Abstract
Chronic intermittent ethanol (CIE) exposure dysregulates glutamatergic and GABAergic neurotransmission, facilitating BLA pyramidal neuron hyperexcitability and the expression of anxiety during withdrawal. It is unknown whether ethanol-induced alterations in nucleus basalis magnocellularis (NBM) cholinergic projections to the BLA mediate anxiety-related behaviors through direct modulation of GABA and glutamate afferents. Following 10 days of CIE exposure and 24 hours of withdrawal, we recorded GABAergic and glutamatergic synaptic responses in BLA pyramidal neurons with electrophysiology, assessed total protein expression of cholinergic markers, and quantified acetylcholine and choline concentrations using a colorimetric assay. We measured α7 nicotinic acetylcholine receptor (nAChR) dependent modulation of presynaptic function at distinct inputs in AIR- and CIE-exposed BLA coronal slices as a functional read-out of cholinergic neurotransmission. CIE/withdrawal upregulates the endogenous activity of α7 nAChRs, facilitating release at both GABAergic’ local’ interneuron and glutamatergic synaptic responses to stria terminalis (ST) stimulation, with no effect at GABAergic lateral paracapsular cells. CIE caused a three-fold increase in BLA acetylcholine concentration, with no changes in α7 nAChR or cholinergic marker expression. These data illustrate that α7 nAChR-dependent changes in presynaptic function serve as a proxy for CIE-dependent alterations in synaptic acetylcholine levels. Thus, cholinergic projections appear to mediate CIE-induced alterations at GABA/glutamate inputs.
Keywords: patch-clamp electrophysiology, presynaptic, withdrawal, acetylcholine, basolateral amygdala
Introduction
Alcohol use disorders (AUD) include neurophysiological and behavioral modifications that lead to cycles of excessive alcohol consumption and abstinence. Withdrawal (WD) symptoms manifest in psychological and physical forms and remain an underlying cause of inflated relapse rates among long-term alcohol users (McCaul et al., 2017; Oliva et al., 2018; Willinger et al., 2002). Anxiety is a principal characteristic of acute and protracted withdrawal that patients commonly cite following relapse. The lateral/basolateral amygdala (BLA) is an emotional center that is sensitive to alcohol-induced alterations in synaptic transmission (Christian et al., 2013; Christian et al., 2012; Diaz et al., 2011; Gioia et al., 2017; Robinson et al., 2016) and governs the progression of withdrawal-associated anxiety.
Glutamatergic pyramidal neurons are the primary projection neurons in the BLA, comprising ~85% of the total cell population (McDonald, 1982, 1992) that receive sensory, cognitive, and memory-related information via glutamatergic afferents from several cortical and subcortical brain regions. Conversely, two anatomically distinct GABAergic interneuron populations, lateral paracapsular cells (LPCs) (Diaz et al., 2011; Marowsky et al., 2005) and ‘local’ interneurons (Woodruff and Sah, 2007), exert robust inhibitory control through feedforward and feedback inhibition, respectively. Chronic ethanol and withdrawal dysregulate these glutamatergic and GABAergic circuits, consequently driving BLA pyramidal neuron hyperexcitability and the expression of anxiety, fear, and reward-seeking behaviors (Christian et al., 2013; Lack et al., 2007; Morales et al., 2018). To date, little is known about whether ethanol-induced alterations in BLA neuromodulatory inputs contribute to the disruptions in the GABA/glutamate balance and generation of withdrawal-induced anxiety. The present study suggests that alterations in cholinergic inputs are involved in producing these complex behaviors.
The nucleus basalis magnocellularis (NBM) of the basal forebrain sends dense cholinergic projections to the basolateral nucleus of the BLA (Carlsen et al., 1985; Lee and Kim, 2019; Woolf and Butcher, 1982) and plays a critical role in memory formation and the consolidation of salient cues (Crouse et al., 2020; Gold, 2003; Power A. E. and McGaugh, 2002b). Activation of this circuit impairs fear-related extinction (Jiang et al., 2016), influences approach and avoidance behaviors (Aitta-Aho et al., 2018), and increases arousal (See Review, McGaugh 2004) in rodent models. In contrast, inhibition of these cholinergic inputs impairs the acquisition and consolidation of aversive memories in the inhibitory avoidance task (Power A. E. and McGaugh, 2002a) and causes deficiencies in fear learning and recall (Jiang et al., 2016). Together, these studies strongly suggest that cholinergic input to the BLA mediates the consolidation of fear- and anxiety-like behaviors. No studies have examined ethanol-induced alterations in the NBM-BLA circuit or the subsequent effects on BLA pyramidal neuron excitability.
Acetylcholine (ACh) binds to nicotinic and muscarinic acetylcholine receptors (n/mAChRs) expressed by GABAergic interneurons (Lee and Kim, 2019; Zhu et al., 2005)by cortical glutamatergic terminals (Jiang and Role, 2008), and by BLA pyramidal neurons (Klein and Yakel, 2006; Muller et al., 2016; Power J. M. and Sah, 2008) to modulate neuronal excitability through fast synaptic transmission and G-protein signaling cascades. Neuronal nAChRs composed of alpha (α2–α10) and beta (β2–β4) subunits are permeable to cations, including Na+, K+, and Ca2+ (See Review, Corradi and Bouzat, 2016). Notably, α7 nAChRs are highly permeable to Ca2+ (Seguela et al., 1993), making them an important facilitator of neurotransmitter release (Cheng and Yakel, 2014). Presynaptic α7 nAChRs on glutamatergic terminals (Jiang and Role, 2008) and local interneurons (Barazangi and Role, 2001) facilitate neurotransmitter release onto BLA pyramidal neurons (Pidoplichko et al., 2013) and mediate anxiety- and depressive-like behaviors (Mineur et al., 2016). Human fMRI data illustrate that α7 nAChR negative allosteric modulators reduce human amygdala reactivity and connectivity, similarly to clinically effective anxiolytics (Wise et al., 2020). These results provide converging evidence in preclinical and clinical models that tonic α7 nAChR activity, indicating enhanced cholinergic transmission, contributes to BLA pyramidal neuron hyperexcitability.
Therefore, we hypothesized that chronic intermittent ethanol (CIE) exposure, an ethanol vapor exposure paradigm that produces ethanol dependence and anxiety-like behaviors in rodent models (McGinnis et al., 2020; Morales et al., 2015; Rogers et al., 1979), would cause an upregulation of α7 nAChR activity in the BLA In the present study, we utilize whole-cell patch clamp electrophysiology to understand how CIE exposure and withdrawal modulates cholinergic circuitry, as measured through α7 nAChR-mediated changes in GABAergic and glutamatergic neurotransmission. Our results indicate that CIE exposure and withdrawal upregulate cholinergic modulation of both glutamate and ‘local’ GABAergic synapses.
Experimental Procedures
Animals
Male Sprague-Dawley rats were purchased from Envigo at 5–6 weeks old (~150g) and pair-housed in an animal care facility under a reverse 12 hour light/dark cycle. Rats were given food and water ad libitum. All animal procedures were performed in accordance with protocols approved by the Wake Forest University School of Medicine Institutional Animal Care and Use Committee and were consistent with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Chronic intermittent ethanol vapor exposure
Rats in their home-cages were placed into large, Plexiglas chambers (Triad Plastics) during the exposure, as described previously (Lack et al., 2009; Lack et al., 2007; McGinnis et al., 2020; Robinson et al., 2016). Ethanol vapor was pumped into the chamber at a constant rate (16 L/min) for the light cycle duration (9:00 P.M.–9:00 A.M.), lasting 12 hrs/day. Ethanol vapor exposure lasted 10 consecutive days. Age-matched AIR-exposed (AIR) controls were similarly housed but exposed to room air only. Blood ethanol concentrations (BECs) were measured twice throughout the exposure using a commercially available alcohol dehydrogenase/NADH (nicotinamide adenine dinucleotide plus hydrogen) enzymatic assay (Carolina Liquid Chemistries). Average BECs during the chronic intermittent ethanol (CIE) exposure were 239.44 ± 4.79 mg/dl. Target blood ethanol concentrations are 150–275 mg/dL to produce dependence (Gilpin et al., 2008). Whole-cell patch clamp electrophysiology studies were completed at 24 hours of withdrawal (WD) following the final ethanol exposure.
Electrophysiology
Slice preparation.
Rats were anesthetized with isoflurane, decapitated, and their brains were quickly removed. Brains were transferred to an ice-cold sucrose artificial cerebral spinal fluid (aCSF) solution for 5 minutes that was equilibrated with 95% O2 and 5% CO2 and contained: 180mM sucrose, 30mM NaCl, 4.5mM KCl, 1mM MgCl2·6H2O, 26mM NaHCO3, 1.2mM NaH2PO4, 0.1mM ketamine, and 10mM glucose. Rodent BLA coronal slices (400μM) were prepared using a BT1200/S Vibrating Blade Microtome (Leica). Slices were incubated (28°C) and oxygenated for ≥ 1 hour in a standard aCSF solution that contained: 126mM NaCl, 3mM KCl, 1.25mM NaH2PO4, 2mM MgSO4·7H2O, 26mM NaHCO3, 10mM D-glucose, and 2mM CaCl2·2H2O. All chemicals were purchased from Sigma-Aldrich or Tocris Bioscience.
Whole-cell patch clamp electrophysiology.
BLA slices were moved into a submersion-type recording chamber perfused with oxygenated, room temperature (~25°C) aCSF at a rate of 2 mL/min. Recording electrodes were filled with an intracellular solution that contained: 145mM CsOH, 10mM EGTA, 5mM NaCl, 1mM MgCl2·6H2O, 10mM HEPES, 4mM Mg-ATP, 0.4mM Na-GTP, 0.4mM QX314, and 1mM CaCl2·2H2O. The pH of the internal solution was adjusted to ~7.2–7.3 with D-gluconic acid, and the osmolarity was adjusted to ~285 Osm/L with sucrose. Putative BLA pyramidal neurons were distinguished from local GABAergic interneurons based on their low access resistance (<25MΩ) and high membrane capacitance (>100pF). A small population of large-soma interneurons is present in the BLA (~0.6% of all BLA neurons) and is unlikely to be included in the data analysis using these inclusion criteria. Glutamatergic excitatory postsynaptic currents (EPSCs) were recorded from basolateral amygdala neurons at a holding potential of −65mV and were pharmacologically isolated by adding GABAA antagonist picrotoxin (100μM) in the external aCSF. In separate recordings, GABAergic inhibitory postsynaptic currents (IPSCs) were recorded at a holding potential of −10mV and were pharmacologically isolated with the glutamate receptor antagonists DNQX (AMPA/kainite receptor antagonist, 20μM) and APV (NMDA receptor antagonist, 50μM). GABAergic IPSCs and glutamatergic EPSCs were electrically evoked with platinum/iridium concentric bipolar stimulating electrodes (FHC Inc., Bowdoinham, ME) with an inner pole of 12.5μm. To activate glutamate synapses, the stimulating electrode was placed several hundred microns away from the basolateral nucleus within the stria terminalis fiber tract, just dorsal to the central amygdala and medial to the lateral/basolateral nuclei (McGinnis et al., 2020; Morales et al., 2018; Fig. 1A). Modest electrical stimulation of the stria typically evokes monosynaptic glutamatergic responses; and, direct activation of antidromic action currents within BLA principal neurons from which we recorded was never observed. Polysynaptic glutamatergic responses, likely due to erroneous spread of electrical stimulation and subsequent activation of ‘local’ circuits, were rarely encountered (n=6 out of 121 responses recording for the current study) and excluded from data analysis. For GABA recordings, LPCs were stimulated by placing the electrode along the external capsule (Diaz et al., 2011b; Silberman et al., 2008; Fig. 2A). To activate synapses from ‘local’ interneurons, the stimulating electrode was placed within the BLA just medial to the recording site (Diaz et al., 2011b; Fig. 3A). The stimulation intensity was normalized in all recordings to elicit synaptic responses with amplitudes of ~100pA. Pyramidal neurons were recorded from the basolateral nucleus as it receives dense cholinergic input (Carlsen et al., 1985; Lee and Kim, 2019; Woolf and Butcher, 1982). Data were acquired at 5 kHz and low-pass filtered at 2 kHz via an Axopatch 700B Amplifier and pClamp10.7 software (Molecular Devices).
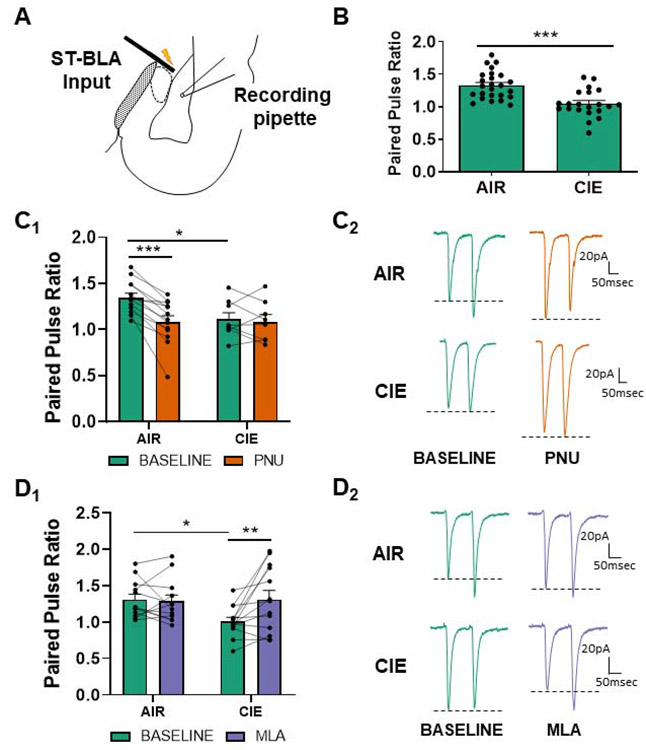
Figure 1.
α7 nAChR activity is upregulated and facilitates the upregulation of glutamate release at stria terminalis (ST) inputs during withdrawal. (A) Schematic showing the typical placement of the stimulating electrode at ST inputs and patch electrode in the basolateral nucleus for electrophysiology recordings. (B) 10 days of CIE and 24 hours of withdrawal (N=21) decreases the paired pulse ratio (see Methods) at ST glutamatergic inputs in comparison to AIR-exposed controls (N=25), as previously reported (t=4.482, p<0.0001; see (Morales et al., 2015)). (C) PNU-282987 (designated PNU in graphs) modulates PPR in AIR-exposed (N=13), but not CIE-exposed BLA neurons (N=8). Bars (C1) represent mean ± SEM of PPRs during baseline and bath application of PNU-282987 (0.5μM). Two-way repeated-measures ANOVA; significant interaction and the main effect of PNU-282987, p<0.05. Bonferroni post-tests confirmed a significant difference between AIR and CIE release probabilities at baseline (t=2.507, * - p<0.05) and following PNU-282987 application in AIR animals (t=6.023, *** - p<0.001). Representative traces (C2) of glutamatergic paired-pulse responses from AIR or CIEneurons at baseline and following drug application. Dashed lines illustrate the drug effect on the second peak amplitude (P2) relative to the first peak amplitude (P1). (D) The α7 nAChR competitive antagonist MLA (10nM) reverses withdrawal-induced decreases in PPR. Two-way repeated-measures ANOVA reveals a main effect of MLA and a significant interaction between exposure and drug (p<0.05). MLA significantly increased PPR in CIE neurons (N=13, D1; Bonferroni post-test, t=3.453, p<0.01) but not in AIR neurons (N=12, p>0.05). Representative traces (D2) of paired-pulse responses from AIR or CIE neurons at baseline and following MLA perfusion.
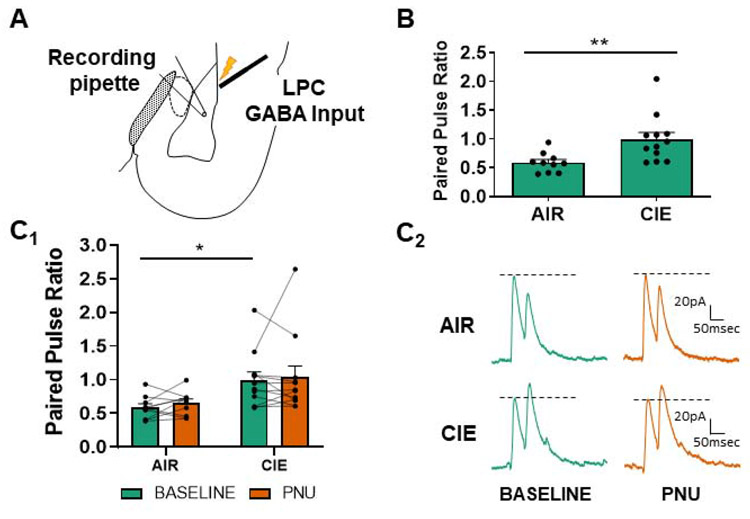
Figure 2.
α7 nAChR activity does not modulate LPC GABAergic release probability. (A) Schematic shows the placement of the stimulating electrode at LPC GABA inputs and patch electrode in the basolateral nucleus for electrophysiology recordings. (B) CIE/withdrawal (N=12) increased the paired pulse ratio (decreased release probability; see Methods) at LPC interneurons relative to AIR-exposed controls (N=10) as previously reported (t-test, t=3.098, ** - p<0.01 see (Diaz et al., 2011)). (C) PNU-282987 (labeled PNU in graphs; 0.5μM) does not alter the release probability in either AIR-exposed or CIE neurons. Bars (C1) represent mean ± SEM of PPRs during baseline and following bath application of PNU-282987 (two-way ANOVA with Bonferroni post-test, p>0.05 for all). Representative traces (C2) of LPC GABAergic paired-pulse responses from AIR or CIE neurons at baseline and following PNU-282987. Dashed lines as in Figure 1.
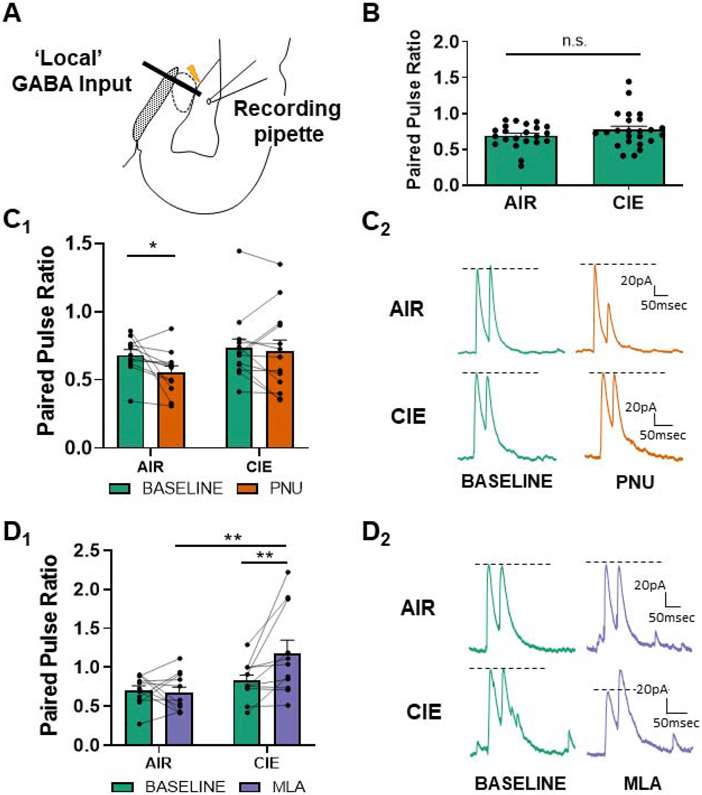
Figure 3.
α7 nAChRs are tonically active at ‘local’ GABAergic synapses during withdrawal. (A) Schematic showing the typical placement of the stimulating electrode at ‘local’ GABAergic interneurons and patch electrode in the basolateral nucleus for electrophysiology recordings. (B) 10 days of CIE and 24 hours of withdrawal (N=24) does not alter the baseline release probability at local interneurons in comparison to AIR-exposed controls, “n.s.” = not significant (N=22; t-test, t=1.328 , p>0.05) as previously reported (Diaz et al., 2011). (C) PNU-282987 (designated PNU in graphs) decreases the PPR (increases release probability) at ‘local’ GABAergic synapses recorded from AIR-exposed control BLA neurons (N=11) with no effect in CIE neurons (N=13; two-way repeated-measures ANOVA, p<0.05). Bars (C1) represent mean ± SEM of PPRs during baseline and following bath application of PNU-282987 (0.5μM). Representative traces (C2) of ‘local’ GABAergic paired-pulse responses from AIR or CIE neurons at baseline and following drug application. (D) The α7 nAChR antagonist MLA (10nM) enhances PPR (decreases release) at ‘local’ GABA synapses following CIE but does not affect AIR-treated control neurons. Average ± SEM (D1) PPRs during baseline and in the presence of α7 nAChR competitive antagonist MLA. Two-way repeated-measures ANOVA reveals a significant interaction between exposure and drug (p<0.05). PPR significantly increased in CIE neurons (N=11) following application of MLA relative to both CIE baseline (**- p<0.01, Bonferroni post-test) and MLA-treated AIR controls (N=11, ** - p<0.01). There was no effect of MLA treatment in AIR-exposed neurons (p>0.05). Representative traces (D2) of paired-pulse responses from AIR or CIE neurons at baseline and following MLA perfusion.
Paired-pulse Ratios (PPR).
Electrical stimulation of equal intensity was delivered with paired stimuli at an interstimulus interval of 50ms. At short interstimulus intervals, the ratio between the first and second EPSC or IPSC response amplitudes serves as a proxy for presynaptic neurotransmitter release probability (Andreasen and Hablitz, 1994; Dobrunz and Stevens, 1997; Fioravante et al., 2011). The paired-pulse ratio (PPR) was calculated using the electrically evoked E/IPSC amplitudes as follows: [(Peak 2 amplitude)/(Peak 1 amplitude)]. For ST glutamate recordings, the Peak 1 and Peak 2 amplitudes are well separated from each other and were measured from the baseline preceding the first EPSC, as previously reported (Christian et al., 2013; McGinnis et al., 2020; Morales et al., 2018). For both ‘local’ and LPC GABA recordings, the second current response typically overlaps with the decay of the first current response. Therefore, peak 2 amplitude was measured as the difference between maximal current at time ‘t’ during the second response and the current amplitude extrapolated from from P1 decay at the time ‘t’.
α7 nAChR pharmacology.
Paired-pulse responses were evoked via electrical stimulation every 30 seconds. Following a 5 minute baseline, α7 nAChR agonist PNU-282987 (0.5μM; Cheng and Yakel, 2014) or α7 nAChR antagonist methyllycaconitine (MLA, 10nM; Drasdo et al., 1992; Palma et al., 1996) was perfused onto BLA coronal slices for 10 minutes. The baseline PPR was compared to the PPR in the final 5 minutes of drug perfusion to ensure maximal drug effect. Pyramidal neurons with ≥ 20% changes in access resistance or membrane capacitance throughout the recording were excluded from data analysis. PNU-282987 (Cat. No. 2303) and MLA (Cat. No. 1029) were purchased from Tocris Biosciences.
Western blotting
Lysis buffer containing tissue protein extraction reagent (TPER, Thermo Fisher Scientific) and protease/phosphatase inhibitors for mammalian tissue (Sigma-Aldrich, 1:100) was added to BLA dissected from AIR and CIE coronal slices at 10 μl/mg tissue, homogenized with gentle sonication, and incubated with agitation at 4°C for 1 hour. The protein yield of AIR- and CIE-exposed rats (N=16) was quantified using the BCA assay (Thermo Fisher Scientific). Protein homogenate (5–30μg protein/lane, depending on the blot) was loaded onto 4–20% 18-well Criterion TGX Precast Midi Protein Gels (catalog #567-8094, Bio-Rad) and transferred to a nitrocellulose membrane with a Bio-Rad Trans-Blot Turbo Transfer System and preassembled membrane stacks (catalog #1704270, Bio-Rad). Total lane protein transfer was detected using the Pierce Reversible Protein Stain Kit (catalog #PI24580, Thermo Fisher Scientific). Membranes were blocked with either 1% or 5% nonfat dry milk in Tris-buffered saline (TBS-T; 150 mM NaCl, 5.2 nM Na2HPO4, 1.7 mM KH2PO4, 0.05% Tween 20) for one hour. Blots were incubated overnight at 4°C in TBS-T/1.0% nonfat dry milk containing each of the following rabbit polyclonal primary antibodies: choline acetyltransferase, 1:1000 dilution (Abcam, ab178850); acetylcholinesterase, 1:1000 dilution (Abcam, ab183591); vesicular acetylcholine transporter, 1:1000 dilution (Abcam, ab235201); α7 nAChR, 1:500 dilution (Abcam, ab182442). Antibodies were validated by the manufacturer and yielded bands of the expected molecular weight. After five TBS-T rinses, the blots were exposed to an anti-rabbit horseradish peroxidase-labeled secondary antibody (1:1500 dilution) for one hour at room temperature. The Super-Signal West Dura Extended Duration Substrate Enhanced Chemiluminescence (catalog #34076, Thermo Fisher Scientific) was used to detect the bound secondary antibody. Immunoreactive band intensity was quantified from digital images captured using a charged coupled device camera (Bio-Rad Chemi-Doc XRS Imaging System) and normalized to total lane protein using Image Lab Analysis software (Bio-Rad). Data were normalized as a percentage of the AIR-exposed controls and analyzed using an unpaired student’s t-test.
Acetylcholine Assay
Choline assay buffer was added to BLA dissected from AIR and CIE coronal slices and processed according to the manufacturer’s instructions. Protein yield from the AIR- and CIE-exposed rats (N=36) was quantified using the BCA assay (Thermo Fisher Scientific) and pooled into 12 groups (N=3 animals/group; 6 groups/condition). A commercially available colorimetric assay (Acetylcholine Assay Kit, Abcam, ab65345) indirectly measured acetylcholine (ACh) levels by quantification of [CholineTOTAL] and [CholineFREE] absorbance levels. Acetylcholinesterase was added to the [CholineTOTAL] master mix to convert available acetylcholine to choline, such that [CholineTOTAL] absorbance values were representative of acetylcholine + choline levels. [CholineFREE] wells were treated with physostigmine (0.5μM, Tocris Biosciences) to minimize enzymatic conversion of acetylcholine to choline. ACh concentration was calculated using the formula: [CholineTOTAL] − [CholineFREE] = [Acetylcholine]. 32μg of pooled total protein was loaded in triplicate wells per [CholineTOTAL] and [CholineFREE] conditions. Each pooled sample was run in duplicate, and the results were comparable across plates. The data shown were representative of one trial of each pooled sample. Data were normalized using Min.–Max. Normalization (Suarez-Alvarez et al., 2012) and expressed as a percentage of AIR-exposed controls and analyzed using a student’s t-test.
Experimental Design and Statistical Analysis
Statistical analyses were analyzed with Prism 5 (Graphpad Software). Data were analyzed with a mixed factorial two-way ANOVA and Bonferroni post hoc tests or with unpaired t-tests depending on the experiment. A value of p<0.05 was considered statistically significant, and statistical significance was demarcated as follows: p<0.05 (*), p<0.01 (**), and p<0.0001 (***). Bar graphs are represented as mean ± SEM. Individual data points with connecting lines indicate paired data at baseline and following drug application.
Results
α7 nAChR activity is upregulated at stria terminalis (ST) inputs during withdrawal and facilitates ‘pathological’ glutamate release
Synaptic responses to stimulation of the stria terminalis (ST) express enhanced presynaptic function following 10 days of CIE exposure and 24 hours of withdrawal, including increased synaptic glutamate concentration and release probability and decreased failure rates (Christian et al., 2013; Morales et al., 2018). To determine whether α7 nAChRs mediate CIE-induced alterations in glutamate release probability, we perfused α7 nAChR selective agonist PNU-282987 (0.5μM; Cheng and Yakel, 2014) or the competitive antagonist MLA (10nM; Palma et al., 1996) onto AIR and CIE-exposed coronal BLA slices while measuring the paired pulse ratio (PPR; see Methods for details).
As previously reported (Lack et al., 2007; Morales et al., 2018), 10-day CIE exposure significantly reduced the PPR at ST synapses, indicating an increase in glutamate release probability (Fig. 1B; AIR - 1.33±0.04 N=25, CIE - 1.05±0.05 N=21; unpaired t-test, t=4.482, p<0.0001). PNU-282987 application significantly decreased the PPR in AIR-exposed, but not CIE neurons (Fig 1C, D). A two-way repeated-measures ANOVA revealed a main effect of CIE exposure (F(1,19)=16.62, p<0.01) and a significant interaction between CIE exposure and PNU-282987 (F(1,19)=11.28, p<0.01). Bonferroni post-tests confirmed a significant difference between AIR (N=13) and CIE (N=8) PPRs at baseline (t=2.507, p<0.05) and following PNU-282987 application in AIR neurons (t=6.023, p<0.001). These data show that exogenous activation of presynaptic α7 nAChRs increases glutamate release probability exclusively in AIR neurons. The absence of PNU-282987 modulation in CIE neurons could either reflect increased endogenous cholinergic activation of α7 nAChRs or a loss of signaling by these receptors.
To differentiate these possible mechanisms, we bath applied the α7 nAChR antagonist MLA (10nM; Klein and Yakel, 2006) in a separate group of recordings. MLA significantly reversed the effects of CIE on PPR but had limited efficacy in AIR-treated controls (Fig. 1E, F). A two-way repeated-measures ANOVA indicated a significant interaction (CIE Χ MLA: F(1,23)=6.668, p<0.05) and a main effect of MLA (F(1,23)=4.849, p<0.05). Bonferroni post-test again revealed a significant difference between AIR (N=12) and CIE (N=13) PPRs at baseline (t=2.338, p<0.05) and a significant effect of MLA only in CIE neurons (t=3.453, p<0.01). Together, these data suggest that CIE upregulates presynaptic function at ST glutamatergic synapses onto BLA neurons and that tonic activity of presynaptic α7 nAChRs contributes to the elevation in glutamate release during withdrawal.
Presynaptic α7 nAChR activity does not modulate lateral paracapsular cell (LPC) release probability
Next, we measured the effect of PNU-282987 on LPC GABAergic release probability. 10 day CIE and 24 hr withdrawal significantly increased PPRs (decreased release) at LPC GABAergic synapses onto BLA pyramidal neurons (Fig. 2B; AIR 0.59±0.05 N=10, CIE 0.99±0.12N=12; Welch’s unpaired t-test, t=3.098, p<0.01), consistent with our previous observations (Diaz et al., 2011). Application of PNU-282987 did not alter LPC GABAergic PPRs in either AIR or CIE neurons (Fig. 2C, D). There was a main effect of CIE exposure (repeated measures two-way ANOVA, F(1,20)=6.632, p<0.05), but no effect of PNU-282987 (F(1,20)=0.551, p>0.05) and no significant interaction between these factors (F(1,20)=0.033, p>0.05). These data illustrate that LPC GABAergic synapses likely do not express presynaptic α7 nAChRs since PNU-282987 does not modify LPC release probability.
α7 nAChRs are tonically active at ‘local’ GABAergic feedback interneuron synapses during withdrawal
Unlike LPC GABAergic synapses, we have previously shown that ‘local’ interneuron GABAergic synapses do not express appreciable changes in baseline PPR following 10 day CIE and withdrawal (Diaz et al., 2011b). We confirmed this in the current study (Fig. 3B; AIR - 0.69±0.04 N=22, CIE - 0.77±0.05 N=24; unpaired t-test, t=1.328 , p>0.05). Since this population of BLA interneurons expresses α7 nAChRs (Pidoplichko et al., 2013), we hypothesized that upregulation of cholinergic neurotransmission following CIE exposure and withdrawal (Fig. 1) might help maintain GABA release probability at these local GABAergic synapses. Consistent with this, bath application of PNU-282987 significantly decreased the local GABAergic PPR in AIR-exposed (N=11) but not CIE-exposed (N=13) neurons (Fig. 3C, D). A two-way repeated-measures ANOVA revealed a main effect of PNU-282987 (F(1,22)=4.767, p<0.05) without a significant effect of exposure (F(1,22)=1.377, p>0.05). The interaction between factors only approached significance (F(1,22)=2.124 , p>0.05). Bonferroni post-tests showed a significant difference in local GABA PPR in only AIR-exposed neurons following PNU-282987 (t=2.473 , p<0.05), with no significant effect of PNU-282987 treatment in CIE neurons (t=0.536 , p>0.05). Similar to ST glutamatergic inputs, the inability of PNU-282987 to modulate GABA release probability in CIE neurons was potentially due to increased endogenous α7 nAChR activation at ‘local’ interneuron synapses.
To test this, we applied the α7 nAChR antagonist and found that MLA significantly increased ‘local’ GABA PPRs (decreased release from ‘local’ interneurons) only in CIE-treated neurons (Fig. 3E, F). There was a significant interaction between MLA and CIE (repeated measures two-way ANOVA, F(1,20)=6.185 , p<0.05). Bonferroni post-tests confirmed a significant increase in PPR measured from CIE-exposed neurons following MLA perfusion (t=3.246, p<0.01) and also found significant differences between both AIR+MLA (N=11) and CIE+MLA (N=11; t=3.447, p<0.01) treatment groups. These data suggest that CIE increases tonic contributions by presynaptic α7 nAChR expressed by ‘local’ GABAergic synapses. This effect may serve as a ‘protective’ compensatory mechanism that acts in opposition to enhanced glutamate release and maintains local GABA release near control levels following CIE.
CIE and withdrawal does not alter the protein expression of α7 nAChR or other cholinergic ‘markers’ in the BLA
To examine potential mechanisms behind CIE-induced alterations in cholinergic projections, we measured total protein expression levels of several ‘markers’ within the BLA cholinergic system. α7 nAChR (Fig. 4A; AIR - 100.0±5.5% N=7; CIE - 92.9±5.7%, N=7; unpaired t-test, t=1.305, p>0.05), vesicular acetylcholine transporter (vAChT, Fig. 4B; AIR - 100.0±14.2% N=8, CIE – 108.8±22.9% N=7; unpaired t-test, t=0.3449, p>0.05), choline acetyltransferase (ChAT, Fig. 4C; AIR - 100.0±10.2% N=8, CIE - 94.3± 6.0% N=8; unpaired t-test, t=0.4623, p>0.05), acetylcholinesterase (AChE, Fig. 4D; AIR - 100.0±7.8% N=8, CIE 96.9±4.1% N=8; unpaired t-test, t=0.5561, p>0.05) total protein levels were not significantly different in BLA homogenates from AIR- and CIE-exposed animals. These data suggest that potentiation of presynaptic α7 nAChR activity does not result from changes in α7 nAChR expression and may not be related to alterations in acetylcholine packaging, biosynthesis, or degradation.
Figure 4.
10 day CIE/24 hr WD does not change the protein expression of cholinergic 'markers' within the BLA. Western blot analysis reveals no significant differences in protein expression of (A) α7 nAChR (~56kD; AIR - 100.0±5.5%, N=7, CIE - 92.9±5.7%, N=7; t-test, t=0.9009, p>0.05), (B) vesicular acetylcholine transporter (vAChT, ~56kD; AIR - 100.0±14.2%, N=8, CIE – 108.8±22.9%, N=7; t=0.3446, p>0.05), (C) choline acetyltransferase (ChAT, ~72kD; AIR - 100.0±10.2%, N=8, CIE - 94.3±6.0%, N=8; t=0.4623, p>0.05), and (D) acetylcholinesterase (AChE, ~68kD; AIR - 100.0±7.8%, N=8, CIE - 96.9±4.1%, N=8; t=0.5561, p>0.05). "n.s." = not significant. Band volumes were normalized to total lane protein and expressed as percent of the group mean from the AIR-exposed controls (see Methods). Each analysis includes a representative blot. Approximate position of molecular weight markers on each gel is shown to the left of the blots. * - indicates band analyzed for the statistical comparisons.
CIE increases the cholineTOTAL and acetylcholine concentration in BLA homogenates
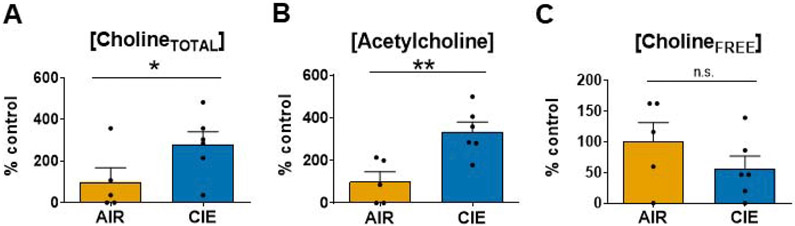
Finally, we performed a colorimetric enzymatic assay to quantify acetylcholine and choline concentrations in BLA dissected from AIR- and CIE-exposed animals. Following CIE, both the normalized cholineTOTAL (acetylcholine + cholineFREE; see Methods) concentration (Fig. 5A; AIR - 100.0±67.2% N=5; CIE - 279.8±60.9% N=6; unpaired t-test, t=1.983, p<0.05) and acetylcholine concentration (Fig. 5B; AIR - 100.0±46.2% N=5, CIE - 334.6±45.6%, N=6; unpaired t-test, t=3.578, p<0.01) were increased 3-fold above levels found in AIR-exposed controls. However, the normalized cholineFREE concentration was not significantly different between AIR and CIE protein homogenates (Fig 5C; AIR - 100.0±31.3%, N=5; CIE - 56.3±20.4%, N=6; unpaired t-test, t=1.209, p>0.05), illustrating that increases in acetylcholine levels (Fig. 5C) drove the cholineTOTAL effect (Fig. 5A). One of the pooled AIR-exposed samples expressed a significantly greater ACh concentration than the rest of the treatment group and was removed from the study (Grubb’s outlier test; p<0.05). These data support our electrophysiology findings and suggest that CIE causes an upregulation of cholinergic neurotransmission in the BLA.
Figure 5.
10 day CIE/24 hr WD significantly increases cholineTOTAL and acetylcholine concentration in the BLA. (A) CholineTOTAL levels (acetylcholine + cholineFREE; see Methods) were significantly higher in CIE homogenates (AIR - 100.0±67.2%, N=5, CIE - 279.8±60.9%, N=6; unpaired t-test, t=1.983, * - p<0.05). (B) Acetylcholine levels were also significantly higher in CIE BLA total homogenates (AIR - 100.0±46.2%, N=5; CIE - 334.6±45.6%, N=6; t-test, t=3.578, ** - p<0.01). (C) CholineFREE levels did not significantly differ between AIR and CIE BLA total homogenates, "n.s." = not significant (AIR - 100.0±31.3%, N=5; CIE - 56.3±20.4%, N=6; t-test, t=1.209, p>0.05). Substrate concentrations were standardized to homogenate total protein, followed by Min.–Max. normalization of raw absorbance values. Values are represent a percentage of the group mean from AIR-exposed controls.
Discussion
The present study sought to understand the effects of chronic ethanol exposure and withdrawal on cholinergic modulation of GABA and glutamate systems in the BLA. Our laboratory has previously shown that CIE dysregulates both glutamatergic and GABAergic synaptic responses measured during withdrawal (Christian et al., 2013; Diaz et al., 2011; Morales et al., 2018). Therefore, we utilized whole-cell patch clamp electrophysiology to dissect the impact of nicotinic cholinergic modulation glutamatergic synaptic responses elicited by electrical stimulation of the stria terminalis (ST) and ‘local'/LPC GABAergic synapses. In total, our findings indicate that ethanol withdrawal enhances the tonic activation of α7 nAChRs at both ST glutamatergic and ‘local’ GABAergic synaptic responses measured from BLA presumptive principal neurons. We should note that there is also a small population of large-soma, GABAergic interneurons within this brain region. These cells express the neuropeptide CCK (McDonald, 1985) and represent approximately half the CCK+ interneurons in the BLA (Mascagni and McDonald, 2003). Given the prevalence of CCK+ neurons, these large-soma interneurons represent ~0.6% of the neurons in the BLA and are unlikely to be represented within the recordings reported here. Regardless, chronic ethanol and withdrawal cause a three-fold increase in BLA acetylcholine concentration (Fig. 5A, B), but no changes in α7 nAChR expression (Fig. 4A), suggesting this enhanced tonic activity likely arises from increased cholinergic neurotransmission. These results also illustrate that α7 nAChR-dependent changes in neurotransmitter release probability serve as a proxy for ethanol-induced alterations in synaptic acetylcholine levels. Considering the dense cholinergic neurons project from the nucleus basalis magnocellularis (NBM) of the basal forebrain (BF) to the BLA (Woolf and Butcher, 1982; Carlsen et al., 1985; Lee and Kim, 2019), our results likely reflect a potentiation of the NBM-BLA circuit during withdrawal.
Our results demonstrate unilateral effects of PNU-282987 (α7 nAChR agonist) and MLA (α7 nAChR antagonist) in AIR-exposed and CIE neurons: α7 nAChR facilitate ST-stimulated glutamatergic responses only in AIR-exposed cells (Cheng and Yakel, 2014; Jiang and Role, 2008) while α7 nAChR antagonism reverses withdrawal-induced increases release from these synapses only in CIE-exposed cells. Since both AIR and CIE neurons are sensitive to α7 nAChR pharmacology, these results strongly suggest that chronic ethanol exposure and withdrawal upregulates cholinergic neurotransmission rather than altering α7 nAChR surface expression or conductance (Charpantier et al., 2005; Cho et al., 2005; Komal et al., 2014; McDaid et al., 2016). Notably, MLA is highly selective for α7 nAChRs over other nicotinic receptor subtypes (Drasdo et al., 1992; Palma et al., 1996) or glutamate and GABA receptors (Alkondon et al., 1992). Similarly, PNU-282987 demonstrates α7 subtype-specificity in both α7 nAChR knock-out and in vitro studies (Chan et al., 2007; Cheng and Yakel, 2014). The pharmacology of these drugs is well-characterized and provides substantial evidence that PNU-282987 and MLA are selective for α7 nAChRs at the concentrations utilized in this study.
Enhanced GABAergic function at LPC synapses decreases anxiety-like behaviors (Silberman et al., 2010), occludes fear/extinction behaviors (Skelly et al., 2016), and reduces appetitive ethanol drinking behaviors (Butler et al., 2014). CIE weakens this control by reducing GABA release from LPC synapses (Fig. 2B). However, our results with PNU-282987 suggest that α7 nAChRs do not significantly contribute to these outcomes (Fig. 2C, D). Regardless, cholinergic activation of inhibitory muscarinic receptors (M2, M4) could potentially drive LPC release probability deficits. LPCs are one of several intercalated cell masses (ICMs) surrounding the BLA classified based on their relative location to the external capsule (de Olmos et al., 1985). The majority of these ICMs, as well as a population of somatostatin-positive ‘local’ interneurons, send GABAergic projections to the basal forebrain (Busti et al., 2011; Marcellino et al., 2012; McDonald et al., 2012; Pare and Smith, 1994). Though reciprocal cholinergic connectivity from basal forebrain to LPCs has not been explored, ICM projections may regulate the activity of basal forebrain neurons by decreasing cholinergic tone in the basolateral nucleus, thereby inhibiting the formation and expression of fear-related memories (Busti et al., 2011; Marcellino et al., 2012). Since CIE attenuates GABA release probability at LPC synapses (Fig. 3B), one possible mechanism for the potentiation of cholinergic neurotransmission is through the disinhibition of cholinergic projections and subsequent facilitation of acetylcholine release. Together, these findings illustrate that the complex mechanisms underlying cholinergic connectivity to inhibitory neuronal populations remain to be precisely identified.
Unlike LPCs, ‘local’ GABAergic interneurons show no change in baseline GABA release probability following 10 days of CIE exposure (Fig. 3B). Our findings with MLA suggest that enhancement of tonic α7 nAChR activity at these ‘local’ GABAergic synapses may help maintain some aspects of GABAergic control of BLA neurons following CIE. Notably, MLA significantly decreases ‘local’ GABA release probability below baseline PPR in 82% of the CIE-exposed cells (Fig. 3E). These findings are consistent with previous work showing that basal forebrain cholinergic neurons form disynaptic connections with BLA pyramidal neurons (Lee and Kim, 2019; Muller et al., 2011) via ‘local’ interneurons and that the majority of these neurons express α7 nAChRs (Pidoplichko et al., 2013). However, it should be noted that the relative expression of α7 nAChR varies considerably among distinct ‘local’ interneuron subtypes, which are classified based on cellular physiology, protein expression, and postsynaptic targets (See Review, Krabbe et al., 2018). Parvalbumin-positive neurons (PV+) express higher levels of α7 nAChRs compared to either cholecystokinin+ and vasoactive intestinal peptide+ interneurons (Rowniak et al., 2017). PV+ interneurons constitute ~50% of the ‘local’ interneuron population and provide an even greater percentage of inhibitory output (McDonald et al., 2002) due to their high-frequency firing patterns (Rainnie et al., 2006) and perisomatic innervation of BLA pyramidal neurons (McDonald and Betette, 2001). Thus, the variability of MLA-induced changes in GABA PPR from ‘local’ synapses likely reflects heterogeneous α7 nAChR expression across different interneuron subpopulations. Cholinergic innervation PV+ interneurons and subsequent activation of α7 nAChRs likely has a significant effect on presynaptic plasticity and regulation of BLA pyramidal neuron excitability.
Potentiation of cholinergic neurotransmission and its ultimate impact on ST glutamatergic and ‘local’ GABAergic synapses occurs without significant changes in acetylcholine-associated protein expression within the BLA (Fig. 4B-D). ChAT, AChE, and vAChT are common cholinergic neuronal markers directly involved in acetylcholine synthesis, degradation, and vesicular packaging, respectively (Arvidsson et al., 1997). Our results suggest that enhanced tonic activity α7 nAChRs is likely not related to these homeostatic activities. This corroborates existing evidence that chronic ethanol does not cause degeneration of NBM projections to the BLA. Specifically, 8 weeks of chronic ethanol exposure does not alter the number of ChAT immunoreactive neurons in the NBM (Fernandes et al., 2002) or ChAT enzymatic activity in the amygdala (Arendt et al., 1989). However, the effect of chronic ethanol on this circuit varies dramatically based on dose, duration, and method of ethanol exposure (Arendt et al., 1989; Miller and Rieck, 1993). Western blots also revealed no significant changes in total α7 nAChR protein expression following chronic ethanol exposure and withdrawal (Fig. 5A). Although previous reports have questioned the selectivity of commercially available α7 nAChR primary antibodies (Garg and Loring, 2017), the primary antibody (Abcam, Ab10096) chosen for this study was previously authenticated using immunoprecipitation and MALDI mass spectrometry (Paulo et al., 2009; Uhlen et al., 2016).
Since our electrophysiology data suggested the upregulation of cholinergic neurotransmission during withdrawal, we independently measured acetylcholine and choline concentrations using an enzymatic colorimetric assay. Following CIE, there was a substantial elevation in cholineTOTAL concentration (Fig. 5A) in the BLA, driven by an increase in acetylcholine levels (Fig. 5B). Together, these results provide converging evidence that CIE causes a substantial elevation in cholinergic neurotransmission in the BLA. The hippocampus also receives cholinergic projections from the basal forebrain and, although these more prominently arise from the medial septum and vertical limb of the diagonal band (Review Ballinger et al., 2016), they are likewise susceptible to the effects of chronic ethanol and withdrawal (Arendt et al., 1989; Mark and Finn, 2002). For example, in vivo microdialysis studies consistently show a robust elevation in hippocampal acetylcholine and choline levels that peak 12–24 hours following chronic ethanol consumption (Celik et al., 2004; Imperato et al., 1998). These studies are comparable to our findings in the BLA (Fig. 5A-B), where acetylcholine concentration is 3–4 times larger than AIR-exposed controls during withdrawal.
The electrophysiology data revealed that CIE increases glutamate and GABA release via tonic α7 nAChR activation (Muller et al., 2016; Unal et al., 2015) suggesting a treatement-dependent increase in cholinergic neurotransmission. However, it is unclear whether potentiation of the NBM-BLA circuit is involved in the formation of alcohol-withdrawal induced anxiety. Previous work shows that endogenous activation of ChAT+ terminals increases BLA pyramidal neuron firing and prolongs fear extinction learning (Jiang et al., 2016), while human fMRI data illustrate basal forebrain activity increases the functional connectivity of the BLA to downstream brain regions in response to emotionally salient stimuli (Gorka et al., 2015). These findings suggest that increasing synaptic acetylcholine would increase anxiety-like behavior, potentially through the combined activity of presynaptic and postsynaptic n/mAChRs at excitatory and inhibitory inputs. We acknowledge that examining CIE-induced dysregulation of the NBM-BLA circuit via α7 nAChR modulation of presynaptic release probability is a gross oversimplification of potential dysregulation of cholinergic signaling in the BLA. BLA principal neurons express postsynaptic M1-like muscarinic receptors (McDonald et al., 2019), which regulate the intrinsic excitability of BLA principal neurons (Power J. M. and Sah, 2008; Washburn and Moises, 1992; Womble and Moises, 1993). M2-like muscarinic receptors are also found on both glutamatergic afferents to BLA neurons (Washburn and Moises, 1992) and on GABAergic terminals from local interneurons (Fajardo-Serrano et al., 2017), where they can inhibit neurotransmitter release. Despite these complexities, this is the first study to examine cholinergic modulation of BLA neurotransmission in the context of chronic ethanol and withdrawal. Our focus on α7 nicotinic receptors was necessary to elucidate both the mechanism of circuit disruption at distinct GABA/glutamate inputs and, ultimately, provides context for any CIE-dependent disruptions m/nAChR receptor function.
In conclusion, chronic ethanol exposure causes neuroadaptations in emotional centers of the brain that facilitate alcohol withdrawal-induced anxiety and subsequent relapse. Our results implicate the NBM-BLA circuit in disrupting the excitatory/inhibitory balance in the BLA, which may profoundly affect behavior during withdrawal. Future studies will bidirectionally manipulate the NBM-BLA circuit to understand the neurophysiological and behavioral effects and in vivo methods to measure extracellular ACh concentrations in AIR and CIE-exposed animals.
Highlights:
CIE causes upregulation of presynaptic α7 nAChR activity at glutamatergic and ‘local’ GABAergic synapses
CIE does not alter the protein expression of α7 nAChR or cholinergic ‘markers’ in the BLA
CIE elevates BLA acetylcholine concentrations three-fold
Acknowledgments
This work was supported by the National Institutes of Health [grants R01AA014445, R01 AA023999, P50 AA026117, R21 AA026572, T32 AA007565]. The authors have no conflict of interest.
Abbreviations
- ACh
acetylcholine
- AChE
acetylcholinesterase
- aCSF
artificial cerebral spinal fluid
- AIR
air-exposed controls
- AUD
Alcohol Use Disorder
- BEC
blood ethanol concentration
- BF
basal forebrain
- BLA
basolateral amygdala
- ChAT
choline acetyltransferase
- CIE
chronic intermittent ethanol
- EC
external capsule
- EPSC
excitatory postsynaptic current
- ICM
intercalated cell mass
- IPSC
inhibitory postsynaptic current
- LPC
lateral paracapsular cell
- mAChR
muscarinic acetylcholine receptor
- MLA
methyllycaconitine
- nAChR
nicotinic acetylcholine receptor
- NBM
nucleus basalis magnocellularis
- PPR
paired-pulse ratio
- PV+
parvalbumin-positive interneurons
- ST
stria terminalis
- vAChT
vesicular acetylcholine transporter
- WD
withdrawal
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: Authors report no conflict of interest
References
- Aitta-Aho T, Hay YA, Phillips BU, Saksida LM, Bussey TJ, Paulsen O, Apergis-Schoute J (2018). Basal forebrain and brainstem cholinergic neurons differentially impact amygdala circuits and learning-related behavior. Curr Biol, 28, 2557–2569.e4. [DOI] [PubMed] [Google Scholar]
- Alkondon M, Pereira EF, Wonnacott S, Albuquerque EX (1992). Blockade of nicotinic currents in hippocampal neurons defines methyllycaconitine as a potent and specific receptor antagonist. Mol Pharmacol, 41, 802–8. [PubMed] [Google Scholar]
- Andreasen M, Hablitz JJ (1994). Paired-pulse facilitation in the dentate gyrus: A patch-clamp study in rat hippocampus in vitro. J Neurophysiol, 72, 326–36. [DOI] [PubMed] [Google Scholar]
- Arendt T, Allen Y, Marchbanks RM, Schugens MM, Sinden J, Lantos PL, Gray JA (1989). Cholinergic system and memory in the rat: Effects of chronic ethanol, embryonic basal forebrain brain transplants and excitotoxic lesions of cholinergic basal forebrain projection system. Neuroscience, 33, 435–62. [DOI] [PubMed] [Google Scholar]
- Arvidsson U, Riedl M, Elde R, Meister B (1997). Vesicular acetylcholine transporter (vacht) protein: A novel and unique marker for cholinergic neurons in the central and peripheral nervous systems. J Comp Neurol, 378, 454–67. [PubMed] [Google Scholar]
- Barazangi N, Role LW (2001). Nicotine-induced enhancement of glutamatergic and GABAergic synaptic transmission in the mouse amygdala. J Neurophysiol, 86, 463–74. [DOI] [PubMed] [Google Scholar]
- Busti D, Geracitano R, Whittle N, Dalezios Y, Manko M, Kaufmann W, Satzler K, Singewald N, Capogna M, Ferraguti F (2011). Different fear states engage distinct networks within the intercalated cell clusters of the amygdala. J Neurosci, 31, 5131–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler TR, Chappell AM, Weiner JL (2014). Effect of beta3 adrenoceptor activation in the basolateral amygdala on ethanol seeking behaviors. Psychopharmacology (Berl), 231, 293–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsen J, Zaborszky L, Heimer L (1985). Cholinergic projections from the basal forebrain to the basolateral amygdaloid complex: A combined retrograde fluorescent and immunohistochemical study. J Comp Neurol, 234, 155–67. [DOI] [PubMed] [Google Scholar]
- Celik T, Kayir H, Ceyhan M, Demirtas S, Cosar A, Uzbay IT (2004). CPP and amlodipine alter the decrease in basal acetylcholine and choline release by audiogenic stimulus in hippocampus of ethanol-withdrawn rats in vivo. Brain Res Bull, 64, 243–9. [DOI] [PubMed] [Google Scholar]
- Chan WK, Wong PT, Sheu FS (2007). Frontal cortical alpha7 and alpha4beta2 nicotinic acetylcholine receptors in working and reference memory. Neuropharmacology, 52, 1641–9. [DOI] [PubMed] [Google Scholar]
- Charpantier E, Wiesner A, Huh KH, Ogier R, Hoda JC, Allaman G, Raggenbass M, Feuerbach D, Bertrand D, Fuhrer C (2005). Alpha7 neuronal nicotinic acetylcholine receptors are negatively regulated by tyrosine phosphorylation and src-family kinases. J Neurosci, 25, 9836–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Q, Yakel JL (2014). Presynaptic alpha7 nicotinic acetylcholine receptors enhance hippocampal mossy fiber glutamatergic transmission via PKA activation. J Neurosci, 34, 124–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho CH, Song W, Leitzell K, Teo E, Meleth AD, Quick MW, Lester RA (2005). Rapid upregulation of alpha7 nicotinic acetylcholine receptors by tyrosine dephosphorylation. J Neurosci, 25, 3712–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian DT, Alexander NJ, Diaz MR, McCool BA (2013). Thalamic glutamatergic afferents into the rat basolateral amygdala exhibit increased presynaptic glutamate function following withdrawal from chronic intermittent ethanol. Neuropharmacology, 65, 134–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian DT, Alexander NJ, Diaz MR, Robinson S, McCool BA (2012). Chronic intermittent ethanol and withdrawal differentially modulate basolateral amygdala AMPA-type glutamate receptor function and trafficking. Neuropharmacology, 62, 2430–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corradi J, Bouzat C (2016). Understanding the bases of function and modulation of alpha7 nicotinic receptors: Implications for drug discovery. Mol Pharmacol, 90, 288–99. [DOI] [PubMed] [Google Scholar]
- Crouse RB, Kim K, Batchelor HM, Girardi EM, Kamaletdinova R, Chan J, Rajebhosale P, Pittenger ST, Role LW, Talmage DA, Jing M, Li Y, Gao XB, Mineur YS, Picciotto MR (2020). Acetylcholine is released in the basolateral amygdala in response to predictors of reward and enhances the learning of cue-reward contingency. Elife, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Olmos J, Alheid GF, Beltramino CA 1985. Amygdala In: Paxinos G, editor. The rat nervous system, Academic Press. [Google Scholar]
- Diaz MR, Christian DT, Anderson NJ, McCool BA (2011). Chronic ethanol and withdrawal differentially modulate lateral/basolateral amygdala paracapsular and local GABAergic synapses. J Pharmacol Exp Ther, 337, 162–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrunz LE, Stevens CF (1997). Heterogeneity of release probability, facilitation, and depletion at central synapses. Neuron, 18, 995–1008. [DOI] [PubMed] [Google Scholar]
- Drasdo A, Caulfield M, Bertrand D, Bertrand S, Wonnacott S (1992). Methyl lycaconitine: A novel nicotinic antagonist. Mol Cell Neurosci, 3, 237–43. [DOI] [PubMed] [Google Scholar]
- Fajardo-Serrano A, Liu L, Mott DD, McDonald AJ (2017). Evidence for m(2) muscarinic receptor modulation of axon terminals and dendrites in the rodent basolateral amygdala: An ultrastructural and electrophysiological analysis. Neuroscience, 357, 349–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes PA, Ribeiro AM, Pereira RF, Marra HL, Pittella JE (2002). Chronic ethanol intake and ageing effects on cortical and basal forebrain cholinergic parameters: Morphometric and biochemical studies. Addict Biol, 7, 29–36. [DOI] [PubMed] [Google Scholar]
- Fioravante D, Chu Y, Myoga MH, Leitges M, Regehr WG (2011). Calcium-dependent isoforms of protein kinase c mediate posttetanic potentiation at the calyx of held. Neuron, 70, 1005–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg BK, Loring RH (2017). Evaluating commercially available antibodies for rat alpha7 nicotinic acetylcholine receptors. J Histochem Cytochem, 65, 499–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Richardson HN, Cole M, Koob GF (2008). Vapor inhalation of alcohol in rats. Curr Protoc Neurosci, Chapter 9, Unit 9.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gioia DA, Alexander N, McCool BA (2017). Ethanol mediated inhibition of synaptic vesicle recycling at amygdala glutamate synapses is dependent upon munc13-2. Front Neurosci, 11, 424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold PE (2003). Acetylcholine modulation of neural systems involved in learning and memory. Neurobiol Learn Mem, 80, 194–210. [DOI] [PubMed] [Google Scholar]
- Gorka AX, Knodt AR, Hariri AR (2015). Basal forebrain moderates the magnitude of task-dependent amygdala functional connectivity. Soc Cogn Affect Neurosci, 10, 501–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imperato A, Dazzi L, Carta G, Colombo G, Biggio G (1998). Rapid increase in basal acetylcholine release in the hippocampus of freely moving rats induced by withdrawal from long-term ethanol intoxication. Brain Res, 784, 347–50. [DOI] [PubMed] [Google Scholar]
- Jiang L, Kundu S, Lederman JD, Lopez-Hernandez GY, Ballinger EC, Wang S, Talmage DA, Role LW (2016). Cholinergic signaling controls conditioned fear behaviors and enhances plasticity of cortical-amygdala circuits. Neuron, 90, 1057–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Role LW (2008). Facilitation of cortico-amygdala synapses by nicotine: Activity-dependent modulation of glutamatergic transmission. J Neurophysiol, 99, 1988–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein RC, Yakel JL (2006). Functional somato-dendritic alpha7-containing nicotinic acetylcholine receptors in the rat basolateral amygdala complex. J Physiol, 576, 865–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komal P, Gudavicius G, Nelson CJ, Nashmi R (2014). T-cell receptor activation decreases excitability of cortical interneurons by inhibiting alpha7 nicotinic receptors. J Neurosci, 34, 22–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krabbe S, Grundemann J, Luthi A (2018). Amygdala inhibitory circuits regulate associative fear conditioning. Biol Psychiatry, 83, 800–809. [DOI] [PubMed] [Google Scholar]
- Lack AK, Christian DT, Diaz MR, McCool BA (2009). Chronic ethanol and withdrawal effects on kainate receptor-mediated excitatory neurotransmission in the rat basolateral amygdala. Alcohol, 43, 25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lack AK, Diaz MR, Chappell A, DuBois DW, McCool BA (2007). Chronic ethanol and withdrawal differentially modulate pre- and postsynaptic function at glutamatergic synapses in rat basolateral amygdala. J Neurophysiol, 98, 3185–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Kim JH (2019). Basal forebrain cholinergic-induced activation of cholecystokinin inhibitory neurons in the basolateral amygdala. Exp Neurobiol, 28, 320–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcellino D, Frankowska M, Agnati L, Perez de la Mora M, Vargas-Barroso V, Fuxe K, Larriva-Sahd J (2012). Intercalated and paracapsular cell islands of the adult rat amygdala: A combined rapid-golgi, ultrastructural, and immunohistochemical account. Neuroscience, 226, 324–47. [DOI] [PubMed] [Google Scholar]
- Mark GP, Finn DA (2002). The relationship between hippocampal acetylcholine release and cholinergic convulsant sensitivity in withdrawal seizure-prone and withdrawal seizure-resistant selected mouse lines. Alcohol Clin Exp Res, 26, 1141–52. [DOI] [PubMed] [Google Scholar]
- Marowsky A, Yanagawa Y, Obata K, Vogt KE (2005). A specialized subclass of interneurons mediates dopaminergic facilitation of amygdala function. Neuron, 48, 1025–37. [DOI] [PubMed] [Google Scholar]
- Mascagni F, McDonald AJ (2003). Immunohistochemical characterization of cholecystokinin containing neurons in the rat basolateral amygdala. Brain Res, 976, 171–84. [DOI] [PubMed] [Google Scholar]
- McCaul ME, Hutton HE, Stephens MA, Xu X, Wand GS (2017). Anxiety, anxiety sensitivity, and perceived stress as predictors of recent drinking, alcohol craving, and social stress response in heavy drinkers. Alcohol Clin Exp Res, 41, 836–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaid J, Abburi C, Wolfman SL, Gallagher K, McGehee DS (2016). Ethanol-induced motor impairment mediated by inhibition of alpha7 nicotinic receptors. J Neurosci, 36, 7768–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AJ (1982). Neurons of the lateral and basolateral amygdaloid nuclei: A golgi study in the rat. J Comp Neurol, 212, 293–312. [DOI] [PubMed] [Google Scholar]
- McDonald AJ (1985). Morphology of peptide-containing neurons in the rat basolateral amygdaloid nucleus. Brain Res, 338, 186–91. [DOI] [PubMed] [Google Scholar]
- McDonald AJ (1992). Projection neurons of the basolateral amygdala: A correlative golgi and retrograde tract tracing study. Brain Res Bull, 28, 179–85. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Betette RL (2001). Parvalbumin-containing neurons in the rat basolateral amygdala: Morphology and co-localization of Calbindin-D(28k). Neuroscience, 102, 413–25. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Jones GC, Mott DD (2019). Diverse glutamatergic inputs target spines expressing m1 muscarinic receptors in the basolateral amygdala: An ultrastructural analysis. Brain Res, 1722, 146349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F, Zaric V (2012). Subpopulations of somatostatin-immunoreactive non-pyramidal neurons in the amygdala and adjacent external capsule project to the basal forebrain: Evidence for the existence of GABAergic projection neurons in the cortical nuclei and basolateral nuclear complex. Front Neural Circuits, 6, 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AJ, Muller JF, Mascagni F (2002). GABAergic innervation of alpha type ii calcium/calmodulin-dependent protein kinase immunoreactive pyramidal neurons in the rat basolateral amygdala. J Comp Neurol, 446, 199–218. [DOI] [PubMed] [Google Scholar]
- McGaugh JL (2004). The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu Rev Neurosci, 27, 1–28. [DOI] [PubMed] [Google Scholar]
- McGinnis MM, Parrish BC, Chappell AM, Alexander NJ, McCool BA (2020). Chronic ethanol differentially modulates glutamate release from dorsal and ventral prefrontal cortical inputs onto rat basolateral amygdala principal neurons. eNeuro, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MW, Rieck RW (1993). Effects of chronic ethanol administration on acetylcholinesterase activity in the somatosensory cortex and basal forebrain of the rat. Brain Res, 627, 104–12. [DOI] [PubMed] [Google Scholar]
- Mineur YS, Fote GM, Blakeman S, Cahuzac EL, Newbold SA, Picciotto MR (2016). Multiple nicotinic acetylcholine receptor subtypes in the mouse amygdala regulate affective behaviors and response to social stress. Neuropsychopharmacology, 41, 1579–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales M, McGinnis MM, McCool BA (2015). Chronic ethanol exposure increases voluntary home cage intake in adult male, but not female, long-evans rats. Pharmacol Biochem Behav, 139, 67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales M, McGinnis MM, Robinson SL, Chappell AM, McCool BA (2018). Chronic intermittent ethanol exposure modulation of glutamatergic neurotransmission in rat lateral/basolateral amygdala is duration-, input-, and sex-dependent. Neuroscience, 371, 277–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller JF, Mascagni F, McDonald AJ (2011). Cholinergic innervation of pyramidal cells and parvalbumin-immunoreactive interneurons in the rat basolateral amygdala. J Comp Neurol, 519, 790–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller JF, Mascagni F, Zaric V, Mott DD, McDonald AJ (2016). Localization of the M2 muscarinic cholinergic receptor in dendrites, cholinergic terminals, and noncholinergic terminals in the rat basolateral amygdala: An ultrastructural analysis. J Comp Neurol, 524, 2400–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva F, Nibbio G, Vizzuso P, Jaretti Sodano A, Ostacoli L, Carletto S, Picci RL (2018). Gender differences in anxiety and depression before and after alcohol detoxification: Anxiety and depression as gender-related predictors of relapse. Eur Addict Res, 24, 163–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palma E, Bertrand S, Binzoni T, Bertrand D (1996). Neuronal nicotinic alpha 7 receptor expressed in xenopus oocytes presents five putative binding sites for methyllycaconitine. J Physiol, 491 (Pt 1), 151–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pare D, Smith Y (1994). GABAergic projection from the intercalated cell masses of the amygdala to the basal forebrain in cats. J Comp Neurol, 344, 33–49. [DOI] [PubMed] [Google Scholar]
- Paulo JA, Brucker WJ, Hawrot E (2009). Proteomic analysis of an alpha7 nicotinic acetylcholine receptor interactome. J Proteome Res, 8, 1849–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pidoplichko VI, Prager EM, Aroniadou-Anderjaska V, Braga MF (2013). Alpha7-containing nicotinic acetylcholine receptors on interneurons of the basolateral amygdala and their role in the regulation of the network excitability. J Neurophysiol, 110, 2358–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power AE, McGaugh JL (2002a). Cholinergic activation of the basolateral amygdala regulates unlearned freezing behavior in rats. Behav Brain Res, 134, 307–15. [DOI] [PubMed] [Google Scholar]
- Power AE, McGaugh JL (2002b). Phthalic acid amygdalopetal lesion of the nucleus basalis magnocellularis induces reversible memory deficits in rats. Neurobiol Learn Mem, 77, 372–88. [DOI] [PubMed] [Google Scholar]
- Power JM, Sah P (2008). Competition between calcium-activated k+ channels determines cholinergic action on firing properties of basolateral amygdala projection neurons. J Neurosci, 28, 3209–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainnie DG, Mania I, Mascagni F, McDonald AJ (2006). Physiological and morphological characterization of parvalbumin-containing interneurons of the rat basolateral amygdala. J Comp Neurol, 498, 142–61. [DOI] [PubMed] [Google Scholar]
- Robinson SL, Alexander NJ, Bluett RJ, Patel S, McCool BA (2016). Acute and chronic ethanol exposure differentially regulate CB1 receptor function at glutamatergic synapses in the rat basolateral amygdala. Neuropharmacology, 108, 474–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J, Wiener SG, Bloom FE (1979). Long-term ethanol administration methods for rats: Advantages of inhalation over intubation or liquid diets. Behav Neural Biol, 27, 466–86. [DOI] [PubMed] [Google Scholar]
- Rowniak M, Kolenkiewicz M, Kozlowska A (2017). Parvalbumin, but not calretinin, neurons express high levels of alpha1-containing GABAA receptors, alpha7-containing nicotinic acetylcholine receptors and D2-dopamine receptors in the basolateral amygdala of the rat. J Chem Neuroanat, 86, 41–51. [DOI] [PubMed] [Google Scholar]
- Seguela P, Wadiche J, Dineley-Miller K, Dani JA, Patrick JW (1993). Molecular cloning, functional properties, and distribution of rat brain alpha 7: A nicotinic cation channel highly permeable to calcium. J Neurosci, 13, 596–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberman Y, Ariwodola OJ, Chappell AM, Yorgason JT, Weiner JL (2010). Lateral paracapsular GABAergic synapses in the basolateral amygdala contribute to the anxiolytic effects of beta 3 adrenoceptor activation. Neuropsychopharmacology, 35, 1886–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberman Y, Shi L, Brunso-Bechtold JK, Weiner JL (2008). Distinct mechanisms of ethanol potentiation of local and paracapsular GABAergic synapses in the rat basolateral amygdala. J Pharmacol Exp Ther, 324, 251–60. [DOI] [PubMed] [Google Scholar]
- Skelly MJ, Chappell AM, Ariwodola OJ, Weiner JL (2016). Behavioral and neurophysiological evidence that lateral paracapsular GABAergic synapses in the basolateral amygdala contribute to the acquisition and extinction of fear learning. Neurobiol Learn Mem, 127, 10–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez-Alvarez MM, Pham D, Prostov MY, Prostov YI (2012). Statistical approach to normalization of feature vectors and clustering of mixed datasets. The Royal Society, 468, 2630–2651. [Google Scholar]
- Uhlen M, Bandrowski A, Carr S, Edwards A, Ellenberg J, Lundberg E, Rimm DL, Rodriguez H, Hiltke T, Snyder M, Yamamoto T (2016). A proposal for validation of antibodies. Nat Methods, 13, 823–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unal CT, Pare D, Zaborszky L (2015). Impact of basal forebrain cholinergic inputs on basolateral amygdala neurons. J Neurosci, 35, 853–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washburn MS, Moises HC (1992). Muscarinic responses of rat basolateral amygdaloid neurons recorded in vitro. J Physiol, 449, 121–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willinger U, Lenzinger E, Hornik K, Fischer G, Schonbeck G, Aschauer HN, Meszaros K (2002). Anxiety as a predictor of relapse in detoxified alcohol-dependent patients. Alcohol Alcohol, 37, 609–12. [DOI] [PubMed] [Google Scholar]
- Wise T, Patrick F, Meyer N, Mazibuko N, Oates AE, van der Bijl AHM, Danjou P, O’Connor SM, Doolin E, Wooldridge C, Rathjen D, Macare C, Williams SCR, Perkins A, Young AH (2020). Cholinergic modulation of disorder-relevant neural circuits in generalized anxiety disorder. Biol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womble MD, Moises HC (1993). Muscarinic modulation of conductances underlying the afterhyperpolarization in neurons of the rat basolateral amygdala. Brain Res, 621, 87–96. [DOI] [PubMed] [Google Scholar]
- Woodruff AR, Sah P (2007). Inhibition and synchronization of basal amygdala principal neuron spiking by parvalbumin-positive interneurons. J Neurophysiol, 98, 2956–61. [DOI] [PubMed] [Google Scholar]
- Woolf NJ, Butcher LL (1982). Cholinergic projections to the basolateral amygdala: A combined evans blue and acetylcholinesterase analysis. Brain Res Bull, 8, 751–63. [DOI] [PubMed] [Google Scholar]
- Zhu PJ, Stewart RR, McIntosh JM, Weight FF (2005). Activation of nicotinic acetylcholine receptors increases the frequency of spontaneous GABAergic ipscs in rat basolateral amygdala neurons. J Neurophysiol, 94, 3081–91. [DOI] [PubMed] [Google Scholar]