Abstract
Cyclic dinucleotide (CDN) agonists of stimulator of interferon genes (STING) hold great therapeutic potential, but their activity is hindered by poor drug-like properties that restrict cytosolic bioavailability. Here, we address this challenge through the synthesis and evaluation of a novel series of PEGMA-co-DEAEMA-co-BMA copolymers with pH-responsive, membrane-destabilizing activity to enhance intracellular delivery of the CDN, cGAMP. We synthesized copolymers with PEGMA of two different molecular weights (300 and 950 Da) and over a range of PEG mass fraction and polymer molecular weight, and elucidated relationships between copolymer structure, self-assembly, endosomal escape, and cGAMP activity. We identified a subset of polymers that self-assembled into 50-800 nm nanoparticles and could be loaded with cGAMP via a simple mixing strategy, resulting in significantly enhanced immunostimulatory activity. We found that increased cGAMP activity was highly correlated with the capacity of carriers to enhance intracellular CDN uptake and to promote endosomal destabilization, findings that establish efficient cytosolic delivery as a criterion for CDN carriers. Additionally, we demonstrated that a lead CDN carrier formulation could enhance STING activation in vivo in a model of intratumoral immunotherapy. Collectively, these investigations demonstrate the utility of PEGMA-co-DEAEMA-co-BMA copolymers as carriers for CDNs and potentially other cytosolically-acting drug cargo.
Keywords: graft copolymer, self-assembly, polymer nanoparticle, endosomal escape, stimulator of interferon genes (STING)
Introduction
Cyclic dinucleotide (CDNs) agonists of the stimulator of interferon genes (STING) pathway have recently emerged as a promising class of therapeutic with broad potential applications as antiviral agents, cancer immunotherapeutics, and vaccine adjuvants.[1] STING recognizes a number of similar but chemically distinct CDNs,[2] and, upon binding, initiates a strong type-I interferon (IFN-I)-driven innate immune response that has been shown to be critical to many immune-mediated responses,[3] including response to immune checkpoint inhibitors,[4] protection from pathogen infection,[1b] and the efficacy of some vaccine adjuvants.[5] CDNs were first discovered as second messengers in bacteria. CDNs, specifically 2’3’-cGAMP, are also produced in mammalian cells by the enzyme cyclic GMP-AMP synthase (cGAS) upon cGAS binding to aberrant, cytosolic double-stranded DNA.[3b, 6] However, with respect to their use as therapeutics, CDNs are highly polar, anionic molecules with poor cellular membrane permeability that restricts both their pharmacological properties (e.g., half-life), and, critically, their ability to access the cytosol for binding to STING, which is localized on the ER membrane.[1c, 7] While direct intratumoral injection of CDNs is being explored in immuno-oncology clinical trials,[1a] the inability of CDNs to diffuse across cell membranes has nonetheless limited the potency, efficacy, and therapeutic utility of this emerging and unique class of immunotherapeutic.
To address this challenge, a number of research groups have recently developed drug carrier platforms to enhance the intracellular delivery of CDNs.[7b, 8] A number of different formulations of liposomal carriers have been explored,[7b, 8i] most commonly exploiting cationic lipids to facilitate loading of anionic CDNs and enhance cytosolic delivery.[8c-e] Several groups have also used biodegradable polymeric nano- and microparticles for improving CDN delivery, including poly(beta amino esters),[8a] acetylated dextran,[8b] and PLGA.[9] Our group has recently described the design of polymer vesicles (i.e., polymersomes) that have an aqueous core and a vesicle membrane comprising amphiphilic diblock copolymer chains with pH-responsive membrane-destabilizing activity.[8g] At physiologic pH, the membrane-destabilizing segments are sequestered in the polymersome bilayer, shielded by a poly(ethylene glycol) (PEG) corona. In response to endosomal acidification, the polymersomes disassemble to release CDNs and reveal membrane lytic domains that mediate endosomal escape of CDNs to the cytosol, resulting in a multiple order of magnitude enhancement of the activity of cGAMP.
Critical to pH-responsive, endosomolytic property of the carrier is a copolymer of 2-diethylaminoethyl methacrylate (DEAEMA), which acts as a pH-sensor, and butyl methacrylate (BMA), which facilitates disruption of lipid bilayer membranes.[8g, 10] Through careful control of both block ratio and overall molecular weight, we found that only relatively low molecular weight chains with a 2kDa mPEG first block and a 5kDa DEAEMA0.6-co-BMA0.4 second block self-assembled into vesicles. However, such short chains displayed weak membrane-destabilizing properties. This prompted us to copolymerize thiol-reactive pyridyl disulfide methacrylate (PDSMA) monomers into the second block to facilitate chain crosslinking, which resulted in increased molecular weight and, hence, endosomolytic capacity and CDN activity. Notably, the in vitro activity of CDNs loaded into crosslinked polymersomes was substantially greater than that achieved using polymer micelles with comparable endosomolytic properties, indicating that both endosomal escape and self-assembled morphology are important determinants of CDN delivery. While this approach offers the advantage of yielding low molecular weight species upon reduction of disulfide crosslinks that may be less toxic, the crosslinking step and the process of removing crosslinking byproducts creates additional steps that adds time and complexity into the formulation process. Furthermore, to achieve high loading efficiency we used a modified direct hydration process in which the CDN in an aqueous phase was added to a highly concentrated, gel-like solution of polymer swollen in ethanol. While this is effective, the scalability of this loading strategy may be a translational concern and, ideally, CDNs could be loaded into nanoparticles via a simple mixing strategy without the need for additional purification. Therefore, the objective of this work was to circumvent these limitations by exploring a novel architecture of endosomolytic polymers as carriers for CDN STING agonists.
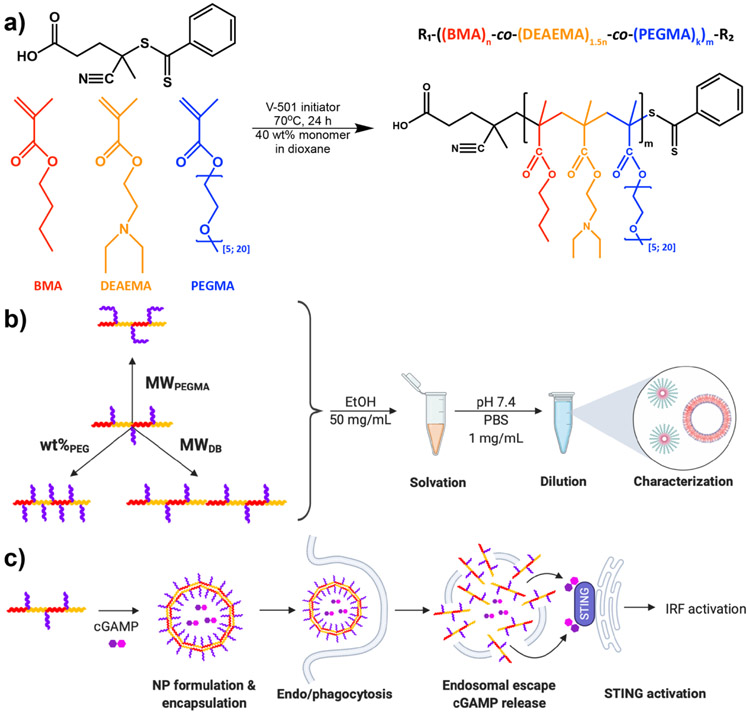
We rationalized that the properties and activities of crosslinked, low molecular weight PEG-block-[DEAMA-co-BMA] chains might be effectively mimicked using a copolymer architecture whereby PEG-functionalized methacylate (PEGMA) monomers were copolymerized with DEAMEA and BMA, yielding PEGMA-co-DEAEMA-co-BMA graft copolymers (Figure 1). By controlling both PEGMA length and composition, the relative hydrophilic mass fraction can be tuned, offering a strategy to modulate self-assembly behavior, while overall polymer molecular weight, a key determinant of membrane-destabilizing activity,[8g, 11] can be controlled through the degree of polymerization. We therefore synthesized a library of 24 different PEGMA-co-DEAEMA-co-BMA polymers comprising PEGMA of two different lengths over a range of composition and overall chain molecular weight, and, by elucidating relationships between polymer properties, endosomal escape, and CDN activity in vitro and in vivo, identified new polymeric carriers for CDN delivery.
Figure 1. Synthesis and evaluation of PEGMA-co-DEAMEA-co-BMA copolymers for cGAMP delivery.
(a) RAFT polymerization scheme used for synthesis of a series of PEGMA-co-DEAMEA-co-BMA of variable PEGMA molecular weight (MWPEGMA), PEG weight fraction (wt%PEG), and molecular weight of the DEAMEA and BMA component of the copolymer (MWDB). (b) Schematic representation of approach used to formulate and evaluate the self-assembly properties of copolymers and to (c) screen the capacity of copolymers to enhancing the intracellular uptake and cytosolic delivery of cGAMP to activate the STING pathway. Image created with Biorender.com.
Materials and Methods
Synthesis of poly(ethylene glycol) methacrylate-co-(2-diethylamino) ethyl methacrylate-co-butyl methacrylate (PEGMA-co-DEAEMA-co-BMA).
Poly(ethylene glycol) methacrylate-co-(2-diethylamino) ethyl methacrylate-co-butyl methacrylate (PEGMA-co-DEAEMA-co-BMA) polymers were synthesized by reversible addition-fragmentation chain transfer (RAFT) polymerization. The monomers poly(ethylene glycol) methyl ether methacrylate (PEGMA, Mn = 950 Da or 300 Da, Millipore-Sigma), 2-(diethylamino) ethyl methacrylate (DEAEMA, TCI Co. Ltd.), and butyl methacrylate (BMA, Millipore-Sigma) were combined in 2,4-dioxane (40 wt% monomer) and polymerized using 4-cyano-4-(phenylcarbonothioylthio) pentanoic acid (CPADB, Millipore-Sigma) as the chain transfer agent (CTA) and 4,4’-Azobis(4-cyanovaleric acid) (V501, MP Biomedical LLC) as the initiator under an inert nitrogen atmosphere at 70°C for 24 h. The molar feed ratio of DEAEMA to BMA was 60:40 and the polymerization was performed using a CTA to initiator ratio of 1:5. The amount in the reaction PEGMA was varied to be between 20-30 wt% of the total monomer content and the theoretical degree of polymerization (DP) for DEAEMA and BMA based on 60:40 monomer ratio was varied between 90 and 600, assuming a 60% conversion rate.
Following the reaction, the mixture was dispersed in acetone, and different purification methods were used for copolymers containing PEGMA of 300 or 950 Da to account for differences in pentane solubility of monomers. Polymers synthesized with 300 Da PEGMA were precipitated in pentane (Millipore-Sigma) three times, and dried in a Symphony vacuum chamber overnight. Polymers containing 950 Da PEGMA were precipitated once in pentane, re-suspended in acetone, and dialyzed (SnakeSkin™ Dialysis Tubing 3kDa or 10kDa MWCO, Thermo-Fisher) in acetone twice and DI water once. The purified product was then lyophilized (Labconco FreeZone 4.5) and stored at 4°C. All polymers were stored at 4°C until use.
The composition of copolymers was analyzed by 1H-NMR spectroscopy (Bruker, AV 400) in CDCl3 (Cambridge Isotope Factories). A representative 1H-NMR spectrum is shown in Figure S1 (Supporting Information). The degree of polymerization (DP) for each monomer was determined based on the ratio of monomer-specific protons to aromatic protons. Polymer molecular weight (Mw) was estimated based on the degree of polymerization and monomer composition.
Formulation and characterization of polymer nanoparticles.
Polymeric nanoparticles were prepared using the solvent displacement method.[12] Briefly, polymers were dissolved in ethanol at 50 mg/mL and allowed to temperature-equilibrate at 37 °C for at least 20 minutes. Ethanolic polymer stock solutions were then rapidly mixed with PBS (pH 7.4, GIBCO) at a 1:50 polymer:buffer ratio to a final polymer concentration of 1 mg/mL. The resultant solution was sonicated for 15 minutes. In the case of cGAMP loaded nanoparticles, polymer solution was diluted into a cGAMP solution in PBS (10 μg/mL). Nanoparticles were characterized using dynamic light scattering (Zetasizer Nano ZS, Malvern Panalytical) and transmission electron microscopy (TEM, FEI Tecnai Osiris). For TEM, colloidal solutions were drop cast onto Carbon Type B grids (TedPella) and stained with a solution of 1% methylamine tungstate before TEM imaging.
Erythrocyte Hemolysis Assay.
The ability of the polymers to induce pH-dependent disruption of lipid bilayer membranes was evaluated using an erythrocyte hemolysis assay as previously described.[13] Briefly, erythrocytes isolated from de-identified human whole blood samples (Vanderbilt Hematology Core Laboratory) were pelleted at 500 RCF and washed three times with PBS (pH 7.4). Thereafter, the erythrocytes were re-suspended in PBS of varying pH associated with endo/lysosomal trafficking (pH 7.4, 6.6 and 5.8). Erythrocytes were treated with polymers at 20 μg/ml and incubated at 37 °C for 2 h. They were then pelleted at 700 RCF and the amount of hemoglobin leakage into the supernatant was quantified via absorbance spectroscopy (Synergy H1, λ = 575 nm) and normalized to 100% erythrocyte lysis with 1% Triton X-100.
Gal8 Recruitment Assay.
Gal8 recruitment assays were performed as previously described[11] with minor modifications as follows. MDA-MB-231 cells stably expressing Gal8-YFP were plated in a half-area 96-well plates (Corning, catalog number 4580) at a confluency of ~40% and allowed to adhere overnight. Media was replaced with 90 μL imaging media (FluoroBrite DMEM supplemented with 25 mM HEPES, 10% FBS, and 1% Pen/Strep). To each well, 10 μL nanoparticle solution in PBS (Gibco) or PBS alone was added for a final well volume of 100 μL and final polymer concentration of 50 μg/mL. Cells were incubated overnight and imaged 17 hours after treatment. Images were acquired with Nikon C1si+ confocal microscope system on a Nikon Eclipse Ti-0E inverted microscope base, Plan Apo VC 20× objective, Galvano scanner, and 408/488/543 dichroic mirror. Images were exported then analyzed using a blinded, automated MATLAB script which measured total florescence intensity with Gal8+ spots, then divided integrated pixel intensity by total cell area per image.
ISG Reporter Cell Assay.
The ability of polymeric carriers to improve cGAMP delivery was assessed using THP1-Blue ISG reporter cells (Invitrogen) as per manufacturer’s instructions. Cells were seeded at 10,000 cells/well in a 96 well plate and treated with free cGAMP or nanoparticle encapsulated cGAMP for 24 hours, over a concentration range of 500-0.1 ng/mL. Relative levels of interferon regulatory factor (IRF) activation was determined through quantification of secreted embryonic alkaline phosphatase (SEAP) in media with QUANTI-Blue reagent (Invitrogen).
Analysis of CDN Uptake.
Nanoparticles were formulated as described above via dissolution of ethanolic polymer stocks into a solution containing 10 μg/mL cGAMP and 5 μg/mL cdGMP-Dy547 (Biolog), a fluorescent CDN analog. THP1 Blue ISG cells were seeded at concentrations of 100,000 cells/well in 12 well plates and incubated with indicated formulations at a concentration of 100 ng/mL cGAMP for 4 hrs. Cells were then harvested, washed, and analyzed on a BD LSR II flow cytometer to evaluate relative levels of cdGMP-Dy547 internalization via quantification of median fluorescent intensity (MFI). Flow cytometry data were analyzed using FlowJo version 10 (Tree Star Inc).
Animal Care and Experimentation.
Female C57BL/6J mice (6-8 weeks old) were obtained from The Jackson Laboratory (Bar Harbor, ME). All animals were maintained at the animal facilities of Vanderbilt University under specific pathogen-free conditions. All animal experiments were approved by the Vanderbilt University Institutional Animal Care and Use Committee (IACUC).
Evaluation of in vivo CDN activity.
6-8 weeks old female C57BL6/J mice (Jackson Labs, Bar Harbor, ME) were inoculated with 5x105 B16-F10 cells subcutaneously into the right flank region. Tumor volume was measured every 3 days via caliper measurements, and volumes were calculated using Vtumor = L × W2 × 0.5, in which Vtumor is tumor volume, L is tumor length and W is tumor width. When tumors reached approximately 100 mm3, mice were intratumorally administered with free cGAMP (10 μg), indicated cGAMP-loaded nanoparticle formulations (10 μg cGAMP), or PBS (vehicle). Mice were euthanized 4 h post-treatment and tumors were harvested and snap frozen until processing. Tumor tissue was homogenized using a gentleMACS™ Octo Dissociator (Miltenyi Biotec) and total RNA was isolated using the RNeasy Mini kit (Qiagen, Germantown, MD). The RNA was reverse transcribed using iScript cDNA synthesis kit (Bio-Rad) as per manufacturer’s instructions. qRT-PCR was performed using Taqman primers (Applied Biosystems) on a CFX96 Thermocycler (Bio-Rad) to evaluate Ifnb1 (Mm00439552_s1), Cxcl10 (Mm00445235_m1), and Tnf (Mm00443258_m1) expression levels and was normalized to Hmbs (Mm01143545_m1) housekeeping gene via the ΔΔCT method.
Evaluation of PEGMA-co-DEAEMA-co-BMA for intratumoral cGAMP administration.
6-8-week-old female C57BL6/J mice (Jackson Labs, Bar Harbor, ME) were inoculated with 5x105 B16-F10 cells subcutaneously into the right flank region. On day 11 (average size of 90 mm3), mice were randomized and treated intratumorally with vehicle or formulations containing 10 μg cGAMP either free of encapsulated into lead nanoparticle formulations. Treatment was repeated on day 14 and day 17. Tumor volume was measured every 3 days via caliper measurements, and mice were sacrificed at humane endpoint when tumor volume reached 2000 mm3.
Statistics.
Statistical analyses were performed using GraphPad Prism (version 8) software. Significance was determined using one-way ANOVA with Tukey’s multiple comparisons test unless otherwise noted. Sample sizes are indicated in Figure legends of the appropriate experiments. Values represent experimental means, and error bars represent S.D. unless otherwise noted. ****P<0.0001, ***P<0.001, **P<0.01, *P<0.05.
Results and Discussion
RAFT synthesis of PEGMA-co-DEAEMA-co-BMA polymers.
Our group, and others, have previously described the synthesis of PEG-block-DEAEMA-co-BMA and related classes of endosomolytic diblock polymers for drug delivery applications.[8g, 14] However, the synthesis of compositionally similar but architecturally distinct PEGMA-co-DEAEMA-co-BMA random copolymers, whereby copolymerization of PEGMA generates pendant PEG grafts interspersed along a DEAEMA-co-BMA backbone, has not been described. We postulated that this grafted copolymer architecture might have unique self-assembly and colloid properties with advantages as carriers for CDNs. We therefore synthesized a library of 24 copolymers using 300 or 950 Da PEGMA monomers and controlled feed ratios and the degree of polymerization to generate polymers with 20, 25, and 30 wt% PEG over a molecular weight range corresponding to 9-60 kDa of the endosomolytic component of the backbone (i.e., DEAEMA and BMA monomers at a 60:40 ratio) (Figure 1). Polymer composition, DEAEMA:BMA ratio, the degree of polymerization of each monomer, and the estimated molecular weight of the endosomolytic component (MWDB) are summarized in Table S1 (Supporting Information).
PEG graft molecular weight and weight fraction dictate graft copolymer self-assembly.
We next evaluated the effect of PEGMA length (300 or 950 Da), graft density (i.e., PEG mass fraction), and overall polymer molecular weight on the size of self-assembled particles formed upon dissolution in aqueous media using dynamic light scattering (Figure 2A, Figure S2 Supporting Information). First, we observed striking differences in the particle size upon self-assembly of otherwise analogous polymers with 300 Da PEGMA grafts relative to those with 950Da PEGMA grafts. At 20, 25, and 30 wt% and across a Mn ranging between 9-60 kDa, all polymers with 950 Da PEGMA assembled into sub-100 nm, and mostly sub-50 nm, particles. By contrast, copolymers comprising 300 Da PEGMA self-assembled into particles ranging between ~30-600 nm depending mostly on PEG wt%. Particle size tended to decrease with increasing PEG wt%, while overall polymer molecular weight had mostly modest effects on particle size for a fixed PEG content. We also evaluated particle morphology by transmission electron microscopy (TEM) to further investigate effects of polymer properties on self-assembly. Polymers with 950 Da PEGMA assembled into small aggregates, potentially spherical or cylindrical micelles, consistent with sizes measured by DLS (Figure S3, Supporting Information), with the notable exception of polymers of 9kDa with 20 wt% PEG, which assembled mostly into vesicles (polymersomes). We postulate that the micelle-dominant morphology can be ascribed to repulsive interactions between the larger 950 Da PEG coronas – however, at lower PEG wt%, a bilayer morphology is preferred to alleviate the poorer shielding of the hydrophobic DEAEMA-co-BMA (DB) segments by the PEG corona. This, in turn, is balanced out by configurational entropic penalties of the bilayer morphology, which grows with larger polymer molecular weight, and thus leads to reversion to micellar morphologies above 9 kDa.[15] By contrast, and in accordance with established phase behavior of diblock copolymers,[15] polymers with 300 Da PEGMA appear to exhibit phase transitions between multicompartment vesicles (MCVs), polymersomes, and micelles at 20, 25, and 30 wt% PEG across 9-30 kDa molecular weight (Figure 2B). Therefore, at least in the range of PEG wt% used here, access to non-micellar morphologies appears to be restricted to the use of PEGMA monomers <950 Da for PEGMA-co-DEAEMA-co-BMA copolymers. We also note that in our previous work[8g] that we did not observe polymersome or MCV assembly with analogous diblock polymers of 20-25 wt% PEG once the size of the second block was >5 kDa. This is further supported by comparing the morphologies of particles self-assembled using graft copolymers and diblock copolymers of equivalent wt% PEG (Figure S4, Supporting Information). Notably, at ~20 wt% PEG and a DB content corresponding to 9kDa, graft copolymers appear to form MCVs whereas diblock polymers form smaller micellar structures. Likewise, at ~25% wt% PEG and ~30kDa DB graft copolymers assemble into vesicles whereas diblock polymers assembled into micelles. Therefore, the grafted architecture appears to enable access to a vesicular or MCV morphology over a larger range of chain molecular weights that is not readily available using diblock copolymers.
Figure 2. Effect of PEGMA-co-DEAEMA-co-BMA properties on colloidal self-assembly properties.
(a) Number average diameter and polydispersity index (PDI) of PEGMA-co-DEAEMA-co-BMA polymers diluted in PBS as measured by dynamic light scattering analysis (n=3 independently-prepared samples). (b) Representative electron micrographs of copolymers of synthesized with 300 Da PEGMA of variable PEG weight percent (wt% PEG) and molecular weight of the endosomolytic DEAEMA and BMA component. Micrographs of the 950 Da PEGMA-containing library can be found in Figure S3 of the SI.
Endosomolytic activity can be modulated via control of copolymer properties.
While the pH-responsive, membrane destabilizing behavior of PEG-block-DEAEMA-co-BMA and similar diblock colymers has been extensively characterized,[8g, 10, 14b] the endosomolytic activity of random PEGMA-co-DEAEMA-co-BMA copolymers, where continuous stretches of membrane-interactive DEAEMA-co-BMA are interrupted with grafted PEG chains, has not been examined. Therefore, we first screened pH-responsive, membrane-destabilizing activity using a commonly employed erythrocyte lysis assay in which polymers are incubated with human red blood cells at different pH and the amount of hemoglobin release is quantified as a metric for the potency of lipid bilayer destabilization (Figure 3A). First, all polymers displayed negligible hemolysis at pH 7.4, suggesting that membrane-interactive DEAEMA-co-BMA segments are sequestered from interaction with erythrocyte membranes. Therefore, even though polymers made with 950 Da PEGMA self-assemble into smaller particles, resulting a higher surface area to core volume, this data suggests that endosomolytic segments are not readily exposed to cell surfaces at pH 7.4. Additionally, regardless of PEGMA molecular weight or wt%, 9 kDa polymers demonstrated minimal hemolysis activity at all pH values, likely due to their low molecular weight as has been reported for diblock copolymers.[8g, 11] Interestingly, for polymers >15kDa, polymers with 950 Da PEGMA tended to have more overall membrane destabilizing activity at pH 5.8 than analogous polymers made with 300 Da PEGMA. However, polymers with 950 Da PEGMA exhibited minimal hemolysis activity at pH 6.6 whereas those with 300 Da PEGMA tended to display their peak activity at this pH. We postulated that this difference in the transition pH is due to the increased steric repulsion associated with the higher density of PEG chains in polymers containing 300 Da PEGMA (approximately 3-4 times as many 300 Da PEGMA monomers to achieve the same wt%, Table S1, Figure S1 Supporting Information), which lowers the energy barrier for protonation-mediated solubilization and exposure of the membrane-interactive segments. However, in evaluating the effect of pH on particle size of two polymers of analogous PEG content (20 wt%) and molecular weight (30 kDa), we found that both polymers underwent a particle-to-unimer transition at pH 6.6, consistent with behavior of diblock polymers (Figure S5). Therefore, we speculate that the weak pH 6.6 hemolysis activity of polymers with 950 Da PEGMA chains likely reflects an insufficient degree of DEAEMA protonation to facilitate electrostatic interactions with the erythrocyte membrane and overcome steric barriers imposed by longer PEG chains. Within polymers >9kDa comprising 950 Da PEGMA, increasing PEG wt% from 0.2-0.3 resulted in a reduction in membrane destabilizing activity, likely reflecting a dilution of the hemolytic DEAEMA and BMA monomers, effectively segmenting the chain into lower molecular weight domains spanning between pendant PEG chains. By contrast, the effect of PEG wt% was less pronounced or predictable within chains synthesized using 300 Da PEGMA, potentially a consequence of their diverse size and self-assembly behavior as well as shorter the 300 Da PEG grafts that create less of a steric barrier to membrane interactions.
Figure 3. Effect of PEGMA-co-DEAEMA-co-BMA properties on membrane-destabilizing and endosomal escape properties.
(a) Relative degree of erythrocyte hemolysis mediated by PEGMA-co-DEAEMA-co-BMA copolymers at different pH values (n=3-4). (b) Schematic illustrating Galectin 8 (Gal8) recruitment assay used to investigate endosomal escape of polymers. (c) Representative fluorescent images of cells expressing Gal8-YFP fusion protein upon treatment with polymers synthesized with 300 Da PEGMA with 15 kDa of DEAEMA-co-BMA (DB) with 25 wt% PEG (highest Gal8 recruitment), 30 wt% (medium), and 20 wt% (lowest), or PBS (NT). (d) Mean intensity of Gal8-YFP vesicles normalized to cell area for indicated polymers (n=6).
We next evaluated the capacity of carriers to promote endosomal escape using an assay that directly measures endosomal disruption, the Gal8-YFP recruitment assay (Figure 3B,C). Gal8-YFP is a fusion protein between Galectin 8 (Gal8), an endogenous cytosolic protein which binds to carbohydrates, such as those inside endosomes, and yellow fluorescent protein (YFP), which allows the quantitative tracking of Gal8 redistribution from the cytosol to endosomes after their membrane is disrupted. Increased Gal8 recruitment directly measures endosomal disruption and has been shown to correlate to increased bioactivity for cytosolic-acting biomacromolecular drugs. Here, we used a Gal8-YFP-expressing MDA-MB-231 breast cancer cell line that has been previously validated as a tool to quantify the disruption of endosomal membranes in response to endosomal acidification following nanoparticle endocytosis.[11] Surprisingly, we found that all polymers synthesized with 950 Da PEGMA exhibited minimal capacity for endosomal escape, despite many having strong hemolytic activity at pH 5.8. Similar discrepancies have been reported in which hemolysis activity is not entirely predictive of endosomal escape.[11] By contrast, nearly all polymers with 300 Da PEGMA resulted in a degree of endosomal disruption above baseline. Interestingly, for 9, 15 and 30 kDa polymers, a bell-shaped relationship existed between Gal8 recruitment and wt% with peak Gal8 recruitment occurring at 25 wt%. Polymers of 15 and 30kDa tended to result in more Gal8 recruitment than 60kDa, potentially reflecting the generally larger particle sizes for 60 kDa that may have resulted in lower cellular uptake or colloidal instability in serum for particles >500 nm. Surprisingly, despite having low hemolytic activity, 9 kDa polymers synthesized with 300 Da PEGMA demonstrated Gal8 recruitment, and therefore may merit future consideration as a low molecular weight, and therefore potentially less toxic, endosomolytic carrier system.
Copolymers enhance cGAMP activity in a composition-dependent manner.
We next evaluated the ability of polymers to enhance the biological activity of cGAMP using a simple “mix and go” formulation strategy whereby polymer self-assembly occurred in a solution of cGAMP without any additional purification steps. We first evaluated the ability of carriers to enhance uptake of a fluorescent CDN in THP-1 cells (Figure 4A). We found that CDN formulated with polymers containing 950 Da PEGMA were taken up to a largely similar extent, corresponding to about ~40-50% of the average CDN uptake achieved using carriers with 300 Da PEGMA. Within 300 Da PEGMA polymers, there was some trend towards increased uptake with increased wt% PEG, potentially owing to reduced particle size at higher wt%, but, overall, differences in CDN uptake were largely subtle. We next screened the ability of all carriers to enhance the activity of cGAMP in THP1-ISG reporter cells (Figure 4B; Figure S6). First, consistent with Gal8 recruitment data, none of the 950 Da PEGMA-containing polymers enhanced cGAMP activity over the range of 0.1-500 ng/mL, consistent with their low activity in the Gal8 recruitment assay. However, all polymers containing 300 Da PEGMA were capable of enhancing cGAMP activity to some extent. As a method to rank the relative effectiveness of these polymers in enhancing cGAMP activity, we determined the cGAMP concentration corresponding to 50% of the maximum IRF induction induced by free cGAMP at 125 μg/mL (concentration at half-maximal activity; CHMA); therefore, the more effective the polymer is as a carrier, the lower concentration of cGAMP necessary to reach this IRF response level. Amongst these polymers, those of higher molecular weight (30 and 60 kDa) tended to have increased activity, with the 2 most active variants being 30 kDa polymers at 20 wt% and 25 wt% PEG.
Figure 4. Effect of PEGMA-co-DEAEMA-co-BMA structure on cGAMP delivery.
(a) Relative uptake (MFI: median fluorescence intensity) of a fluorescently-labeled CDN (cdGMP-Dy547) formulated with indicated polymer by THP-1 monocytes (n=1). (b) Dose-response curves for cGAMP formulated with indicated copolymer in THP-1 ISG cells with an IFN regulatory factor (IRF)-inducible reporter construct (n=4 for each dose). The horizontal line represents the half-maximal activity of free cGAMP at a concentration of 125 μg/mL; the dose response curve for free cGAMP is shown in Figure S6. (c) Summary of the cGAMP concentration at which the half-maximal maximal activity of cGAMP was achieved for each polymer formulation. Note that for carriers that did not induce a response between 0.1-500 ng/mL cGAMP that this value was set at 500 ng/mL.
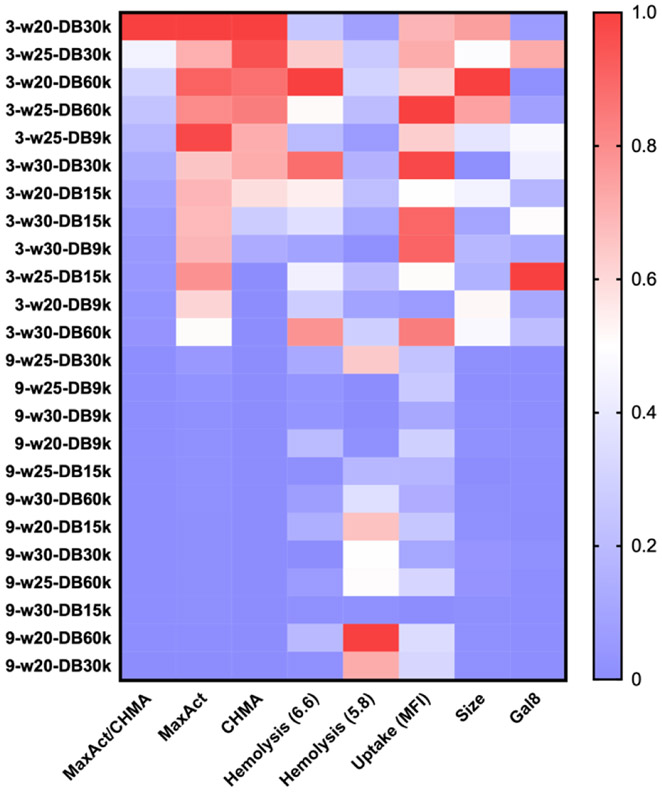
To develop a better understanding of relationships between carrier properties and cGAMP delivery efficiency, we generated a heat map that integrates data regarding size, hemolysis at pH 6.6 and 5.8, cellular uptake, Gal8 recruitment, and cGAMP activity as defined by CHMA, maximum overall activity (MaxAct), and the ratio of MaxAct/CHMA (Figure 5). As also supported by Spearman’s correlation analysis (Table S2), we observe that cGAMP activity tends to correlate most strongly with larger particle size, increased cellular uptake, hemolytic activity at pH 6.6 (but not 5.8), and Gal8 recruitment. Collectively, this reinforces our previous findings that efficient cellular uptake and endosomal escape are important design criteria for CDN carriers.
Figure 5. Heat map summarizing relationships between formulation properties, endosomal escape, and cGAMP activity.
Maximal level of cGAMP activity (MaxAct), the cGAMP concentration at half-maximal activity (CHMA), and the ratio of MaxAct and CMHA are representative of carrier activity, which tends to correlate with CDN uptake, particle size, hemolysis at pH 6.6, and level of Gal8 recruitment. Note that copolymers are identified using the following convention: the first number of the PEGMA molecular weight (3 for 300 Da and 9 for 950 Da)- the weight (w) percent PEGMA-the molecular weight in kilodaltons of the DEAEMA and BMA (DB) component (e.g., 9-w20-DB15k). All properties were normalized to the lowest and highest values on a 0.0-1.0 scale.
Delivery of cGAMP with lead carriers enhances STING activation in vivo.
Having screened polymers for their ability to enhance in vitro cGAMP activity, we next evaluated lead cGAMP carriers for their ability to activate the STING pathway in mice. Here, we employed a model system of intratumoral administration, which is being explored clinically for CDNs, and used qRT-PCR to evaluate levels of the STING-driven genes, Ifnb1, Cxcl10, and Tnf. We evaluated the two carriers that were most active in vitro based on their nearly identical and low CHMA: 30 kDa polymers with 20 and 25 wt% PEG with 300 Da PEGMA (referred to as, 3-w20-30k and 3-w25-30k, respectively). Additionally, an entirely inactive cGAMP carrier, a 30 kDa polymer with 20 wt% 950 Da PEGMA (9-w20-30k), free cGAMP, and PBS (vehicle) were administered as controls. Consistent with in vitro findings, the inactive carrier with 950 Da PEGMA (9-w20-30k) did not enhance STING activation in vivo, whereas the carriers synthesized with 300 Da PEGMA tended to increase gene expression levels relative to free cGAMP, which had no effect relative to vehicle (Figure 6a). While no statistical differences were observed between the two active carriers, only the polymer containing 20 wt% PEG with 300 Da PEGMA (3-w20-30k) resulted in a significant increase in STING activation relative to controls, validating this as the lead carrier. While both carriers have similar in vitro activity, this difference may be attributed to the slightly larger particle size generated using the 3-w20-30k polymer (600 vs. 400 nm) that could have prolonged intratumoral nanoparticle retention or perhaps be a consequence of increased stability of a multicompartment morphology[16] that appears to form when polymers are synthesized with 20 wt% PEG with 300 Da PEGMA, a possibility that merits future investigation.
Figure 6. In vivo evaluation of PEGMA-co-DEAEMA-co-BMA as cGAMP carriers.
(a) qPCR analysis of Ifnb1, Cxcl10, and Tnf expression in B16.F10 tumors 4 h after intratumoral administration of indicated copolymers formulated with cGAMP, free cGAMP or PBS as a vehicle control (n=7-10 biologically independent samples, one-way ANOVA with Tukey’s post-hoc test; *P<0.05, ** P<0.01). (b) Mice were treated intratumorally with cGAMP formulated with indicated carrier, free cGAMP, or vehicle control three times, three days apart. Mean tumor volume in response to intratumoral administration of free cGAMP or cGAMP formulated with indicated copolymer (n=6-7 biologically independent samples, one-way ANOVA with Tukey’s post-hoc test on days 15 and 18; *P<0.05). cGAMP dose in all studies was 10 μg per injection.
We, and others, have demonstrated that activation of STING signaling in a number of different tumor types stimulates innate and adaptive immune responses that inhibit tumor growth.[8g, 17] Therefore, we next sought to confirm that the lead carrier (3-w20-30k) also could enhance the therapeutic activity of cGAMP. Using a B16.F10 melanoma model, we performed intratumoral injections of cGAMP loaded into the 3-w20-30k carrier, free cGAMP, and PBS and monitored tumor volume. Consistent with gene expression data, delivery of cGAMP with the carrier resulted in inhibition of tumor growth whereas free cGAMP did not (Figure 6b). These data further demonstrate the ability of optimized PEGMA-co-DEAEMA-co-BMA to improve cGAMP delivery in vivo. As the objective of these studies was not to determine an optimized carrier for local administration, but instead to test activity in an in vivo setting, further investigation (e.g., dose response studies) will be necessary to determine if any of the other carriers may offer advantages for this specific application of intratumoral immunotherapy. Likewise, as we utilized intratumoral administration only as a test bed for carrier efficacy, we cannot rule out that other carriers may have advantages for other CDN delivery routes; for example, carriers with 30 wt% PEG with 300 Da PEGMA have a relatively high capacity to enhance cGAMP activity based on their CHMA values, and are smaller in size (~30-50 nm) and may therefore be better suited to exploit the enhanced permeation and retention (EPR) effect when administered intravenously[18] or to exploit lymphatic drainage as vaccine adjuvants.[19] Nonetheless, these studies validate PEGMA-co-DEAEMA-co-BMA copolymers for in vivo delivery of CDNs and further reinforce the importance of copolymer structure on achieving efficient intracellular drug delivery for this new carrier architecture.
Conclusion
CDN STING agonists are promising immunostimulatory agents with a wide range of potential applications, but their activity is limited by intracellular drug delivery barriers. Here, we describe the design, synthesis, and evaluation of a novel series of PEGMA-co-DEAEMA-co-BMA random copolymers as carriers to enhance the intracellular delivery and activity of CDNs. Using a library of 24 polymers comprising two different PEGMA monomers (300 and 950 Da), three different PEG wt% (20, 25, 30 wt%), and four different molecular weights, we established relationships between polymer structure, self-assembly properties, endosomal escape, and CDN activity. This resulted in the identification of several carriers synthesized using 300 Da PEGMA that strongly enhanced CDN activity, an effect that correlated with particle size, cellular uptake, and endosomal escape. Additionally, this increased activity was achieved via a simple mixing strategy by which polymers we dissolved in a CDN-containing aqueous solution without any additional purification or processing steps. Importantly, we demonstrated the ability of lead CDN carrier formulations to enhance STING signaling in vivo, studies that highlight the potential for this new class of carriers to increase the efficacy and utility of this emerging class of therapeutic agent. Furthermore, these investigations demonstrated the potential utility of PEGMA-co-DEAEMA-co-BMA copolymers as a facile strategy for fabricating self-assembled drug carriers of variable morphology, particle size, and endosomolytic properties via control of PEGMA molecular weight and content that may be tuned for specific CDN delivery applications or for delivery of other types of drug cargo.
Supplementary Material
Acknowledgements
We thank the core facilities of the Vanderbilt Institute of Nanoscale Sciences and Engineering (VINSE) for use of dynamic light scattering and electron microscopy and the Vanderbilt Technologies for Advanced Genomics (VANTAGE) for providing human blood for hemolysis studies. 2’3’-cGAMP was provided by the Vanderbilt Institute of Chemical Biology Chemical Synthesis Core. This research was supported by grants from the National Science Foundation CBET-1554623 (JTW), the National Institutes of Health R01 CA245134 (JTW) a Vanderbilt Ingram Cancer Center (VICC) Ambassador Discovery Grant (JTW), a VICC-Vanderbilt Center for Immunobiology Pilot Grant (JTW), the National Institutes of Health Integrated Training in Engineering and Diabetes Training Grant (T32 DK101003; LEP), the Melanoma Research Alliance 503565 (JTW), and also supported by a Stand Up To Cancer Innovative Research Grant, Grant Number SU2C-AACR-IRG 20-17 (JTW). Stand Up To Cancer (SU2C) is a program of the Entertainment Industry Foundation. Research grants are administered by the American Association for Cancer Research, the scientific partner of SU2C. MW acknowledges a postdoctoral fellowship from the Canadian Institutes of Health Research (CIHR) and DCN acknowledges the support of the SyBBURE Searle Undergraduate Research Program at Vanderbilt University.
References
- [1].a) Flood BA, Higgs EF, Li S, Luke JJ, Gajewski TF, Immunol Rev 2019, 290, 24; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Li X-D, Wu J, Gao D, Wang H, Sun L, Chen ZJ, Science 2013, 341, 1390; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Dubensky TW Jr., Kanne DB, Leong ML, Ther Adv Vaccines 2013, 1, 131; [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Gutjahr A, Papagno L, Nicoli F, Kanuma T, Kuse N, Cabral-Piccin MP, Rochereau N, Gostick E, Lioux T, Perouzel E, Price DA, Takiguchi M, Verrier B, Yamamoto T, Paul S, Appay V, JCI Insight 2019, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].a) Khoo LT, Chen LY, EMBO Rep 2018, 19; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Wu JJ, Zhao L, Hu HG, Li WH, Li YM, Med Res Rev 2020, 40, 1117. [DOI] [PubMed] [Google Scholar]
- [3].a) Corrales L, McWhirter SM, Dubensky TW Jr., Gajewski TF, J Clin Invest 2016, 126, 2404; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Chen Q, Sun L, Chen ZJ, Nat Immunol 2016, 17, 1142. [DOI] [PubMed] [Google Scholar]
- [4].Wang H, Hu S, Chen X, Shi H, Chen C, Sun L, Chen ZJ, Proc Natl Acad Sci U S A 2017, 114, 1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Carroll EC, Jin L, Mori A, Munoz-Wolf N, Oleszycka E, Moran HBT, Mansouri S, McEntee CP, Lambe E, Agger EM, Andersen P, Cunningham C, Hertzog P, Fitzgerald KA, Bowie AG, Lavelle EC, Immunity 2016, 44, 597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Berger G, Marloye M, Lawler SE, Trends Mol Med 2019, 25, 412. [DOI] [PubMed] [Google Scholar]
- [7].a) Hubbell JA, Swartz MA, Nat Nanotechnol 2019, 14, 196; [DOI] [PubMed] [Google Scholar]; b) Hanson MC, Crespo MP, Abraham W, Moynihan KD, Szeto GL, Chen SH, Melo MB, Mueller S, Irvine DJ, The Journal of Clinical Investigation 2015, 125, 2532; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Vanpouille-Box C, Hoffmann JA, Galluzzi L, Nat Rev Drug Discov 2019, 18, 845. [DOI] [PubMed] [Google Scholar]
- [8].a) Wilson DR, Sen R, Sunshine JC, Pardoll DM, Green JJ, Kim YJ, Nanomedicine 2018, 14, 237; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Collier MA, Junkins RD, Gallovic MD, Johnson BM, Johnson MM, Macintyre AN, Sempowski GD, Bachelder EM, Ting JP, Ainslie KM, Mol Pharm 2018, 15, 4933; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Cheng N, Watkins-Schulz R, Junkins RD, David CN, Johnson BM, Montgomery SA, Peine KJ, Darr DB, Yuan H, McKinnon KP, Liu Q, Miao L, Huang L, Bachelder EM, Ainslie KM, Ting JP, JCI Insight 2018, 3; [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Nakamura T, Miyabe H, Hyodo M, Sato Y, Hayakawa Y, Harashima H, J Control Release 2015, 216, 149; [DOI] [PubMed] [Google Scholar]; e) Koshy ST, Cheung AS, Gu L, Graveline AR, Mooney DJ, Advanced Biosystems 2017, 1, 1600013; [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Liu Y, Crowe WN, Wang L, Lu Y, Petty WJ, Habib AA, Zhao D, Nat Commun 2019, 10, 5108; [DOI] [PMC free article] [PubMed] [Google Scholar]; g) Shae D, Becker KW, Christov P, Yun DS, Lytton-Jean AKR, Sevimli S, Ascano M, Kelley M, Johnson DB, Balko JM, Wilson JT, Nat Nanotechnol 2019, 14, 269; [DOI] [PMC free article] [PubMed] [Google Scholar]; h) An M, Yu C, Xi J, Reyes J, Mao G, Wei WZ, Liu H, Nanoscale 2018, 10, 9311; [DOI] [PMC free article] [PubMed] [Google Scholar]; i) Wang J, Li P, Yu Y, Fu Y, Jiang H, Lu M, Sun Z, Jiang S, Lu L, Wu MX, Science 2020, 367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lin LC, Huang CY, Yao BY, Lin JC, Agrawal A, Algaissi A, Peng BH, Liu YH, Huang PH, Juang RH, Chang YC, Tseng CT, Chen HW, Hu CJ, Adv Funct Mater 2019, 29, 1807616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Manganiello MJ, Cheng C, Convertine AJ, Bryers JD, Stayton PS, Biomaterials 2012, 33, 2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kilchrist KV, Dimobi SC, Jackson MA, Evans BC, Werfel TA, Dailing EA, Bedingfield SK, Kelly IB, Duvall CL, ACS Nano 2019, 13, 1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Reis CP, Neufeld RJ, Ribeiro AJ, Veiga F, Nanomedicine 2006, 2, 8. [DOI] [PubMed] [Google Scholar]
- [13].Evans BC, Nelson CE, Yu SS, Beavers KR, Kim AJ, Li H, Nelson HM, Giorgio TD, Duvall CL, J Vis Exp 2013, DOI: 10.3791/50166e50166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].a) Baljon JJ, Dandy A, Wang-Bishop L, Wehbe M, Jacobson ME, Wilson JT, Biomater Sci 2019, 7, 1888; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Nelson CE, Kintzing JR, Hanna A, Shannon JM, Gupta MK, Duvall CL, ACS Nano 2013, DOI: 10.1021/nn403325f; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Berguig GY, Convertine AJ, Frayo S, Kern HB, Procko E, Roy D, Srinivasan S, Margineantu DH, Booth G, Palanca-Wessels MC, Baker D, Hockenbery D, Press OW, Stayton PS, Mol Ther 2015, 23, 907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Mai Y, Eisenberg A, Chem Soc Rev 2012, 41, 5969. [DOI] [PubMed] [Google Scholar]
- [16].Bobbala S, Allen SD, Yi S, Vincent M, Frey M, Karabin NB, Scott EA, Nanoscale 2020, 12, 5332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].a) Wang-Bishop L, Wehbe M, Shae D, James J, Hacker BC, Garland K, Chistov PP, Rafat M, Balko JM, Wilson JT, J Immunother Cancer 2020, 8; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Sivick KE, Desbien AL, Glickman LH, Reiner GL, Corrales L, Surh NH, Hudson TE, Vu UT, Francica BJ, Banda T, Katibah GE, Kanne DB, Leong JJ, Metchette K, Bruml JR, Ndubaku CO, McKenna JM, Feng Y, Zheng L, Bender SL, Cho CY, Leong ML, van Elsas A, Dubensky TW Jr., McWhirter SM, Cell Rep 2018, 25, 3074; [DOI] [PubMed] [Google Scholar]; c) Sallets A, Robinson S, Kardosh A, Levy R, Blood Adv 2018, 2, 2230; [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Moore E, Clavijo PE, Davis R, Cash H, Van Waes C, Kim Y, Allen C, Cancer Immunol Res 2016, 4, 1061; [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Corrales L, Glickman LH, McWhirter SM, Kanne DB, Sivick KE, Katibah GE, Woo SR, Lemmens E, Banda T, Leong JJ, Metchette K, Dubensky TW Jr., Gajewski TF, Cell Rep 2015, 11, 1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Maeda H, Nakamura H, Fang J, Adv Drug Deliv Rev 2013, 65, 71. [DOI] [PubMed] [Google Scholar]
- [19].Thomas SN, Schudel A, Curr Opin Chem Eng 2015, 7, 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.