1038/sj.jid.5701184. [DOI] [PubMed] [Google Scholar]
- [36].Cook PW, Piepkorn M, Clegg CH, Plowman GD, DeMay JM, Brown JR, Pittelkow MR, Transgenic expression of the human amphiregulin gene induces a psoriasis-like phenotype, J. Clin. Invest 100 (1997) 2286–2294. 10.1172/JCI119766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ben-Bassat H, Klein BY, Inhibitors of tyrosine kinases in the treatment of psoriasis, Curr. Pharm. Des 6 (2000) 933–942. [DOI] [PubMed] [Google Scholar]
- [38].Overbeck TR, Griesinger F, Two cases of psoriasis responding to erlotinib: time to revisiting inhibition of epidermal growth factor receptor in psoriasis therapy?, Dermatology (Basel). 225 (2012) 179–182. 10.1159/000342786. [DOI] [PubMed] [Google Scholar]
- [39].Wierzbicka E, Tourani JM, Guillet G, Improvement of psoriasis and cutaneous side-effects during tyrosine kinase inhibitor therapy for renal metastatic adenocarcinoma. A role for epidermal growth factor receptor (EGFR) inhibitors in psoriasis?, Br. J. Dermatol 155 (2006) 213–214. 10.1111/j.1365-2133.2006.07299.x. [DOI] [PubMed] [Google Scholar]
- [40].Selam M, Psoriasis aggravation due to lapatinib, BMJ Case Rep. 2013 (2013). 10.1136/bcr-2012-007592. [DOI] [PMC free article] [PubMed]
- [41].Lichtenberger BM, Gerber PA, Holcmann M, Buhren BA, Amberg N, Smolle V, Schrumpf H, Boelke E, Ansari P, Mackenzie C, Wollenberg A, Kislat A, Fischer JW, Röck K, Harder J, Schröder JM, Homey B, Sibilia M, Epidermal EGFR controls cutaneous host defense and prevents inflammation, Sci Transl Med. 5 (2013) 199ra111 10.1126/scitranslmed.3005886. [DOI] [PubMed] [Google Scholar]
- [42].Thatcher N, Nicolson M, Groves RW, Steele J, Eaby B, Dunlop J, McPhelim J, Nijjar R, Ukachukwu I, Erlotinib UK Skin Toxicity Management Consensus Group, Expert consensus on the management of erlotinib-associated cutaneous toxicity in the u.k, Oncologist. 14 (2009) 840–847. 10.1634/theoncologist.2009-0055. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


