Abstract
Objective:
The optimal neoadjuvant therapy for resectable pancreatic ductal adenocarcinoma (PDA) and the impact on surgical outcomes remains unclear.
Methods:
S1505 (NCT02562716) was a randomized phase II study of perioperative chemotherapy with mFOLFIRINOX (Arm 1) or gemcitabine/nab-paclitaxel (Arm 2). Measured parameters included resection rate, margin positivity, pathologic response, and toxicity.
Results:
Between 2015 and 2018, 147 patients were randomized. Of these, 44 (30%) were deemed ineligible (43 by central review). Of the 103 eligible patients, 77 (76%) completed preoperative therapy and underwent surgery; reasons patients did not undergo surgery included toxicity related to preoperative therapy (n=9), progression (n=9), or other (n=7). Of the 77, 73 (95%) underwent successful resection; 21 (29%) required vascular reconstruction, 62 (85%) had negative (R0) margins, and 24 (33%) had a complete or major pathologic response to therapy. The Grade 3–5 postoperative complication rate was 16%. Of the 73 patients completing surgery, 57 (78%) started and 46 (63%) completed postoperative therapy. This study represents the first prospective trial evaluating modern systemic therapy delivered in a neoadjuvant/perioperative format for resectable PDA.
Conclusions:
We have demonstrated: 1) Based on the high percentage of enrolled, but ineligible patients, it is clear that adherence to strict definitions of resectable PDA is challenging; 2) Patients can tolerate modern systemic therapy and undergo successful surgical resection without prohibitive perioperative complications; 3) Completion of adjuvant therapy in the perioperative format is difficult; 4) Major pathologic response rate of 33% is encouraging.
Introduction
Prospective clinical trials have validated adjuvant therapy as a treatment modality that improves overall survival in patients with resectable pancreas cancer 1,2. Unfortunately, the great majority of patients with pancreas cancer have occult systemic disease 3 at presentation and, therefore, preoperative delivery of systemic therapy is an attractive strategy that has been recommended by a growing number of investigators. Furthermore, a surgery-first approach can lead to the omission of adjuvant therapy. Evaluation of the American College of Surgeons National Surgical Quality Improvement Program and the National Cancer Data Base (NCDB) has demonstrated that adjuvant therapy is omitted in upwards of 58% of patients undergoing a surgery-first approach secondary to post-operative surgical complications 4. Based on such considerations, the role of neoadjuvant therapy and treatment sequencing for resectable pancreas cancer has gained momentum over the last decade. The recently completed Dutch randomized phase III PREOPANC Trial 5 is one example of a prospective trial of neoadjuvant therapy. Although this study did not demonstrate a survival benefit, preoperative therapy was associated with improvement in disease-free survival, improved local control, and lower rates of nodal disease. More recently, the use of multi-agent chemotherapy for the management of both advanced and localized pancreas cancer has also been demonstrated to improve survival when compared to the historical standard of single-agent gemcitabine 2. To date, these newer multi-agent regimens have not been prospectively evaluated in a neoadjuvant format in a multi-institutional clinical trial. With this background, the current SWOG 1505 study aimed to study the feasibility and efficacy of two different multi-agent regimens delivered using a neoadjuvant and adjuvant (perioperative) design. SWOG 1505 was the first prospective randomized trial within the National Clinical Trials Network (NCTN) to assess the safety and efficacy of two aggressive chemotherapy regimens- mFOLFIRINOX (5-flurouracil, irinotecan, and oxaliplatin) and gemcitabine/ nab-paclitaxel for resectable PDA. This paper summarizes the accrual, surgical, and peri-operative outcomes of this study.
Patients and Methods
Study Design and Eligibility Criteria
S1505 was a randomized phase II clinical trial of perioperative chemotherapy for resectable pancreatic adenocarcinoma. Eligibility criteria included a confirmed histologic or cytologic diagnosis of pancreatic adenocarcinoma (histologies other than adenocarcinoma, or mixed histologies, were excluded); measurable disease on imaging, per RECIST 1.1 criteria; no prior therapy of any kind for the index cancer; age ≥ 18 years and ≤ 75 years; ECOG Performance Status (PS) of 0 or 1, assessed within 28 days of registration; non-pregnant, non-lactating women; and no uncontrolled medical or psychiatric illness, or social situation, leading to undue risk or incomplete compliance with study requirements. Furthermore, the following laboratory evaluations, within 14 days of registration, were required: adequate hematologic function (absolute neutrophil count [ANC] > 1,500/mm3; platelets > 100,000/mm3; hemoglobin > 9 g/dL); adequate hepatic function (total bilirubin < 1.5 × Institutional Upper Limit of Normal [IULN]; AST and ALT both < 2.5 × IULN; serum albumin > 3 g/dL); adequate renal function as evidenced by serum creatinine < IULN; baseline CA19.9 value. Resectability was evaluated using cross-sectional imaging which included either contrast-enhanced CT or MRI scans of the chest, abdomen, and pelvis – obtained within 28 days prior to registration. Resectable disease was defined based on the Intergroup definition 6. Staging requirements included no interface of the tumor with the celiac, common hepatic, or superior mesenteric arteries (and, if present, arterial anatomy variants); <180° interface between tumor and vessel wall, of the portal vein or superior mesenteric vein (SMV) patent portal vein/splenic vein confluence; no metastases and no lymphadenopathy outside the surgical basin. For tumors of the body and tail of the pancreas, interface with the splenic artery and splenic vein of any degree was considered resectable disease.
Central Radiology Review
Baseline imaging studies were uploaded to a central server, and a specialized abdominal radiologist blinded to treatment assignments performed reviews using a 6-point checklist (visible pancreatic mass; measurable disease; absence of arterial interface; venous interface of less than or equal to 180°; patent portal-splenic confluence; absence of metastatic disease, including lymphadenopathy outside the surgical basin). Patients not meeting all criteria were deemed ineligible; however, since this determination was not in real-time, enrolling sites were not notified of this status and all patients were allowed to proceed with protocol-defined therapy based on local determination. After a temporary closure for planned interim analysis to assess safety of neoadjuvant therapy, an institutional checklist was developed for all participating centers. This checklist was identical to the one used by the central radiologist and was mandated for completion by the enrolling site radiologist in an effort to minimize the enrollment of ineligible patients.
Treatments
After registration to the study, patients were randomized in equal proportions to one of the 2 treatment arms with stratification for ECOG PS 0 vs. 1. Arm 1 patients were treated with 3 months pre-operative and 3 months postoperative therapy using a modified FOLFIRINOX: oxaliplatin 85 mg/m2, followed by irinotecan 180 mg/m2, followed by 5-fluorouracil 2400 mg/m2, infused over 46 hours. This treatment was administered every 2 weeks, for a total of 6 neoadjuvant doses and 6 adjuvant doses. Arm 2 patients were treated with nab-paclitaxel 125 mg/m2, followed by gemcitabine 1000 mg/m2. This treatment was administered every week, 3 weeks on and 1 week off, for a total of 9 (3 months) neoadjuvant doses and 9 adjuvant doses.
After 3 months of neoadjuvant chemotherapy, patients underwent repeat cross-sectional imaging. In the absence of disease progression (by RECIST 1.1 criteria), patients were taken to surgical resection within 4–8 weeks following the last dose of neoadjuvant chemotherapy. Patients were to start adjuvant chemotherapy within 4–8 weeks after resection, with a maximum limit of up to 12 weeks post-surgery. The final neoadjuvant chemotherapy dose levels were used as the initial adjuvant chemotherapy dose levels.
Surgery
Pancreatectomy occurred within 4–8 weeks after the last dose of preoperative chemotherapy. Staging laparoscopy was allowed but not mandated. Based on primary tumor location, standard or pylorus preserving pancreaticoduodenectomy, distal subtotal pancreatectomy, or total pancreatectomy were performed. Surgical drains and enteral tubes were allowed at the discretion of the attending surgeon. For patients undergoing a pancreaticoduodenectomy, a peri-adventitial dissection of the right lateral aspect of the superior mesenteric artery was highly recommended. Vascular resection and /or reconstruction of the superior mesenteric vein, SMV/Portal vein confluence, or hepatic artery was allowed at the discretion of the attending surgeon in order to obtain a margin negative resection (R0). A standard lymphadenectomy was recommended to be performed at the time of the pancreatectomy.
Pathology
Surgeons were asked to orient the surgical specimen for the pathologist. Any segment of resected vascular structure was identified and marked. Relevant margins evaluated by intra-operative frozen section (bile duct and pancreas) were identified. The superior mesenteric artery (SMA) margin was separately inked and marked using the principles defined in the 2009 AJCC staging system 7. Pathological evaluation occurred by local pathologists at the institution at which the surgery was performed. Three primary margins were evaluated: these included the bile duct, pancreas neck, and the SMA. The specimen and margins were inked and evaluated according to the American Joint Commission on Cancer 7th edition staging system and the College of the American Pathologists guidelines 2012 edition 8.
Outcomes and Assessments
The overall primary outcome of this study was overall survival (OS), however, this endpoint is not yet mature and will be reported subsequently. Secondary outcomes included overall resection rate, R0 resection rate, pathologic response rates, and toxicity. Additional metrics assessed included lymph node status, reasons for protocol deviation, and surgical training. Surgical and pathologic reports were centrally reviewed by a study surgical oncologist (SAA). Pathologic response was graded using the College of American Pathologists criteria, as follows: 0: Complete response – no residual tumor; 1: Moderate response – minimal residual cancer (single cells or small groups of cancer cells); 2: Minimal response – residual cancer outgrown by fibrosis; 3: Poor or no response – no definite response identified (minimal or no tumor kill; extensive residual cancer)9. Pathologic assessments were local; there was no retrospective central pathologic review. Follow up included cross-sectional imaging, every 3 months following completion of treatment, with the first post-treatment scan within 2 weeks of completion of all therapy, until disease progression, symptomatic deterioration, or death. Radiologic response after neoadjuvant chemotherapy was defined by RECIST 1.1 criteria. At each treatment visit, chemotherapy toxicities were assessed using CTCAE v5.0. Postoperative complications were also assessed and noted. All resected surgical specimens were banked in a central biorepository.
Statistical Analyses
This study utilized a randomized Phase II “pick the winner” design, with minimum activity requirements, to address the objective of choosing a chemotherapy regimen for further study in resectable PDA. For each arm, the observed 2-year OS will first be compared to the null hypothesis of 40%, assuming a 58% alternative hypothesis, 88% power, and a 1-sided significance of 0.05. If OS rates in both arms meet this threshold, then a sample size of 100 patients (50 per arm) provides a 90% probability of selecting the better regimen with an OS hazard ratio of at least 1.4. An initial sample size of 112 was determined, to allow for approximately 10% ineligible patients. A revised sample size of 150 was allowed to ensure 100 eligible cases for final analysis. Descriptive statistics were applied to summarize surgical findings.
Results
Patients
SWOG 1505 was opened through the National Clinical Trials Network (NCTN). From October 2015 through April 2018, 147 patients were enrolled to this study. The study completed enrollment in April 2018. Forty-four patients were ineligible, including 43 deemed non-resectable by central review. Baseline patient and tumor characteristics of eligible and ineligible patients are shown in Table 1. For the 103 eligible patients, the median age of enrolled patients was 64 years, 42% of patients were female, and one patient had venous involvement based on preoperative imaging.
Table 1.
Patient and Tumor Characteristics
| Eligible (n=103) | Ineligible (n=44) | |
|---|---|---|
| Age | ||
| Median (range) | 64.2 (40–76) | 66.5 (45–75.8) |
| Primary Tumor Size | ||
| N | 73 | 23 |
| Median (range) | 2.7 cm (0.1–6.7 cm) | 2.6 cm (0–4.5 cm) |
| Sex | ||
| Females | 43 (42%) | 22 (50%) |
| Males | 60 (58%) | 22 (50%) |
| ECOG Performance Status | ||
| 0 | 65 (63%) | 27 (61%) |
| 1 | 38 (37%) | 17 (39%) |
| Race | ||
| White | 91 (88%) | 38 (87%) |
| Black | 7 (6%) | 4 (9%) |
| Unknown | 5 (4%) | 2 (5%) |
| Venous Involvement | ||
| Yes | 1 (1%) | 15 (34%) |
| No | 102 (99%) | 29 (66%) |
| Arterial Involvement | ||
| Yes | 0 (0%) | 22 (50%) |
| No | 103 (100%) | 22 (50%) |
| Metastatic Disease | ||
| Yes | 0 (0%) | 28 (64%) |
| No | 103 (100%) | 16 (36%) |
Surgical Results
Of the 103 eligible patients, 77 patients reached surgery (75%). The reasons patients did not reach surgery are listed in Table 2. These (n=25) included toxicity related to preoperative therapy (n=9), progression on therapy (n=9), or other (n=4). This latter category included patients found to have elevated CA 19–9 tumor marker, screening failure, withdrawal of consent and inability to gain insurance approval for the surgery. Of the 77 patients reaching surgery, 73 (95%) underwent successful surgical extirpation. The main reason for not undergoing resection at the time of exploration was the finding of occult metastatic disease.
Table 2.
Surgical Resection
| Eligible (n=102*) | |
|---|---|
| Did Not Reach Surgery | 25 (25%) |
| Patient Refused | 2/25 (8%) |
| Progression of Disease | 9/25 (36%) |
| Symptomatic Deterioration | 1/25(4%) |
| Toxicity | 9/25 (36%) |
| Other | 4/25 (16%) |
| Reached Surgery | 77 (75%) |
| Not Resected Due to Complication | 1/77 (1%) |
| Metastatic Disease | 3/77 (4%) |
| Resected | 73/77 (95%) |
1 patient who stopped treatment secondary to diarrhea was not included in the surgery data.
Details of the operative approach are listed in Table 3. Successful resections were most commonly performed by a general surgeon (45%), followed by specialists in surgical oncology (44%) and hepatobiliary surgery (7%). The most common operation performed was a standard pancreaticoduodenectomy (51%), followed by pylorus preserving pancreaticoduodenectomy (30%). Eleven distal pancreatectomies and two total pancreatectomies were also performed. Despite requiring documentation of the performance of a peri-adventitial dissection of the right lateral wall of the superior mesenteric artery (SMA), this technical aspect of the operation was documented in only 44% of the cases. Twenty-one (29%) patients required vascular resection and reconstruction. Most (n=19) were venous resections and/or reconstructions, and two were hepatic artery resections and reconstructions.
Table 3.
Surgical Characteristics in Patients with Successful Resection
| Eligible (n=73) | |
|---|---|
| Type of Operation | |
| Standard Pancreaticoduodenectomy | 37 (51%) |
| Pylorus Preserving PD | 22 (30%) |
| Distal Pancreatectomy | 11 (15%) |
| Total Pancreatectomy | 2 (3%) |
| Unknown | 1 (1%) |
| Periadventitial Dissection | |
| Yes | 32 (44%) |
| No | 36 (49%) |
| Unknown | 5 (7%) |
| Diagnostic Laparoscopy | |
| Yes | 36 (49%) |
| No | 37 (51%) |
| Vascular Resection | |
| Yes | 21 (29%) |
| No | 52 (71%) |
| Vascular Structure Involvement | |
| None | 52 (71%) |
| SMV | 9 (12%) |
| Portal Vein | 7 (10%) |
| SMV/ Portal Vein confluence | 3 (4%) |
| Hepatic Artery | 2 (3%) |
| Surgical Training | |
| General Surgeon | 33 (45%) |
| Surgical Oncology Fellowship | 32 (44%) |
| Hepatobiliary Fellowship | 5 (7%) |
| Transplant Fellowship | 1 (1%) |
| MIS Fellowship | 0 |
| Unknown | 2 (3%) |
Pathological Outcomes
Pathologic outcomes are listed in Table 4. Overall, for the subset of patients undergoing surgery, 85% underwent a margin negative resection (R0). Eleven patients had positive margins; these included, retroperitoneal (n=6), pancreas (n=6), and bile duct (n=1). The median number of nodes harvested was 18 (range 1–56); of these, 31 (42%) were node negative. Response to neoadjuvant therapy was measured using the College of American Pathology Protocol for the examination of specimens. Major pathologic response (Grades 0 and 1) was found in 24 patients (33%).
Table 4.
Pathologic Outcomes in Patients with Successful Resection
| Eligible (n=73) | |
|---|---|
| Resection | |
| R0 | 62 (85%) |
| R1 | 11 (15%) |
| Total nodes (Median, range) | 18 (1 – 56) |
| Positive nodes (Median, range) | 1 (0 – 26) |
| 0 nodes positive | 31 (42%) |
| 1–3 nodes positive | 24 (33%) |
| >3 nodes positive | 18 (25%) |
| Pathologic response | |
| 0 | 5 (7%) |
| 1 | 19 (26%) |
| 2 | 21 (29%) |
| 3 | 28 (38%) |
Post-operative Toxicity
In total 11 out of 68 patients had post-operative Grade 3 or 4 adverse events; no Grade 5 events were noted. The most common post-operative adverse events included anemia (n=6), abnormal LFTs (n=5), anorexia/ nausea/ vomiting (n=5), and dehydration/ diarrhea (n=3) (Table 5).
Table 5.
Surgery Related Adverse Events
| Eligible Patients (n=68) Grade | |||
|---|---|---|---|
| Adverse Event | 3 | 4 | 5 |
| Anemia | 6 | 0 | 0 |
| Anorexia/ Nausea/ Vomiting | 5 | 0 | 0 |
| Abnormal LFTs | 4 | 1 | 0 |
| Dehydration/ Diarrhea | 3 | 0 | 0 |
| Maximum Grade of Any AE* | 10 | 1 | 0 |
Includes other less frequent Grade 3 and 4 adverse events.
Discussion
SWOG 1505 was the first NCTN-sponsored study evaluating the two most active regimens that are used to treat pancreas cancer in a peri-operative format. The study accrued 147 patients over a two-and-a-half-year period (2015–2018), including a three-month accrual hiatus. There are several important findings in this paper: (1) Based on the high percentage of enrolled, but ineligible patients based on local determination, it is clear that adherence to strict definitions of “resectable” PDA remains challenging and that central radiology review should be incorporated into all future trials, (2) Patients can tolerate modern systemic therapy and then undergo successful surgical resection without prohibitive perioperative complications, (3) Completion of adjuvant therapy in the perioperative period is difficult, and (4) Major pathologic response rate of 33% is encouraging especially in the absence of upfront radiation.
In this study, the difficulty of accurately performing locoregional staging of pancreas cancer was highlighted. A total of 147 patients were enrolled in this study, of whom 44 (30%) were eventually found to be ineligible. Ineligibility was mainly due to inaccurate local staging, with 22 patients having arterial involvement of the SMA or hepatic artery. The second subset was those patients with underappreciated locoregional nodal disease outside the resection basin. For this study, we utilized the Intergroup definition of what constitutes resectable disease 6. This definition was proposed by Katz et al. in an effort to distinguish resectable disease from borderline resectable cancers in the context of the Alliance A021101 clinical trial 9. This distinction was proposed based on the understanding that locoregional staging could predict the risk of margin positivity and that negative margin resections were associated with improved long-term survival. In this regard, locoregional staging is also utilized by many for treatment sequencing. For this study and in an effort to improve quality control, baseline imaging was uploaded to a central server for a dedicated radiologist to review staging based on a 6-point checklist. These characteristics included documenting the presence of a radiologically visible pancreatic mass, absence of arterial involvement/ interface, venous involvement of less than 180 degrees, and absence of metastatic disease including lymphadenopathy outside the surgical basin. The local enrolling institutions were charged with following these preset guidelines. Unfortunately, this assessment was not performed in real time by our central radiologist and enrolled patients were allowed to continue with assigned therapy. At a planned interim analysis, the study was halted and patient characteristics were reviewed. After determining the proportion of ineligible patients, we mandated that all local institutions utilize the six-point check list that was used by our central radiologist. This resulted in only marginal improvement in patient selection, however. This difficulty with locoregional staging highlights the importance of quality control in surgical trials. Ideally, real-time review of baseline imaging would have prevented this from occurring, but we were limited by infrastructure availability at this scale within the cooperative group setting. Real-time review was utilized successfully within the Alliance 021101 and 021501 studies evaluating neoadjuvant therapy for patients with borderline resectable pancreas cancer 9,10, demonstrating the feasibility of staging quality control.
In this study, of the 103 eligible patients, 77 (75%) completed preoperative therapy and underwent surgery. Of those not undergoing surgery (n=25), 9 failed to undergo surgical extirpation secondary to progression of their cancer and 9 (36%) secondary to toxicity related to the preoperative therapy. Our study compares favorably to several other previous reports, however, with different study schemas. Murphy et al 11 reported on a series of 48 patients undergoing total neoadjuvant therapy with either 4 or 8 cycles of preoperative FOLFIRINOX. In this study, 39 out of 48 patients were able to complete at least 4 cycles of assigned therapy prior to radiation and surgery. The authors did comment that 4 out of 43 patients assigned to 8 cycles were unable to complete preoperative therapy secondary to toxicity of therapy. This rate of toxicity is similar to our rate of 10%. Similarly, in a separate report, Katz et al reported on the results from the Alliance 021101 study 9. This was a smaller feasibility study focused on patients with borderline resectable cancers. Patients were assigned to receive four cycles of modified FOLFIRINOX prior to chemoradiation and surgery. Out of 23 registered patients, 22 patients started and completed all assigned chemotherapy treatments and toxicity of preoperative therapy did not limit the patients’ ability to proceed to surgery. Several retrospective analyses have compared neoadjuvant gemcitabine and nab-paclitaxel to FOLFIRINOX, however, none of these studies commented on the toxicity of gemcitabine/nab-paclitaxel limiting the ability of the patient to undergo surgery 12, 13,14. Our current study demonstrated that a subset of patients had toxicity that prevented them from proceeding to surgery. This highlights the diligence that is required in the multi-disciplinary monitoring of these patients and the need to prospectively identify patients who are most suited to tolerate preoperative therapy.
One of the most encouraging findings in this study was the major pathologic response rate of 33% to preoperative therapy. Specifically, 24 out of 73 patients achieved either a tumor regression grade 0 (complete response) or grade 1 (moderate response- minimal residual cancer) response. Our results are encouraging when compared to previous reports. In the previous study by Katz et al. 9, 7 out of 15 patients achieved a grade 0 or 1 response. However, a closer evaluation of the study results indicates that only half of the responses were achieved with systemic therapy, the remaining secondary to preoperative chemoradiation. In the study by Murphy et al 11, tumor response was evaluated radiographically and then after radiotherapy and surgery. After induction chemotherapy, 44% of patients achieved partial response by RECIST criteria, 30% had stable disease and 5% had progression of their disease. Pathologic evaluation, unfortunately, demonstrated no pathologic complete responses after surgery despite the majority of patients receiving 8 cycles of pre-operative FOLFIRINOX. Several retrospective reviews have also evaluated response rates to both regimens. Mecedo et al 14 evaluated a total of 274 patients (FOLFIRINOX n=183, gemcitabine/ nab-paclitaxel n=91) receiving neoadjuvant therapy for pancreas cancer. In this study, 6% of patients receiving both regimens achieved a complete pathologic response, and partial response was described in 56% of patients. In a separate study by Chapman et al 12, 83 patients were treated with FOLFIRINOX and 37 received neoadjuvant gemcitabine and nab-paclitaxel. No patients achieved pathologic complete response. Both of these studies were retrospective and did not perform intention to treat analysis, thus, the reported response rates were most likely over-estimated. Based upon these prospective and retrospective reports, the response rates achieved in our current study are very encouraging and need to be further analyzed with regard to their relationship to survival outcomes.
In the modern era, two multi-agent regimens have been demonstrated to improve overall survival for advanced pancreas cancer 15, 16. One of these regimens (FOLFIRINOX) has proven to be of benefit in the adjuvant setting 2, the other regimen (gemcitabine/nab-paclitaxel) is also commonly utilized in the real world adjuvant setting, however the recent APACT trial did not show a benefit over gemcitabine alone17. S1505 was designed to determine if either regimen was superior to the historical standard in a peri-operative format. We utilized the peri-operative approach based on the concerns of many regarding the tolerability of these regimens in the adjuvant setting. Secondary endpoints for this study focused on understanding if patients could tolerate these regimens and then undergo successful surgery and additional adjuvant therapy. This initial report from SWOG 1505 has demonstrated that both regimens are tolerated by patients when delivered prior to surgery, and that most patients are not limited by the toxicity of therapy with regard to undergoing successful surgery. A small number of patients experience toxicity limiting their ability to undergo surgical exploration, and as our data matures this subset of patients needs further characterization. Furthermore, we have demonstrated encouraging pathologic response rates, especially when evaluated in the context of previous reports. Whether this response rate translates into an improvement in overall survival will need to be determined as data mature. We have also demonstrated that after surgery, patients continue to have difficulty with completing adjuvant therapy. This finding has been reported previously 4, and randomized studies evaluating the benefit of delivering all of the systemic therapy upfront are needed. A longer course of neoadjuvant FOLFIRINOX (8 cycles) is planned in the upcoming Alliance for Clinical Trials in Oncology study A02186 which will compare perioperative to adjuvant FOLFIRINOX for resectable pancreatic cancer.
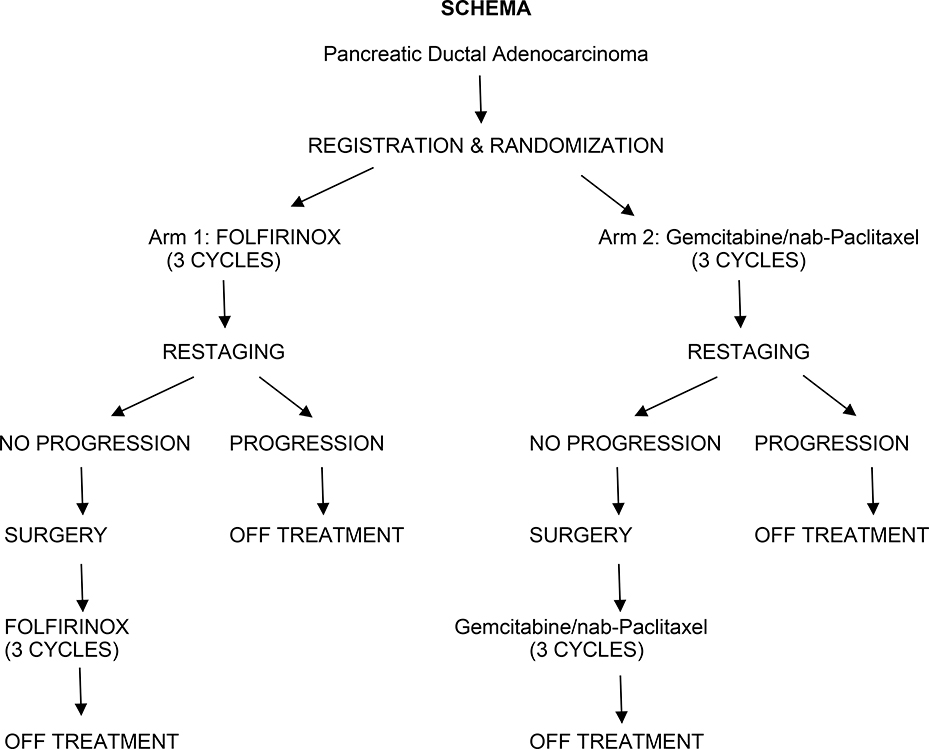
Figure 1.
Randomization and treatment schema for SWOG 1505.
Acknowledgments
Funding: National Institutes of Health, National Cancer Institute grants CA180888, CA180819, CA180820, CA180821, CA189830, CA180801, CA189953, CA189957, CA239767, CA189821, CA189972, CA233230, CA189858, CA189958, CA189822, CA189848, CA189971, CA13612, CA189873, CA189856, CA180798, CA189861, and CA189954. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Neoptolemos JP, Palmer DH, Ghaneh P, et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet. 2017;389(10073):1011–1024. [DOI] [PubMed] [Google Scholar]
- 2.Conroy T, Hammel P, Hebbar M, et al. FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. N Engl J Med 2018; 379: 2395–2406. [DOI] [PubMed] [Google Scholar]
- 3.Yachida S, Jones S, Bozic I, et al. Distant Metastases Occurs Late During the Genetic Evolution of Pancreatic Cancer. Nature. 2010; 467(7319): 1114–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Merkow RP, Bilimoria KY, Tomlinson JS, et al. Postoperative complications reduce adjuvant chemotherapy use in resectable pancreatic cancer. Ann Surg. 2014; 260(2): 372–7. [DOI] [PubMed] [Google Scholar]
- 5.Verstreijne E, Suker M, Groothuis K, et al. Preoperative chemoradiotherapy versus immediate surgery for resectable and borderline resectable pancreatic cancer: Results of the Dutch Randomized Phase III PREOPANC Trial. J Clin Oncol 2020; 38: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Katz MH, Marsh R, Herman JM, et al. Borderline resectable pancreatic cancer: need for standardization and methods for optimal clinical trial design. Ann Surg Oncol 2013; 20(8): 2787–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chun YS, Pawlik TM, Vauthey JN. 8th Edition of the AJCC Staging Manuel: Pancreas and Hepatobiliary Cancers. Ann Surg Oncol 2018; 25(4): 845–847. [DOI] [PubMed] [Google Scholar]
- 8.Adsay NV, Basturk O, Saka B, et al. Whipple made simple for surgical pathologists: orientation, dissection, and sampling of pancreaticoduodenectomy specimens for a more practical and accurate evaluation of pancreatic, distal common bile duct, and ampullary tumors. Am J Surg Pathol 2014; 38(4): 480–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Katz MH, Shi Q, Ahmad SA, et al. Preoperative modified FOLFIRINOX treatment followed by capecitabine based chemoradiation for borderline resectable pancreatic cancer: Alliance for Clinical Trials in Oncology Trial A021101. JAMA Surg 2016; 151(8): e161137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katz MHG, Ou FS, Herman JM, et al. Alliance trial A021501: preoperative extended chemotherapy vs. chemotherapy plus hypofractionated radiation therapy for borderline resectable adenocarcinoma of the head of the pancreas. BMC Cancer. 2017; 17(1): 505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murphy JE, Wo JY, Ryan DP, et al. Total neoadjuvant therapy with FOLFIRINOX followed by individualized chemoradiotherapy for borderline resectable pancreatic adenocarcinoma. A Phase 2 Clinical Trial. JAMA Oncol 2018; 4(7): 963–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chapman BC, Geisner A, Rigg D, et al. Perioperative survival outcomes following neoadjuvant FOLFIRINOX versus gemcitabine abraxane in patients with pancreatic adenocarcinoma. JOP. 2018; 19(2): 75–85. [PMC free article] [PubMed] [Google Scholar]
- 13.Dhir M, Zenati MS, Hamad A, et al. FOLFIRINOX versus gemcitabine/ nab-paclitaxel for neoadjuvant treatment of resectable and borderline resectable pancreatic head adenocarcinoma. Ann Surg Oncol 2018; 25(7): 1896–1903. [DOI] [PubMed] [Google Scholar]
- 14.Macedo FL, Ryon E, Maithel SK, et al. Survival outcomes associated with clinical and pathological response following neoadjuvant FOLFIRINOX or gemcitabine/ nab-paclitaxel chemotherapy in resected pancreatic cancer. Ann Surg 2019; 270(3): 400–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011; 364: 1817–25. [DOI] [PubMed] [Google Scholar]
- 16.Von Hoff DD, Ervine T, Arena FP, et al. Increased survival in pancreas cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013; 369–1691–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tempero MA, Reni M, Riess H, et al. APACT: phase III, multicenter, international, open-label, randomized trial of adjuvant nab-paclitaxel plus gemcitabine (nab-P/G) vs gemcitabine (G) for surgically resected pancreatic adenocarcinoma. J Clin Oncol 2019; 37 (15). [Google Scholar]