Abstract
Background.
Pancreatoduodenectomy (PD) for duodenal adenoma (DA) resection may be associated with excessive surgical risk for patients with potentially benign lesions, given the absence of pancreatic duct obstruction. We examined factors associated with final malignant pathology and evaluated the postoperative course of patients with DA versus pancreatic ductal adenocarcinoma (PDAC).
Methods.
We retrospectively analyzed patients with DA who underwent PD from 2008 to 2018 and assessed the accuracy rate of preoperative biopsy and factors associated with final malignant pathology. Complications for DA patients were compared with those of matched PDAC patients.
Results.
Forty-five consecutive patients who underwent PD for DA were identified, and the preoperative biopsy false negative rate was 29. Factors associated with final malignant pathology included age over 70 years, preoperative biliary obstruction, and common bile duct diameter > 8 mm (p < 0.05). Compared with patients with PDAC (n = 302), DA patients experienced more major complications (31% vs. 15%, p < 0.01), more grade C postoperative pancreatic fistulas (9% vs. 1%, p < 0.01), and greater mortality (7% vs. 2%, p < 0.05). Propensity score matched patients with DA had more major complications following PD (32% vs. 12%, p < 0.05).
Conclusions.
Preoperative biopsy of duodenal adenomas is associated with a high false-negative rate for malignancy, and PD for DA is associated with higher complication rates than PD for PDAC. These results aid discussion among patients and surgeons who are considering observation versus PD for DA, especially in younger patients without biliary obstruction, who are less likely to harbor malignancy.
Duodenal adenomas (DA) are premalignant tumors, representing < 0.12% of upper endoscopy findings, and are typically identified incidentally.1 Adenomas larger than 20 mm, those with biopsy-proven high-grade dysplasia, and those involving the ampulla of Vater carry an increased risk of malignancy and obstructive complications.2,3 Endoscopic verification of underlying malignancy in adenomas can be challenging, as biopsies of large adenomas are associated with false-negative rates ranging from 16 to 54.5%.3-5 The natural history of duodenal adenomas is poorly understood, and there is no consensus regarding the acceptable time frame for observation.6
Gastroenterologists and surgeons generally recommend resection, given the potential for malignant transformation, particularly for symptomatic adenomas, large adenomas, those with high-grade dysplasia, and those involving the ampulla of Vater. Smaller adenomas without concerning histologic features and those without involvement of the ampulla may be amenable to endoscopic removal techniques, including snare polypectomy and mucosal resection, which can provide curative treatment while avoiding the high morbidity and mortality rates of surgical resection. Large adenomas that do not involve the ampulla may be addressed with pancreas-sparing duodenectomy or local excision.7
Lesions with multifocal high-grade dysplasia and those involving the ampulla and/or the second portion of the duodenum often require pancreatoduodenectomy (PD), which is associated with perioperative morbidity ranging from 30 to 60% and overall mortality ranging from 1 to 5%.8 Complications following PD are increased in patients with benign neoplasms due to the absence of pancreatic and bile duct dilatation and the presence of soft pancreatic parenchyma—two factors associated with development of a postoperative pancreatic fistula (POPF).8-10
In the absence of a preoperative cancer diagnosis, patients with duodenal adenomas not amenable to endoscopic or local resection are faced with the decision of whether to undergo PD and a potentially complicated postoperative course, which may represent a higher risk benefit ratio than desired. This study examined the preoperatively and intraoperatively identifiable factors that may predict a final malignant diagnosis and the postoperative complication profile for patients who underwent PD for DA. To assess the increased risk of PD for DA over that of PD for pancreatic adenocarcinoma (PDAC), we compared the morbidity and mortality following PD for DA against a cohort of patients who underwent PD for PDAC without receiving neoadjuvant therapy or vascular resection. The goal was to equip surgeons and patients with DA with a basis for choosing to proceed with PD versus continued endoscopic observation.
PATIENTS AND METHODS
Study Population
Approval for this study was first obtained from the Emory University institutional review board. All patients who underwent PD within the Emory Healthcare System for a biopsy-proven diagnosis of DA between January 1, 2008 and December 31, 2018 were identified from previously consolidated data of all pancreas resections.
The outcomes of these patients were compared with those of patients with a confirmed preoperative diagnosis of PDAC who underwent PD from 2013 to 2018, used in a benchmark analysis of PD.11 This subset of patients was intentionally chosen from the larger PD database for comparative analysis, as they did not receive preoperative chemotherapy and/or radiation and did not require an intraoperative vascular resection. The authors felt that these specific exclusion criteria were crucial in creating a control group with similar preoperative patient characteristics and surgical considerations to the patients with DA.
Study Variables
Patient, tumor, operative, and postoperative data were obtained from patient electronic medical records. Data examined included patient demographics (age, gender, race), patient comorbidities, preoperative tumor information (tumor size, location, histology, dysplasia), operative information (length of surgery, estimated blood loss (EBL), pancreatic and bile duct sizes, pancreas texture, intraoperative transfusion requirement, drain placement), final pathology report, postoperative complications, and 30-day and in-hospital mortality.
Definitions
Age-adjusted Charlson comorbidity index (ACCI) was utilized to characterize patient preoperative comorbidities.12,13 For the purpose of comparing the preoperative overall health of the patients with DA and PDAC, the two points allotted for ‘history of malignancy’ were only assigned to patients with a history of solid tumor in the last 5 years, excluding the current diagnosis of PDAC. Genetic predisposition was defined as any hereditary syndrome predisposing one to develop duodenal adenomas, including but not limited to Lynch syndrome, familial adenomatous polyposis (FAP), and Peutz–Jeghers syndrome. Spigelman scoring for duodenal adenomas was not calculated, as the majority of DA patients did not have a predisposing syndrome.14
The Clavien–Dindo (CD) classification of surgical complications was applied to standardize postoperative complication severity. 15, 16For the purpose of analyses, CD grades I and II were classified as “minor” complications, and CD grades III-V were classified as “major” complications. The POPF grading is based on the 2005 International Study Group of Pancreatic Fistula (ISGPF) definition.17
Statistical Analysis
Frequency distributions and summary statistics were calculated for all variables. Continuous variables are expressed as median and range, and categorical variables are expressed as frequency and percentage. Bivariate associations were evaluated using Pearson Chi squared tests for dichotomous variables and independent t tests for continuous variables. Multivariate analysis was conducted utilizing a binary logistic regression model for variables approaching statistical significance (p value ≤ 0.10) on bivariate analysis. Patients with PDAC and DA patients were matched 1:1 based on the propensity score using a greedy 5–1 digit match algorithm, with respect to age, BMI, and ACCI.18 These covariates were chosen for patient matching as prior literature has demonstrated consistent associations with poor postoperative outcomes following PD.12,19-21 A standardized difference of < 0.10 was accepted as a balanced match.22 A p value of < 0.05 was accepted to indicate a statistically significant association. All analyses were performed using SPSS software version 26.0 (SPSS, IBM Corp., Armonk, NY, USA) and SAS 9.4 (SAS Institute Inc., Cary, NC, USA).
RESULTS
Demographic and Clinicopathologic Characteristics of Patients with DA
A total of 1241 PD resections were performed in the Emory Healthcare System between 2008 and 2018. Forty-five (3.6%) of these were performed for the biopsy-proven preoperative diagnosis of DA and 302 (24.3%) were for resectable PDAC who did not receive preoperative chemotherapy or radiation (Table 1). Focusing on the DA patients, the median age was 64 years (range 41–89), and 56% (n = 25) were female. The median BMI was 28.0 kg/m2 (range 17.9–44.7), and the median ACCI score was 4 (range 0–9). The median tumor size was 34 mm (range 4–100), and 20% (n = 9) of patients required a preoperative biliary stent for biliary obstruction. Primary factors involved in the decision to pursue PD included symptomatic biliary obstruction (n = 7), tumor size greater than 20 mm (n = 19), biopsy-proven high-grade dysplasia (n = 12), and genetic predisposition (n = 5).
TABLE 1.
Duodenal adenoma (DA) and pancreatic ductal adenocarcinoma (PDAC) patient and tumor characteristics
| DA n = 45 (%) |
PDAC n = 302 (%) |
p value | |
|---|---|---|---|
| Age at surgery (years) | 64 (41–89) | 67 (33–89) | 0.10 |
| Female | 25 (56) | 155 (51) | 0.60 |
| Body mass index | 28.0 (17.9–44.7) | 25.7 (13.8–51.7) | 0.35 |
| Age-adjusted Charlson index | 4 (0–9) | 4 (0–10) | 0.17 |
| Preoperative biliary stent | 9 (20) | 189 (63) | < 0.01 |
| Tumor size (mm) | 34 (4–100) | 30 (2–87) | < 0.05 |
| Ampullary involvement | 32 (71) | 89 (30) | < 0.01 |
| Operation length (min) | 185 (109–480) | 197 (98–633) | 0.73 |
| Estimated blood loss (ml) | 150 (20–700) | 150 (0–2000) | 0.83 |
| Pancreatic duct (mm) | 2.75 (± 0.92) | 3.46 (± 2.71) | < 0.01 |
| Bile duct (mm) | 8.93 (± 4.25) | 11.77 (± 5.12) | < 0.01 |
| Soft pancreas | 21 (47) | 23 (8) | < 0.01 |
| Intraoperative drain | 10 (22) | 62 (21) | 0.90 |
Continuous variables are expressed as median (range) with the exception of pancreatic and bile duct sizes, which are expressed as mean (SD)
Categorical variables are expressed as n (%)
Bold values denote p values < 0.05
Comparative Analyses of Patients with Duodenal Adenomas Based on Final Pathologic Diagnosis
We compared outcomes of the DA patients who ultimately possessed benign pathology on final histologic analysis with those who had malignant findings. Twenty-nine percent (n = 13) of DA patients harbored adenocarcinoma on final pathology, representing a biopsy accuracy rate of 71% for benign pathology. Of these patients, the median age was 68 (range 50–89), 46% (n = 6) were female, and the median BMI and ACCI scores were 26.6 (range 18.4–37.1) and 4 (range 1–7), respectively. The median tumor size was 25 mm (range 10–50), 46% (n = 6) required a preoperative biliary stent, and 38% (n = 5) had preoperatively identified high-grade dysplasia.
DA patients with benign final pathology were more likely to suffer from a major complication compared with those with malignancy (44% vs. 0%; p < 0.01). The grade C POPF rate for patients with final benign pathology was 13% (n = 4), compared with 0% among patients with final malignant pathology, though this did not reach statistical significance (p = 0.18). The median length of stay for patients with final benign pathology was 9 days (range 2–90; p < 0.05), compared with 7 days (range 5–12) for patients with malignant pathology (p < 0.05). There were no significant differences in 30-day mortality and/or readmission rates. These factors are delineated in Table 2.
TABLE 2.
Perioperative outcomes of patients with preoperatively diagnosed duodenal adenomas stratified by final pathology
| Benign n = 32 (71) |
Malignant n = 13 (29) |
p value | |
|---|---|---|---|
| Age at surgery (years) | 59 (41–80) | 68 (50–89) | < 0.05 |
| Female | 19 (59) | 6 (46) | 0.42 |
| Race | |||
| Caucasian/White | 20 (63) | 12 (92) | < 0.05 |
| African-American/Black | 12 (37) | 1 (8) | < 0.05 |
| Body mass index (kg/m2) | 28.3 (17.9–44.7) | 26.6 (18.4–37.1) | 0.83 |
| Alcohol use within the last 5 yearsa | 2 (6) | 3 (23) | 0.10 |
| Tobacco use within the last 5 yearsb | 5 (16) | 2 (15) | 0.98 |
| Age-adjusted Charlson index | 2 (0–7) | 4 (1–7) | 0.05 |
| Preoperative biliary stent | 3 (9) | 6 (46) | < 0.01 |
| Asymptomatic at diagnosis | 12 (38) | 2 (15) | 0.15 |
| High-grade dysplasia on biopsy | 7 (22) | 5 (38) | 0.25 |
| Tumor size (mm) | 35 (4–100) | 25 (10–50) | < 0.05 |
| Pancreatic duct (mm) | 2.71 (± 0.97) | 2.85 (± 0.80) | 0.66 |
| Bile duct (mm) | 7.52 (± 3.19) | 12.31 (± 4.64) | < 0.01 |
| Estimated blood loss (ml) | 100 (20–700) | 200 (25–600) | 0.78 |
| Length of surgery (min) | 193 (109–480) | 172 (125–397) | 0.18 |
| Major complicationsc | 14 (44) | 0 (0) | < 0.01 |
| Postoperative pancreatic fistula | 7 (22) | 1 (8) | 0.26 |
| Grade C | 4 (13) | 0 (0) | 0.18 |
| Length of stay (days) | 9 (2–90) | 7 (5–12) | < 0.05 |
| Readmission | 6 (19) | 2 (15) | 0.79 |
| Patient death | 3 (9) | 0 (0) | 0.36 |
Continuous variables are expressed as median (range); categorical variables are expressed as n (%) Bold values denote p values < 0.05
Alcohol use is defined as > 3 drinks/day or > 7 drinks/week for women and > 4 drinks/day or > 14 drinks/week for men (National Institute on Alcohol Abuse and Alcoholism)
Tobacco use is defined as daily use of any tobacco product (Substance Abuse and Mental Health Services Administration)
Major complications (Clavien–Dindo grade ≥ III)
Univariate analysis demonstrated significant associations between final malignancy and increased age, Caucasian/White race, preoperative biliary stent, and increased bile duct diameter (Table 2). With the exception of race, these factors remained independently associated with malignancy on multivariate analysis, accounting for recent alcohol use, presence of symptoms at diagnosis, and larger tumor size (Fig. 1). Male gender, substance abuse, genetic predisposition, larger tumor size, and the presence of high-grade dysplasia on the preoperative biopsy did not significantly predict malignancy on final pathology.
FIG. 1.
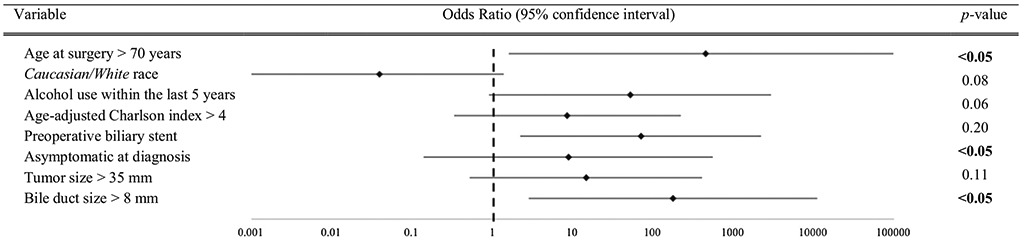
Multivariate analysis of select duodenal adenoma preoperatively and intraoperatively identified variables and association with malignancy on final pathology. (Multivariate analysis was conducted utilizing a binary logistic regression model for variables approaching statistical significance (p value ≤ 0.10) in univariate analysis. The odds ratios and 95% confidence intervals for each of the variables are depicted, where 1 represents no effect and values > 1 indicate a positive independent association between the variable and a final pathologic diagnosis of malignancy. p values < 0.05 were accepted as statistically significant)
Postoperative Outcomes of Patients with DA
Ninety-six percent (n = 43) of DA patients suffered from at least one postoperative complication. Sixty-seven percent (n = 29) were classified as minor and 31% (n = 14) were major. Of these major complications, 71% (n = 10) resulted in ICU admission or patient death (CD grade > 4). Eighteen percent of patients (n = 8) developed a POPF, 50% (n = 4) of which were classified as grade C. The 30-day mortality and readmission rates were 7% (n = 3) and 18% (n = 8), respectively. None of the preoperatively known patient and tumor characteristics, including age, gender, genetic predisposition, BMI, ACCI, presence of high-grade dysplasia, or biliary stent requirement, were associated with a worse postoperative outcome. These findings are displayed in Table 3.
TABLE 3.
Duodenal adenoma patient and tumor characteristics stratified by postoperative complication severity
| Complication severitya |
p value | ||
|---|---|---|---|
| Non-major n = 31 (69) |
Major n = 14 (31) |
||
| Age at surgery (years) | 58 (41–89) | 66 (55–80) | 0.12 |
| Female | 19 (61) | 6 (43) | 0.25 |
| Race | |||
| Caucasian/White | 22 (71) | 10 (71) | 0.98 |
| African-American/Black | 9 (29) | 4 (29) | 0.98 |
| Body mass index (kg/m2) | 26.7 (17.9–44.7) | 29.8 (18.5–35.8) | 0.66 |
| Age-adjusted Charlson index | 3 (0–7) | 3 (2–7) | 0.17 |
| Asymptomatic presentation | 9 (29) | 5 (36) | 0.65 |
| Preoperative biliary stent | 8 (26) | 1 (7) | 0.15 |
| High-grade dysplasia on biopsy | 7 (23) | 3 (21) | 0.59 |
| Tumor size (mm) | 35 (4–100) | 32 (16–70) | 0.52 |
| Ampullary involvement | 24 (77) | 8 (57) | 0.17 |
| Soft pancreas parenchymal texture | 13 (42) | 8 (57) | 0.34 |
| Operation length (min) | 189 (109–447) | 183 (133–480) | 0.92 |
| Pancreatic duct (mm) | 2.8 (± 0.8) | 2.7 (± 1.2) | 0.86 |
| Bile duct (mm) | 9.4 (± 4.9) | 7.9 (± 2.0) | 0.14 |
| Estimated blood loss (ml) | 150 (20–700) | 200 (50–600) | 0.34 |
Continuous variables are expressed as median (range) with the exception of pancreatic and bile duct size, which are expressed as mean (standard deviation); categorical variables are expressed as n (%)
Non-major complications (Clavien–Dindo grade < III); major complications (Clavien–Dindo grade ≥ III)
Comparative Analyses of Postoperative Outcomes Between DA and PDAC Cohorts
As demonstrated in Table 1, the PDAC patients (n = 302) possessed statistically similar demographics to the DA patients, with a median age of 67 (range 33–89), 51% female predominance (n = 155), and median ACCI score of 4 (range 0–10). The median operation length for DA and PDAC patients was 185 min (range 109–480) and 197 min (range 98–633), respectively (p = 0.73). The mean pancreatic and bile duct sizes were smaller in DA patients at 2.75 mm (± 0.92) and 8.93 mm (± 4.25), compared with PDAC patients (3.46 mm ± 2.71 and 11.77 mm ± 5.12, respectively; p < 0.01). DA patients were more likely to have soft pancreatic parenchymal tissue than PDAC patients (47% vs. 8%; p < 0.01). An intraoperative drain was placed at a similar rate for DA and PDAC patients (22% vs. 21%; p = 0.90). All operative characteristics are delineated in Table 1.
DA patients were more likely to develop a major complication postoperatively (31% vs. 15%; p < 0.01) and grade C POPF (9% vs. 1%; p < 0.01) than PDAC patients (Table 4). DA patients experienced a significantly longer mean length of hospital stay (13 ± 16 vs. 10 ± 7 days, p < 0.05) and a higher mortality rate (7% vs. 2%, p < 0.01). Readmission rates were similar at 18% (n = 8) and 14% (n = 43; p = 0.53), respectively (Table 5). Utilizing 1:1 propensity score matching with respect to age, BMI, and ACCI, DA patients were significantly more likely to experience a major postoperative complication (32% vs. 12%, p < 0.05). All matched outcomes are reported in Table 6.
TABLE 4.
Perioperative outcomes stratified by preoperative diagnosis of duodenal adenoma (DA) and pancreatic ductal adenocarcinoma (PDAC)
| DA n = 45 (%) |
PDAC n = 302 (%) |
p value | |
|---|---|---|---|
| Estimated blood loss (ml) | 150 (20–700) | 150 (0–2000) | 0.83 |
| Major complicationsa | 14 (31) | 46 (15) | < 0.01 |
| Postoperative pancreatic fistula | 8 (18) | 20 (7) | < 0.05 |
| Grade C | 4 (9) | 4 (1) | < 0.01 |
| Patient death | 3 (7) | 5 (2) | < 0.05 |
| Length of stay (days) | 13.02 (± 15.74) | 9.72 (± 6.75) | < 0.05 |
| Readmission | 8 (18) | 43 (14) | 0.53 |
Continuous variables are expressed as median (range) except length of stay, which is expressed as mean (standard deviation); categorical variables are expressed as n (%)
Bold values denote p values < 0.05
Major complications (Clavien–Dindo grade ≥ III)
TABLE 5.
Balance check: propensity score matching of patients with duodenal adenoma (DA) and pancreatic ductal adenocarcinoma (PDAC)
| Covariates | Statistics | DA n = 41 |
PDAC n = 41 |
p valuea | Standardized difference |
|---|---|---|---|---|---|
| Age | Mean (SD) | 63.44 (10.39 | 62.68 (11.11) | 0.751 | 0.071 |
| Body mass index (kg/m2) | Mean (SD) | 27.39 (5.67) | 26.99 (5.96) | 0.755 | 0.069 |
| Age-adjusted Charlson index | Mean (SD) | 4.63 (1.65) | 4.51 (2.12) | 0.772 | 0.064 |
The p value is calculated by analysis of variance (ANOVA) for numerical covariates and Chi square test for categorical covariates
TABLE 6.
Comparison of perioperative outcomes between matched patients with duodenal adenoma (DA) and pancreatic ductal adenocarcinoma (PDAC)
| DA n = 41 (%) |
PDAC n = 41 (%) |
p valuea | |
|---|---|---|---|
| Estimated blood loss (ml) | 150 (20–700) | 150 (0–1200) | 0.84 |
| Major complicationsb | 13 (32) | 5 (12) | < 0.05 |
| Postoperative pancreatic fistula | 7 (17) | 2 (5) | 0.10 |
| Grade C | 4 (10) | 1 (2) | 0.18 |
| Patient death | 3 (7) | 1 (2) | 0.32 |
| Length of stay (days) | 13.29 (± 16.41) | 9.59 (± 6.57) | 0.18 |
| Readmission | 8 (20) | 4 (10) | 0.25 |
Continuous variables are expressed as median (range) except length of stay, which is expressed as mean (SD); categorical variables are expressed as n (%)
Bold values denote p values < 0.05
The p value was calculated by a paired t test for numerical covariates, and McNemar’s test for two-level categorical covariates
Major complications (Clavien–Dindo grade ≥ III)
Discussion
The decision to proceed with PD for resection of a benign lesion can be difficult, as it subjects a frequently asymptomatic patient with a slow-growing lesion to an extensive operation with high perioperative morbidity and mortality rates. We examined the incidence of a postoperative diagnosis of adenocarcinoma in patients with DA, as well as perioperative outcomes following PD resection. We then compared 30-day postoperative morbidity and mortality after PD for a preoperative diagnosis of DA versus PDAC, with the goal of identifying the subset of patients who should more likely proceed with PD for DA. The PDAC patients in the comparison group were selectively chosen to best represent similar preoperative characteristics to the DA patients.
The accuracy of endoscopic biopsy for preoperative confirmation of malignancy in duodenal adenomas in the literature ranges from 45 to 84%.3, 5, 11 In this study, preoperative endoscopic biopsies failed to identify malignant pathology in 29% of patients. This is important, as DA patients with malignant final pathology had no major postoperative complications, or clinically significant POPFs. These patients had a significantly shorter length of hospital stay (7 vs. 9 days, p < 0.05) compared with their benign counterparts. The pathophysiology involved in this observation is not readily apparent, though it likely relates to the well-established association between the pancreatic parenchymal fibrosis and dilated ducts observed with malignant lesions and resulting decreased POPF development.23,24
The 29% of DA patients who were found to harbor malignancy on final pathology were statistically more likely to be over the age of 70, require a preoperative biliary stent, and have a bile duct diameter greater than 8 mm. These findings are similar to those reported by Yarandi et al.,25 where jaundice and ductal dilation were most associated with malignancy. Tumor size and presence of high-grade dysplasia on biopsy were less predictive of malignancy on final pathology, contrary to findings reported by Okada et al.,3 where tumor size > 20 mm and high-grade dysplasia were most predictive.
Compared with the cohort of PDAC patients, DA patients were more likely to have an increased BMI, and their tumors were more likely to involve the ampulla of Vater. DA patients also had smaller pancreatic and bile ducts (2.75 mm ± 0.92 and 8.93 mm ± 4.25) compared with PDAC patients (3.46 mm ± 2.71 and 11.77 mm ± 5.12, p < 0.01) and were more likely to have a soft pancreatic texture (47% vs. 8%, p < 0.01). In addition, DA patients were more likely than PDAC patients to develop a major postoperative complication (31% vs. 15%; p < 0.01) and clinically significant POPF (9% vs. 1%; p < 0.01). Furthermore, DA patients were more likely to require a longer length of hospital stay (13 ± 16 vs. 10 ± 7 days, p < 0.05) and experienced a higher mortality rate (7% vs. 2%, p < 0.01). The difference in fistula rates is consistent with Lee et al.,26 who reported a 28.9% POPF rate, half of which were grade C, in patients with preoperative duodenal tumors, compared with 14.5% among patients with all other pathologies following PD. This is also consistent with several studies that have correlated soft pancreas texture with an increased risk for POPF development.23, 24
Propensity score matching was conducted to further analyze the postoperative outcomes of patients with DA and PDAC, given the disparity in sample sizes between the two cohorts. Utilizing 1:1 matching, the significantly increased major complication rate experienced by patients with DA remained present after accounting for age, BMI, and ACCI (32% vs. 12%, p < 0.05). Age, BMI, and ACCI were chosen for propensity score matching given their known independent correlations with worse outcomes following PD.12,19-21
Multiple studies have reported prognostic scoring systems for predicting postoperative complication risk following PD. Braga et al.27 reported and validated a 0- to 15-point scale that determines postoperative minor and major complication risk based on functional status, pancreatic texture, pancreatic duct size, and EBL. Wiltberger et al.28 identified poor functional status, obesity, and cardiovascular and pulmonary comorbidities with increased major postoperative complications following PD. In our study, we did not identify any preoperative or intraoperative factors among DA patients that predicted postoperative complication severity, including age, BMI, Charlson score, preoperatively identified high-grade dysplasia, pancreatic or biliary duct size, or pancreatic texture (Table 3).
The strengths of this study include the inclusion of 10 years of patients within our health system who have undergone PD for DA with the granularity of the extracted patient, tumor, and outcome data. This is one of few studies to directly compare PD for benign adenoma with PDAC resection, as the majority of the literature is derived from chronic pancreatitis and PDAC comparisons between benign and chronic lesion resection outcomes. This is important, as patients undergoing PD for chronic pancreatitis frequently have reduced POPF and morbidity rates due to the increased fibrosis of the pancreas parenchyma compared with patients with other benign lesions of the duodenum and pancreas.29,30 Finally, this study provides surgeons and patients with additional guidance for informed decision-making on whether or not to pursue PD, particularly in younger patients without ductal obstruction.
The limitations of the study include its retrospective design and small number of patients with DA, resulting in potential sampling bias and reduced power. Given the rare nature of DA, a prospective, randomized study with a larger patient sample size is not feasible. We selected all patients who underwent PD for DA over the last 10 years in order to be most representative of the patients and experiences of similar large academic centers where the majority of PD for duodenal adenomas are performed. Given the small DA sample size, propensity score matching was also limited to 1:1, resulting in small patient sample sizes in both the DA and PDAC categories. However, this additional analysis was deemed crucial for bolstering the comparison of postoperative outcomes.
CONCLUSIONS
Preoperative biopsies for duodenal adenoma may miss a cancer diagnosis roughly one-third of the time, particularly among older patients with biliary obstruction. Patients who undergo pancreatoduodenectomy for duodenal adenoma resection experience a higher rate of major postoperative complications compared with those with pancreatic ductal adenocarcinoma, even after matching for age, BMI, and comorbidities. Furthermore, patients with duodenal adenoma may experience higher rates of postoperative pancreatic fistulas, longer hospital stays, and increased mortality. These data emphasize the need for thorough risks and benefits discussions between healthcare providers and patients when determining surgical management of duodenal adenomas, particularly among younger patients presenting without biliary obstruction.
ACKNOWLEDGMENTS
We would like to acknowledge the following surgeons for providing access to their patient data: Mihir M. Shah MD, Kenneth Cardona MD, Juan M. Sarmiento MD, Maria C. Russell MD, Shishir K. Maithel MD, and David A. Kooby MD. Research reported in this publication was supported in part by the Biostatistics and Bioinformatics Shared Resource of Winship Cancer Institute of Emory University and NIH/NCI under award number P30CA138292. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
REFERENCES
- 1.Ma MX, Bourke MJ. Management of duodenal polyps. Best Pract Res Clin Gastroenterol. 2017;31:389–99. [DOI] [PubMed] [Google Scholar]
- 2.Perzin KH, Bridge MF. Adenomas of the small intestine: a clinicopathologic review of 51 cases and a study of their relationship to carcinoma. Cancer. 1981;48:799–819. [DOI] [PubMed] [Google Scholar]
- 3.Okada K, Fujisaki J, Kasuga A et al. Sporadic nonampullary duodenal adenoma in the natural history of duodenal cancer: a study of follow-up surveillance. Am J Gastroenterol. 2011;106:357–64. [DOI] [PubMed] [Google Scholar]
- 4.Heidecke CD, Rosenberg R, Bauer M et al. Impact of grade of dysplasia in villous adenomas of Vater’s papilla. World J Surg. 2002;26:709–14. [DOI] [PubMed] [Google Scholar]
- 5.Bellizzi AM, Kahaleh M, Stelow EB. The assessment of specimens procured by endoscopic ampullectomy. Am J Clin Pathol. 2009;132:506–13. [DOI] [PubMed] [Google Scholar]
- 6.Adler DG, Qureshi W, Davila R et al. The role of endoscopy in ampullary and duodenal adenomas. Gastrointest Endosc. 2006;64:849–54. [DOI] [PubMed] [Google Scholar]
- 7.Culver E, McIntyre A. Sporadic duodenal polyps: classification, investigation, and management. Endoscopy. 2011;43:144–55. [DOI] [PubMed] [Google Scholar]
- 8.Ho CK, Kleeff J, Friess H, Büchler MW. Complications of pancreatic surgery. HPB. 2005;7:99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yeo CJ, Cameron JL, Lillemoe KD et al. Does prophylactic octreotide decrease the rates of pancreatic fistula and other complications after pancreaticoduodenectomy? Results of a prospective randomized placebo-controlled trial. Ann Surg. 2000;232:419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hogg ME, Zenati M, Novak S et al. Grading of surgeon technical performance predicts postoperative pancreatic fistula for pancreaticoduodenectomy independent of patient-related variables. Ann Surg. 2016;264:482–91. [DOI] [PubMed] [Google Scholar]
- 11.Sánchez-Velázquez P, Muller X, Malleo G et al. Benchmarks in pancreatic surgery: a novel tool for unbiased outcome comparisons. Ann Surg. 2019;270:211–8. [DOI] [PubMed] [Google Scholar]
- 12.Dias-Santos D, Ferrone CR, Zheng H et al. The Charlson age comorbidity index predicts early mortality after surgery for pancreatic cancer. Surgery. 2015;157:881–7. [DOI] [PubMed] [Google Scholar]
- 13.Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47:1245–51. [DOI] [PubMed] [Google Scholar]
- 14.Spigelman A, Talbot I, Williams C et al. Upper gastrointestinal cancer in patients with familial adenomatous polyposis. Lancet. 1989;334:783–5. [DOI] [PubMed] [Google Scholar]
- 15.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. In Ann Surg. 2004;205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clavien PA, Barkun J, De Oliveira ML et al. The Clavien–Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187–96. [DOI] [PubMed] [Google Scholar]
- 17.Bassi C International study group on pancreatic fistula definition; postoperative pancreatic fistula; an international study group (ISGPF) definition. Surgery. 2005;138:8–13. [DOI] [PubMed] [Google Scholar]
- 18.Parsons LS. Using SAS software to perform a case-control match on propensity score in an observational study. In: Proceedings of the twenty-fifth annual SAS users group international conference; 2000, p. 1166–71. [Google Scholar]
- 19.Noun R, Riachy E, Ghorra C et al. The impact of obesity on surgical outcome after pancreaticoduodenectomy. JOP. J Pancreas. (Online) 2008;9:468–76. [PubMed] [Google Scholar]
- 20.Benns M, Woodall C, Scoggins C et al. The impact of obesity on outcomes following pancreatectomy for malignancy. Ann Surg Oncol. 2009;16:2565–9. [DOI] [PubMed] [Google Scholar]
- 21.Faraj W, Alameddine R, Mukherji D et al. Postoperative outcomes following pancreaticoduodenectomy: how should age affect clinical practice? World J Surg Oncol. 2013;11:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Austin PC, Grootendorst P, Anderson GM. A comparison of the ability of different propensity score models to balance measured variables between treated and untreated subjects: a Monte Carlo study. Stat Med. 2007;26:734–53. [DOI] [PubMed] [Google Scholar]
- 23.Wellner UF, Kayser G, Lapshyn H et al. A simple scoring system based on clinical factors related to pancreatic texture predicts postoperative pancreatic fistula preoperatively. HPB 2010;12:696–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Callery MP, Pratt WB, Kent TS et al. A prospectively validated clinical risk score accurately predicts pancreatic fistula after pancreatoduodenectomy. J Am Coll Surgeons. 2013;216:1–14. [DOI] [PubMed] [Google Scholar]
- 25.Yarandi SS, Runge T, Wang L et al. Increased incidence of benign pancreatic pathology following pancreaticoduodenectomy for presumed malignancy over 10 years despite increased use of endoscopic ultrasound. Diagn Therap Endosc. 2014;2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee CHA, Shingler G, Mowbray NG et al. Surgical outcomes for duodenal adenoma and adenocarcinoma: a multicentre study in Australia and the United Kingdom. Anz J Surg. 2018;88:E157–61. [DOI] [PubMed] [Google Scholar]
- 27.Braga M, Capretti G, Pecorelli N et al. A prognostic score to predict major complications after pancreaticoduodenectomy. Ann Surg. 2011;254:702–8. [DOI] [PubMed] [Google Scholar]
- 28.Wiltberger G, Muhl B, Benzing C et al. Preoperative risk stratification for major complications following pancreaticoduodenectomy: identification of high-risk patients. Int J Surg. 2016;31:33–9. [DOI] [PubMed] [Google Scholar]
- 29.Winter JM, Cameron JL, Campbell KA et al. 1423 pancreaticoduodenectomies for pancreatic cancer: a single-institution experience. J Gastrointest Surg. 2006;10:1199–211. [DOI] [PubMed] [Google Scholar]
- 30.Hamanaka Y, Nishihara K, Hamasaki T et al. Pancreatic juice output after pancreatoduodenectomy in relation to pancreatic consistency, duct size, and leakage. Surgery. 1996;119:281–7. [DOI] [PubMed] [Google Scholar]


