Abstract
Tocilizumab (TCZ), an IL-6 receptor blocker, is approved for relapsing, refractory giant cell arteritis (GCA). We report real-life clinical experience with TCZ in GCA including assessment of responses on imaging (ultrasound (US) and 18F-Fluorodeoxyglucose Positron Emission Tomography-computed Tomography (18FDG-PET-CT)) during the first year of treatment. We included 22 consecutive patients with GCA treated with TCZ where EULAR core data set on disease activity, quality of life (QoL) and treatment-related complications were collected. Pre-TCZ US and 18FDG-PET/CT findings were available for 21 and 4 patients, respectively, where we determined the effect on US halo thickness, temporal and axillary artery Southend Halo Score and Total Vascular Score on 18FDG-PET-CT. The 22 patients with GCA (10 cranial, 10 large vessel, 2 both) had a median disease duration of 58.5 (range, 1–370) weeks prior to initiation of TCZ. Half had used prior conventional synthetic disease-modifying antirheumatic drug (csDMARDs). TCZ was initiated for refractory (50%), ischaemic (36%) or relapsing (14%) disease. Median follow-up was 43 (12–52) weeks. TCZ was discontinued due to serious adverse events (SAEs) in two patients. On treatment with TCZ, 4 discontinued prednisolone, 11 required doses ≤2.5 mg, 2 required daily dose of 2.5–5 mg and 5 needed prednisolones ≥5 mg daily. QoL improved by 50%. Total US halo thickness decreased in 38 arterial segments, median temporal artery Halo Score decreased from 11 to 0, axillary artery Halo Score remained stable. Median Total Vascular Score on FDG-PET/CT reduced from 11.5 to 6.5. In our experience, TCZ showed an excellent response with acceptable safety in GCA, with improvement on US and FDG-PET/CT imaging.
Keywords: Giant Cell Arteritis, Ultrasonography, Systemic vasculitis, Glucocorticoids
INTRODUCTION
INTRODUCTION
Giant cell arteritis (GCA) is a vasculitis associated with sight loss, jaw and limb claudication, headaches, polymyalgia rheumatica and vascular damage.1 Glucocorticoids (GC) are the mainstay of long-term therapy. GiACTA study showed that tocilizumab (TCZ), an interleukin-6 receptor (IL-6R) antagonist, is more effective than placebo plus blinded prednisone taper for inducing sustained remission at 52 weeks. The European medicines agency (EMA) and the Food and Drug Administration (FDA) approved TCZ for the GCA. National Institute for Health and Care Excellence (NICE) approved its use in relapsing and refractory GCA from July 2018 for a maximum of 1 year.2
Assessing real-life efficacy and safety of TCZ is difficult due to its cost, limited availability and absence of a GCA registry. It is also challenging to identify groups with highest unmet need. This involves disease stratification (severity, extent and damage) into subgroups according to response to GC: remitting, relapsing, refractory disease and patients with adverse effects/GC-intolerance.3 Large vessel GCA (LV-GCA) has a more relapsing course and requires higher cumulative doses of GC.4
Our experience from a NHS Hospital emphasises several aspects of GCA disease management. It underlines the role of imaging (ultrasound (US) and 18F-Fluorodeoxyglucose Position Emission Tomography-computed Tomography (18FDG PET-CT)) and histology for providing secure diagnosis and stratifying disease type (cranial, large vessel (LV) or combined), pre-approval of TCZ. Disease assessment is problematic due to TCZ suppression of inflammatory markers. US (including the quantitative Southend Halo score) and 18PET-CT imaging during the follow-up of some TCZ-treated patients were included here. We also emphasise the need for nursing support to monitor adverse events, patient safety and logistic issues.
We hope that this case series enables improved standards of care, monitoring and use of other agents in GCA.
Key messages.
What is already known about the study?
Tocilizumab (TCZ) is approved to treat refractory and relapsing giant cell arteritis (GCA); vascular ultrasound and FDG-PET/CT are recommended imaging tools to recognise GCA.
What does the study add?
This real-life study shows Tocilizumab has an excellent response with acceptable safety and improves quality of life in GCA.
Ultrasound Halo Score may be a promising marker in addition to halo thickness and PET-CT total vascular score in assessing the GCA activity.
How might this impact on clinical practice or future developments?
These findings indicate that TCZ treatment is efficacious and safe outside the clinical trial setting; our real-life data indicate that vascular abnormalities on ultrasound (Halo Score) and FDG-PET/CT imaging improve during treatment and may be used to monitor response.
METHODS
Patients
We included 22 consecutive patients with GCA treated with TCZ at Southend University Hospital from July 2018 to February 2020. Our academic centre is a tertiary referral centre in the East of England for GCA.
The diagnosis was based on clinical, laboratory and imaging findings5 with a confirmatory imaging test or temporal artery (TA) biopsy required. Diagnosis was confirmed by at least 6 months follow-up in all patients.
EULAR core data items6 were prospectively collected (online supplemental table S1) including demographics, clinical symptoms and signs, ophthalmology report, GC use, disease-modifying antirheumatic drugs (DMARDs) therapy, comorbidities, clinical outcomes, imaging findings and laboratory results (online supplemental table S2).
rmdopen-2020-001417supp002.PDF (474.1KB, PDF)
rmdopen-2020-001417supp001.PDF (99.2KB, PDF)
GCA disease activity and adverse events were assessed by an expert rheumatologist (BD). ‘Remission’ was defined as the absence of signs and symptoms.7 For ‘refractory’ and ‘relapsing’ disease, we used the NHSE Blueteq form, a web-based approval system which notifies a person starting TCZ.8 ‘Refractory disease’ is inability to induce remission in a patient who has (i) confirmatory diagnosis of GCA with (ii) ischaemic signs or symptoms with a significant risk of end organ or vascular damage, despite optimal standard care (ie, GC safe doses in compliance with accepted Guidelines). ‘Relapsing disease’ is a patient previously responded to treatment with confirmatory evidence of currently active or progressive GCA with (a) definite ischaemic complications and/or (b) clear recurrence of symptoms with/without increased inflammatory markers without another explanation.
Start of TCZ was not always associated with an increment of GC and remission was not always obtained by GC prior to initiate TCZ.
‘Cranial GCA’ was defined as a disease limited to TAs and their branches. ‘LV-GCA’ was defined as a disease involving extra-cranial large vessels (thoracic and abdominal aorta; carotid, subclavian, axillary arteries (AAs)).
Imaging of temporal and axillary arteries
US scans were performed or supervised by an experienced ultrasonographer (BD) with an Esaote MyLabTwice, Esaote US machine using a linear probe LA435 (frequency 18 MHz or 22 MHz), colour Doppler frequency 9 MHz and a pulse repetition frequency of 2–3 kHz.9 The common superficial TA, its frontal and parietal branches and/or the AA were examined bilaterally in the long and short axis. Halo was measured at the point of maximum thickness in the longitudinal plane. A halo sign was morphologically defined as a US finding of a dark hypoechoic, non-compressible area around the vessel lumen.10–13 An abnormal vessel wall thickness was defined as >0.29–0.42 mm in TA segments and >1.0 mm in AA.14 The Southend Halo Score was determined as described.15 In addition, a provisional AA Halo Score was assessed.5 Baseline US scans were performed within 4 weeks of TCZ start.
PET-CT: modified total vascular score
PET scans were all combined with low-dose CT. Interval between FDG injection and image acquisition was 60 min. Vascular FDG uptake was visually graded compared to liver uptake (0: no uptake; 1: less than liver; 2: equal to liver; 3: greater than liver).16 For each patient, a modified total vascular score (TVS) was calculated including the AAs compared to previously published TVS.17 In total, 11 vascular regions (ascending aorta, aortic arch, descending thoracic aorta, abdominal aorta, innominate artery and bilateral carotid, subclavian and AAs) were assessed. Modified TVS ranged from 0 to 33 (previous TVS ranges 0–27), with higher scores indicating more intense and extensive vascular inflammation. Baseline PET scans were performed within 4 weeks of TCZ start. All 18PET-CT scans were reviewed and TVS was calculated by an experienced radiologist (JM).
Statistical analysis
Statistical analysis was performed using SPSS Statistics for Windows, version 18.0 (SPSS Inc. Chicago, IL, USA). All continuous variables were tested for normality, and results were expressed as means±SD/SEM or as the median and range as appropriate. The comparison of continuous variables among time periods was performed using the Wilcoxon signed-rank test. A two-sided p value <0.05 was considered statistically significant.
RESULTS
Patients’ characteristics
Among 22 patients treated with TCZ, 10 had cranial GCA, 10 LV-GCA and 2 with both. Indications for TCZ treatment were refractory (50%), ischaemic (36%) and relapsing (14%) disease. The median age of patients was 71 (range, 54–88) years, with 64% of them being females (table 1). Median duration after GCA diagnosis to initiate TCZ was 58.5 (range, 1–370) weeks. Temporal headache (73%), scalp tenderness (41%), jaw claudication (46%), constitutional symptoms (82%), polymyalgic symptoms (55%) and visual disturbance (59%) were the main clinical symptoms at the initial presentation (table 2). With visual symptoms, the patients complained of blurred vision (69%), diplopia (23%) and amaurosis (8%). Six (27%) patients had prior permanent sight loss due to ischaemic optic neuropathy, central retinal artery occlusion or both at presentation. Half had used a conventional synthetic disease-modifying antirheumatic drug (csDMARD) prior to initiation of TCZ, including methotrexate, leflunomide, azathioprine or mycophenolate mofetil (mean duration, 23 weeks). In all cases, DMARD was continued after TCZ start.
Table 1.
Main features at diagnosis of 22 patients with giant cell arteritis treated with tocilizumab
| GCA patients treated with TCZ (n=22) | |
|---|---|
| Demographic data | |
| Age, years (mean±SD) | 72.1±7.0 |
| Female sex, n (%) | 14 (63.6) |
| Weeks from GCA diagnosis to TCZ start, median (range) | 58.5 (1–370) |
| GCA subset | |
| Cranial, n (%) | 10 (45.5) |
| LVV, n (%) | 10 (45.5) |
| Both, n (%) | 2 (9) |
| Cranial manifestations | |
| Temporal headache, n (%) | 16 (72.7) |
| Scalp tenderness, n (%) | 9 (40.9) |
| Jaw claudication, n (%) | 10 (45.5) |
| Visual disturbances, n (%) | 13 (59.0) |
|
1/13 (7.7) |
|
9/13 (69.2) |
|
3/13 (23.1) |
| Vision loss, n (%) | 6 (27.3) |
|
3/6 (50.0) |
|
2/6 (33.3) |
|
1/6 (16.7) |
| Systemic manifestations | |
| PMR symptoms, n (%) | 12 (54.5) |
| Constitutional symptoms, n (%) | 18 (81.8) |
|
4/18 (22.2) |
|
14/18 (77.8) |
|
13/18 (72.2) |
| Diagnosis confirmatory test | |
| Ultrasound, n (%) | 21 (95.4) |
|
7/21 (33.3) |
|
8/21 (38.1) |
|
5/21 (23.8) |
|
1/21 (4.8) |
| PET/CT alone, n (%) | 1 (4.5) |
| Comorbidities | |
| Hypertension, n (%) | 9 (40.9) |
| Diabetes mellitus, n (%) | 5 (22.7) |
| Osteoporosis, n (%) | 3 (13.6) |
| Patients treated with DMARDs, n (%) | 11 (50.0) |
| Leflunomide, n (%) | 6 (27.3) |
| Mycophenolate mofetil, n (%) | 2 (9.1) |
| Methotrexate, n (%) | 4 (18.2) |
| Azathioprine, n (%) | 1 (4.5) |
AION, anterior ischaemic optic neuritis; CRAO, central retinal artery occlusion; CT, computed tomography; GCA, giant cell arteritis; LVV, large vessel vasculitis; PET, position emission tomography; PMR, polymyalgia rheumatica; TCZ, tocilizumab.
Table 2.
Demographics, clinical, imaging and treatment details of 22 patients with giant cell arteritis treated with tocilizumab
| No. | Age | Sex | GCA type | Confirmatory test | Prior csDMARD |
Disease duration prior TCZ (months) | TCZ indication | TCZ treatment | Outcome | Prednisolone, dose at TCZ start | Prednisolone, lowest dose during TCZ |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 66 | F | LVV | US+PET | MMF | 19 | Refractory | Continuous | Remission | 40 mg | 2.5 mg |
| 2 | 73 | F | LVV | US+PET | MTX +AZA | 39 | Relapse | Continuous | Remission | 20 mg | 3.75 mg |
| 3 | 70 | F | LVV | US+PET | LEF | 14 | Relapse | Continuous | Remission | 15 mg | 2.5 mg |
| 4 | 84 | M | Cranial | US | 2 | Ischaemia | Continuous | Remission | 20 mg | 6 mg | |
| 5 | 72 | F | Both | US+TAB | 1 | Ischaemia | Continuous | Remission | 20 mg | 2.5 mg | |
| 6 | 70 | F | LVV | US+PET | LEF | 28 | Refractory | Discontinued | Remission | 20 mg | 1 mg |
| 7 | 75 | M | Cranial | US | 4 | Ischaemia | Continuous | Remission | 60 mg | 2.5 mg | |
| 8 | 70 | M | Cranial | US | 3 | Ischaemia | Interrupted | Remission | 60 mg | 15 mg | |
| 9 | 71 | F | LVV | US+PET | MTX | 35 | Refractory | Completed | Active | 60 mg | 2.5 mg |
| 10 | 77 | F | Cranial | US | 2 | Refractory | Continuous | Remission | 50 mg | 2.5 mg | |
| 11 | 59 | F | Cranial | US | 2 | Ischaemia | Completed | Remission | 60 mg | 2.5 mg | |
| 12 | 60 | M | LVV | US+PET | LEF | 15 | Refractory | Continuous | Remission | 10 mg | 0 mg |
| 13 | 75 | F | Both | US+TAB | MTX | 16 | Refractory | Discontinued | Active | 60 mg | 8.75 mg |
| 14 | 84 | F | Cranial | US+TAB | 0 | Ischaemia | Completed | Remission | 40 mg | 5 mg | |
| 15 | 69 | M | LVV | US+TAB+PET | MMF | 42 | Refractory | Completed | Remission | 30 mg | 0 mg |
| 16 | 68 | F | Cranial | US+TAB | MTX+LEF | 92 | Relapse | Continuous | Remission | 20 mg | 2 mg |
| 17 | 66 | M | LVV | US+PET | LEF | 20 | Refractory | Continuous | Remission | 20 mg | 15 mg |
| 18 | 76 | F | Cranial | US | 4 | Ischaemia | Continuous | Remission | 60 mg | 0 mg | |
| 19 | 71 | M | Cranial | US | 37 | Ischaemia | Continuous | Remission | 20 mg | 2.5 mg | |
| 20 | 88 | M | Cranial | US+TAB | 12 | Refractory | Continuous | Remission | 30 mg | 12.5 mg | |
| 21 | 71 | F | LVV | US+PET | LEF | 21 | Refractory | Continuous | Remission | 7.5 mg | 7.5 mg |
| 22 | 70 | F | LVV | PET | 3 | Refractory | Continuous | Remission | 30 mg | 0 mg |
AZA, azathioprine; csDMARD, conventional synthetic disease-modifying antirheumatic drug; GCA, giant cell arteritis; LEF, leflunomide; LVV: large vessel vasculitis; MTX, methotrexate; MMF, mycophenolate mofetil; PET: position emission tomography; TCZ: tocilizumab; US: ultrasound.
Efficacy and safety of tocilizumab in real-life practice
All patients received TCZ subcutaneously (162 mg weekly); it was initiated intravenously (8 mg/kg) in four patients (18%) with critical ischaemic presentation.
Four patients (18%) have completed 12 months of treatment. Three patients were in remission at this time point, with a maximum daily dose of 5 mg (range 0–5) prednisolone. One had an acute LV-GCA flare (confirmed by 18FDG-PET) within a month of stopping TCZ.
Fifteen patients (68%) are on TCZ (median duration of TCZ—43 weeks) and all remain in remission at their most recent follow-up evaluation with a median daily dose of 2.5 mg (range, 0–12.5) prednisolone.
In one patient, TCZ is currently withheld due to a leg ulcer. He is in remission with 15 mg of prednisolone (table 3). Six patients developed adverse events leading to a brief discontinuation of TCZ (2–8 weeks). In two patients (9%), TCZ had to be permanently discontinued. The first patient had a severe allergic reaction to TCZ, and the disease was in remission with 1 mg of prednisolone. In the second patient, TCZ was discontinued after six injections due to detection of new bladder cancer. At the last review, she was on prednisolone 8.75 mg daily.
Table 3.
Adverse events on TCZ
| Patient | Adverse event | TCZ outcome | Duration of TCZ suspension |
|---|---|---|---|
| Patient 1 | Varicella Zoster infection | Restarted | 6 weeks |
| Patient 3 | Chicken pox | Restarted | 4 weeks |
| Patient 4 | Dental abscess | Restarted | 8 weeks |
| Patient 5 | Myocardial infarction | Restarted | 2 weeks |
| Patient 6 | Severe allergic reaction | Discontinued | – |
| Patient 8 | Leg ulcer | On Hold | 3 weeks |
| Patient 10 | Injection site reaction | Restarted | 7 weeks |
| Patient 13 | Bladder cancer | Discontinued | – |
| Patient 16 | Urinary tract infection | Restarted | 2 weeks |
TCZ: tocilizumab.
There were no new reports of vision loss on any patient after TCZ was started.
Effect of tocilizumab on patient-reported outcomes
Subjective measures of Quality of life (QoL), specifically mood level, sleep hygiene and fatigue, were evaluated by a questionnaire at the most recent follow-up visit (online supplemental table S3). Patients were asked whether these parameters had improved, remained similar or worsened since TCZ treatment was started. Eight (36%) patients reported that all three measures had improved, one (5%) patient reported that two had improved, three (14%) patients reported that one had improved and nine (41%) patients reported that all three remained similar. Only one patient reported worsening in two measures.
rmdopen-2020-001417supp003.PDF (24.7KB, PDF)
Fifteen out of 22 patients contacted the Rheumatology advice line during their treatment, for a total of 28 calls (online supplemental table S4). Queries were mainly related to drug administration and possible adverse effects.
rmdopen-2020-001417supp004.PDF (20KB, PDF)
Effect of tocilizumab on imaging findings (online supplemental table S5)
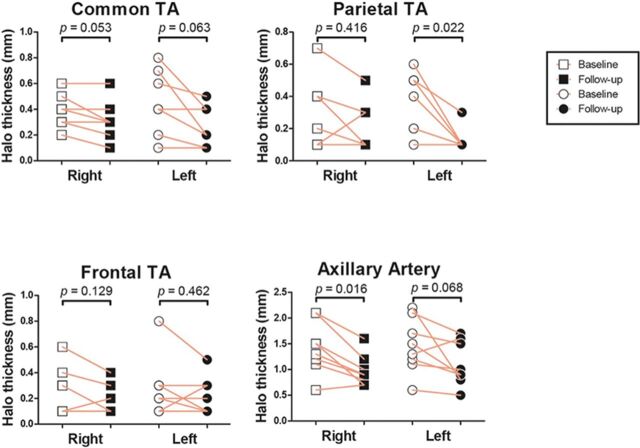
Change in halo thickness on US was assessed in 21 patients. Halo thickness in a total of 92 and 66 arterial segments was recorded pre and post TCZ, respectively. Among the 54 segments with a positive halo before TCZ start, 38 showed a reduction during follow-up (range 3–12 months) of treatment (figure 1).
Figure 1.
Halo thickness in individual patients at TA branches and AA. AA, axillary artery; TA, temporal artery.
rmdopen-2020-001417supp005.PDF (41.4KB, PDF)
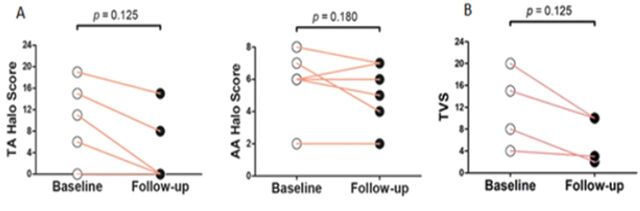
The effect of TCZ on the TA and AA Halo Score was evaluated in five and eight patients, respectively. TA Halo Score showed a marked improvement on follow-up (range 3–12 months). The AA Halo Score remained stable during the follow-up period (range 3–12 months) (figure 2).
Figure 2.
(A) TA and AA Halo Score pre and post TCZ. (B) PET-CT TVS before and after TCZ. AA, axillary artery; TA, temporal artery; TCZ, tocilizumab; TVS, total vascular score.
Baseline and follow-up 18FDG-PET/CT scans were available for four patients. The modified TVS decreased after 8 months (range 2–8 months) of TCZ treatment.
Online supplemental table S5 gives more information regarding US and PET-CT results with timelines of assessments.
DISCUSSION
Our real-life experience shows that TCZ is efficacious and safe outside the clinical trial setting in the treatment of relapsing and refractory GCA. It leads to a significant reduction in GC dose and improves QoL. Imaging abnormalities on US and FDG-PET/CT scan improve during treatment and may be used to monitor response and assess GCA disease activity.
Our study results indicate that TCZ has an effective steroid sparing effect in GCA patients, and this supports evidence from GiACTA.2 Our data showed a remission rate of 91% on TCZ with a significant reduction in GC from a median daily dose of 30 mg to 2.5 mg. The cumulative GC dose reduction is noted here, as in published data.18 Daily maintenance dose of GC (IQR 1–5 mg) was achieved within a shorter period as compared to EULAR and British Society for Rheumatology (BSR) recommendations.19–21 Clinical remission was defined as the absence of signs and symptoms of GCA. In addition, we included objective measures such as stability or improvement of US halo thickness or PET-CT uptake. Inflammatory parameters such as C-Reactive Protein (CRP) were not considered in our study to assess the disease activity as CRP level normalises with TCZ due to IL-6 blockade.22
NICE approval of TCZ in the UK is limited to 12 months. Among the four patients (18%) who completed the 12 months TCZ to date, one had an acute LV-GCA flare (confirmed by FDG-PET) within a month of stopping TCZ. A similar aortic flare after 12 months led to a death with a ruptured aortic aneurysm.23 Valvular heart diseases, aortic aneurysm and dissection are well-known complications of LV-GCA.24
Current NICE TA highlights the unmet need for effective GC sparing agents beyond 12 months of therapy in relapsing and refractory GCA. There is lack of quality evidence for maintenance of cDMARDs in GCA. A meta-analysis suggests a modest effect for methotrexate and there are case series and an open non-randomised study of leflunomide.25–27 We feel that TCZ retreatment should be permitted and even continued long term with aortic/large vessel disease. There is also a need to recruit TCZ unresponsive or post-TCZ flares to further clinical trials in GCA.
Permanent visual loss is a significant complication of GCA.28 Our study found six (27%) had lost sight before referral to our service. This data is higher than previously published data of GCA-related blindness of 15–20%29 30 but it is expected since NHSE guidelines include ischaemic vascular complications as refractory disease and hence as an eligibility criterion for TCZ.8
Diverticulitis and bowel perforation have been reported in TCZ-treated rheumatoid arthritis patients.31 In our study, two had to permanently discontinue TCZ due to severe adverse events. However, our findings suggest that overall TCZ is well tolerated.
Ninety-five per cent of patients had US as first imaging investigation to diagnose GCA. This complies with the EULAR recommendation of GCA.9 Halo thickness reduced in most TA and AA segments, with statistically significant reduction seen in two. The Southend Halo Score,15 particularly the TA Halo Score, showed considerable improvement in follow-up scans. A single patient with persisting AA halo has completed 12 months treatment and remains clinical remission. Our ongoing HAS GCA (HAlo Score GCA) study5 should provide definitive answers for the role of quantitative Southend Halo Score in the prognosis and monitoring of GCA.
The study demonstrated a good correlation between the PET-CT TVS and the disease activity. Four in clinical remission (18%) had a PET-CT before and after TCZ treatment with significant reduction in TVS at follow-up. Grading of vascular uptake against the liver uptake16 32 previously did not include the AAs.7 We feel that AA inclusion in a modified TVS is essential in LV-GCA. A limitation of PET-CT is decreased FDG uptake after high-dose GC treatment.33 In our patients 1, 12, 17 and 21 who had a follow-up PET-CT, the daily dose of prednisolone was 2.5, 0, 1.5 and 7.5 mg, respectively, which was withheld 2 weeks before the scan.
Nursing support played a major role in initiating and monitoring TCZ drug therapy. Nearly half of the patients’ queries could be resolved by specialist nursing staff. Patients were comfortable sharing their subjective measures and queries through the telephone helpline. Minor adverse events, blood monitoring abnormalities or logistic problems were identified promptly and brought to immediate attention of the treating clinician.
Among our strengths, this is the first case series with data collection based on the EULAR core dataset, towards a GCA registry. The use of US and PET-CT to diagnose and monitor our cohort along with nursing input seems a requirement for using biologic agents in GCA.
Among limitations is the retrospective real-life study design with small sample size. Not all the patients had completed the 12 months of follow-up. Follow-up US results were not available in all the segments. However, the available Halo Score results bear promise. Follow-up PET-CT and TVS were available in four patients but showed significant improvement and suggest PET-CT as an effective monitoring modality for LV-GCA.
In conclusion, our data suggests that TCZ is efficacious and safe in GCA. US is a valuable imaging tool for diagnosis and follow-up of GCA disease activity. Quantitative Southend Halo Score may be superior to halo thickness in assessment of GCA. PET-CT is a useful investigation, particularly in LV-GCA. Nursing support is vital and plays a pivotal role in GCA services.
Footnotes
Twitter: Bhaskar Dasgupta @profbdasgupta.
Acknowledgements: All authors would like to thank the Research and Development department at Southend University hospital and the East of England ENRAD MDT led by Dr Frances Hall.
Contributors: AS, DPP, MW and BD contributed to the conception or design of the work. DPP, AK, AT and BD contributed to acquisition of data. All US scans were done or supervised by BD. All PET-CT scans were reported and TVS calculated by JM. AS, DPP, KSMvdG and BD contributed to the analysis or the interpretation of data. All authors were involved in drafting the work or revising it critically for relevant intellectual content. All authors provided final approval of the version published.
Funding: AS received an international educational (Bresnihan-Molloy) fellowship grant from the Royal College of Physicians of Ireland.
Competing interests: BD reports grants and personal fees from Roche, personal fees from GSK, BMS, Sanofi and Abbie, outside the submitted work. KSMvdG reports grants from the Mandema Stipend and the Dutch Society for Rheumatology, and personal fees from Roche, outside the submitted work. All other authors have nothing to declare.
Patient consent for publication: Not required.
Ethics approval: This is a retrospective study where Research Ethics Committee approval is not required according to the local ethics guidelines.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All data relevant to the study are included in the article or available as supplemental material.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
REFERENCES
- 1. Dejaco C, Duftner C, Buttgereit F, et al. The spectrum of giant cell arteritis and polymyalgia rheumatica: revisiting the concept of the disease. Rheumatology (UK) 2017. 10.1093/rheumatology/kew273 [DOI] [PubMed] [Google Scholar]
- 2. Coath F, Gillbert K, Griffiths B, et al. Giant cell arteritis: new concepts, treatments and the unmet need that remains. Rheumatology (United Kingdom) 2019;58:1123–5. 10.1093/rheumatology/key326 [DOI] [PubMed] [Google Scholar]
- 3. Kermani TA, Dasgupta B. Current and emerging therapies in large-vessel vasculitis. Rheumatol (UK) 2018. 10.1093/rheumatology/kex385 [DOI] [PubMed] [Google Scholar]
- 4. Muratore F, Kermani TA, Crowson CS, et al. Large-vessel giant cell arteritis: A cohort study. Rheumatol (United Kingdom) 2015. 10.1093/rheumatology/keu329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sebastian A, Kayani A, Dasgupta B. Excellent response to leflunomide in a case of large-vessel giant cell arteritis demonstrated simultaneously by clinical, laboratory, ultrasound, and positron emission tomography/computed tomography parameters. JCR J Clin Rheumatol 2020;Publish Ahead of Print 10.1097/RHU.0000000000001393 [DOI] [PubMed] [Google Scholar]
- 6. Ehlers L, Askling J, Bijlsma HWJ, et al. 2018 EULAR recommendations for a core data set to support observational research and clinical care in giant cell arteritis. Ann Rheum Dis 2019;78:1160–6. 10.1136/annrheumdis-2018-214755 [DOI] [PubMed] [Google Scholar]
- 7. Stone JH, Tuckwell K, Dimonaco S, et al. Trial of tocilizumab in giant-cell arteritis. N Engl J Med 2017;377:317–28. 10.1056/NEJMoa1613849 [DOI] [PubMed] [Google Scholar]
- 8. NHS England Specialised services circular_SSC1894. Blueteq form. Technology appraisal 518: tocilizumab for treating giant cell arteritis. Issued July 2018. [Internet]. Available http://www.moz-extension://aa362167-b248-4b0b-904d-52bbf5bafc6d/enhanced-reader.html?openApp&pdf=https%3A%2F%2Fwww.nice.org.uk%2Fguidance%2Fta518%2Fresources%2Ftocilizumab-for-treating-giant-cell-arteritis-pdf-82606786726597 (accessed 17 Jul 2020)
- 9. Dejaco C, Ramiro S, Duftner C, et al. EULAR recommendations for the use of imaging in large vessel vasculitis in clinical practice. Ann Rheum Dis 2018;77:636–43. 10.1136/annrheumdis-2017-212649 [DOI] [PubMed] [Google Scholar]
- 10. Schmidt WA, Kraft HE, Vorpahl K, et al. Color duplex ultrasonography in the diagnosis of temporal arteritis. N Engl J Med 1997;337:1336–42. 10.1056/NEJM199711063371902 [DOI] [PubMed] [Google Scholar]
- 11. Chrysidis S, Duftner C, Dejaco C, et al. Definitions and reliability assessment of elementary ultrasound lesions in giant cell arteritis: a study from the OMERACT large vessel vasculitis ultrasound working group. RMD Open 2018;4:e000598 10.1136/rmdopen-2017-000598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Aschwanden M, Daikeler T, Kesten F, et al. Temporal artery compression sign - a novel ultrasound finding for the diagnosis of giant cell arteritis. Ultraschall Der Medizin 2013. 10.1055/s-0032-1312821 [DOI] [PubMed] [Google Scholar]
- 13. Aschwanden M, Imfeld S, Staub D, et al. The ultrasound compression sign to diagnose temporal giant cell arteritis shows an excellent interobserver agreement. Clin Exp Rheumatol 2015. [PubMed] [Google Scholar]
- 14. Schäfer VS, Juche A, Ramiro S, et al. Ultrasound cut-off values for intima-media thickness of temporal, facial and axillary arteries in giant cell arteritis. Rheumatol (UK) 2017. 10.1093/rheumatology/kex143 [DOI] [PubMed] [Google Scholar]
- 15. Van Der Geest KSM, Borg F, Kayani A, et al. Novel ultrasonographic halo score for giant cell arteritis: assessment of diagnostic accuracy and association with ocular ischaemia. Ann Rheum Dis 2019. 10.1136/annrheumdis-2019-216343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Slart RHJA, Glaudemans AWJM, Chareonthaitawee P, et al. FDG-PET/CT(A) imaging in large vessel vasculitis and polymyalgia rheumatica: Joint procedural recommendation of the EANM, SNMMI, and the PET Interest Group (PIG), and endorsed by the ASNC. Eur J Nucl Med Mol Imaging 2018. 10.1007/s00259-018-3973-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Grayson PC, Alehashemi S, Bagheri AA, et al. 18F-fluorodeoxyglucose: positron emission tomography as an imaging biomarker in a prospective, longitudinal cohort of patients with large vessel vasculitis. Arthritis Rheumatol 2018. 10.1002/art.40379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Calderón-Goercke M, Loricera J, Aldasoro V, et al. Tocilizumab in giant cell arteritis. Observational, open-label multicenter study of 134 patients in clinical practice. Semin Arthritis Rheum 2019. 10.1016/j.semarthrit.2019.01.003 [DOI] [PubMed] [Google Scholar]
- 19. Strehl C, Bijlsma JWJ, De Wit M, et al. Defining conditions where long-term glucocorticoid treatment has an acceptably low level of harm to facilitate implementation of existing recommendations: viewpoints from an EULAR task force. Ann Rheum Dis 2016;75:952–7. 10.1136/annrheumdis-2015-208916 [DOI] [PubMed] [Google Scholar]
- 20. Hellmich B, Agueda A, Monti S, et al. Update of the EULAR recommendations for the management of large vessel vasculitis. Ann Rheum Dis 2018;2020 10.1136/annrheumdis-2019-215672 [DOI] [PubMed] [Google Scholar]
- 21. Mackie SL, Dejaco C, Appenzeller S, et al. British society for rheumatology guideline on diagnosis and treatment of giant cell arteritis. Rheumatology 2020. 10.1093/rheumatology/kez664 [DOI] [PubMed] [Google Scholar]
- 22. Villiger PM, Adler S, Kuchen S, et al. Tocilizumab for induction and maintenance of remission in giant cell arteritis: a phase 2, randomised, double-blind, placebo-controlled trial. Lancet 2016. 10.1016/S0140-6736(16)00560-2 [DOI] [PubMed] [Google Scholar]
- 23. M C, S J A case for continuing tocilizumab beyond 12 months in giant cell arteritis? Rheumatol Adv Pract. 2019 doi: 10.1093/rap/rkz028.024. [DOI] [Google Scholar]
- 24. Robson JC, Kiran A, Maskell J, et al. The relative risk of aortic aneurysm in patients with giant cell arteritis compared with the general population of the UK. Ann Rheum Dis 2015. 10.1136/annrheumdis-2013-204113 [DOI] [PubMed] [Google Scholar]
- 25. Adizie T, Christidis D, Dharmapaliah C, et al. Efficacy and tolerability of leflunomide in difficult-to-treat polymyalgia rheumatica and giant cell arteritis: a case series. Int J Clin Pract 2012. 10.1111/j.1742-1241.2012.02981.x [DOI] [PubMed] [Google Scholar]
- 26. Diamantopoulos AP, Hetland H, Myklebust G. Leflunomide as a corticosteroid-sparing agent in giant cell arteritis and polymyalgia rheumatica: a case series. Biomed Res Int 2013. 10.1155/2013/120638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hočevar A, Ješe R, Ž R, et al. Does leflunomide have a role in giant cell arteritis? An open-label study. Clin Rheumatol 2019. 10.1007/s10067-018-4232-x [DOI] [PubMed] [Google Scholar]
- 28. Patil P, Williams M, Maw WW, et al. Fast track pathway reduces sight loss in giant cell arteritis: results of a longitudinal observational cohort study. Clin Exp Rheumatol 2015. [PubMed] [Google Scholar]
- 29. Salvarani C, Cimino L, Macchioni P, et al. Risk factors for visual loss in an Italian population-based cohort of patients with giant cell arteritis. Arthritis Care Res 2005. 10.1002/art.21075 [DOI] [PubMed] [Google Scholar]
- 30. González-Gay MA, García-Porrúa C, Llorca J, et al. Visual manifestations of giant cell arteritis: trends and clinical spectrum in 161 patients. Medicine (Baltimore) 2000. 10.1097/00005792-200009000-00001 [DOI] [PubMed] [Google Scholar]
- 31. Strangfeld A, Richter A, Siegmund B, et al. Risk for lower intestinal perforations in patients with rheumatoid arthritis treated with tocilizumab in comparison to treatment with other biologic or conventional synthetic DMARDs. Ann Rheum Dis 2017. 10.1136/annrheumdis-2016-209773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Soussan M, Nicolas P, Schramm C, et al. Management of large-vessel vasculitis with FDG-PET. Medicine (United States) 2015. 10.1097/MD.0000000000000622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nielsen BD, Gormsen LC, Hansen IT, et al. Three days of high-dose glucocorticoid treatment attenuates large-vessel 18F-FDG uptake in large-vessel giant cell arteritis but with a limited impact on diagnostic accuracy. Eur J Nucl Med Mol Imaging 2018. 10.1007/s00259-018-4021-4 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2020-001417supp002.PDF (474.1KB, PDF)
rmdopen-2020-001417supp001.PDF (99.2KB, PDF)
rmdopen-2020-001417supp003.PDF (24.7KB, PDF)
rmdopen-2020-001417supp004.PDF (20KB, PDF)
rmdopen-2020-001417supp005.PDF (41.4KB, PDF)