Abstract
Objective:
Heavy alcohol consumption is associated with poorer cognitive function in older adults. Although understudied in middle-aged adults, the relationship between alcohol and cognition may also be influenced by genetics such as the apolipoprotein (ApoE) ε4 allele, a risk factor for Alzheimer’s disease. We examined the relationship between alcohol consumption, ApoE genotype, and cognition in middle-aged adults and hypothesized that light and/or moderate drinkers (≤2 drinks per day) would show better cognitive performance than heavy drinkers or non-drinkers. Additionally, we hypothesized that the association between alcohol use and cognitive function would differ by ApoE genotype (ε4+ vs. ε4−).
Method:
Participants were 1,266 men from the Vietnam Era Twin Study of Aging (VETSA; M age = 56; range 51-60) who completed a neuropsychological battery assessing seven cognitive abilities: general cognitive ability, episodic memory, processing speed, executive function, abstract reasoning, verbal fluency, and visuospatial ability. Alcohol consumption was categorized into five groups: never, former, light, moderate, and heavy.
Results:
In fully adjusted models, there was no significant main effect of alcohol consumption on cognitive functions. However, there was a significant interaction between alcohol consumption and ApoE ε4 status for general cognitive ability and episodic memory, such that the relationship of alcohol consumption and cognition was stronger in ε4 carriers. The ε4+ heavy drinking subgroup had the poorest general cognitive ability and episodic memory.
Conclusions:
Presence of the ε4 allele may increase vulnerability to the deleterious effects of heavy alcohol consumption. Beneficial effects of light or moderate alcohol consumption were not observed.
Keywords: Middle aged, male, aging, apolipoprotein E4 (APOE), alcohol drinking, cognitive abilities, risk factors
Excessive consumption of alcohol is among the top five risk factors for deteriorating health, functional disability, and premature death throughout the world (World Health Organization, 2014). Alcohol misuse is also a well-established risk factor for developing cognitive impairment and dementia during aging (Kuerbis, Moore, Sacco, & Zanjani, 2017). However, the impact of alcohol on specific domains of cognition are not entirely clear, especially for those who do not drink heavily (Hassing, 2018; Kuerbis et al., 2017; Panza et al., 2012). The apolipoprotein E (ApoE) ε4 allele is the strongest genetic risk factor for Alzheimer’s disease (Riedel, Thompson, & Brinton, 2016) and ApoE genotype may moderate potential effects of alcohol consumption on cognition (Anttila et al., 2004; Downer, Zanjani, & Fardo, 2014; Dufouil et al., 2000).
Heavy consumption of alcohol is associated with lower cognitive ability, cognitive decline or impairment, and increased risk for dementia (Neafsey & Collins, 2011; Sabia et al., 2014). Sabia et al. (2014) found that men who drank heavily (defined as >36 g/day; equivalent to >2 or 3 drinks/day) experienced a more rapid decline over 10 years in global cognition, memory, and executive function than those with light to moderate consumption (defined as <20 g/day). Although most researchers have found negative effects of heavy alcohol consumption, Richard et al. (2017) reported that moderate and heavy consumption (up to 3 drinks/day for women and for men 65 years and older, up to 4 drinks/day for men under 65 years) was associated with a 2-fold higher likelihood of living to age 85 without impairment on the Mini Mental Status Examination than the non-drinking group. In a rare study of alcohol consumption and change in cognitive abilities across age ranges, Zanjani, Downer, Kruger, Willis, and Schaie (2013) examined three age groups (middle-aged: 45-64, young-old: 65-75, and old-old: 75+), three alcohol consumption groups (abstinent, moderate (up to seven drinks/week), at-risk (more than seven drinks/week) and six cognitive abilities (memory, reasoning, spatial, verbal number, and speed). They observed relative stability of verbal and spatial ability for at-risk drinkers; however, for men, perceptual speed declined over time with increasing alcohol consumption. Although heavy or excessive consumption has been associated with worse cognitive outcomes, many studies have reported an inverted J- or U-shaped dose-response type relationship between alcohol consumption in cognitive functioning, in which light or moderate consumption was associated with better cognition than non-drinking or heavy drinking (for reviews, see Kim et al., 2012; Neafsey & Collins, 2011; Panza et al., 2012). However, some recent studies observed no associations (Kalapatapu, Ventura, & Barnes, 2017; Kumari et al., 2014; Topiwala et al., 2017). Thus, whether consumption of lower amounts of alcohol may be beneficial remains controversial and requires further investigation.
One key source of the conflicting findings may be that many studies do not consider the moderating role of genetic risk factors for cognitive impairment, such as ApoE genotype. The effects of ApoE genotype on cognitive aging are documented (Riedel et al., 2016); however, interactions between alcohol consumption and the ApoE genotype on cognition have been examined far less, especially in middle-aged adults and across multiple types of cognitive measures. This relationship may be important because both ApoE and alcohol are related to cerebrovascular health and neurodegeneration (Jack et al., 2015; Riedel et al., 2016). The ApoE gene is of great significance for the transportation of cholesterol and is abundantly concentrated in the liver and brain (Jack et al., 2015; Riedel et al., 2016). The ε4 allele of ApoE is associated with increased risk of Alzheimer’s disease (Riedel et al., 2016). Alcohol consumption in ε4+ men has been found to be positively associated with low-density lipoprotein cholesterol concentration (Corella et al., 2001). In addition, ε4 carriers may be more vulnerable to the toxic effects of alcohol consumption on the brain, thereby indirectly influencing cognition (Kim et al., 2012). Although ApoE genotype has been found to moderate the relationship between alcohol consumption and cognition in some studies (Anttila et al., 2004; Downer et al., 2014; Dufouil et al., 2000; Panza et al., 2012; Reas et al., 2019), a large, systematic review concluded that evidence for ApoE moderation of the interaction between alcohol consumption and cognition was still unclear (Neafsey & Collins, 2011).
Age may also be an important consideration in assessing ApoE moderation of the alcohol-cognition interaction. In a retrospective study, Downer, Zanjani and Fardo (2014) examined the relationship between alcohol consumption, ApoE genotype, and cognition from midlife to late life (M ages 44 and 77; N = 610) using data from the Framingham Heart Study. They observed a moderating effect of ApoE genotype for the association of alcohol consumption with late life on a composite of learning and memory. When compared to abstention, moderate alcohol consumption (defined as 1-2 drinks/day) in late life was associated with higher composite scores among those who were ε4− (β = 0.03) and lower scores among those who were ε4+ (β = −0.04). In contrast, there were no significant effects of alcohol consumption on midlife learning and memory (Downer et al., 2014). In addition, a cross-sectional study of 818 older adults (M age = 81.56, SD = 7.17), showed independent effects of moderate alcohol consumption (defined as up to 2 drinks/day) and ApoE genotype for three out of four cognitive domains (executive function, memory, and visuospatial), but found no interaction between ε4 status and alcohol consumption on cognitive performance (Herring & Paulson, 2018). In contrast to the null interaction effect observed by Herring and Paulson (2018), Reas et al. (2019) observed significant interactive effects of ApoE genotype on verbal episodic memory decline based on the total recall score on the Buschke Selective Reminding test (Buschke & Fuld, 1974). Their results indicated greater decline in memory for ε4+ who reported no alcohol consumption than among ε4+ participants who consumed alcohol.
These conflicting findings may be due to the method of cognitive assessment. Studies are limited by use of a single assessment of general cognitive function, usually the Mini Mental State Examination (MMSE), or by use of only a single test within a cognitive domain (Carmelli, Swan, Reed, Schellenberg, & Christian, 1999; Dufouil et al., 2000; Elwood et al., 1999; Lindeman, Wayne, Baumgartner, & Garry, 2005; Ngandu et al., 2007; Park, Park, Jun, Choi, & Suh, 2013). Neafsey and Collins (2011) found systematic differences in outcomes between studies that used standard neuropsychological tests and those that used assessment of mental status. Studies that used standard neuropsychological tests were more likely to find no significant associations between alcohol intake and cognitive function, whereas those that used mental status tests were more likely to find positive associations (Neafsey & Collins, 2011). However, it is important to note that the latter studies more often involved older study samples than the former. Generally, the MMSE is a less sensitive method for determining cognitive impairment with several weaknesses, including poor assessment of executive function and floor and/or ceiling effects due to its limited testing of cognition (Neafsey & Collins, 2011). In addition, more comprehensive measures may provide more robust and reliable assessments of cognition (Skinner et al., 2012).
Two other issues to consider are the abstainer bias and socioeconomic status bias. The abstainer bias occurs because many people who do not drink in late adulthood are previous alcohol consumers who have quit drinking for various reasons (e.g., health problems, medications, abuse) (Fillmore, Kerr, Stockwell, Chikritzhs, & Bostrom, 2006; Hassing, 2018). Including a “non-drinking” group, without differentiating abstainers from former drinkers could result in lower cognitive scores for the “non-drinking group” that may be due in part to characteristics (i.e., health deficits) of the former drinkers (Hassing, 2018). Analyses of the relationship between alcohol consumption and cognition also may be confounded by socioeconomic factors, as alcohol consumption may be associated with socioeconomic status (SES) (Kim et al., 2012; Sabia et al., 2014). People with lower SES are more susceptible to being stigmatized as having the highest rates of alcohol use because they tend to experience greater burden of the negative effects of alcohol (Bloomfield, Grittner, Kramer, & Gmel, 2006; Collins, 2016). However, studies have shown that higher SES is related to higher rates of alcohol consumption and fewer negative alcohol-related outcomes, such as alcohol-related morality (for review see Collins, 2016). This may be due to several factors, such as being able to afford to consume alcohol and engage in alcohol-related social events, higher initial cognitive ability, better health earlier in life, and more resources for health care that mitigate problematic aspects of alcohol consumption (Collins, 2016).
The present study takes a unique look at relationships between alcohol consumption, ApoE genotype and cognition. We examined these associations in a large, well-characterized middle-aged male sample that makes use of data on a wide variety of cognitive abilities, allows separation of the former and never drinkers, uses multiple tests to assess several different cognitive domains instead of single tests within a domain, and tests whether ApoE genotype moderates associations between alcohol consumption and cognitive function. In addition, general cognitive ability was measured by the same method at two time points, age 20 and age 56, so the current study is able to control for preexisting/early cognitive ability.
The study had two main goals. First, we predicted that light and moderate drinking groups would show significantly better cognitive function than the never, former, and heavy drinking groups, especially for general cognitive ability, processing speed, executive function, and episodic memory (Downer et al., 2014; Herring & Paulson, 2018; Ngandu et al., 2007; Sabia et al., 2014). We also investigated whether this may be true for other less studied domains, such as abstract reasoning, verbal fluency, and visuospatial ability. Second, we hypothesized that presence of at least one ApoE ε4 allele would moderate the association between alcohol consumption and cognition. For cognitive abilities with a significant alcohol by ApoE interaction effect, we examined comparisons within the ApoE subgroups. We expected that negative associations of heavy drinking with cognitive function would be stronger among ε4 carriers than non-carriers.
Method
Participants
Participants consisted of 1291 individuals who participated in the baseline assessment of a longitudinal study of risk and protective factors of cognitive and brain aging: the Vietnam Era Twin Study of Aging (VETSA) (Kremen, Franz, & Lyons, 2013; Kremen et al., 2006). VETSA participants were randomly recruited from the twin pairs who participated in the Harvard Drug Study (HDS; Tsuang et al., 1996), which had recruited all available twins from the Vietnam Era Twin Registry (VETR). The VETR is a sample of community-dwelling male twins who served in the United States military at some time during the Vietnam era between 1965–1975 (Goldberg, Curran, Vitek, Henderson, & Boyko, 2002). VETSA participants were not selected on the basis of substance use or any other diagnoses. To be eligible to participate in VETSA, both twins had to agree to participate and be between the ages of 51 and 59 (M = 55.89 years; SD = 2.44) at enrollment. Approximately 80% of the sample did not experience combat exposure during their time in the military. Although the VETSA sample is predominantly White non-Hispanic (88%), they are a reasonably representative sample with regard to demographic and health characteristics for American men in this age range (Schoenborn & Heyman, 2009).
VETSA participants traveled to one of two testing sites for assessment, Boston University (BU) or the University of California, San Diego (UCSD), or in rare circumstances, staff traveled to participant’s place of residence. Written informed consent was obtained from all participants. The research protocol was approved by the UCSD Human Research Protection Program and the BU Charles River Campus Institutional Review Board.
Measures
Alcohol Consumption.
Alcohol consumption groups were operationalized based on recommendations for alcohol consumption for men (U.S. Department of Health and Human Services and U.S. Department of Agriculture, 2015), see Table 1. At the VETSA visit, participants were asked if they had consumed more than 20 drinks in their lifetime. Those who responded yes were asked how many days in the past 2 weeks they consumed beer, and how many beers they had on days in which they drank beer. These questions were repeated for wine and hard liquor. We summed across beverage types to yield the number of alcoholic beverages consumed in the past two weeks. We were able to separate former drinkers from those who never drank by using information on alcohol use collected previously in the HDS (Tsuang et al., 1996) when participants were 44 years old, on average. Participants were categorized in the never drinking group (n=118; 9.14%) if they: reported during HDS that there was no period in which they consumed at least one drink a month for 6 or more months; reported consuming less than 20 drinks in their lifetime; and reported no alcohol consumption within the two weeks prior to the VETSA visit. Those who did not consume alcoholic beverages in the two weeks prior to VETSA testing, yet consumed more than 20 drinks in their lifetime, were categorized in the former drinking group (n=324; 25.10%). Individuals who reported consuming 1-14 drinks in the past 2 weeks (up to 1 drink/day) were categorized in the light drinking group (n=531; 41.13%). Individuals who reported consuming 15-28 drinks in the past 2 weeks (more than 1 drink/day and up to 2 drinks/day) were categorized in the moderate drinking group (n=130; 10.07%). Individuals who reported consuming more than 28 drinks in the past 2 weeks (more than 2 drinks/day) were categorized in the heavy drinking group (n=188; 14.56%).
Table 1.
Criteria for Alcohol Consumption Groups
| Alcohol Group | HDS: “Consumed at least one drink a month for 6 or more months?” | VETSA: “Consume more than 20 drinks in lifetime?” | VETSA: “Total number of drinks in past 2 weeks.” |
|---|---|---|---|
| Never | No | No | 0 |
| Former | Yes | Yes | 0 |
| Light | Yes | 1-14 | |
| Moderate | Yes | >14-28 | |
| Heavy | Yes | >28 |
Note: HDS = Harvard Drug Study; VETSA = Vietnam Era Twin Study of Aging.
Apolipoprotein E (ApoE) Genotype.
ApoE genotype was determined as previously described (Lyons et al., 2013; Schultz et al., 2008). For the present study, participants with at least one ε4 allele present were classified as being ε4 positive (ε4+) and all other participants were classified as ε4 negative (ε4−). There were 1266 (98.06%) out of 1291 participants with ApoE genotype data. Of the 1266, 380 (30%) were ε4+ and 886 (70%) were ε4−.
Cognitive Measures.
General Cognitive Ability (GCA) was assessed with the Armed Forces Qualification Test (AFQT). The AFQT is a 50-min paper-and-pencil test and has 100 multiple-choice items (Uhlaner & Bolanovich, 1952). The same measure of GCA was administered at military induction at average age 20 (referred to as age 20 GCA) and VETSA at average age 56 (referred to as age 56 GCA). The AFQT is a valid and reliable measure of general cognition as it is correlated highly (r = 0.84) with Wechsler IQ and the AFQT scores of VETSA participants were highly correlated across 35 years (r = 0.74) (Lyons et al., 2009).
The VETSA neurocognitive battery included 13 tests that assessed 6 specific cognitive domains: processing speed, episodic memory, abstract reasoning, verbal fluency, visuospatial ability, and executive function. When multiple tests were used, individual test scores were standardized (z-scored) and averaged to create the domain scores; executive function, however, was based on a factor score (Gustavson et al., 2018). All scores were reverse coded where appropriate such that high scores represent better performance. These cognitive measures have been previously described in detail (Franz et al., 2011; Gustavson et al., 2018).
The processing speed domain was a composite of the number correctly completed on the word and color conditions of the Stroop (Golden & Freshwater, 2002; Stroop, 1935) and time to complete the number sequencing and letter sequencing conditions of the Delis-Kaplan Executive Function System (D-KEFS) Trail Making Test (Delis, Kaplan, & Kramer, 2001). Episodic memory domain comprised the short-delay recall, long-delay recall, and total of trials 1-5 of the California Verbal Learning Test (CVLT; Delis, Kramer, Kaplan, & Ober, 2000), the immediate and delayed recall of the Logical Memory and Visual Reproductions subtests of the WMS-III (Wechsler, 1997). Abstract reasoning was measured using the Matrix Reasoning subtest of the Wechsler Abbreviated Scale of Intelligence (WASI; Wechsler, 1999). Verbal fluency domain was measured by the total correct words generated on the phonemic (F, A, S) and semantic (animals, boy’s names) trials of the D-KEFS (Delis et al., 2001). Visuospatial ability domain included Thurstone’s adaptation of the Gottschaldt Hidden Figures Test (Thurstone, 1944), and the Card (Mental) Rotation Test (Ekstrom, Dermen, & Harman, 1976).
Executive function domain was based on a factor score derived from a latent variable with seven indicators (Gustavson et al., 2018) that measured inhibition, set-switching, and working memory. Inhibition measures included the residualized Stroop color-word interference, adjusted for performance on word and color conditions (Golden & Freshwater, 2002; Stroop, 1935) and the AX-Continuous Performance Test (AX-CPT; Servan-Schreiber, Cohen, & Steingard, 1996). Switching measures included the residualized time on the switching condition of the D-KEFS Trail Making, adjusted for time on number sequencing and letter sequencing conditions and the residualized D-KEFS category switching, adjusted for category fluency (Delis et al., 2001). Working memory measures included the Reading Span Test (Daneman & Carpenter, 1980) and the Digit Span and Letter-Number Sequencing subtests of the Wechsler Memory Scale (WMS-III; Wechsler, 1997).
Covariates.
Covariates included in the analyses were identified based on literature review and included age, race/ethnicity, education, age 20 GCA, current smoking status, objective and subjective health, depressive symptoms, income, and current work status. Race/ethnicity was categorized as non-Hispanic white or other. Education was defined as the number of years of education completed. Age 20 GCA was measured with the AFQT. Smoking was categorized as current smoker vs. non-smoker. Objective health counted the presence of 15 major chronic conditions from the Charlson Comorbidity index: diabetes, emphysema, asthma, cancer, osteoarthritis, rheumatoid arthritis, stroke, heart attack, heart failure, heart surgery, angina, hypertension, peripheral vascular disease, cirrhosis, and AIDS (Charlson, Pompei, Ales, & MacKenzie, 1987). Subjective health was self-reported using a scale from 1 (excellent) to 5 (poor). Depressive symptoms were assessed with the Center for Epidemiologic Studies Depression Scale (CESD; Radloff, 1977). Personal income was assessed on a scale ranging from 1 (<$10,000 per year) to 13 (>= $120,000 per year) in increments of $10,000 and current work status was categorized as working full-time or not.
Statistical Analysis
Using SAS software, Version 9.4 (SAS Institute, Cary, NC), comparisons of categorical demographic variables across alcohol consumption groups were conducted using the Chi-Square Test and mixed models (Proc Mixed) were used for continuous demographic variables. Drinking groups were treated as nominal variables. Although never, light, moderate, and heavy alcohol use could be treated a continuous distribution, the inclusion of former drinkers makes it difficult to classify these individuals who may be qualitatively dissimilar to the other groups. To examine the relationship between cognition, alcohol consumption, ApoE genotype, and the alcohol consumption by ApoE genotype interaction, generalized linear mixed models (Proc Mixed) were conducted. Separate models were run for each cognitive domain with alcohol consumption (0 = never, 1 = former, 2 = light, 3 = moderate, and 4 = heavy) and ApoE status (0 = ε4−, 1 = ε4+) as main effects, as well as their interaction, and controlled for family (clustering of twins within pairs) as a random effect. Fully adjusted models also included all covariates: age, race/ethnicity, education, age 20 GCA, current smoking, objective health, subjective health, depressive symptoms, income, and working full-time.
The final analytic sample for the fully adjusted model analyses ranged from 1,220 to 1,227 due to missing data for some variables. Results are presented as estimates of Type III fixed effects. Results are reported as two-tailed and significance was set at p < 0.05. Specific predictions were made for four of the seven cognitive outcomes (general cognitive ability, processing speed, episodic memory, and executive function) and the remaining were exploratory. Uncorrected p-values for tests of the a priori hypotheses for the specific group comparisons for the main effects of alcohol consumption are provided, but results are interpreted in terms of Bonferroni adjusted alpha levels of .008 (.05/6). Similarly, uncorrected p-values for tests of the a priori hypotheses for the specific comparisons for the interactions are presented, but findings are interpreted in terms of Bonferroni adjusted alpha levels of .0025 (.05/20).
Results
Descriptive Statistics and Preliminary Analyses
Comparisons of demographic and lifestyle characteristics by alcohol consumption group are presented in Table 2. There were significant between group differences in age 20 GCA, number of years of education, annual income, and proportion of individuals working full-time. The light drinking group had higher age 20 GCA than the former and heavy drinking groups. The former and heavy drinking groups completed fewer years of education than the light and never drinking group. The heavy drinking group had lower annual income than the light and moderate drinking groups, and the former drinking group had less annual income than all other groups. Those in the former drinking group were less likely to be working full-time compared to all other groups.
Table 2.
Sample Characteristics by Alcohol Consumption Group
| Mean (SE) or N (%) | N | All | Never (n = 118) | Former (n = 342) | Light (n = 531) | Moderate (n = 130) | Heavy (n = 188) | Overall Test |
|---|---|---|---|---|---|---|---|---|
| ApoE ε4+ | 1266 | 380 (30.02%) | 43 (36.75%) | 88 (27.50%) | 146 (28.29%) | 37 (28.91%) | 66 (35.68%) | χ2 (4) = 7.12, p = 0.130 |
| Age (years) | 1291 | 55.93 (0.10) | 55.92 (0.10) | 55.89 (0.10) | 55.87 (0. 10) | 55.90 (0. 10) |
F(4, 637) = 3.81,
p = 0.005 *N, F > L, M *H > M |
|
| Ethnicity (Non-Hispanic White) | 1291 | 1160 (89.85%) | 107 (90.68%) | 276 (85.19%) | 495 (93.22%) | 117 (90.00%) | 165 (87.77%) | χ2 (4) = 15.34, p = 0.004 |
| Education (years) | 1291 | 14.20 (0.19) | 13.62 (0.12) | 14.05 (0.09) | 13.83 (0.17) | 13.52 (0.15) |
F(4, 637) = 5.01,
p < 0.001 *N, L > F, H |
|
| Age 20 GCA | 1274 | 0.33 (0.06) | 0.27 (0.04) | 0.42 (0.03) | 0.37 (0.05) | 0.29 (0.05) |
F(4, 620) = 3.91,
p = 0.004 *L > F, H |
|
| Current smokers | 1289 | 306 (23.74%) | 11 (9.32%) | 73 (22.60%) | 126 (23.77%) | 29 (22.31%) | 67 (35.64%) | χ2 (4) = 28.63, p < 0.0001 |
| Objective health | 1291 | 1.02 (0.11) | 1.23 (0.07) | 1.02 (0.05) | 1.16 (0.10) | 1.06 (0.09) |
F(4, 637) = 1.91,
p = 0.11 *F > L |
|
| More than one chronic illness | 1291 | 375 (29.05%) | 33 (27.96%) | 120 (37.04%) | 139 (26.18%) | 37 (28.46%) | 46 (24.47%) | χ2 (4) = 14.16, p = 0.007 |
| Subjective health | 1283 | 2.35 (0.08) | 2.69 (0.05) | 2.44 (0.04) | 2.56 (0.08) | 2.58 (0.07) |
F(4, 629) = 5.42,
p < 0.001 *F, H > N *F > L |
|
| Report health as excellent or very good | 1283 | 624 (48.64%) | 74 (63.25%) | 133 (41.05%) | 277 (52.36%) | 59 (46.45%) | 81 (43.55%) | χ2 (4) = 22.57, p < 0.001 |
| Depressive symptoms | 1284 | 7.13 (0.77) | 10.01 (0.47) | 7.68 (0.37) | 8.88 (0.70) | 8.18 (0.61) |
F(4, 630) = 5.24,
p < 0.001 *F > N, L, H |
|
| Annual self-income | 1281 | 5.96 (0.28) | 5.26 (0.17) | 6.38 (0.13) | 6.61 (0.25) | 5.88 (0.22) |
F(4, 627) = 8.88,
p < 0.001 *N, L, M, H > F *L, M > H |
|
| Working full-time | 1279 | 994 (77.72%) | 95 (80.51%) | 224 (70.00%) | 421 (79.89%) | 102 (80.31%) | 152 (81.28%) | χ2 (4) = 14.84, p = 0.005 |
| Currently married | 1284 | 1013 (78.71%) | 99 (83.90%) | 245 (75.85%) | 430 (80.98%) | 102 (79.69%) | 137 (73.26%) | χ2 (4) = 8.49, p = 0.075 |
| Hypertension | 1291 | 512 (39.66%) | 48 (40.68%) | 127 (39.20%) | 195 (36.72%) | 58 (44.62%) | 84 (44.68%) | χ2 (4) = 5.31, p = 0.257 |
| Diabetes | 1290 | 149 (11.56%) | 13 (11.02%) | 56 (17.34%) | 58 (10.94%) | 11 (8.46%) | 11 (5.85%) | χ2 (4) = 17.99, p = 0.001 |
Note: F-tests also include significant t-test comparisons. Significant p values are highlighted in bold (p < .05). SE = Standard Error; Age 20 general cognitive ability (GCA) was based on the Armed Forces Qualifications Test and standardized to military norms; Ethnicity = % of White/ Non-Hispanic; Objective health = Number of chronic health conditions (diabetes, emphysema, asthma, cancer, osteoarthritis, rheumatoid arthritis, stroke, heart attack, heart failure, heart surgery, angina, hypertension, peripheral vascular disease, cirrhosis, AIDS); Subjective health = self-reported health on a scale from 1 (excellent) to 5 (poor). Depressive symptoms are based on the Center for Epidemiologic Studies Depression Scale (CESD); Annual self-income = 1 (< $10,000/year) to 13 (>=$120,000/year) in increments of $10,000.
= Significant specific t-test comparisons: i.e., L > F means that the light drinking group (L) had significantly higher scores than the former drinking group (F) (p < 0.05); N = never drinking group, M = moderate drinking group, and H = heavy drinking group.
There were also significant group differences in current smoking, subjective health rating, proportion of rating health as “excellent” or “very good”, self-reported depressive symptoms, and diabetes. Those in the heavy drinking group were significantly more likely to smoke tobacco than all other groups, while those in the never drinking group were significantly less likely to smoke compared with all other groups. The never and light drinking groups rated their health higher than the former drinking group, and the never drinking group also rated their health higher than the heavy drinking group. Furthermore, the never and light drinking groups were significantly more likely to rate their health as “excellent” or “very good” than the former, moderate, and heavy drinking groups. The former drinking group had significantly more depressive symptoms than the never, light, and heavy drinking group. The former drinking group was significantly more likely to report a diabetes diagnosis than the light drinking group. The proportion of ApoE ε4 carriers, objective health, the proportion of individuals with hypertension, and the proportion of individuals currently married did not differ by alcohol consumption group. In the simple models with just alcohol consumption, ApoE genotype, and the alcohol consumption by ApoE genotype interaction controlling for family, there was a significant overall F at the Bonferroni-adjusted p value of p=.007 (.05/7) for three out of seven cognitive measures (GCA, processing speed, abstract reasoning). There also appears to be a trend for positive outcomes for those in the light and moderate drinking group for all cognitive measures, excluding visuospatial ability for the moderate consumption group. See Supplemental Table 1 for the simple model results of the individual cognitive variables by alcohol consumption group.
Main Effects of Alcohol Consumption on Cognition
In fully adjusted models with alcohol consumption, ApoE genotype, and the alcohol consumption by ApoE genotype interaction controlling for family, age, ethnicity, education, age 20 GCA, current smoking status, objective health, subjective health, depressive symptoms, income, and working full-time there were no significant main effects of alcohol consumption on cognition. In examination of the predicted comparisons between light or moderate consumption with never, former, and heavy consumption, there were instances of significant differences for specific comparisons between groups for age 56 GCA and processing speed, but none survived Bonferroni corrections (Table 3). For instance, the light drinking group had significantly higher GCA than the heavy drinking group, [t(567) = 2.02, p = 0.044]. The light drinking group also had significantly faster processing speed than the never drinking group [t(565) = −2.06, p = 0.040] and the former drinking group [t(565) = −2.62, p = 0.009].
Table 3.
Type III Tests of Fixed Effects for the Main Effect of Alcohol Consumption on Cognition at Age 56
| Cognitive Domain Estimate (SE) | N | Never (n=118) | Former (n=324) | Light (n=531) | Moderate (n=130) | Heavy (n=188) | F-test |
|---|---|---|---|---|---|---|---|
| Age 56 GCA | 1225 | 0.34 (0.05) | 0.33 (0.04) | 0.31 (0.03) | 0.29 (0.05) | 0.23 (0.04) | F(4, 567) = 1.55, p = 0.186 |
| Processing Speed | 1221 | −0.23 (0.08) | −0.21 (0.06) | −0.07 (0.05) | −0.13 (0.07) | −0.16 (0.06) | F(4, 565) = 2.20, p = 0.068 |
| Executive Function | 1227 | −0.01 (0.03) | −0.02 (0.02) | −0.03 (0.02) | 0.01 (0.03) | −0.05 (0.02) | F(4, 569) = 1.38, p = 0.240 |
| Episodic Memory | 1226 | 0.04 (0.07) | −0.02 (0.05) | −0.05 (0.05) | 0.01 (0.07) | −0.07 (0.06) | F(4, 568) = 0.75, p = 0.561 |
| Abstract Reasoning | 1220 | −0.13 (0.10) | −0.07 (0.07) | −0.01 (0.06) | −0.05 (0.09) | −0.19 (0.08) | F(4, 564) = 1.55, p = 0.187 |
| Verbal Fluency | 1220 | −0.14 (0.09) | −0.17 (0.07) | −0.07 (0.06) | 0.09 (0.09) | −0.06 (0.08) | F(4, 564) = 2.06, p = 0.085 |
| Visuospatial Ability | 1225 | −0.13 (0.09) | −0.16 (0.06) | −0.12 (0.05) | −0.21 (0.08) | −0.25 (0.07) | F(4, 567) = 1.44, p = 0.218 |
Note: Models are run separately for each cognitive domain and presented p-values are not Bonferroni-corrected (p < .05). After Bonferroni corrections, no specific comparisons between light or moderate and other groups were significant. All estimates and standard errors presented are from the full model analyses with alcohol consumption, ApoE genotype, and the alcohol consumption by ApoE genotype interaction controlling for family, age, ethnicity, education, age 20 general cognitive ability (GCA), current smoking status, objective health, subjective health, depressive symptoms, income, and working full-time. Cognitive domain scores were z-score composites, except for age 56 GCA which was standardized based on military norms.
In follow-up hierarchical analyses we entered the covariates in stepwise fashion, first with demographics (race/ethnicity), second step with education and age 20 GCA, third step with health measures, and a final step with income and work status (final results shown in Table 4). With the exception of processing speed, the main effect of alcohol consumption on cognition became non-significant at the second step when adjustments for education and GCA occurred. The main effect of alcohol consumption on processing speed became non-significant at the final step with the adjustments for income and work status.
Table 4.
Type III Tests of Fixed Effects for the Main Effects, Interaction, and Covariates on Cognition at Age 56
| Main Effects, Interaction, and Covariates | Age 56 GCA | Processing Speed | Executive Function | Episodic Memory | Abstract Reasoning | Verbal Fluency | Visuospatial Ability |
|---|---|---|---|---|---|---|---|
| (F, p) | (F, p) | (F, p) | (F, p) | (F, p) | (F, p) | (F, p) | |
| Independent Variables | |||||||
| Alcohol Consumption | 1.55 (0.19) | 2.20 (0.07) | 1.38 (0.24) | 0.75 (0.56) | 1.55 (0.19) | 2.06 (0.08) | 1.44 (0.22) |
| ApoE | 0.16 (0.69) | 1.77 (0.18) | 4.04 (0.045) | 0.74 (0.39) | 0.84 (0.36) | 0.78 (0.38) | 0.00 (0.99) |
| Alcohol x ApoE | 2.68 (0.031) | 0.35 (0.85) | 1.57 (0.18) | 2.85 (0.023) | 0.44 (0.78) | 1.57 (0.18) | 2.18 (0.07) |
| Covariates | |||||||
| Age | 21.10 (<.0001) | 30.34 (<.0001) | 20.86 (<.0001) | 27.30 (<.0001) | 20.30 (<.0001) | 22.52 (<.0001) | 14.42 (<.001) |
| Ethnicity | 39.88 (<.0001) | 7.65 (0.006) | 19.28 (<.0001) | 0.88 (0.35) | 5.08 (0.025) | 2.09 (0.15) | 15.54 (<.0001) |
| Education | 1.73 (0.19) | 22.03 (<.0001) | 22.08 (<.0001) | 23.69 (<.0001) | 22.88 (<.0001) | 24.34 (<.0001) | 4.16 (0.042) |
| Age 20 GCA | 911.96 (<.0001) | 37.41 (<.0001) | 140.19 (<.0001) | 116.08 (<.0001) | 203.09 (<.0001) | 35.25 (<.0001) | 172.51 (<.0001) |
| Current smoking | 0.10 (0.76) | 5.76 (0.017) | 0.83 (0.36) | 0.00 (0.96) | 1.04 (0.31) | 4.19 (0.041) | 0.83 (0.36) |
| Objective health | 0.53 (0.47) | 1.00 (0.32) | 2.32 (0.13) | 0.66 (0.42) | 0.99 (0.32) | 0.59 (0.44) | 0.48 (0.49) |
| Subjective health | 0.46 (0.50) | 4.55 (0.033) | 2.15 (0.14) | 1.42 (0.23) | 3.73 (0.05) | 1.21 (0.27) | 3.60 (0.06) |
| Depressive symptoms | 6.61 (0.01) | 2.22 (0.14) | 9.81 (0.002) | 0.06 (0.81) | 1.22 (0.27) | 0.56 (0.46) | 0.00 (0.99) |
| Income | 2.16 (0.14) | 4.79 (0.029) | 0.91 (0.34) | 3.58 (0.06) | 9.07 (0.003) | 1.12 (0.29) | 1.48 (0.22) |
| Working full-time | 1.80 (0.18) | 1.67 (0.20) | 0.01 (0.91) | 0.95 (0.33) | 0.09 (0.76) | 0.90 (0.34) | 2.67 (0.10) |
Note: Models are run separately for each cognitive domain (i.e., each column represents a separate regression model). Significant p values are highlighted in bold and are not Bonferroni-corrected (p < .05). GCA= General Cognitive Ability; Age 20 and Age 56 GCA were based on the Armed Forces Qualifications Test and standardized to military norms; Ethnicity = % of White/ Non-Hispanic; Objective health = Number of chronic health conditions (diabetes, emphysema, asthma, cancer, osteoarthritis, rheumatoid arthritis, stroke, heart attack, heart failure, heart surgery, angina, hypertension, peripheral vascular disease, cirrhosis, AIDS); Depressive symptoms = the Center for Epidemiologic Studies Depression Scale (CESD). Subjective health = self-reported health on a scale from 1 (excellent) to 5 (poor).
Main Effects of ApoE Genotype and Alcohol Consumption by ApoE Genotype Interactions on Cognition
The main effect of ApoE genotype on executive functioning (p = 0.045) was not significant after Bonferroni correction (Table 4). There were no significant main effects of ApoE genotype for any of the other cognitive measures. Alcohol consumption by ApoE genotype interactions were observed for age 56 GCA [F(4, 567) = 2.68, p = 0.031, eta-squared = 0.004], and episodic memory [F(4, 568) = 2.85, p = 0.023, eta-squared = 0.010] (Table 4).
Age 56 GCA did not significantly differ among any of the ε4− subgroups but did significantly differ among the ε4+ subgroups (Figure 1). As predicted, the ε4+ heavy drinking subgroup (M = 0.17, SE = 0.06) had the lowest GCA, significantly lower than the ε4+ never drinking subgroup [M = 0.44, SE = 0.07; t(567) = 3.07, p = 0.002], which had the highest age 56 GCA and remained significant after Bonferroni corrections. The ε4+ heavy drinking subgroup also had lower GCA than the ε4+ former drinking subgroup [M = 0.35, SE = 0.05; t(567) = 2.52, p = 0.012]; however, this was not significant with Bonferroni correction.
Figure 1. Interaction of Alcohol Consumption and ApoE Genotype on age 56 General Cognitive Ability.
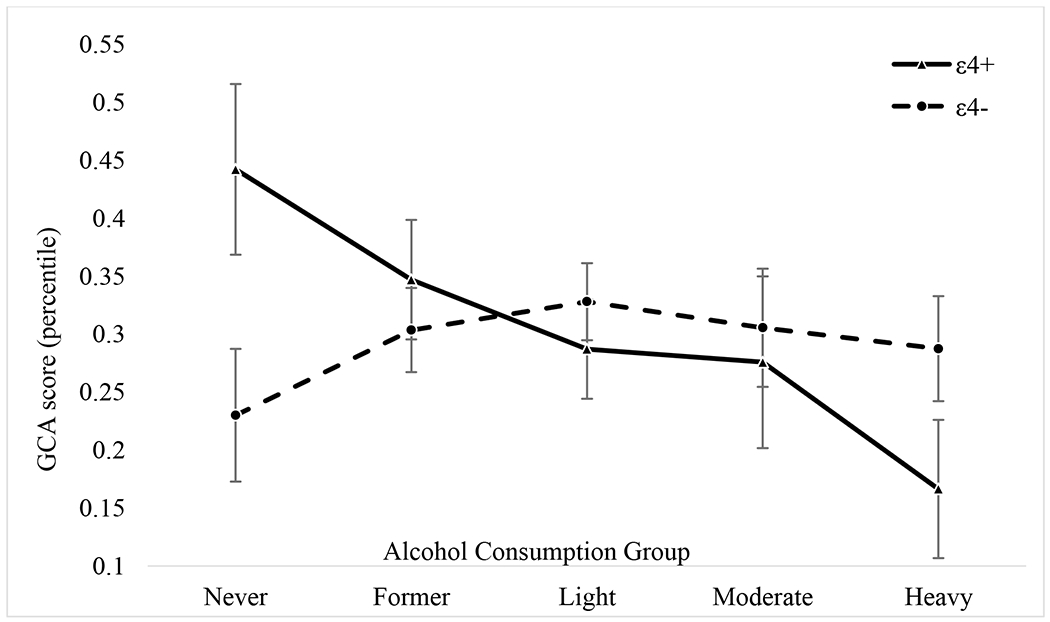
All means and standard errors presented are from the full model analyses controlling for family, age, ethnicity, education, age 20 general cognitive ability (GCA), current smoking status, objective health, subjective health, depressive symptoms, income, and working full-time. GCA is based on the Armed Forces Qualifications Test and percentiles were standardized based on military norms. Solid line is ε4+ and the dashed line is ε4−.
Comparable to the interaction pattern for GCA, episodic memory (Figure 2) did not significantly differ among any of the ε4− subgroups. Among the ε4+s, the heavy drinking subgroup (M = −0.13, SE = 0.09) had the lowest episodic memory and never drinking group had the highest episodic memory [M = 0.18, SE = 0.11; t(568) = 2.31, p = 0.021]. The ε4+ never drinking subgroup also had significantly higher episodic memory than the ε4+ light drinking subgroup [M = −0.11, SE = 0.06; t(568) = 2.51, p = 0.012]. However, these comparisons were no longer significant with Bonferroni corrections. There were no significant interactions for the other cognitive abilities.
Figure 2. Interaction of Alcohol Consumption and ApoE Genotype on age 56 Episodic Memory.
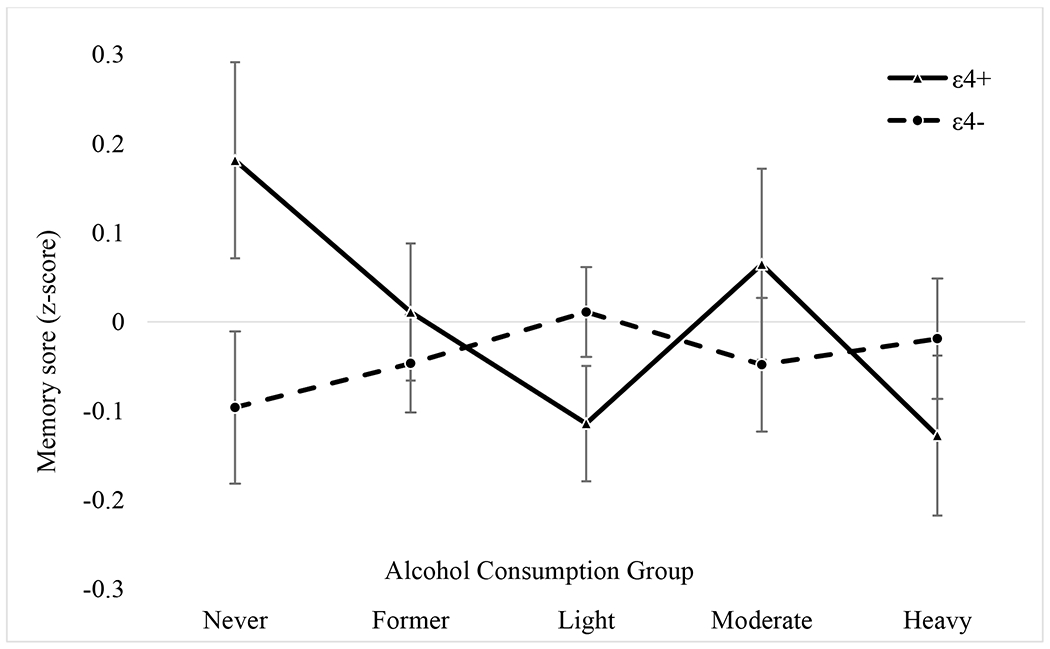
All means and standard errors presented are from the full model analyses controlling for family, age, ethnicity, education, age 20 general cognitive ability (GCA), current smoking status, objective health, subjective health, depressive symptoms, income, and working full-time. Solid line is ε4+ and the dashed line is ε4−.
Covariates
Type III effects for all measures are shown in Table 4. Age and age 20 GCA both contributed significantly to all cognitive measures at age 56. Education contributed significantly to all cognitive measures, except for GCA. Race/ethnicity was associated with all cognitive measures, except episodic memory and verbal fluency. Other covariates were less consistently associated with cognitive measures. Smoking, income, subjective health, and depression symptoms were variably related to cognition, but many associations would not survive multiple test correction. Additionally, objective health and working full-time were not significantly related to any cognitive measures.
Discussion
In this study of a national sample of middle-aged men, we observed no significant main effect of alcohol consumption on any of the cognitive measures when controlling for multiple testing. It may be that our ability to separate never drinkers from former drinkers and to adjust for early cognitive ability as well as many other covariates (i.e., SES, health) allowed us to differentiate between effects due to alcohol or by other factors (Collins, 2016). Although alcohol is a vascular risk factor (Dufouil et al., 2000), it may also be that the effects of alcohol are not yet apparent in this relatively young sample (mean age 56).
The ApoE ε4 allele is a recognized risk factor for dementia and cognitive performance, yet few studies have examined this risk in relation to alcohol consumption in midlife adults. We hypothesized that ApoE genotype would moderate the association between alcohol consumption and cognition. Significant alcohol consumption by ApoE polymorphism interactive effects were observed for both age 56 GCA and episodic memory. There were no differences in cognitive performance among the ε4− groups. However, the ε4+ nondrinking subgroup had better GCA and episodic memory than ε4+ heavy drinking subgroup. These results supported the prediction that those who were in the ε4+ heavy drinking subgroup would have the worst cognition, at least for GCA and episodic memory. Presence of the ε4 allele may confer added vulnerability to the deleterious effects of heavy alcohol consumption.
Some previous studies have found an interaction between ApoE genotype and alcohol consumption on cognition in older samples (Anttila et al., 2004; Downer et al., 2014; Dufouil et al., 2000). In one study with midlife adults, though, Downer, Zanjani, and Fardo (2014) observed no interaction between midlife alcohol consumption and ApoE genotype on learning and memory. However, that study had younger participants at baseline (35–59 years), a small sample size (only six ε 4+ abstainers), no separation of former drinkers from the never drinkers, did not control for early cognitive ability, and included both males and females (Downer et al., 2014).
The ApoE genotype by alcohol consumption group interaction for age 56 GCA and episodic memory showed that the ε4+ never drinking subgroup had the best performance and the ε4+ heavy drinking subgroup the poorest. It appears that, in midlife men, being at high genetic risk (ε4+) may exacerbate the effects of heavy drinking on cognition. More controversially, the results suggest that the ApoE ε4 allele could in some way be beneficial for overall cognitive functioning and episodic memory for middle-aged men who are lifetime abstainers. Although the ApoE ε4 allele has been shown to have favorable outcomes in certain situations (Oria et al., 2010; Oria, Patrick, Blackman, Lima, & Guerrant, 2007; Riedel et al., 2016; Wright et al., 2003), it is clearly harmful in others (Eichner et al., 2002; Lamar et al., 2019; Riedel et al., 2016). This result was not predicted and requires further investigation considering that ApoE ε4 allele is a risk factor for Alzheimer’s disease; it will be necessary to replicate these findings in other samples. Additionally, longitudinal studies could potentially shed more light on these findings; for example, ε4+ nondrinkers may have more normal declines in cognition in aging but ε4+ drinkers may experience exacerbated decline.
A strength of our study is that we were able to separate never drinkers from former drinkers. Although this study observed no difference in cognitive function between the never and former drinkers, the former drinking group looked significantly worse compared to the other alcohol consumption groups in most of the health factors and demographic characteristics. A possible explanation for this phenomenon is that rather than prior consumption, current, continued alcohol consumption status may be key for understanding potential beneficial or debilitating effects on cognition. It has been hypothesized that the putative benefits of alcohol could be due to its cardiovascular effects. In this study, however, SES-related variables and prior cognitive ability were more strongly associated with consumption and cognitive performance than health-related variables.
There were some limitations in our study. Alcohol consumption was assessed using self-report measures; therefore, participants may have under- or over-reported their consumption. In addition, it is possible that participants may have experienced an unusual consumption pattern in the two weeks before they were assessed, and this pattern may or may not reflect their regular consumption. However, there is a strong correlation (r = 0.76) between the baseline and follow-up alcohol consumption groupings, so we are confident in the reliability of the self-report measurement. The sample in the present study was all male and a majority (nearly 90%) were non-Hispanic white; although the VETSA population is representative of the male population in their age range in most respects, it is not representative of females or other races/ethnicities. Finally, we used illnesses from the Charlson Comorbidity Index to develop a measure of major chronic health problems in order to control for these in analyses. These health problems do not contribute equally to disease burden; however, the influence of disease burden was not the primary goal of the study.
Our study provides further evidence for the importance of assessing genetic moderators for the interaction of possible lifestyle and health factors and cognitive outcomes early in the aging process. In addition, our study did not support previous literature proposing beneficial effects of light or moderate alcohol consumption. Further research is necessary to examine these effects longitudinally while continuing to control for important confounding variables, such as genetic and environmental risk factors related to both alcohol and cognition.
Supplementary Material
Acknowledgments
The content is the responsibility of the authors and does not necessarily represent official views of the NIA, NIH, or VA. The U.S. Department of Veterans Affairs, Department of Defense; National Personnel Records Center, National Archives and Records Administration; Internal Revenue Service; National Opinion Research Center; National Research Council, National Academy of Sciences; and the Institute for Survey Research, Temple University provided invaluable assistance in the conduct of the VET Registry. The Cooperative Studies Program of the U.S. Department of Veterans Affairs provided financial support for development and maintenance of the Vietnam Era Twin Registry. We would also like to acknowledge the continued cooperation and participation of the members of the VET Registry and their families. Ms. Slayday was supported by the Advancing Diversity in Aging Research (ADAR) program at San Diego State University. This work was supported by grants from the National Institute on Aging at the National Institute of Health: (W.S.K., M.J.L., C.E.F., grant number R01AG050595), (W.S.K., grant number R01AG022381), (C.E.F. consortia PI, grant number P01AG055367-01A1, R01AG059329), (L.K.M., grant number R01AG062483), and (R.E.S., grant number R25AG043364). The authors report no conflict of interest.
References
- Anttila T, Helkala EL, Viitanen M, Kareholt I, Fratiglioni L, Winblad B, … Kivipelto M (2004). Alcohol drinking in middle age and subsequent risk of mild cognitive impairment and dementia in old age: A prospective population based study. BMJ, 329(7465), 539. doi: 10.1136/bmj.38181.418958.BE [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomfield K, Grittner U, Kramer S, & Gmel G (2006). Social inequalities in alcohol consumption and alcohol-related problems in the study countries of the EU concerted action ‘Gender, Culture and Alcohol Problems: a Multi-national Study’. Alcohol and Alcoholism, 41(suppl_1), i26–i36. [DOI] [PubMed] [Google Scholar]
- Buschke H, & Fuld PA (1974). Evaluating storage, retention, and retrieval in disordered memory and learning. Neurology, 24(11), 1019–1025. doi: 10.1212/wnl.24.11.1019 [DOI] [PubMed] [Google Scholar]
- Carmelli D, Swan GE, Reed T, Schellenberg GD, & Christian JC (1999). The effect of apolipoprotein E epsilon4 in the relationships of smoking and drinking to cognitive function. Neuroepidemiology, 18(3), 125–133. doi: 10.1159/000026204 [DOI] [PubMed] [Google Scholar]
- Charlson ME, Pompei P, Ales KL, & MacKenzie CR (1987). A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. Journal of Chronic Diseases, 40(5), 373–383. [DOI] [PubMed] [Google Scholar]
- Collins SE (2016). Associations between socioeconomic factors and alcohol outcomes. Alcohol research: current reviews, 38(1), 83. [PMC free article] [PubMed] [Google Scholar]
- Corella D, Tucker K, Lahoz C, Coltell O, Cupples LA, Wilson PW, … Ordovas JM (2001). Alcohol drinking determines the effect of the APOE locus on LDL-cholesterol concentrations in men: the Framingham Offspring Study. Am J Clin Nutr, 73(4), 736–745. doi: 10.1093/ajcn/73.4.736 [DOI] [PubMed] [Google Scholar]
- Daneman M, & Carpenter PA (1980). Individual differences in working memory and reading. Journal of Verbal Learning and Verbal Behavior, 19(4), 450–466. [Google Scholar]
- Delis DC, Kaplan E, & Kramer JH (2001). Delis-Kaplan executive function system: technical manual: Psychological Corporation. [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, & Ober BA (2000). California verbal learning test–second edition. Adult version. Manual The Psychological Corporation: San Antonio, TX. [Google Scholar]
- Downer B, Zanjani F, & Fardo DW (2014). The relationship between midlife and late life alcohol consumption, APOE e4 and the decline in learning and memory among older adults. Alcohol and Alcoholism, 49(1), 17–22. doi: 10.1093/alcalc/agt144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufouil C, Tzourio C, Brayne C, Berr C, Amouyel P, & Alperovitch A (2000). Influence of apolipoprotein E genotype on the risk of cognitive deterioration in moderate drinkers and smokers. Epidemiology, 11(3), 280–284. [DOI] [PubMed] [Google Scholar]
- Eichner JE, Dunn ST, Perveen G, Thompson DM, Stewart KE, & Stroehla BC (2002). Apolipoprotein E polymorphism and cardiovascular disease: a HuGE review. American journal of epidemiology, 155(6), 487–495. [DOI] [PubMed] [Google Scholar]
- Ekstrom RB, Dermen D, & Harman HH (1976). Manual for kit of factor-referenced cognitive tests (Vol. 102): Educational testing service; Princeton, NJ. [Google Scholar]
- Elwood PC, Gallacher JE, Hopkinson CA, Pickering J, Rabbitt P, Stollery B, … Bayer A (1999). Smoking, drinking, and other life style factors and cognitive function in men in the Caerphilly cohort. Journal of Epidemiology and Community Health, 53(1), 9–14. doi: 10.1136/jech.53.1.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillmore KM, Kerr WC, Stockwell T, Chikritzhs T, & Bostrom A (2006). Moderate alcohol use and reduced mortality risk: Systematic error in prospective studies. Addiction Research & Theory, 14(2), 101–132. [DOI] [PubMed] [Google Scholar]
- Franz CE, Lyons MJ, O’Brien R, Panizzon MS, Kim K, Bhat R, … Kremen WS (2011). A 35-year longitudinal assessment of cognition and midlife depression symptoms: the Vietnam Era Twin Study of Aging. The American Journal of Geriatric Psychiatry, 19(6), 559–570. doi: 10.1097/JGP.0b013e3181ef79f1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg J, Curran B, Vitek ME, Henderson WG, & Boyko EJ (2002). The Vietnam era twin registry. Twin Research and Human Genetics, 5(5), 476–481. [DOI] [PubMed] [Google Scholar]
- Golden C, & Freshwater S (2002). Stroop Color and Word Test Adult Version A manual for clinical and experimental uses.(2a ed.). Wood Dale, Illinois: Stoelting. [Google Scholar]
- Gustavson DE, Panizzon MS, Elman JA, Franz CE, Reynolds CA, Jacobson KC, … Kremen WS (2018). Stability of genetic and environmental influences on executive functions in midlife. Psychology and Aging, 33(2), 219–231. doi: 10.1037/pag0000230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassing LB (2018). Light alcohol consumption does not protect cognitive function: A longitudinal prospective study. Frontiers in Aging Neuroscience, 10, 81. doi: 10.3389/fnagi.2018.00081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring D, & Paulson D (2018). Moderate alcohol use and apolipoprotein E-4 (ApoE-4): Independent effects on cognitive outcomes in later life. Journal of Clinical and Experimental Neuropsychology, 40(4), 326–337. doi: 10.1080/13803395.2017.1343803 [DOI] [PubMed] [Google Scholar]
- Jack CR Jr., Wiste HJ, Weigand SD, Knopman DS, Vemuri P, Mielke MM, … Petersen RC (2015). Age, Sex, and APOE epsilon4 Effects on Memory, Brain Structure, and beta-Amyloid Across the Adult Life Span. JAMA Neurology, 72(5), 511–519. doi: 10.1001/jamaneurol.2014.4821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalapatapu RK, Ventura MI, & Barnes DE (2017). Lifetime alcohol use and cognitive performance in older adults. Journal of Addictive Diseases, 36(1), 38–47. doi: 10.1080/10550887.2016.1245029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JW, Lee DY, Lee BC, Jung MH, Kim H, Choi YS, & Choi IG (2012). Alcohol and cognition in the elderly: A review. Psychiatry Investigation, 9(1), 8–16. doi: 10.4306/pi.2012.9.1.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremen WS, Franz CE, & Lyons MJ (2013). VETSA: the Vietnam Era Twin Study of Aging. Twin Research and Human Genetics, 16(1), 399–402. doi: 10.1017/thg.2012.86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremen WS, Thompson-Brenner H, Leung YM, Grant MD, Franz CE, Eisen SA, … Lyons MJ (2006). Genes, environment, and time: the Vietnam Era Twin Study of Aging (VETSA). Twin Research and Human Genetics, 9(6), 1009–1022. doi: 10.1375/183242706779462750 [DOI] [PubMed] [Google Scholar]
- Kuerbis A, Moore AA, Sacco P, & Zanjani F (2017). Alcohol and aging: Clinical and public health perspectives: Springer. [Google Scholar]
- Kumari M, Holmes MV, Dale CE, Hubacek JA, Palmer TM, Pikhart H, … Bobak M (2014). Alcohol consumption and cognitive performance: A Mendelian randomization study. Addiction, 109(9), 1462–1471. doi: 10.1111/add.12568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamar M, Yu L, Rubin LH, James BD, Barnes LL, Farfel JM, … Schneider JA (2019). APOE genotypes as a risk factor for age-dependent accumulation of cerebrovascular disease in older adults. Alzheimer’s & Dementia, 15(2), 258–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindeman RD, Wayne SJ, Baumgartner RN, & Garry PJ (2005). Cognitive function in drinkers compared to abstainers in the New Mexico elder health survey. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 60(8), 1065–1070. [DOI] [PubMed] [Google Scholar]
- Lyons MJ, Genderson M, Grant MD, Logue M, Zink T, McKenzie R, … Jerskey B (2013). Gene‐environment interaction of ApoE genotype and combat exposure on PTSD. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 162(7), 762–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons MJ, York TP, Franz CE, Grant MD, Eaves LJ, Jacobson KC, … Kremen WS (2009). Genes determine stability and the environment determines change in cognitive ability during 35 years of adulthood. Psychological Science, 20(9), 1146–1152. doi: 10.1111/j.1467-9280.2009.02425.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neafsey EJ, & Collins MA (2011). Moderate alcohol consumption and cognitive risk. Neuropsychiatric Disease and Treatment, 7, 465–484. doi: 10.2147/NDT.S23159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngandu T, Helkala EL, Soininen H, Winblad B, Tuomilehto J, Nissinen A, & Kivipelto M (2007). Alcohol drinking and cognitive functions: Findings from the Cardiovascular Risk Factors Aging and Dementia (CAIDE) Study. Dementia and Geriatric Cognitive Disorders, 23(3), 140–149. doi: 10.1159/000097995 [DOI] [PubMed] [Google Scholar]
- Oria RB, Patrick P, Oriá M, Lorntz B, Thompson M, Azevedo O, … Lima A (2010). ApoE polymorphisms and diarrheal outcomes in Brazilian shanty town children. Brazilian Journal of Medical and Biological Research, 43(3), 249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oria RB, Patrick PD, Blackman JA, Lima AA, & Guerrant RL (2007). Role of apolipoprotein E4 in protecting children against early childhood diarrhea outcomes and implications for later development. Medical Hypotheses, 68(5), 1099–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panza F, Frisardi V, Seripa D, Logroscino G, Santamato A, Imbimbo BP, … Solfrizzi V (2012). Alcohol consumption in mild cognitive impairment and dementia: Harmful or neuroprotective? International Journal of Geriatric Psychiatry, 27(12), 1218–1238. doi: 10.1002/gps.3772 [DOI] [PubMed] [Google Scholar]
- Park B, Park J, Jun JK, Choi KS, & Suh M (2013). Gender differences in the association of smoking and drinking with the development of cognitive impairment. PLoS One, 8(10), e75095. doi: 10.1371/journal.pone.0075095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS (1977). The CES-D scale: a self-report depression scale for research in the general population. Applied Psychological Measurement, 1(3), 385–401. [Google Scholar]
- Reas ET, Laughlin GA, Bergstrom J, Kritz-Silverstein D, Barrett-Connor E, & McEvoy LK (2019). Effects of APOE on cognitive aging in community-dwelling older adults. Neuropsychology, 33(3), 406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard EL, Kritz-Silverstein D, Laughlin GA, Fung TT, Barrett-Connor E, & McEvoy LK (2017). Alcohol Intake and Cognitively Healthy Longevity in Community-Dwelling Adults: The Rancho Bernardo Study. Journal of Alzheimer’s disease, 59(3), 803–814. doi: 10.3233/JAD-161153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedel BC, Thompson PM, & Brinton RD (2016). Age, APOE and sex: Triad of risk of Alzheimer’s disease. The Journal of Steroid Biochemistry and Molecular Biology, 160, 134–147. doi: 10.1016/j.jsbmb.2016.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabia S, Elbaz A, Britton A, Bell S, Dugravot A, Shipley M, … Singh-Manoux A (2014). Alcohol consumption and cognitive decline in early old age. Neurology, 82(4), 332–339. doi: 10.1212/WNL.0000000000000063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenborn CA, & Heyman KM (2009). Health characteristics of adults aged 55 years and over: United States, 2004–2007. National Health Statistics Reports, 16, 1–31. [PubMed] [Google Scholar]
- Schultz MR, Lyons MJ, Franz CE, Grant MD, Boake C, Jacobson KC, … Kremen WS (2008). Apolipoprotein E genotype and memory in the sixth decade of life. Neurology, 70(19 Part 2), 1771–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servan-Schreiber D, Cohen JD, & Steingard S (1996). Schizophrenic deficits in the processing of context: A test of a theoretical model. Archives of General Psychiatry, 53(12), 1105–1112. [DOI] [PubMed] [Google Scholar]
- Skinner J, Carvalho JO, Potter GG, Thames A, Zelinski E, Crane PK, … Alzheimer’s Disease Neuroimaging Initiative. (2012). The Alzheimer’s disease assessment scale-cognitive-plus (ADAS-Cog-Plus): An expansion of the ADAS-Cog to improve responsiveness in MCI. Brain Imaging and Behavior, 6(4), 489–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroop JR (1935). Studies of interference in serial verbal reactions. Journal of Experimental Psychology, 18(6), 643. [Google Scholar]
- Thurstone LL (1944). A factorial study of perception. Chicago, IL, US: University of Chicago Press. [Google Scholar]
- Topiwala A, Allan CL, Valkanova V, Zsoldos E, Filippini N, Sexton C, … Ebmeier KP (2017). Moderate alcohol consumption as risk factor for adverse brain outcomes and cognitive decline: Longitudinal cohort study. BMJ, 357, j2353. doi: 10.1136/bmj.j2353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuang MT, Lyons MJ, Eisen SA, Goldberg J, True W, Lin N, … Eaves L (1996). Genetic influences on DSM-III-R drug abuse and dependence: a study of 3,372 twin pairs. American Journal of Medical Genetics, 67(5), 473–477. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services and U.S. Department of Agriculture. (2015). 2015 – 2020 Dietary Guidelines for Americans: Skyhorse Publishing Inc. [Google Scholar]
- Uhlaner J, & Bolanovich DJ (1952). Development of Armed Forces Qualification Test and Predecessor Army Screening Tests, 1946-1950. Washington, DC: Personnel Research Section, Department of the Army. [Google Scholar]
- Wechsler D (1997). Manual for the Wechsler Memory Scale—Third Edition (WMS-III). The Psychological Corporation: San Antonio, TX. [Google Scholar]
- Wechsler D (1999). Manual for the Wechsler abbreviated scale of intelligence. San Antonio, TX: Psychological Corporation. [Google Scholar]
- World Health Organization. (2014). Global status report on alcohol and health, 2014: World Health Organization. [Google Scholar]
- Wright RO, Hu H, Silverman EK, Tsaih SW, Schwartz J, Bellinger D, … Hernandez-Avila M (2003). Apolipoprotein E genotype predicts 24-month bayley scales infant development score. Pediatric Research, 54(6), 819. [DOI] [PubMed] [Google Scholar]
- Zanjani F, Downer BG, Kruger TM, Willis SL, & Schaie KW (2013). Alcohol effects on cognitive change in middle-aged and older adults. Aging & Mental Health, 17(1), 12–23. doi: 10.1080/13607863.2012.717254 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


