Abstract
Objectives
To assess the long-term effectiveness of pirfenidone in idiopathic pulmonary fibrosis (IPF) treatment and to establish its adverse effects profile.
Methods
Retrospective observational study in patients with IPF who initiated treatment with pirfenidone between 2011 and 2016. We collected demographic variables (age, sex); date of first and last treatment; reason for discontinuation; pulmonary function measures (forced vital capacity (FVC), carbon monoxide diffusion capacity (DLCO), and 6 min walk test (6MWT)) at treatment initiation (baseline) and at 1, 2 and 3 year follow-up; adherence to pirfenidone treatment; recorded adverse effects; and mortality.
Results
Thirty-one patients treated with pirfenidone were included; mean±SD age was 69±8 years, 74% were men, and 59% had a smoking history. Mean baseline values were: FVC 2.43±0.66 L (61.8±12.1%); DLCO 46.1±19.4%; and 6MWT 334±125 m. Median duration of treatment was 14±13 months, and treatment was discontinued in 58% of patients. The most frequently observed adverse effects were gastrointestinal disturbances and photosensitivity. Twenty (65%) patients were evaluated at 1 year, when mean FVC was 2.41±0.86 L (64.7±20.3%); DLCO 50.8±26.8%; and 6MWT 341±139 m. At 2 years’ follow-up, 11 patients (36%) who were still taking pirfenidone were evaluated. Mean FVC was 2.34±0.79 L (66.2±14.7%); DLCO 50.0±28.3%; and 6MWT 265±121 m. At 3 years, five patients were still taking the treatment. Mean FVC was 2.71±0.84 L (71.0±24.7%); DLCO 52.6±26.7%; and 6MWT 286±139 m. Nineteen per cent of patients were non-adherent to treatment.
Conclusions
Pirfenidone seems to be effective for long-term control of IPF despite substantial variability in response among individual patients. The most frequent adverse effects were digestive and cutaneous, prompting in some cases a reduction in dose or even discontinuation of the treatment.
Keywords: pirfenidone, effectiveness, safety, idiopathic pulmonary fibrosis, long-term study, adherence
Introduction
Idiopathic pulmonary fibrosis (IPF) is a rare disease with unknown aetiology and poor prognosis, characterised by the gradual fibrosis of the pulmonary interstitium. Its clinical course is variable, but the disease is associated with high morbimortality.1 Prevalence is estimated at 3 to 9 cases per 100 000 people,2 and mean survival is 2 to 5 years from onset of symptoms.3 There are few therapeutic options; the only treatment that leads to a substantial improvement in function and survival is a lung transplant. Failing that, currently available drug treatments (pirfenidone and nintedanib) aim to stabilise the course of the disease or at least slow the speed of its progression.4
The European Medicines Agency approved pirfenidone in February 2011 for the treatment of mild to moderate IPF, defined as forced vital capacity (FVC) of 50% or more and carbon monoxide diffusion capacity (DLCO) of 35% or more. Pirfenidone has anti-inflammatory, antifibrotic, and antioxidant properties5 that have proven beneficial for slowing the progression of IPF and the deterioration of FVC, and for improving mortality outcomes; however, the drug may also cause adverse effects that lead to discontinuation of treatment, mainly gastrointestinal problems such as nausea, vomiting and dyspepsia.4 6 7
This study aims to assess the long-term effectiveness of pirfenidone for treating IPF and to establish its adverse effects profile in patients receiving treatment in a tertiary hospital. Primary outcomes were related to lung function (FVC and DLCO), measured over 3 years from the start of treatment. Secondary outcomes were also measured over 3 years: (a) adverse effects; (b) rate of treatment discontinuation; (c) reasons for treatment discontinuation; (d) mortality in patients receiving treatment; (e) exercise capacity, measured using the 6 min walk test (6MWT); and (f) short-term evolution of lung function (FVC and DLCO) and exercise capacity (6MWT), using 1 and 2 year follow-up as intermediate time points.
Methods
This was a retrospective observational study in patients with IPF who were started on pirfenidone treatment from 2011 to December 2016. We included all patients with IPF whose clinical records included baseline measures (ie, at treatment initiation) for FVC, DLCO and 6MWT. As pirfenidone’s data sheet indicates this drug for the treatment of mild to moderate IPF, our patients had, at the time they started pirfenidone, FVC ≥50% and DLCO ≥35%. We collected the following data: demographic variables (age, sex, comorbidities); date of initiation and end of treatment; reason for discontinuing treatment; lung function tests (FVC and DLCO as absolute values and as the relative percentage compared with the reference values established by formulae described in existing clinical practice guidelines) and exercise test (6MWT) at baseline, 1, 2 and 3 year follow-up; adherence to pirfenidone treatment, measured according to hospital pharmacy dispensing records (patients who stopped collecting their prescriptions from the hospital pharmacy were considered non-adherent); adverse effects; and mortality.
To evaluate the qualitative evolution of the disease with regard to FVC (predicted values), we defined stability between assessment time points as a variation of up to 10% from the previously recorded value. Values that increased by >10% were considered improvements, and those that decreased by >10% were considered to show worsening. For DLCO (predicted values), the reference value to define stability, improvement, or worsening was 15%. For 6MWT, 50 m was the cut-off for establishing differences.
All statistical analyses were performed with SPSS software (version 25.0). Continuous data were recorded as mean±SD. The Shapiro-Wilk test was used to test for normality of these data. Lung function values were compared through time with the Mann-Whitney U test.
Results
Demographic characteristics
We included 31 patients (74% men; 59% had a smoking history) with a mean age of 69±8 years. Complete information on the baseline demographic characteristics of our patients is provided in online supplementary file 1. Mean baseline values were: FVC 2.43±0.66 L (61.8±12.1%); DLCO 46.1±19.4%; and 6MWT 334±125 m (table 1). Median length of time between IPF diagnosis and the start of pirfenidone treatment was 13.61±15.22 months. Median duration of pirfenidone treatment was 14±13 months at study end, by which time treatment had been discontinued in 18 (58%) patients (1–49 months). These patients stopped treatment due to digestive complaints (39%), death (28%), treatment inefficacy (17%), photosensitivity (11%), and non-adherence (5%).
Table 1.
Baseline lung function and exercise values.
| Baseline values | |
| Mean±SD FVC, L | 2.43±0.66 |
| Mean±SD FVC, % | 61.8±12.1 |
| Mean±SD DLCO, % | 46.1±19.4 |
| Mean±SD 6MWT, m | 334±125 |
DLCO, carbon monoxide diffusion capacity;FVC, forced vital capacity; 6MWT, 6 min walk test.
ejhpharm-2018-001806supp001.pdf (59.2KB, pdf)
Figure 1 represents mean FVC in patients throughout time. Lung function measured as FVC remains with no statistically significant change at the end of the 3 year observational period. In addition, no changes were observed at the intermediate points of evaluation (1 and 2 years). Table 2 summarises all the data on lung function and exercise capacity in the patients throughout the study period.
Figure 1.
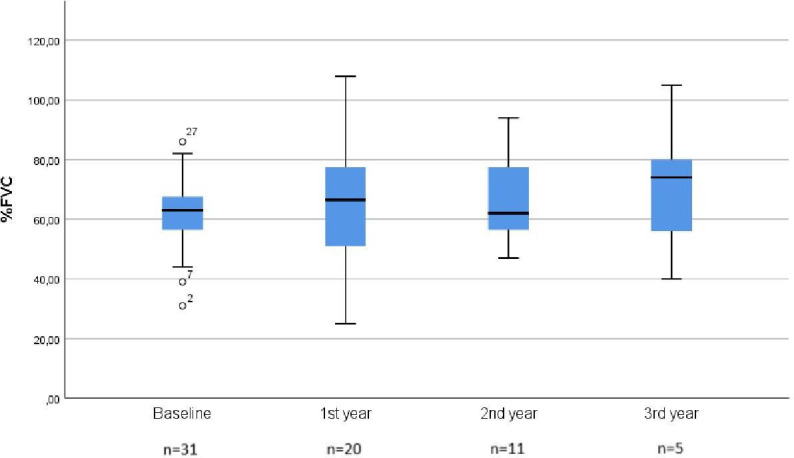
Mean forced vital capacity (%FVC) throughout the 3 year observational period.
Table 2.
Evolution of lung function and exercise capacity over follow-up.
| Baseline | 1 year | 2 years | 3 years | ||
| No. patients assessed (%) | 31 (100%) | 20 (65%) | 11 (36%) | 5 (16%) | |
| Mean±SD FVC (% pred) | 61.8±12.1% | 64.7±20.3% | 66.2±14.7% | 71.0±24.7% | |
| DLCO±SD (% pred) | 46.1±19.4% | 50.8±26.8% | 50.0±28.3% | 52.6±26.7% | |
| 6MWT±SD (m) | 334±12 | 341±139 | 265±121 | 286±139 | |
| Change from baseline* | |||||
| FVC±SD (% pred) | — | +2.7±12.1% | +2.2±13.0% | +8.8±18.1% | |
| DLCO±SD (% pred) | — | +3.6±13.4% | +4.2±17.9% | +7.4±21.9% | |
| 6MWT±SD (m) | — | +2±99 | −63±121 | +21±129 | |
| Qualitative evolution (% patients) | |||||
| FVC | Improved | — | 30% | 18% | 40% |
| Stabilised | — | 60% | 82% | 60% | |
| Worsened | — | 10% | 0% | 0% | |
| DLCO | Improved | — | 50% | 18% | 20% |
| Stabilised | — | 45% | 73% | 80% | |
| Worsened | — | 5% | 9% | 0% | |
| 6MWT | Improved | — | 15% | 27% | 60% |
| Stabilised | — | 70% | 18% | 20% | |
| Worsened | — | 15% | 55% | 20% | |
*The change from baseline is referred to the change from the baseline of the patients assessed.
DLCO, carbon monoxide diffusion capacity;FVC, forced vital capacity; 6MWT, 6 min walk test.
Primary outcomes
At 3 years, five patients were still taking the treatment. Mean FVC was 2.71±0.84 L (71.0±24.7%); DLCO 52.6±26.7%; and 6MWT 286±139 m. Change from baseline for FVC was +0.01±0.36 L (+8.8±18.1%); DLCO +7.4±21.9%; and 6MWT+21±129 m. For FVC, there was a qualitative improvement in 40% of cases and stabilisation in 60%; for DLCO, improvement in 20% and stabilisation in 80%; and for 6MWT, improvement in 60%, stabilisation in 20%, and worsening in 20%. Nineteen per cent of patients were non-adherent to treatment.
Secondary outcomes
At 3 years, eight (26%) patients had died, all of them due to respiratory causes. Pirfenidone treatment had been stopped in two of these cases due to lack of efficacy. Table 3 analyses mean FVC at baseline and each year comparing survivors and non-survivors. As this table shows, mean FVC at any time was lower in patients who died than in survivors, but it only had statistical significance in the first year of treatment.
Table 3.
Statistical analysis of survivors versus non-survivors FVC at baseline and throughout time
| n | Mean±SD (%) | Statistical significance | ||
| FVC (%) baseline | Survivors | 24 | 64.92±9.63 | p=0.770 |
| Non-survivors | 7 | 51.43±14.63 | ||
| FVC (%) first year | Survivors | 15 | 70.60±18.43 | p=0.033 |
| Non-survivors | 5 | 46.80±15.29 | ||
| FVC (%) second year | Survivors | 9 | 68.78±15.05 | p=0.491 |
| Non-survivors | 2 | 54.50±4.95 | ||
| FVC (%) third year | Survivors | 4 | 78.75±20.25 | p=1.00 |
| Non-survivors | 1 | 40.00±0.00 |
Significance p<0.05 (95% CI). A non-survivor was a patient who died at any time.
FVC, forced vital capacity.
Table 4 compares mean FVC at baseline and yearly in patients who discontinued pirfenidone and patients who did not. As expected, those who discontinued pirfenidone had a lower FVC at baseline and every following year than those who continued pirfenidone. This fact appeared to be more relevant in patients at the baseline and in the first year of treatment.
Table 4.
Statistical analysis of dropouts versus non-dropouts FVC throughout time.
| n | Mean±SD (%) | Statistical significance | ||
| FVC (%) baseline | Non-dropout | 14 | 67.64±10.40 | p=0.021 |
| Dropout | 17 | 57.12±11.58 | ||
| FVC (%) first year | Non-dropout | 11 | 76.00±18.39 | p=0.005 |
| Dropout | 9 | 50.78±12.69 | ||
| FVC (%) second year | Non-dropout | 6 | 76.00±12.44 | p=0.061 |
| Dropout | 5 | 54.40±5.55 | ||
| FVC (%) third year | Non-dropout | 3 | 86.33±16.44 | p=0.400 |
| Dropout | 2 | 4800±11.31 |
Significance p<0.05 (95% CI). A dropout was a patient who discontinued pirfenidone at any time.
FVC, forced vital capacity.
Six of the 31 (19%) patients were non-adherent. All of these patients stopped treatment, in 67% of the cases due to adverse effects: three patients experienced gastrointestinal disorders and one patient experienced phototoxicity. In one case, the patient stopped treatment due to perceived inefficacy, and the other became non-adherent after reducing the dosage following adverse gastrointestinal effects.
Table 5 shows the adverse effects reported in the patients as well as the treatment modifications arising from them. There were a total 45 adverse effects, or an average of 1.45 per patient. Ten (22%) effects led to a reduction in dose, and 16 (35%) prompted the definitive discontinuation of treatment. Regarding short-term evolution of lung function and exercise capacity, results at intermediate time points were as follows.
Table 5.
Adverse reactions to treatment and subsequent dosage adjustment
| Adverse effects | N (%) | Dosage reduction | Discontinuation |
| Musculoskeletal | 2 (6%) | 0 | 0 |
| Myalgia | 1 (3%) | 0 | 0 |
| Arthralgia | 1 (3%) | 0 | 0 |
| General | 2 (6%) | 0 | 0 |
| Tiredness | 1 (3%) | 0 | 0 |
| Fatigue | 1 (3%) | 0 | 0 |
| Gastrointestinal | 19 (61%) | 4 (13%) | 7 (23%) |
| Non-specific gastrointestinal disturbances | 6 (19%) | 0 | 1 (3%) |
| Dyspepsia | 1 (3%) | 0 | 0 |
| Aerophagy | 1 (3%) | 0 | 0 |
| Abdominal pain | 6 (19%) | 2 (6%) | 3 (10%) |
| Nausea | 9 (29%) | 2 (6%) | 6 (19%) |
| Vomiting | 3 (10%) | 0 | 3 (10%) |
| Diarrhoea | 4 (13%) | 3 (10%) | 1 (3%) |
| Constipation | 1 (3%) | 1 (3%) | 0 |
| Heartburn | 1 (3%) | 1 (3%) | 0 |
| Cutaneous | 5 (16%) | 0 | 2 (6%) |
| Phototoxicity | 3 (10%) | 0 | 1 (3%) |
| Exanthema | 1 (3%) | 0 | 1 (3%) |
| Pruritus | 1 (3%) | 0 | 0 |
| Psychiatric | 2 (6%) | 0 | 0 |
| Insomnia | 1 (3%) | 0 | 0 |
| Moodiness | 1 (3%) | 0 | 0 |
| Central nervous system | 2 (6%) | 1 (3%) | 0 |
| Headache | 1 (3%) | 0 | 0 |
| Dizziness | 1 (3%) | 1 (3%) | 0 |
Twenty (65%) patients were followed up at 1 year, when mean FVC was 2.41±0.86 L (64.7±20.3%); DLCO 50.8±26.8%; and 6MWT 341±139 m. Mean change from baseline for FVC was −0.05±0.38 L (+2.7±12.1%); DLCO +3.6±13.4%; and 6MWT +2±99 m. Qualitative measures were: for FVC, improvement in 30% of patients, stabilisation in 60%, and worsening in 10%; for DLCO, improvement in 50%, stabilisation in 45%, and worsening in 5%; and for 6MWT, improvement in 15%, stabilisation in 70%, and worsening in 15%.
At 2 years’ follow-up, 11 patients (36%) who were still taking pirfenidone were evaluated. Mean FVC was 2.34±0.79 L (66.2±14.7%); DLCO 50.0±28.3%; and 6MWT 265±121 m. Change from baseline for FVC was −0.04±0.29 L (+2.2±13.0%); DLCO +4.2±17.9%; and 6MWT −63±121 m. Qualitative indicators for FVC showed improvement in 18% of patients and stabilisation in 82%; for DLCO: improvement in 18%, stabilisation in 73%, and worsening in 9%; and for 6MWT: improvement in 27%, stabilisation in 18%, and worsening in 55%.
Discussion
Historically, treatment of IPF has focused on the use of anti-inflammatory and immunomodulating agents, but data supporting this approach were weak. A definitive answer to the use of these agents was provided by the PANTHER- IPFP study. It was a randomised, double-blind, placebo-controlled trial of IPF patients randomised to prednisone+azathioprine and N-acetylcysteine (NAC), NAC alone, or placebo. Increased rate of hospitalisation and death was found in the combination group when compared with the placebo group, leading to definitive advice against their use.6
Another past treatment for IPF patients was NAC in monotherapy, as its antioxidant properties were thought to provide some benefits in the disease. Again, data supporting its use were weak. A definitive answer was provided by the NAC arm of the PANTHER-IPF study, demonstrating that NAC was not effective in slowing down the rate of progression of the disease.7 A decrease in FVC is the best predictor of imminent mortality in this disease,8–10 with patients showing a decrease in FVC of 10% or more over 24 weeks having a 4.8-fold greater risk of death at 1 year; in those with a 5–10% decrease, the risk of death is 2.1 times higher.11 For that reason, the international guidelines for IPF consider an absolute decrease of 10% of FVC to be evidence of significant disease progression.1 Nevertheless, neither this 10% reduction nor admission to hospital necessarily indicate treatment failure with pirfenidone, with some data indicating that continuing treatment in these cases reduces the risk for continued deterioration of FVC or death at 6 months.12 Similarly, diminished FVC has been shown to be a predictor for early mortality but not future deterioration of FVC.12 Another predictor of mortality was a decrease of 15% or more in DLCO over 6 to 12 months, so this measure is also considered a criterion of disease progression.13
The use of antifibrotic drugs, such as pirfenidone, was a definitive milestone in the management of IPF. Pirfenidone has slowed disease progression and has decreased mortality compared with placebo in phase III clinical trials.14 15 Before effective treatments had been used, median survival of patients with IPF was only 2 to 3 years (with only 20–30% of subjects alive 5 years after diagnosis), and mean annual rate of decline in FVC ranged from 150 mL to 200 mL.16 17 This reduction tends to be smaller under pirfenidone treatment.
Long-term data on the effectiveness of pirfenidone is available from the RECAP trial, with an annualised rate of FVC decline of 144±6.0 mL, a mean change in percent predicted FVC at 180 weeks of −9.6%, and a median survival of 77.2 months from the beginning of the treatment.18
In a randomised trial, the annual reduction in FVC was 80–90 mL.19 In a retrospective multicentre study, mean FVC decreased by just 30 mL in the first year and then by an average of 158 mL and 201 mL in the second and third year, respectively,20 suggesting that the benefits of pirfenidone treatment are limited to the first year. More recent studies in a clinical practice setting point to an attenuation in the degree of annual reduction of FVC. Some compare results with measures taken before initiation of treatment, confirming this trend.21–23 Another multicentre study performed in Denmark confirmed that pirfenidone lessened the mean reduction in parameters such as the FVC, DLCO and 6MWT, helping to maintain change scores below the thresholds established for clinically significant disease progression.24 In a study in China, mean FVC and DLCO values had improved by 3.5% and 1.1% in the first 6 months while controls worsened these values by 2.3% and 4.7%; although this improvement was lost after a year, it still stabilised lung function.25
Another important ‘real-world’ study, conducted in Greece, demonstrated functional improvement and functional stabilisation for a large group of patients in the first 3 years of treatment with pirfenidone.26 The same group of authors tried to demonstrate that pirfenidone could be used in patients with severe IPF, and pirfenidone decreased functional decline compared with 6 months before treatment initiation, but this benefit did not last for 1 year after initiation.27
The high length of time between IPF diagnosis and pirfenidone use is remarkable in our population. Patients included were diagnosed with IPF during the period 2008–2016, but pirfenidone was not officially approved in Spain for IPF until late 2014. Before 2014, most of our patients were using other available treatments, such as prednisone or azathioprine. Pirfenidone was available for the first time in Spain in 2011, when it could be requested in a compassionate use programme, which accounts for the delay in beginning treatment. In the present study, our patients receiving pirfenidone had a significantly smaller mean annual reduction in FVC, of up to 50 mL, than the majority of patients in the above cited studies, with similar change scores even at 3 years among patients who continued treatment in the long term. Furthermore, mean functional parameters had slightly increased in our patients after 1, 2 and 3 years of treatment. In our population we could corroborate that lung function tended to stabilise through the years in most patients, as previously seen.26 During a follow-up period of up to 3 years, minor changes were observed in lung function in our IPF patients. These minor changes are very relevant from a clinical point of view.
Adverse effects were the most common reason for discontinuing treatment, which is consistent with findings from similar studies.20 The most frequent effects in our population were gastrointestinal and cutaneous, with values similar to those reported in the CAPACITY study14 and in studies similar to ours.21 24 26 We also obtained similar adverse effects to those in a recent safety study.28 In some cases, adverse effects could be controlled with a reduction in dose, and in others treatment had to be stopped altogether. Once an adverse effect appears, the dose adjustment leads to a smaller percentage of patients who have to stop treatment due to adverse effects and other causes, including death.29
We measured adherence using the hospital pharmacy outpatient dispensing registry. This method reveals which patients are collecting their prescriptions on time, allowing inferences regarding adherence. The administration of pirfenidone follows a complex dosing regimen, starting with an initial period to build up tolerance, followed by a maintenance period with three daily doses of three capsules each. Given this complexity, and the fact that treatment mainly occurs in the context of polypharmacy, real adherence could be lower than that reflected in the hospital dispensing records.
Data from this study seem to indicate that pirfenidone has a positive effect by stabilising (and even improving) the natural course of IPF, even if the predicted trend would be a slight worsening of function. This is consistent with data from pivotal studies and real-world studies. Now efforts are focused on the development of new drugs and targets that allow these health outcomes to be improved.30 However, the study does have certain limitations, chief among them the retrospective collection of data, which in the case of aspects like drug adherence did not permit us to obtain real data but only absolute non-adherence data. Another important limitation was the small sample size, which makes it difficult to draw firm conclusions regarding the effectiveness of the drug, especially in light of the high variation in individual responses. The one-centre model was also a major flaw.
Conclusions
In our population, pirfenidone managed to stabilise lung function, as measured by both FVC and DLCO, in all patients who took it for the whole 3 year follow-up period. Aerobic capacity and endurance, as measured in the 6MWT, worsened in 20% of the patients treated at 3 years, although it remained stable or improved in the rest of the patients. Pirfenidone was generally well tolerated, despite the need to adjust the dose in some cases. The main adverse effects were gastrointestinal followed by cutaneous effects. These reactions were the main reason for discontinuing treatment. During the study period, 26% of the patients died, all of them due to respiratory causes; this outcome was most apparent at 2 years. At the intermediate time points (1 and 2 years after initiation of treatment), lung function improved or stabilised in most of the patients. Regarding the exercise test, the 6MWT showed a reduction in aerobic capacity and endurance at 2 years in most of the patients assessed (55%).
What this paper adds.
What is already known on this subject
The use of pirfenidone has been shown to be atherapeutic approach to idiopathic pulmonary fibrosis that is able to slow the progression of the disease in its earlier stages
Further studies about its use long term and in a real-world environment are needed.
What this study adds
Pirfenidone could be an effective option tostabilise the course of idiopathic pulmonaryfibrosis in long-term treatment.
ejhpharm-2018-001806supp002.pdf (190.2KB, pdf)
Footnotes
Contributors: All the authors participated in the conception and design of the work, all the authors believe that the manuscript represents valid work, and have carefully read and fully approve of it.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Xaubet A, Ancochea J, Bollo E, et al. . Normativa sobre El diagnóstico Y tratamiento de la fibrosis pulmonar idiopática. Arch Bronconeumol 2013;49:343–53. 10.1016/j.arbres.2013.03.011 [DOI] [PubMed] [Google Scholar]
- 2. Hutchinson J, Fogarty A, Hubbard R, et al. . Global incidence and mortality of idiopathic pulmonary fibrosis: a systematic review. Eur Respir J 2015;46:795–806. 10.1183/09031936.00185114 [DOI] [PubMed] [Google Scholar]
- 3. Fisher M, Nathan SD, Hill C, et al. . Predicting life expectancy for pirfenidone in idiopathic pulmonary fibrosis. J Manag Care Spec Pharm 2017;23(3-b Suppl):S17–S24. 10.18553/jmcp.2017.23.3-b.s17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Raghu G, Rochwerg B, Zhang Y, et al. . An official ATS/ERS/JRS/ALAT clinical practice guideline: treatment of idiopathic pulmonary fibrosis. An update of the 2011 clinical practice guideline. Am J Respir Crit Care Med 2015;192:e3–19. 10.1164/rccm.201506-1063ST [DOI] [PubMed] [Google Scholar]
- 5. Nakazato H, Oku H, Yamane S, et al. . A novel anti-fibrotic agent pirfenidone suppresses tumor necrosis factor-alpha at the translational level. Eur J Pharmacol 2002;446:177–85. 10.1016/S0014-2999(02)01758-2 [DOI] [PubMed] [Google Scholar]
- 6. Raghu G, Anstrom KJ, King TE, et al. . Prednisone, azathioprine, and N-acetylcysteine for pulmonary fibrosis. N Engl J Med 2012;366:1968–77. 10.1056/NEJMoa1113354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Martinez FJ, de Andrade JA, Anstrom KJ, et al. . Randomized trial of acetylcysteine in idiopathic pulmonary fibrosis. N Engl J Med 2014;370:2093–101. 10.1056/NEJMoa1401739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Collard HR, King TE, Bartelson BB, et al. . Changes in clinical and physiologic variables predict survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2003;168:538–42. 10.1164/rccm.200211-1311OC [DOI] [PubMed] [Google Scholar]
- 9. Zappala CJ, Latsi PI, Nicholson AG, et al. . Marginal decline in forced vital capacity is associated with a poor outcome in idiopathic pulmonary fibrosis. Eur Respir J 2010;35:830–6. 10.1183/09031936.00155108 [DOI] [PubMed] [Google Scholar]
- 10. Richeldi L, Ryerson CJ, Lee JS, et al. . Relative versus absolute change in forced vital capacity in idiopathic pulmonary fibrosis. Thorax 2012;67:407–11. 10.1136/thoraxjnl-2011-201184 [DOI] [PubMed] [Google Scholar]
- 11. du Bois RM, Weycker D, Albera C, et al. . Forced vital capacity in patients with idiopathic pulmonary fibrosis: test properties and minimal clinically important difference. Am J Respir Crit Care Med 2011;184:1382–9. 10.1164/rccm.201105-0840OC [DOI] [PubMed] [Google Scholar]
- 12. Nathan SD, Albera C, Bradford WZ, et al. . Effect of continued treatment with pirfenidone following clinically meaningful declines in forced vital capacity: analysis of data from three phase 3 trials in patients with idiopathic pulmonary fibrosis. Thorax 2016;71:429–35. 10.1136/thoraxjnl-2015-207011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nathan SD, Shlobin OA, Weir N, et al. . Long-term course and prognosis of idiopathic pulmonary fibrosis in the new millennium. Chest 2011;140:221–9. 10.1378/chest.10-2572 [DOI] [PubMed] [Google Scholar]
- 14. Noble PW, Albera C, Bradford WZ, et al. . Pirfenidone in patients with idiopathic pulmonary fibrosis (capacity): two randomised trials. The Lancet 2011;377:1760–9. 10.1016/S0140-6736(11)60405-4 [DOI] [PubMed] [Google Scholar]
- 15. King TE, Bradford WZ, Castro-Bernardini S, et al. . A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med 2014;370:2083–92. 10.1056/NEJMoa1402582 [DOI] [PubMed] [Google Scholar]
- 16. Raghu G, Collard HR, Egan JJ, et al. . An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011;183:788–824. 10.1164/rccm.2009-040GL [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ley B, Collard HR, King TE. Clinical course and prediction of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2011;183:431–40. 10.1164/rccm.201006-0894CI [DOI] [PubMed] [Google Scholar]
- 18. Costabel U, Albera C, Lancaster LH, et al. . An open-label study of the long-term safety of pirfenidone in patients with idiopathic pulmonary fibrosis (recap). Respiration 2017;94:408–15. 10.1159/000479976 [DOI] [PubMed] [Google Scholar]
- 19. Taniguchi H, Ebina M, Kondoh Y, et al. . Pirfenidone in idiopathic pulmonary fibrosis. Eur Respir J 2010;35:821–9. 10.1183/09031936.00005209 [DOI] [PubMed] [Google Scholar]
- 20. Bando M, Yamauchi H, Ogura T, et al. . Clinical experience of the long-term use of pirfenidone for idiopathic pulmonary fibrosis. Intern Med 2016;55:443–8. 10.2169/internalmedicine.55.5272 [DOI] [PubMed] [Google Scholar]
- 21. Hughes G, Toellner H, Morris H, et al. . Real world experiences: pirfenidone and nintedanib are effective and well tolerated treatments for idiopathic pulmonary fibrosis. J Clin Med 2016;5 10.3390/jcm5090078. [Epub ahead of print: 02 Sep 2016]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Harari S, Caminati A, Albera C, et al. . Efficacy of pirfenidone for idiopathic pulmonary fibrosis: an Italian real life study. Respir Med 2015;109:904–13. 10.1016/j.rmed.2015.04.010 [DOI] [PubMed] [Google Scholar]
- 23. Ogawa K, Miyamoto A, Hanada S, et al. . The efficacy and safety of long-term pirfenidone therapy in patients with idiopathic pulmonary fibrosis. Intern Med 2018;57:2813–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Salih GN, Shaker SB, Madsen HD, et al. . Pirfenidone treatment in idiopathic pulmonary fibrosis: nationwide Danish results. Eur Clin Respir J 2016;3 10.3402/ecrj.v3.32608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yan YJ, Fan YL, SW Y, et al. . Real-life experience with pirfenidone in idiopathic pulmonary fibrosis. Zhonghua Jie He He Hu Xi Za Zhi 2018;41:327–32. [DOI] [PubMed] [Google Scholar]
- 26. Tzouvelekis A, Karampitsakos T, Ntolios P, et al. . Longitudinal “real-world” outcomes of pirfenidone in idiopathic pulmonary fibrosis in Greece. Front Med 2017;4 10.3389/fmed.2017.00213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tzouvelekis A, Ntolios P, Karampitsakos T, et al. . Safety and efficacy of pirfenidone in severe idiopathic pulmonary fibrosis: A real-world observational study. Pulm Pharmacol Ther 2017;46:48–53. 10.1016/j.pupt.2017.08.011 [DOI] [PubMed] [Google Scholar]
- 28. Galli JA, Pandya A, Vega-Olivo M, et al. . Pirfenidone and nintedanib for pulmonary fibrosis in clinical practice: tolerability and adverse drug reactions. Respirology 2017;22:1171–8. 10.1111/resp.13024 [DOI] [PubMed] [Google Scholar]
- 29. Cottin V, Maher T. Long-term clinical and real-world experience with pirfenidone in the treatment of idiopathic pulmonary fibrosis. Eur Respir Rev 2015;24:58–64. 10.1183/09059180.00011514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Aryal S, Nathan SD. An update on emerging drugs for the treatment of idiopathic pulmonary fibrosis. Expert Opin Emerg Drugs 2018;23:159–72. 10.1080/14728214.2018.1471465 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ejhpharm-2018-001806supp001.pdf (59.2KB, pdf)
ejhpharm-2018-001806supp002.pdf (190.2KB, pdf)


