Abstract
Background:
Intermittent fasting (IF) strategies have emerged as viable alternatives to traditional calorie-restricted diets. A key predictor of metabolic health and response to diet is cardiometabolic fitness, including intrinsic aerobic capacity. In a contrasting rat model of aerobic capacity—high- and low-capacity runners (HCR, LCR)—we found that the lean and physically active HCR were also more responsive to a standard calorie-restricted diet. Here, we assessed the ability of IF to induce weight loss on a background of high and low aerobic fitness accompanied by different levels of daily physical activity.
Methods:
Female HCR and LCR (8 per line) were subjected to IF (alternate-day fasting) for 14 weeks. Outcomes included changes in body weight, fat and lean mass, daily physical activity, and food and water intake. After initial measurements, IF was continued, and measurements were repeated after one year of IF.
Results:
All rats lost weight with IF, and LCR lost significantly more weight than HCR. This difference was primarily due to differential fat loss; loss of lean mass, on the other hand, was similar between HCR and LCR. Total food intake decreased with IF, and LCR showed lower intake than HCR only during the first 5 weeks of IF. Physical activity was suppressed by long-term IF. Physical activity increased on fed days compared to fasted days, and this pattern was more pronounced in HCR. The differential effects of IF in HCR and LCR persisted after one year of IF, with IF preventing the marked weight gain seen in ad libitum fed LCR during this time.
Conclusion:
Weight and fat loss from IF was more pronounced in obesity-prone, low-aerobic capacity LCR, despite the low activity levels seen in these rats. The possibility that aerobic capacity modulates response to IF in human participants remains unexplored.
Keywords: Alternate-day fasting, physical activity, HCR, LCR, aerobic capacity
1. Introduction
As an alternative to standard caloric restriction based on the amount of daily calorie intake, there is growing evidence that approaches using timed feeding provide health benefits (1–3). Instead of limiting the number of calories each day as in standard calorie restriction, the timing of food availability can be modified using intermittent fasting (IF) strategies, such as alternate-day fasting in which food is limited every-other day. While there are numerous variations to meal timing in IF procedures, they generally consist of fasting cycles interspersed with periods of ad libitum feeding. This strategy decreases metabolic risk biomarkers in mice and humans, supporting its use to promote lifespan (4). A recent meta-analysis of randomized controlled trials supports the safety and efficacy of IF procedures, with the potential for some benefit in insulin sensitivity over daily calorie restriction (1). With the rising popularity of IF strategies, it is important to discern approaches that are most effective for long-term weight loss as well as the promotion of health-span, especially for those with or at risk for obesity or metabolic syndrome.
One influential predictor of metabolic health is intrinsic aerobic capacity, a complex trait linked to multiple facets of health and lifespan, including glycemic control, lipid metabolism, and cardiovascular health (5). With respect to human health, aerobic capacity and its proxy, cardiorespiratory fitness, are strong predictors of lifespan and health-span (6–11). Based on the idea that disease risk is polygenic and based in energy transfer and non-equilibrium thermodynamics, intrinsic aerobic capacity was modeled using rats, resulting in high- and low-aerobic capacity phenotypes—HCR and LCR (12). Consistent with the underlying hypothesis, LCR have higher risk for metabolic and related disease as well as decreased longevity (5, 12, 13). In addition to diverging in disease risk and physiology, HCR and LCR also exhibit differences in behavior (14) including physical activity. Elevated physical activity, along with greater activity-related energy expenditure, is part of the lean, high aerobic capacity phenotype (15). Using 3 weeks of 50% calorie restriction, we demonstrated that the inherently lean and active HCR lost proportionally more weight than LCR, even in the case of female rats which did not show a marked phenotypic difference in baseline body weight (16). Food restriction in HCR and LCR altered physical activity, where short-term restriction increased activity, but this was followed by suppression of locomotor activity as food restriction progressed (16). This long-term suppression of physical activity served to further dampen energy expenditure and prime the animal for weight recovery upon resumption of ad libitum feeding. Diet strategies that might circumvent this suppression of physical activity with long-term weight loss would be beneficial for sustained maintenance of the reduced body weight.
With increasing interest in time-restricted feeding strategies (17), the question remains as to whether different populations show varying responses to timed feeding (18). Here, we used the HCR/LCR rat model to investigate weight loss with IF. In addition, we examined how IF affects daily physical activity in rats known to be highly active compared to sedentary (15, 16, 19, 20). Lastly, the long-term viability of IF for weight loss was also considered.
2. Methods
2.1. Animals.
Procedures were approved by the Kent State University Animal Care and Use committee, and animals were cared for in accordance with the Guide to the Care and Use of Laboratory Animals. Female HCR/LCR rats (N=26; generation 21) were housed at 72°F on a 12:12 light-dark cycle with lights-on at 0700 Eastern Standard Time, with tap water available ad libitum. Food (Prolab RMH 3000 – 5P00; T.R. Last Co. Inc.) was available ad libitum except during alternating fasting days. Chow pellets were comprised of 26% protein, 14% porcine and plant oil fat, and 60% carbohydrate, with a physiological fuel value of 3.46 kcal/gram.
A model of rats artificially selected from the large, genetically heterogeneous N:NIH founder population into two lines for their divergent aerobic capacity for endurance running on a treadmill—HCR and LCR—has been generationally maintained at the University of Michigan (now at the University of Toledo). This selection based on the ability to utilize oxygen, phenotypically assessed using treadmill endurance running, separates the rats into two distinct and contrasting but genetically intact phenotypic strains with their own unique metabolic and behavioral characteristics; this approximates a gradient of a human population containing both lean and obese phenotypes (5). Female rats were chosen because the small phenotypic difference in size between HCR and LCR minimized potential effects of baseline body weight on weight loss. Estrous cycles were not monitored here; previously, we detected small but predictable effects of cycle on physical activity in these rats (16), with no HCR-LCR differences noted there or in the context of blood glucose control (21). Aerobic capacity phenotype was assessed using treadmill running at about 4 months of age; out of 5 running trials, the IF-fed HCR had significantly longer best run times (HCR, 73±2min; LCR, 20±1 min), best distance (HCR, 2052±101m; LCR, 288±21m), and Joules (HCR, 916±74J; LCR, 143±14J) compared to LCR. Aerobic capacity did not differ between IF-fed and control-fed rats within line for best run time (control: HCR, 74±2min; LCR, 20±1 min), best distance (control: HCR, 2073±81m; LCR, 294±24m), and Joules (control: HCR, 951±29J; LCR, 156±12J). Rats were divided into four groups. At about 7 months of age, group of 8 rats from each selected line was placed in individual activity monitor chambers (ordered among a total of 16 activity monitors to avoid bias in placement by selected line) and subjected to IF where food was available ad libitum every other day, alternating fed and fasted days. A group of 5 rats from each line remained individually housed in standard caging under normal ad libitum-fed conditions to serve as an age-matched normative control for unmanipulated conditions. Rats in the activity monitors underwent daily measurements of body weight, food and water intake, and physical activity. Activity monitors were reset each day during an afternoon window for measurements of body weight and food. Once a week, body composition was determined using an EchoMRI-700. For body composition measurements taken 16 days before the first day of fasting, IF-fed HCR weighed 235 ± 11 g (22 ± 2 g fat, 182 ± 8 g lean mass) with IF-fed LCR weighing 276 ± 12 g (54 ± 7 g fat, 186 ± 11 g lean mass); at the same time, control-fed HCR weighed 235 ± 15 g (24 ± 2 g fat, 184 ±7 g lean mass) and control-fed LCR weighed 277 ± 2 g (48 ± 10 g fat, 194 ± 4 g lean mass). IF-fed and control-fed rats did not differ in body weight or composition within line, but LCR had significantly higher body weight and fat mass than HCR (2-tialed t-tests), with no difference in lean mass, consistent with their phenotype (15, 16, 19, 20).
For comparison, another contrasting selected line was subjected to intermittent fasting, rats artificially selected (i.e., selectively bred) for high and low response to training, which were also derived from N:NIH stock (22). Female high-response trainer (HRT, n=8) and low-response trainer (LRT, n=8) underwent activity measurement before and during 11 weeks of alternate-day fasting after 19 days of baseline measurement.
2.2. Intermittent fasting.
Intermittent fasting was accomplished by removing and weighing all food from the cage for 24 hrs, and replacing the food the following day at the same time. HCR and LCR rats underwent IF for 14 weeks while housed in the activity monitors, with daily measurements taken starting at 1200 EST. Subsequently, rats continued IF in standard housing with body weight and composition measured weekly; during this time, daily measurements began at 1700 EST. Body composition was measured in all rats in week 46. Beginning on the 56th week of IF, physical activity, food and water intake, and body weight were measured daily for an additional 7 weeks to assess the effects of long-term IF on activity levels. Body weight and food intake measured in control ad libitum-fed rats during the third of the seven weeks.
2.3. Physical activity.
Physical activity was measured using activity monitors (Opto-M Animal Activity Meters; Columbus Instruments, Columbus, OH), as previously described (16). Briefly, the 16X16-inch housing area is surrounded by infrared beans to collect horizontal, ambulatory, and vertical activity data. Activity measurements were stopped each day for 1–2 hrs during the light phase to measure body weight and download activity data. Physical activity variables calculated included distance traveled (cm/min); time spent resting (sec/min); time spent in each stereotypic activity and ambulatory activity (sec/min); and horizontal, ambulatory, and vertical activity counts (counts/min). Note that running wheels were not used to measure activity because of the ability of wheels to independently skew energy balance and increase activity levels (23).
2.4. Statistical analyses.
Analyses were conducted using IBM SPSS software. Food intake was compared by calculating daily intake for control rats; for IF-fed rats, fed-day intake/2 was used to calculate effective daily food intake. Data through 14 weeks of IF were subjected to repeated-measures ANOVAs examining effects of IF (vs control-fed), time on IF (from baseline day through IF), and selected line (HCR/LCR) on body weight, fat mass, lean mass, add food intake in separate analyses (Table S1). Fed days and fasted days were also analyzed separately for physical activity (i.e., over IF only on fed days, or only on fasted days; Table S2). For data after prolonged IF, one fed and one fasted day body composition (fat and lean mass) were analyzed separately using 2X2 ANOVAS, then body weight and composition and water intake were compared between fed and fasted days in HCR vs LCR using 2X2 ANOVAs (Table S3). Physical-activity data analyses compared the mean fed and mean fasted day activity between HCR and LCR using 2X2 ANOVAs (Table S4). Food intake after one year of IF or ad libitum food intake was also analyzed using analysis of covariance (ANCOVA) with body weight as the covariate. Change in body weight, fat mass, lean mass, and food intake were also compared between HCR and LCR using 2-tailed t-tests, as were simple comparisons between HCR and LCR baseline, mean fed day, and mean fasted day, illustrated in inset bar graphs (Figures 1, 3, and 4). Based on prior identification of acute fasting-induced changes in activity (16), a 1-tailed paired t-test was performed comparing distance traveled on the day before IF and the first fasting day to examine acute effects of fasting on activity. Significance was determined as p < 0.05.
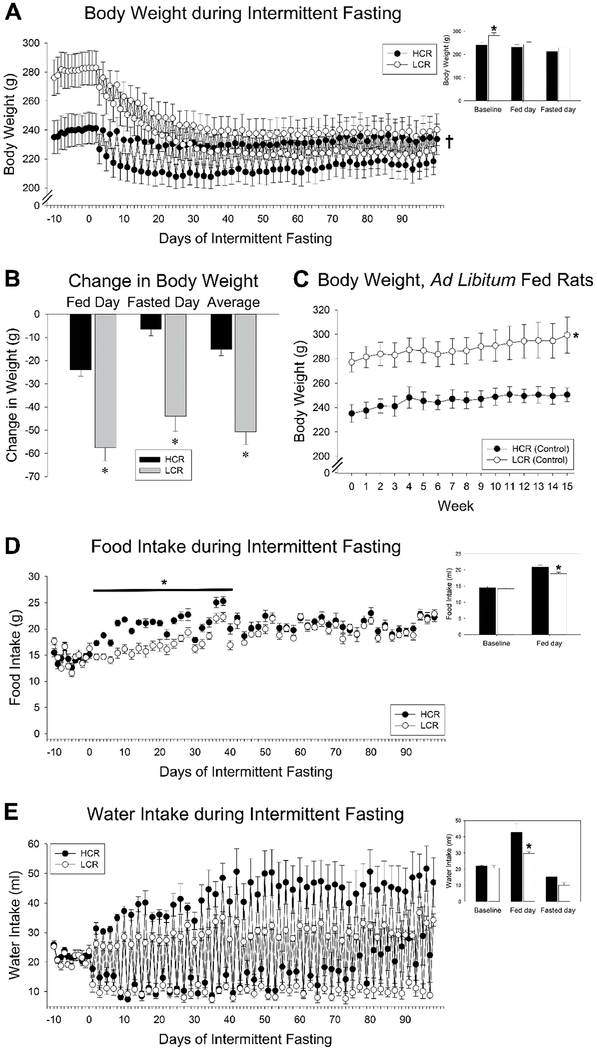
Fig 1. Intermittent fasting (IF) in high-capacity runners (HCR) and low-capacity runners (LCR).
(A) 14 weeks of IF induced significant weight loss in female rats, but this was more pronounced in LCR. (B) Body weight fluctuated day-to-day with food intake; the overall loss of body weight was significantly higher in LCR when considering weight on the day of food availability, the fasting day, or the average of these. (C) Ad libitum fed female HCR and LCR from the same cohort showed no weight loss over the same period. (D) There was a phenotypic difference in caloric intake where food intake on fed days was higher in HCR for the first 6 weeks of IF, but then did not differ between phenotypes by week 6 of IF. (E) Water intake mirrored food intake, where rats drank more water on fasted days, with no difference between HCR and LCR. *HCR ≠ LCR, p<0.05, †significant interaction where LCR (n=8) lost more weight than HCR (n=8), p<0.05. Inset bar graphs show ad libitum-fed baseline, mean of all fed days, and mean of all fasting days; *p<0.05, 2-tailed t-test, HCR vs. LCR.
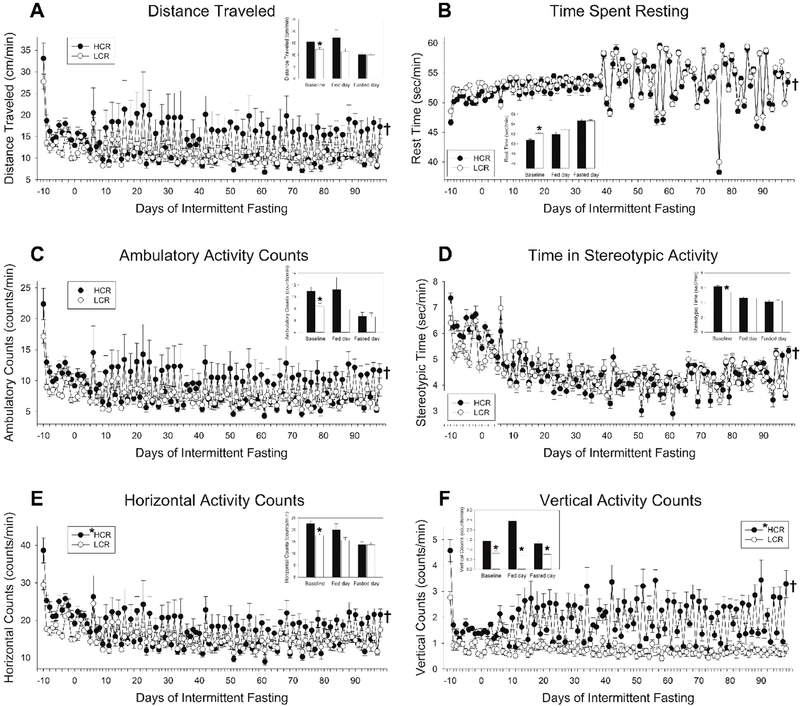
Fig 3. Intermittent fasting (IF) altered physical activity in high-capacity runners (HCR) and low-capacity runners (LCR).
(A) Distance traveled showed significant day-to-day changes, with activity elevated on fed days relative to fasted days, and an overall suppression of activity due to IF detectable only on fasted days (n = 8/group). (B) For time spent resting, the day-to-day change in activity from fed days to fasted days was similar between HCR and LCR, and an overall suppression in activity levels due to IF. Ambulatory activity counts (C) were affected similar to distance traveled, whereas stereotypic activity (D) was similar to time spent resting where HCR and LCR showed similar day-to-day changes in activity. For both horizontal (E) and vertical (F) activity counts, phenotypic differences in activity were apparent on fed days. Overall, the differential response to IF between HCR and LCR was more evident in more energy-consuming activities (e.g., ambulation, vertical activity) than less energy-consuming activities (e.g., stereotypic activity or resting). *HCR (in legend), main effect on fed day, HCR>LCR, p<0.05, n = 5 rats/group; †significant interaction where HCR and LCR responded differently to IF from baseline on fasted days (distance traveled, time spent resting, ambulatory and horizontal counts), or both fed and fasted days (time in stereotypic activity, vertical counts). Inset bar graphs show ad libitum-fed baseline, mean of all fed days, and mean of all fasting days; *p<0.05, 2-tailed t-test, HCR vs. LCR.
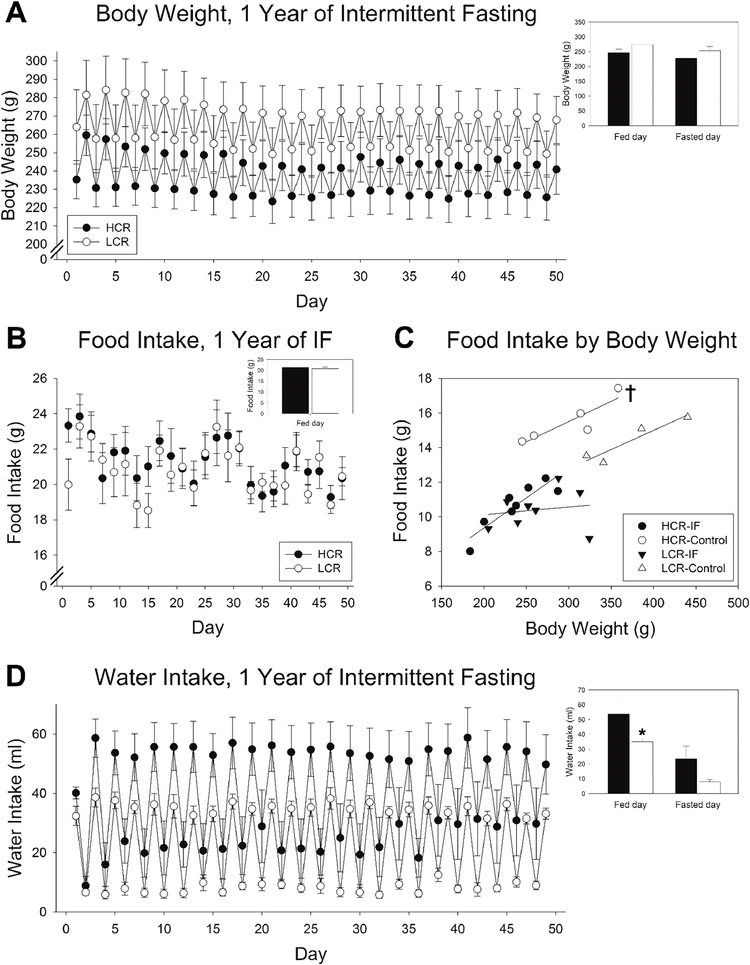
Fig 4. Response to prolonged intermittent fasting (IF) in high-capacity runners (HCR) and low-capacity runners (LCR).
(A) Body weight of HCR and LCR subjected to IF continued to fluctuate day to day after prolonged (>1 year) of IF, with no significant difference between HCR and LCR. (B) Food intake on fed days did not significantly differ between HCR and LCR. (C) When considered alongside body weight, ad libitum intake was higher in HCR than LCR, whereas daily food intake (fed day/2) during prolonged IF did not significantly differ between HCR and LCR; For a given body weight, IF suppressed intake relatively more in HCR than LCR. (D) Water intake mirrored food intake, where rats drank more on days when they were provided food, with no difference (no main effect) between HCR and LCR. †significant interaction, p<0.05; n = 8 rats/group. Inset bar graphs show mean of all fed and fasting days; *p<0.05, 2-tailed t-test, HCR vs. LCR.
3. Results
3.1. LCR lost more weight than HCR over 14 weeks of intermittent fasting.
For all analyses, see Tables S1–S4 for detailed statistical results. As shown in Fig 1 A–B, there was an interaction in weight loss between HCR and LCR where LCR lost significantly more weight than HCR over 14 weeks of IF (p<0.001). The typical phenotypic difference in body weight observed during baseline ad libitum feeding (where LCR weigh more than HCR) abated by the third week of IF, resulting in no overall main effect of body weight between HCR and LCR. Taking the average of fed and fasted days on week 14, HCR lost 15.26 g (±2.71 g), while LCR lost 50.82 g (±5.37 g; p<0.0001). This contrasted with body weight of HCR and LCR from the same cohort that did not undergo IF; as shown in Fig 1C, control-fed rats showed small but steady weight gain over 14 weeks. For food intake, though hyperphagia occurred every-other day in response to the previous day’s fast, the 2-day intake did not fully compensate for the missed intake the previous day (Fig 1D). While HCR ate more than LCR on their fed day for the first 5 weeks of IF, after this the food intake did not significantly differ between HCR and LCR. Water intake generally mirrored food intake (Fig 1E), with greater water intake on fed days compared to fasted days, but no main effect of selected line and no interaction.
As shown in Fig 2A, there was significant fat loss over 14 weeks of IF (main effect of fat mass over time, p<0.001). At baseline, LCR had greater fat mass than HCR, and there was a phenotypic difference in fat loss over time on IF (p<0.001). As shown in Fig 2A, 14 weeks of IF decreased LCR fat mass, with little change in HCR fat mass; there was no significant phenotypic difference in fat mass by week 4–5 of IF (2-tailed t-tests; week 4, p=0.174; week 5, p=0.182). By the end of 14 weeks, HCR and LCR showed similar fat mass, in contrast to the phenotypic disparity in fat mass typical of these lines. IF-fed HCR were similar to control HCR, showing significantly less fat mass than their control-fed counterparts. Thus, IF eliminated the elevated adiposity typically seen in LCR. As shown in Fig 2B, lean mass showed a different pattern, with no overall difference (i.e., no main effect) between HCR and LCR (p=0.778). Both HCR and LCR lost lean mass by the second week of IF (1-tailed paired t-tests, p<0.05). Over 14 weeks of IF (p<0.001), with no significant difference in the HCR vs LCR response to IF (i.e., no significant interaction, p=0.165). In summary, LCR and HCR showed similar loss of lean mass due to IF. Body weight, fat mass, and lean mass each fluctuated between fed and fasted days, but the daily fluctuation was more marked when observing lean mass than fat mass.
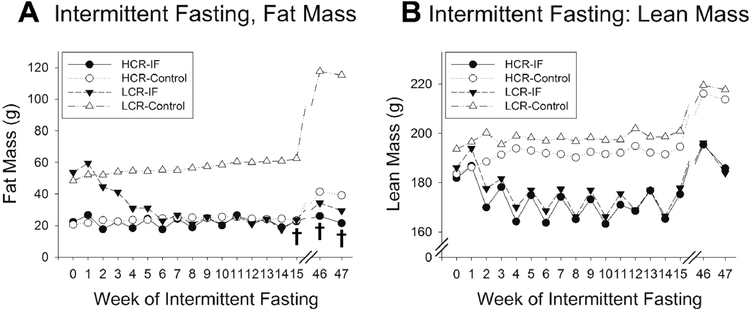
Fig 2. Body composition of high-capacity runners (HCR) and low-capacity runners (LCR) with intermittent fasting (IF).
(A) Fat mass in LCR significantly decreased with alternate-day fasting, reaching levels of fat mass typical of HCR (n = 8/group). Ad libitum-fed LCR showed chronically elevated levels of body fat compared to HCR. After 46–47 weeks of IF, IF-fed LCR maintained lower adiposity compared to ad libitum-fed LCR (n = 5 ad libitum-fed rats/group). (B) Lean mass was lost in both LCR and HCR subjected to IF relative to ad libitum-fed LCR and HCR, with no significant difference between phenotypes. Lean mass showed more day-to-day variation than fat mass in IF-fed rats. After 46–47 weeks, both IF- and ad libitum-fed rats gained lean mass, with no differences between phenotypes. Significant interaction where LCR showed greater fat loss after 14 weeks of IF as well as after prolonged IF (both fed and fasted days).
3.2. Physical activity decreased with 14 weeks of IF, with a larger decrease in HCR.
IF significantly affected multiple aspects of physical activity. As previously demonstrated (16), rats showed high activity levels upon introduction to the new environment (i.e., the activity monitor), and HCR were more active than LCR during and after acclimation (Fig 3). With the onset of fasting, an acute increase in distance traveled was seen in HCR (p=0.022) but not LCR (p=0.103; 1-tailed paired t-tests comparing distance traveled on final day of ad libitum feeding with first fasting day). As shown in Fig 3, IF significantly affected physical activity over time. First, physical activity depended on food availability, where rats were more active on fed days than on fasted days. When considered separately, time spent resting, time in stereotypic activity, and time in ambulatory activity, as well as horizontal and vertical counts, all showed significant changes over IF on both fed and fasted days. Distance traveled and ambulatory counts were significantly suppressed only on fasted days (Fig 3A, C). Second, the effect of IF differed between HCR and LCR. There was a significant interaction where HCR showed larger changes in activity due to IF when considering only fasted days for all activity variables, and significantly greater decrease in activity when considering only fed days for time in stereotypic activity and vertical counts. The day-to-day change in activity was significantly more pronounced in HCR; for example, HCR and LCR traveled similar distances on fasted days, but the HCR showed a much steeper increase on fed days (Fig 3A). The day-to-day change in activity was more pronounced in HCR than in LCR for vertical counts, in contrast to time spent resting or stereotypic activity in which HCR and LCR showed similar day-to-day changes (Fig 3F vs B, D). The daily (fed-to-fasting day) change in rest time increased markedly in both HCR and LCR 3–4 days following a temporary change in room environmental controls (Fig 3B), but this was not evident in other indices of activity.
3.3. The effects of IF on body weight, body composition, and physical activity persisted after 1 year.
An additional seven weeks of activity measurements were collected after one year of continuous IF, with body weight and food intake measured in control ad libitum-fed rats during the third of the seven weeks, and body composition measured during week 46. Rats subjected to IF maintained a reduced body weight after one year on the diet (Fig 4A). Body weight significantly differed between fed and fasted days in both HCR and LCR, but there was no significant difference between body weight in IF-fed HCR and LCR at 1 year after IF began (one-tailed t-test, p=0.092). The control, ad libitum-fed LCR were significantly heavier than HCR (one-tailed t-test, p=0.033). The weight loss in IF-fed rats contrasts to the increase in body weight over time seen in ad libitum-fed HCR and LCR; during the third week of the second activity measurement, HCR weighed 300g (±21g) and LCR weighed 372g (±27g).
Relative to ad libitum intake, food intake remained significantly decreased after one year of continuous IF, but did not differ between HCR and LCR (Fig 4B). When food intake at 1 year of IF was compared using body weight as the covariate (Fig 4C), body weight was a significant predictor of food intake, with larger rats eating more food. Rats fed ad libitum ate more than IF-fed rats, and HCR ate more than LCR (i.e., significant main effects). There was a significant interaction in which ad libitum-fed HCR of a given body weight ate significantly more than LCR (e.g., HCR weighing 300 ± 21 g ate 15.50 ± 0.55 g, and LCR weighing 372 ± 27 g ate 14.38 ± 0.63 g), but on IF, there was no phenotypic difference in food intake once body weight was taken into account (HCR weighing 237 ± 12 g ate 10.65± 0.47 g, and LCR weighing 264 ± 15 g ate 10.41 ± 0.40 g). As before, water intake showed day-to-day changes between fed and fasted days, with no difference between HCR and LCR (main effect, p=0.054).
For body composition, compared to their ad libitum-fed counterparts, HCR and LCR had significantly lower fat mass after 1 year of IF, with a significant interaction where LCR showed significantly more fat loss than HCR (Fig 2B); these results were found on both fed days and fasting days. This differed from the effect of 1 year of IF on lean mass, where IF caused a decrease in lean mass over time relative to control-fed rats, but with no significant interactions found between HCR and LCR.
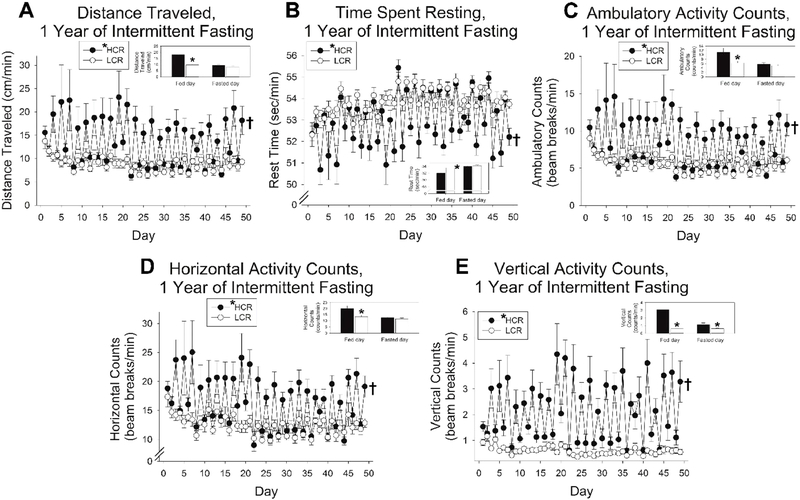
When rats were placed in the physical activity monitors for 7 weeks after a year of continuous IF, activity variables monitored (distance traveled; times spent resting; horizontal, ambulatory, and vertical counts) were similar to what was observed at the beginning of IF (Fig 5). The elevated activity on fed days relative to fasted days persisted, with HCR more active than LCR overall, and a significant interaction where the day-to-day change in activity was more pronounced in HCR than in LCR. On fasted days, HCR and LCR were similarly inactive; on fed days, HCR increased their physical activity while LCR showed much less day-to-day change. Compared to the first 14 weeks of IF (Fig 3), the phenotypic difference in the day-to-day variation in activity was more pronounced after prolonged IF. There was a significant difference between fed and fasting days, and a significant interaction between HCR and LCR, for each activity variable measured.
Fig 5. Physical activity levels with prolonged intermittent fasting (IF) in high-capacity runners (HCR) and low-capacity runners (LCR).
For all measurements of physical activity, rats were more active on fed days than fasted days, HCR were more active than LCR, and the day-to-day volatility in activity was significantly more pronounced in HCR than LCR. This applied to (A) distance traveled, (B) time spent resting, and (C) ambulatory, (D) horizontal, and (E) vertical activity counts. *HCR (in legend), main effect, HCR>LCR, p<0.05; †significant interaction between fed/fasted day and HCR/LCR, p<0.05; n = 8 rats/group. Inset bar graphs show mean of all fed and fasting days; *p<0.05, 2-tailed t-test, HCR vs. LCR.
3.4. IF did not differentially affect body weight in rats artificially selected for response to training.
Artificial selection has also been performed for response to aerobic training, yielding high-response trainer and low-response trainer phenotypes (i.e., HRT and LRT rats)(22). Based on statistical modeling evidence, the response to training phenotype is independent of intrinsic capacity and body weight (22). As shown in Table 1, three weeks before the onset of IF, baseline body weight differed between female HRT and LRT groups (HRT, 252.8 g ± 4.9 g; LRT, 309.3 g ± 5.7 g; p=0.018). For body composition, baseline lean mass differed between HRT (172.2 g ± 1.7 g) and LRT (198.3 g ± 30.7 g; p=0.045), but fat mass did not reach significance in a 2-tailed t-test comparing HRT (52.7 g ± 4.1 g) and LRT (78.9 g ± 2.7 g). Eleven weeks of IF induced significant loss of body weight, fat mass, and lean mass in these rats, but HRT and LRT did not significantly differ in any parameter measured (see Table 1); loss of body weight, fat mass, and lean mass did not show any difference between HRT and LRT. Food and water intake changed with IF, as predicted, also with no difference between HRT and LRT. Though activity changed over IF, this was mostly due to day-to-day changes in activity in response to acute food availability rather than an overall suppression of activity levels (Table 1); there was no difference between HRT and LRT in physical activity.
Table 1.
Response of high-response trainers (HCR) and low-response trainers (LRT) to intermittent fasting (IF). Data are expressed as mean (SEM), and statistical outputs are expressed as F-values (degrees of freedom) above p-values.
| HRT | LRT | Main effects | Interaction | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Before IF | During IF | Before IF | During IF | Time | HRT vs LRT | Time vs Line | |||
| Fed | Fasted | Fed | Fasted | ||||||
| Body weight (g) | 252.4 (13.5) | 232.1 (7.0) | 209.0 (7.1) | 308.7 (16.8) | 279.3 (11.2) | 253.5 (10.6) | 23.38(14,140)p<0.001 | 5.49 (1,10)p=0.041 | 0.51(14,140) p=0.932 |
| Fat mass (g) | 50.9 (10.5) | 28.3 (3.3) | 23.4 (3.5) | 79.7 (7.3) | 51.6 (5.9) | 45.7 (5.1) | 16.17(14,140) p<0.001 | 5.23(1,10) p=0.044 | 0.19(14,140) p=0.999 |
| Lean mass (g) | 173.3 (4.1) | 171.9 (5.5) | 159.7 (5.1) | 198.0 (11.7) | 193.7 (9.3) | 177.3 (8/9) | 12.70(14,140) p<0.001 | 2.02(1,10) p=0.185 | 0.19(14,140) p=0.783 |
| Food intake (g) | 10.9 (0.4) | 18.7 (0.6) | 0 | 11.6 (0.4) | 19.7 (0.7) | 0 | 79.66(98,1274) p<0.001 | 0.874(1,13) p=0.367 | 0.60(98,1274) p=0.999 |
| Water intake (ml) | 26.4 (1.7) | 43.2 (2.7) | 9.4 (2.7) | 20.0 (0.7) | 36.5 (1.2) | 6.0 (0.8) | 575.07(98,1372) p<0.001 | 10.27(1,14) p=0.006 | 1.10(98,1372) p=0.249 |
| Physical Activity | |||||||||
| Distance traveled (cm/min) | 8.6 (0.6) | 12.2 (1.6) | 7.6 (0.5) | 8.8 (0.8) | 15.3 (3.2) | 7.8 (1.0) | 8.29(98,1274) p<0.001 | 0.51(1,13) p=0.486 | 0.92(98,1274) p=0.697 |
| Time spent resting (sec/min) | 52.7 (0.3) | 52.3 90.5) | 54.4 (0.2) | 53.5 (0.3) | 52.4 (0.6) | 54.6 (0.4) | 16.79(98,1372) p<0.001 | 0.32(1,14) p=0.583 | 0.93(98,1372) p=0.679 |
| Horizontal counts (per min) | 13.5 (0.8) | 15.4 (1.4) | 10.4 (0.6) | 12.8 (1.0) | 17.6 (3.0) | 10.5 (1.1) | 9.14(98,1274) p<0.001 | 0.11(1,13) p=0.748 | 0.78(98,1274) p=0.544 |
| Ambulatory counts (per min) | 5.1 (0.4) | 7.2 (0.9) | 4.3 (0.3) | 5.5 (0.6) | 9.6 (2.2) | 4.8 (0.7) | 5.18(98,1274) p<0.001 | 0.77(1,13) p=0.398 | 0.92(98,1274) p=0.686 |
| Vertical counts (per min) | 0.32 (0.04) | 0.69 (0.19) | 0.48 (0.06) | 0.44 (0.06) | 0.74 (0.09) | 0.64 (0.25) | 6.09(98,1274) p<0.001 | 0.34(1,12) p=0.574 | 1.07(98,1176) p=.0304 |
For body weight, fat and lean mass: baseline values immediately before start of IF; fed-day is after 9 weeks of IF, and fasted day is after 10 weeks of IF. Food, water, and activity through 11 weeks of IF; For activity, 17 days of baseline activity before onset of IF calculated. Main effect of time includes all measurements before and after IF without differentiating between fed and fasted days.
4. Discussion
As time-restricted eating strategies become increasingly common, evidence including clinical trials supports the safety and efficacy of many of these diets, including alternate-day fasting (1, 24, 25). We hypothesized that a key physiological predictor of long-term metabolic health—intrinsic aerobic capacity (5)—would modulate responsiveness to IF. Here, we employed an animal model system with contrasting levels of adiposity and physical activity to investigate phenotypic differences in response to IF. Alternate-day fasting induced a marked loss of weight and fat in the obesity-prone, low-fitness LCR (Fig 1). The differential weight loss was due primarily to greater loss of body fat in LCR (Fig 2A). Little difference was seen in loss of lean mass outside of the anticipated decrease in lean mass with fasting (Fig 2B). Low-fitness LCR lost weight and fat despite their low levels of daily physical activity (Fig 3). This contrasts markedly with the proportionally enhanced weight loss in HCR seen with daily calorie restriction (16). Altogether, this suggests that alternate-day fasting may be particularly effective for weight loss in the obesity-prone, low-aerobic capacity phenotype.
Under ad libitum conditions, LCR show a consistently obese phenotype, with high body weight and adiposity relative to their lean, HCR counterparts (15). The striking weight loss seen in LCR subjected to IF (Fig 1A, B) could result from loss of fat, lean mass, or both. Indeed, human weight loss, even with concurrent exercise, comes with reduced lean mass along with lower adiposity (26). Here, IF resulted in loss of body fat and lean mass (Fig 2), however, HCR and LCR differed in the contribution of fat and lean mass to overall weight loss. In the first 5 weeks of IF, LCR showed a significant decrease in adiposity, while HCR showed very little change in fat mass over time (Fig 2A). Baseline lean mass showed less phenotypic difference between HCR and LCR, although the greater overall body mass in LCR is commonly accompanied by higher baseline fat-free mass, at least in males (27). Both HCR and LCR lost lean mass with IF, as would be expected with negative energy balance and weight loss (Fig 2B). There was no significant difference in the amount of lean mass lost between HCR and LCR on IF (Figure 2B). Therefore, of the additional weight lost by LCR over HCR, most of this was due to decreased adiposity, not loss of lean mass. In essence, subjecting LCR to IF removed the phenotypic difference in body weight and body composition typically seen in these rats. Given the greater phenotypic divergence in body composition seen between male HCR and LCR (16), the possibility that IF may be more effective for weight and fat loss in male LCR is particularly intriguing. Lastly, the day-to-day changes in body weight (Fig 1A) was primarily accounted for by the change in lean mass between fed and fasted days, which was accompanied by changes in total water (data not shown) as well as water intake (Fig 1E), which generally mirrored food intake (e.g., higher on fed days). Although evidence from humans and mice links IF with improved glycemic control (28, 29), potential deleterious effects are also possible. For example, in young female Wistar rats, 12 weeks of IF leads to pancreatic beta-cell dysfunction and hyperinsulinemia (30).
The greater weight loss seen in the LCR relative to HCR could stem from differential changes in food intake, energy expenditure, or both. Total food intake was markedly reduced by alternate-day fasting (Fig 1D). On their fed day, rats ate more food than on a baseline (pre-IF) day, but the additional intake was insufficient to offset the fasting days. Therefore, rats decreased their total food intake upon introduction of IF, with the most marked change in the first week of IF and a gradual partial recovery thereafter (Fig 1D). These HCR and LCR did not have significantly different caloric intake at baseline. At the onset of IF, while all rats ate less overall, HCR intake was higher than LCR for the first six weeks of IF; this likely contributed to the relatively enhanced weight loss in LCR in the first weeks of IF. By about the sixth week of IF, however, there was no longer any phenotypic difference in food intake (Fig 1D). This paralleled weight loss and weight maintenance, where most of the decrease in body weight was seen by week 3 in the HCR, and by week 5 in the LCR. While HCRs’ more gradual decline in food intake may have limited their weight loss, LCR maintained their reduced body weight and food intake despite free availability of food on the feeding days. In other words, despite the steady weight loss upon introduction to IF, LCR did not adjust food intake sufficiently to compensate and maintain their body weight—or achieve a body weight low enough to be maintained by the existing food intake—for several weeks into IF.
The altered physical activity may have also contributed to phenotypic differences in energy balance during IF. HCR are consistently more physically active than LCR (15, 16, 19, 20), as seen in these rats before the onset of IF (Fig 3). Daily food restriction (50% calorie restriction) has a biphasic effect on physical activity, increasing activity in the first 1–3 days of restriction, then decreasing activity levels thereafter (16). This suggested that IF may be able to mimic the onset of food restriction and promote physical activity. Close examination of physical activity during IF, however, showed that this was not the case. Although HCR showed an acute increase in activity on the first day of fasting, by the end of the first week of IF, both HCR and LCR were more active on fed days than on fasting days (Fig 3). For many aspects of activity, this day-to-day volatility in physical activity was more marked in HCR than in LCR. For vertical activity counts, the elevation in HCR activity is particularly salient (Fig 3F), especially in comparison to time spent resting or in stereotypic activities like grooming which show similar day-to-day changes in HCR and LCR (Fig 3 B, D). With relatively elevated activity on fed days, particularly in activities that are more calorically costly like vertical activity (e.g., rearing), this would promote negative energy balance in the HCR. Yet, HCR were relatively resistant to weight loss on IF. Negative energy balance is accompanied by a marked suppression of energy expenditure beyond what is accounted for by the lower body size; this has been documented in humans (31, 32) as well as in laboratory animals including rats (33). Measurement of energy expenditure during IF in these rats would clarify whether differences in weight loss are accompanied by differentially suppressed energy expenditure during IF. Interestingly, the rats that lost less weight on IF—the HCR—would be predicted to have higher EE at baseline (15) as well as higher activity-associated energy expenditure during IF due to their elevated activity levels (Fig 3, Fig 5). Future examination of energy expenditure in HCR and LCR with IF should include a detailed analysis of caloric use during activity.
Food intake was compared relative to body weight in HCR and LCR with comparable weight and body composition after IF (Fig 4C). Under ad libitum-fed conditions, HCR ate more than LCR once body weight was considered (Fig 4C), consistent with our previous demonstration that ad libitum-fed HCR show higher energy expenditure than LCR (15). After prolonged IF, however, there was little difference in food intake between HCR and LCR of similar body weights (Fig 4C). In other words, HCR subjected to IF ate less than ad libitum-fed HCR of the same body size, while the lower food intake in IF-fed LCR was accompanied by a significantly smaller body size; this may contribute to the widely disparate response of HCR and LCR to daily calorie restriction and IF (16). Focusing on female rats subjected to daily calorie restriction (see Table 1 of (16)), compared to LCR, HCR lose slightly more body weight and a similar amount of absolute body fat. This contrasts markedly with the rats’ relative response to IF (Fig 2), where LCR lose well over half of their body fat while HCR lose very little. Future investigations should examine the relative adaptation in energy expenditure by daily food restriction and IF in these phenotypes.
We next questioned if LCR weight loss would be sustained by long-term IF. Fig 4A demonstrates that 1 year of IF maintained weight loss, with total weight loss in LCR greater than HCR. Food intake on fed days did not significantly differ between HCR and LCR after one year of IF, with water intake mirroring food intake (Fig 4B, D). Comparing food intake between HCR and LCR, ad libitum fed HCR of a given body weight ate more than LCR; this difference is not apparent in rats subjected to IF, however (Fig 4C). Similarly, the phenotypic difference in fat loss persisted after nearly one year of IF (Fig 2A). Ad libitum fed HCR and LCR, on the other hand, showed gains in fat mass by week 46–47, and this was especially salient in LCR (Fig 2A). Gains of lean mass, on the other hand, were similar between IF-fed and ad libitum-fed rats, with no difference in lean mass between HCR and LCR subjected to IF (Fig 2B). Altogether, this reinforces the conclusion that LCR (compared to HCR) respond to IF with substantial weight loss, primarily due to greater fat loss, and that continued IF prevents appreciable gains in weight and fat over time (Fig 2).
Changes in physical activity identified with IF persisted with long-term IF (Fig 5). Activity varied day to day, increasing on fed days and decreasing on fasted days. This pattern was significantly more prevalent in the HCR than the LCR (Fig 5). In more energetically expensive activities like vertical activity (e.g., rearing), LCR showed relatively consistent low activity levels, while HCR were significantly more active on fed days (Fig 5E). Whereas the overall levels of each type of activity in which HCR or LCR engaged were in the same range between short-term and long-term IF, the acute changes in activity with daily alterations in food availability were further dampened in LCR with long-term IF. The ability of IF to alter habitual daily activity was recently investigated by Beaulieu et al (2020) in a randomized controlled trial of women subjected to intermittent energy restriction (modified alternate-day fasting). Objectively measured light physical activity was significantly lower on the alternating fasted days, without an overall suppression of activity during IF compared to baseline conditions (34). It remains possible that further differences in human habitual activity could be detected if participants were compared based on intrinsic aerobic capacity.
As a secondary test of whether there is a significant relationship between aerobic capacity and the phenotypic response to IF, we examined changes in body weight and composition with IF in another contrasting rat model system that was selectively bred for a low and high adaptive response to aerobic training—HRT and LRT (22). Briefly, these rat models were developed using the same genetically heterogeneous stock as the HCR/LCR (22), but selected on gain in exercise capacity as a result of 8 weeks of endurance treadmill training, rather than on intrinsic capacity. Similar to the LCR rats, LRT rats show metabolic abnormalities like insulin resistance and dyslipidemia (35). While the baseline phenotype was consistent with previous findings that LRT had a higher body weight (35), there was no phenotypic difference in the weight loss induced by IF (Table 1). Indeed, while IF decreased total food intake, body weight, and fat and lean mass, with day-to-day changes in physical activity in response to acute food availability, there were no differences between HRT and LRT in any of these responses to IF. With respect to statistical power, the same number of rats were compared in both contrasted selected lines (i.e., 8 HCR/8 LCR and 8 HRT/8 LRT). Therefore, the differential phenotypic weight loss seen with IF in these selected lines is likely to be inherent to the LCR.
While intermittent fasting strategies have been deemed safe, tolerable, and effective for weight loss in clinical studies (36–39), less interest has focused on physical activity patterns. Descriptions of methods and outcomes regarding physical-activity data collection are typically cursory (e.g., activity diaries), with broad temporal resolution in the timing of activity; commonly, participants are instructed to maintain habitual activity or exercise habits (40–42). Studies that have measured physical activity, for example using step counts, do not indicate that IF strategies appreciably alter physical activity in humans, however (42–45). In participants with obesity, physical activity was maintained over 4 weeks of modified alternate-day fasting, and activity did not differ between fed and fasted days (44). Similarly, Hoddy (2016) found no change in steps per day with modified alternate-day fasting in participants recruited based on lower activity levels (45). Aside from the results of Beaulieu et al (2020) described above (34), there is little indication whether IF strategies negatively impact activity levels, or if people who are more active compared to less active show differential benefits of IF. While some interest has focused on differential effects of IF strategies varying in macronutrient composition, or comparing timing schedules of IF diets, less attention is given to individual differences in response to IF. The marked difference in the metabolic and behavioral outcomes of IF based on metabolic phenotype reported here—intrinsic aerobic capacity—suggest that this phenotypic difference may extend to humans as well. This comparison could be accomplished using a relatively straightforward comparison based on aerobic fitness, especially in non-exercising populations.
4.1. Conclusion
Overall, alternate-day fasting induced marked weight and fat loss in rats, but this effect depended on metabolic phenotype, with a more pronounced loss seen in the rats with low aerobic fitness (i.e., LCR). In the phenotypically lean HCR, on the other hand, weight loss stemmed predominately from loss of lean mass rather than fat mass. The differential effect of IF on body weight and composition endured after prolonged IF of one year. Alternating food availably also impacted physical activity in a predictable manner, where activity was higher on days when food was available, then suppressed on fasting days. This was particularly pronounced in the rats that showed less weight loss—the lean HCR, which are more physically active at baseline (15, 16, 19, 20). Given the importance of exercise capacity in estimating the risk for human metabolic and cardiovascular health risk (5–11), and the favorable weight-loss effect of IF over daily calorie restriction in the LCR (16), aerobic capacity may prove to be a relevant predictive factor in the success of human weight loss with IF.
Supplementary Material
Highlights.
Alternate-day fasting induced weight and fat loss in female rats
Obesity-prone, low-aerobic-fitness rats lost weight & fat with intermittent fasting
Intermittent fasting suppressed physical activity more in lean, high-fitness rats
Activity fluctuated between fed and fasted days, especially in aerobically fit rats
The weight-loss effects of intermittent fasting are modulated by aerobic capacity
Acknowledgements
This body of work is dedicated to Christopher Paul Smyers for his unfailing support and his faith in Mark.
We thank Emily Welch and Ashley Davis for assistance with proofreading the manuscript. There are no conflicts of interest to disclose.
Funding: This work was supported by the National Institutes of Health R15DK108668 (to CMN) and R15DK097644 (to CMN). The LCR-HCR and LRT-HRT rat models were funded in part by the NIH P40OD021331 (LGK and SLB) and are maintained as an animal model resource for researchers at the University of Toledo, Toledo, OH. Contact LGK
Lauren.Koch2@UToledo.Edu or SLB brittons@umich.edu for information. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. https://www.nih.gov/ https://www.niddk.nih.gov/
Abbreviations
- ANCOVA
analysis of covariance
- ANOVA
analysis of variance
- HCR
high-capacity runner
- HRT
high-response trainer
- IF
intermittent fasting
- LCR
low-capacity runner
- LRT
low-response trainer
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Cho Y, Hong N, Kim KW, Cho SJ, Lee M, Lee YH, et al. The Effectiveness of Intermittent Fasting to Reduce Body Mass Index and Glucose Metabolism: A systematic review and meta-analysis. J Clin Med. 2019;8(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chaix A, Zarrinpar A, Miu P, Panda S. Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metab. 2014;20(6):991–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Longo VD, Panda S. Fasting, circadian rhythms, and time-restricted feeding in healthy lifespan. Cell Metab. 2016;23(6):1048–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brandhorst S, Choi IY, Wei M, Cheng CW, Sedrakyan S, Navarrete G, et al. A periodic diet that mimics fasting promotes multi-system regeneration, enhanced cognitive performance, and healthspan. Cell Metab. 2015;22(1):86–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koch LG, Britton SL. Theoretical and biological evaluation of the link between low exercise eapacity and disease risk. Cold Spring Harb Perspect Med. 2018;8(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Artero EG, Jackson AS, Sui X, Lee DC, O’Connor DP, Lavie CJ, et al. Longitudinal algorithms to estimate cardiorespiratory fitness: associations with nonfatal cardiovascular disease and disease-specific mortality. J Am Coll Cardiol. 2014;63(21):2289–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Earnest CP, Artero EG, Sui X, Lee DC, Church TS, Blair SN. Maximal estimated cardiorespiratory fitness, cardiometabolic risk factors, and metabolic syndrome in the aerobics center longitudinal study. Mayo Clin Proc. 2013;88(3):259–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McAuley PA, Artero EG, Sui X, Lee DC, Church TS, Lavie CJ, et al. The obesity paradox, cardiorespiratory fitness, and coronary heart disease. Mayo Clin Proc. 2012;87(5):443–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kodama S, Saito K, Tanaka S, Maki M, Yachi Y, Asumi M, et al. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA. 2009;301(19):2024–35. [DOI] [PubMed] [Google Scholar]
- 10.Rankinen T, Church TS, Rice T, Bouchard C, Blair SN. Cardiorespiratory fitness, BMI, and risk of hypertension: the HYPGENE study. Med Sci Sports Exerc. 2007;39(10):1687–92. [DOI] [PubMed] [Google Scholar]
- 11.Dvorak RV, Tchernof A, Starling RD, Ades PA, DiPietro L, Poehlman ET. Respiratory fitness, free living physical activity, and cardiovascular disease risk in older individuals: a doubly labeled water study. J Clin Endocrinol Metab. 2000;85(3):957–63. [DOI] [PubMed] [Google Scholar]
- 12.Koch LG, Britton SL. Rat models of exercise for the study of complex disease. Methods Mol Biol. 2019;2018:309–17. [DOI] [PubMed] [Google Scholar]
- 13.Koch LG, Britton SL, Wisloff U. A rat model system to study complex disease risks, fitness, aging, and longevity. Trends Cardiovasc Med. 2012;22(2):29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burghardt PR, Flagel SB, Burghardt KJ, Britton SL, Gerard-Koch L, Watson SJ, et al. Risk-assessment and coping strategies segregate with divergent intrinsic aerobic capacity in rats. Neuropsychopharmacology. 2011;36(2):390–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gavini CK, Mukherjee S, Shukla C, Britton SL, Koch LG, Shi H, et al. Leanness and heightened nonresting energy expenditure: role of skeletal muscle activity thermogenesis. Am J Physiol Endocrinol Metab. 2014;306(6):E635–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smyers ME, Bachir KZ, Britton SL, Koch LG, Novak CM. Physically active rats lose more weight during calorie restriction. Physiol Behav. 2015;139:303–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Cabo R, Mattson MP. Effects of intermittent fasting on health, aging, and disease. N Engl J Med. 2019;381(26):2541–51. [DOI] [PubMed] [Google Scholar]
- 18.Stockman MC, Thomas D, Burke J, Apovian CM. Intermittent fasting: Is the wait worth the weight? Curr Obes Rep. 2018;7(2):172–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Novak CM, Escande C, Burghardt PR, Zhang M, Barbosa MT, Chini EN, et al. Spontaneous activity, economy of activity, and resistance to diet-induced obesity in rats bred for high intrinsic aerobic capacity. Horm Behav. 2010;58(3):355–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Novak CM, Escande C, Gerber SM, Chini EN, Zhang M, Britton SL, et al. Endurance capacity, not body size, determines physical activity levels: role of skeletal muscle PEPCK. PLoS One. 2009;4(6):e5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karvinen SM, Silvennoinen M, Ma H, Tormakangas T, Rantalainen T, Rinnankoski-Tuikka R, et al. Voluntary running aids to maintain high body temperature in rats bred for high aerobic capacity. Front Physiol. 2016;7:311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koch LG, Pollott GE, Britton SL. A selectively bred rat model system for low and high response to exercise training. Physiol Genomics. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Novak CM, Burghardt PR, Levine JA. The use of a running wheel to measure activity in rodents: Relationship to energy balance, general activity, and reward. Neurosci Biobehav Rev. 2012;36(3):1001–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rynders CA, Thomas EA, Zaman A, Pan Z, Catenacci VA, Melanson EL. Effectiveness of intermittent fasting and time-restricted feeding compared to continuous energy restriction for weight loss. Nutrients. 2019;11(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harris L, Hamilton S, Azevedo LB, Olajide J, De Brun C, Waller G, et al. Intermittent fasting interventions for treatment of overweight and obesity in adults: a systematic review and meta-analysis. JBI Database System Rev Implement Rep. 2018;16(2):507–47. [DOI] [PubMed] [Google Scholar]
- 26.Redman LM, Heilbronn LK, Martin CK, Alfonso A, Smith SR, Ravussin E. Effect of calorie restriction with or without exercise on body composition and fat distribution. J Clin Endocrinol Metab. 2007;92(3):865–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gavini CK, Britton SL, Koch LG, Novak CM. Inherently lean rats have enhanced activity and skeletal muscle response to central melanocortin receptors. Obesity (Silver Spring). 2018;26(5):885–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Antoni R, Johnston KL, Collins AL, Robertson MD. Intermittent v. continuous energy restriction: differential effects on postprandial glucose and lipid metabolism following matched weight loss in overweight/obese participants. Br J Nutr. 2018;119(5):507–16. [DOI] [PubMed] [Google Scholar]
- 29.Wei S, Zhao J, Bai M, Li C, Zhang L, Chen Y. Comparison of glycemic improvement between intermittent calorie restriction and continuous calorie restriction in diabetic mice. Nutr Metab (Lond). 2019;16:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Munhoz AC, Vilas-Boas EA, Panveloski-Costa AC, Leite JSM, Lucena CF, Riva P, et al. Intermittent Fasting for Twelve Weeks Leads to Increases in Fat Mass and Hyperinsulinemia in Young Female Wistar Rats. Nutrients. 2020;12(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosenbaum M, Leibel RL. Models of energy homeostasis in response to maintenance of reduced body weight. Obesity (Silver Spring). 2016;24(8):1620–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muller MJ, Enderle J, Bosy-Westphal A. Changes in energy expenditure with weight gain and weight loss in humans. Curr Obes Rep. 2016;5(4):413–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Almundarij TI, Gavini CK, Novak CM. Suppressed sympathetic outflow to skeletal muscle, muscle thermogenesis, and activity energy expenditure with calorie restriction. Physiol Rep. 2017;5(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beaulieu K, Casanova N, Oustric P, Hopkins M, Varady K, Finlayson G, et al. An exploratory investigation of the impact of ‘fast’ and ‘feed’ days during intermittent energy restriction on free-living energy balance behaviours and subjective states in women with overweight/obesity. Eur J Clin Nutr. 2020. [DOI] [PubMed] [Google Scholar]
- 35.Lessard SJ, Rivas DA, Alves-Wagner AB, Hirshman MF, Gallagher IJ, Constantin-Teodosiu D, et al. Resistance to aerobic exercise training causes metabolic dysfunction and reveals novel exercise-regulated signaling networks. Diabetes. 2013;62(8):2717–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patterson RE, Sears DD. Metabolic Effects of Intermittent Fasting. Annu Rev Nutr. 2017;37:371–93. [DOI] [PubMed] [Google Scholar]
- 37.Hoddy KK, Kroeger CM, Trepanowski JF, Barnosky AR, Bhutani S, Varady KA. Safety of alternate day fasting and effect on disordered eating behaviors. Nutr J. 2015;14(1):44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stekovic S, Hofer SJ, Tripolt N, Aon MA, Royer P, Pein L, et al. Alternate Day Fasting Improves Physiological and Molecular Markers of Aging in Healthy, Non-obese Humans. Cell Metab. 2019;30(3):462–76 e5. [DOI] [PubMed] [Google Scholar]
- 39.Catenacci VA, Pan Z, Ostendorf D, Brannon S, Gozansky WS, Mattson MP, et al. A randomized pilot study comparing zero-calorie alternate-day fasting to daily caloric restriction in adults with obesity. Obesity (Silver Spring). 2016;24(9):1874–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Halberg N, Henriksen M, Soderhamn N, Stallknecht B, Ploug T, Schjerling P, et al. Effect of intermittent fasting and refeeding on insulin action in healthy men. J Appl Physiol (1985). 2005;99(6):2128–36. [DOI] [PubMed] [Google Scholar]
- 41.Harvie M, Wright C, Pegington M, McMullan D, Mitchell E, Martin B, et al. The effect of intermittent energy and carbohydrate restriction v. daily energy restriction on weight loss and metabolic disease risk markers in overweight women. Br J Nutr. 2013;110(8):1534–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stote KS, Baer DJ, Spears K, Paul DR, Harris GK, Rumpler WV, et al. A controlled trial of reduced meal frequency without caloric restriction in healthy, normal-weight, middle-aged adults. Am J Clin Nutr. 2007;85(4):981–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beaulieu K, Casanova N, Oustric P, Turicchi J, Gibbons C, Hopkins M, et al. Matched weight loss through intermittent or continuous energy restriction does not lead to compensatory increases in appetite and eating behavior in a randomized controlled trial in women with overweight and obesity. J Nutr. 2019. [DOI] [PubMed] [Google Scholar]
- 44.Klempel MC, Bhutani S, Fitzgibbon M, Freels S, Varady KA. Dietary and physical activity adaptations to alternate day modified fasting: implications for optimal weight loss. Nutr J. 2010;9:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hoddy KK, Gibbons C, Kroeger CM, Trepanowski JF, Barnosky A, Bhutani S, et al. Changes in hunger and fullness in relation to gut peptides before and after 8 weeks of alternate day fasting. Clin Nutr. 2016;35(6):1380–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.