Abstract
Acanthamoeba keratitis (AK) is a rare protozoal infection of the cornea. At least eight species of Acanthamoeba are known to cause this sight-threatening disease of the ocular surface. Acanthamoeba spp. exist in a wide array of niches ranging from thermal springs to under ice and every conceivable habitat in between. Contact lens wear is the leading risk factor for AK and is practiced by over 30 million individuals in the United States, yet the incidence of AK is less than 33 cases per one million contact lens wearers. Serological studies have reported that 90% to 100% of individuals with no history of AK possess antibodies specific for Acanthamoeba antigens indicating that exposure to this organism is commonplace, yet disease is remarkably rare. Animal studies have shed light on the pathobiology and immunobiology of AK and indicate that a constellation of factors including the ocular surface microbiome and the microbiome of Acanthamoeba itself contribute to the pathogenesis of AK. Interesting, secretory antibodies produced by the adaptive immune response can prevent the initiation of corneal infection, but once Acanthamoeba trophozoites breach the corneal epithelium the adaptive immune system is helpless in altering the course of AK. It has been almost 50 years since AK was first described, yet many questions remain unanswered about this curious and enigmatic disease of the ocular surface.
Keywords: Acanthamoeba, Keratitis, Cornea, Microbiome, Immunology, Pathology, Contact Lens
1. Introduction
Acanthamoeba spp. are free-living amoebae that reside in a remarkably wide range of habitats including hot springs, under ice, in soil, air and heating ducts, fresh vegetables, bottled water, eyewash stations, and in the nasopharyngeal washes from asymptomatic individuals (Alizadeh, 1996; Niederkorn et al., 1999). Although Acanthamoeba spp. primarily exist as free-living heterotrophs that feed on bacteria and fungi, they can on occasion be facultative parasites and produce corneal infections.
Acanthamoebae exist as either vegetative trophozoites (10–25 μm) or as resistant cysts (8–12 μm). The cyst stage can withstand remarkably harsh conditions including high doses of ultraviolet irradiation and gamma-irradiation (Aksozek et al., 2002). In fact, cysts remain viable even after a dose of 250,000 rads of gamma irradiation, a dose that is more than 100 times the lethal dose for humans (Aksozek et al., 2002). Cysts can remain viable after 20 years of storage at room temperature with no food source (Mazur et al., 1995). Cysts can also remain in a dormant state in corneal tissues for up to 31 months and can produce recrudescence of keratitis (Kremer et al., 1994; Mathers et al., 1987).
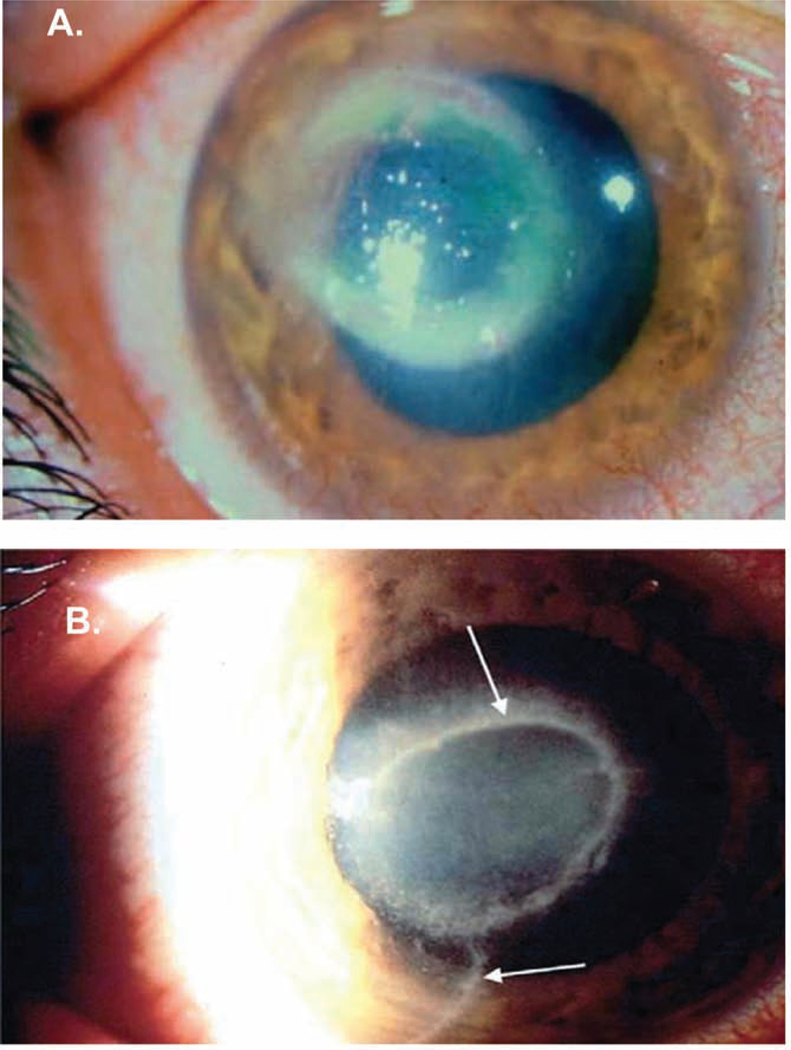
Acanthamoeba keratitis (AK) is a sight-threatening corneal disease that is caused by at least eight species of Acanthamoeba: A. castellanii, A. culbertsoni, A. polyphaga, A. hatchetti, A. rhysodes, A. lugdunesis, A. quina, and A. griffini (Lorenzo-Morales et al., 2015). Acanthamoeba classification is based on rRNA gene sequencing, which divides Acanthamoeba into 20 different evolutionary lines or clades (T1 through T20) (Corsaro et al., 2017; Fuerst, 2014). Most clinical isolates are the T4 genotype (Visvesvara et al., 2007). Although the T4 genotype is most commonly associated with clinical disease (Maciver et al., 2013), AK produced by non-T4 genotypes is more severe and has a poorer response to therapy (Arnalich-Montiel et al., 2014). Although the clinical presentation of AK is frequently confused with Herpes Simplex Virus (HSV) keratitis, corneal lesions in AK often display a characteristic ring-like infiltrate (Figure 1A) and develop radial keratoneuritis that is associated with exquisite pain that is not commensurate with the degree of tissue damage (Figure 1B).
Figure 1.
Clinical appearance of Acanthamoeba keratitis. A. Ring-like infiltrate of the cornea that is a characteristic clinical lesion in Acanthamoeba infections of the cornea. B. Radial keratoneuritis (arrows) is associated with trophozoites juxtaposed to corneal nerves. Images courtesy of James P. McCulley, M.D. and reproduced with permission (Clarke and Niederkorn, 2006).
Contact lens wear is the leading risk factor for AK and accounts for >90% of the AK in western countries (Carvalho et al., 2009; McAllum et al., 2009; Page and Mathers, 2013). However, in Asian countries a growing number of cases of AK occur in non-contact lens wearers (Buerano et al., 2014; Carvalho et al., 2009; Manikandan et al., 2004; McAllum et al., 2009; Page and Mathers, 2013; Watt and Swarbrick, 2007). The incidence of bilateral AK in contact lens wearers is rare and occurs in only 4% to 11% of the AK patients (McKelvie et al., 2018; Randag et al., 2019). There are an estimated 30 million contact lens wearers in the United States, yet the incidence of AK is less than 33 cases per one million contact lens wearers (Maycock and Jayaswal, 2016). Serological surveys have reported that 90% to 100% of the adult population with no history of Acanthamoeba infections has serum antibodies specific for Acanthamoeba antigens, which suggests that exposure to Acanthamoeba is commonplace while infection is rare (Alizadeh, 2001; Cursons et al., 1980). The low incidence of bilateral AK combined with the enormous population at risk for AK suggests that risk factors other than contact lens wear contribute to AK. Understanding those risk factors and the pathogenic processes that lead to AK are questions best addressed in prospective studies in animal models.
2. Animal models of AK: “One size does not fit all”
One common misconception in designing animal models is that mice can serve as universal hosts for any human infectious disease and that simply injecting infectious microorganisms into laboratory mice will recapitulate the human disease. The attractiveness of using mice for such experiments is obvious: a) they are readily available and inexpensive; b) inbred and genetically defined backgrounds are available from commercial sources; and c) reagents and monoclonal antibodies for mouse immune cells are exquisitely specific and relatively inexpensive. However, in developing an animal model it is important to realize that “one size does not fit all”. One must always consider host specificity and the method used by the pathogen for entering the body when developing an animal model. With some infectious diseases such as toxoplasmosis, there is essentially no host specificity and virtually any warm-blooded animal (including birds) can be infected with T. gondii. By contrast, Mycobacterium leprae, the bacterium that causes leprosy, is exquisitely host-specific (Adams et al., 2012; Avci et al., 2013). In fact, the nine-banded armadillo is the only known natural host for M. leprae and the only other animal model of leprosy relies on injecting the bacterium into the footpads of mice, which are largely resistant to developing classical leprosy (Adams et al., 2012; Avci et al., 2013).
In an effort to develop an animal model of AK, we attempted to induce corneal infections in various inbred mouse strains using clinical isolates of Acanthamoeba. We found that we could not produce keratitis in mice that was any different than the transient inflammation produced by directly injecting killed Acanthamoeba trophozoites into the cornea. Similar results were found using rats, cotton rats, and rabbits (Niederkorn et al., 1992). Since >90% of AK occurs in contact lens-wearers it stands to reason that infectious trophozoites are transmitted to the corneal surface by binding to contact lenses, which serve as vectors to deliver infectious trophozoites to the ocular surface. Therefore, trophozoite binding to the corneal epithelium is the obligatory first step in the infectious process of AK. With this in mind we evaluated the capacity of Acanthamoeba trophozoites to bind to the corneas of 11 different animal species and found that trophozoites bound to the corneas of only three species: a) human; b) Chinese hamster; and c) pig (Niederkorn et al., 1992) Accordingly, we evaluated the pig and Chinese hamster as potential animal models of AK transmitted via Acanthamoeba-laden contact lenses (He et al., 1992; van Klink et al., 1993). These models facilitated prospective studies that revealed the roles of contact lenses, ocular trauma, and the immune system in the pathogenesis of AK (Neelam and Niederkorn, 2017). The sequence of events in the pathogenesis of AK begins with the wearing of contact lenses, which induces an upregulation of mannosylated proteins on the corneal epithelium and conditions the ocular surface to make it more receptive to trophozoite binding. Although contact lens wear upregulates mannosylated protein expression the corneal simple abrasions to the cornea have the same effect. For example, mild abrasions to the corneas of Chinese hamsters leads to an 18-fold increase in the binding of trophozoites to the corneal epithelium (van Klink et al., 1993). The increase on AK in non-contact lens wearers in Asia might be explained by presence of corneal abrasions in these patients (Buerano et al., 2014; Carvalho et al., 2009; Manikandan et al., 2004; McAllum et al., 2009; Page and Mathers, 2013; Watt and Swarbrick, 2007). Engagement of the mannose binding protein on the trophozoite cell membrane elicits the release of a 133 KDa protease called mannose-induced protease 133 (MIP-133), which induces apoptosis and cytolysis of corneal epithelial cells and facilitates trophozoite invasion of the corneal surface and erosion of the underlying stroma. Pathogenic strains of Acanthamoeba produce additional proteases that affect the corneal stroma including a plasminogen activator (Alizadeh et al., 2007). MIP-133 and the other proteases elaborated by Acanthamoeba spp. are therapeutic targets for managing AK.
It is widely believed that the majority of Acanthamoeba spp. exist as heterotrophs that feed on bacteria and fungi in a wide range of habitats. A sampling of soil isolates of Acanthamoeba revealed that none of the environmental isolates produced MIP-133 and they also failed to produce AK in the Chinese hamster model (Hurt et al., 2003a).
Investigations using soil isolates of Acanthamoeba also revealed that trophozoites of nonpathogenic soil isolates of A. castellanii curiously produced significantly more cytolysis of corneal epithelial cells in vitro than trophozoites from pathogenic isolates of A. castellanii (van Klink et al., 1992a). However, the binding of soil isolate trophozoites to corneal epithelial cells in vitro was profoundly less than the binding by pathogenic isolates of A. castellanii (van Klink et al., 1992a). We concluded that the in vitro cytolysis of corneal cells by trophozoites from non-pathogenic soil isolates was produced by contact-independent processes mediated by proteases released by trophozoites in vitro. However, at the ocular surface cytopathic effects would be eliminated by the diluting effects of the tear film combined with the mechanical shear forces of the blink reflex, which would rid the eye of soil isolate trophozoites, which have little or no capacity to bind to the corneal epithelium. Moreover, soil isolates also have reduced chemotactic responses to corneal cell extracts and do not express fibrinolytic enzymatic activity that is present in ocular isolates and is involved in corneal invasion (van Klink et al., 1992b). Thus, relying solely on in vitro cytolytic activity Acanthamoeba trophozoites is misleading and underscores the value of validating in vitro findings in vivo; that is, “in vivo veritas” (paraphrased from Latin - “in a living thing there is truth”).
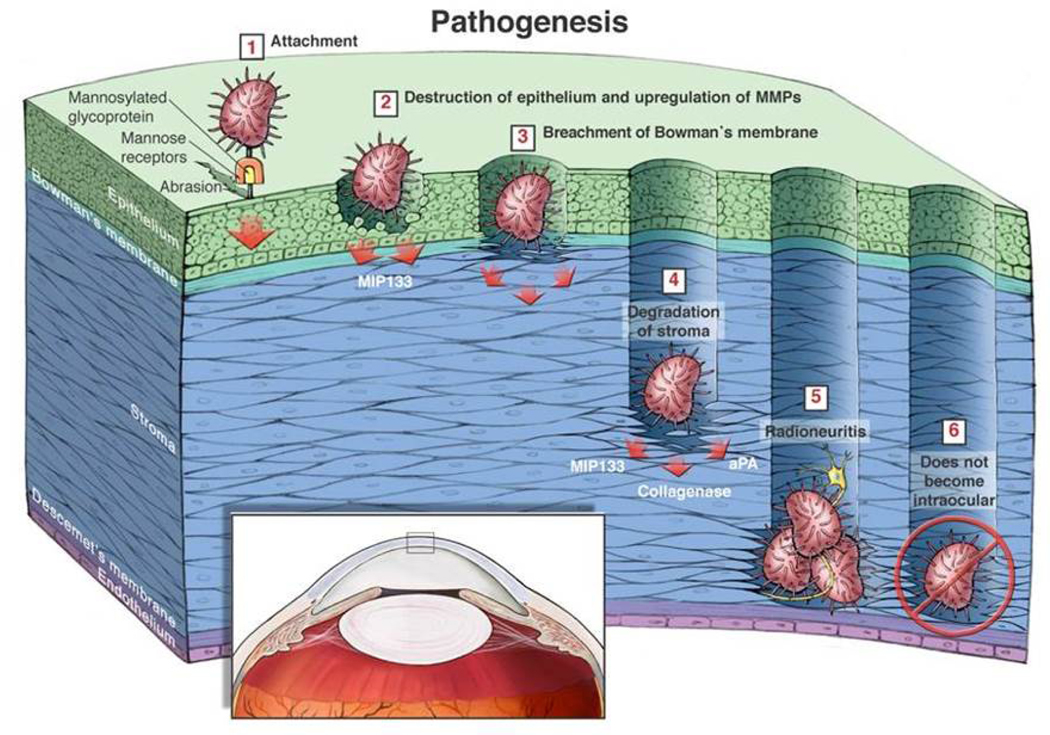
Thus, the pathogenic cascade of AK involves several factors that occur in a sequential manner beginning with contact lens-induced upregulation of mannosylated proteins on the corneal epithelium and culminating in melting of the corneal stroma (Clarke and Niederkorn, 2006) (Figure 2).
Figure 2.
Schematic depiction of the pathophysiology of Acanthamoeba keratitis. 1. Trophozoites bind to mannosylated proteins that are upregulated in response to corneal abrasions or contact lens wear. 2. Engagement of mannosylated proteins with the mannose-binding receptor on trophozoites stimulates trophozoites to release mannose-induced protease 133 (MIP-133), which lyses corneal cells. 3. MIP-133 facilitates trophozoite penetration of Bowman’s membrane. 4. MIP-133 leads to “stromal melting” by degradation of the collagenous stromal matrix. 5. Trophozoites accumulate around corneal nerves and are associated with radial keratoneuritis and exquisite pain. 6. Trophozoites almost never progress to the posterior regions of the eye. Reproduced with permission (Clarke and Niederkorn, 2006).
3. The microbiome inside and out
The microbiome has emerged as one of the most provocative and important topics in biomedical research in recent years. “Microbiome” is the collective term for the genomes of all of the microorganisms residing in and on the body. It has been estimated that over 90% of the cells in the human body are bacterial and only 10% are mammalian. At least 1,000 different bacterial species reside in the human gut, and some estimates are as high as 35,000. Virtually every aspect of human health and disease is influence by the microorganisms that live within us and on our integumentary tissues. The microbiome has a profound impact on pathological and immunological process relating to infectious diseases (Baumler and Sperandio, 2016; Honda and Littman, 2016; Thaiss et al., 2016). Years before the microbiome was at the forefront of biomedical research, investigators noted an intriguing association between the presence of certain bacterial species found on the ocular surface and the occurrence of AK. Badenoch et al. observed an unusually high frequency of the Gram positive bacterium Corynebacterium xerosis in cultures from the eyes of patients diagnosed with AK and proposed that the bacterium served as a food source and co-factor for the development of AK (Badenoch et al., 1990). These investigators found that the severity of AK was exacerbated when trophozoites were fed C. xerosis prior to injecting them into the corneas of rats. However, subsequent studies offered a different explanation. The bacterial flora of the ocular surface includes many bacteria that could serve as food sources for Acanthamoeba trophozoites, however C. xerosis stands apart from all of these bacteria by having the highest concentration of mannose in its cell wall (Alizadeh et al., 2005). As mentioned earlier, the binding of mannose to its receptor on the cell membrane of Acanthamoeba trophozoites elicits the release of MIP-133, which amplifies cytolysis of corneal cells and facilitates melting of the corneal stroma, which in turn exacerbates AK in the Chinese hamster model. Moreover, trophozoites exposed to C. xerosis in vitro prior to infecting Chinese hamster display a profound increase in their cytolytic properties against corneal epithelial cells in vitro and exacerbates the pathology of AK in Chinese hamsters (Alizadeh et al., 2005). Supernatants from trophozoites cultures incubated with C. xerosis display increased levels of proteolytic enzymes (e.g., MIP-133), and express enhanced cytolytic activity against corneal cells (Alizadeh et al., 2005). These effects might explain the enhanced pathogenic properties of Acanthamoeba trophozoites exposed to mannose on the cell walls of C. xerosis. Importantly, supernatants from axenic cultures of C. xerosis did not display detectable protease or cytolytic activity indicating that the exacerbation of AK by C. xerosis was due to the bacterium’s effect on trophozoites and not due to bacterial toxicity (Alizadeh et al., 2005).
It bears noting that constituents of the ocular microbiome such as Corynebacterium can have remarkably different effects on pathogenesis of different categories of microbial keratitis. C. mastitidis elicits a commensal-specific interleukin-17 response that drives neutrophil recruitment to the ocular mucosa and is crucial for resistance to Candida albicans and Pseudomonas aeruginosa infections (St Leger et al., 2017). By contrast, C. xerosis exacerbates, rather than mitigates, AK by directly modifying the trophozoites’ release of pathogenic proteases, which facilitate invasion of the stroma and by increasing tropohozoite-mediated cytolysis of corneal cells (Alizadeh et al., 2005). Thus, the microbiome’s effect on microbial keratitis is contextual and complex and reveals that “one size does not fit all” when it comes to microbiome’s impact on microbial infections of the ocular surface.
Just like the mammalian host harbors a unique microbiome, Acanthamoeba trophozoites have their own microbiome in the form of endosymbiotic bacteria. Although Acanthamoeba trophozoites ingest a wide variety of bacteria and fungi as food sources, some bacterial species can evade intracellular digestion by trophozoites and can establish long-term residence within the cytoplasm of Acanthamoeba spp. The presence of such bacterial endosymbionts was recognized over 35 years ago (Hall and Voelz, 1985). The relationship between the bacterial endosymbionts can evolve into a permanent obligatory condition in which the endosymbiotic bacteria cannot reside outside of the cytoplasm of the trophozoites and efforts to culture such endosymbionts have consistently met with failure (Fritsche et al., 1998). Some of the endosymbionts found in Acanthamoeba trophozoites are notable human pathogens including Mycobacteria, Legionella, Pseudomonas, and Chlamydia (Fritsche et al., 1993). The endosymbionts can influence the pathogenicity and resistance of Acanthamoeba to therapeutic agents. A clinical study of 23 AK patients found that over half of the Acanthamoeba isolates from corneal lesions contained at least one endosymbiont (Iovieno et al., 2010). Bacterial endosymbionts can lead to enhanced in vitro cytopathogenicity of Acanthamoeba trophozoites (Fritsche et al., 1998; Iovieno et al., 2010). The molecular basis for enhanced cytopathogenicity that is associated with bacterial endosymbionts remains unknown but it is tempting to speculate that molecules such as mannose that are embedded in the cell wall of the endosymbiont might stimulate the elaboration of pathogenic proteases by the trophozoites who serve as hosts for the endosymbionts. Thus, in an ironic twist of fate, the parasite (i.e., Acanthamoeba) has become the host – a condition that is reminiscent of the Jonathan Swift quote “… Great fleas have little fleas upon their backs to bite ‘em and little fleas have lesser fleas and so ad infinitum…”.
4. The immunobiology of AK: Role of the innate immune system
The immune system is composed of two functionally distinct components: a) the innate immune response and b) the adaptive immune response. Elements of the innate response are the “first responders” and are characterized by their nimble response to microorganisms through their recognition of pathogen-associated molecular patterns (PAMPS) expressed on the cell membranes of microorganisms. Neutrophils and macrophages are the primary cellular elements of the innate immune system. Macrophages, especially those activated with interferon-γ (IFN-γ), are capable of killing Acanthamoeba trophozoites (Marciano-Cabral and Toney, 1998; Stewart et al., 1992) and cysts (Hurt et al., 2003b). Depletion of periocular macrophages by subconjunctival injection of liposomes loaded with the macrophagicidal drug clodronate profoundly exacerbates AK in the Chinse hamster model of this disease (van Klink et al., 1996). Neutrophils are a second innate cell population that exerts significant resistance to AK. Neutrophils are consistently found in AK lesions in both animals and humans. Neutrophils demonstrate significant killing of both cysts and trophozoites in vitro (Stewart et al., 1994). Animal studies have provided compelling evidence that neutrophils control the progression of AK. In vivo inhibition of neutrophil migration through the injection of anti-MIP-2 antibody results in a severe exacerbation of AK in Chinese hamsters (Hurt et al., 2001). Likewise, intracorneal injection of MIP-2, a potent chemoattractant of neutrophils, produces a significant mitigation of AK (Hurt et al., 2001).
One of the curious conditions in AK is the remarkable absence of intraocular infections. Of the hundreds of cases of AK reported in the literature, only six cases have progressed to produce infection in the posterior regions of the eye (Heffler et al., 1996; Jones et al., 1975a; Moshari et al., 2001). One explanation might be that trophozoites cannot breach Descemet’s membrane and enter the anterior chamber. However, in vitro studies revealed that trophozoites rapidly penetrate Descemet’s membrane (Clarke et al., 2005). The trophozoites’ capacity to penetrate Descemet’s membrane raised the possibility that the aqueous humor (AH) in the anterior chamber exerts anti-microbial effects leading to the death or encystment of the trophozoites. In vitro studies revealed that AH induced trophozoites to encyst. However, it was subsequently found that the encystment was due to nutrient deprivation and trophozoites incubated in AH in the presence of a food source such as iris/ciliary body cells, did not encyst and the trophozoites remained viable as long as a food source was available (Clarke et al., 2005). Moreover, Acanthamoeba trophozoites require intact cells as food sources as cell homogenates fail to support the in vitro survival of Acanthamoeba trophozoites (Stopak et al., 1991). Thus, the anterior chamber and AH did not have deleterious effects on Acanthamoeba trophozoites. Prospective studies in the Chinese hamster model of AK revealed that injection of as many as one million trophozoites into the anterior chamber elicited a robust neutrophil response that resulted in the elimination of the enormous trophozoite inoculum within 15 days of the injection (Clarke et al., 2005). Moreover, within 24 hours of intracameral injection, the trophozoites were incarcerated by neutrophils and at no time were trophozoites ever detected in the posterior regions of the eye (Clarke et al., 2005).
The complement system interfaces with both innate and adaptive immune systems and is a crucial element in the resistance to many bacterial infections. The complement system is comprised of over 30 proteins and protein fragments, which act in a cascade like manner to kill bacterial and protozoal pathogens by membrane lysis. Bacterial products can activate the complement cascade through the alternative pathway that is independent of the adaptive immune response. This pathway is also invoked by mannosylated glycoproteins on the surface of Acanthamoeba trophozoites. The classical pathway for complement activation is initiated by antibodies that bind to antigens expressed on microorganisms and as such represents an arm of the adaptive immune response. Trophozoites from non-pathogenic strains of Acanthamoeba are vulnerable to cytolysis by complement activated by the alternative pathway (Ferrante and Rowan-Kelly, 1983). However, pathogenic strains of Acanthamoeba resist lysis by complement due to their expression of complement regulatory proteins that inactivate the complement cascade (Toney and Marciano-Cabral, 1998). This resistance to the anti-microbial properties of complement may explain why animals immunized against Acanthamoeba trophozoites and expressing complement-fixing antibodies are not protected against AK (Van Klink et al., 1997).
5. The immunobiology of AK: Role of the adaptive immune response
Unlike the innate immune system, which mounts a nimble response, the adaptive immune response requires a lag time in which T and B lymphocytes undergo clonal expansion. However, once engaged, the adaptive immune system produces a durable resistance to infectious agents and displays memory such that subsequent encounters with pathogens are met with a swift and robust response. There is considerable evidence that Acanthamoeba spp. can arouse the adaptive immune system. Acanthamoeba spp. are ubiquitous and are found in virtually every terrestrial, aquatic, and marine environment. Thus, exposure to Acanthamoeba spp. is inevitable as demonstrated by serological surveys indicating that 90 to 100 percent of the adult population with no history of Acanthamoeba infections expresses serum IgG antibodies specific for Acanthamoeba antigens (Alizadeh, 2001; Cursons et al., 1980) and 50% of normal asymptomatic individuals display T cell responses to Acanthamoeba antigens (Tanaka et al., 1994). The high incidence of T and B lymphocyte activation in individuals with no history of Acanthamoeba infections reinforces the notion environmental exposure to Acanthamoeba is common.
Animal models have facilitated prospective studies for determining if active immunization can protect or mitigate AK. Experiments in both the pig and Chinese hamster models of AK indicated that subcutaneous immunization with Acanthamoeba antigens elicited robust T cell responses in the form of delayed-type hypersensitivity (DTH) and B cell responses in the form serum IgG antibodies (Alizadeh et al., 1995; Niederkorn, 1994; Van Klink et al., 1997). In spite of the presence of T and B cell immunity, both pigs and Chinese hamsters are susceptible to AK and show no evidence that immunization prevents or mitigates corneal infection with Acanthamoeba. It is important to note that in the pig and Chinese hamster models AK is induced through the application of contact lenses laden with Acanthamoeba trophozoites. A recent study in a mouse model of AK induced by intracorneal injection of trophozoites found compelling evidence for Th17 cells in mitigating the severity of AK by promoting neutrophil activation and migration into the infected cornea (Suryawanshi et al., 2015).
It is widely believed that AK is initiated when a contact lens with adhering Acanthamoeba trophozoites is applied to the ocular surface. Animal studies have demonstrated that upregulation of mannosylated glycoproteins on the corneal epithelium in response to mild ocular trauma or the wearing of contact lenses facilitates binding of trophozoites to the corneal epithelium (Alizadeh et al., 2005; van Klink et al., 1993). The ocular surface is regularly exposed to a wide range of environmental insults most of which are from non-infectious agents. However, infectious microorganism occasionally pose a risk to the well-being of the cornea. These threats are addressed by a myriad of anti-microbial molecules in the tear film that bathes the ocular surface. Among these molecules are secretory IgA antibodies. Although there are 5 isotypes of antibodies produced by mammals, IgA is far and away the most abundant and is produced in quantities higher than all four of the other antibody isotypes combined. Although elements of the adaptive immune response in the form of serum IgG antibodies or DTH fail to protect or mitigate AK, secretory IgA antibodies provide a strong level of protection against the initial stages of corneal infection with parasite-laden contact lenses. Animals immunized with Acanthamoeba antigens in the presence of a mucosal adjuvant (i.e., neutralized cholera toxin) produced Acanthamoeba-specific IgA antibodies in the tears and were protected against corneal infections so long as immunization was initiated before exposure to infectious trophozoites. However, IgA antibodies were ineffectual if immunization was delayed until after the initial infection was established (Leher et al., 1998a; Leher et al., 1998d). The IgA antibodies present in the tears of the mucosally immunized animals did not affect the viability of the trophozoites, but strongly inhibited their binding to the corneal epithelium. Moreover, passive transfer of a monoclonal IgA antibodies specific for Acanthamoeba antigens protected Chinese hamsters against AK produced via Acanthamoeba-laden contact lenses (Leher et al., 1999).
In addition to blocking trophozoite binding to the corneal epithelium, IgA antibodies might influence the development of AK by interfering with the trophozoites after they have penetrated the corneal epithelium and have entered the stroma. Using a rabbit model, Feng et al. bypassed the initial adhesion step to the corneal epithelium and injected trophozoites directly into the stroma and found evidence that secretory IgA antibodies mitigated AK (Feng et al., 2015). Secretory IgA antibodies can interact with the Fc receptor on neutrophils or activate the complement cascade via the alternate/lectin pathways. Activation of the alternative pathway of complement enhances the anti-microbial activity of neutrophils. In this study, it is possible the IgA antibodies activated neutrophils, which have the capacity to kill trophozoites (Stewart et al., 1994).
6. Acanthamoeba cysts, scleritis and ischemic posterior segment inflammation
Acanthamoeba is one of the rare protozoal parasites that forms true cysts in human tissue. Acanthamoeba cysts can remain viable in the absence of a food source for over 20 years (Mazur et al., 1995) and can persist in the human eye for over two years and produce recrudescent infections (Kremer et al., 1994; Mathers et al., 1987). The persistence of cysts in human tissue is not due to weak immunogenicity that would allow them to “fly under the radar of the immune system”. Animal studies have demonstrated that cysts can elicit robust DTH and IgG antibody responses (McClellan et al., 2002). Cysts also induce inflammatory DTH responses when injected into immune hosts (McClellan et al., 2002). Thus, Acanthamoeba cysts are both immunogenic and antigenic. Patients whose active Acanthamoeba infections have been brought under control sometimes require corneal transplants to restore their vision. The presence of dormant cysts in ocular tissues in these patients poses a risk for the emergence of trophozoites and recrudescence of AK. This is especially problematic as the corticosteroids that are routinely used to prevent immune rejection of corneal transplants also have an unexpected deleterious effect on Acanthamoeba cysts. In vitro treatment with dexamethasone results in a 4 to 6 fold increase in excystment and a doubling in the proliferation of the emergent trophozoites (McClellan et al., 2001). Moreover, dexamethasone activates Acanthamoeba trophozoites and significantly increases their cytolysis of corneal cells. These exacerbating effects also occur in vivo as Chinese hamsters treated with dexamethasone have profoundly more severe AK (McClellan et al., 2001). These in vitro and animal studies are supported by clinical studies, which have reported that AK patients treated with corticosteroids before initiating antimicrobial therapy have a significantly poorer outcome than when steroid treatment was initiated after anti-microbial therapy had been implemented (Carnt et al., 2016; Robaei et al., 2014).
The capacity of cysts to persist in ocular tissues combined with their immunogenicity has led some to suspect that the inflammatory response to cysts is the underlying cause for chronic scleritis that occurs in 10 to 18% of the AK patients (Bacon et al., 1993; Iovieno et al., 2010; Lee et al., 2002; Mannis et al., 1986). One study reported the histological presence of degenerated and necrotic Acanthamoeba cysts, yet cultures of the tissues failed to reveal viable Acanthamoeba (Iovieno et al., 2010). The presence of chronic scleritis in the absence of viable organisms is consistent with an immune-mediated inflammatory response to cysts. As mentioned earlier Acanthamoeba cysts are highly immunogenic and antigenic (McClellan et al., 2002). Chronic inflammation might be due to the persistence of the highly resistant cysts or through the process of “molecular mimicry” in which a pathogen expresses antigenic epitopes similar to epitopes present on some host tissues. A classic example of molecular mimicry occurs when scarlet fever elicits T cell and B cell responses to epitopes displayed on Streptococcus pyogenes. Antigenic epitopes similar to those on S. pyogenes are also expressed on heart valves, which are subsequently damaged by the immune response to S. pyogenes antigens. It is possible that a similar condition may explain a curious form ischemic inflammation that occurred in the retinas of four patients diagnosed with persistent AK (Awwad et al., 2007). Acanthamoeba cysts were detected in the corneas of the patients, yet there was no histopathological evidence of either trophozoites or cysts in the retinas or anywhere in the posterior regions of the eye. Interestingly, the ischemic inflammation in the retinas of all four patients displayed perivascular lymphocytic cuffing, thrombosis of the retinal arteries and ischemia, which are reminiscent of the histopathological features of classic DTH lesions. Since Acanthamoeba, cysts are capable of inducing strong DTH responses it is tempting to speculate that the scleritis and the severe ischemic inflammation in these four AK patients are examples of “molecular mimicry” of Acanthamoeba antigens with antigens expressed in the eye. There is tantalizing evidence that Acanthamoeba antigens may also initiate molecular mimicry in brain. Mice infected with A. castellanii develop T cells that in addition to recognizing Acanthamoeba antigens also react with myelin antigens in the brain leading to inflammation in the brain (Massilamany et al., 2014).
7. Mucosal immunity and an anti-disease vaccine for AK
A crucial step in the pathogenic cascade of AK is the binding of trophozoites to mannosylated proteins on the corneal epithelium. Blocking the initial adherence of trophozoites to the corneal epithelium by IgA antibodies in the tears that are directed against surface epitopes on the trophozoite cell membrane is highly effective in preventing infection of the cornea (Leher et al., 1998a; Leher et al., 1998d). However, once the trophozoites penetrate the ocular surface, IgA antibodies directed at the trophozoite cell membrane no longer mitigate AK (Leher et al., 1998a; Leher et al., 1998c).
Engagement of the mannose-binding protein on the surface of the trophozoite triggers the release of a pathogenic serine protease, MIP-133, which not only lyses corneal cells but degrades the stromal matrix (Garate et al., 2006; Leher et al., 1998b). Acanthamoeba-derived proteases are potential therapeutic targets for mitigating the pathological events at the ocular surface. Secretory immune system is ideally designed for this challenge. Oral immunization with MIP-133 conjugated with neutralized cholera toxin preferentially stimulates the production of secretory IgA antibodies that appear in the tears. The anti-MIP-133 IgA antibodies block the cytopathic effects of MIP133 on corneal cells in vitro and mitigate the severity of AK in Chinese hamsters (Garate et al., 2006). The tears containing anti-MIP-133 IgA antibodies continuously bathe the ocular surface and provide a sustained delivery of the “therapeutic agent”. As mentioned earlier, IgA antibodies directed against structural molecules expressed on the cell membrane of trophozoites must be present in the tears before the initial binding of trophozoites to the ocular surfaced and are ineffectual after the amoebae have penetrated the corneal epithelium. By contrast, the “anti-disease” vaccine can be initiated after the initial infection has occurred and exerts a therapeutic effect by neutralizing the ongoing pathological processes throughout the course of the disease and accordingly could be combined with anti-microbial agents that target the trophozoites.
8. Conclusions and conundrums
It has been almost a half century since AK was first described as a distinct clinical disease, yet a number of perplexing questions remain (Jones et al., 1975b). Acanthamoeba spp. are ubiquitous and are found in every conceivable environmental niche from thermal springs to under ice and everywhere in between. Contact lens wear is the leading risk factor and is practiced by over 30 million individuals in the United States, yet the frequency of AK in contact lens wearers is less than 33 cases per one million (i.e., 0.003%)(Maycock and Jayaswal, 2016). Exposure to Acanthamoeba spp. is commonplace with 90% to 100% of the population with no previous history of AK developing serum antibodies against Acanthamoeba antigens (Alizadeh, 2001; Cursons et al., 1980). The highly evolved adaptive immune response can mount robust T and B cell responses to Acanthamoeba antigens, yet neither DTH nor IgG antibodies provide any resistance and reinfection is always a threat. AK is characterized by an aggressive relentless destruction of the corneal epithelium and melting of the stroma, yet with the exception of six cases, the AK infection never progresses to the posterior regions of the eye. Animal studies suggest that this resistance to Acanthamoeba infections in the posterior chamber is due to a robust neutrophil response. If the same condition applies to humans, why is the neutrophil response effective inside the eye but ineffectual at the ocular surface?
Highlights for “Biology of Acanthamoeba Keratitis”.
Jerry Y. Niederkorn
Contact lens wear stimulates the release of pathogenic proteases by Acanthamoeba.
Microbiomes of the eye and Acanthamoeba influence pathogenesis of Acanthamoeba keratitis.
Ocular infections with Acanthamoeba do not produce immunity to reinfection.
IgA antibodies in the tears prevent adhesion of Acanthamoeba to the cornea.
Acknowledgments
Funding
Supported in part by P30-EY030413 and an unrestricted grant from Research to Prevent Blindness,
Footnotes
Propriety interest statement
The author has no commercial or proprietary interest in this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams LB, Pena MT, Sharma R, Hagge DA, Schurr E, Truman RW, 2012. Insights from animal models on the immunogenetics of leprosy: a review. Mem Inst Oswaldo Cruz 107 Suppl 1, 197–208. [DOI] [PubMed] [Google Scholar]
- Aksozek A, McClellan K, Howard K, Niederkorn JY, Alizadeh H, 2002. Resistance of Acanthamoeba castellanii cysts to physical, chemical, and radiological conditions. J Parasitol 88, 621–623. [DOI] [PubMed] [Google Scholar]
- Alizadeh H, Apte S, El-Agha M-SH, Li L, Hurt M, Howard K, Cavanagh HD, McCulley JP, Niederkorn JY, 2001. Tear IgA and serum IgG antibodies against Acanthmaoeba in patients with Acanthamoeba keratitis. Cornea. [DOI] [PubMed] [Google Scholar]
- Alizadeh H, He Y, McCulley JP, Ma D, Stewart GL, Via M, Haehling E, Niederkorn JY, 1995. Successful immunization against Acanthamoeba keratitis in a pig model. Cornea 14, 180–186. [PubMed] [Google Scholar]
- Alizadeh H, Neelam S, Hurt M, Niederkorn JY, 2005. Role of contact lens wear, bacterial flora, and mannose-induced pathogenic protease in the pathogenesis of amoebic keratitis. Infect Immun 73, 1061–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alizadeh H, Neelam S, Niederkorn JY, 2007. Effect of immunization with the mannose-induced Acanthamoeba protein and Acanthamoeba plasminogen activator in mitigating Acanthamoeba keratitis. Invest Ophthalmol Vis Sci 48, 5597–5604. [DOI] [PubMed] [Google Scholar]
- Alizadeh H, Niederkorn JY, McCulley JP, 1996. Acanthamoebic Keratitis, in: Pepose J, Holland GN, Wilhelmus KR (Ed.), Ocular Infection and Immunity. Mosby, St. Louis, Missouri, pp. 1062–1071. [Google Scholar]
- Arnalich-Montiel F, Lumbreras-Fernandez B, Martin-Navarro CM, Valladares B, Lopez-Velez R, Morcillo-Laiz R, Lorenzo-Morales J, 2014. Influence of Acanthamoeba genotype on clinical course and outcomes for patients with Acanthamoeba keratitis in Spain. J Clin Microbiol 52, 1213–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avci P, Sadasivam M, Gupta A, De Melo WC, Huang YY, Yin R, Chandran R, Kumar R, Otufowora A, Nyame T, Hamblin MR, 2013. Animal models of skin disease for drug discovery. Expert Opin Drug Discov 8, 331–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awwad ST, Heilman M, Hogan RN, Parmar DN, Petroll WM, McCulley JP, Cavanagh HD, 2007. Severe reactive ischemic posterior segment inflammation in acanthamoeba keratitis: a new potentially blinding syndrome. Ophthalmology 114, 313–320. [DOI] [PubMed] [Google Scholar]
- Bacon AS, Frazer DG, Dart JK, Matheson M, Ficker LA, Wright P, 1993. A review of 72 consecutive cases of Acanthamoeba keratitis, 1984–1992. Eye (Lond) 7 ( Pt 6), 719–725. [DOI] [PubMed] [Google Scholar]
- Badenoch PR, Johnson AM, Christy PE, Coster DJ, 1990. Pathogenicity of Acanthamoeba and a Corynebacterium in the rat cornea. Arch Ophthalmol 108, 107–112. [DOI] [PubMed] [Google Scholar]
- Baumler AJ, Sperandio V, 2016. Interactions between the microbiota and pathogenic bacteria in the gut. Nature 535, 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buerano CC, Trinidad AD, Fajardo LS, Cua IY, Baclig MO, Natividad FF, 2014. Isolation of acanthamoeba genotype t4 from a non-contact lens wearer from the Philippines. Trop Med Health 42, 145–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnt N, Robaei D, Watson SL, Minassian DC, Dart JK, 2016. The Impact of Topical Corticosteroids Used in Conjunction with Antiamoebic Therapy on the Outcome of Acanthamoeba Keratitis. Ophthalmology 123, 984–990. [DOI] [PubMed] [Google Scholar]
- Carvalho FR, Foronda AS, Mannis MJ, Hofling-Lima AL, Belfort R Jr., de Freitas D, 2009. Twenty years of acanthamoeba keratitis. Cornea 28, 516–519. [DOI] [PubMed] [Google Scholar]
- Clarke DW, Alizadeh H, Niederkorn JY, 2005. Failure of Acanthamoeba castellanii to produce intraocular infections. Invest Ophthalmol Vis Sci 46, 2472–2478. [DOI] [PubMed] [Google Scholar]
- Clarke DW, Niederkorn JY, 2006. The pathophysiology of Acanthamoeba keratitis. Trends Parasitol 22, 175–180. [DOI] [PubMed] [Google Scholar]
- Corsaro D, Kohsler M, Montalbano Di Filippo M, Venditti D, Monno R, Di Cave D, Berrilli F, Walochnik J, 2017. Update on Acanthamoeba jacobsi genotype T15, including full-length 18S rDNA molecular phylogeny. Parasitol Res 116, 1273–1284. [DOI] [PubMed] [Google Scholar]
- Cursons RT, Brown TJ, Keys EA, Moriarty KM, Till D, 1980. Immunity to pathogenic free-living amoebae: role of humoral antibody. Infect Immun 29, 401–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng X, Zheng W, Wang Y, Zhao D, Jiang X, Lv S, 2015. A Rabbit Model of Acanthamoeba Keratitis That Better Reflects the Natural Human Infection. Anat Rec (Hoboken) 298, 1509–1517. [DOI] [PubMed] [Google Scholar]
- Ferrante A, Rowan-Kelly B, 1983. Activation of the alternative pathway of complement by Acanthamoeba culbertsoni. Clin Exp Immunol 54, 477–485. [PMC free article] [PubMed] [Google Scholar]
- Fritsche TR, Gautom RK, Seyedirashti S, Bergeron DL, Lindquist TD, 1993. Occurrence of bacterial endosymbionts in Acanthamoeba spp. isolated from corneal and environmental specimens and contact lenses. J Clin Microbiol 31, 1122–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsche TR, Sobek D, Gautom RK, 1998. Enhancement of in vitro cytopathogenicity by Acanthamoeba spp. following acquisition of bacterial endosymbionts. FEMS Microbiol Lett 166, 231–236. [DOI] [PubMed] [Google Scholar]
- Fuerst PA, 2014. Insights from the DNA databases: approaches to the phylogenetic structure of Acanthamoeba. Exp Parasitol 145 Suppl, S39–45. [DOI] [PubMed] [Google Scholar]
- Garate M, Alizadeh H, Neelam S, Niederkorn JY, Panjwani N, 2006. Oral immunization with Acanthamoeba castellanii mannose-binding protein ameliorates amoebic keratitis. Infect Immun 74, 7032–7034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J, Voelz H, 1985. Bacterial endosymbionts of Acanthamoeba sp. J Parasitol 71, 89–95. [PubMed] [Google Scholar]
- He YG, McCulley JP, Alizadeh H, Pidherney M, Mellon J, Ubelaker JE, Stewart GL, Silvany RE, Niederkorn JY, 1992. A pig model of Acanthamoeba keratitis: transmission via contaminated contact lenses. Invest Ophthalmol Vis Sci 33, 126–133. [PubMed] [Google Scholar]
- Heffler KF, Eckhardt TJ, Reboli AC, Stieritz D, 1996. Acanthamoeba endophthalmitis in acquired immunodeficiency syndrome. Am J Ophthalmol 122, 584–586. [DOI] [PubMed] [Google Scholar]
- Honda K, Littman DR, 2016. The microbiota in adaptive immune homeostasis and disease. Nature 535, 75–84. [DOI] [PubMed] [Google Scholar]
- Hurt M, Apte S, Leher H, Howard K, Niederkorn J, Alizadeh H, 2001. Exacerbation of Acanthamoeba keratitis in animals treated with anti-macrophage inflammatory protein 2 or antineutrophil antibodies. Infect Immun 69, 2988–2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurt M, Neelam S, Niederkorn J, Alizadeh H, 2003a. Pathogenic Acanthamoeba spp secrete a mannose-induced cytolytic protein that correlates with the ability to cause disease. Infect Immun 71, 6243–6255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurt M, Proy V, Niederkorn JY, Alizadeh H, 2003b. The interaction of Acanthamoeba castellanii cysts with macrophages and neutrophils. J Parasitol 89, 565–572. [DOI] [PubMed] [Google Scholar]
- Iovieno A, Ledee DR, Miller D, Alfonso EC, 2010. Detection of bacterial endosymbionts in clinical acanthamoeba isolates. Ophthalmology 117, 445–452, 452 e441–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DB, Visvesvara GS, Robinson NM, 1975a. Acanthamoeba polyphaga keratitis and Acanthamoeba uveitis associated with fatal meningoencephalitis. Trans Ophthalmol Soc U K 95, 221–232. [PubMed] [Google Scholar]
- Jones DB, Visvesvara GS, Robinson NM, 1975b. Acanthamoeba polyphaga keratitis and Acenthamoeba uveitis associated with fatal meningoencephalitis. Trans Ophthalmol Soc U K 95, 221–232. [PubMed] [Google Scholar]
- Kremer I, Cohen EJ, Eagle RC Jr., Udell I, Laibson PR, 1994. Histopathologic evaluation of stromal inflammation in Acanthamoeba keratitis. Clao J 20, 45–48. [PubMed] [Google Scholar]
- Lee GA, Gray TB, Dart JK, Pavesio CE, Ficker LA, Larkin DF, Matheson MM, 2002. Acanthamoeba sclerokeratitis: treatment with systemic immunosuppression. Ophthalmology 109, 1178–1182. [DOI] [PubMed] [Google Scholar]
- Leher H, Kinoshita K, Alizadeh H, Zaragoza FL, He Y, Niederkorn J, 1998a. Impact of oral immunization with Acanthamoeba antigens on parasite adhesion and corneal infection. Invest Ophthalmol Vis Sci 39, 2337–2343. [PubMed] [Google Scholar]
- Leher H, Silvany R, Alizadeh H, Huang J, Niederkorn JY, 1998b. Mannose induces the release of cytopathic factors from Acanthamoeba castellanii. Infect Immun 66, 5–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leher H, Zaragoza F, Taherzadeh S, Alizadeh H, Niederkorn JY, 1999. Monoclonal IgA antibodies protect against Acanthamoeba keratitis. Exp Eye Res 69, 75–84. [DOI] [PubMed] [Google Scholar]
- Leher HF, Alizadeh H, Taylor WM, Shea AS, Silvany RS, Van Klink F, Jager MJ, Niederkorn JY, 1998c. Role of mucosal IgA in the resistance to Acanthamoeba keratitis. Invest Ophthalmol Vis Sci 39, 2666–2673. [PubMed] [Google Scholar]
- Leher HF, Alizadeh H, Taylor WM, Shea AS, Silvany RS, Van Klink F, Jager MJ, Niederkorn JY, 1998d. Role of mucosal IgA in the resistance to Acanthamoeba keratitis. Invest Ophthalmol Vis Sci 39, 2666–2673. [PubMed] [Google Scholar]
- Lorenzo-Morales J, Khan NA, Walochnik J, 2015. An update on Acanthamoeba keratitis: diagnosis, pathogenesis and treatment. Parasite 22, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciver SK, Asif M, Simmen MW, Lorenzo-Morales J, 2013. A systematic analysis of Acanthamoeba genotype frequency correlated with source and pathogenicity: T4 is confirmed as a pathogen-rich genotype. Eur J Protistol 49, 217–221. [DOI] [PubMed] [Google Scholar]
- Manikandan P, Bhaskar M, Revathy R, John RK, Narendran V, Panneerselvam K, 2004. Acanthamoeba keratitis - a six year epidemiological review from a tertiary care eye hospital in south India. Indian J Med Microbiol 22, 226–230. [PubMed] [Google Scholar]
- Mannis MJ, Tamaru R, Roth AM, Burns M, Thirkill C, 1986. Acanthamoeba sclerokeratitis. Determining diagnostic criteria. Arch Ophthalmol 104, 1313–1317. [DOI] [PubMed] [Google Scholar]
- Marciano-Cabral F, Toney DM, 1998. The interaction of Acanthamoeba spp. with activated macrophages and with macrophage cell lines. J Eukaryot Microbiol 45, 452–458. [DOI] [PubMed] [Google Scholar]
- Massilamany C, Marciano-Cabral F, Rocha-Azevedo B, Jamerson M, Gangaplara A, Steffen D, Zabad R, Illes Z, Sobel RA, Reddy J, 2014. SJL mice infected with Acanthamoeba castellanii develop central nervous system autoimmunity through the generation of cross-reactive T cells for myelin antigens. PLoS One 9, e98506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathers W, Stevens G Jr., Rodrigues M, Chan CC, Gold J, Visvesvara GS, Lemp MA, Zimmerman LE, 1987. Immunopathology and electron microscopy of Acanthamoeba keratitis. Am J Ophthalmol 103, 626–635. [DOI] [PubMed] [Google Scholar]
- Maycock NJ, Jayaswal R, 2016. Update on Acanthamoeba Keratitis: Diagnosis, Treatment, and Outcomes. Cornea 35, 713–720. [DOI] [PubMed] [Google Scholar]
- Mazur T, Hadas E, Iwanicka I, 1995. The duration of the cyst stage and the viability and virulence of Acanthamoeba isolates. Trop Med Parasitol 46, 106–108. [PubMed] [Google Scholar]
- McAllum P, Bahar I, Kaiserman I, Srinivasan S, Slomovic A, Rootman D, 2009. Temporal and seasonal trends in Acanthamoeba keratitis. Cornea 28, 7–10. [DOI] [PubMed] [Google Scholar]
- McClellan K, Howard K, Mayhew E, Niederkorn J, Alizadeh H, 2002. Adaptive immune responses to Acanthamoeba cysts. Exp Eye Res 75, 285–293. [PubMed] [Google Scholar]
- McClellan K, Howard K, Niederkorn JY, Alizadeh H, 2001. Effect of steroids on Acanthamoeba cysts and trophozoites. Invest Ophthalmol Vis Sci 42, 2885–2893. [PubMed] [Google Scholar]
- McKelvie J, Alshiakhi M, Ziaei M, Patel DV, McGhee CN, 2018. The rising tide of Acanthamoeba keratitis in Auckland, New Zealand: a 7-year review of presentation, diagnosis and outcomes (2009–2016). Clin Exp Ophthalmol 46, 600–607. [DOI] [PubMed] [Google Scholar]
- Moshari A, McLean IW, Dodds MT, Damiano RE, McEvoy PL, 2001. Chorioretinitis after keratitis caused by Acanthamoeba: case report and review of the literature. Ophthalmology 108, 2232–2236. [DOI] [PubMed] [Google Scholar]
- Neelam S, Niederkorn JY, 2017. Pathobiology and Immunobiology of Acanthamoeba Keratitis: Insights from Animal Models. Yale J Biol Med 90, 261–268. [PMC free article] [PubMed] [Google Scholar]
- Niederkorn J, Alizadeh H, Taylor W, He Y-G, McCulley JP, Stewart GL, Haehling E, Van Klink F, 1994. Oral immunization induces protective immunity against Acanthamoeba keratitis, in: Nussenblatt R, Whitcup SM, Caspi RR, Gery I (Ed.), Advances in Ocular Immunology. Elsevier, Amsterdam, pp. 281–284. [Google Scholar]
- Niederkorn JY, Alizadeh H, Leher H, McCulley JP, 1999. The pathogenesis of Acanthamoeba keratitis. Microbes Infect 1, 437–443. [DOI] [PubMed] [Google Scholar]
- Niederkorn JY, Ubelaker JE, McCulley JP, Stewart GL, Meyer DR, Mellon JA, Silvany RE, He YG, Pidherney M, Martin JH, et al. , 1992. Susceptibility of corneas from various animal species to in vitro binding and invasion by Acanthamoeba castellanii. Invest Ophthalmol Vis Sci 33, 104–112. [PubMed] [Google Scholar]
- Page MA, Mathers WD, 2013. Acanthamoeba keratitis: a 12-year experience covering a wide spectrum of presentations, diagnoses, and outcomes. J Ophthalmol 2013, 670–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randag AC, van Rooij J, van Goor AT, Verkerk S, Wisse RPL, Saelens IEY, Stoutenbeek R, van Dooren BTH, Cheng YYY, Eggink CA, 2019. The rising incidence of Acanthamoeba keratitis: A 7-year nationwide survey and clinical assessment of risk factors and functional outcomes. PLoS One 14, e0222092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robaei D, Carnt N, Minassian DC, Dart JK, 2014. The impact of topical corticosteroid use before diagnosis on the outcome of Acanthamoeba keratitis. Ophthalmology 121, 1383–1388. [DOI] [PubMed] [Google Scholar]
- St Leger AJ, Desai JV, Drummond RA, Kugadas A, Almaghrabi F, Silver P, Raychaudhuri K, Gadjeva M, Iwakura Y, Lionakis MS, Caspi RR, 2017. An Ocular Commensal Protects against Corneal Infection by Driving an Interleukin-17 Response from Mucosal gammadelta T Cells. Immunity 47, 148–158 e145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart GL, Kim I, Shupe K, Alizadeh H, Silvany R, McCulley JP, Niederkorn JY, 1992. Chemotactic response of macrophages to Acanthamoeba castellanii antigen and antibody-dependent macrophage-mediated killing of the parasite. J Parasitol 78, 849–855. [PubMed] [Google Scholar]
- Stewart GL, Shupe K, Kim I, Silvany RE, Alizadeh H, McCulley JP, Niederkorn JY, 1994. Antibody-dependent neutrophil-mediated killing of Acanthamoeba castellanii. Int J Parasitol 24, 739–742. [DOI] [PubMed] [Google Scholar]
- Stopak SS, Roat MI, Nauheim RC, Turgeon PW, Sossi G, Kowalski RP, Thoft RA, 1991. Growth of acanthamoeba on human corneal epithelial cells and keratocytes in vitro. Invest Ophthalmol Vis Sci 32, 354–359. [PubMed] [Google Scholar]
- Suryawanshi A, Cao Z, Sampson JF, Panjwani N, 2015. IL-17A-mediated protection against Acanthamoeba keratitis. J Immunol 194, 650–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y, Suguri S, Harada M, Hayabara T, Suzumori K, Ohta N, 1994. Acanthamoeba-specific human T-cell clones isolated from healthy individuals. Parasitol Res 80, 549–553. [DOI] [PubMed] [Google Scholar]
- Thaiss CA, Zmora N, Levy M, Elinav E, 2016. The microbiome and innate immunity. Nature 535, 65–74. [DOI] [PubMed] [Google Scholar]
- Toney DM, Marciano-Cabral F, 1998. Resistance of Acanthamoeba species to complement lysis. Journal of Parasitology 84, 338–344. [PubMed] [Google Scholar]
- van Klink F, Alizadeh H, He Y, Mellon JA, Silvany RE, McCulley JP, Niederkorn JY, 1993. The role of contact lenses, trauma, and Langerhans cells in a Chinese hamster model of Acanthamoeba keratitis. Invest Ophthalmol Vis Sci 34, 1937–1944. [PubMed] [Google Scholar]
- van Klink F, Alizadeh H, Stewart GL, Pidherney MS, Silvany RE, He Y, McCulley JP, Niederkorn JY, 1992a. Characterization and pathogenic potential of a soil isolate and an ocular isolate of Acanthamoeba castellanii in relation to Acanthamoeba keratitis. Curr Eye Res 11, 1207–1220. [DOI] [PubMed] [Google Scholar]
- van Klink F, Alizadeh H, Stewart GL, Pidherney MS, Silvany RE, He Y, McCulley JP, Niederkorn JY, 1992b. Characterization and pathogenic potential of a soil isolate and an ocular isolate of Acanthamoeba castellanii in relation to Acanthamoeba keratitis. Curr Eye Res 11, 1207–1220. [DOI] [PubMed] [Google Scholar]
- Van Klink F, Leher H, Jager MJ, Alizadeh H, Taylor W, Niederkorn JY, 1997. Systemic immune response to Acanthamoeba keratitis in the Chinese hamster. Ocul Immunol Inflamm 5, 235–244. [DOI] [PubMed] [Google Scholar]
- van Klink F, Taylor WM, Alizadeh H, Jager MJ, van Rooijen N, Niederkorn JY, 1996. The role of macrophages in Acanthamoeba keratitis. Invest Ophthalmol Vis Sci 37, 1271–1281. [PubMed] [Google Scholar]
- Visvesvara GS, Moura H, Schuster FL, 2007. Pathogenic and opportunistic free-living amoebae: Acanthamoeba spp., Balamuthia mandrillaris, Naegleria fowleri, and Sappinia diploidea. FEMS Immunol Med Microbiol 50, 1–26. [DOI] [PubMed] [Google Scholar]
- Watt KG, Swarbrick HA, 2007. Trends in microbial keratitis associated with orthokeratology. Eye Contact Lens 33, 373–377; discussion 382. [DOI] [PubMed] [Google Scholar]