Abstract
Objective:
Imagined walking has yielded insights into normal locomotor control and could improve understanding of neurologic gait dysfunction. This study evaluated brain activation during imagined walking in chronic stroke.
Methods:
Ten persons with stroke and 10 matched controls completed a walking test battery and a magnetic resonance imaging session including imagined walking and knee extension tasks. Brain activations were compared between tasks and groups. Associations between activations and composite gait score were also calculated, while controlling for lesion load.
Results:
Stroke and worse gait score were each associated with lesser overall brain activation during knee extension but greater overall activation during imagined walking. During imagined walking, the stroke group significantly activated the primary motor cortex lower limb region and cerebellar locomotor region. Better walking function was associated with less activation of these regions and greater activation of medial superior frontal gyrus area 9.
Conclusions:
Compared with knee extension, imagined walking was less sensitive to stroke-related deficits in brain activation but better at revealing compensatory changes, some of which could be maladaptive.
Keywords: Locomotion, gait, imagery, brain, magnetic resonance imaging
1. Introduction
Walking dysfunction is a common and devastating consequence of stroke and other neurologic disorders.(Hill et al., 1997; Mayo et al., 1999; Middleton et al., 2015) Although efficacious rehabilitation strategies to improve locomotion have emerged,(Hornby et al., 2019; Hornby et al., 2016; Ada et al., 2013) mechanisms of walking dysfunction and recovery remain poorly understood. For example, damage to the primary motor cortex or corticospinal tract is a plausible cause of post-stroke gait impairment that is strongly implicated in upper limb dysfunction and recovery processes,(Stinear et al., 2007; Favre et al., 2014) but has shown inconsistent association with locomotor outcomes.(Dawes et al., 2008; Peters et al., 2018; Smith et al., 2017) This discrepancy may relate to the more distributed nature of normal locomotor control. While cortical motor regions contribute to human walking,(Miyai et al., 2001; Knaepen et al., 2015; Gwin et al., 2011) key subcortical walking centers have also been evolutionarily preserved, including the midbrain and cerebellar locomotor regions (MLR and CLR).(Jahn, Deutschlander, Stephan, Kalla, Hufner et al., 2008; Jahn, Deutschlander, Stephan, Kalla, Wiesmann et al., 2008; la Fougere et al., 2010)
Preliminary evidence suggests that these subcortical regions play important roles in normal and pathological human walking function.(Yeo et al., 2011; Fraix et al., 2013; Boyne et al., 2018; Fasano et al., 2017; Jahn et al., 2008; la Fougere et al., 2010; Jahn et al., 2008) For example, better gait function among healthy adults has been shown to correlate with greater resting-state functional connectivity between the MLR, cerebellum and a novel locomotor region in the medial superior frontal gyrus portion of Brodmann area 9 (SFG9m).(Boyne et al., 2018) In addition, it has been suggested that upregulation of the CLR and primary motor cortex could be a generic compensatory response to gait impairment, which could theoretically reduce gait automaticity.(Boyne et al., 2018; Peterson and Horak, 2016) It remains unclear how adaptive or maladaptive such a compensation could be, particularly after stroke, where less is known about neural control of locomotion.
Since brain network dynamics differ between the resting state and task performance,(Di et al., 2013) task-based imaging protocols may be needed to fully evaluate brain locomotor function. Unfortunately, portable imaging modalities which can record brain activity during actual walking have insufficient penetration to reach subcortical locomotor regions.(Bohnen and Jahn, 2013) Consequently, post-stroke locomotor function and plasticity have most commonly been assessed using task-based functional magnetic resonance imaging (fMRI) of unilateral single-joint leg movements (e.g. paretic knee extension),(Dobkin et al., 2004; Luft et al., 2005; Luft et al., 2004; Luft et al., 2002; Burke et al., 2014) and it is uncertain how well these tasks represent walking function.
Given these issues, imagined walking during fMRI may be able to provide novel insights about mechanisms of walking dysfunction and recovery after stroke. It is well established that imagined and actual movements activate similar (albeit not identical) brain regions across a multitude of tasks, including walking.(Lafleur et al., 2002; Jahn et al., 2008; la Fougere et al., 2010; Miyai et al., 2001; Sharma and Baron, 2013) Imagined walking during fMRI has also been shown to activate subcortical locomotor centers in healthy adults(Wang et al., 2008; la Fougere et al., 2010; Sacco et al., 2006; Jahn et al., 2008) and persons with Parkinson’s Disease.(Peterson et al., 2014; Cremers et al., 2012) Mental imagery ability is not usually affected by stroke, including imagined walking.(Sharma et al., 2006; Malouin et al., 2008a; Dickstein et al., 2004) However, no previous studies have tested imagined walking fMRI in this population.
This study aimed to assess brain activation during imagined walking in chronic stroke, including its association with walking function, in comparison with a paretic knee extension fMRI task and compared with non-stroke control participants. We hypothesized that:
Brain activation during imagined walking would significantly differentiate stroke and control groups and would be significantly associated with walking function;
Compared with paretic knee extension, imagined walking would elicit significantly greater stroke-control differences in brain activation and associations with walking function, since it is designed to more closely mimic brain activity during gait;
Compared with matched controls, participants with stroke would show significantly greater activations of the primary motor cortex lower limb region (M1-LL) and CLR during imagined walking, as a possible compensation for gait impairment;
Walking function would be positively associated with activations in the MLR, cerebellum and SFG9m during imagined walking, and negatively associated with activations in M1-LL and CLR, based on prior resting functional connectivity findings among healthy adults.(Boyne et al., 2018)
2. Methods
This study was approved by the University of Cincinnati Institutional Review Board, followed U.S. policy for the protection of human research participants and was prospectively registered (ClinicalTrials.gov Identifier NCT0285834). Using a cross-sectional design, participants with stroke and 1:1 matched control participants without stroke each underwent eligibility screening(Boyne et al., 2016; Boyne et al., 2015) and phenotypic assessments of gait and mental imagery ability, followed by standardized MRI task practice and MRI testing.
2.1. Participants
Participants were recruited from the community and provided written informed consent prior to participation. General eligibility criteria for both stroke and control participants were: age 30–90 years; MRI compatibility; able to communicate with investigators and correctly answer consent comprehension questions; able to perform mental imagery (time-dependent motor imagery screening test);(Malouin et al., 2008b) no recent drug or alcohol abuse or significant mental illness; and not pregnant.
Additional eligibility criteria for participants with stroke were: unilateral stroke in middle cerebral artery territory experienced >6 months prior to enrollment; walking speed <1.0 m/s on the 10 meter walk test;(Bohannon and Williams Andrews, 2011) able to walk 10m over ground with assistive devices as needed and no physical assistance; no evidence of significant arrhythmia or myocardial ischemia on treadmill electrocardiographic (ECG) stress test, nor significant baseline ECG abnormalities that would make an exercise ECG uninterpretable;(American College of Sports Medicine, 2014) no recent cardiopulmonary hospitalization; no significant ataxia or neglect (National Institutes of Health Stroke Scale item scores ≤ 1);(Brott et al., 1989) no severe lower limb hypertonia (Ashworth ≤ 2);(Ashworth, 1964) no major post-stroke depression (Patient Health Questionnaire-9 <10),(Williams et al., 2005) unless being managed by a health care provider;(Duncan et al., 2007) not currently participating in physical therapy or an interventional research study; no recent paretic lower limb botulinum toxin injection; and no concurrent progressive neurologic disorder or other major medical conditions affecting gait.
Additional eligibility criteria for non-stroke control participants were: demographic match for a participant with stroke (same sex, age difference ≤5 years); and no known neurologic, orthopedic or medical condition affecting gait.
2.2. Phenotypic assessments
Participants used any habitual orthotic and assistive devices during testing and were guarded as needed by a physical therapist, who only provided assistance if required to prevent a fall or injury.
Gait speed was measured with the 10-meter walk test,(Tilson et al., 2010) a reliable and valid measure of overall mobility and health status, including after stroke.(Middleton et al., 2015) The test was performed twice at comfortable speed and twice at fastest speed with trial pairs averaged for analysis.
Spatiotemporal gait parameters were measured with a sensor-embedded electronic walkway, which provides reliable and valid assessment of cadence, step lengths and step times.(Bilney et al., 2003; Cutlip et al., 2000) Two passes across the walkway at comfortable speed were combined and gait parameters were averaged across gait cycles.
Walking capacity was measured with the 6-minute walk test, where the participant is instructed to walk as far as possible in 6 minutes, using a standardized course, instructions and encouragement.(ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories, 2002) The distance walked provides a reliable and valid measure of walking capacity and is associated with community ambulation after stroke.(Fulk et al., 2010; Fulk et al., 2008)
Subjective mental imagery ability was measured with the Kinesthetic and Visual Imagery Questionnaire 10,(Malouin et al., 2007) where the participant performs, then imagines performing 5 different movements, rating both the visual clarity and kinesthetic intensity of the imagery on separate 5-point scales after each movement. Scores were averaged across all movements and both subscales to provide a total score. This test is reliable and valid after stroke.(Malouin et al., 2007)
2.3. Functional MRI task paradigm
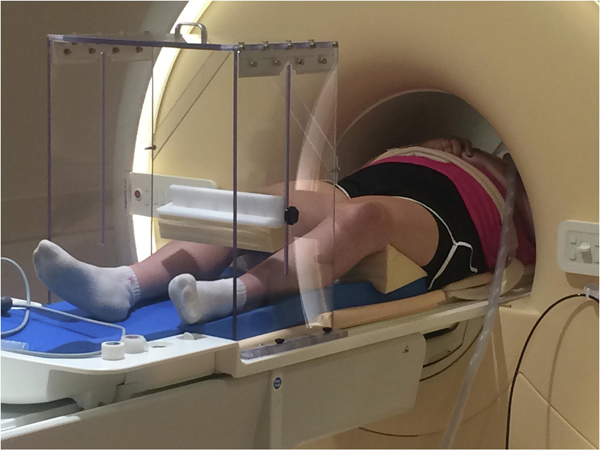
During MRI scanning, participants were positioned in a custom scaffold (Fig 1) based on a previous design,(Luft et al., 2005) to control leg movement during the knee extension task. Foam wedges were placed far enough under each thigh to avoid contact with the calf, which helped minimize superior-inferior translation of the head during knee extension cycles in pilot testing. The starting point for the knee extension motion was set at 30° of flexion by adjusting the amount of padding under the heels. The ending point was set at 15° of flexion by moving the position of the shin pad on the scaffold.
Figure 1.
Functional magnetic resonance imaging (fMRI) task setup.
The task fMRI protocol used a 4-phase block design, including: 1) knee extension with metronome pacing; 2) rest; 3) imagined walking; and 4) rest with metronome audio. Each block lasted 30 seconds, including a 3-second audiovisual stimulus to initiate each 27 second task. Five cycles of each task condition were performed, resulting in a 10 minute total sequence. Participants were trained to keep eyes closed when performing each task and were continuously monitored during the scan for compliance with task performance.
The knee extension task followed the protocol used in several previous stroke fMRI studies.(Luft et al., 2005; Luft et al., 2002; Luft et al., 2004; Luft et al., 2008; Luft et al., 1998) Participants repeatedly extended the paretic knee against gravity then returned to the starting position at a metronome-paced rate of 1 cycle every 3 seconds (0.33 Hz). Control participants used the same limb as their matched participant with stroke.
The imagined walking task was based on previous studies among healthy adults and persons with Parkinson’s Disease.(Jahn et al., 2008; Jahn et al., 2004; Cremers et al., 2012) Participants imagined walking again the same way they had just walked during gait testing, while focusing on motor and kinesthetic aspects of walking. Small overt movements were permitted, since the goal was to approximate actual locomotor function, rather than studying the neural basis of motor imagery.
2.4. Gait imagery and fMRI task practice
Between gait testing and MRI scanning, participants had standardized gait imagery and fMRI task practice. For initial imagery training, participants alternated between actual walking and seated imagined walking in 30 second bouts for 2–3 practice cycles. Standardized instructions emphasized motor and kinesthetic over visual aspects of imagery and emphasized actual rather than optimal gait (see script in the Supplementary File 1 - Appendix). Participants then practiced the whole task MRI sequence (knee extension, rest, imagined walking, rest) in supine inside a mock scanner, first with standardized coaching for each task, then using the audiovisual cues and timings for the actual MRI (see script in the Supplementary File 1 - Appendix). This practice continued until the participant demonstrated competence, which typically took about 10 minutes.
2.5. MRI data acquisition
A 3.0T Philips Ingenia MRI system with a 32-channel head coil was used to acquire: 1) a T1-weighted MPRAGE with 1×1×1 mm spatial resolution, 8.1 ms TR, 3.7 ms TE, 8° flip angle and SENSE factor 2; and 2) a gradient-echo echo-planar task fMRI image sequence with 3×3×3.5 mm spatial resolution, 2000 ms TR, 30 ms TE, 75° flip angle, and SENSE factor 2.5.
2.6. MRI data processing
Initial preprocessing generally followed the approach of the Human Connectome Project,(Glasser et al., 2013) with adaptations for stroke lesions and available MRI sequences. For participants with right hemispheric stroke and their matched control participants, images were flipped across the x-axis so that all lesions were on the left in MRI space.
2.6.1. Structural preprocessing
Automated stroke lesion masks were created from the T1-weighted MRI (T1w) with the Lesion GNB Toolbox,(Griffis et al., 2016) then were manually edited as needed following published guidelines.(Liew et al., 2018) The Functional MRI of the Brain Software Library (FSL) (Jenkinson et al., 2012) was used for T1w bias field correction, tissue type segmentation and non-linear registration to the MNI152 template, using the lesion mask to improve registration. Lesioned voxels in the native-space T1w image were temporarily filled with MNI template voxels(Siegel et al., 2017) to enable surface-based cortical modeling and subcortical labelling with the standard freesurfer pipeline (Fischl, 2012). The left and right native cortical surface meshes were registered to the Conte69 population average meshes(Van Essen et al., 2012) by aligning cortical folding patterns using multimodal surface matching with higher order smoothness constraints.(Robinson et al., 2018)
To estimate the extent of motor projection fiber disruption, weighted corticofugal motor tract lesion load (wCMT-LL) was calculated. Publicly-available volumetric masks of the normal projections from each of the left cortical motor regions (primary motor cortex, supplemental and pre-supplemental motor areas, dorsal and ventral premotor cortices(Archer et al., 2018) were combined and binarized to create a canonical CMT mask in MNI152 space. Since lesions in narrower portions of a tract will tend to cause greater connectivity disruption, weighting factors were calculated for each axial slice of the CMT mask by dividing the maximum CMT cross-sectional area by its area at that slice.(Zhu et al., 2010) This gives higher weight to lesion voxels in narrower portions of the CMT. The area of each participant’s (MNI152 registered) lesion mask overlap with the canonical CMT was calculated at each slice, multiplied by the weighting factor for that slice, and summed across slices to obtain the wCMT-LL measurement.
2.6.2. Task fMRI preprocessing
FSL software(Jenkinson et al., 2012) was used for fMRI slice timing correction, motion correction, white matter boundary-based registration to the native space T1w image and concatenation with the T1w-derived MNI warp field for one step interpolation to MNI152 template space. Connectome Workbench(Glasser et al., 2013) was then used to: 1) resample the cortical fMRI data onto the T1w-derived, Conte69-registered surface meshes; 2) resample the subcortical fMRI data from freesurfer-labelled structures onto the Conte69 subcortical gray matter template;(Glasser et al., 2013) and 3) combine the subcortical volumetric fMRI data and cortical surface fMRI data into a single file in the standard Connectivity Informatics Technology Initiative (CIFTI) 91,282 gray-ordinate space.(Glasser et al., 2013) Spatial averaging was performed within parcels from a custom atlas (see details below) and a 120 second high pass temporal filter was applied.
2.6.3. CIFTI brain atlas and core locomotor regions of interest (ROIs)
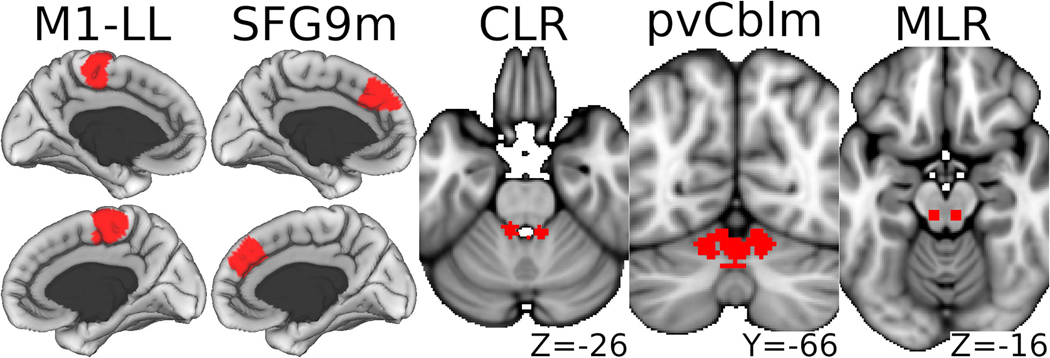
A custom 300-parcel CIFTI brain atlas was created by combining the 246 cortical surface and subcortical volumetric parcels of the Brainnetome atlas(Fan et al., 2016) with subcortical volumetric MLR & CLR labels(Fox et al., 2014; Jaeger et al., 2014; Boyne et al., 2018) and the 50 parcels of the Shen-Finn atlas(Shen et al., 2013; Finn et al., 2015) that overlapped the brainstem and cerebellum in the standard CIFTI template.(Glasser et al., 2013) Five bilateral core locomotor ROIs were selected from a previous meta-analysis and normative study of resting functional connectivity related to walking capacity.(Boyne et al., 2018) These ROIs included the M1-LL, SFG9m, CLR, paravermal cerebellum area VI (pvCblm) and MLR (Fig 2).
Figure 2. Core locomotor regions of interest.
Volumetric structures (right most 3 panels) are magnified by 30%. M1-LL, Primary motor cortex lower limb region; SFG9m, Superior frontal gyrus medial area 9; CLR, cerebellar locomotor region; pvCblm, Paravermal cerebellum area VI; MLR, midbrain locomotor region.
2.7. First level (within-participant) task analysis
The design variable for each task (knee extension with metronome, rest, imagined walking, rest with metronome) had a 3 second onset delay to account for the task cue and lasted 27 seconds in each cycle. An additional design variable to account for the effect of the task cues (3 second duration at the beginning of each block) was also included as a covariate. Each design variable was convolved with a double-gamma hemodynamic response function. Noise confound regressors, calculated from the dense volumetric data, were also added to the design matrix. These included a linear trend, the mean time series within white matter and cerebrospinal fluid masks and motion-related independent components identified by ICA-AROMA (independent components analysis based automatic removal of motion artifacts).(Pruim, Mennes, Buitelaar et al., 2015; Pruim, Mennes, van Rooij et al., 2015) ICA-AROMA noise components were orthogonalized to the ICA-AROMA signal components for non-aggressive denoising.(Pruim et al., 2015; Pruim et al., 2015) The task and confound design matrix was also temporally filtered to match the CIFTI fMRI data.
For each participant, the fMRI time series in each parcel was modeled as a function of the design matrix. Contrast estimates were obtained using FSL film_gls, with prewhitening.(Woolrich et al., 2001) Task contrasts included: 1) paretic knee extension minus rest with metronome; 2) imagined walking minus rest without metronome; and 3) imagined walking minus paretic knee extension (contrast 2 minus contrast 1).
2.8. Second level (between-participant) statistical analysis
To summarize walking function with a single variable, principle components analysis (singular value decomposition) was performed among all participants with the following (centered) gait measures: 6-minute walk test, cadence, left-right (L-R) mean step length, L-R mean single-support time, fastest gait speed, comfortable gait speed, absolute step time difference, absolute single-support difference and absolute step length difference. The primary component was used as a composite gait measure and individual participant scores were obtained. Characteristics and phenotypic variables were compared between participants with stroke and matched controls using independent t-tests and chi-squared tests. In the stroke group, the Pearson correlation between composite gait score and wCMT-LL was also calculated.
FSL flameo was used for group level mixed effects task fMRI analysis.(Woolrich et al., 2004) For each task contrast, mean activation estimates (Z statistics) were obtained for the stroke & control groups and the between-group difference. Associations between task activations and composite gait scores were also calculated among all participants combined, with wCMT-LL as a covariate to adjust for stroke/control status and stroke severity.
For hypotheses 1 and 2, the primary analyses pooled results across all 300 brain parcels to calculate associations and between-task comparisons at the level of whole brain activation. To assess brain activation associations with stroke/control status and gait score for the imagined walking task (hypothesis 1), Wilcoxon signed rank tests were used to determine whether the median Z statistic (across brain parcels) was significantly different from zero (p<0.05) for each association. To compare these brain activation associations between tasks (hypothesis 2), Wilcoxon signed rank tests determined whether the median Z statistic was significantly different between imagined walking and knee extension for each association. Sensitivity analyses utilized subsets of brain parcels, including parcels that were found to be related to walking in a previous meta-analysis,(Boyne et al., 2018) cortical parcels, cortical sensorimotor parcels, subcortical, basal ganglia, cerebellar and brainstem parcels.
For hypotheses 3 and 4, brain activation associations with stroke/control status and gait score were examined within the five bilateral, a priori, core locomotor ROIs. Since we were testing specific a priori hypotheses for each ROI, an unadjusted significance threshold of p<0.05 was applied.
2.8.1. Sample size calculation
Based on previous studies,(Luft et al., 2008; Enzinger et al., 2009) an a priori target sample size of 10 participants with stroke and 10 controls was selected for 80% estimated power to detect: 1) a within-group Cohen’s d effect size as small as 1.00; 2) a between-group effect size as small as 1.32; and 3) a phenotypic-fMRI correlation as low as 0.58. These calculations were based on a two-sided significance level of 0.05 and were performed with the R package ‘pwr’.(Champely et al., 2020)
3. Results and Discussion
Ten participants with stroke and 10 matched controls were enrolled and all participants completed data collection. The matching process resulted in very similar demographics between the stroke and control groups (Table 1; Supplementary File 2). Similar to previous study,(Malouin et al., 2008) groups were also similar in self-rated mental imagery ability, which lessens the possibility that group differences in mental imagery ability could confound comparisons of imagined walking activations. All participants were able to perform the knee extension task and reported that they were able to perform the imagined walking task. As expected, participants with stroke had significantly lower function than controls for nearly every gait measure (Table 1). A map of lesion overlap across participants is shown in Fig 3. Despite several very large strokes, no participants had lesions in any of the core locomotor ROIs.
Table 1.
Phenotypic variables and stroke characteristics
| Participants with Stroke (N=10) | Matched Controls (N=10) | |
|---|---|---|
| Participant Characteristics | ||
| Age, years | 59.8 (6.8) | 58.9 (7.8) |
| Females, N (%) | 4 (40%) | 4 (40%) |
| Body mass index (kg/m2) | 30.2 (4.2) | 30.8 (4.2) |
| Walking Function | ||
| Composite gait score† | −103.4 (150.1)* | 282.0 (76.4) |
| Comfortable overground gait speed, m/s | 0.41 (0.33)* | 1.36 (0.16) |
| Comfortable overground gait speed, % predicted | 30.4 (24.2)* | 100.5 (7.3) |
| Fastest overground gait speed, m/s | 0.57 (0.56)* | 1.94 (0.24) |
| 6-minute walk test, m | 156 (147)* | 537 (77) |
| 6-minute walk test, % predicted | 28.2 (24.8)* | 101.6 (15.7) |
| Spatiotemporal Gait Parameters | ||
| Cadence, steps per minute | 62.9 (31.5)* | 109.0 (7.4) |
| Absolute step time difference, s | 0.78 (0.71)* | 0.01 (0.01) |
| Mean step length, cm | 34.8 (12.2)* | 71.1 (9.8) |
| Absolute step length difference, cm | 10.7 (11.9) | 2.7 (2.3) |
| Paretic‡ step length, cm | 39.0 (11.8)* | 71.7 (9.5) |
| Non-paretic‡ step length, cm | 30.5 (15.9)* | 70.4 (10.4) |
| Mean single support, % gait cycle | 22.0 (8.9)* | 35.4 (1.7) |
| Absolute single-support difference, % gait cycle | 9.3 (6.4)* | 1.3 (2.4) |
| Paretic‡ single support, % gait cycle | 17.3 (9.1)* | 35.6 (2.2) |
| Non-paretic‡ single support, % gait cycle | 26.7 (9.8)* | 35.1 (2.1) |
| Subjective Mental Imagery Ability | ||
| Kinesthetic & Visual Imagery Questionnaire, 1–5 | 3.7 (0.7) | 4.0 (0.6) |
| Stroke Characteristics | ||
| Left hemispheric stroke, N (%) | 5 (50%) | NA |
| Stroke chronicity, years | 2.4 (1.7) | NA |
| Lesion volume, mL | 121.7 (105.0) | 0 (0) |
| Weighted corticofugal motor tract lesion load, AU | 10.9 (7.5) | 0 (0) |
Values are mean (SD) or N (%).
Significant (uncorrected p<0.05) difference between participants with stroke and controls.
Composite gait score is the primary component from a principle components analysis combining information from nine gait measures (see text for details).
Individual participant data provided in the Supplementary File 2.
Matched side for control group. AU, arbitrary units.
Figure 3. Lesion overlap across stroke sample (N=10).
Percentage of participants with lesion at each cortical surface vertex (top panel) and brain voxel (bottom panel). Right sided lesions (N=5) were reflected over the x-axis so that all primary lesions are on the left.
In the principal components analysis to obtain a composite gait measure, the primary component explained 99.0% of the variability among the following variables (correlation with primary component in parentheses): 6-minute walk test (0.9999), cadence (0.86), L-R mean step length (0.95), L-R mean single-support time (0.88), fastest gait speed (0.98), comfortable gait speed (0.98), absolute step time difference (−0.76), absolute single-support difference (−0.49) and absolute step length difference (−0.42). Importantly, measures where higher numbers indicate worse function (i.e. asymmetry measures) had negative associations with the primary component. Thus, the primary component appeared to be a valid measure of composite gait function.
3.1. Brain activation during imagined walking: Controls vs. previous study
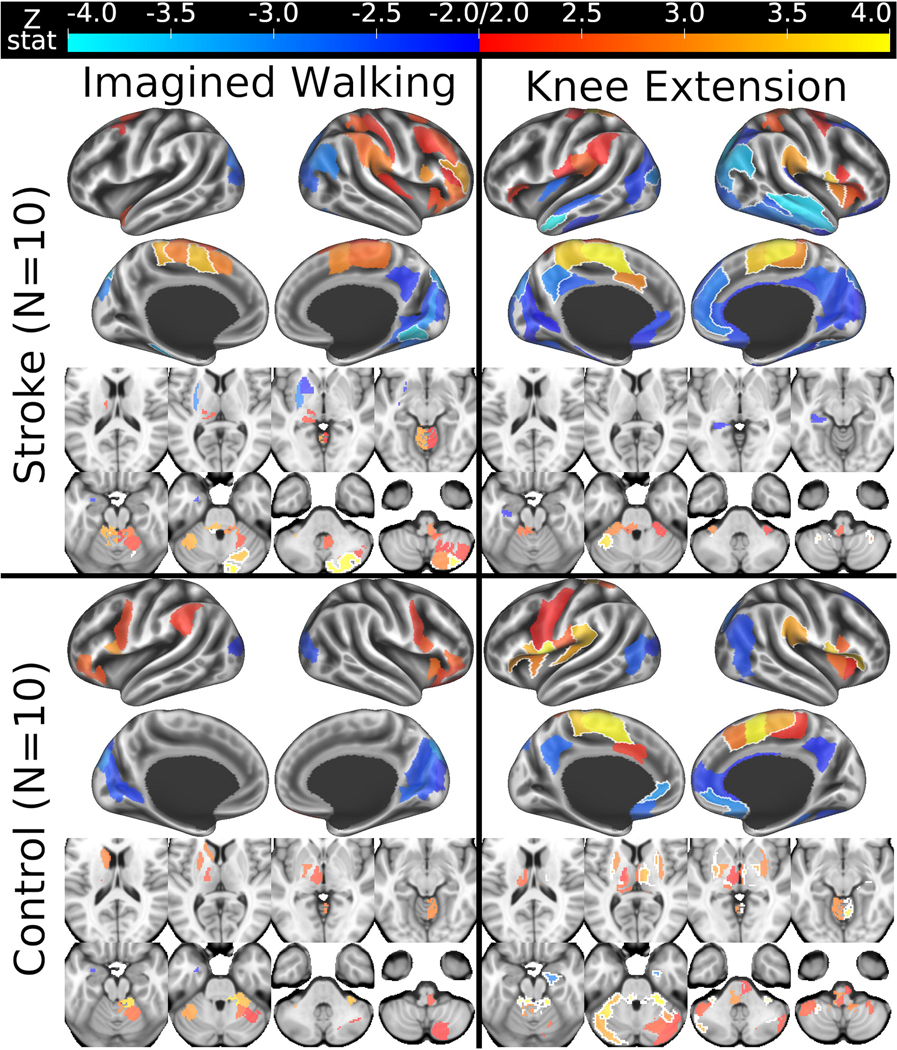
Similar to previous imagined walking fMRI studies among healthy adults,(Jahn et al., 2008; la Fougere et al., 2010; Jahn et al., 2008) control participants tended to activate the bilateral anterior insula and inferior frontal gyrus during imagined walking (activations thought to be related to the cognitive aspects of motor imagery(la Fougere et al., 2010)), as well as the premotor cortex and parts of the basal ganglia, thalamus, brainstem and cerebellum (Fig 4, bottom left panel; puncorrected<0.05 results provided for descriptive purposes). Unlike some previous studies which showed activation of the supplementary motor area, visual cortex and navigation-related parts of the cortex (e.g. parahippocampal gyri) during imagined walking,(Jahn et al., 2008; la Fougere et al., 2010; Jahn et al., 2008) our control participants tended to not activate or deactivate in those regions. We suspect that at least some of these differences were because our imagined walking protocol targeted motor & kinesthetic (vs. visual) imagery, whereas these previous studies were less specific or emphasized visual aspects of imagined walking.
Figure 4. Parcellated whole-brain fMRI activation contrasts by task and group.
Thresholded at uncorrected p < 0.05 with black outlines surrounding regions that were also statistically significant after false discovery rate correction for 300 parcels within each contrast. Since images were flipped across the x-axis for participants with right hemispheric stroke and their matched controls, the left side of MRI space is ipsilesional and contralateral to the paretic/matched knee extension. fMRI, functional magnetic resonance imaging.
3.2. Brain activation during imagined walking: Associations with stroke and walking function (hypothesis 1)
Consistent with hypothesis 1, overall brain activation during imagined walking significantly differed between the stroke and control groups (p=0.003) and was significantly associated with composite gait score while adjusting for stroke lesion load (p=0.0008; Table 2, first row). Interestingly, overall brain activation was higher for the stroke group vs. controls, which suggests that imagined walking preferentially reveals compensatory changes in brain activation after stroke. Also, greater brain activation was associated with worse walking function, raising the possibility that some of these compensatory changes could be maladaptive. More specific testing of this possibility is reported in section 3.5 below.
Table 2.
Overall associations with stroke and walking function by fMRI task
| Stroke - control activation differences | Activation associations with composite gait score (adjusted for wCMT-LL) | |||
|---|---|---|---|---|
| Imagined walking | Paretic knee extension | Imagined walking | Paretic knee extension | |
| Primary Analysis Using All Brain Parcels (300) | 0.16 ± 1.26* | −0.49 ± 1.29*† | −0.26 ± 1.82*† | 0.24 ± 1.91* |
| Sensitivity Analyses Using Subsets of Brain Parcels | ||||
| From gait fMRI meta-analysis (85) | 0.20 ± 1.22 | −0.78 ± 1.23*† | −0.40 ± 1.73*† | 0.23 ± 2.22* |
| Cortical (210) | 0.21 ± 1.27* | −0.38 ± 1.25*† | −0.30 ± 1.82*† | −0.08 ± 1.69 |
| Cortical sensorimotor (42) | 0.61 ± 1.02* | 0.25 ± 1.16* | −1.13 ± 1.84*† | −0.68 ± 1.56* |
| Subcortical (47) | −0.27 ± 1.24 | −1.01 ± 1.31*† | −0.35 ± 1.61 | 0.97 ± 1.69*† |
| Basal ganglia (10) | −0.92 ± 1.96* | −1.42 ± 1.52* | −0.25 ± 2.00 | 1.14 ± 0.89*† |
| Cerebellum (43) | 0.24 ± 1.32* | −0.69 ± 1.08*† | −0.06 ± 1.73 | 1.51 ± 1.98*† |
| Brainstem (11) | 0.37 ± 1.12 | −0.53 ± 0.96*† | −0.35 ± 1.08 | 0.66 ± 1.16† |
Values are the median ± interquartile range Z statistic across brain parcels. Negative Z statistics for stroke – control differences indicate that activation was lower for the stroke group vs. the control group.
Significantly different from zero.
Significant (p<0.05) difference between tasks (imagined walking vs. knee extension) from Wilcoxon Signed Rank tests. fMRI, functional magnetic resonance imaging; wCMT-LL, weighted corticofugal motor tract lesion load.
In the sensitivity analysis using subsets of brain parcels (Table 2, all rows except first), results were generally consistent with the primary whole-brain analysis, especially in cortical sensorimotor regions. However, basal ganglia activation during imagined walking was significantly lower in the stroke group vs. controls (the opposite of the whole-brain results). In addition, the negative association between brain activation during imagined walking and gait function appeared to be fairly specific to cortical sensorimotor regions. These findings suggest that the cortex may be particularly capable of compensatory upregulation after stroke. We further speculate that the reduced basal ganglia activation for the stroke group could be related to previous observations of lower gait automaticity in this population,(Kal et al., 2016; Orrell et al., 2009; Hyndman et al., 2006) since the basal ganglia contribute to subconscious locomotor control mechanisms.(Takakusaki et al., 2004; Peterson and Horak, 2016). Alternatively, these findings could also reflect a higher relative subcortical vs. cortical lesion burden.
3.3. Comparison of imagined walking and knee extension tasks (hypothesis 2)
Compared with knee extension, imagined walking elicited significantly greater overall brain activation in the stroke group vs. controls (p=3.7 × 10−15; Table 2), consistent with hypothesis 2. In fact, the stroke-control differences were opposite between the two tasks, with knee extension eliciting lesser activation in the stroke group vs. controls (p=3.9 × 10−16). This finding suggests that knee extension tends to reveal stroke-related deficits in brain activation. In the sensitivity analysis, this was consistent across brain regions, except for cortical sensorimotor areas. These areas showed the opposite pattern (more active in the stroke vs. control group), similar to the imagined walking task.
Contrary to hypothesis 2, the overall association between brain activation and walking function was significantly more negative for imagined walking vs. knee extension (p=3.8 × 10−8; Table 2). The direction of the association was opposite between the two tasks, such that better walking function was generally associated with less brain activation during imagined walking and greater activation during knee extension (p=0.0001). While the negative association for the imagined walking task was fairly specific to cortical sensorimotor regions, the positive association for the knee extension task was fairly specific to the basal ganglia and cerebellum. Similar to the imagined walking task, knee extension activation in cortical sensorimotor regions was negatively associated with walking function (Table 2).
Taken together, these results indicate that imagined walking and knee extension reflect very different aspects of locomotor control. Yet, the shared cortical sensorimotor activation & associations between the two tasks reinforce the possibility that the cortex is particularly capable of compensatory upregulation after stroke. The negative associations between walking function and cortical sensorimotor activations for both tasks also further elevate the possibility of maladaptive compensation.
3.4. Core locomotor ROI activation: Stroke vs. controls
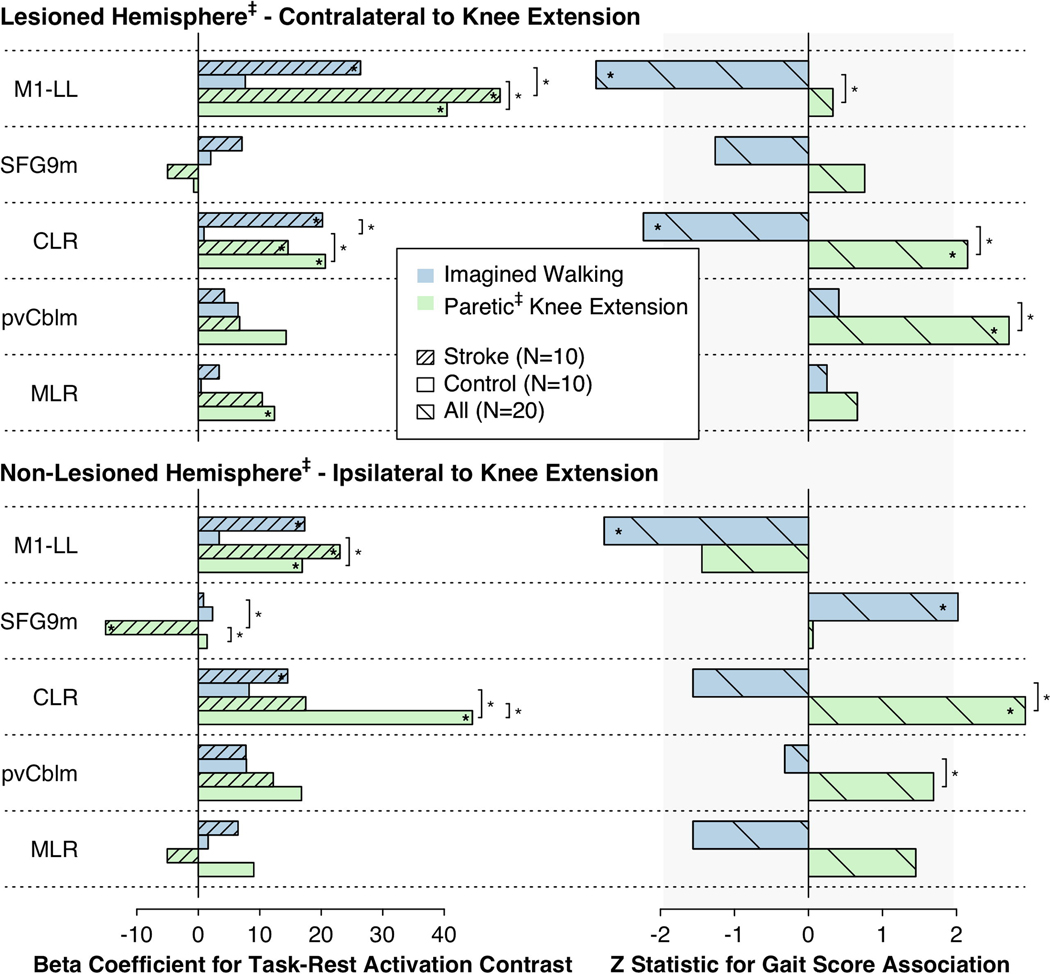
Consistent with hypothesis 3, only the stroke group showed significant activation of the bilateral M1-LL and CLR during imagined walking (p=0.009–0.0002; Fig 5). Although the stroke-control difference was only statistically significant for the ipsilesional/matched CLR (p=0.005 vs. 0.35–0.11 for the contralesional CLR and bilateral M1-LL), these results provide additional evidence that M1-LL/CLR upregulation may be a compensatory response to gait impairment.(Boyne et al., 2018)
Figure 5. Task fMRI activation contrasts and associations with walking function in core locomotor ROIs.
The left panel shows task minus rest activation contrasts for each core ROI separately for the stroke and control groups. The right panel shows associations between task activations and composite gait scores across all participants while controlling for wCMT-LL. The vertical gray shaded area shows the non-significant region (p ≥ 0.05 uncorrected). *Asterisks within bars indicate significant activations or associations. *Asterisks next to square braces indicate significant differences between tasks (imagined walking vs. paretic‡ knee extension) or groups (stroke vs. control). ‡Matched side for control group. fMRI, functional magnetic resonance imaging; ROI, region of interest; M1-LL, Primary motor cortex lower limb region; SFG9m, Superior frontal gyrus medial area 9; CLR, cerebellar locomotor region; pvCblm, Paravermal cerebellum area VI; MLR, midbrain locomotor region.
Knee extension significantly activated the bilateral M1-LL and ipsilesional CLR (contralateral to knee extension) in both groups (p=0.02 to 2.0 × 10−7; Fig 5). In the control group, it also significantly activated the matched contralesional CLR (ipsilateral to knee extension) and the matched ipsilesional MLR (p=4.1 × 10−5 and 0.04, respectively) Contralesional/ipsilateral CLR activation was significantly lower in the stroke group (p=0.04). These findings are consistent with the whole brain results suggesting that knee extension preferentially highlighted stroke-related deficits in brain activation, rather than compensatory upregulation.
3.5. Core locomotor ROI activation: Associations with walking function
The directional associations proposed under hypothesis 4 were based on previous findings among healthy adults across the lifespan, which tested associations between six-minute walk distance and resting-state functional connectivity among the same ROIs used for this study (while adjusting for age, sex, height and body mass index).(Boyne et al., 2018) Compared with knee extension, the overall pattern of gait function associations for imagined walking more closely resembled these previous findings from resting-state connectivity. For example, gait function in this study was positively associated with contralesional SFG9m activation and negatively associated with bilateral M1-LL and ipsilesional CLR activation during imagined walking (p=0.004–0.04; Fig 5). However, the hypothesized positive gait function association with pvCblm activation was only observed for the knee extension task (p=0.007 & 0.09), suggesting that imagined movement may recruit the cerebellum in a different way than actual movement. Although group mean cerebellar activations are generally similar between imagined and actual movements,(Sharma and Baron, 2013; la Fougere et al., 2010) individual cerebellar activation during imagined walking is known to vary based on motor imagery ability,(van der Meulen et al., 2014) which could obscure the relationship between pvCblm activation and actual gait function. Another null finding was that neither task showed significant evidence of the hypothesized positive gait function association for the MLR (p=0.12–0.81). This might indicate that the magnitude of task-related MLR activation is less relevant than its connectivity, but it could also be due to differences in MLR importance between stroke survivors & healthy adults, or between-study differences in covariates or sample size.
Altogether, the current results indicate that imagined walking and knee extension fMRI may each provide complementary insights into neurologic gait function. Given that previous studies among healthy adults have largely used imagined walking fMRI to assess locomotor control,(Wang et al., 2008; la Fougere et al., 2010; Sacco et al., 2006; Jahn et al., 2008) similar future studies should consider including a lower limb motor task, particularly if interested in subcortical or cerebellar ROIs. Likewise, future stroke studies interested in locomotion-related activation should consider adding an imagined walking task to the commonly used paretic knee extension or ankle dorsiflexion paradigm, particularly if interested in cortical sensorimotor ROIs.
The present finding that gait function was negatively associated with M1-LL and CLR activation during imagined walking (while controlling for lesion load) also supports the counterintuitive possibility that M1-LL and CLR upregulation may be maladaptive. Similarly, in the previously referenced study, greater resting functional connectivity between M1-LL and CLR was associated with lower six-minute walk distance among healthy adults, while adjusting for known confounders.(Boyne et al., 2018) Likewise, in two studies among young healthy adults, increased oxygenated hemoglobin concentration in M1-LL during actual treadmill walking has been associated with increased gait variability (decreased consistency).(Kurz et al., 2012; Berger et al., 2019) Taken together, these results suggest that M1-LL/CLR upregulation may be a common and potentially maladaptive response to gait disturbance that is not specific to stroke.
However, these findings cannot determine the order of events (e.g. [M1-LL/CLR upregulation → lower gait function] vs. [lower gait function → M1-LL/CLR upregulation]), nor can they completely rule out a common cause of both M1-LL/CLR upregulation and lower gait function (e.g. gait impairment due to stroke or subclinical pathology, episodes of random movement error) as a non-causal explanation for the association (i.e. incomplete control of confounding). For the present study, higher stroke lesion load was a plausible confounding factor that could theoretically cause both worse walking function and a compensatory increase in M1-LL/CLR activation. Indeed, lesion load (wCMT-LL) was negatively correlated with the composite gait score (full sample (N=20) r=−0.73, p=0.0003; stroke group (N=10) r=−0.30, p=0.40). Yet, the negative association between M1-LL/CLR activation and walking function was present even when controlling for lesion load, which suggests that these findings may not be attributable to stroke/control status or differences in stroke severity. Thus, it is possible that M1-LL/CLR upregulation is maladaptive, but longitudinal and experimental studies are needed to establish causation and better elucidate the role of M1-LL/CLR upregulation in locomotor recovery.
It is also intriguing that greater contralesional/matched SFG9m activation during imagined walking was associated with better walking function (Fig 5). Although SFG9m has not typically been considered as a motor region, this finding contributes to a growing body of evidence that this region is more relevant than previously recognized for locomotor function.(Boyne et al., 2018) Future studies are now needed to further elucidate the role of SFG9m in walking.
3.6. Limitations
A primary study limitation described above is that the cross-sectional design limits the causal inference that can be drawn from the identified associations. Another important limitation is that the sample size was relatively small (N=20 total). By analyzing repeated fMRI volumes and task cycles within each participant, the first level fMRI analyses effectively increased the degrees of freedom for the between-participant (second level) analysis. While this appeared to provide sufficient statistical power for hypothesis testing, the results should still be viewed as preliminary since they could be more prone to fluctuate with the addition of other participants in future studies. The low sample size also precluded any dense voxel-wise or vertex-wise hypothesis testing to more specifically localize any potential associations within or outside of our a priori ROIs. Such an analysis requires a higher sample size to have adequate statistical power due to the increased need for multiple comparisons adjustment resulting from hypothesis tests at every gray-ordinate.
4. Conclusions
Imagined walking and knee extension each elicit brain activation related to walking ability, but seem to assess different aspects of locomotor function. Imagined walking appeared to better reveal compensatory responses to stroke and to generally provide better differentiation of gait function in cortical sensorimotor regions. On the other hand, knee extension was better at identifying stroke-related deficits in brain activation and appeared to generally provide better differentiation of gait function in the subcortex & cerebellum. Thus, future studies may want to consider including both fMRI tasks, depending on the aims. M1-LL & CLR upregulation seems to be a common neural compensation for gait impairment. The current cross-sectional results suggest that this compensation could be maladaptive, but experimental and longitudinal studies are needed for further evaluation. In addition, SFG9m appears to play an important but poorly understood role in human walking function, which warrants further study.
Supplementary Material
Supplementary File 1. Appendix.docx. Standardized instructions for gait imagery and task fMRI practice.
Supplementary File 2. Table 1. Individual Data.xlsx. Individual participant characteristics.
Significance:
The identified associations for imagined walking suggest potential neural mechanisms of locomotor adaptation after stroke, which could be useful for future intervention development and prognostication.
Highlights.
Imagined walking evokes greater overall brain activation in stroke survivors vs. controls, likely revealing compensatory changes.
Greater activation during imagined walking is associated with worse gait function, raising the possibility of maladaptation.
Compared with unilateral knee extension, imagined walking shows reverse associations with stroke and gait function.
Acknowledgements
The authors thank Hamza Sultan, Susan Patel, Daniel Tyson and the teams in the UC Medical Center Cardiovascular Stress Laboratory and Cincinnati Children’s Hospital Imaging Research Center for their assistance with data collection.
Funding Sources
This work was supported by the National Institutes of Health [grant numbers KL2TR001426, UL1TR001425, R01HD093694]; and the American Heart Association [grant number 17MCPRP33670446]. Funding sources were not involved in the design, execution or reporting of the study.
Abbreviations
- CIFTI
connectivity informatics technology initiative
- CLR
cerebellar locomotor region
- ECG
electrocardiogram
- fMRI
functional magnetic resonance imaging
- FSL
functional MRI of the brain software library
- ICA-AROMA
independent components analysis based automatic removal of motion artifacts
- MLR
midbrain locomotor region
- M1-LL
primary motor cortex lower limb region
- pvCblm
paravermal cerebellum area VI
- ROI
region of interest
- SFG9m
superior frontal gyrus medial area 9
- T1w
T1-weighted MRI
- wCMT-LL
weighted corticofugal motor tract lesion load
Footnotes
Declaration of Interest
The authors declare no conflicts of interest.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ada L, Dean CM, Lindley R. Randomized trial of treadmill training to improve walking in community-dwelling people after stroke: the AMBULATE trial. Int.J.Stroke 2013;8:436–444. [DOI] [PubMed] [Google Scholar]
- American College of Sports Medicine. ACSM’s guidelines for exercise testing and prescription. 9th ed Philadephia, PA: Lippincott Williams & Wilkins, 2014. [Google Scholar]
- Archer DB, Vaillancourt DE, Coombes SA. A Template and Probabilistic Atlas of the Human Sensorimotor Tracts using Diffusion MRI. Cereb.Cortex 2018;28:1685–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashworth B. Preliminary Trial of Carisoprodol in Multiple Sclerosis. Practitioner 1964;192:540–542. [PubMed] [Google Scholar]
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am.J.Respir.Crit.Care Med 2002;166:111–117. [DOI] [PubMed] [Google Scholar]
- Berger A, Horst F, Steinberg F, Thomas F, Muller-Eising C, Schollhorn WI, et al. Increased gait variability during robot-assisted walking is accompanied by increased sensorimotor brain activity in healthy people. J.Neuroeng Rehabil 2019;16:161–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilney B, Morris M, Webster K. Concurrent related validity of the GAITRite walkway system for quantification of the spatial and temporal parameters of gait. Gait Posture 2003;17:68–74. [DOI] [PubMed] [Google Scholar]
- Bohannon RW, Williams Andrews A. Normal walking speed: a descriptive meta-analysis. Physiotherapy 2011;97:182–189. [DOI] [PubMed] [Google Scholar]
- Bohnen NI, Jahn K. Imaging: What can it tell us about parkinsonian gait? Mov.Disord. 2013;28:1492–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyne P, Dunning K, Carl D, Gerson M, Khoury J, Kissela B. Within-session responses to high-intensity interval training in chronic stroke. Med.Sci.Sports Exerc 2015;47:476–484. [DOI] [PubMed] [Google Scholar]
- Boyne P, Dunning K, Carl D, Gerson M, Khoury J, Rockwell B, et al. High-Intensity Interval Training and Moderate-Intensity Continuous Training in Ambulatory Chronic Stroke: A Feasibility Study. Phys.Ther 2016;96:1533–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyne P, Maloney T, DiFrancesco M, Fox MD, Awosika O, Aggarwal P, et al. Resting-state functional connectivity of subcortical locomotor centers explains variance in walking capacity. Hum.Brain Mapp 2018;39:4831–4843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brott T, Adams HP Jr, Olinger CP, Marler JR, Barsan WG, Biller J, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke 1989;20:864–870. [DOI] [PubMed] [Google Scholar]
- Burke E, Dobkin BH, Noser EA, Enney LA, Cramer SC. Predictors and biomarkers of treatment gains in a clinical stroke trial targeting the lower extremity. Stroke 2014;45:2379–2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champely S, Ekstrom C, Dalgaard P, Gill J, Weibelzahl S, Ford C, et al. Basic Functions for Power Analysis (pwr) v1.3.0. 2020. [Google Scholar]
- Cremers J, D’Ostilio K, Stamatakis J, Delvaux V, Garraux G. Brain activation pattern related to gait disturbances in Parkinson’s disease. Mov.Disord. 2012;27:1498–1505. [DOI] [PubMed] [Google Scholar]
- Cutlip RG, Mancinelli C, Huber F, DiPasquale J. Evaluation of an instrumented walkway for measurement of the kinematic parameters of gait. Gait Posture 2000;12:134–138. [DOI] [PubMed] [Google Scholar]
- Dawes H, Enzinger C, Johansen-Berg H, Bogdanovic M, Guy C, Collett J, et al. Walking performance and its recovery in chronic stroke in relation to extent of lesion overlap with the descending motor tract. Exp.Brain Res 2008;186:325–333. [DOI] [PubMed] [Google Scholar]
- Di X, Gohel S, Kim EH, Biswal BB. Task vs. rest-different network configurations between the coactivation and the resting-state brain networks. Front.Hum.Neurosci 2013;7:493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickstein R, Dunsky A, Marcovitz E. Motor imagery for gait rehabilitation in post-stroke hemiparesis. Phys.Ther 2004;84:1167–1177. [PubMed] [Google Scholar]
- Dobkin BH, Firestine A, West M, Saremi K, Woods R. Ankle dorsiflexion as an fMRI paradigm to assay motor control for walking during rehabilitation. Neuroimage 2004;23:370–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan PW, Sullivan KJ, Behrman AL, Azen SP, Wu SS, Nadeau SE, et al. Protocol for the Locomotor Experience Applied Post-stroke (LEAPS) trial: a randomized controlled trial. BMC Neurol. 2007;7:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enzinger C, Dawes H, Johansen-Berg H, Wade D, Bogdanovic M, Collett J, et al. Brain activity changes associated with treadmill training after stroke. Stroke 2009;40:2460–2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan L, Li H, Zhuo J, Zhang Y, Wang J, Chen L, et al. The Human Brainnetome Atlas: A New Brain Atlas Based on Connectional Architecture. Cereb.Cortex 2016;26:3508–3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasano A, Laganiere SE, Lam S, Fox MD. Lesions causing freezing of gait localize to a cerebellar functional network. Ann.Neurol 2017;81:129–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favre I, Zeffiro TA, Detante O, Krainik A, Hommel M, Jaillard A. Upper limb recovery after stroke is associated with ipsilesional primary motor cortical activity: a meta-analysis. Stroke 2014;45:1077–1083. [DOI] [PubMed] [Google Scholar]
- Finn ES, Shen X, Scheinost D, Rosenberg MD, Huang J, Chun MM, et al. Functional connectome fingerprinting: identifying individuals using patterns of brain connectivity. Nat.Neurosci 2015;18:1664–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B. FreeSurfer. Neuroimage 2012;62:774–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Buckner RL, Liu H, Chakravarty MM, Lozano AM, Pascual-Leone A. Resting-state networks link invasive and noninvasive brain stimulation across diverse psychiatric and neurological diseases. Proc.Natl.Acad.Sci.U.S.A 2014;111:4367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraix V, Bastin J, David O, Goetz L, Ferraye M, Benabid AL, et al. Pedunculopontine nucleus area oscillations during stance, stepping and freezing in Parkinson’s disease. PLoS One 2013;8:e83919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulk GD, Echternach JL, Nof L, O’Sullivan S. Clinometric properties of the six-minute walk test in individuals undergoing rehabilitation poststroke. Physiother.Theory Pract 2008;24:195–204. [DOI] [PubMed] [Google Scholar]
- Fulk GD, Reynolds C, Mondal S, Deutsch JE. Predicting home and community walking activity in people with stroke. Arch.Phys.Med.Rehabil 2010;91:1582–1586. [DOI] [PubMed] [Google Scholar]
- Glasser MF, Sotiropoulos SN, Wilson JA, Coalson TS, Fischl B, Andersson JL, et al. The minimal preprocessing pipelines for the Human Connectome Project. Neuroimage 2013;80:105–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffis JC, Allendorfer JB, Szaflarski JP. Voxel-based Gaussian naive Bayes classification of ischemic stroke lesions in individual T1-weighted MRI scans. J.Neurosci.Methods 2016;257:97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwin JT, Gramann K, Makeig S, Ferris DP. Electrocortical activity is coupled to gait cycle phase during treadmill walking. Neuroimage 2011;54:1289–1296. [DOI] [PubMed] [Google Scholar]
- Hill K, Ellis P, Bernhardt J, Maggs P, Hull S. Balance and mobility outcomes for stroke patients: a comprehensive review. Aust J Physiother 1997;43:173–80. [DOI] [PubMed] [Google Scholar]
- Hornby TG, Henderson CE, Plawecki A, Lucas E, Lotter J, Holthus M, et al. Contributions of Stepping Intensity and Variability to Mobility in Individuals Poststroke. Stroke 2019;50:2492–2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornby TG, Holleran CL, Hennessy PW, Leddy AL, Connolly M, Camardo J, et al. Variable Intensive Early Walking Poststroke (VIEWS): A Randomized Controlled Trial. Neurorehabil.Neural Repair 2016;30:440–50. [DOI] [PubMed] [Google Scholar]
- Hyndman D, Ashburn A, Yardley L, Stack E. Interference between balance, gait and cognitive task performance among people with stroke living in the community. Disabil.Rehabil 2006;28:849–856. [DOI] [PubMed] [Google Scholar]
- Jaeger L, Marchal-Crespo L, Wolf P, Riener R, Michels L, Kollias S. Brain activation associated with active and passive lower limb stepping. Front.Hum.Neurosci 2014;8:828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn K, Deutschlander A, Stephan T, Kalla R, Hufner K, Wagner J, et al. Supraspinal locomotor control in quadrupeds and humans. Prog.Brain Res 2008;171:353–362. [DOI] [PubMed] [Google Scholar]
- Jahn K, Deutschlander A, Stephan T, Kalla R, Wiesmann M, Strupp M, et al. Imaging human supraspinal locomotor centers in brainstem and cerebellum. Neuroimage 2008;39:786–792. [DOI] [PubMed] [Google Scholar]
- Jahn K, Deutschlander A, Stephan T, Strupp M, Wiesmann M, Brandt T. Brain activation patterns during imagined stance and locomotion in functional magnetic resonance imaging. Neuroimage 2004;22:1722–1731. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. Fsl. Neuroimage 2012;62:782–790. [DOI] [PubMed] [Google Scholar]
- Kal E, Houdijk H, Van Der Wurff P, Groet E, Van Bennekom C, Scherder E, et al. The inclination for conscious motor control after stroke: Validating the Movement-Specific Reinvestment Scale for use in inpatient stroke patients. Disabil.Rehabil 2016;38:10971–106. [DOI] [PubMed] [Google Scholar]
- Knaepen K, Mierau A, Tellez HF, Lefeber D, Meeusen R. Temporal and spatial organization of gait-related electrocortical potentials. Neurosci.Lett 2015;599:75–80. [DOI] [PubMed] [Google Scholar]
- Kurz MJ, Wilson TW, Arpin DJ . Stride-time variability and sensorimotor cortical activation during walking. Neuroimage 2012;59:1602–1607. [DOI] [PubMed] [Google Scholar]
- la Fougere C, Zwergal A, Rominger A, Forster S, Fesl G, Dieterich M, et al. Real versus imagined locomotion: a [18F]-FDG PET-fMRI comparison. Neuroimage 2010;50:1589–1598. [DOI] [PubMed] [Google Scholar]
- Lafleur MF, Jackson PL, Malouin F, Richards CL, Evans AC, Doyon J. Motor learning produces parallel dynamic functional changes during the execution and imagination of sequential foot movements. Neuroimage 2002;16:142–157. [DOI] [PubMed] [Google Scholar]
- Liew S, Anglin JM, Banks NW, Sondag M, Ito KL, Kim H, et al. A large, open source dataset of stroke anatomical brain images and manual lesion segmentations. Scientific Data 2018;5:180011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luft AR, Forrester L, Macko RF, McCombe-Waller S, Whitall J, Villagra F, et al. Brain activation of lower extremity movement in chronically impaired stroke survivors. Neuroimage 2005;26:184–194. [DOI] [PubMed] [Google Scholar]
- Luft AR, Macko RF, Forrester LW, Villagra F, Ivey F, Sorkin JD, et al. Treadmill Exercise Activates Subcortical Neural Networks and Improves Walking After Stroke. A Randomized Controlled Trial. Stroke 2008;39:3341–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luft AR, Skalej M, Stefanou A, Klose U, Voigt K. Comparing motion- and imagery-related activation in the human cerebellum: a functional MRI study. Hum.Brain Mapp 1998;6:105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luft AR, Smith GV, Forrester L, Whitall J, Macko RF, Hauser TK, et al. Comparing brain activation associated with isolated upper and lower limb movement across corresponding joints. Hum.Brain Mapp 2002;17:131–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luft AR, Waller S, Forrester L, Smith GV, Whitall J, Macko RF, et al. Lesion location alters brain activation in chronically impaired stroke survivors. Neuroimage 2004;21:924–935. [DOI] [PubMed] [Google Scholar]
- Malouin F, Richards CL, Durand A, Doyon J. Clinical assessment of motor imagery after stroke. Neurorehabil.Neural Repair 2008a;22:330–340. [DOI] [PubMed] [Google Scholar]
- Malouin F, Richards CL, Durand A, Doyon J. Reliability of mental chronometry for assessing motor imagery ability after stroke. Arch.Phys.Med.Rehabil 2008b;89:311–319. [DOI] [PubMed] [Google Scholar]
- Malouin F, Richards CL, Jackson PL, Lafleur MF, Durand A, Doyon J. The Kinesthetic and Visual Imagery Questionnaire (KVIQ) for assessing motor imagery in persons with physical disabilities: a reliability and construct validity study. J.Neurol.Phys.Ther 2007;31:20–29. [DOI] [PubMed] [Google Scholar]
- Mayo NE, Wood-Dauphinee S, Ahmed S, Gordon C, Higgins J, McEwen S, et al. Disablement following stroke. Disabil.Rehabil 1999;21:258–268. [DOI] [PubMed] [Google Scholar]
- Middleton A, Fritz SL, Lusardi M. Walking speed: the functional vital sign. J.Aging Phys.Act 2015;23:314–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyai I, Tanabe HC, Sase I, Eda H, Oda I, Konishi I, et al. Cortical mapping of gait in humans: a near-infrared spectroscopic topography study. Neuroimage 2001;14:1186–1192. [DOI] [PubMed] [Google Scholar]
- Orrell AJ, Masters RSW, Eves FF. Reinvestment and Movement Disruption Following Stroke. Neurorehabil.Neural Repair 2009;23:177–183. [DOI] [PubMed] [Google Scholar]
- Peters DM, Fridriksson J, Stewart JC, Richardson JD, Rorden C, Bonilha L, et al. Cortical disconnection of the ipsilesional primary motor cortex is associated with gait speed and upper extremity motor impairment in chronic left hemispheric stroke. Hum.Brain Mapp 2018;39:120–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson DS, Horak FB. Neural Control of Walking in People with Parkinsonism. Physiology (Bethesda) 2016;31:95–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson DS, Pickett KA, Duncan RP, Perlmutter JS, Earhart GM. Brain activity during complex imagined gait tasks in Parkinson disease. Clin.Neurophysiol 2014;125:995–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruim RHR, Mennes M, Buitelaar JK, Beckmann CF. Evaluation of ICA-AROMA and alternative strategies for motion artifact removal in resting state fMRI. Neuroimage 2015;112:278–287. [DOI] [PubMed] [Google Scholar]
- Pruim RHR, Mennes M, van Rooij D, Llera A, Buitelaar JK, Beckmann CF. ICA-AROMA: A robust ICA-based strategy for removing motion artifacts from fMRI data. Neuroimage 2015;112:267–277. [DOI] [PubMed] [Google Scholar]
- Robinson EC, Garcia K, Glasser MF, Chen Z, Coalson TS, Makropoulos A, et al. Multimodal surface matching with higher-order smoothness constraints. Neuroimage 2018;167:453–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacco K, Cauda F, Cerliani L, Mate D, Duca S, Geminiani GC. Motor imagery of walking following training in locomotor attention. The effect of “the tango lesson”. Neuroimage 2006;32:1441–1449. [DOI] [PubMed] [Google Scholar]
- Sharma N, Baron JC. Does motor imagery share neural networks with executed movement: a multivariate fMRI analysis. Front.Hum.Neurosci 2013;7:564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma N, Pomeroy VM, Baron JC. Motor imagery: a backdoor to the motor system after stroke? Stroke 2006;37:1941–1952. [DOI] [PubMed] [Google Scholar]
- Shen X, Tokoglu F, Papademetris X, Constable RT. Groupwise whole-brain parcellation from resting-state fMRI data for network node identification. Neuroimage 2013;82:403–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JS, Shulman GL, Corbetta M. Measuring functional connectivity in stroke: Approaches and considerations. J.Cereb.Blood Flow Metab 2017;37:2665–2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MC, Barber PA, Stinear CM. The TWIST Algorithm Predicts Time to Walking Independently After Stroke. Neurorehabil.Neural Repair 2017;31:955–964. [DOI] [PubMed] [Google Scholar]
- Stinear CM, Barber PA, Smale PR, Coxon JP, Fleming MK, Byblow WD. Functional potential in chronic stroke patients depends on corticospinal tract integrity. Brain 2007;130:170–180. [DOI] [PubMed] [Google Scholar]
- Takakusaki K, Oohinata-Sugimoto J, Saitoh K, Habaguchi T. Role of basal ganglia-brainstem systems in the control of postural muscle tone and locomotion. Prog.Brain Res 2004;143:231–237. [DOI] [PubMed] [Google Scholar]
- Tilson JK, Sullivan KJ, Cen SY, Rose DK, Koradia CH, Azen SP, et al. Meaningful gait speed improvement during the first 60 days poststroke: minimal clinically important difference. Phys.Ther 2010;90:196–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meulen M, Allali G, Rieger SW, Assal F, Vuilleumier P. The influence of individual motor imagery ability on cerebral recruitment during gait imagery. Hum.Brain Mapp 2014;35:455–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen DC, Glasser MF, Dierker DL, Harwell J, Coalson T. Parcellations and hemispheric asymmetries of human cerebral cortex analyzed on surface-based atlases. Cereb.Cortex 2012;22:2241–2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Wai Y, Kuo B, Yeh YY, Wang J. Cortical control of gait in healthy humans: an fMRI study. J.Neural Transm.(Vienna) 2008;115:1149–1158. [DOI] [PubMed] [Google Scholar]
- Williams LS, Brizendine EJ, Plue L, Bakas T, Tu W, Hendrie H, et al. Performance of the PHQ-9 as a screening tool for depression after stroke. Stroke 2005;36:635–638. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Behrens TE, Beckmann CF, Jenkinson M, Smith SM. Multilevel linear modelling for FMRI group analysis using Bayesian inference. Neuroimage 2004;21:1732–1747. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Ripley BD, Brady M, Smith SM. Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage 2001;14:1370–1386. [DOI] [PubMed] [Google Scholar]
- Yeo SS, Ahn SH, Choi BY, Chang CH, Lee J, Jang SH. Contribution of the pedunculopontine nucleus on walking in stroke patients. Eur.Neurol 2011;65:332–337. [DOI] [PubMed] [Google Scholar]
- Zhu LL, Lindenberg R, Alexander MP, Schlaug G. Lesion load of the corticospinal tract predicts motor impairment in chronic stroke. Stroke 2010;41:910–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary File 1. Appendix.docx. Standardized instructions for gait imagery and task fMRI practice.
Supplementary File 2. Table 1. Individual Data.xlsx. Individual participant characteristics.