Abstract
Background:
Environmental phenols, such as parabens, bisphenol A, and triclosan, are ubiquitous in indoor environments because of their use in packaging, plastics, personal care products, and as anti-microbials. The primary pathways of exposure, as well as habits and behaviors that may lead to greater exposure, are still unclear.
Objectives:
Herein, we investigate the relationships between phenols found in residential environments by comparing levels in paired samples of house dust and hand wipes with children’s urine. In addition, phenols were analyzed in a novel exposure tool, the silicone wristband to investigate which external matrix best correlates with individual exposure based on urinary phenol biomarkers.
Methods:
Children aged 3–6 years in central North Carolina, United States, provided paired hand wipe (n = 202), wristband (n = 76), and three spot urine samples that were pooled (n=180), while legal guardians completed questionnaires on habits and behaviors. House dust samples (n = 186) were collected from the main living area during home visits completed between 2014–2016.
Results:
Environmental phenols were detected frequently in all matrices investigated. Ethyl, methyl, and propylparaben levels observed in hand wipes, dust, and on wristbands were significantly correlated to their associated urinary biomarkers. In addition, intra-paraben correlations were noted, with biomarkers of ethyl, methyl, and propylparabens generally positively and significantly correlated, suggesting co-application of parabens in products. Triclosan levels in dust were positive and significantly correlated with levels in hand wipes and wristbands and with urinary concentrations, suggesting non-personal care product sources may be important in children’s overall triclosan exposure. Generally, chemicals on wristbands were more highly correlated with urinary biomarkers than with chemicals in hand wipes or house dust. In addition, more frequent lotion use was positively associated with urinary concentrations of paraben biomarkers.
Conclusions:
Our results suggest that the home environment is an important source of exposure which has been under-investigated for some environmental phenols (e.g. triclosan in house dust). Associations between wristbands and biomarkers of exposure, which were stronger than for hand wipes and house dust, suggest that silicone wristbands may provide a suitable exposure assessment tool for some phenols.
Introduction
Environmental phenols, including parabens and triclosan, are regularly used in personal care products (PCPs) and household items. Biomarkers of these semi-volatile organic compounds (SVOCs) are, therefore, commonly detected in the majority of the United States population (CDC, 2019). Phenols are some of the most abundant chemicals found in the indoor environment and can be detected at higher concentrations than many other classes of chemicals measured in U.S. indoor dust (Mitro et al., 2016). Though the ubiquity of exposure to environmental phenols in residential environments is undisputed, some disagreements in the literature exist as to the extent to which environmental phenols are associated with adverse health outcomes, or whether they represent a health risk. However, results from in vitro, animal, and human studies have linked a range of environmental phenols to endocrine system modulations, including thyroid disruption, testosterone and estrogen antagonism, carcinogenicity, and childhood growth, amongst other health effects (Aker et al., 2018; Bledzka, Gromadzińska, & Wasowicz, 2014; Gao & Kannan, 2020; Koeppe, Ferguson, Colacino, & Meeker, 2013). Because of the possible health effects associated with exposure to environmental phenols, an accurate measurement of residential exposure to these compounds is relevant to formulating a risk assessment.
Environmental phenols cover a large range of compounds. Uses of environmental phenols widely vary across PCPs and household products, and are found in objects such as room deodorizers, flame retardants, antimicrobials, plastics, toothpaste, and building materials. The compounds discussed in this manuscript and their uses are detailed in Table S 1. Human exposure to environmental phenolic chemicals or their precursors is thought to occur via several pathways. Because many of these chemicals are applied directly to the skin in adults and children alike, particularly in the case of parabens (i.e., as PCPs and cosmetics), both inhalation and dermal exposure are of particular relevance when estimating overall exposure. For some compounds, such as parabens, 2,4-dichlorophenol, and BPA, stratum corneum to gas partitioning coefficients have been determined and range from 107.4 to 1011.3 (Weschler & Nazaroff, 2012; Weschler & Nazaroff, 2014), highlighting the importance of both the dermal and inhalation exposure routes for all ages. Exposures to environmental phenols may also occur through ingestion due to their presence in food packaging and drinking water (e.g., BPA, BPS, BPF, 2,4-dichlorophenol, and 2,5-dichlorophenol) (Liu et al., 2019; Park & Kim, 2018).
Herein, we sought to investigate the associations between urinary phenol biomarkers with ambient measurements in residential settings among a cohort of children aged 3–6 years in North Carolina, United States to increase our understanding of exposure pathways besides diet. Paired environmental samples (hand wipes, house dust, and silicone wristbands) were compared to urinary biomarkers quantified in pooled samples in order to further understanding of children’s environmental phenol exposure pathways. Additionally, children’s habits and behaviors were investigated to determine if they contribute to an increase in exposure to phenols or their precursors. Environmental phenols of particular interest, and relevant to PCPs, in this study include: 2,4-dichlorophenol, 2,5-dichlorophenol, 2,4,6-tribromophenol, bisphenol A (BPA), bisphenol S (BPS), bisphenol F (BPF), butylparaben, methylparaben, ethylparaben, propylparaben, and triclosan. Although triclocarban is not a phenol, we have included it within this study because of its similar use as triclosan as an anti-microbial. To our knowledge, this is the first quantitative report of many of these environmental phenols on hand wipes and wristbands, and the first report to compare environmental samples of three matrices to environmental phenol urinary biomarkers for children.
Materials and Methods
Study Population
Mothers who participated in the Newborn Epigenetics STudy (NEST), a prospective pregnancy cohort study Durham, North Carolina, were invited to participate in the Toddler’s Exposure to SVOCs in the Indoor Environment (TESIE) study with their children (Hoffman et al., 2018; Hoyo et al., 2011). A detailed description of recruitment and enrollment procedures for the TESIE study was included in Hoffman et al., 2018. In summary, 203 children aged 3–6 from 190 families participated in the TESIE study from September 2014 to April 2016. Study team members completed home visits with each family enrolled in the TESIE study to collect biospecimens and environmental samples. In addition, the study team collected information about the home environment as well as children’s health and behavior through questionnaires. Study protocols and related materials were reviewed and approved by the Duke Medicine Institutional Review Board. The Centers for Disease Control and Prevention (CDC) laboratory’s participation did not constitute engagement in human subject research. All legal guardians provided informed consent before participation in the TESIE study, and all mothers previously provided informed consent to participate in NEST.
Home Environment Characteristics
Research personnel administered questionnaires to parents or legal guardians during home visits. This questionnaire focused on housing characteristics, children’s health and behavior, and the use of PCPs in the home. Information collected included the frequency of child’s product use (such as the use of nail polish, baby wipes, and lotion) and familial habits (such as how often children consumed food microwaved in plastic containers).
Hand Wipe Collection and Extraction
Families were instructed to not wash children’s hands for at least 1 hour prior to the study team’s visit. During this home visit, research personnel collected a single hand wipe sample from each child using cotton twill wipes (4 × 4 in., MG Chemicals) that were solvent extracted and cleaned, as previously described (Phillips et al., 2018). In summary, gloved research staff soaked the wipe with 3 mL of isopropyl alcohol and wiped the entire surface area of each of the child’s hands. Hand wipes were assessed on a per-wipe basis, as previous work has indicated that normalizing to the surface area of hands does not reduce variability in the hand wipe measurements (Stapleton et al., 2008). Hand wipes were then wrapped in aluminum foil and stored at −20°C until analysis. Full details of the hand wipe analysis was described previously (Phillips et al., 2018). In summary, wipes were spiked with the following internal standards: 13C12-BPA (53.6 ng), 13C12-triclosan (178.6 ng), and d5-ethylparaben (40.4 ng). All analytical standards, both labelled and unlabeled, were sourced from either Cambridge Isotope Laboratories, Inc. (Tewskbury, MA) or Wellington Laboratories (Guelph, Ontario). Wipes were extracted in a 1:1 hexane/dichloromethane (v/v) solution using sonication. Extracts were concentrated to approximately 1 mL using a SpeedVac Concentrator then fractionated using Florisil solid-phase extraction (SPE) cartridges (Supel-clean ENVI-Florisil, 6 mL, 500 mg; Supelco). F3 fractions were eluted with 6 mL methanol and concentrated to approximately 1 mL prior to analysis using liquid chromatography-tandem mass spectrometry (LC/MS/MS). LC/MS/MS conditions and ions monitored can be found in the Supporting Information (Item S1, Table S 5). Recovery of internal standards was assessed using 13C6-triclocarban (10 ng) for all of the internal standards. Field blanks (n = 13) were analyzed alongside the samples for quality assurance and control (Table S 6).
Wristband Collection and Extraction
As described in detail in Hammel et al., 2020, adjustable silicone wristbands were purchased in an array of colors (diasstro adjustable silicone wristband bracelets, Amazon.com) and prepared for deployment to TESIE children. Briefly, wristbands were cleaned using sequential Soxhlet extractions and dried passively in a fume hood, then individually wrapped in pre-cleaned aluminum foil and stored in an air-tight amber 40 mL glass jar until deployment. TESIE children were asked to wear their wristbands continuously 7 days during all daily activities, including sleeping and bathing. At the end of the sampling period, wristbands were wrapped in clean foil, returned to the amber jar, and stored at −20°C until extraction.
A detailed description of the wristband extraction procedure can be found in Hammel et al., 2020. To summarize, about one-third of each wristband, lab blank (n = 5), and field blank (n = 8) was removed from the total wristband for analysis, with the remainder re-wrapped and returned to storage for future analyses. This segment (~1.5 g) was accurately weighed and placed in a glass centrifuge tube. After spiking the internal standards 13C12-BPA (50.0 ng), 13C12-triclosan (100.0 ng), and d5-ethylparaben (100.0 ng), the samples were extracted via sonication using 1:1 hexane/dichloromethane (v/v). Like the hand wipe and dust extracts, the wristband extract was then concentrated to approximately 1 mL using a Thermo Scientific SpeedVac Concentrator. Extracts were fractionated using Florisil SPE cartridges and sequential solvent elution to obtain 3 fractions, which were then concentrated again to approximately 1 mL. The F2 fractions, which was eluted using 10 mL ethyl acetate, were solvent exchanged to hexane (for analyses detailed in Hammel et al., 2020) and then to methanol for analysis here. Extracts were filtered then analyzed for phenols and parabens via LC/MS/MS. For quality assurance and quality control (QA/QC), laboratory blanks (n = 5) and field blanks (n=8) were analyzed alongside the samples (Table S 6). Recovery of internal standards was evaluated using 13C6-triclocarban (10 ng) (Table S 2).
Dust Collection and Extraction
Families were instructed to not vacuum their homes for at least two days prior to the scheduled study team visit. To collect the house dust sample, the entire exposed floor area in the room in which the child or children spent the most time active and awake was vacuumed by a study team member using a Eureka Mighty Mite vacuum fitted with a cellulose thimble within the hose attachment (Stapleton et al., 2012). Thimbles were wrapped in aluminum foil and stored at −20°C until analysis.
Before extraction, each dust sample was sieved to < 500 μm. Dust extraction is described in detail in Phillips et al., 2018. Briefly, dust extracts were first split by mass into aliquots for various analyses. Half of the original dust sample was used for the targeted analysis described herein. Internal standards, 13C12-BPA (51.7 ng), 13C12-triclosan (172.4 ng), and d5-ethylparaben (77.9 ng), were spiked before extraction. The F3 fraction, which was eluted in the SPE step using 6 mL methanol and concentrated to approximately 1mL, was analyzed for the phenols and parabens via LC/MS/MS. Recovery of internal standards was assessed using 13C6-triclocarban (10 ng) for all of the internal standards. For quality assurance and quality control, analysis of laboratory blanks (n = 6) and house dust standard reference materials (n = 5; SRM 2585 National Institute of Standards and Technology (NIST), Gaithersburg, MD) were included in each batch. Measurements of phenols and parabens in SRM 2585 are included in the supplementary information (as well as our comparisons to the literature).
Urine Collection and Analysis
TESIE families received urine sample collection kits during home visits. Three spot urine samples from each child were collected over a 48 h period. Samples were stored in freezers in the families’ home during the sampling period and were transported to the Duke University research laboratory on ice where they were then stored at −20°C. Before analysis, individual samples were thawed and thoroughly mixed. Equal volumes of each of the three urine samples were pooled and composite samples were used for all analyses. The composite urine samples were analyzed for phenolic biomarkers by the CDC laboratory(Ye et al., 2006; Ye, et al., 2005), as described previously in Hoffman et al., 2018. Specific gravity (SG) of pooled samples was measured using a digital handheld refractometer (Atago) and all analyses were conducted with specific gravity corrected urinary biomarker concentrations (Boeniger et al., 1993).
Statistical Analysis
All analyses were performed using SAS statistical software (version 9.4; SAS Institute Inc., Cary, NC) for analytes detected in > 60% of the samples. All values in samples were blank corrected by subtracting the average laboratory or field blank. Method detection limits (MDLs) for dust, hand wipes, and wristbands were calculated using three times the standard deviation of lab blank concentrations. Urinary MDLs were calculated as three times the standard deviation as the concentration approaches zero (Taylor, 1987). For chemicals detected in >60% of samples (e.g., urine, hand wipes, dust or wristbands), values that were less than MDL replaced with MDL/2 in statistical analyses (Antweiler & Taylor, 2008).
Spearman correlations were first used to assess relationships within and between matrices. To examine predictors of phenol biomarkers in urine, generalized estimating equations were used to account for residual intra-family correlations that may occur due to the inclusion of a small number of siblings in our study sample. Analyses were conducted for questionnaire data: child’s nail polish use, child’s hand lotion use, frequency of child’s food consumption from microwaved plastic, frequency of eating out, child’s handwashing frequency, frequency with which child eats out of a plastic bag, and child’s use of scented and unscented wipes. In addition, parent compounds on hand wipes, wristbands and in dust were categorized into quartiles for analyses. Urinary biomarkers were adjusted for specific gravity to account for dilution and log10-transformed before analysis to account for skewed distributions.
Covariates
Covariates included in regression analyses were based on a priori expectations of association with outcomes and predictor variables of interest. Previous work within the TESIE study observed changes in exposure biomarkers based on temperature (Hoffman et al., 2018; Phillips et al., 2018). Average outdoor temperature information from the National Weather Service website was collected based on the week of sample collection. Models that examined predictors of urinary biomarkers included mother’s race/ethnicity, mother’s education level at the time of child’s birth, and average outdoor temperature (modeled as a continuous variable), child’s age and sex.
Though participants were asked to provide all dust, hand wipe, and urine samples, particular circumstances arose in which certain samples could either not be collected (e.g. a family unwilling to collect urine samples). In addition, wristband collection began in the second half to the TESIE study (starting April 2016), and as a result, not all children were asked to wear a wristband. Thus, there is not a complete overlap in the number of participants with each sample type. Relationships were evaluated for the maximum number of available paired samples. However, to ensure conclusions were not driven by our use of different sample sizes, all statistical analyses were repeated limiting to children with complete data for all matrices (Figure S 1).
Results and Discussion
Demographic characteristics of the TESIE study population as well as characteristics of children’s homes are described in Table 1 and were discussed extensively in Hoffman et al., 2018. In brief, the TESIE study contained 203 children from 190 unique households. Slightly more than half of TESIE children were male (56%). Children’s age ranged from 38 to 73 months, with a median age of 54 months (4.5 years). Mothers mostly self-identified as non-Hispanic White (41%) or non-Hispanic Black (37%), while the remaining mothers identified as Hispanic (20%) or other race/ethnicity (2%). Those mothers identifying as other race/ethnicity (n = 3) were excluded from the adjusted analyses. Nearly half of all mothers had at least a four-year college degree (44%) at the time of their child’s birth. Data collected from the questionnaire included information on product use and behavioral characteristics of children within the household. Questionnaire responses and frequencies are also included in Table 1.
Table 1.
Select demographic characteristics of children participating in the TESIE study (2014–2016), select product use patterns, and household characteristics of the TESIE study participants.
| Characteristic | N | % |
| Child Sex | ||
| Male | 113 | 56 |
| Female | 90 | 44 |
| Age | ||
| 38–47 months | 34 | 17 |
| 48–59 months | 130 | 64 |
| 60–73 months | 39 | 19 |
| Ethnicity | ||
| Non-Hispanic white | 84 | 41 |
| Non-Hispanic black | 75 | 37 |
| Hispanic white | 41 | 20 |
| Other | 3 | 1 |
| Maternal education | ||
| Less than college graduate | 113 | 56 |
| College graduate or more | 90 | 44 |
| Mean | range | |
| Child age | 53.9 | 38–73 |
| Average outdoor temp (°C) | 15.5 | −4.4–29.4 |
| Product Use information | N | % |
| Do not use baby wipes | 98 | 48 |
| Use baby wipes (scented) | 33 | 16 |
| Use baby wipes (unscented) | 72 | 36 |
| Do not use nail polish | 132 | 65 |
| Use nail polish | 71 | 35 |
| Microwave plastic | 105 | 52 |
| Do not microwave plastic | 97 | 48 |
| Child never uses lotion | 56 | 28 |
| Child uses lotion 1–5 times/month | 40 | 20 |
| Child uses lotion 6–29 times/month | 29 | 14 |
| Child uses lotion daily | 78 | 38 |
| Child never eats from plastic bag | 49 | 25 |
| Child eats from plastic bag once a month | 32 | 16 |
| Child eats from plastic bag 1–3 times a month | 36 | 18 |
| Child eats from plastic bag >4 times a month | 81 | 41 |
| Child’s Behavioral Habits | ||
| Child washes hands 1–4 times per day | 66 | 33 |
| Child washes hands 5–6 times per day | 72 | 36 |
| Child washes hands more than 6 times per day | 64 | 32 |
| Child never eats out | 18 | 9 |
| Child eats out maybe once a week | 73 | 37 |
| Child eats out 1–2 times a week | 65 | 33 |
| Child eats out >3 times a week | 42 | 21 |
As previously described in Hammel et al., 2020, children began wearing wristbands during the second half of the recruitment phase. As a consequence, we have a smaller number of participants with paired wristbands (n = 77), and these children tended to be older than those in the larger TESIE cohort, ranging in age from 50 to 67 months (median = 57 months), as shown in Table S 3. Children wearing wristbands were more likely to identify as non-Hispanic Black (31%) or Hispanic (43%) than children in the TESIE study as a whole (37% identified as non-Hispanic Black, 20% identified as Hispanic), due in part to our ability to recruit Spanish speakers in the second half of the study. As a sensitivity analysis, all statistical models were additionally evaluated for the subset of children with data available for all exposure matrices (Table S 4). Results were quite similar, and we focus our presentation of results on analyses using the largest samples size available.
Phenol Measurements
Urinary biomarkers were quantified at the CDC’s laboratory, and measurements in abiotic samples were conducted at Duke University. As a result, there is not complete overlap in the target analytes measured in urine and the abiotic matrices. For example, chlorophenols and benzophenone-3 were measured in urine, but not in the hand wipes, wristbands or dust. In total, 7 environmental phenols were quantified in all matrices and are therefore the primary focus here.
Hand wipes.
To our knowledge, this is the first report of phenol measurements on hand wipes and our results suggest phenols are commonly detected on children’s hands. Methylparaben, ethylparaben, propylparaben, and triclosan, were detected in > 60% of hand wipes (Table 2), while 2,4,6-tribromophenol, BPA, butylparaben, and triclocarban were detected less frequently. Methylparaben had the highest median concentration (84 ng/wipe), while triclosan had the largest 95th percentile (3,149 ng/wipe). Both methylparaben and propylparaben were found in 100% of samples. Due to the high detection frequency of ethylparaben, methylparaben, and propylparaben, as well as triclosan on hand wipes, hand-to-mouth behavior and dermal absorption are likely important pathways of exposure for these compounds in particular.
Table 2.
Descriptive statistics for phenols and associated urinary biomarkers as well as triclocarban.
| Matrix and Compound | Det. Freq | MDL | Median | 95th Percentile |
|---|---|---|---|---|
| Dust (ng/g) n = 186 | ||||
| 2,4,6-tribromophenol | 77 | 0.3 | 46 | 1,967 |
| Bisphenol A | 83 | 27 | 3,816 | 27,784 |
| Butylparaben | 72 | 0.3 | 18 | 381 |
| Ethylparaben | 73 | 0.9 | 100 | 1,095 |
| Methylparaben | 91 | 5.7 | 1,874 | 13,788 |
| Propylparaben | 98 | 1.4 | 1,048 | 9,750 |
| Triclocarban | 46 | 0.5 | ND | 431 |
| Triclosan | 99 | 0.2 | 787 | 4,175 |
| Hand Wipe (ng/wipe) n = 202 | ||||
| 2,4,6-tribromophenol | 38 | 1.0 | ND | 129 |
| Bisphenol A | 57 | 7.6 | 17 | 193 |
| Butylparaben | 44 | 0.5 | ND | 10 |
| Ethylparaben | 84 | 0.7 | 3.7 | 89 |
| Methylparaben | 100 | 1.9 | 84 | 1,358 |
| Propylparaben | 100 | 1.0 | 40 | 429 |
| Triclocarban | 37 | 0.2 | ND | 14 |
| Triclosan | 85 | 1.2 | 39 | 3,149 |
| Wristband (ng/g wristband) n = 76 | ||||
| 2,4,6-tribromophenol | 70 | 0.7 | 2.9 | 158 |
| Bisphenol A | 100 | 1.1 | 20 | 67 |
| Butylparaben | 95 | 0.4 | 3.9 | 44 |
| Ethylparaben | 72 | 2.7 | 7.3 | 179 |
| Methylparaben | 100 | 0.8 | 99 | 816 |
| Propylparaben | 100 | 0.7 | 157 | 987 |
| Triclocarban | 92 | 0.9 | 14.2 | 1,081 |
| Triclosan | 99 | 1.6 | 180 | 3,920 |
|
SG-corrected Urine (ng/mL) n = 180 | ||||
| 2,4-dichlorophenol | 97 | 0.10 | 1.0 | 32 |
| 2,5-dichlorophenol | 98 | 0.10 | 6.6 | 1,277 |
| Benzophenone-3 | 99 | 0.40 | 25 | 1,274 |
| Bisphenol A | 100 | 0.20 | 2.1 | 12 |
| Bisphenol F | 45 | 0.20 | ND | 11 |
| Bisphenol S | 99 | 0.10 | 0.94 | 7 |
| Butylparaben | 44 | 0.10 | ND | 7 |
| Ethylparaben | 66 | 1.0 | 1.4 | 123 |
| Methylparaben | 100 | 1.0 | 57 | 1,801 |
| Propylparaben | 100 | 0.10 | 8.5 | 269 |
| Triclocarban | 44 | 0.10 | ND | 7 |
| Triclosan | 76 | 1.7 | 5.7 | 70 |
Wristbands.
Phenol detection frequencies in wristbands were the highest among all abiotic matrices analyzed (Table 2). Triclosan was measured in the greatest abundance with a median of 180 ng/g wristband, followed closely by propylparaben with a median of 157 ng/g wristband. As observed in hand wipes, methylparaben and propylparaben were both detected in 100% of samples. In addition, BPA was found in all wristbands analyzed (detection frequency = 100%), though found in far fewer samples of dust or hand wipes (detection frequency = 84% and 57%, respectively) analyzed in this study. To our knowledge, this is the first investigation to quantify these phenols and parabens in silicone wristbands.
House dust.
Phenols were commonly detected in house dust, with a majority detected in > 70% of all samples (n = 186), as shown in Table 2 (see Table S 6 for phenols measured in dust SRM 2585 used for QA/QC). BPA was the most abundant compound measured in the house dust. In the TESIE study homes, the median BPA level in dust was 3,816 ng/g, which is higher than previously reported worldwide median values (Shin et al., 2019; Wilson et al., 2007) but similar to values reported in Korea and Japan (Liao et al., 2012) and substantially higher than values recently reported in China (Zhu et. al., 2020). Propylparaben was the next most abundant compound in dust samples collected in our study (median = 1,048 ng/g dust). Overall, indoor dust levels of parabens were in line with previously reported dust levels (Bledzka et al., 2014; Chen et al., 2018), though they trend towards the higher end of these worldwide median ranges. Triclosan was found in 100% of all dust samples analyzed, and triclocarban was least commonly detected in house dust, with only a 46% detection frequency. Our median triclosan level of 787 ng/g dust was similar to medians reported in other studies across Asia, Europe, and North America which have been reported to be between 200 – 880 ng/g (Canosa et al., 2007; Chen et al., 2018) but higher than levels in China (Zhu et al., 2020). As described by Chen et al., 2018, phenol abundance in indoor dust may be influenced not only by PCP use, but may also be driven by the different usage of building materials, textiles, and paints that incorporate anti-microbial compounds (Halden et al., 2017).
Urinary Biomarkers.
Twelve phenol biomarkers were quantified in urine samples (Table 2). Similar to wristbands, urinary BPA, methylparaben, and propylparaben were detected in 100% of samples analyzed. Of these, methylparaben was found at the greatest concentrations (median = 57 ng/mL). Associations of urinary biomarkers with demographic variables relevant to this population have been discussed previously (Hoffman et al., 2018). Briefly, concentrations of many of these urinary biomarkers (benzophenone-3, triclosan, and the four parabens) were similar to that observed in the overall U.S. general population between 2008 and 2012 (Calafat et al., 2008; Ferguson et al., 2017) and were generally similar to the median values reported in the 2013–2016 U.S. National Health and Nutrition Examination Survey for older children aged 6–19 years (Jacobson et al., 2019; Lehmler et al., 2018). Urinary biomarker concentrations in our study were also similar to those reported in a previous study of female children aged 6–8 for 2,4-dichlorophenol, 2,5-dichlorophenol, BPA, benzophenone-3, and triclosan (Wolff et al., 2007). Similarly, in a convenience group of 122 3–5 year old children in the United States, median butylparaben, methylparaben, ethylparaben, propylparaben, benzophenone-3, BPA, triclosan, 2,4-dichlorophenol, and 2,5-dichlorophenol volumetric values were all reported as similar to median volumetric urinary biomarker concentrations reported here, with similar detection frequency per compound (Calafat et al., 2017). Note that these comparisons to this dataset were made based on unadjusted concentrations, as different methods were used by Calafat et al. (2017) to account for urine dilution.
Associations between Environmental Samples and Urine.
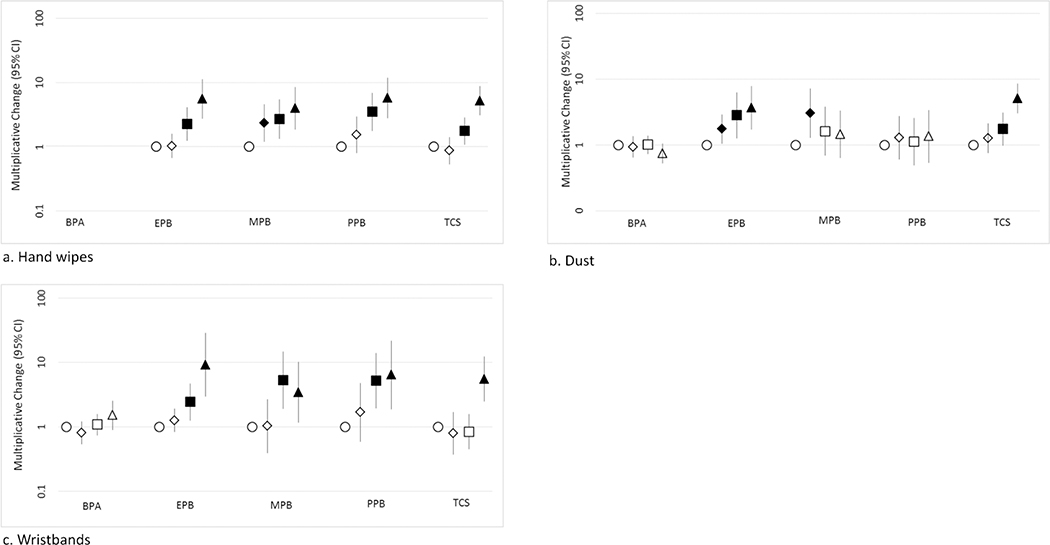
Correlation coefficients for phenols in dust, hand wipes, wristbands and their urinary biomarkers are listed in Table 3. Correlations were generally greater for hand wipes as compared to dust. Correlations between parent phenol and associated urinary biomarker were generally larger for wristbands than correlations for dust, and were similar to or greater than hand wipe correlations. Similar to correlation analyses, significant associations were observed between parent compound concentrations on hand wipes and urinary biomarker for all parabens and triclosan in adjusted regression models. This trend held for parent compound concentrations in wristbands and urinary biomarkers as well (Figure 1; Table S 7 – Table S 9).
Table 3.
Spearman correlation coefficients for phenols and associated urinary biomarkers in paired hand wipes (n = 178 with hand wipes and urine), dust (n = 174 with dust and urine), wristbands (n = 74 children with wristbands and urine) and urinary biomarkers (n=180 with urine samples).
| Urinary Metabolite | ||||||||
|---|---|---|---|---|---|---|---|---|
| 2,4-dichlorophenol | 2,5-dichlorophenol | BPA | Ethylparaben | Methylparaben | Propylparaben | Triclosan | ||
| 2,4,6-tribromophenol | −0.10 | −0.20# | −0.14 | 0.004 | −0.01 | 0.05 | 0.16* | |
| BPA | 0.10 | 0.19* | −0.07 | 0.04 | 0.02 | 0.01 | −0.08 | |
| Dust | Ethylparaben | 0.15 | 0.09 | 0.03 | 0.34† | 0.25# | 0.20# | 0.12 |
| Methylparaben | 0.15 | 0.19* | 0.10 | 0.25# | 0.25# | 0.21# | 0.09 | |
| Propylparaben | 0.17* | 0.23# | 0.11 | 0.29# | 0.26# | 0.26# | 0.03 | |
| Triclosan | 0.14 | −0.09 | −0.15 | 0.02 | −0.07 | −0.11 | 0.47† | |
| Ethylparaben | 0.02 | 0.07 | 0.19* | 0.48† | 0.22# | 0.18* | −0.09 | |
| Hand Wipes | Methylparaben | −0.04 | 0.07 | 0.19* | 0.33† | 0.41† | 0.39† | 0.005 |
| Propylparaben | 0.06 | 0.07 | 0.19# | 0.34† | 0.42† | 0.48† | 0.08 | |
| Triclosan | 0.19* | −0.06 | 0.07 | −0.01 | −0.02 | −0.003 | 0.50† | |
| 2,4,6-tribromophenol | 0.12 | 0.15 | −0.01 | −0.12 | −0.09 | −0.11 | −0.15 | |
| BPA | −0.17 | −0.11 | 0.23* | 0.21 | 0.15 | 0.13 | −0.15 | |
| Wristbands | Ethylparaben | −0.01 | 0.10 | 0.08 | 0.66† | 0.33# | 0.21 | −0.01 |
| Methylparaben | −0.08 | −0.04 | 0.04 | 0.40# | 0.56† | 0.51† | 0.20 | |
| Propylparaben | −0.17 | −0.06 | 0.11 | 0.33# | 0.51† | 0.64† | 0.02 | |
| Triclosan | 0.11 | −0.05 | −0.06 | 0.04 | 0.19 | 0.02 | 0.51† | |
| 2,4-dichlorophenol | 1.00 | 0.65† | 0.23# | 0.07 | 0.11 | 0.05 | 0.19* | |
| 2,5-dichlorophenol | 1.00 | 0.28# | 0.01 | 0.15* | 0.15* | −0.20# | ||
| Urinary biomarker | BPA | 1.00 | 0.23# | 0.29† | 0.26 | 0.01 | ||
| Ethylparaben | 1.00 | 0.54† | 0.42† | 0.03 | ||||
| Methylparaben | 1.00 | 0.81† | 0.05 | |||||
| Propylparaben | 1.00 | −0.08 | ||||||
| Triclosan | 1.00 | |||||||
p<0.05
p <0.01
p<0.0001; dark gray cells represent parent and biomarker pairs.
Figure 1.
Multiplicative change in urinary biomarker quartiles versus parent compound in hand wipes, dust, and wristbands and 95% confidence interval (n = 179 for hand wipes, 174 for dust and 74 for wristbands Quartiles defined by ○ (reference/first quartile), ◊ (second quartile), □ (third quartile), and Δ (fourth quartile). Analyses not conducted for BPA on hand wipes due to its low detection frequency.
Note: solid symbols are significant at least at p<0.05; BPA: Bisphenol-A, EPB: Ethylparaben, MPB: Methylparaben, PPB: Propylparaben, TCS: Triclosan
Ethylparaben, methylparaben, and propylparaben all displayed similar correlations (rs = 0.48, 0.41, and 0.48, respectively, all p<0.0001) between parent compound in hand wipes and associated urinary biomarker. Wristband ethylparaben was positively correlated with its urinary biomarker (rs = 0.66, p<0.0001), as were propylparaben, methylparaben, and triclosan (rs = 0.64, 0.56, and 0.51 respectively, all p<0.0001). In adjusted regression models using dust levels as the predictor, only ethylparaben and triclosan were positively associated with their respective urinary biomarker concentrations (Figure 1; Table S 8).
In dust samples, the largest correlations between parent phenol and associated urinary biomarker were observed for triclosan (rs = 0.47, p<0.0001) and ethylparaben (rs = 0.34, p<0.001). The largest correlation across all matrices observed for triclosan in hand wipes and its associated urinary biomarker (rs = 0.50, p<0.0001). Children with the highest levels of triclosan on their wristbands had urinary triclosan concentrations approximately 4.5 times higher than those with the lowest levels on their wristbands (10β = 5.49; 95% Confidence Interval (CI): 2.47, 12.21; p<.0001) (Table S 9).
Importantly, BPA was not detected frequently in hand wipes and as a result, correlation analyses were not conducted Despite frequent detection of BPA in dust and on wristbands, BPA levels on wristbands and in dust were not associated with urinary BPA in children after adjusting for demographic factors and outdoor temperature. These findings could be explained by BPA’s common presence in foodstuffs and may reflect the importance of the ingestion exposure pathway for BPA.
Environmental phenols, particularly parabens and triclosan, can be found in many PCPs applied to skin and/or that employ anti-microbial properties. Results presented here suggest that the parent concentrations measured in hand wipes and wristbands are most strongly associated with urinary biomarkers measured in the children in this study, as compared to measuring the chemical levels in dust. hand wipes and wristbands may both be better able to integrate exposures across multiple microenvironments where a child spends time, compared to dust which is only representative of potential exposure in one microenvironment. Taken together, correlation analyses suggest that exposure to some phenols, parabens or their precursors can be effectively captured using wristbands or hand wipes. We generally observed slightly higher correlations for wristbands as compared to hand wipes. One possible explanation for this pattern is that hand wipes are more variable due to handwashing behaviors.
Correlations of triclosan on hand wipes, wristbands, and dust were significantly correlated with urinary triclosan concentrations. Though exposure to triclosan is primarily considered to occur through the use of PCPs, this finding suggests that the indoor environment important and plays a role in children’s overall exposure. We would not expect to see a significant correlation between external exposures such as house dust (from the main living area) and urinary concentrations of triclosan if PCPs such as hand soap were the primary source. Because of the increased potential for dust exposure in children compared to adults, predominantly due to their high rates of hand-to-mouth behavior, exposure to triclosan via external exposure routes such as dust may be of particular interest for future investigations regarding children’s exposure.
Wristbands in particular are thought to integrate both inhalation and dermal exposure of semi-volatile organic compounds or SVOCs (Wang et al., 2019), which may provide a better predictor of the urinary biomarker concentrations observed for children, particularly for parabens given their relatively high octanol-air partitioning coefficients (Koa = 107.6 – 108.9) (Weschler & Nazaroff, 2014). Previous modeling of SVOCs suggests that the dermal exposure route is thought to have been severely underestimated in the past and may contribute to overall environmental exposure burdens at levels equal to that of the inhalation pathways for SVOCs (Weschler & Nazaroff, 2014). For parabens, capturing the dermal pathway of exposure may be of particular interest because they are often used in PCPs frequently applied to the skin, which may result in dermal absorption. Dermal absorption of parabens has been demonstrated in humans and animals, though absorption through human skin is thought to be higher than through animal skin (Darbre et al., 2004; Darbre & Harvey, 2014; Janjua et al., 2008; Janjua et al., 2007). Therefore, using hand wipes and/or wristbands may assist in assessing the dermal pathway of exposure for parabens in children.
Associations between Environmental Samples.
Spearman correlations were also calculated between parent phenol concentrations found in dust and hand wipe samples (Table 4). Triclosan was most strongly correlated between the two exposure matrices (rs = 0.37, p<0.0001), followed by propylparaben (rs = 0.34, p<0.0001). When evaluating correlations between wristbands and either dust or hand wipes (Table 5), ethylparaben, methylparaben, and propylparaben were most strongly and significantly correlated between hand wipes and wristband measurements (rs = 0.55, 0.44, 0.54, respectively; p<0.0001). In addition, positive correlations were observed between dust, hand wipes, and wristband matrices for all parabens. Finally, triclosan in both dust and hand wipes significantly and positively correlated with triclosan on wristbands (rs = 0.44, p<0.0001; rs = 0.36, p<0.01, respectively).
Table 4.
Spearman correlation coefficients for phenols measured in paired hand wipe and dust (n=197 with both samples).
| Dust | |||||||
|---|---|---|---|---|---|---|---|
| BPA | Butyl Paraben | Ethylparaben | Methylparaben | Propylparaben | Triclosan | ||
| Hand Wipes | Ethylparaben | 0.01 | 0.01 | 0.26# | 0.12 | 0.16* | −0.04 |
| Methylparaben | 0.06 | −0.05 | 0.14* | 0.20# | 0.24# | −0.05 | |
| Propylparaben | 0.08 | 0.03 | 0.17* | 0.28† | 0.34† | 0.03 | |
| Triclosan | −0.03 | 0.11 | 0.20# | 0.23# | 0.19# | 0.37† | |
p<0.05
p <0.01
p<0.0001; dark gray cells denote the relationship between the same compound in dust and hand wipes.
Table 5.
Spearman correlation coefficients for phenols in paired hand wipes (n = 76), dust (n = 75).
| Wristbands | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 2,4,6-tribromophenol | BPA | Butylparaben | Ethylparaben | Methylparaben | Propylparaben | Triclocarban | Triclosan | ||
| BPA | −0.03 | −0.15 | −0.14 | −0.09 | 0.09 | 0.12 | −0.05 | 0.16 | |
| Butylparaben | 0.06 | −0.01 | 0.23* | 0.17 | 0.14 | 0.17 | 0.05 | 0.08 | |
| Dust | Ethylparaben | 0.05 | 0.06 | 0.17 | 0.40# | 0.37# | 0.28* | 0.01 | 0.10 |
| Methylparaben | −0.15 | 0.08 | 0.02 | 0.28* | 0.36# | 0.34# | 0.17 | 0.16 | |
| Propylparaben | −0.15 | 0.09 | 0.08 | 0.23* | 0.33# | 0.38# | 0.17 | 0.05 | |
| Triclosan | −0.07 | −0.02 | −0.01 | 0.17 | 0.21 | 0.12 | 0.04 | 0.44† | |
| Hand Wipes | Ethylparaben | −0.15 | 0.21 | 0.38# | 0.55† | 0.23* | 0.16 | 0.12 | −0.02 |
| Methylparaben | −0.26* | 0.03 | 0.07 | 0.29* | 0.44† | 0.37# | 0.12 | 0.04 | |
| Propylparaben | −0.30# | 0.15 | 0.22 | 0.27* | 0.44† | 0.54† | 0.05 | 0.01 | |
| Triclosan | 0.01 | −0.15 | −0.09 | −0.02 | 0.06 | −0.02 | 0.06 | 0.36# | |
p<0.05
p <0.01
p<0.0001; dark gray cells denote the relationship between the same compound in wristbands and dust/hand wipes.
As shown in Table 3–5, there are a number of phenols that are correlated within and between matrices, suggesting that exposure sources and pathways may be similar (including physical chemical properties and metabolism). For example, the correlations amongst ethylparaben, methylparaben, and propylparaben were particularly strong across all three exposure matrices (p<0.05), except for ethylparaben on wristbands and urinary propylparaben which were not significantly correlated. Correlation patterns likely relate to the use of parabens together in many products, such as lotions and other cosmetics, as has been described previously (Calafat et al., 2010; Guo & Kannan, 2013; Ma et al., 2016). This strengthens the evidence that co-exposure or co-occurrence of parent parabens in residential products is occurring (Bledzka et al., 2014).
Product Use and Exposure Matrices.
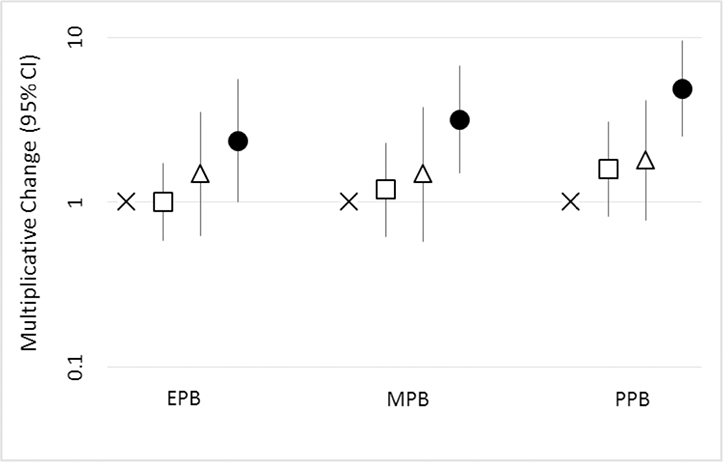
Associations of the urinary biomarkers of ethylparaben, methylparaben, and propylparaben, as well as biomarkers of triclocarban and triclosan were compared to hand lotion use frequency, nail polish use, use of baby wipes, hand washing frequency and frequency of eating out. In these analyses, the results were largely null (Table S 10 – Table S 16). However, lotion use frequency was positively associated with paraben urinary biomarkers. As shown in Figure 2, propylparaben urinary concentrations in children who used lotion daily were around 5 times as high as in those who did not use lotion (10β = 4.9, 95% CI = 2.5–9.6, p<0.0001); similarly, concentrations of ethylparaben and methylparaben biomarkers were also significantly higher among this highest lotion use frequency group (10β = 2.4, 95% CI = 1.0–5.6, p<0.05; 10β = 3.2, 95% CI = 1.5–6.8, p<0.01, respectively). Similar to our reported values herein, Braun et al. (2014) found that users of lotion had higher propylparaben concentrations (approximately 2.5 times higher) than non-users. Because there is evidence that both methylparaben and propylparaben are present in lotion (Guo et al., 2014), the higher exposure for methylparaben and propylparaben based on children who use lotion most frequently is understandable. One study also found that an intervention successfully decreased paraben urinary biomarker concentrations by changing personal care products, including lotions, to include fewer or no potential endocrine disrupting compounds in adolescents (Harley et al., 2016).
Figure 2.
Multiplicative change in children’s paraben urinary biomarker based on child’s hand lotion use frequency and 95% confidence interval from adjusted model (n=180). Child’s hand lotion frequency defined by x (child never uses hand lotion), □ (child uses hand lotion 1–5 times a month), Δ (child uses hand lotion 6–29 times a month), and ○ (child uses hand lotion daily). Analyses were adjusted for child age and sex, maternal race/ethnicity and education, and average outdoor temperature at the time of collection.
Note: solid symbols are significant at least at p<0.05; EPB: Ethylparaben, MPB: Methylparaben, PPB: Propylparaben
Limitations and Strengths.
Our study included a large population size used for an exposure study of a diverse group of children, and included paired samples of dust, hand wipes, wristbands and urine. Furthermore, three urine samples were collected over 48 hours and then pooled. Nonetheless, our study does have a few potential limitations that should be considered. Home environments could only be measured at a single point in time, which limited our ability to evaluate long-term exposures. Dust was only sampled from the main living area, which may have left out potential exposures of interest that originated in other areas of the home or outside the home, such as at school or daycare. No assessments of personal diet were conducted during these home visits and we cannot estimate how much of the urinary concentrations were attributable to diet. No analyses were conducted on particular products used to verify the presence or absence of particular environmental phenols, which may result in misclassification bias. Importantly, this type of misclassification may have biased associations to the null, suggesting there may be a stronger association between the use of paraben containing lotion and paraben exposure. Finally, the study population was a convenience sample derived from a previous pregnancy cohort and may not be generalizable to the broader population, though we do not expect this to impact the internal validity of the study.
Conclusions
Overall, we found that a number of phenols and phenols biomarkers measured in paired samples of dust, hand wipes, wristbands and urine were moderately to strongly correlated, suggesting that the ambient indoor environment, and PCPs use are primary sources of exposure. Based on correlations with urinary biomarkers, both wristbands and hand wipes demonstrated better estimates of ambient environmental phenols exposures in the TESIE children than house dust. Our results suggest wristbands and hand wipes appear to capture the primary pathways of exposure for several environmental phenols or their precursors where diet is not the main pathway, and particularly parabens. In contrast, while it appears that BPA exposure was detectable on the wristbands, diet is likely the major exposure pathway and explains the poorer correlation with urinary BPA. However, wristbands may ultimately provide better utility than hand wipes because of the increased ease of deployment and collection of an exposure monitoring matrix and due to their ability to measure an aggregate exposure over a set time period. Particularly for environmental phenols, which tend to have a short metabolic half-life, wristbands may better capture aggregate exposure to these compounds than other environmental matrices.
Supplementary Material
Highlights.
Paired hand wipe, wristband, house dust and urine samples were analyzed for phenols
Exposure matrices and urinary biomarkers were positively correlated
Triclosan in dust, wristbands and hand wipes was correlated with urinary biomarkers
Lotion use was associated with ethyl, methyl, and propylparaben biomarkers
Acknowledgements
Funding for this research was provided by grants from the US Environmental Protection Agency (Grant 83564201) and NIEHS (R01 ES016099). Additional support for ALP was provided by NIEHS (T32-ES021432). T. Webster was supported in part by NIH/NIEHS R01ES028800 and R01ES027813. Thanks to Albert Chen for help with home data collection. We also thank our participants for opening their homes to our study team and helping us gain a better understanding of children’s exposures to SVOCs.
Footnotes
The authors declare no competing financial interests.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention (CDC). Use of trade names is for identification only and does not imply endorsement by the CDC, the Public Health Service, or the US Department of Health and Human Services.
Associated Content
Supporting Information
Additional information on the study sample, sensitivity analyses and analytic methods is provided in supplemental information.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aker AM, Johns L, McElrath TF, Cantonwine DE, Mukherjee B, & Meeker JD (2018). Associations between maternal phenol and paraben urinary biomarkers and maternal hormones during pregnancy: A repeated measures study. Environment International, 113, 341–349. 10.1016/j.envint.2018.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antweiler RC, & Taylor HE (2008). Evaluation of statistical treatments of left-censored environmental data using coincident uncensored data sets: I. Summary statistics. Environmental Science and Technology, 42(10), 3732–3738. 10.1021/es071301c [DOI] [PubMed] [Google Scholar]
- Aschenbeck KA, & Warshaw EM (2017). Allergenic Ingredients in Personal Hygiene Wet Wipes. Dermatitis, 28(5), 317–322. 10.1097/DER.0000000000000275 [DOI] [PubMed] [Google Scholar]
- Bledzka D, Gromadzińska J, & Wasowicz W (2014). Parabens. From environmental studies to human health. Environment International, 67, 27–42. 10.1016/j.envint.2014.02.007 [DOI] [PubMed] [Google Scholar]
- Boeniger MF, Lowry LK, & Rosenberg J (1993). Interpretation of urine results used to assess chemical exposure with emphasis on creatinine adjustments: a review. American Industrial Hygiene Association Journal, 54(10), 615–627. [DOI] [PubMed] [Google Scholar]
- Braun JM, Just AC, Williams PL, Smith KW, Calafat AM, & Hauser R (2014). Personal care product use and urinary phthalate metabolite and paraben concentrations during pregnancy among women from a fertility clinic. Journal of Exposure Science and Environmental Epidemiology, 24(5), 459–466. 10.1038/jes.2013.69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai S, Rao X, Ye J, Ling Y, Mi S, Chen H, … Li Y (2020). Relationship between urinary bisphenol a levels and cardiovascular diseases in the US adult population, 2003–2014. Ecotoxicology and Environmental Safety, 192(February), 110300 10.1016/j.ecoenv.2020.110300 [DOI] [PubMed] [Google Scholar]
- Calafat AM, Ye X, Valentin-blasini L, Li Z, Mortensen ME, & Wong L (2017). Co-exposure to non-persistent organic chemicals among American pre-school aged children : A pilot study. International Journal of Hygiene and Environmental Health, 220(2), 55–63. 10.1016/j.ijheh.2016.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat AM, Ye X, Wong LY, Bishop AM, & Needham LL (2010). Urinary concentrations of four parabens in the US Population: NHANES 2005–2006. Environmental Health Perspectives, 118(5), 679–685. 10.1289/ehp.0901560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat AM, Ye X, Wong LY, Reidy JA, & Needham LL (2008). Exposure of the US population to Bisphenol A and 4-tertiary-octylphenol: 2003–2004. Environmental Health Perspectives, 116(1), 39–44. 10.1289/ehp.10753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canosa P, Rodríguez I, Rubí E, & Cela R (2007). Determination of parabens and triclosan in indoor dust using matrix solid-phase dispersion and gas chromatography with tandem mass spectrometry. Analytical Chemistry, 79(4), 1675–1681. 10.1021/ac061896e [DOI] [PubMed] [Google Scholar]
- CDC. (2019). Fourth National Report on Human Exposure to Environmental Chemicals. Retrieved from https://www.cdc.gov/exposurereport/index.html [PubMed]
- Champmartin C, Marquet F, Chedik L, Décret MJ, Aubertin M, Ferrari E, … Cosnier F (2020). Human in vitro percutaneous absorption of bisphenol S and bisphenol A: A comparative study. Chemosphere, 252 10.1016/j.chemosphere.2020.126525 [DOI] [PubMed] [Google Scholar]
- Chen J, Hartmann EM, Kline J, Van Den Wymelenberg K, & Halden RU (2018). Assessment of human exposure to triclocarban, triclosan and five parabens in US indoor dust using dispersive solid phase extraction followed by liquid chromatography tandem mass spectrometry. Journal of Hazardous Materials, 360(August), 623–630. 10.1016/j.jhazmat.2018.08.014 [DOI] [PubMed] [Google Scholar]
- Cherian P, Zhu J, Bergfeld WF, Belsito DV, Hill RA, Klaassen CD, Liebler DC, Marks JG, Shank RC, Slaga TJ, Snyder PW, Heldreth B (2020). Amended Safety Assessment of Parabens as Used in Cosmetics. International Journal of Toxicology. 39(Supplement 1), 5S–97S. 10.1177/1091581820925001 [DOI] [PubMed] [Google Scholar]
- Darbre PD, Aljarrah A, Miller WR, Coldham NG, Sauer MJ, & Pope GS (2004). Concentrations of Parabens in human breast tumours. Journal of Applied Toxicology, 24(1), 5–13. 10.1002/jat.958 [DOI] [PubMed] [Google Scholar]
- Darbre Philippa D. (2019). Review: The history of endocrine-disrupting chemicals.pdf. Elsevier. [Google Scholar]
- Darbre Philippa D., & Harvey PW (2014). Parabens can enable hallmarks and characteristics of cancer in human breast epithelial cells: A review of the literature with reference to new exposure data and regulatory status. Journal of Applied Toxicology, 34(9), 925–938. 10.1002/jat.3027 [DOI] [PubMed] [Google Scholar]
- Ferguson KK, Colacino JA, Lewis RC, & Meeker JD (2017). Personal care product use among adults in NHANES: associations between urinary phthalate metabolites and phenols and use of mouthwash and sunscreen. J Expo Sci Environ Epidemiol, 27(3), 326–332. 10.1038/jes.2016.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao CJ, & Kannan K (2020). Phthalates, bisphenols, parabens, and triclocarban in feminine hygiene products from the United States and their implications for human exposure. Environment International, 136(September 2019), 105465 10.1016/j.envint.2020.105465 [DOI] [PubMed] [Google Scholar]
- Gribble GW (2000). The natural production of organobromine compounds. Environmental Science and Pollution Research, 7, 37–49. [DOI] [PubMed] [Google Scholar]
- Guo Y, & Kannan K (2013). A survey of phthalates and parabens in personal care products from the United States and its implications for human exposure. Environmental Science and Technology, 47(24), 14442–14449. 10.1021/es4042034 [DOI] [PubMed] [Google Scholar]
- Guo Y, Wang L, & Kannan K (2014). Phthalates and parabens in personal care products from China: Concentrations and human exposure. Archives of Environmental Contamination and Toxicology, 66(1), 113–119. 10.1007/s00244-013-9937-x [DOI] [PubMed] [Google Scholar]
- Halden RU (2014). On the need and speed of regulating triclosan and triclocarban in the United States. Environmental Science and Technology, 48(7), 3603–3611. 10.1021/es500495p [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halden RU, Lindeman AE, Aiello AE, Andrews D, Arnold WA, Fair P, … Blum A (2017). The florence statement on triclosan and triclocarban. Environmental Health Perspectives, 125(6), 1–13. 10.1289/EHP1788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammel SC, Levasseur JL, Hoffman K, Phillips AL, Lorenzo AM, Calafat AM, … Stapleton HM (2019). Children’s exposure to phthalates and non-phthalate plasticizers in the home: The TESIE study. Environment International, 132 10.1016/j.envint.2019.105061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammel Stephanie C., Hoffman K, Phillips AL, Levasseur JL, Lorenzo AM, Webster TF, & Stapleton HM (2020). Comparing the Use of Silicone Wristbands, Hand Wipes, And Dust to Evaluate Children’s Exposure to Flame Retardants and Plasticizers. Environmental Science & Technology, 54(7), 4484–4494. 10.1021/acs.est.9b07909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley KG, Kogut K, Madrigal DS, Cardenas M, Vera IA, Meza-Alfaro G, … Parra KL (2016). Reducing phthalate, paraben, and phenol exposure from personal care products in adolescent girls: Findings from the hermosa intervention study. Environmental Health Perspectives, 124(10), 1600–1607. 10.1289/ehp.1510514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines EP, Mendola P, von Ehrenstein OS, Ye X, Calafat AM, & Fenton SE (2015). Concentrations of environmental phenols and parabens in milk, urine and serum of lactating North Carolina women. Reproductive Toxicology, 54, 120–128. 10.1016/j.reprotox.2014.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman K, Hammel SC, Phillips AL, Lorenzo AM, Chen A, Calafat AM, … Stapleton HM (2018). Biomarkers of exposure to SVOCs in children and their demographic associations: The TESIE Study. Environment International, 119(June), 26–36. 10.1016/j.envint.2018.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyo C, AP M, JM S, MR F, B. C, W. D-W, … RL J (2011). Folic acid supplementation before and during pregnancy in the Newborn Epigenetics STudy (NEST). BMC Public Health, 11(1), 46 Retrieved from http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=emed12&NEWS=N&AN=560065423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson MH, Woodward M, Bao W, Liu B, & Trasande L (2019). Urinary Bisphenols and Obesity Prevalence Among US Children and Adolescents. Journal of the Endocrine Society, 3(9), 1715–1726. 10.1210/js.2019-00201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janjua NR, Frederiksen H, Skakkebæk NE, Wulf HC, & Andersson AM (2008). Urinary excretion of phthalates and paraben after repeated whole-body topical application in humans. International Journal of Andrology, 31(2), 118–130. 10.1111/j.1365-2605.2007.00841.x [DOI] [PubMed] [Google Scholar]
- Janjua NR, Mortensen GK, Andersson AM, Kongshoj B, Skakkebæk NE, & Wulf HC (2007). Systemic uptake of diethyl phthalate, dibutyl phthalate, and butyl paraben following whole-body topical application and reproductive and thyroid hormone levels in humans. Environmental Science and Technology, 41(15), 5564–5570. 10.1021/es0628755 [DOI] [PubMed] [Google Scholar]
- Koeppe ES, Ferguson KK, Colacino JA, & Meeker JD (2013). Relationship between urinary triclosan and paraben concentrations and serum thyroid measures in NHANES 2007–2008. Science of the Total Environment, 445–446, 299–305. 10.1016/j.scitotenv.2012.12.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao C; Liu F; Guo Y; Moon HB; Nakata H; Wu Q; Kannan K Occurrence of eight bisphenol analogues in indoor dust from the United States and several Asian countries: implications for human exposure. Environ. Sci. Technol 563 2012, 46 (16), 9138–9145. [DOI] [PubMed] [Google Scholar]
- Larsson K, Ljung Björklund K, Palm B, Wennberg M, Kaj L, Lindh CH, … Berglund M (2014). Exposure determinants of phthalates, parabens, bisphenol A and triclosan in Swedish mothers and their children. Environment International, 73, 323–333. 10.1016/j.envint.2014.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmler HJ, Liu B, Gadogbe M, & Bao W (2018). Exposure to Bisphenol A, Bisphenol F, and Bisphenol S in US Adults and Children: The National Health and Nutrition Examination Survey 2013–2014. ACS Omega. 10.1021/acsomega.8b00824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonetti C, Butt CM, Hoffman K, Lynn M, & Stapleton HM (2016). Concentrations of polybrominated diphenyl ethers ( PBDEs ) and 2, 4, 6-tribromophenol in human placental tissues. Environment International, 88, 23–29. 10.1016/j.envint.2015.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Lehmler H, Sun Y, Xu G, Sun Q, Snetselaar LG, & Wallace RB (2019). Association of Bisphenol A and Its Substitutes, Bisphenol F and Bisphenol S, with Obesity in United States Children and Adolescents Study population. 59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma WL, Zhao X, Lin ZY, Mohammed MOA, Zhang ZF, Liu LY, … Li YF (2016). A survey of parabens in commercial pharmaceuticals from China and its implications for human exposure. Environment International, 95, 30–35. 10.1016/j.envint.2016.07.013 [DOI] [PubMed] [Google Scholar]
- Matwiejczuk N, Galicka A, & Brzóska MM (2020). Review of the safety of application of cosmetic products containing parabens. Journal of Applied Toxicology, 40(1), 176–210. 10.1002/jat.3917 [DOI] [PubMed] [Google Scholar]
- Meeker JD, Cantonwine DE, Rivera-González LO, Ferguson KK, Mukherjee B, Calafat AM, … Cordero JF (2013). Distribution, variability, and predictors of urinary concentrations of phenols and parabens among pregnant women in puerto rico. Environmental Science and Technology, 47(7), 3439–3447. 10.1021/es400510g [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitro SD, Dodson RE, Singla V, Adamkiewicz G, Elmi AF, Tilly MK, & Zota AR (2016). Consumer Product Chemicals in Indoor Dust: A Quantitative Meta- analysis of US Studies. 10.1021/acs.est.6b02023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss T, Howes D, & Williams FM (2000). Percutaneous penetration and dermal metabolism of triclosan (2,4,4’- trichloro-2’-hydroxydiphenyl ether). Food and Chemical Toxicology, 38(4), 361–370. 10.1016/S0278-6915(99)00164-7 [DOI] [PubMed] [Google Scholar]
- Parada H, Gammon MD, Ettore HL, Chen J, Calafat AM, Neugut AI, … Teitelbaum SL (2019). Urinary concentrations of environmental phenols and their associations with breast cancer incidence and mortality following breast cancer. Environment International, 130(May). 10.1016/j.envint.2019.05.084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H, & Kim K (2018). Concentrations of 2,4-dichlorophenol and 2,5-dichlorophenol in Urine of Korean adults. International Journal of Environmental Research and Public Health, 15(4). 10.3390/ijerph15040589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philips EM, Santos S, Steegers EAP, Asimakopoulos AG, Kannan K, Trasande L, & Jaddoe VWV (2020). Maternal bisphenol and phthalate urine concentrations and weight gain during pregnancy. Environment International, 135(November 2019), 105342 10.1016/j.envint.2019.105342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips AL, Hammel SC, Hoffman K, Lorenzo AM, Chen A, Webster TF, & Stapleton HM (2018). Children’s residential exposure to organophosphate ester flame retardants and plasticizers: Investigating exposure pathways in the TESIE study. Environment International, 116(April), 176–185. 10.1016/j.envint.2018.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman KJ (1990). Rothman 1990 No Adjustments Are Needed for Multiple Comparisons.pdf. Epidemiology, (January), 43–46. [PubMed] [Google Scholar]
- Sandanger TM, Huber S, Moe MK, Braathen T, Leknes H, & Lund E (2011). Plasma concentrations of parabens in postmenopausal women and self-reported use of personal care products: The NOWAC postgenome study. Journal of Exposure Science and Environmental Epidemiology, 21(6), 595–600. 10.1038/jes.2011.22 [DOI] [PubMed] [Google Scholar]
- Sandborgh-Englund G, Adolfsson-Erici M, Odham G, & Ekstrand J (2006). Pharmacokinetics of triclosan following oral ingestion in humans. Journal of Toxicology and Environmental Health - Part A: Current Issues, 69(20), 1861–1873. 10.1080/15287390600631706 [DOI] [PubMed] [Google Scholar]
- Shin HM, Moschet C, Young TM, & Bennett DH (2019). Measured concentrations of consumer product chemicals in California house dust: Implications for sources, exposure, and toxicity potential. Indoor Air. 10.1111/ina.12607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton HM, Eagle S, Sjödin A, & Webster TF (2012). Serum PBDEs in a North Carolina toddler cohort: Associations with handwipes, house dust, and socioeconomic variables. Environmental Health Perspectives, 120(7), 1049–1054. 10.1289/ehp.1104802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton HM, Kelly SM, Allen JG, McClean MD, & Webster TF (2008). Measurement of Polybrominated Diphenyl Ethers on Hand Wipes: Estimating Exposure from Hand-to-Mouth Contact. Environmental Science and Technology, 42(9), 3329–3334. 10.1021/es7029625 [DOI] [PubMed] [Google Scholar]
- Taylor JK (1987). Quality Assurance of Chemical Measurements. Analytical Chemistry, 53(14), 1588–1596. 10.1021/ac00237a001 [DOI] [Google Scholar]
- United States Environmental Protection Agency. (2020a). Triclosan CASRN: 3380–34-5. Retrieved May 6, 2020, from ACTor: Triclosan website: https://actor.epa.gov/cpcat/faces/chemicalUse.xhtml?casrn=3380-34-5
- United States Environmental Protection Agency. (2020b). Triclocarbon CASRN: 101–20-2. Retrieved May 6, 2020, from ACTor: Triclosan website: https://actor.epa.gov/cpcat/faces/chemicalUse.xhtml?casrn=101-20-2
- Völkel W, Colnot T, Csanády GA, Filser JG, & Dekant W (2002). Metabolism and kinetics of bisphenol a in humans at low doses following oral administration. Chemical Research in Toxicology, 15(10), 1281–1287. 10.1021/tx025548t [DOI] [PubMed] [Google Scholar]
- Wang S, Romanak KA, Stubbings WA, Arrandale VH, Hendryx M, Diamond ML, … Venier M (2019). Silicone wristbands integrate dermal and inhalation exposures to semi-volatile organic compounds (SVOCs). Environment International, 132(August), 105104 10.1016/j.envint.2019.105104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weschler CJ, & Nazaroff WW (2012). SVOC exposure indoors: Fresh look at dermal pathways. Indoor Air, 22(5), 356–377. 10.1111/j.1600-0668.2012.00772.x [DOI] [PubMed] [Google Scholar]
- Weschler Charles J., & Nazaroff WW (2014). Dermal uptake of organic vapors commonly found in indoor air. Environmental Science and Technology, 48(2), 1230–1237. 10.1021/es405490a [DOI] [PubMed] [Google Scholar]
- Wilson NK, Chuang JC, Morgan MK, Lordo RA, & Sheldon LS (2007). An observational study of the potential exposures of preschool children to pentachlorophenol, bisphenol-A, and nonylphenol at home and daycare. Environmental Research, 103(1), 9–20. 10.1016/j.envres.2006.04.006 [DOI] [PubMed] [Google Scholar]
- Wolff MS, Teitelbaum SL, Windham G, Pinney SM, Britton JA, Chelimo C, … Calafat AM (2007). Pilot study of urinary biomarkers of phytoestrogens, phthalates, and phenols in girls. Environmental Health Perspectives, 115(1), 116–121. 10.1289/ehp.9488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue J, Liu W, & Kannan K (2017). Bisphenols, Benzophenones, and Bisphenol A Diglycidyl Ethers in Textiles and Infant Clothing. Environmental Science and Technology, 51(9), 5279–5286. 10.1021/acs.est.7b00701 [DOI] [PubMed] [Google Scholar]
- Ye X, Kuklenyik Z, Bishop AM, Needham LL, & Calafat AM (2006). Quantification of the urinary concentrations of parabens in humans by on-line solid phase extraction-high performance liquid chromatography–isotope dilution tandem mass spectrometry. Journal of Chromatography B, 884(1), 53–59. [DOI] [PubMed] [Google Scholar]
- Ye X, Kuklenyik Z, Needham LL, & Calafat AM (2005). Automated On-Line Column-Switching HPLC-MS/MS Method with Peak Focusing for the Determination of Nine Environmental Phenols in Urine. Analytical Chemistry, 77(16), 5407–5413. [DOI] [PubMed] [Google Scholar]
- Ye X, Wong LY, Jia LT, Needham LL, & Calafat AM (2009). Stability of the conjugated species of environmental phenols and parabens in human serum. Environment International, 35(8), 1160–1163. 10.1016/j.envint.2009.07.011 [DOI] [PubMed] [Google Scholar]
- Zhu Q, Wang M, Jia J, Hu Y, Wang X, Liao C & Jiang G (2020). Occurrence, distribution and human exposure of several endocrine disrupting chemicals in indoor dust: A nationwide study. Environ. Sci. Technol DOI: 10.1021/acs.est.0c04299 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.