Abstract
Objective:
To examine the allocation of daily activities over a 24-hour period in caregivers (CG) with and without a probable affective disorder.
Methods:
Participants were 192 older dementia CGs (mean age= 72.9 years, 70% female) who participated in the National Study of Caregiving. Time diary data were used to measure the duration and timing that caregivers were doing: hygienic self-care; eating/drinking; household care; physical caregiving; medical caregiving; socializing; and television viewing. Affective status was assessed using the two-item Patient Health Questionnaire and the Generalized Anxiety Disorder screeners.
Results:
CGs were more likely to screen positive for depression/anxiety symptoms if they: started hygienic self-care later in the AM (OR=1.76, 95% CI=1.10–2.83) and started medical caregiving later in the AM (OR=2.34, 95% CI=1.24–4.41). Hygienic self-care start times attenuated the effects of medical caregiving on affective status.
Discussion:
Later timing of hygienic self-care may be an important behavioral response that contributes to affective disorder risk in dementia CGs.
Keywords: aging, daily diary, time use, family caregivers
Caring for a loved one with dementia is one of the most demanding types of family caregiving (Pinquart & Sörensen, 2007). Studies show that family dementia caregivers (CG) experience significant caregiver burden, depression, anxiety compared to their peers (Adelman, Tmanova, Delgado, Dion, & Lachs, 2014; Cooper, Balamurali, & Livingston, 2007; Ma, Dorstyn, Ward, & Prentice, 2018; Mausbach, Chattillion, Roepke, Patterson, & Grant, 2013; Ory, Hoffman, Yee, Tennstedt, & Schulz, 1999; Pinquart & Sorensen, 2003; Sallim, Sayampanathan, Cuttilan, & Ho, 2015). Not surprisingly, the incidence of affective disorders is high in dementia CGs: prospective studies have shown that 25–50% of dementia CGs developed clinically significant affective symptoms in less than two years (Ballard, Eastwood, Gahir, & Wilcock; Joling et al., 2012; Joling et al., 2015).
The stress-health model is widely used to understand the health effects of dementia caregiving (Schulz & Martire, 2004). In this model, affective disorders like depression and anxiety arise from individual differences in CGs emotional and behavioral responses to caregiving stressors. Primary stressors of caregiving are often uncontrollable and include care recipient (CR) disability, disruptive CR behaviors, exposure to CR suffering, and financial difficulties. Secondary stressors are a consequence of primary stressor and include declines in relationship quality, family conflict, and anticipatory grief, among others.
A substantial literature shows that individual differences in behavioral responses to caregiving stress (i.e., engaged versus withdrawn/disengaged coping styles) are associated with depression and anxiety in older CGs (Garcia-Alberca, Cruz, Lara, Garrido, Gris, et al., 2012; Garcia-Alberca, Cruz, Lara, Garrido, Lara, et al., 2012; Garcia-Alberca et al., 2013; Huang et al., 2015; Khalaila & Cohen, 2016; Lim, Griva, Goh, Chionh, & Yap, 2011; Mausbach et al., 2006; Spira et al., 2007; Wright, Lund, Caserta, & Pratt, 1991). For example, disengaging from caregiving stress via passive reaction and avoidance behaviors is associated with lower perceived control of the situation that, in turn, reduces psychological wellbeing. In dementia CGs, the association between disengagement coping and depression/anxiety are independent of caregiving intensity suggesting that individual differences to caregiving stress that are disengaged may uniquely shape depression/anxiety risk. (Garcia-Alberca, Cruz, Lara, Garrido, Gris, et al., 2012; Garcia-Alberca, Cruz, Lara, Garrido, Lara, et al., 2012; Garcia-Alberca et al., 2013; Mausbach et al., 2006; Spira et al., 2007).
Recent evidence suggests that behavioral disengagement that occurs at specific times, measured using objective 24-hour actigraphy recording, is related to mood symptoms in dementia CGs. Slower transitions between resting and active states uniquely correlated with depression symptoms in one study of dementia CGs (Smagula et al., 2017). Another study found that lower levels of actigraphy-measured activity from 8AM-10AM, correlated with depression symptom persistence over time (Smagula et al., 2019). These findings regarding the timing of behavioral engagement related and depression/anxiety are consistent with large-scale studies in other populations. In younger adults, advances in sleep-wake timing (a shift towards “morningness”) correlates with decreased depression severity (Druiven et al., 2020). In middle aged and older women, compared with those self-reporting being an “intermediate type,” those considering themselves “evening types” had similar depression incidence rates, while “morning types” had lower incident rates (Céline Vetter et al., 2018). These findings suggest that behavioral engagement in the morning may be related to depression resilience.
However, evidence is sorely needed from population-based studies of dementia CGs regarding how individuals with and without depression/anxiety symptoms differentially engage in daily activities; e.g., what activities they spend more or less time on and at what time of the day. Daily patterns of behavioral engagement in common activities that are associated with depression/anxiety symptoms could represent markers of, or contributors to, a larger context of vulnerability which interventions for dementia caregivers could be tailored address. The National Study of Caregiving (NSOC) Time Diary Study provides the opportunity to identify such potential differences in behavioral engagement. In a sample designed to be nationally representative of CGs in the United States, the NSOC Time Diary Study gathered detailed information on what CGs were doing, for how long, and at what time of the day.
Here, we characterize the duration and timing of seven common daily activities that are plausibly affected by and/or contribute to depression/anxiety symptoms in older adults (e.g., see Fiske, Wetherell, and Gatz (2009)): bathing and grooming, food preparation and eating, household care and financial management, socializing and personal communication, viewing television, physical care and assistance to others, and medical care for others. We hypothesized that dementia CGs with depression/anxiety symptoms would spend less time participating in self-care and recreational type activities and would initiate these activities later (reflecting greater behavioral disengagement/withdrawal as reviewed above).
METHOD
Participants
Data for this study were drawn from the National Study of Caregiving (NSOC), a large nationally representative study of family CGs in the United States (Freedman & Cornman, 2019). The NSOC was conducted in tandem with the National Health and Aging Trends Study (NHATS), a longitudinal study of Medicare beneficiaries ages 65 and older. Starting in 2011 (NSOC 1) and again in 2015 (NSOC II) and 2017 (NSOC III), caregivers to NHATS participants were invited to participate in the NSOC via in-person assessments with trained interviewers. A total of 3,210 individuals participated in the NSOC III, of whom 2,605 were eligible for the time diary study. Of these, 2,136 (82%) completed the time diary. To limit the impact of contextual differences on our study, we created a relatively homogeneous sample by restricting analyses to caregivers who were: (a) providing care for a CR with possible or probable dementia (n=963) (see NHATS technical paper #5 for dementia diagnosis testing and as in Wolff, Spillman, Freedman, and Kasper (2016); (b) at least 60 years of age (n=351); (c) the spouse or adult child CG to the CR (n=317); and (d) living in the same household as the CR (n=220). The final analytic sample was further restricted to 192 CGs who completed the outcome measures and models were further restricted based on covariate data availability (see Table 2).
Table 2.
Associations between the duration and timing of daily activities with affective disorder status (n=166)
| Positive Screen Models | Symptom Severity Models | |||||
|---|---|---|---|---|---|---|
| Activity domain | Odds Ratio | 95% Confidence Interval | p-value | β | Standard Error | p-value |
| Duration (in minutes): | ||||||
| Washing/dressing/grooming (per 48 minutes) | 0.91 | (0.54–1.53) | 0.71 | −0.02 | 0.25 | 0.95 |
| Eating (per 57 minutes) | 0.99 | (0.7–1.39) | 0.94 | 0.08 | 0.21 | 0.72 |
| Chores (per 136 minutes) | 1.04 | (0.66–1.63) | 0.87 | 0.16 | 0.26 | 0.54 |
| Physical caregiving (per 77 minutes) | 0.98 | (0.6–1.6) | 0.93 | 0.27 | 0.32 | 0.4 |
| Medical caregiving (per 24 minutes) | 0.98 | (0.47–2.03) | 0.94 | −0.05 | 0.28 | 0.85 |
| Socializing (per 110 minutes) | 1.14 | (0.71–1.86) | 0.58 | −0.28 | 0.17 | 0.11 |
| Television (per 168 minutes) | 0.68 | (0.37–1.22) | 0.19 | −0.37 | 0.25 | 0.14 |
| Time activity started: | ||||||
| Washing/dressing/grooming (per 4.7 hours)a | 1.76 | (1.1–2.83) | 0.02 | 0.44 | 0.18 | 0.02 |
| Eating (per 4.0 hours)b | 1.12 | (0.67–1.89) | 0.65 | −0.03 | 0.22 | 0.88 |
| Chores (per 3.0 hours)c | 1.53 | (0.95–2.48) | 0.08 | 0.47 | 0.26 | 0.09 |
| Physical caregiving (per 4.5 hours)d | 0.81 | (0.54–1.21) | 0.28 | −0.25 | 0.22 | 0.26 |
| Medical caregiving (per 5.6 hours)e | 2.34 | (1.24–4.41) | 0.008 | 0.42 | 0.08 | <0.0001 |
| Socializing (per 4.4 hours)f | 1.3 | (0.81–2.09) | 0.27 | 0.18 | 0.23 | 0.45 |
| Television (per 5.1 hours)g | 0.87 | (0.48–1.57) | 0.63 | −0.03 | 0.24 | 0.89 |
| Time activity ended: | ||||||
| Washing/dressing/grooming (per 5.9 hours)a | 1.07 | (0.65–1.76) | 0.80 | −0.13 | 0.24 | 0.58 |
| Eating (per 3.0 hours)b | 0.81 | (0.55–1.18) | 0.26 | −0.34 | 0.29 | 0.25 |
| Chores (per 3.7 hours)c | 1.01 | (0.59–1.71) | 0.98 | 0.13 | 0.28 | 0.65 |
| Physical caregiving (per 5.3 hours)d | 1.06 | (0.46–2.41) | 0.89 | 0.1 | 0.23 | 0.66 |
| Medical caregiving (per 5.4 hours)e | 2.45 | (1.78–3.37) | <0.0001 | 0.16 | 0.07 | 0.03 |
| Socializing (per 4.3 hours)f | 1.27 | (0.8–2.03) | 0.29 | 0.02 | 0.23 | 0.91 |
| Television (per 3.3 hours)g | 0.97 | (0.6–1.58) | 0.90 | −0.07 | 0.24 | 0.78 |
Notes. Results from logistic (screening results) and linear (symptom severity) regression models adjusted for sampling weights. Odds ratio indicate the likelihood of a positive screen (negative screen is the reference value). Each time diary variable was standardized so effect sizes could be compared (see table for standard deviation values). Covariates included age, sex, relationship to care recipient, day of week the diary was recorded, the number of household caregiving tasks, number of personal care assistance tasks, number of mobility related caregiving tasks, number of medical caregiving tasks, and care-related sleep interruptions.
n = 147,
n = 148,
n = 161,
n = 102,
n = 63,
n = 121,
n = 151
Procedures
NSOC III participants who reported providing care to an older adult in the last month were asked to complete a 24-hour time diary over the phone. The instrument was designed as a 30–40 minute diary and modeled after the Panel Study of Income Dynamic’s Disability and Use of Time supplement (Freedman & Cornman, 2019). Eighty percent of the time diary interviews took place within two weeks of the NSOC interview, with almost half occurring within a week. The diary captured all activities, beginning at 4 AM the previous day, and continuing until 4AM of the interview. Respondents were asked what they were doing starting at 4:00AM; then they were asked follow-up questions about the activity including 1) how long it took; 2) where they were; 3) who was doing the activity with them; and 4) who else was there. In the NSOC time diary, an attempt was made to distinguish sequential activities from simultaneous activities (i.e., separating main activities from secondary activities). After recording main and secondary activities and confirming the duration of main activities (start and end times in HH:MM), trained interviewers assigned codes to each activity (work, household chores, and travel, among others) to help probe additional reporting details to allow CGs to make corrections and/or adjust their end times. Days of the week were randomly assigned to CGs so that time diaries represented all days of the week and the weekend.
Measures
Time Diary.
We processed time diary data by coding activities that are plausibly related to and/or affected by depression/anxiety (e.g., see Fiske et al. (2009)): (1) hygienic self-care (including washing, dressing, and grooming; activities 121–129 from time diary supplement user guide); (2) eating and drinking (activities 131, 137, and 139); (3) household care and financial management (activities 421–479); (4) physical care and assistance to others (activities 511–519); (5) health and medical care to others (activities 521–529); (6) socializing and personal communication (activities 611–619); and (7) viewing television (activities 621–629). Five domains were considered personal activities (hygienic self-care, eating/drinking, household care, socializing, and television viewing); and two domains were considered caregiving activities (physical care/assistance to others and health/medical care to others). For each activity, we calculated the total number of minutes spent, the time the first instance of the activity began (start time), and the time the last instance began (end time).
Affective status.
Our primary outcome was probable affective status, based on the 2-item Patient Health Questionnaire (PHQ-2), (Kroenke, Spitzer, & Williams, 2003) and the 2-item Generalized Anxiety Disorder scale (Kroenke, Spitzer, Williams, Monahan, & Lowe, 2007). These measures were obtained at NSOC III. The PHQ-2 questions were “Over the last month, how often have you: 1) had little interest or pleasure in doing things; 2) felt down, depressed, or hopeless.” The GAD-2 questions were “Over the last month, how often have you: 1) felt nervous, anxious, or on edge; 2) been unable to stop or control worrying?” Response categories and item scores are: 0 = not at all; 1= several days; 2=more than half the days; 3=nearly every day). Scores range from 0–6 for each measure and are calculated by summing item scores. The optimal cut-off for both scales, in terms of the ability to screen for their respective affective disorders, is a score ≥3. Using the cut-point, the PHQ-2 has sensitivity of 83% and specificity of 92% for detecting major depressive disorder; and the GAD-2 has a sensitivity of 86% and specific of 83% for detecting generalized anxiety disorder. We considered scores of ≥3 on either or both scales as a positive affective disorder screen. To verify that the associations detected were due to differences in symptom severity, and not related to the categorization of these scales, we also examined the total severity on both scales combined (possible range: 0–12). Cronbach’s alpha for this continuous scale, indicating internal consistency for these items in this sample, was good (0.76).
Correlates of affective status.
Analyses controlled for variables known to be associated with depression/anxiety in older dementia CGs including sociodemographic variables (e.g., age, sex, CGs relationship to the CR), caregiving intensity, and disruptive CR night-time sleep behaviors. CG relationship to their CR was categorized as spouse or adult child. We estimated caregiving intensity as the total number of activities of daily living (ADL) and instrumental activities of daily living (IADL) for which the CG provided care to the CR including chores (shopping, ordering medications, and banking: range=0–3); personal caregiving activities (personal care, teeth cleaning, and foot care: range=0–3); mobility tasks e.g., (helping the CR get around the house, lifting the CR from a seated position, letting the CR lean on the CG for support, and holding the CR when they walked or stood, range=0–4); and medical caregiving tasks (giving medications, injections, managing other medical tasks, and caring for wounds or sores, range=0–4). We also examined CR night-time interruptions and whether CGs’ sleep was interrupted to deliver care; responses to this item ranged from 0 (never) to 4 (every night). These covariates were included to test whether associations between time diary variables and affective status were independent of caregiving intensity. Overall measures of overall perceived stress were not included as covariates because they are typically highly correlated with, and conceptually proximal and/or a proxy for, depression/anxiety symptoms (the outcome variable).
Statistical analyses
We compared demographic, caregiving, and time diary variables between CGs who did and did not screen positive for a probable affective disorder using the t-test for continuous variables and the chi-square statistics for categorical variables. For our main analysis, we used separate logistic regression models for each time diary variable (the independent variable) with affective disorder screening results (positive or negative) as the dependent variable. We also used linear regression to examine the relation between time diary variables and symptom severity of depression/anxiety. Each time diary variable was standardized to a mean of zero and a standard deviation of one to facilitate effect size comparison. To illustrate our findings, we report the weighted prevalence of positive screens in strata by tertile values of significant time diary variables. All models accounted for the diary sampling weights and adjusted for the covariates listed above. We also control for the day of the week that the time diary was completed to control for potential weekend-weekday effects.
RESULTS
Descriptive statistics
Table 1 reports sample descriptive information. CGs mean age was 73 years (SD = 8.5 years). The sample was 70% women. Twenty-four percent screened positive for a probable affective disorder. Compared to CGs who screened negative, CGs who screened positive for an affective disorder were more likely to report CR night-time sleep disruptions. Caregiving intensity did not significantly differ by affective disorder screening results.
Table 1.
Characteristics of caregivers by affective disorder screening results (n=192).
| Positive screen (24%) | Negative screen (76%) | p-value | |
|---|---|---|---|
| CG demographics | |||
| Age, years | 73.9 (9.1) | 72.7 (8.3) | 0.39 |
| Sex, female, % (n) | 68 (32) | 70 (101) | 0.84 |
| CG relationship to CR, % (n) | 0.22 | ||
| Spouse | 66 (31) | 56 (81) | |
| Adult child | 34 (16) | 44 (64) | |
| Race, % (n)1,2 | 0.73 | ||
| White | 59 (27) | 63 (87) | |
| Black | 37 (17) | 35 (48) | |
| Other | 4 (2) | 3 (4) | |
| Employed for pay, % (n)1 | 9 (4) | 19 (28) | 0.11 |
| Caregiving intensity | |||
| Help with household tasks, no. | 3.6 (0.6) | 3.5 (0.8) | 0.38 |
| Help with personal care tasks, no. | 1.5 (1.0) | 1.5 (1.0) | 0.91 |
| Help with mobility tasks, no. | 2.8 (1.0) | 2.6 (1.2) | 0.41 |
| Help with medical tasks, no. | 1.4 (0.9) | 1.4 (0.9) | 0.81 |
| Care-related sleep interruptions, % (n) | 0.03 | ||
| Never | 28 (13) | 43 (62) | |
| Rarely | 25 (12) | 30 (44) | |
| Some nights | 38 (18) | 18 (26) | |
| Often/every night | 9 (4) | 9 (13) |
Notes. Means (standard deviations) shown unless otherwise noted. P-values are from t-tests for continuous variables and Chi-Squared tests for categorical variables.
Fischer’s Exact Test;
n=185 due to missing data
CG=caregiver; CR=care recipient.
Duration of daily activities and affective status
The number of minutes spent in each activity was not associated with affective status (Table 2). While approximately 90%+ of CGs reported at least some time participating in most activities, other activities were less commonly reported. These included medical caregiving (57% reported some time); physical caregiving (34% reported some time); and socializing (73% reported some time). We therefore additionally modeled whether having any versus no time in these three activities related to affective status. Results were not substantively altered from those presented in Table 2 (i.e., no p<0.05).
Timing of daily activities and affective status
The timing of CGs hygienic self-care and timing of CG’s provision of medical care to CRs were significantly associated with affective status (Table 2). For each standard deviation (SD) later that CGs started hygienic self-care, the odds of screening positive were 76% higher. In addition, for each SD later that CGs started medical care in the AM and ended medical care in the PM, the odds of screening positive for an affective disorder were over two times higher. The timing of these activities also correlated with the symptom severity outcome (Table 2, right columns). Note that these associations were independent of CGs’ relationship to their CR, caregiving intensity, and CR night-time sleep disruptions. Consistent findings were obtained when also adjusting for race, employment status, education, or whether the participant agreed the time diary interview was about a “typical day” (69% agreed).
Follow-up analyses
We computed additional models to test whether hygienic self-care start times and medical caregiving times were associated with affective status independent of each other. In these models, the statistical effects of both medical caregiving timing variables were attenuated and no longer statistically significant, whereas the statistical effects of hygienic self-care start times were not attenuated and remained statistically significant. Multicollinearity was not detected in these models (variance inflation factors all <2.6). To illustrate the association between hygienic self-care and affective status, Figure 1 shows affective status by tertile of hygienic self-care start times. Positive affective disorder screens were more common in CGs who started hygienic self-care later (Rao-Scott Chi-Square p=0.001).
Figure 1.
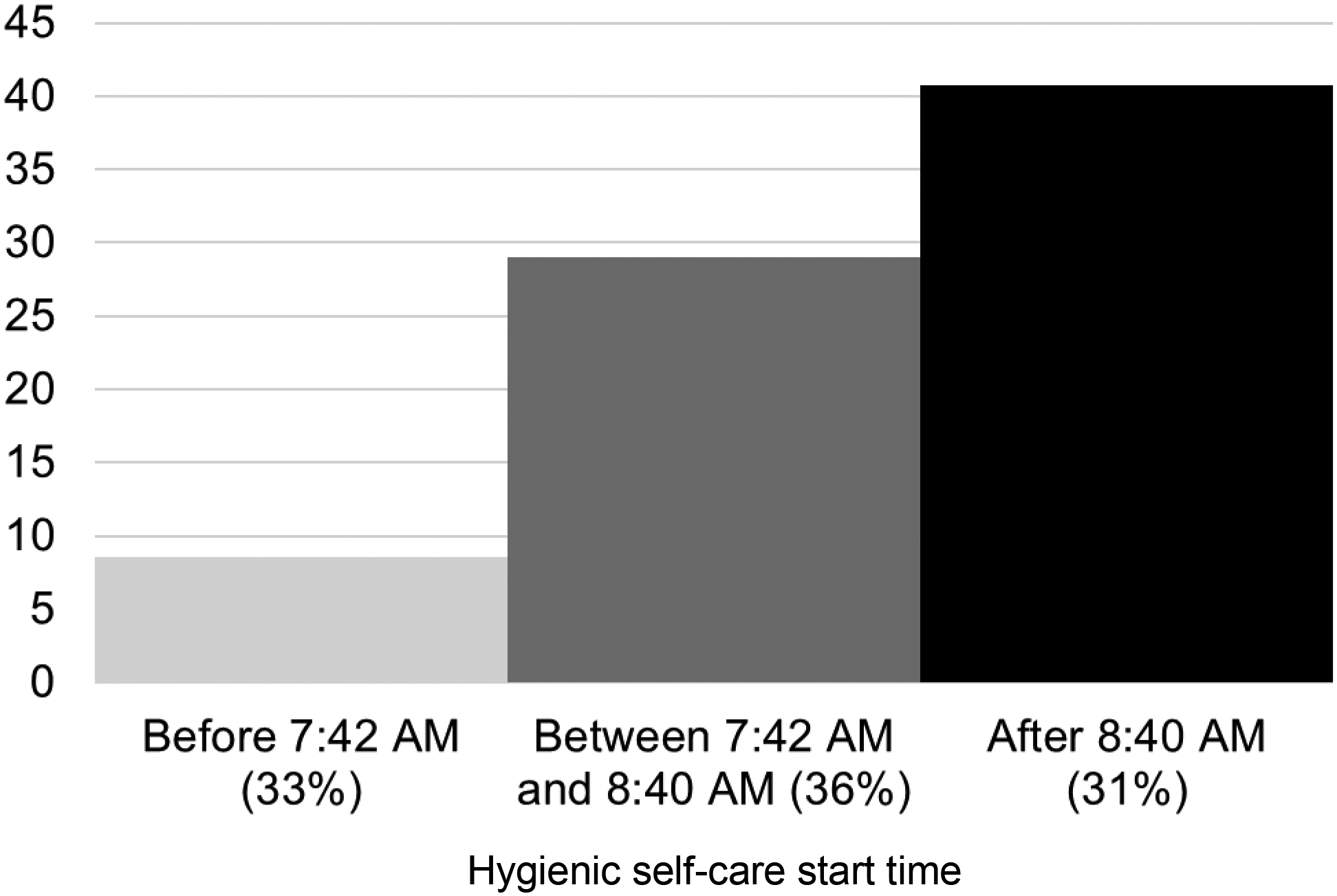
Percentage of caregivers who screened positive for an affective disorder stratified by tertile of hygienic self-care start times (n=192)
Given that hygienic self-care start times could be due to later sleep offset times, we evaluated the correlation between sleep offset times and affective status. Activity codes 111 and 119 in the NSOC captured sleeping. The average sleep end time was just after 7 AM (n=192; mean=7.1; standard deviation=2.5; inter-quartile range=7–8). Hygienic self-care onset and sleep offset times were poorly correlated (n=169, Spearman r=0.12, p=0.13). We found that sleep offset times were not associated with symptom severity (β per standard deviation later sleep offset=−0.03, standard error=0.30, t-value=−.10, p=0.92) or the likelihood of having screened positive for a probably affective disorder (odds ratio per standard deviation later sleep offset=0.98, 95% confidence interval: 0.69, 1.39, Wald Chi-Square=0.01, p=0.91). Additionally, adjusting for sleep offset times as a covariate did not alter the estimates of associations between self-care start times and depression/anxiety symptoms.
DISCUSSION
In a nationally representative sample of older co-residing dementia CGs, we found that CGs were more likely to screen positive for a probable affective disorder if they started hygienic self-care later in the morning, started medical caregiving later in the morning, and ended medical caregiving later in the evening. Later timing of CG self-care attenuated the statistical effects of medical caregiving timing, suggesting that later morning self-care start times mark an initial, unique activity timing correlate of depression/anxiety symptoms in dementia CGs. These time diary-based findings add a new behavioral-timing dimension to prior literature on the relevance of individual responses characterized by disengagement coping (Garcia-Alberca, Cruz, Lara, Garrido, Gris, et al., 2012; Garcia-Alberca, Cruz, Lara, Garrido, Lara, et al., 2012; Garcia-Alberca et al., 2013; Huang et al., 2015; Khalaila & Cohen, 2016; Lim et al., 2011; Mausbach et al., 2006; Spira et al., 2007; Wright et al., 1991). Later initiation of hygienic self-care may be an important correlate of depression to consider in efforts to reduce depression/anxiety symptoms and their consequences in dementia CGs.
There are several possible reasons why earlier start times may relate to depression/anxiety symptoms in older dementia CGs. Higher levels of perceived stress related to depression and anxiety symptoms could serve as barriers that delay self-care activities like washing and grooming. At the same time, delays to the timing of these activities could have consequences. For example, it is plausible that earlier self-care earlier provides: a task for CGs to focus on, thereby interrupting ruminating thoughts and negative emotions, potentially generating more neutral or positive cognitions; the chance to feel relaxed, relieving stress, and increasing circulation earlier in the morning; and facilitating comfort with social and/or physical engagement starting earlier in the day. This potential direction of temporal relations, from earlier self-care to better mood, is consistent with studies that show that morning inactivity correlates with depression symptoms (Smagula et al., 2019); and that being a “morning type” individual prospectively protects against depression (C. Vetter et al., 2018). But it remains unclear why being a “morning type” appears protective against depression. Since activity timing and affective status were both only measured once, we cannot be sure of the directionality of effects between activity timing and affective status. Additionally, measures of perceived stress upon waking, the availability of respite care and support at different times, and the temporal dynamics of these processes were not available. Future studies are required to confirm the temporal relations, underlying mechanisms, and modifiability of the association between morning activity and depression/anxiety symptoms in dementia CGs.
Several other limitations should be noted. First, there is the potential of measurement error: the 24-hour time diary and measures of depression/anxiety symptoms were collected only once each (and not at the exact same time). Note, however, that: limitations to our time diary and outcome measures would theoretically introduce measurement error resulting in an under-estimate of the true effect; and we obtained consistent results with categorical and continuous expressions of the symptom measures. But future research is required to specify the particular domains of emotional health related to activity timing in dementia CGs. In addition, although we statistically adjusted for several key caregiving characteristics, as with all observational research, there remains a risk of unmeasured (residual) confounding. As stated above, relations between morning self-care, perceptions of stress, and mood are complex and plausibly bi-directional processes. Finally, although the sample was designed to be representative of older co-residing family dementia CGs in the United States, these findings will not necessarily generalize to other nationalities and cultures in which different patterns of daily activities ensue.
In conclusion, our findings show that later initiation of hygienic self-care relates to affective status in older dementia CGs. Future experimental studies are needed to determine if modifying the start times of activities, like bathing and grooming, reduces depression and anxiety symptoms in dementia CGs. In such efforts, existing CG interventions could be adapted to boost behavioral engagement in the morning such as the activity scheduling components of behavioral activation (Kanter et al., 2010) and/or timing the delivery of support services so that CGs can start their day off “right.”
Funding sources:
Supported in part by grants MH118270 and K01MH112683 from the National Institute of Mental Health.
Footnotes
Financial disclosures: The authors have no conflicts to disclose.
Ethics statement: The data examined in this study (the 2011 National Health and Aging Trends Study (NHATS) and National Study of Caregiving (NSOC) are publicly available, do not contain individual identifiers, and are therefore exempt from institutional review board review.
REFERENCES
- Adelman RD, Tmanova LL, Delgado D, Dion S, & Lachs MS (2014). Caregiver Burden: A Clinical Review. Jama, 311(10), 1052–1060. doi: 10.1001/jama.2014.304 [DOI] [PubMed] [Google Scholar]
- Ballard CG, Eastwood C, Gahir M, & Wilcock G (1996). A follow up study of depression in the carers of dementia sufferers. BMJ : British Medical Journal, 312(7036), 947–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper C, Balamurali TB, & Livingston G (2007). A systematic review of the prevalence and covariates of anxiety in caregivers of people with dementia. Int Psychogeriatr, 19(2), 175–195. doi: 10.1017/s1041610206004297 [DOI] [PubMed] [Google Scholar]
- Druiven SJM, Hovenkamp-Hermelink JHM, Knapen SE, Kamphuis J, Haarman BCM, Penninx BWJH, … Riese H (2020). Stability of chronotype over a 7-year follow-up period and its association with severity of depressive and anxiety symptoms. Depression and anxiety, 37(5), 466–474. doi: 10.1002/da.22995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiske A, Wetherell JL, & Gatz M (2009). Depression in older adults. Annu Rev Clin Psychol, 5, 363–389. doi: 10.1146/annurev.clinpsy.032408.153621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman V, & Cornman JC (2019). National Study of Caregiving III Time Diary Use Guide. Retrieved from Retrieved from www.nhats.org.:
- Garcia-Alberca JM, Cruz B, Lara JP, Garrido V, Gris E, Lara A, & Castilla C (2012). Disengagement coping partially mediates the relationship between caregiver burden and anxiety and depression in caregivers of people with Alzheimer’s disease. Results from the MALAGA-AD study. J Affect Disord, 136(3), 848–856. doi: 10.1016/j.jad.2011.09.026 [DOI] [PubMed] [Google Scholar]
- Garcia-Alberca JM, Cruz B, Lara JP, Garrido V, Lara A, & Gris E (2012). Anxiety and depression are associated with coping strategies in caregivers of Alzheimer’s disease patients: results from the MALAGA-AD study. Int Psychogeriatr, 24(8), 1325–1334. doi: 10.1017/s1041610211002948 [DOI] [PubMed] [Google Scholar]
- Garcia-Alberca JM, Cruz B, Lara JP, Garrido V, Lara A, Gris E, & Gonzalez-Herero V (2013). The experience of caregiving: the influence of coping strategies on behavioral and psychological symptoms in patients with Alzheimer’s disease. Aging Ment Health, 17(5), 615–622. doi: 10.1080/13607863.2013.765833 [DOI] [PubMed] [Google Scholar]
- Huang MF, Huang WH, Su YC, Hou SY, Chen HM, Yeh YC, & Chen CS (2015). Coping Strategy and Caregiver Burden Among Caregivers of Patients With Dementia. Am J Alzheimers Dis Other Demen, 30(7), 694–698. doi: 10.1177/1533317513494446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joling KJ, Smit F, van Marwijk HW, van der Horst HE, Scheltens P, Schulz R, & van Hout HP (2012). Identifying target groups for the prevention of depression among caregivers of dementia patients. Int Psychogeriatr, 24(2), 298–306. doi: 10.1017/s1041610211001633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joling KJ, van Marwijk HW, Veldhuijzen AE, van der Horst HE, Scheltens P, Smit F, & van Hout HP (2015). The two-year incidence of depression and anxiety disorders in spousal caregivers of persons with dementia: who is at the greatest risk? Am J Geriatr Psychiatry, 23(3), 293–303. doi: 10.1016/j.jagp.2014.05.005 [DOI] [PubMed] [Google Scholar]
- Kanter JW, Manos RC, Bowe WM, Baruch DE, Busch AM, & Rusch LC (2010). What is behavioral activation?: A review of the empirical literature. Clin Psychol Rev, 30(6), 608–620. doi: 10.1016/j.cpr.2010.04.001 [DOI] [PubMed] [Google Scholar]
- Khalaila R, & Cohen M (2016). Emotional suppression, caregiving burden, mastery, coping strategies and mental health in spousal caregivers. Aging Ment Health, 20(9), 908–917. doi: 10.1080/13607863.2015.1055551 [DOI] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, & Williams JB (2003). The Patient Health Questionnaire-2: validity of a two-item depression screener. Med Care, 41(11), 1284–1292. doi: 10.1097/01.mlr.0000093487.78664.3c [DOI] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, Williams JB, Monahan PO, & Lowe B (2007). Anxiety disorders in primary care: prevalence, impairment, comorbidity, and detection. Ann Intern Med, 146(5), 317–325. [DOI] [PubMed] [Google Scholar]
- Lim J, Griva K, Goh J, Chionh HL, & Yap P (2011). Coping strategies influence caregiver outcomes among Asian family caregivers of persons with dementia in Singapore. Alzheimer Dis Assoc Disord, 25(1), 34–41. doi: 10.1097/WAD.0b013e3181ec18ae [DOI] [PubMed] [Google Scholar]
- Ma M, Dorstyn D, Ward L, & Prentice S (2018). Alzheimers’ disease and caregiving: a meta-analytic review comparing the mental health of primary carers to controls. Aging Ment Health, 22(11), 1395–1405. doi: 10.1080/13607863.2017.1370689 [DOI] [PubMed] [Google Scholar]
- Mausbach BT, Aschbacher K, Patterson TL, Ancoli-Israel S, von Känel R, Mills PJ, … Grant I (2006). Avoidant Coping Partially Mediates the Relationship Between Patient Problem Behaviors and Depressive Symptoms in Spousal Alzheimer Caregivers. The American Journal of Geriatric Psychiatry, 14(4), 299–306. doi: 10.1097/01.JGP.0000192492.88920.08 [DOI] [PubMed] [Google Scholar]
- Mausbach BT, Chattillion EA, Roepke SK, Patterson TL, & Grant I (2013). A comparison of psychosocial outcomes in elderly Alzheimer caregivers and noncaregivers. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry, 21(1), 5–13. doi: 10.1016/j.jagp.2012.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ory MG, Hoffman RR 3rd, Yee JL, Tennstedt S, & Schulz R (1999). Prevalence and impact of caregiving: a detailed comparison between dementia and nondementia caregivers. Gerontologist, 39(2), 177–185. [DOI] [PubMed] [Google Scholar]
- Pinquart M, & Sorensen S (2003). Differences between caregivers and noncaregivers in psychological health and physical health: a meta-analysis. Psychol Aging, 18(2), 250–267. [DOI] [PubMed] [Google Scholar]
- Sallim AB, Sayampanathan AA, Cuttilan A, & Ho R (2015). Prevalence of Mental Health Disorders Among Caregivers of Patients With Alzheimer Disease. J Am Med Dir Assoc, 16(12), 1034–1041. doi: 10.1016/j.jamda.2015.09.007 [DOI] [PubMed] [Google Scholar]
- Smagula SF, Hasler BP, Schulz R, Graves JL, Reynolds CF, Aizenstein HJ, … Hall MH (2019). Activity patterns related to depression symptoms in stressed dementia caregivers. Int Psychogeriatr, 1–8. doi: 10.1017/s1041610219001601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smagula SF, Krafty RT, Taylor BJ, Martire LM, Schulz R, & Hall MH (2017). Rest-activity rhythm and sleep characteristics associated with depression symptom severity in strained dementia caregivers. J Sleep Res, 26(6), 718–725. doi: 10.1111/jsr.12549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spira AP, Beaudreau SA, Jimenez D, Kierod K, Cusing MM, Gray HL, & Gallagher-Thompson D (2007). Experiential Avoidance, Acceptance, and Depression in Dementia Family Caregivers. Clin Gerontol, 30(4), 55–64. doi: 10.1300/J018v30n04_04 [DOI] [Google Scholar]
- Vetter C, Chang S-C, Devore EE, Rohrer F, Okereke OI, & Schernhammer ES (2018). Prospective study of chronotype and incident depression among middle- and older-aged women in the Nurses’ Health Study II. J Psychiatr Res, 103, 156–160. doi: 10.1016/j.jpsychires.2018.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff JL, Spillman BC, Freedman VA, & Kasper JD (2016). A national profile of family and unpaid caregivers who assist older adults with health care activities. JAMA Intern Med, 176(3), 372–379. doi: 10.1001/jamainternmed.2015.7664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright SD, Lund DA, Caserta MS, & Pratt C (1991). Coping and Caregiver Well-Being: The Impact of Maladaptive Strategies. Journal of Gerontological Social Work, 17(1–2), 75–91. doi: 10.1300/J083v17n01_07 [DOI] [Google Scholar]


