Abstract
Microglia are one of the first responders to ischemic injury. Aged microglia acquire a senescent phenotype and produce more inflammatory cytokines after stroke. Depletion of microglia in young mice worsens post-ischemic damage by increasing inflammation. However, young mice do not have dysfunctional microglia. Hence, we hypothesized that depletion of microglia in older mice will contribute to improved early recovery after ischemic stroke injury. Aged (18–19 month) mice were fed with either control chow diet (CD) or PLX5622 chow diet (PLXD) for 21 days. On day 22, a 60-minute middle cerebral artery occlusion (MCAo) surgery or sham surgery was performed. Twenty-four and 72 hours after stroke immunohistochemistry and flow cytometry were performed. AFS98, a monoclonal antibody against CSF1R was used to specifically deplete brain macrophages by injection into the right hemisphere. Two days after AFS98 injections, mice underwent one-hour MCAo. Twenty-four hours later mice were euthanized and flow cytometry was performed. An increase in infarct volume (p<0.05) was seen in the PLXD versus CD after stroke in aged mice at 24 and 72 hours. An increase (p<0.05) in infiltrating monocytes was observed after microglial depletion in aged stroke mice suggesting a differential monocyte response. An increase in astrocyte numbers was evident in the PLXD sham mice compared to CD sham, reflecting the off-target effects of PLX5622 treatment. In conclusion, PLX5622 and AFS98 treatment depleted microglia in aged animals but resulted in increased neuroinflammation after ischemic stroke.
Keywords: Age, Stroke, Microglia, Colony-stimulating factor 1R, Inflammation
1. Introduction
Stroke is a disease of aging and approximately two-thirds of all strokes occur in older adults (Feigin et al., 2014). Older mice exhibit a differential response to stroke and have worse outcomes despite smaller infarcts (Chauhan et al., 2018). In response to cerebral injury, microglia are some of the first responders, quickly developing an activated phenotype, generating reactive oxygen species, phagocytizing, and producing pro-inflammatory cytokines and proteases (Ritzel et al., 2018). With aging, microglia acquire a dysfunctional phenotype characterized by dystrophic morphology, impaired phagocytosis, reduced motility, and exaggerated response to injury (Niraula et al., 2017). Microglia in aged mice produce higher levels of reactive oxygen species and have an exaggerated inflammatory response after ischemic stroke compared to young animals (Ritzel et al., 2018). Studies have shown that depletion of microglia reduces neuroinflammation and improves disease phenotype in intracranial hemorrhage, and Alzheimer’s disease models (Li et al., 2017; Sosna et al., 2018). However, microglia depletion worsened damage by increasing inflammation and triggering neuronal death in ischemic stroke models (Szalay et al., 2016). A major limitation of all of these studies is that they were performed in young animals, although stroke is a disease of aging, and aged mice demonstrate heightened inflammatory responses and dysfunctional microglia. Hence, we hypothesized that reducing microglia would dampen the exaggerated neuroinflammatory responses seen in aged mice and improve early recovery after ischemic stroke.
2. Materials and methods
Ethics statement
All animal procedures were performed under NIH guidelines for the care and use of laboratory animals and approved by the Animal Care Committee of the University of Texas Health Science Center at Houston, McGovern Medical School. The mice were randomly allocated to the study groups.
Experimental animals
C57BL/6J male mice 8–12 weeks (young) and 18–19 months (aged) of age were pair-housed in a specific pathogen-free facility (light cycle 12/12 h light/dark). Food and water were provided ad libitum.
Microglia depletion
The depletion of microglia was achieved by using CSF1R inhibitor, PLX5622, and AFS98 a monoclonal antibody against CSF1R. Details of these procedures are provided in supplementary methods.
Middle cerebral artery occlusion
On day 22, transient focal ischemia was induced under isoflurane anesthesia in aged mice for 60 minutes by occlusion of the right middle cerebral artery (Chauhan et al., 2018). Details of procedures including neurological deficit score (NDS), infarct analysis are provided in supplementary methods
Immunohistochemistry staining
The brain sections were mounted and immunohistochemistry was performed as described earlier (Chauhan et al., 2018). Details of procedures are provided in supplementary methods.
Flow cytometry
The flow cytometry procedure was followed as described earlier (Ritzel et al., 2018). Details of procedures are provided in supplementary methods.
Statistical Analysis
Two-group comparisons were performed by the by Two-sample t test with Welch’s correction using Graphpad prism 7. Two-way ANOVA followed by post hoc Tukey test was used to multiple comparisons between groups. The data is presented as an interleaved box and whisker with min to max and showing all points except NDS where the interquartile range is provided (Mann-Whitney). Statistical significance was set at p<0.05.
3. Results
PLX5622 treatment reduced microglia counts in aged mice
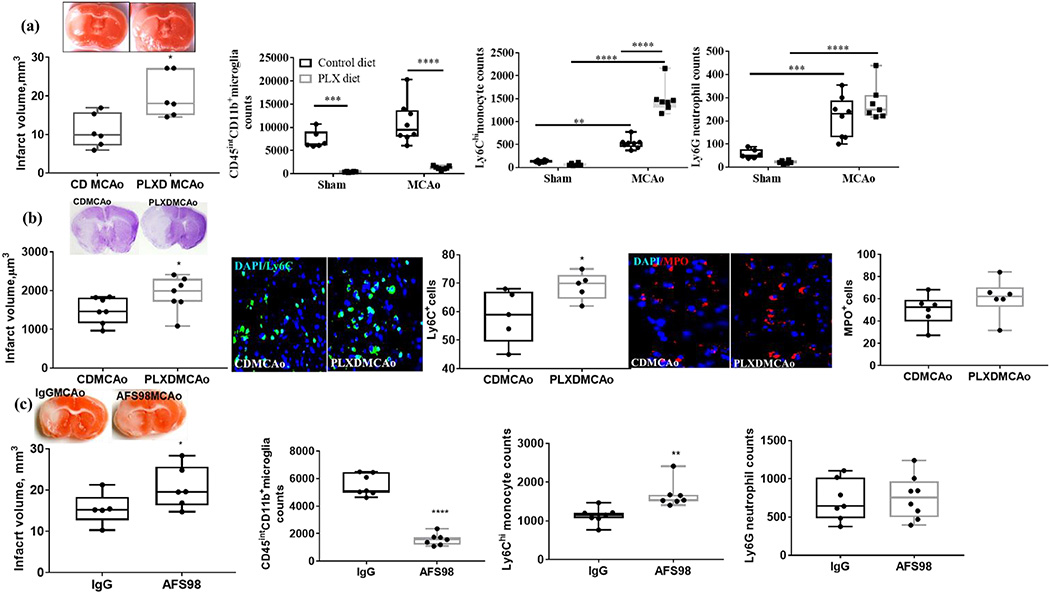
Microglia, macrophages, and monocytes depend upon colony-stimulating factor 1 receptor (CSF1R) signaling for survival (Elmore et al., 2018). We administered the CSF1R inhibitor PLX5622 or CD in chow for 7, 14, and 21 days to both young and aged male mice to investigate the reduction in microglia over time. PLX5622 treatment reduced microglia in both young and aged mice (Supple fig 1) however; the depletion was less effective in aged brains. We observed a significant reduction in cortical and striatum Iba1+ cells in the young and aged brain as compared to day 0 (p<0.05). There was significant reduction in Iba1+ cells in striatum of the young brain compared to day 0 at all observed time points, including day 7 (p<0.001), 14 (p<0.001), and 21 (p<0.001). In aged mice, striatal Iba1+ cells decreased at day 7 (p<0.05), day 14 (p<0.001), and day 21 (p<0.001). Flow cytometry confirmed the decline in brain microglia with PLXD versus CD in the aged mice (Fig 1a).
Figure 1.
Microglia depletion increased injury at 24 and 72 hours after stroke in aged animals. (a) Increase in infarct volume, decrease in CD45intCD11b+ microglia with PLXD, and increase in infilterating Ly6Chi monocytes and Ly6G+neutrophils in the aged mice at 24 hours. (b) Increase in infarct volume, increase in Ly6C+ cells and no difference in MPO+ cells in the PLXD MCAo mice at 72 hours. (c) Increase in infarct volume, decrease in CD45intCD11b+ microglia with AFS98, and infilterating Ly6Chi monocytes with no difference in Ly6G+ neutrophils in the aged mice at 24 hours. ***p<0001, ** p<0.01, * p<0.05
PLX5622 treatment lead to an increase in infarct volume and infiltration of myeloid cells at 24 hours and 72 hours post MCAo
Depletion of microglia resulted in an increased infarct volume in young and aged male mice (Suppl. fig 2a and Fig. 1a and 1b) (Szalay et al., 2016). To understand how peripheral immune cells respond to microglial depletion, we assessed infiltrating monocytes and neutrophils in the brain 24 hours after stroke in aged animals. We found that infiltrating monocyte numbers increased (p<0.05) in both CD and PLXD MCAo groups versus their respective shams. Higher monocyte counts were observed in the PLXD MCAo compared to CD MCAo (Fig. 1a) suggesting differential monocyte response with microglia reduction in the PLXD stroke group. An increase (p<0.05) in infiltrating neutrophils was observed in both stroke groups as compared to their respective shams (Fig. 1a). Similarly, at 72 hour, an increase (p<0.05) in infarct volume was observed in the PLXD MCAo mice compared to CD MCAo (Fig 1b). Ly6C+ cells were higher (p<0.05) in the PLXD MCAo compared to CD MCAo. No difference in the MPO+ cells was seen between PLXD MCAo and CD MCAo animals at 72 hours (Fig 1b). No difference in NDS was observed between the treatment groups (Suppl. fig. 3a and 3b). No mortality was observed in the 24-hour cohort however, one mouse died in the PLXD at 72 hour.
Reduction in microglia resulted in a reduction in neurons and astrocytes at 24 hours MCAo
Prior studies have shown that depletion of microglia in ischemic stroke resulted in increased neuronal injury (Szalay et al., 2016). Neuronal nuclear antigen (NeuN) quantification revealed a decrease in of NeuN+ cells in the PLXD MCAo group compared to the PLXD sham group (Suppl. fig 4a). Furthermore, we observed an increase in the glial fibrillary acidic protein (GFAP) + cells in the PLXD sham compared to CD sham in both young and aged mice (Suppl. fig 4b). After stroke, GFAP+ cell counts were lower in the PLXD MCAo compared to PLXD treated mice (Suppl. fig 4b) suggesting that interaction between astrocytes and microglia could be essential for demarcation of the damaged area and peripheral immune cell extravasation. At 72 hours after MCAo, there was no difference between GFAP+ cells between PLXD MCAo and CD MCAo mice (Suppl. fig 4c).
Depletion of Iba1+ microglia by AFS98 also resulted in an increase in infarct volume and higher infiltration of inflammatory monocytes
To confirm the efficacy of AFS98 to deplete microglia, Iba1+ cells were quantified. There was significant decline (p<0.01) in Iba1+ cells in the AFS98 group compared to IgG (Supple fig 5). However, no difference in the GFAP+ cells was seen between AFS98 (144.25 ± 27.11) and IgG (133 ± 16.21) treated mice. At 24 hours after MCAo, the infarct volume was higher in the AFS98 treated mice compared to IgG treated animals (Fig 1c). Further, an increase in the Ly6chi monocytes was seen in the AFS98 compared to IgG mice (Fig 1c). No difference in neutrophil infiltration was seen between the two treatment groups after MCAo (Fig 1c). No mortality was observed any treatment group. No difference in NDS was observed between IgG and AFS98 MCAo groups (Suppl. fig 3c).
4. Discussion
Earlier studies have shown that PLX5622 depletes microglia in both young and aged mice (Elmore et al., 2018). In our study, we observed that PLX5622 treatment indeed reduced microglia in young and aged mice. However, the aged brain had more surviving microglia likely due to the known reduced penetration of the PLX5622 into the aged brain (Elmore et al., 2018). Consistent with previous findings in young mice (Szalay et al., 2016), a reduction in microglia increased infarct volume in aged mice. Further, brain specific depletion of microglia with a monoclonal antibody against CSF1R also lead to increased infarct volume in aged mice. This observation suggests that despite the dysregulated state of aged microglia, they appear to contribute to beneficial effects in the early phases of ischemic stroke in aged animals. However, their role in later repair at more chronic endpoints remains to be investigated.
Infiltrating immune cells contribute to the progression of brain injury after ischemic stroke. We observed an increase in infiltrating monocytes and neutrophils after stroke in both PLXD and CD aged mice. No preferential neutrophil recruitment was observed in aged PLXD stroke mice vs aged CD groups as reported by others (Otxoa-de-Amezaga et al., 2018). The difference in the neutrophil responses could partly be explained by the use of young mice by Otxoa-de-Amezaga et al (Otxoa-de-Amezaga et al., 2018). Young stroke mice have shown to recruit monocytes after ischemic stroke, unlike aged mice that recruit neutrophils (Ritzel et al., 2018). However, neutrophilia in both young and aged mice has shown to be correlated with worse functional outcomes (Ritzel et al., 2018). Differential monocyte infiltration was observed in the aged PLXD MCAo group. Potentially, the higher number of infiltrating monocytes could be recruited to replace the available niche created by the lost microglia. Incidentally, higher number of infiltrating monocytes were present in both the depletion models, PLXD and anti CSF1R antibody. Interestingly, we also observed an increase in the number of astrocytes in the PLXD sham group in mice of both ages. Punal et al. demonstrated that genetic depletion of microglia in the retina resulted in inhibition of astrocyte death and an increase in astrocyte population size, however; this needs further validation (Punal et al., 2019). However, we did not observe an increase in GFAP +cells in the AFS98 treated mice. This could be partly explained as the diet led to prolonged and consistent depletion of microglia, which might be needed to promote astrocyte population. We only administered AFS98 once and the mice were euthanized at 72 hours, this was done to avoid repopulation of microglia.
Finally, these findings demonstrate that reduction of microglia resulted in an increase in peripheral myeloid cell infiltration and larger infarcts in aged male mice. However, this study has several limitations. First, we did observe the effects of microglia depletion at acute time points after stroke, so the role of microglia in recovery needs further exploration. Second, microglia display inflammatory and anti-inflammatory phenotype after stroke. However, how these different phenotypes affect the injury progression or recovery needs further investigation. Third, more sensitive behavioral test were not performed as this was an acute study however, with the larger infarcts in PLXD and AFS98 MCAo groups one would assume more functional deficits. In summary, we show that microglia are essential modulators of neuroinflammation and peripheral immune cell infiltration in ischemic stroke in aged animals.
Supplementary Material
Supplementary figure 1. PLX-5622 treatment effectively depleted microglia after 21 days of treatment in both young and aged animals. ****p<0.001 vs young day 0; ##p<001 vs old day 0
Supplementary figure 2. Microglia depletion increased injury at 24 hours after MCAo in young animals. (a) Increase in infarct volume in the PLXD MCAo young mice. (b) Increase in GFAP+ cells in the PLXD treated sham young mice. * p<0.05
Supplementry figure 3. No difference in the NDS at (a) 24 and (b) 72 hours in the CD MCAo and PLXD MCAo mice. No difference in the NDS (c) between IgG MCAo and AFS98 MCAo groups at 24 hours.
Supplementary figure 4. (a) Decrease in NeuN+ cells in PLXD MCAo at 24 hours. (b) A decrease in GFAP+ cells in the PLXD group at 24 hours. Representative images showing NeuN and GFAP staining at 24 hours after MCAo. 63X confocal images. Scale bar 20 μm. (c) No difference in the GFAP+ cells in the CD MCAo and PLXD MCAo mice at 72 hours. Representative images showing GFAP staining. 20X images. Scale bar 50 μm.***p<0001, ** p<0.01.
Supplementary figure 5. AFS98 treatment depletes microglia. ** p<0.01.
Supplementary figure 6. Gating stratergy for immune cells in the brain.
Acknowledgements
AHA post-doctoral grant to AC (16POST27490032) and RO1 (5R01NS094543) to LDM.
Footnotes
Conflict of Interest
The authors declare no conflicts
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Chauhan A, Al Mamun A, Spiegel G, Harris N, Zhu L, McCullough LD, 2018. Splenectomy protects aged mice from injury after experimental stroke. Neurobiol Aging, 61, 102–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmore MRP, Hohsfield LA, Kramar EA, Soreq L, Lee RJ, Pham ST, Najafi AR, Spangenberg EE, Wood MA, West BL, Green KN, 2018. Replacement of microglia in the aged brain reverses cognitive, synaptic, and neuronal deficits in mice. Aging Cell, 17, e12832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigin VL, Forouzanfar MH, Krishnamurthi R, Mensah GA, Connor M, Bennett DA, Moran AE, Sacco RL, Anderson L, Truelsen T, O'Donnell M, Venketasubramanian N, Barker-Collo S, Lawes CM, Wang W, Shinohara Y, Witt E, Ezzati M, Naghavi M, Murray C, Global Burden of Diseases, I., Risk Factors, S., the, G. B. D. S. E. G., 2014. Global and regional burden of stroke during 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet, 383, 245–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Li Z, Ren H, Jin WN, Wood K, Liu Q, Sheth KN, Shi FD, 2017. Colony stimulating factor 1 receptor inhibition eliminates microglia and attenuates brain injury after intracerebral hemorrhage. J Cereb Blood Flow Metab, 37, 2383–2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niraula A, Sheridan JF, Godbout JP, 2017. Microglia Priming with Aging and Stress. Neuropsychopharmacology, 42, 318–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otxoa-de-Amezaga A, Miro-Mur F, Pedragosa J, Gallizioli M, Justicia C, Gaja-Capdevila N, Ruiz-Jaen F, Salas-Perdomo A, Bosch A, Calvo M, Marquez-Kisinousky L, Denes A, Gunzer M, Planas AM, 2018. Microglial cell loss after ischemic stroke favors brain neutrophil accumulation. Acta Neuropathol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punal VM, Paisley CE, Brecha FS, Lee MA, Perelli RM, Wang J, O'Koren EG, Ackley CR, Saban DR, Reese BE, Kay JN, 2019. Large-scale death of retinal astrocytes during normal development is non-apoptotic and implemented by microglia. PLoS Biol, 17, e3000492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritzel RM, Lai YJ, Crapser JD, Patel AR, Schrecengost A, Grenier JM, Mancini NS, Patrizz A, Jellison ER, Morales-Scheihing D, Venna VR, Kofler JK, Liu F, Verma R, McCullough LD, 2018. Aging alters the immunological response to ischemic stroke. Acta Neuropathol, 136, 89–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosna J, Philipp S, Albay R 3rd, Reyes-Ruiz JM, Baglietto-Vargas D, LaFerla FM, Glabe CG, 2018. Early long-term administration of the CSF1R inhibitor PLX3397 ablates microglia and reduces accumulation of intraneuronal amyloid, neuritic plaque deposition and pre-fibrillar oligomers in 5XFAD mouse model of Alzheimer's disease. Mol Neurodegener, 13, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szalay G, Martinecz B, Lenart N, Kornyei Z, Orsolits B, Judak L, Csaszar E, Fekete R, West BL, Katona G, Rozsa B, Denes A, 2016. Microglia protect against brain injury and their selective elimination dysregulates neuronal network activity after stroke. Nat Commun, 7, 11499. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figure 1. PLX-5622 treatment effectively depleted microglia after 21 days of treatment in both young and aged animals. ****p<0.001 vs young day 0; ##p<001 vs old day 0
Supplementary figure 2. Microglia depletion increased injury at 24 hours after MCAo in young animals. (a) Increase in infarct volume in the PLXD MCAo young mice. (b) Increase in GFAP+ cells in the PLXD treated sham young mice. * p<0.05
Supplementry figure 3. No difference in the NDS at (a) 24 and (b) 72 hours in the CD MCAo and PLXD MCAo mice. No difference in the NDS (c) between IgG MCAo and AFS98 MCAo groups at 24 hours.
Supplementary figure 4. (a) Decrease in NeuN+ cells in PLXD MCAo at 24 hours. (b) A decrease in GFAP+ cells in the PLXD group at 24 hours. Representative images showing NeuN and GFAP staining at 24 hours after MCAo. 63X confocal images. Scale bar 20 μm. (c) No difference in the GFAP+ cells in the CD MCAo and PLXD MCAo mice at 72 hours. Representative images showing GFAP staining. 20X images. Scale bar 50 μm.***p<0001, ** p<0.01.
Supplementary figure 5. AFS98 treatment depletes microglia. ** p<0.01.
Supplementary figure 6. Gating stratergy for immune cells in the brain.