Abstract
Purpose:
Metastasis-directed therapy (MDT) is increasingly used in castration-sensitive oligometastatic prostate cancer because it prolongs progression-free survival (PFS) and androgen deprivation free survival. Here we describe patterns of recurrence and identify modes of progression after MDT using SABR.
Methods and Materials:
Two hundred fifty-eight patients with castration-sensitive oligometastatic prostate cancer (≤5 lesions at staging) were retrospectively identified from a multi-institutional database. Descriptive patterns of recurrence and modes of progression were reported. Other outcomes including median time to prostate-specific antigen (PSA) recurrence, time to next intervention, distant metastasis–free survival, overall survival, and biochemical PFS (bPFS) were reported. Survival analysis was performed using the Kaplan-Meier method, and multivariable analysis was performed.
Results:
Median follow-up was 25.2 months, and 50.4% of patients received concurrent androgen deprivation. Median time to PSA recurrence was 15.7 months, time to next intervention was 28.6 months, distant metastasis–free survival was 19.1 months, and bPFS was 16.1 months. Two-year overall survival was 96.8%. On multivariable analysis, factors associated with bPFS included age (hazard ratio [HR], 1.03; P = .04), N1 disease at diagnosis (HR, 2.00; P = .02), M1 disease at diagnosis (HR, 0.44; P = .01), initial PSA at diagnosis (HR, 1.002; P = <.001), use of androgen deprivation therapy (HR, 0.41; P < .001), pre-SABR PSA (HR, 1.02; P = .01), and use of enhanced imaging for staging (HR, 2.81; P = .001). Patterns of progression favored an osseous component at recurrence; in patients initially treated to a bone lesion alone, the vast majority (86.5%) experienced a recurrence that included an osseous site. Patients treated initially to a nodal site alone tended to recur in a node only (64.5%); however, there was also a significant minority with an osseous component of recurrence at progression (32.3%). Modes of progressors were class I (patients with long term control [no recurrence ≥18 months after therapy]) occurring in 40.9%, class II (oligoprogressors [≤3 lesions at recurrence]) occurring in 36% (including 7.9% of patients with PSA recurrence but no metastatic disease), and class III (polyprogressors [>3 lesions]) occurring in 23.1% of patients.
Conclusions:
After MDT, the majority of patients have long-term control or oligoprogression (class I or II). Recurrence tended to occur in osseous sites. These findings, if validated, have implications for future integration of MDT and clinical trial design.
Introduction
The oligometastatic hypothesis postulates that metastasis represents a continuum spanning a single lesion to widely metastatic disease. Along this continuum, and between these 2 states, lies what is considered oligometastatic disease, whereby individuals have a limited number of metastatic deposits.1 The implication of the oligometastatic hypothesis is that those in this disease state might benefit from prolonged periods of recurrence-free survival or even be cured with local therapies to the primary tumor and oligometastatic sites in conjunction with systemic agents.2
Several studies have demonstrated improvements in progression-free survival (PFS) and overall survival (OS) through aggressive treatment of oligometastatic disease, thus establishing a body of literature to provide support the use of metastasis-directed therapy (MDT).3–5 Within the oligometastatic prostate cancer literature, the Surveillance or Metastasis-Directed Therapy for Oligometastatic Prostate Cancer Recurrence (STOMP) and Observation vs Stereotactic Ablative Radiation for Oligometastatic Prostate Cancer (ORIOLE) trials reported prolonged androgen deprivation therapy (ADT)–free survival and PFS after MDT compared with observation.6,7 These findings are also supported by several retrospective studies showing sustained periods of PFS, ADT-free survival, and time to next intervention (TTNI) with MDT.8–15 Collectively, these results have led to an increasing trend to treat oligometastatic lesions with MDT to improve OS and PFS, delay initiation of systemic therapies with unfavorable toxicity profiles, and offer treatment breaks for individuals amassing toxicity from systemic therapy.
With growing evidence to support the use of MDT in oligometastatic disease, its use will continue to expand. Therefore, a better understanding of which subpopulations most benefit from MDT, and conversely which patients recur and their patterns and modes of progression, are needed to guide future treatment strategies.16–18 Herein we report a retrospective multi-institutional study of 258 men treated to 474 total lesions and present patterns of recurrence and modes of progression after MDT in oligometastatic castration-sensitive prostate cancer.
Methods and Materials
Patient population
After obtaining institutional review board approval from both institutions, we reviewed our retrospectively collected databases of patients with oligometastatic prostate cancer treated with SABR at the Johns Hopkins Hospital and Mayo Clinic from January 2013 through October 2019. Inclusion criteria included men with histologically confirmed prostate cancer and imaging features consistent with metastatic disease (pelvic nodes, extrapelvic nodes, bone, and viscera) who received definitive-intent radiation therapy to all known metastatic lesions. Typically, men with ≤5 lesions seen on imaging were considered oligometastatic and appropriate for MDT. However, those with >5 lesions were included in the analysis if all sites of disease were treated with definitive-intent radiation. Individuals without any follow-up data, either in the form of prostate-specific antigen (PSA) or repeat imaging, were excluded from the analysis.
Follow-up was not mandated by a specific protocol; however, patients were typically seen every 3 to 6 months after SABR with repeat history, physical examination, PSA, and testosterone. Imaging was also often repeated at 6- to 12-month intervals or sooner if warranted by symptoms or change in PSA dynamics. Imaging was classified as either conventional (computed tomography [CT], magnetic resonance imaging [MRI], bone scan) or enhanced (choline, fluciclovine, sodium fluoride, or prostate-specific membrane antigen [PSMA] positron emission tomography [PET]). Interpretations of disease progression, decision regarding changes to a patient’s treatment paradigm, and use of ADT after SABR was typically conducted in a multidisciplinary manner. Additional specifics regarding radiation techniques have been described previously for both institutions.8,19
Statistical analysis
Analysis was performed at both the patient and lesion level. Summary statistics were calculated for patients and lesions. Descriptive patterns of recurrence and modes of progression were reported. Subsequent stratification was based on the use of ADT and imaging technique (conventional [CT, MRI, bone scan] or enhanced [choline, fluciclovine, sodium fluoride, PSMA PET]). Survival analysis was conducted for PSA recurrence, distant metastasis-free survival (DMFS), TTNI, OS, and biochemical PFS (bPFS). PSA recurrence was defined per Scher et al20 and used in previous work as follows: (1) an initial decline from baseline PSA was observed (a PSA increase of ≥25% and ≥2 ng/mL above the nadir, or an increase of ≥25% and greater than the pretreatment PSA value, as confirmed with a second value ≥3 weeks later); (2) no initial decline from baseline (if the baseline PSA was ≥2 ng/mL, a PSA increase of ≥25% and ≥2 ng/mL above baseline after 3 months, or a PSA increase of ≥2 ng/mL after 3 months if the baseline PSA was <2 ng/mL); or (3) initiation of systemic therapy before meeting the 2 previous criteria.19,20 Death before PSA recurrence was censored.
Events for other endpoints of interest were as follows: TTNI was a change in therapy after SABR (including repeat SABR); DMFS was the development of new metastatic lesions (noted on CT/MRI or uptake noted on bone scan or PET-based imaging) or death; and bPFS was PSA recurrence (as defined earlier), initiation of systemic therapy, local or distant recurrence, or death. Times to endpoints were calculated from the end of MDT using the Kaplan-Meier method and compared using the log rank test.
Univariable Cox regression analysis was conducted to identify clinical variables associated with endpoints of interest, followed by multivariable (MVA) models including all variables. Rates of local recurrence and factors associated with local recurrence after SABR were calculated using competing risk and Fine-Gray analysis and were assessed on an individual lesion basis. Local recurrence was defined as any increase in size or radiotracer avidity of the treated lesion from nadir, subsequent use of a secondary local salvage therapy to the treated site, or the development of a new lesion within the initial 50% isodose line. Marginal failure was defined as recurrence directly adjacent to the original treated lesions, but outside the 50% isodose line. All statistical analyses were conducted using R.
Results
Baseline characteristics
A total of 258 patients were included in the analysis, and Table 1 describes baseline characteristics of the population. Median follow-up time for the population was 25.2 months (interquartile range [IQR], 14.4–37 months); 68% of patients were treated at the Johns Hopkins Hospital and 32% were treated at the Mayo Clinic. The median age of the population was 67 years (IQR, 61–72 years). The median number of metastases initially treated was 1, and the majority of patients (62.4%) had only osseous lesions. Half of patients (50.4%) received concurrent ADT with radiation with median duration of 18.7 months (IQR, 12.4–27 months). All patients who received ADT were treated with luteinizing hormone–releasing hormone agonists/antagonists. A small percentage of patients also received additional androgen receptor axis agents, such as enzalutamide or abiraterone (1.6%), and 1 patient (0.4%) was also treated with concurrent docetaxel. Finally, 4.3% of patients received nelfinavir during SABR. Initial Gleason groups (GG) were as follows: GG1 (5.4%), GG2 (20.5%), GG3 (24.4%), GG4 (18.6%), and GG5 (29.8%). The majority of patients (74.4%) underwent enhanced imaging at the time of metastatic staging and at follow-up (53.9%), with 20.9% of patients not having any follow-up imaging because of stable PSA.
Table 1.
Baseline characteristics
| Variable | Value (median) |
|---|---|
| Age, y (IQR) | 67 (61–72) |
| iPSA, ng/mL (IQR) | 7.35 (5.4–15.8) |
| Metastases, n (IQR) | 1 (1–2) |
| PSA nadir, ng/mL (IQR) | 0 (0–1.9) |
| Follow-up, mo (IQR) | 25.2 (14.4–37) |
| T stage, n | |
| T1 | 12 (4.7%) |
| T2 | 98 (38%) |
| T3 | 138 (53.5%) |
| T4 | 4 (1.6%) |
| Tx | 6 (2.3%) |
| N stage, n | |
| N0 | 193 (74.8%) |
| N1 | 57 (22.1%) |
| Nx | 8 (3.1%) |
| M stage, n | |
| M0 | 138 (53.5%) |
| M1 | 66 (25.6%) |
| Mx | 54 (20.9%) |
| Gleason grade group, n | |
| 6 | 14 (5.4%) |
| 7 | 117 (45.3%) |
| 8 | 48 (18.6%) |
| 9 | 70 (27.1%) |
| 10 | 7 (2.7%) |
| N/A | 2 (0.8%) |
| Gleason grade group, n | |
| 1 | 14 (5.4%) |
| 2 | 53 (20.5%) |
| 3 | 63 (24.4%) |
| 4 | 48 (18.6%) |
| 5 | 77 (29.8%) |
| N/A | 3 (1.2%) |
| ADT treatment site, n | |
| Bone | 161 (62.4%) |
| Node | 71 (27.5%) |
| Mixture | 26 (10.1%) |
Abbreviations: ADT = androgen deprivation therapy; iPSA = initial PSA; IQR = interquartile range; N/A = not applicable; PSA = prostate specific antigen.
In total 474 lesions were treated, of which 289 (61%) were osseous lesions, 182 (38.4%) were nodal lesions, and 3 (0.6%) were nodular local recurrences adjacent to the prostatic bed or rectum. Median biologic equivalent dose (using alpha/beta of 3, BED3) was 123.9 Gy. The most common areas for treatment were in the pelvis (48.7%), spine (15.8%), and abdomen (11.8%).
Patterns and modes of recurrence
A total of 113 patients experienced distant recurrence at last follow-up, with osseous recurrence representing the most common pattern of progression after MDT. Fifty patients (44.2%) had a recurrence in a bone site alone, whereas 28 patients (24.8%) had recurrence in multiple locations of which bone was a component. Thirty patients (26.5%) experienced recurrence in a nodal site alone. A local recurrence, although uncommon, occurred in 4 (3.6%) patients; a visceral recurrence alone occurred in 1 patient (0.9%).
When stratified by original treatment location, there appeared to be a shift toward bone recurrence after MDT. In patients initially treated to a bone lesion alone, the vast majority (86.5%) experienced a recurrence that included an osseous site. Patients treated initially to a nodal site alone tended to recur again in a node only (64.5%); however, there was also a significant minority with an osseous component of recurrence at progression (32.3%). These distributions in recurrence sites were statistically different (Table 2; P < .001).
Table 2.
Patterns of recurrence after metastasis-directed therapy
| Original treatment site | ||||
|---|---|---|---|---|
| Failure location | Bone (N = 74) | Node (N = 31) | Bone/Node (N = 8) | P value |
| Bone component, n | 64 (86.5%) | 10 (32.3%) | 4 (50%) | <.001 |
| Node, n | 7 (9.5%) | 20 (64.5%) | 3 (37.5%) | |
| Other, n | 3 (4.0%) | 1 (3.2%) | 1 (12.5%) | |
We then attempted to stratify patients into modes of progression after therapy (Fig. 1A, Table 3),16 which appeared to fall into 3 classes. Class I included patients with long-term control (defined as no recurrence ≥18 months after therapy) of their disease after MDT and represented 40.9% of patients. Class II included patients with oligometastatic recurrence (either local or distant) after MDT (≤3 metastatic lesions) and represented 36% of patients (including 7.9% of patients with a PSA recurrence but no metastatic disease on follow-up imaging). Class III included men with polyprogression after MDT (>3 lesions) and represented 23.1% of patients.
Fig. 1.
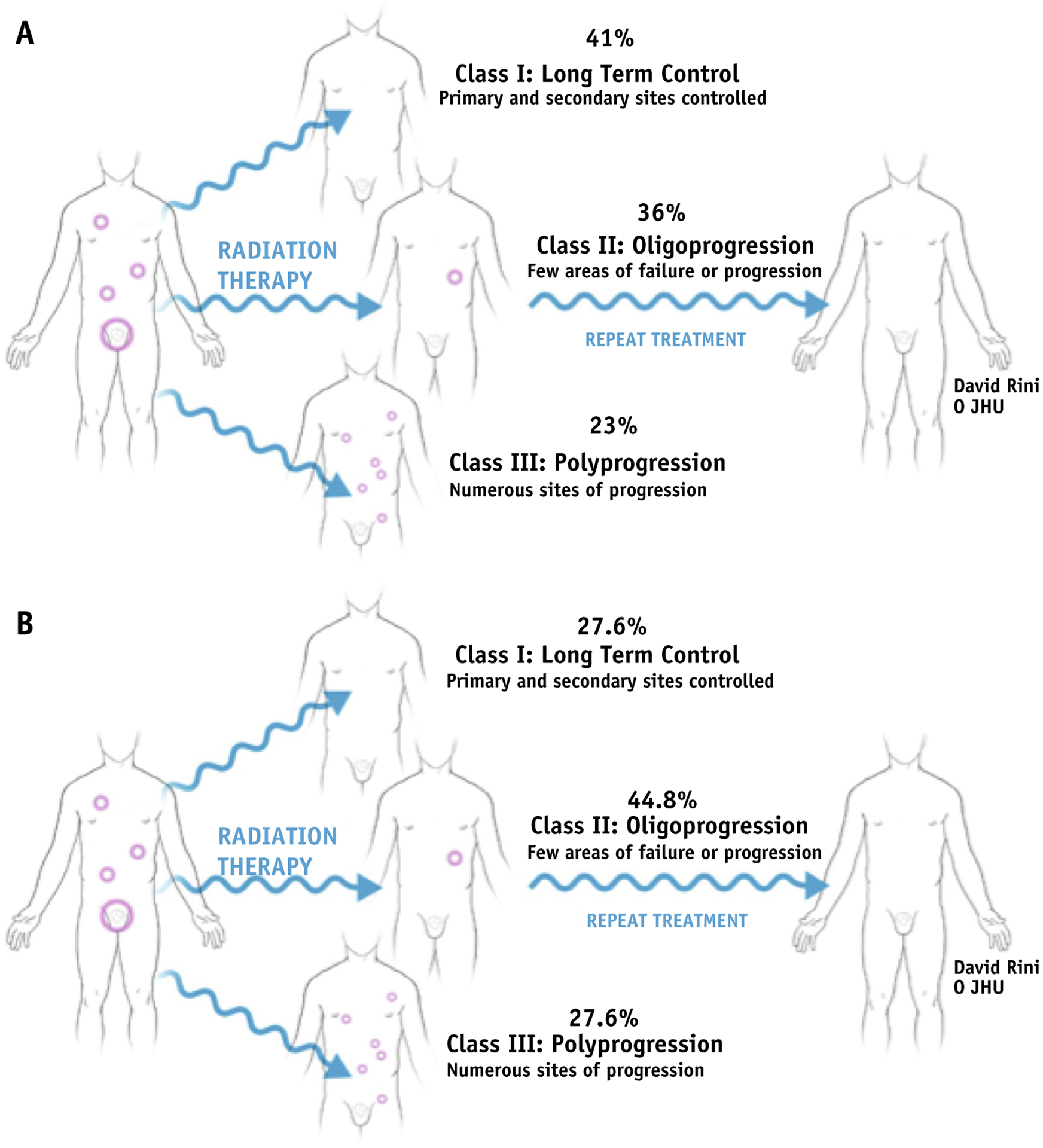
(A) Modes of progression after metastasis-directed therapy (MDT). (B) Modes of progression after MDT in men not treated with androgen deprivation therapy.
Table 3.
Modes of progression
| LTC | Oligoprogressor | Polyprogressor | |
|---|---|---|---|
| Total population | 40.9% | 36% | 23.1% |
| No ADT | 27.6% | 44.8% | 27.6% |
| Conventional imaging | 46.1% | 36.2% | 17.7% |
| Enhanced imaging | 36.3% | 37.7% | 26% |
Abbreviations: ADT = androgen deprivation therapy; LTC = long-term control.
Class distribution was different in the subgroup of men treated without ADT (Fig. 1B, Table 3): 27.6% in class I, 44.8% in class II (10.5% with PSA recurrence only), and 27.6% in class III. Nine of the patients with oligoprogression were retreated with SABR (without ADT) with median time to second PSA recurrence of 11.7 months. Finally, the use of advanced imaging for surveillance (choline, fluciclovine, sodium fluoride, PSMA PET) was associated with differences in class distribution compared with conventional imaging (Table 3). Lower percentages of class I (36.3% vs 46.1%), similar percentages of class II (37.7% vs 36.2%), and higher percentages of class III (26% vs 17.7%) progression were identified with advanced imaging.
Systemic oncologic outcomes
At last follow-up, only 10 deaths had occurred, corresponding to a 2-year OS of 96.8% (Fig. 2A). Median time to PSA recurrence was 15.7 months (95% confidence interval [CI], 13.9–23.4), TTNI was 28.6 months (95% CI, 24.6–42.9), and DMFS was 19.1 months (95% CI, 14.8–27.8) (Fig. 2B–D). For the composite endpoint bPFS, median time to recurrence was 16.1 months (95% CI, 13.9–24.0; Fig. 2E). Fifty-seven patients underwent repeat SABR after progression with a median DMFS of 11.7 months (95% CI, 7.7–16.5 months) after another round of MDT.
Fig. 2.
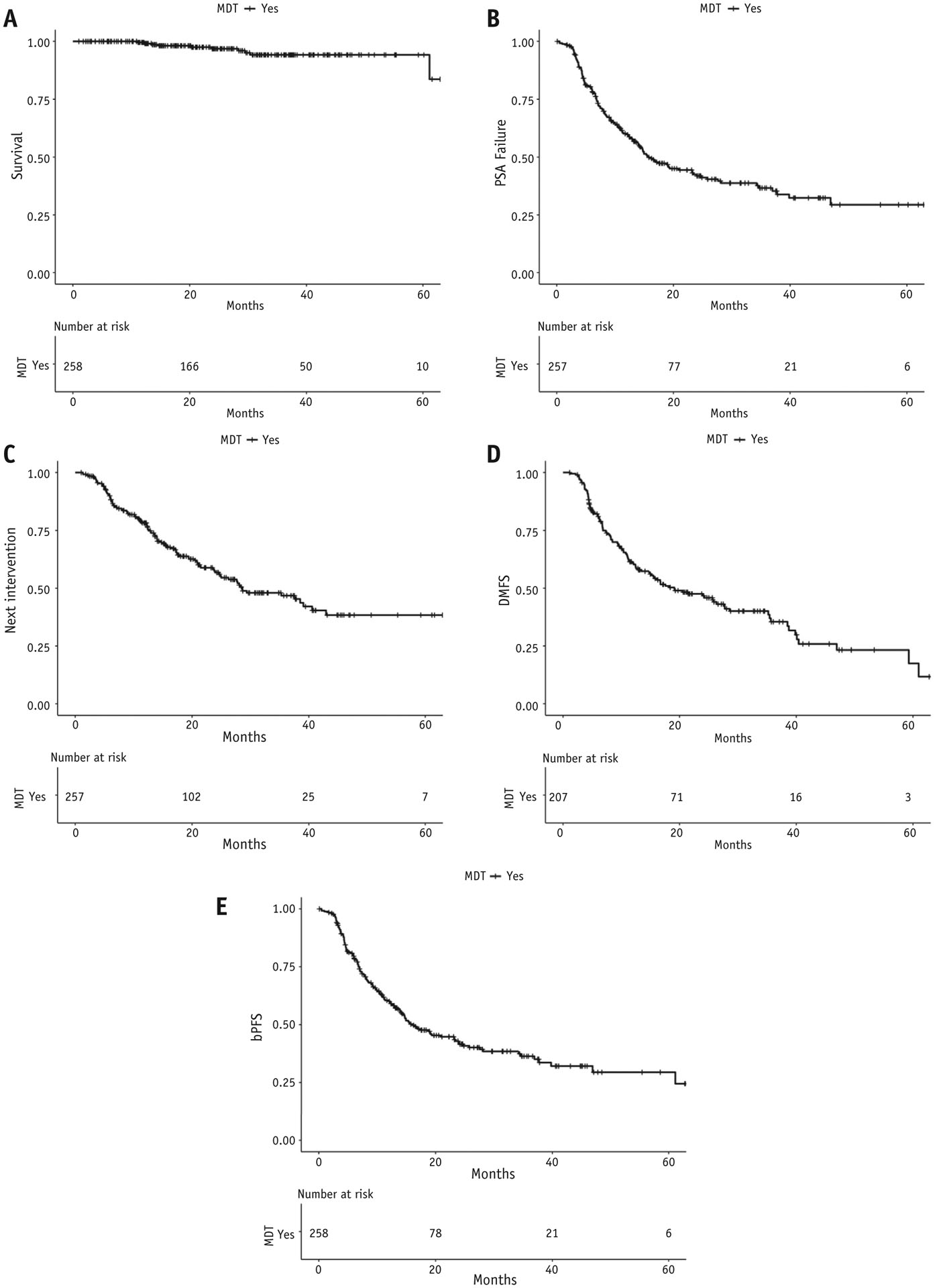
(A) Overall survival after metastasis-directed therapy (MDT). (B) Time to prostate specific antigen (PSA) recurrence after MDT. (C) Time to next intervention after MDT. (D) Distant metastasis–free survival (DMFS) after MDT. (E) Biochemical progression-free survival (bPFS) after MDT.
Factors (Table E1) associated with bPFS on univariable analysis included increasing age (HR, 1.04; 95% CI, 1.01–1.06; P = .001), initial presence of M1 disease at diagnosis (HR, 0.36; 95% CI, 0.22–0.57; P < .001), PSA at diagnosis (HR, 1.001; 95% CI, 1.0004–1.002; P = .04), use of ADT (HR, 0.41; 95% CI, 0.29–0.58; P < .001), and use of enhanced imaging for staging (HR, 2.93; 95% CI, 1.70–5.06; P < .001). On MVA (Table 4) factors associated with bPFS included age (HR, 1.03; 95% CI, 1.001–1.05; P = .04), N1 disease at diagnosis (HR, 2.00; 95% CI, 1.12–3.55; P = .02), M1 disease at diagnosis (HR, 0.44; 95% CI, 0.23–0.83; P = .01), initial PSA at diagnosis (HR, 1.002; 95% CI, 1.0008–1.003; P ≤ .001), use of ADT (HR, 0.41; 95% CI, 0.26–0.67; P < .001), pre-SABR PSA (HR, 1.02; 95% CI, 1.01–1.04; P = .01), and use of enhanced imaging for staging (HR, 2.81; 95% CI, 1.55–5.12; P = .001).
Table 4.
Multivariable analysis for factors associated with biochemical progression-free survival
| Variable | Comparison | HR (95% CI) | P value |
|---|---|---|---|
| Age | 1.03 (1.001–1.05) | .04 | |
| T stage | Tx vs T1/2 | 0.35 (0.08–1.50) | .16 |
| T3/4 vs T1/2 | 1.33 (0.90–2.00) | .15 | |
| N stage | Nx vs N0 | 1.40 (0.54–3.57) | .49 |
| N1 vs N0 | 2.00 (1.12–3.55) | .02 | |
| M stage | Mx vs M0 | 0.86 (0.55–1.35) | .51 |
| M1 vs M0 | 0.44 (0.23–0.83) | .01 | |
| Gleason grade group | 1.01 (0.83–1.24) | .91 | |
| iPSA | 1.002 (1.0008–1.003) | <.001 | |
| ADT | Yes vs No | 0.41 (0.26–0.67) | <.001 |
| Number met | 0.99 (0.83–1.20) | .94 | |
| Tx location | Bone vs Node | 1.42 (0.93–2.17) | .11 |
| Multiple vs Node | 0.50 (0.22–1.12) | .09 | |
| Pre-SABR PSA | 1.02 (1.01–1.04) | .01 | |
| Enhanced imaging | 2.81 (1.55–5.12) | .001 |
Abbreviations: ADT = androgen deprivation therapy; CI = confidence interval; HR = hazard ratio; iPSA = initial PSA; PSA = prostate-specific antigen; Tx= treatment.
Impact of ADT
One hundred twenty-eight patients underwent treatment without concurrent ADT. Median time to PSA recurrence in this group was 10.9 months (95% CI, 8.2–14.1), TTNI was 21.4 months (95% CI, 14.8–28.7), DMFS was 12.4 months (95% CI, 10.8–19.1), and bPFS was 10.9 months (95% CI, 8.2–14.1; Fig. E1). Twenty men were treated with a defined course of concurrent ADT and experienced testosterone recovery after stopping ADT. The median bPFS of this group was 17.6 months after stopping ADT; however, compared with those not treated with ADT, the differences in bPFS did not reach statistical significance (P = .21; Fig. 2).
Lesion data
The vast majority of patients were treated with ≥5 Gy per fraction; however, a small minority (7%) received less, typically because of organ at risk dose constraints. The most common fractionation schemes were as follows: 15 to 20 Gy × 1 fraction (36.1%), 8 to 12 Gy × 3 fractions (22.4%), and 6 to 10 Gy × 5 fractions (19.2%). In absolute numbers, rates of local recurrence were low, with only 20 in total; this translated to a cumulative incidence of local recurrence at 12 and 24 months of 3.4% and 7%, respectively (Fig. E3). In addition, only 1 marginal failure occurred, with a 3-year cumulative incidence of 2.8%. The small number of events limited analysis for factors associated with local recurrence. On univariable analysis age (P = .58), BED3 (P = .16), GG (P = .34), and treatment location (node vs bone, P = .48) did not affect rates of local recurrence. In addition, the concurrent use of ADT did not affect rates of recurrence, with cumulative incidence of local recurrence at 12 months of 5.9% (n = 12) versus 3.7% (n = 8; P = .71).
Discussion
In this article, we describe a retrospective multi-institution experience in treating oligometastatic prostate cancer with SABR. In our cohort of 258 patients and 474 lesions, median bPFS after MDT was 16.1 months. Patterns of recurrence after MDT favor progression in osseous sites. In addition, we identified 3 modes of progression, which showed that a majority of patients appeared to experience either long-term control (class I) or few sites of progression after MDT (class II).
Our study reports on a classification for modes of progression after MDT in oligometastatic castration-sensitive prostate cancer. Class I, representing 30% to 40% of patients, appears to have long-term control. Class II, representing 30% to 40%, experiences oligoprogression. Class III, comprising 20% to 25%, has polyprogression after MDT. These findings have several important implications. First, they suggest that MDT is an effective therapy for a significant proportion of patients and provide more evidence to support its integration as a standard intervention. Six-month PFS in our cohort not treated with ADT was 70% and was higher in those treated with ADT, in line with prospective trials of similar patient populations.6,7 Second, the findings demonstrate that many patients who experience progression have only a small number of new lesions. These patients could feasibly be retreated with MDT18 and potentially experience long-term control or freedom from systemic therapy. Finally, we identified 20% to 25% of patients as polyprogressors who are unlikely to have benefited from MDT. This finding suggests that improved efforts to identify these patients before MDT are needed and could be aided by the integration of more sensitive imaging techniques such as choline, fluciclovine, sodium fluoride, and PSMA PET or a better understanding of the influence of tumor biology and genomics on disease behavior.21–24
This study represents one of the largest reports on MDT in castration-sensitive prostate cancer available in the literature, allowing for a detailed and accurate analysis of patterns and modes of progression. We noted recurrences tended to occur in new osseous structures after MDT. The vast majority (>85%) of patients who underwent treatment for a bone lesion had another osseous site as a component of progression at time of recurrence. Patients who underwent initial treatment at nodal sites alone tended to have recurrence in a node alone; however, there seemed to be a shift to an osseous element of recurrence, with >30% of patients experiencing a component of bony progression. These findings appear in line with a report on a smaller cohort of patients by Soldatov et al,25 which found mostly bone recurrence after MDT for oligometastatic prostate cancer using advanced imaging techniques. This finding indicates that subclinical micrometastatic disease might exist within osseous structures for a large number of patients with oligometastatic castration-sensitive disease and raises questions regarding how to manage these men. The currently accruing RAVENS trial (NCT04037358) is randomizing men with oligometastatic castration-sensitive prostate cancer and at least 1 bone metastasis to SABR with or without radium-223 with the primary outcome of improving PFS.26 If the result is positive, it might suggest a role for radium-223 in the management of subclinical micrometastatic osseous disease and another treatment option in the armamentarium for oligometastatic prostate cancer.
ADT with gonadotropin hormone-releasing antagonist/agonists and intensification with a number of supracastrating agents or docetaxel chemotherapy is the first-line standard-of-care therapy in metastatic castration-sensitive disease.27–30 However, most MDT research to date for oligometastatic castration-sensitive disease has attempted to avoid ADT and its associated unfavorable adverse effect profile.31 This was the goal of the STOMP6 and ORIOLE7 trials, which randomized men with castration-sensitive prostate cancer to MDT versus observation and reported a prolonged ADT-free interval and improved PFS in the MDT arms. Therefore, the optimal way to integrate MDT and systemic therapy into the management of oligometastatic prostate cancer, and whether the combination might result in improvements in outcomes, is still not clear. Our cohort did not show a clear benefit in bPFS in men with oligorecurrent disease treated with combined MDT and a course of ADT compared with MDT alone; however, the small number of patients who underwent combined therapy and the retrospective nature of this study warrants prospective evaluation.
There are several limitations to our study—most prominent is that this is a retrospective report, which makes it susceptible to several biases. We attempted account for such bias using an MVA model; however, this method cannot account for unrecognized confounders. Therefore, this report is hypothesis generating, and the information gleaned from it will be tested and validated in a more controlled manner. Nevertheless, this study contributes to the literature surrounding oligometastatic castration-sensitive prostate cancer and it might spur future studies to continue advancement in the disease.
Conclusion
MDT can result in sustained disease control for 30% to 40% of patients. Among patients who experience progression, approximately half are oligorecurrent and possibly amenable to repeat MDT. Patterns of recurrence tend to favor osseous progression. These findings, if validated, have implications for future clinical trial design.
Supplementary Material
Acknowledgments
Phuoc T. Tran was funded by the Ronald Rose and Joan Lazar, Nesbitt-McMaster Foundation, Movember-PCF, Babara’s Fund, and the National Institutes of Health and National Cancer Institute (grants U01CA212007, U01CA231776, and R21CA223403).
Footnotes
Disclosures: none.
Research data are stored in an institutional repository and will be shared upon request to the corresponding author.
Supplementary material for this article can be found at https://doi.org/10.1016/j.ijrobp.2020.08.030.
References
- 1.Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol 1995;13:8–10. [DOI] [PubMed] [Google Scholar]
- 2.Hughes KS, Simon R, Songhorabodi S, et al. Resection of the liver for colorectal carcinoma metastases: A multi-institutional study of patterns of recurrence. Surgery 1986;100:278–284. [PubMed] [Google Scholar]
- 3.Palma DA, Olson R, Harrow S, et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): A randomised, phase 2, open-label trial. Lancet 2019;393:2051–2058. [DOI] [PubMed] [Google Scholar]
- 4.Iyengar P, Wardak Z, Gerber DE, et al. Consolidative radiotherapy for limited metastatic non-small-cell lung cancer: A phase 2 randomized clinical trial. JAMA Oncol 2018;4:e173501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gomez DR, Blumenschein GR Jr, Lee JJ, et al. Local consolidative therapy versus maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer without progression after first-line systemic therapy: A multicentre, randomised, controlled, phase 2 study. Lancet Oncol 2016;17:1672–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ost P, Reynders D, Decaestecker K, et al. Surveillance or metastasis-directed therapy for oligometastatic prostate cancer recurrence: A prospective, randomized, multicenter phase II trial. J Clin Oncol 2018; 36:446–453. [DOI] [PubMed] [Google Scholar]
- 7.Phillips R, Shi WY, Deek M, et al. Outcomes of observation vs stereotactic ablative radiation for oligometastatic prostate cancer: The ORIOLE phase 2 randomized clinical trial. JAMA Oncol 2020;6:650–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deek MP, Yu C, Phillips R, et al. Radiation therapy in the definitive management of oligometastatic prostate cancer: The Johns Hopkins experience. Int J Radiat Oncol Biol Phys 2019;105:948–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moyer CL, Phillips R, Deek MP, et al. Stereotactic ablative radiation therapy for oligometastatic prostate cancer delays time-to-next systemic treatment. World J Urol 2019;37:2623–2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muldermans JL, Romak LB, Kwon ED, Park SS, Olivier KR. Stereotactic body radiation therapy for oligometastatic prostate cancer. Int J Radiat Oncol Biol Phys 2016;95:696–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ost P, Jereczek-Fossa BA, As NV, et al. Progression-free survival following stereotactic body radiotherapy for oligometastatic prostate cancer treatment-naive recurrence: A multi-institutional analysis. Eur Urol 2016;69:9–12. [DOI] [PubMed] [Google Scholar]
- 12.Ahmed KA, Barney BM, Davis BJ, Park SS, Kwon ED, Olivier KR. Stereotactic body radiation therapy in the treatment of oligometastatic prostate cancer. Front Oncol 2012;2:215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berkovic P, De Meerleer G, Delrue L, et al. Salvage stereotactic body radiotherapy for patients with limited prostate cancer metastases: Deferring androgen deprivation therapy. Clin Genitourin Cancer 2013;11:27–32. [DOI] [PubMed] [Google Scholar]
- 14.Ponti E, Ingrosso G, Carosi A, et al. Salvage stereotactic body radiotherapy for patients with prostate cancer with isolated lymph node metastasis: A single-center experience. Clin Genitourin Cancer 2015;13:e279–e284. [DOI] [PubMed] [Google Scholar]
- 15.Jereczek-Fossa BA, Beltramo G, Fariselli L, et al. Robotic image-guided stereotactic radiotherapy, for isolated recurrent primary, lymph node or metastatic prostate cancer. Int J Radiat Oncol Biol Phys 2012;82:889–897. [DOI] [PubMed] [Google Scholar]
- 16.Deek MP, Phillips R, Tran PT. Radiotherapy in the management of metastatic hormone-sensitive prostate cancer: What is the standard of care? Cancer J 2020;26:87–93. [DOI] [PubMed] [Google Scholar]
- 17.Ost P, Jereczek-Fossa BA, Van As N, et al. Pattern of progression after stereotactic body radiotherapy for oligometastatic prostate cancer nodal recurrences. Clin Oncol (R Coll Radiol) 2016;28:e115–e120. [DOI] [PubMed] [Google Scholar]
- 18.Decaestecker K, De Meerleer G, Lambert B, et al. Repeated stereotactic body radiotherapy for oligometastatic prostate cancer recurrence. Radiat Oncol 2014;9:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao RW, Olivier KR, Park SS, et al. Single-fraction stereotactic body radiation therapy versus conventionally fractionated radiation therapy for the treatment of prostate cancer bone metastases. Adv Radiat Oncol 2019;4:314–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scher HI, Halabi S, Tannock I, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: Recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol 2008;26:1148–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCarthy M, Francis R, Tang C, Watts J, Campbell A. A multicenter prospective clinical trial of (68)gallium PSMA HBED-CC PET-CT restaging in biochemically relapsed prostate carcinoma: Oligometastatic rate and distribution compared with standard imaging. Int J Radiat Oncol Biol Phys 2019;104:801–808. [DOI] [PubMed] [Google Scholar]
- 22.Artigas C, Flamen P, Charlier F, et al. (68)Ga-PSMA PET/CT-based metastasis-directed radiotherapy for oligometastatic prostate cancer recurrence after radical prostatectomy. World J Urol 2019;37:1535–1542. [DOI] [PubMed] [Google Scholar]
- 23.Guler OC, Engels B, Onal C, et al. The feasibility of prostate-specific membrane antigen positron emission tomography(PSMA PET/CT)-guided radiotherapy in oligometastatic prostate cancer patients. Clin Transl Oncol 2018;20:484–490. [DOI] [PubMed] [Google Scholar]
- 24.Henkenberens C, von Klot CA, Ross TL, et al. (68)Ga-PSMA ligand PET/CT-based radiotherapy in locally recurrent and recurrent oligometastatic prostate cancer: Early efficacy after primary therapy. Strahlenther Onkol 2016;192:431–439. [DOI] [PubMed] [Google Scholar]
- 25.Soldatov A, von Klot CAJ, Walacides D, et al. Patterns of progression after (68)Ga-PSMA-Ligand PET/CT-guided radiation therapy for recurrent prostate cancer. Int J Radiat Oncol Biol Phys 2019;103:95–104. [DOI] [PubMed] [Google Scholar]
- 26.Hasan H, Deek MP, Phillips R, et al. A phase II randomized trial of RAdium-223 dichloride and SABR Versus SABR for oligomEtastatic prostate caNcerS (RAVENS). BMC Cancer 2020;20:492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chi KN, Agarwal N, Bjartell A, et al. Apalutamide for metastatic, castration-sensitive prostate cancer. N Engl J Med 2019;381:13–24. [DOI] [PubMed] [Google Scholar]
- 28.Kyriakopoulos CE, Chen YH, Carducci MA, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer: Long-term survival analysis of the randomized phase III E3805 CHAARTED Trial. J Clin Oncol 2018;36:1080–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fizazi K, Tran N, Fein L, et al. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med 2017;377:352–360. [DOI] [PubMed] [Google Scholar]
- 30.Davis ID, Martin AJ, Stockler MR, et al. Enzalutamide with standard first-line therapy in metastatic prostate cancer. N Engl J Med 2019;381:121–131. [DOI] [PubMed] [Google Scholar]
- 31.Nguyen PL, Alibhai SM, Basaria S, et al. Adverse effects of androgen deprivation therapy and strategies to mitigate them. Eur Urol 2015;67:825–836. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


