Abstract
A growing literature has shown that volatile anesthetics are promiscuous molecules targeting multiple molecules, some of which are critical for immunological functions. We focused on studies that delineated target molecules of volatile anesthetics on immune cells and summarized the effects of volatile anesthetics on immune functions. We also presented the perspectives of studying volatile anesthetics-mediated immunomodulation.
Keywords: Volatile anesthetics, Immunomodulation, Surgery
Introduction
Surgery elicits innate immune responses via several mechanisms. Our body is equipped with a number of defense systems including protective barriers (for example, the skin and mucosa) and effector immune cells. Surgery involves the incision and the dissection of these protective barriers and allows microorganisms to invade otherwise sterile sites of the body. The host effector immune cells respond to microbial components called pathogen associated molecular patterns (PAMPs) via their pattern recognition receptors (PRRs). For example, Toll-like receptor (TLR)2 responds to gram positive bacterial and fungal components and TLR4 responds to gram negative bacterial components. Damaged tissues and cells release endogenous peptides called damage associated molecular patterns (DAMPs) to alert the host to the danger. The host PRRs also bind to DAMPs to recognize the danger and activate effector immune cells. TLR2 and TLR4 recognize the majority of DAMPs and lie at the interface of microbial and sterile inflammation 1.
Neutrophils and monocytes/macrophages are the primary responders for host defense in the early perioperative period and have various PRRs including TLR2 and TLR4. The stimulation of these professional phagocytes by DAMPs and PAMPs at the site of infection and/or inflammation activates a cascade of intracellular signaling pathways to produce an array of proinflammatory mediators and enhance phagocytosis 2. In addition, TLR4 stimulation induces the activation of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, which leads to the production of reactive oxygen species (ROS) 1. PAMPs and DAMPs can enter the systemic circulation and affect circulating neutrophils and monocytes. TLR2 and TLR4 stimulation induces the adhesion of these phagocytes to the endothelium via the activation of adhesion molecules integrins 3,4.
Volatile anesthetics are widely used drugs to provide general anesthesia during surgery. Volatile anesthetics in current clinical use include halogenated ethers; isoflurane, sevoflurane and desflurane. They are promiscuous small molecules and possess immunomodulatory properties 5–7. We reviewed the effect of volatile anesthetics on immune cells both in the systemic circulation and at local inflammatory sites by including mechanistic considerations.
Methods
We searched electronic databases:
MEDLINE/PubMed until April 30, 2020. We focused to identify studies describing molecular targets of volatile anesthetics on neutrophils and monocytes/macrophages. “Isoflurane”, “Sevoflurane”, “Desflurane”, “Halothane”, “Ether”, “Volatile anesthetics”, “Inhaled anesthetics”, “Anesthesia”, “Neutrophil”, “Monocyte”, “Macrophage”, “Structure”, “X-ray crystallography”, “Nuclear magnetic resonance (NMR)”, “Photolabeling”, “Photoactivatable anesthetics”, “Mass spectrometry”, “Docking” and “Mutagenesis” were chosen for search.
Volatile anesthetic targets on immune cells
The majority of volatile anesthetic targets in the central nervous system for anesthesia (“canonical targets”) are ion channels including γ-aminobutyric acid type A (GABAA) receptor, N-methyl-D-aspartate (NMDA) receptor and two-pore-domain K+ (K2P) channel 8. A growing literature has shown that volatile anesthetics also target the following non-ion channel molecules on immune cells (“non-canonical targets”).
TLRs
TLRs are one of major PRRs. So far 10 human TLRs (TLR1-TLR10) and 12 mouse TLRs (TLR1–9, TLR11–13) have been identified. TLRs have characteristic leucine-rich-repeats (LRRs), responsible for their horseshoe structures 9. Particularly TLR2 and TLR4 are structurally alike among them. TLR2 forms a heterodimer either with TLR1 or TLR6. The activation of TLR2 by TLR1/2 ligand Pam3CSK4 was attenuated by sevoflurane, not by isoflurane using a reporter cell assay 10. In contrast, the activation of TLR2 by TLR2/6 ligand lipoteichoic acid (LTA) was attenuated by neither isoflurane nor sevoflurane. The in silico structural analysis estimated that sevoflurane shared the binding pocket on TLR2 with Pam3CSK4. Supporting this prediction, sevoflurane displaced Pam3CSK4 from TLR2, demonstrating that sevoflurane competed with Pam3CSK4 for TLR2 to attenuate TLR2 activation 10. Lipopolysaccharide (LPS) is a cell wall component of gram negative bacteria that contributes to TLR4 activation. LPS does not directly bind to TLR4. Instead TLR4 activation by LPS occurs as follows; 1) Adaptor protein myeloid differentiation protein-2 (MD-2) binds to TLR4 to form a heterodimer, 2) LPS binds to MD-2 via the lipophilic part of LPS (lipid A), and 3) Once LPS-MD-2-TLR4 complex is formed, the complex binds to another LPS-MD-2-TLR4 complex to form a dimer for TLR4 activation 11. Both isoflurane and sevoflurane attenuated the activation of TLR4 by LPS using a reporter cell assay 12. The experiments using photoactivatable anesthetics to delineate anesthetic binding demonstrated that both isoflurane and sevoflurane bound to MD-2/TLR4 complex 12. Isoflurane bound to residues at TLR4-MD-2 interface as well as TLR4-TLR4 dimer interface. Sevoflurane bound to residues at LPS-MD-2 interface, TLR4-MD-2 interface and TLR4-TLR4 dimer interface. These results indicated that both isoflurane and sevoflurane directly bound to TLR4 complex with the modification of its activation.
β2 integrins
Integrins are heterodimeric adhesion molecules consisting of α- and β- subunits 13. 18 different α subunits and 8 different β subunits have been reported so far, forming at least 25 αβ heterodimers. β2 integrins consist of four members; αLβ2, αMβ2, αXβ2 and αDβ2, exclusively expressed on leukocytes. Among them, αLβ2 (leukocyte function-associated antigen-1; LFA-1) and αMβ2 (macrophage 1-antigen; Mac-1) are major β2 integrins on neutrophils and monocytes/macrophages. Each β2 integrin contains a von Willebrand factor type A domain called the αI domain in the α subunit. This domain serves as a ligand binding domain, whereas other domains play regulatory roles 14. Ligand binding to the αI domain is governed by its conformation. Ligands only bind to the αI domain of activated β2 integrin when the domain has experienced extensive conformational changes. The αI domain has a large pocket known as “allosteric pocket” (known LFA-1 allosteric inhibitors such as lovastatin and BIRT377 bind to this pocket and inactivate LFA-1) at resting (inactivated) state of β2 integrin 15,16. This pocket is strongly tied with the function of β2 integrin. The loss of this pocket reorganizes the structure of the αI domain for β2 integrin activation. Intercellular adhesion molecule-1 (ICAM-1) is a major LFA-1 ligand. Both isoflurane and sevoflurane reduced the binding of ICAM-1 to LFA-1 in cell-based as well as protein-based binding assays 17–19. The direct binding of isoflurane to the allosteric pocket of LFA-1 was demonstrated by X-ray crystallography, NMR as well as photolabeling experiments, showing that isoflurane worked as a LFA-1 allosteric inhibitor 18,19. Similarly, the direct binding of sevoflurane to LFA-1 at the same pocket was demonstrated by NMR experiment, showing that sevoflurane would also serve as a LFA-1 allosteric inhibitor 17. Mac-1 also uses ICAM-1 as its ligand. The binding of ICAM-1 to Mac-1 was inhibited by isoflurane, not by sevoflurane in a cell-based binding assay 20. The in silico structural analysis predicted that isoflurane would bind to Mac-1 at the allosteric pocket of the αI domain, as it bound to that of LFA-1. The experiment using mutants at residues of the pocket supported that isoflurane bound to it, suggesting the idea of isoflurane as an allosteric Mac-1 inhibitor 20. In addition to β2 integrins, both isoflurane and sevoflurane also bind to and inhibit critical platelet adhesion molecule αIIbβ3 (glycoprotein IIb/IIIa)21.
Ras-related protein 1
Ras-related protein 1 (Rap1) is a Ras-like small guanosine triphosphate (GTP) ase and located at the hub of intracellular signaling for adhesion and phagocytosis 22–24. The low molecular weight GTPase cycles between an inactive, guanosine diphosphate (GDP)-bound conformation and an active, GTP-bound conformation. Using a macrophage cell line, both isoflurane and sevoflurane were shown to attenuate Rap1 activation 25. Sevoflurane was shown to bind directly to Rap1 using the photolabeling experiment at residues critical for Rap1 activation, while isoflurane did not bind to 25. The attenuation of Rap1 activation by isoflurane was presumably explained by its inhibition of Mac-1 based on Mac-1-Rap1 functional interaction.
Volatile anesthetics potently attenuate neutrophil and macrophage functions in vivo 26,27. Because volatile anesthetics target TLR2, TLR4, LFA-1, Mac-1 and Rap1 in addition to canonical target molecules, we proposed that volatile anesthetics affect neutrophil and monocyte/macrophage functions through their direct interactions with these molecules in the following sections.
The effect of volatile anesthetics on innate immune cells in the systemic circulation
In the setting of inflammation and/or infection, neutrophils and monocytes in the systemic circulation are activated and recruited to the active site. The process of neutrophil and monocyte recruitment to the site of infection and inflammation consists of 1) tethering/rolling, 2) adhesion, 3) crawling, 4) transmigration and 5) detachment 28. Integrins play a major role in this process. Among the integrins, β2 integrins LFA-1 and Mac-1 as well as VLA4 (very late antigen-4, α4β1) are responsible for neutrophil and monocyte recruitment (Table. 1)28. The signaling pathway leading to the activation of these integrins has been studied mostly in neutrophils and T cells and to a limited extent in monocytes.
Table 1.
Integrins involved in neutrophil and monocyte recruitment [ref. 27]
| Rolling | Adhesion | Crawling | Transmigration | Detachment | |
|---|---|---|---|---|---|
| Neutrophils | LFA-1 | LFA-1 | Mac-1 | LFA-1 | LFA-1 |
| Monocytes | VLA4 | VLA4, LFA-1, Mac-1 | LFA-1, Mac-1 | LFA-1 | LFA-1 |
Neutrophils are uniquely sensitive to a vast array of chemoattractants such as a lipid mediator leukotriene B4 (LTB4), chemokines and bacterial products (N-formylated peptides) 29. LTB4 is produced by neutrophils and macrophages. LTB4 stimulation can lead to the release of diacyl glycerol (DAG) via phospholipase C (PLC), followed by DAG-regulated guanine exchange factor-1 (CalDAG-DEFI)-dependent Rap1 activation 30. N-formylmethionine-leucyl-phenylalanine (fMLP) released from bacteria is a known molecule that induces LTB4 production often under LPS priming via TLR4 12,31. LTB4 is produced in a significantly lower quantity in the setting of sterile inflammation 32. In trauma-induced sterile inflammation, LTB4 is considered an early phase of chemoattractant to amplify neutrophil recruitment 33. Neutrophils produce proinflammatory cytokines at their recruited site. These cytokines trigger the production of chemokines for C-C motif chemokine receptor 1 (CCR1) and C-X-C motif chemokine receptor 2 (CXCR2). Chemokines activate these G-protein coupled receptors (GPCRs) on neutrophils, leading into the activation of Rap 1 via PLC 28. Rap 1 then activates LFA-1 via an inside-out signaling for neutrophil adhesion (Fig. 1). Although the detailed molecular events involved in the transition from adhesion to crawling remain to be determined, the activation of LFA-1 induces outside-in signaling, leading to Mac-1-dependent crawling 34. Circulating TLR2 and TLR4 ligands can stimulate TLR2 and TLR4, respectively, which leads to the activation of Rap1 and then β2 integrins for firm adhesion 3,4 (Fig. 1). Non-canonical volatile anesthetic target molecules TLR2, TLR4, Rap1, LFA-1 and Mac-1 are located in the same signaling cascade leading to neutrophil recruitment. Taken together, volatile anesthetics can attenuate this signaling cascade by directly interacting with multiple molecules in a sequential manner. In vivo, 2% isoflurane exposure reduced the number of recruited neutrophils by 90% in a skin inflammation model induced by the Arthus reaction 26. In the polymicrobial abdominal sepsis model induced by cecal ligation and puncture surgery, 1% isoflurane exposure attenuated the number of recruited neutrophils to the peritoneal cavity by 70%. Compared to these profound in vivo effects, 1% isoflurane attenuated the binding of LFA-1 to ICAM-1 only by 25% 18. Similarly, 2% isoflurane attenuated only 50% of Mac-1 binding to its ligand 20. 1% isoflurane attenuated TLR4-mediated nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) activation by 60% 12. Although the direct binding between isoflurane and TLR4 complex was shown by photolabeling experiment 12, there is no data describing how potently isoflurane impairs MD-2-TLR4 complex formation and TLR4 dimer formation. It is not known if isoflurane or sevoflurane has additional anesthetic targets at the downstream of TLR4 signaling cascade. Furthermore, the contribution of lipid-cytokine-chemokine cascade to recruit neutrophils should be considered (Fig. 1). Isoflurane attenuated LTB4 production by interfering TLR4-mediated LPS priming 12. The reduction of mediator production by volatile anesthetics can thereby suppress neutrophil recruitment. It is possible that additional anesthetic targets other than non-canonical targets described above exist. The expression of canonical anesthetic target GABAA receptor on human and rodent neutrophils has not been reported6. Although NMDA receptor expression on human neutrophils has not been reported, NR1 and NR2 subunit expression on rodents stimulated with zymosan was reported35. NR1 subunit binds to glycine, while NR2 subunit binds to glutamate. Both subunits are sensitive to isoflurane 36,37. However, the role of NMDA receptors in neutrophil function has not been clearly delineated yet. The mechanism of neutrophil recruitment impairment by volatile anesthetics remains to be examined furthermore. Monocytes are classified by their surface expression pattern of a LPS receptor complex component CD14 and FcγRIII CD16 into classical (CD14+/CD16−), intermediate (CD14+/CD16+) and nonclassical (CD14dim/CD16+) monocytes 38–40. Classic monocytes have proinflammatory and anti-microbial functions, consisting of ~90% of monocytes. Intermediate and nonclassical monocytes constitute 2–3% and 7–8% of monocytes, respectively 41. Nonclassical monocytes are known as patrolling monocytes, constantly surveying the vasculature to trigger early responses to inflammation and infection via LFA-1 and pose anti-inflammatory effects 42. Intermediate monocytes are considered as monocytes in transition from classical to nonclassical monocytes 43. Once monocytes migrate into the tissues from the systemic circulation, they are likely to be transformed into macrophages. A very few studies have examined the effect of anesthetics on monocyte recruitment. In the experimental abdominal sepsis model, isoflurane exposure reduced the number of macrophages in the peritoneal cavity27. Classic monocytes are recruited to the site of infection as macrophages. They adhere to the endothelium via integrin VLA4 for their recruitment. Because isoflurane did not inhibit the binding of VLA4 to its ligand vascular cell adhesion molecule 1 (VCAM-1) 27, different molecule(s) should serve as anesthetic target(s). Given that intracellular signaling cascades leading to VLA4 activation during monocyte recruitment are less studied, further biological studies are needed for monocyte recruitment before understanding the mechanism of volatile anesthetics-mediated modulation of monocyte recruitment. Of note, the expression of NMDA receptors on monocytes and macrophages has not been reported 6. Regarding GABAA receptor, α1 and β2 subunits were expressed on human monocytes and macrophages. In rodents, α1–2, β3 and δ subunits were expressed. α1, α2 and β3 subunits were potentiated by volatile anesthetics 44–48. GABA receptor regulates Cl− permeability, which can affect Ca2+ influx. However, the role of these GABAA receptors in monocytes/macrophages has not been clearly delineated yet.
Figure 1. Signaling cascade for neutrophil recruitment and the effect of volatile anesthetics.
(A) Lipid-chemokine interaction. LTB4 is released from injured tissues (left) and attracts neutrophils. Neutrophils recruited to the tissues release chemokines, which further attract neutrophils in the blood vessel (right).
(B) Signaling cascades activated by chemoattractants and the effect of volatile anesthetics.
The effect of volatile anesthetics on immune cells at local inflammatory sites
The effect of volatile anesthetics on microbial phagocytosis and killing by neutrophils
Professional phagocytes, initially neutrophils, then macrophages are recruited to the surgical site to control invading microbes. They are loaded with phagocytic receptors such as Fc receptors (FcRs) and complement receptors49. Monocytes/macrophages have a greater repertoire of phagocytic receptors than neutrophils 50. In an ex vivo study of children undergoing cardiac catheterization, volatile anesthetic-based anesthetics impaired neutrophil phagocytosis, but intravenous anesthetics did not 41. In contrast, monocyte phagocytosis was not affected by volatile anesthetics. In the in vivo sepsis model, volatile anesthetic exposure also attenuated neutrophil phagocytosis 27. Although phagocytosis by macrophages was attenuated by volatile anesthetics in vitro 25, there is limited data available in vivo in this regard. Based on these facts, we reviewed the mechanism of how volatile anesthetics affect neutrophil phagocytosis.
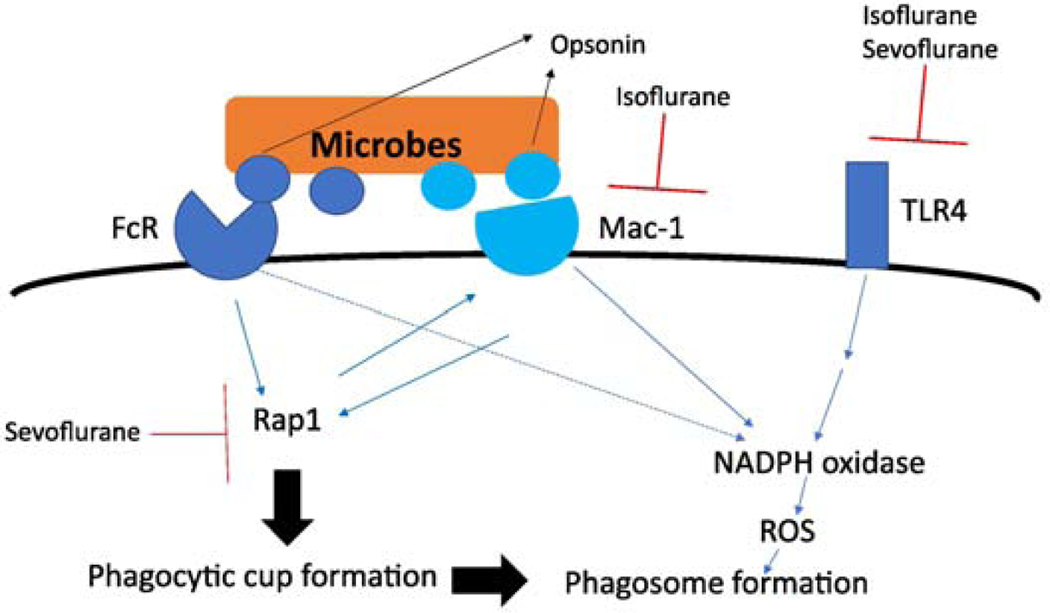
In neutrophil phagocytosis, complement receptors (such as Mac-1) and FcRs play a major role. FcRs and Mac-1 do not necessarily work independently and usually cross-talk to augment the overall phagocytic process 51. Rap1 is activated by signals from both FcRs and Mac-1 to form a phagocytic cup, which is an invagination of the cell membrane that subsequently closes to form a phagosome. Then, ROS generated by NADPH oxidase is released into the phagosome for microbial elimination 52. The activation of NADPH oxidase in neutrophils occurs through a number of receptors including Mac-1, FcRs and TLR4 (Fig. 2). Thus, volatile anesthetics can impair neutrophil phagocytosis and subsequent microbial killing by inhibiting multiple molecules including Mac-1, Rap1 and TLR4. Whether or not volatile anesthetics target other molecules to modulate neutrophil phagocytosis and microbial killing needs further investigation.
Figure 2. Signaling cascade for neutrophil phagocytosis and microbial killing and the effect of volatile anesthetics.
Rap1 assists the crosstalk between Fc receptor (FcR) and Mac-1.
The effect of volatile anesthetics on proinflammatory mediators by neutrophils
Both neutrophils and macrophages produce an array of cytokines. A number of studies have examined the effect of volatile anesthetics on blood cytokine levels and demonstrated that they could attenuate proinflammatory cytokine levels 53,54. However, a very few studies have examined the mechanism of these volatile anesthetics-mediated anti-inflammatory effects. As we focused on the effect of volatile anesthetics on neutrophil recruitment and phagocytosis above, we commented on their effect on cytokine production by neutrophils here.
A large number of neutrophil receptors including GPCRs, FcRs, Mac-1 and TLRs trigger cytokine production 55. Among them, Mac-1, TLR2 and TLR4 are known volatile anesthetic targets. TLR2 and TLR4 reportedly bind to a diverse array of exogenous and endogenous ligands. TLR2 binds to gram positive bacterial components (lipoprotein, lipoteichoic acid), fungal component (zymosan) and hepatitis virus capsid 56. Endogenous TLR2 ligands include high mobility group protein 1 (HMGB-1) and heat shock proteins 57. The effect of anesthetics on TLR1/TLR2- and TLR2/TLR6-mediated proinflammatory cytokine production has not been reported. Aside from LPS, TLR4 binds to endogenous ligands such as hyaluronan, heparan sulfate, HMGB1 and heat shock proteins. So far the effect of anesthetics on TLR4-mediated proinflammatory cytokine production is largely done only in the context of LPS 12. Based on the effect of volatile anesthetics on TLR4 complex, it is intuitive that they attenuate proinflammatory cytokine production. Then, volatile anesthetics have additional targets to modulate cytokine production? Signaling pathways to produce cytokines via TLR2 and TLR4 activation are well described. TLR2 and TLR4 are monomeric at the baseline. Their dimer formation activates intracellular signaling pathways solely through myeloid differentiation primary response 88 (MyD88) and leads to the induction of proinflammatory gene expression via activation of Interleukin-1 receptor associated kinase (IRAK) and tumor necrosis factor receptor associated factor 6 (TRAF6) (Fig. 3). Dimerization of TLR4 also activates TIR-domain-containing adaptor-inducing interferon-β (TRIF) and leads to the induction of interferon (IFN) regulatory factor 3 (IRF3) nuclear translocation to promote the production of type I IFN (Fig. 3). However, whether or not volatile anesthetics affect intracellular molecules in these signaling cascades is not well studied. Mac-1 stimulation can also lead into NFκB activation for proinflammatory cytokine production. However, the effect of volatile anesthetics on Mac-1 specific cytokine production has not been studied.
Figure 3. Signaling cascade for proinflammatory cytokine production by neutrophils and the effect of volatile anesthetics.
Mac-1, TLR2 and TLR4 activation induces gene expression of cytokines and chemokines such as tumor necrosis factor (TNF)-α, IL1-β, IL-6, IL-12 and IFN-β.
Since neutrophils have a repertoire of receptors that contribute to the production of proinflammatory cytokines, studies to examine the effect of volatile anesthetics on individual pathways need to be done. In vivo, a number of ligands likely exist and activate many receptors. Thus, it is possible that volatile anesthetics can affect the production of proinflammatory mediators differently, depending on the combination of existing ligands. Thorough and extensive studies need to be done to delineate the effect of volatile anesthetics on cytokine production and its mechanism.
The effect of anesthetics on brain-immune communication
So far, we have reviewed the direct effect of volatile anesthetics on immune cells and/or peripheral immune system. However, the crosstalk between brain and immune system has been increasingly recognized. During surgical stress, the hypothalamic-pituitary-adrenal axis (HPA axis) is activated via afferent nerves to produce endogenous steroids and glucocorticoids. These steroids inhibit the expression of proinflammatory cytokines and induce the overexpression of anti-inflammatory cytokines 58,59. Furthermore, the efferent fibers of vagus nerve are activated by inflammatory stimuli to transmit to the central nervous system, which in turn activates vagus and splenic nerves for the recruitment of anti-inflammatory immune cells 60. The use of volatile anesthetics was associated with increased cortisol and adrenocorticotrophic hormone (ACTH) levels 61,62. Studies on the effect of anesthetics on vagus-mediated anti-cholinergic pathway are not done extensively. The study by Picq et al. compared the effect of isoflurane and pentobarbital on a rat model of vagus nerve stimulation and found that TNF-α levels were much lower in isoflurane arm 63. However, it is unclear if isoflurane augmented the signaling of this pathway. Because brain-immune communication is in part responsible for cytokine production, which volatile anesthetics can affect, studies on anesthetic interaction with neuro-immune axis are critical to understand the effect of anesthetics on cytokine production.
Future perspectives
Based on available data, we hypothesized that volatile anesthetics suppress neutrophil recruitment, phagocytosis and microbial killing by modulating multiple molecules in signaling cascades. Because in vivo techniques to directly evaluate the interaction between volatile anesthetics and proteins are not available, testing our hypothesis is difficult at this moment. The development of such modalities will allow us to explore the mechanism of anesthetics-mediated immunomodulation in vivo in detail. Delineation of the effects of volatile anesthetics on neuro-immune interaction needs further work in addition to studies focusing on their effects on peripheral immune system. Further elucidation of anesthetic effects on immune network and identification of target proteins in vivo will help to develop anesthetics devoid of unwanted side effects.
Conclusions
Here we summarized our current knowledge about the effect of volatile anesthetics on innate immune cells, particularly on neutrophils. Based on the existing literature, we hypothesized that volatile anesthetics significantly reduce neutrophil recruitment and phagocytosis by targeting multiple molecules in surgical patients.
Highlights.
Volatile anesthetics target immunological molecules in neutrophils.
Volatile anesthetics can target multiple molecules in the same immunological signaling cascade.
Understanding the detailed mechanism of anesthetics on immune system will allow us to redesign anesthetics.
Acknowledgments
Financial Support: This work was in part supported by CHMC Anesthesia Foundation, NIH R01GM118277 (K.Y.), R01GM127600 (K.Y.) and R21HD099194 (K.Y., S.K.)
Footnotes
Conflict of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Xiang M, Fan J. & Fan J. Association of Toll-like receptor signaling and reactive oxygen species: a potential therapeutic target for posttrauma acute lung injury. Mediators Inflamm 2010, doi: 10.1155/2010/916425 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pinheiro CDS et al. Short-Term Regulation of FcgammaR-Mediated Phagocytosis by TLRs in Macrophages: Participation of 5-Lipoxygenase Products. Mediators Inflamm 2017, 2086840, doi: 10.1155/2017/2086840 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chung KJ et al. A novel pathway of rapid TLR-triggered activation of integrin-dependent leukocyte adhesion that requires Rap1 GTPase. Mol Biol Cell 25, 2948–2955, doi: 10.1091/mbc.E14-04-0867 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schmidt A, Caron E. & Hall A. Lipopolysaccharide-induced activation of beta2-integrin function in macrophages requires Irak kinase activity, p38 mitogen- activated protein kinase, and the Rap1 GTPase. Mol Cell Biol 21, 438–448, doi: 10.1128/MCB.21.2.438-448.2001 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eckenhoff RG Promiscuous ligands and attractive cavities: how do the inhaled anesthetics work? Mol Interv 1, 258–268 (2001). [PubMed] [Google Scholar]
- 6.Yuki K. & Eckenhoff RG Mechanisms of the Immunological Effects of Volatile Anesthetics: A Review. Anesth Analg 123, 326–335, doi: 10.1213/ANE.0000000000001403 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yuki K. & Murakami N. Sepsis pathophysiology and anesthetic consideration. Cardiovasc Hematol Disord Drug Targets 15, 57–69, doi: (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Franks NP General anaesthesia: from molecular targets to neuronal pathways of sleep and arousal. Nat Rev Neurosci 9, 370–386, doi: 10.1038/nrn2372 (2008). [DOI] [PubMed] [Google Scholar]
- 9.Botos I, Segal DM & Davies DR The structural biology of Toll-like receptors. Structure 19, 447–459, doi: 10.1016/j.str.2011.02.004 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mitsui Y. et al. Volatile Anesthetic Sevoflurane Attenuates Toll-Like Receptor 1/2 Activation. Anesth Analg, doi: 10.1213/ANE.0000000000004741 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park BS & Lee JO Recognition of lipopolysaccharide pattern by TLR4 complexes. Exp Mol Med 45, e66, doi: 10.1038/emm.2013.97 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okuno T. et al. Volatile anesthetics isoflurane and sevoflurane directly target and attenuate Toll-like receptor 4 system. FASEB J 33, 14528–14541, doi: 10.1096/fj.201901570R (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hynes RO Integrins: bidirectional, allosteric signaling machines. Cell 110, 673–687, doi: 10.1016/s0092-8674(02)00971-6 (2002). [DOI] [PubMed] [Google Scholar]
- 14.Shimaoka M, Takagi J. & Springer TA Conformational regulation of integrin structure and function. Annu Rev Biophys Biomol Struct 31, 485–516, doi: 10.1146/annurev.biophys.31.101101.140922 (2002). [DOI] [PubMed] [Google Scholar]
- 15.Yuki K, Bu W, Xi J, Shimaoka M. & Eckenhoff R. Propofol shares the binding site with isoflurane and sevoflurane on leukocyte function-associated antigen-1. Anesth Analg 117, 803–811, doi: 10.1213/ANE.0b013e3182a00ae0 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tazawa K, Koutsogiannaki S, Chamberlain M. & Yuki K. The effect of different anesthetics on tumor cytotoxicity by natural killer cells. Toxicol Lett 266, 23–31, doi: 10.1016/j.toxlet.2016.12.007 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yuki K, Astrof NS, Bracken C, Soriano SG & Shimaoka M. Sevoflurane binds and allosterically blocks integrin lymphocyte function-associated antigen-1. Anesthesiology 113, 600–609, doi: 10.1097/ALN.0b013e3181e89a77 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yuki K. et al. The volatile anesthetic isoflurane perturbs conformational activation of integrin LFA-1 by binding to the allosteric regulatory cavity. FASEB J 22, 4109–4116, doi: 10.1096/fj.08-113324 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yuki K. et al. Isoflurane binds and stabilizes a closed conformation of the leukocyte function-associated antigen-1. FASEB J 26, 4408–4417, doi: 10.1096/fj.12-212746 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jung S. & Yuki K. Differential effects of volatile anesthetics on leukocyte integrin macrophage-1 antigen. J Immunotoxicol 13, 148–156, doi: 10.3109/1547691X.2015.1019596 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yuki K, Bu W, Shimaoka M. & Eckenhoff R. Volatile anesthetics, not intravenous anesthetic propofol bind to and attenuate the activation of platelet receptor integrin alphaIIbbeta3. PLoS One 8, e60415, doi: 10.1371/journal.pone.0060415 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caron E, Self AJ & Hall A. The GTPase Rap1 controls functional activation of macrophage integrin alphaMbeta2 by LPS and other inflammatory mediators. Curr Biol 10, 974–978, doi: 10.1016/s0960-9822(00)00641-2 (2000). [DOI] [PubMed] [Google Scholar]
- 23.Chung J. et al. Rap1 activation is required for Fc gamma receptor-dependent phagocytosis. J Immunol 181, 5501–5509, doi: 10.4049/jimmunol.181.8.5501 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bos JL, de Rooij J. & Reedquist KA Rap1 signalling: adhering to new models. Nat Rev Mol Cell Biol 2, 369–377, doi: 10.1038/35073073 (2001). [DOI] [PubMed] [Google Scholar]
- 25.Zha H. et al. Volatile anesthetics affect macrophage phagocytosis. PLoS One 14, e0216163, doi: 10.1371/journal.pone.0216163 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carbo C, Yuki K, Demers M, Wagner DD & Shimaoka M. Isoflurane inhibits neutrophil recruitment in the cutaneous Arthus reaction model. J Anesth 27, 261–268, doi: 10.1007/s00540-012-1508-1 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koutsogiannaki S. et al. From the Cover: Prolonged Exposure to Volatile Anesthetic Isoflurane Worsens the Outcome of Polymicrobial Abdominal Sepsis. Toxicol Sci 156, 402–411, doi: 10.1093/toxsci/kfw261 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herter J. & Zarbock A. Integrin Regulation during Leukocyte Recruitment. J Immunol 190, 4451–4457, doi: 10.4049/jimmunol.1203179 (2013). [DOI] [PubMed] [Google Scholar]
- 29.McDonald B. & Kubes P. Chemokines: sirens of neutrophil recruitment-but is it just one song? Immunity 33, 148–149, doi: 10.1016/j.immuni.2010.08.006 (2010). [DOI] [PubMed] [Google Scholar]
- 30.Bergmeier W. et al. Mice lacking the signaling molecule CalDAG-GEFI represent a model for leukocyte adhesion deficiency type III. J Clin Invest 117, 1699–1707, doi: 10.1172/JCI30575 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Surette ME, Palmantier R, Gosselin J. & Borgeat P. Lipopolysaccharides prime whole human blood and isolated neutrophils for the increased synthesis of 5-lipoxygenase products by enhancing arachidonic acid availability: involvement of the CD14 antigen. J Exp Med 178, 1347–1355, doi: 10.1084/jem.178.4.1347 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oyoshi MK et al. Leukotriene B4-driven neutrophil recruitment to the skin is essential for allergic skin inflammation. Immunity 37, 747–758, doi: 10.1016/j.immuni.2012.06.018 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lammermann T. et al. Neutrophil swarms require LTB4 and integrins at sites of cell death in vivo. Nature 498, 371–375, doi: 10.1038/nature12175 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Phillipson M. et al. Vav1 is essential for mechanotactic crawling and migration of neutrophils out of the inflamed microvasculature. J Immunol 182, 6870–6878, doi: 10.4049/jimmunol.0803414 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bryushkova EA, Vladychenskaya EA, Stepanova MS & Boldyrev AA Effect of homocysteine on properties of neutrophils activated in vivo. Biochemistry (Mosc) 76, 467–472, doi: 10.1134/s0006297911040109 (2011). [DOI] [PubMed] [Google Scholar]
- 36.Ogata J. et al. Effects of anesthetics on mutant N-methyl-D-aspartate receptors expressed in Xenopus oocytes. J Pharmacol Exp Ther 318, 434–443, doi: 10.1124/jpet.106.101691 (2006). [DOI] [PubMed] [Google Scholar]
- 37.Hollmann MW, Liu HT, Hoenemann CW, Liu WH & Durieux ME Modulation of NMDA receptor function by ketamine and magnesium. Part II: interactions with volatile anesthetics. Anesth Analg 92, 1182–1191, doi: 10.1097/00000539-200105000-00020 (2001). [DOI] [PubMed] [Google Scholar]
- 38.Cros J. et al. Human CD14dim monocytes patrol and sense nucleic acids and viruses via TLR7 and TLR8 receptors. Immunity 33, 375–386, doi: 10.1016/j.immuni.2010.08.012 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mukherjee R. et al. Non-Classical monocytes display inflammatory features: Validation in Sepsis and Systemic Lupus Erythematous. Sci Rep 5, 13886, doi: 10.1038/srep13886 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stansfield BK & Ingram DA Clinical significance of monocyte heterogeneity. Clin Transl Med 4, 5, doi: 10.1186/s40169-014-0040-3 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koutsogiannaki S, Shimaoka M. & Yuki K. The Use of Volatile Anesthetics as Sedatives for Acute Respiratory Distress Syndrome. Transl Perioper Pain Med 6, 27–38, doi: 10.31480/2330-4871/084 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Auffray C. et al. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science 317, 666–670, doi: 10.1126/science.1142883 (2007). [DOI] [PubMed] [Google Scholar]
- 43.Kratofil RM, Kubes P. & Deniset JF Monocyte Conversion During Inflammation and Injury. Arterioscler Thromb Vasc Biol 37, 35–42, doi: 10.1161/ATVBAHA.116.308198 (2017). [DOI] [PubMed] [Google Scholar]
- 44.Nishikawa K. & Harrison NL The actions of sevoflurane and desflurane on the gamma-aminobutyric acid receptor type A: effects of TM2 mutations in the alpha and beta subunits. Anesthesiology 99, 678–684, doi: 10.1097/00000542-200309000-00024 (2003). [DOI] [PubMed] [Google Scholar]
- 45.Borghese CM et al. An isoflurane- and alcohol-insensitive mutant GABA(A) receptor alpha(1) subunit with near-normal apparent affinity for GABA: characterization in heterologous systems and production of knockin mice. J Pharmacol Exp Ther 319, 208–218, doi: 10.1124/jpet.106.104406 (2006). [DOI] [PubMed] [Google Scholar]
- 46.Schofield CM & Harrison NL Transmembrane residues define the action of isoflurane at the GABAA receptor alpha-3 subunit. Brain Res 1032, 30–35, doi: 10.1016/j.brainres.2004.11.002 (2005). [DOI] [PubMed] [Google Scholar]
- 47.Sonner JM et al. Alpha 1 subunit-containing GABA type A receptors in forebrain contribute to the effect of inhaled anesthetics on conditioned fear. Mol Pharmacol 68, 61–68, doi: 10.1124/mol.104.009936 (2005). [DOI] [PubMed] [Google Scholar]
- 48.Rau V. et al. Gamma-aminobutyric acid type A receptor beta3 subunit forebrain-specific knockout mice are resistant to the amnestic effect of isoflurane. Anesth Analg 113, 500–504, doi: 10.1213/ANE.0b013e3182273aff (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Underhill DM & Goodridge HS Information processing during phagocytosis. Nat Rev Immunol 12, 492–502, doi: 10.1038/nri3244 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kobzik L. & Swirski FK MARCOing monocytes for elimination. Sci Transl Med 6, 219fs214, doi: 10.1126/scitranslmed.3008448 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ortiz-Stern A. & Rosales C. Cross-talk between Fc receptors and integrins. Immunol Lett 90, 137–143, doi: 10.1016/j.imlet.2003.08.004 (2003). [DOI] [PubMed] [Google Scholar]
- 52.Nguyen GT, Green ER & Mecsas J. Neutrophils to the ROScue: Mechanisms of NADPH Oxidase Activation and Bacterial Resistance. Front Cell Infect Microbiol 7, 373, doi: 10.3389/fcimb.2017.00373 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Helmy SA, Wahby MA & El-Nawaway M. The effect of anaesthesia and surgery on plasma cytokine production. Anaesthesia 54, 733–738, doi: 10.1046/j.1365-2044.1999.00947.x (1999). [DOI] [PubMed] [Google Scholar]
- 54.Stollings LM et al. Immune Modulation by Volatile Anesthetics. Anesthesiology 125, 399–411, doi: 10.1097/ALN.0000000000001195 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tecchio C, Micheletti A. & Cassatella MA Neutrophil-derived cytokines: facts beyond expression. Front Immunol 5, 508, doi: 10.3389/fimmu.2014.00508 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Oliveira-Nascimento L, Massari P. & Wetzler LM The Role of TLR2 in Infection and Immunity. Front Immunol 3, 79, doi: 10.3389/fimmu.2012.00079 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schaefer L. Complexity of danger: the diverse nature of damage-associated molecular patterns. J Biol Chem 289, 35237–35245, doi: 10.1074/jbc.R114.619304 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Besnier E, Clavier T. & Compere V. The Hypothalamic-Pituitary-Adrenal Axis and Anesthetics: A Review. Anesth Analg 124, 1181–1189, doi: 10.1213/ANE.0000000000001580 (2017). [DOI] [PubMed] [Google Scholar]
- 59.Yuki K. et al. Pediatric Perioperative Stress Responses and Anesthesia. Transl Perioper Pain Med 2, 1–12 (2017). [PMC free article] [PubMed] [Google Scholar]
- 60.Dantzer R. Neuroimmune Interactions: From the Brain to the Immune System and Vice Versa. Physiol Rev 98, 477–504, doi: 10.1152/physrev.00039.2016 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kostopanagiotou G. et al. The differential impact of volatile and intravenous anaesthetics on stress response in the swine. Hormones (Athens) 9, 67–75, doi: 10.14310/horm.2002.1255 (2010). [DOI] [PubMed] [Google Scholar]
- 62.Mizutani A, Hattori S, Yoshitake S, Kitano T. & Noguchi T. Effect of additional general anesthesia with propofol, midazolam or sevoflurane on stress hormone levels in hysterectomy patients, receiving epidural anesthesia. Acta Anaesthesiol Belg 49, 133–139 (1998). [PubMed] [Google Scholar]
- 63.Picq CA, Clarencon D, Sinniger VE, Bonaz BL & Mayol JF Impact of Anesthetics on Immune Functions in a Rat Model of Vagus Nerve Stimulation. PLoS One 8, e67086, doi: 10.1371/journal.pone.0067086 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]