Abstract
Background:
Opioid Use Disorder (OUD) is a significant public health problem associated with severe morbidity and mortality. While effective pharmacotherapies are available, limitations exist with each. Induction onto extended-release naltrexone (XR-NTX) is more difficult than initiation of buprenorphine or methadone, even in inpatient settings, as it is recommended that patients remain abstinent for at least 7 days prior to initiating XR-NTX. The purpose of this trial was to determine if lorcaserin, a 5HT2c agonist, improves outpatient XR-NTX induction rates.
Methods:
An 8-week trial beginning with a brief detoxification period and induction onto XR-NTX. Sixty participants with OUD were enrolled in the trial, with 49 participants at the initiation of detoxification randomized to lorcaserin or placebo for 39 days. Additionally, ancillary medications were provided. The primary outcome was the proportion of participants inducted onto the first XR-NTX injection. Secondary outcomes were withdrawal severity (measured by COWS and SOWS) prior to the first injection and the proportion of participants receiving the second XR-NTX injection.
Results:
The proportion of participants inducted onto the first (lorcaserin: 36%; placebo: 44%; p=.67) and the second XR-NTX injection (lorcaserin: 27%; placebo: 31%; p=.77) was not significantly different between treatment arms. Prior to the first injection, withdrawal scores did not significantly differ between treatment arms over time (treatment*time interaction COWS: p=.11; SOWS: p=.39).
Conclusions:
Lorcaserin failed to improve outpatient XR-NTX induction rates. Although this study is small, the findings do not support the use of lorcaserin in promoting induction onto XR-NTX or in mitigating withdrawal symptoms.
Keywords: opioid use disorder, extended-release naltrexone, lorcaserin, treatment, clinical trial, induction
1. Introduction
In the past decade there has been a steep rise in opioid overdose deaths, with more than 47,000 opioid-related deaths in 2018 (Centers for Disease Control and Prevention (CDC), 2020; Wilson et al., 2020). It has been estimated that approximately 2.0 million Americans suffer from an opioid use disorder (OUD) (Substance Abuse and Mental Health Services Administration (SAMHSA), 2019a). Repeatedly, data have demonstrated that maintenance treatment with buprenorphine and methadone reduces overdose risk by 70–80% (Kakko et al., 2003; Lee et al., 2018; Sordo et al., 2017). While overdose reduction with extended-release naltrexone (XR-NTX) is less clear (Morgan et al., 2019), this may be due to poorer retention on XR-NTX than agonist treatments (Morgan et al., 2018).
While the number of individuals prescribed a medication for OUD (MOUD) has increased in the past several years (SAMHSA, 2019b) there are many that still go untreated. Moreover, many who start MOUD, drop-out of treatment within 3–6 months (Hser et al., 2014; Morgan et al., 2018). The reasons for this are multifactorial and include limited reimbursement or time-limited reimbursement, lack of available prescribers, lack of additional behavioral health services, patients’ perception that long-term maintenance is unnecessary, and stigma (Jones et al., 2015; Marino et al., 2019).
Each of the 3 FDA-approved medications (buprenorphine, methadone and XR-NTX) have their advantages and possible disadvantages (Koehl et al., 2019). Unlike buprenorphine and methadone, XR-NTX has no risk of diversion, potentially less stigma associated with its use, and no withdrawal if doses are missed. However, whereas buprenorphine can be initiated after 12–24 hours of opioid abstinence, prescribing guidelines for XR-NTX suggest that it needs to be administered only after an OUD patient has been successfully withdrawn from opioids and an adequate washout period has occurred (generally 7–14 days). Having individuals achieve abstinence for a prolonged period in an outpatient setting can be quite difficult. Frequently, OUD patients will relapse or be lost to treatment. Thus, XR-NTX is more likely to be initiated on an inpatient detoxification unit or inpatient rehabilitation program, restricting the clinical utility of this effective medication. Moreover, the cost of XR-NTX is much greater than buprenorphine. Thus, it is not surprising that in 2018, there were over 680,000 individuals with OUD treated with buprenorphine and only 75,000 treated with XR-NTX (SAMHSA, 2019b).
Over the past several years our research group has developed an outpatient induction approach to efficiently induct OUD patients onto XR-NTX within eight days (Bisaga et al., 2018; Sullivan et al., 2017). Despite this, less than 50% of heroin users are successfully inducted onto XR-NTX, although individuals with OUD who use prescription painkillers achieve higher rates of induction, ranging from 60–70%. A more recent study evaluated a slighter shorter 5-day induction approach with the hope to reduce relapse and drop-out (Sibai et al., 2020). This approach proved to be feasible, albeit rates of XR-NTX induction were only slightly better than earlier trials. Although speculative, subacute withdrawal symptoms, craving, and insomnia can persist during the first several weeks after the first XR-NTX dose and may contribute to early dropout (after 1–2 injections).
While ancillary medications (i.e. clonidine, zolpidem, chlorpromazine) are often provided to mitigate withdrawal during the induction period onto XR-NTX (Bisaga et al., 2018; Sullivan et al., 2017), additional medications, such as memantine or dronabinol (Bisaga et al., 2014; 2015) have also been tested to determine if they improve XR-NTX rates. Unfortunately, neither medications were found superior to placebo in improving XR-NTX induction rates. Thus, we were interested in finding an additional medication, with a different mechanism of action, that might help facilitate the induction onto XR-NTX and enhance retention once XR-NTX is administered.
One possible pharmacologic approach is the use of an agent that modulates serotonin (5-hydrotryptamine; 5-HT) neurotransmission by acting as an agonist on the 5-HT2C receptor. Numerous lines of evidence, reviewed by Cunningham and Anastasio (2014), support how 5-HT modulates drug reward. Specifically, lorcaserin, a high-affinity 5-HT2C receptor agonist that was approved by the FDA in 2012 for treating obesity, might prove useful. Supporting this concept, there are promising preclinical data demonstrating that lorcaserin suppresses behavioral sensitization, opioid withdrawal symptoms, and self- administration in mice and rats (Neelakantan et al., 2017; Wu et al., 2015) as well as heroin self-administration in nonhuman primates (Kohut and Bergman, 2018). While at high doses lorcaserin can produce hallucinatory effects, mediated via its modest binding to the 5HT2a receptor, it has low abuse potential among recreational polydrug users (Brandt et al., 2020; Schram et al., 2011), making it an attractive medication for those suffering with an OUD.
While there are encouraging findings from a recent large positive treatment trial (n=603) evaluating lorcaserin for smoking cessation (Shanahan et al., 2017), to our knowledge there are no placebo-controlled trials in other treatment-seeking substance use disorder populations.
Thus, we initiated a randomized, double-blind, pilot trial comparing lorcaserin to placebo. We were interested in determining if lorcaserin had the potential to improve XR-NTX initiation during a 5-day induction by mitigating withdrawal symptoms and opioid use. Our primary hypothesis is that OUD individuals randomized to lorcaserin would be more likely than placebo to be inducted onto XR-NTX. Secondary hypotheses were that those that received lorcaserin would be more likely to have less withdrawal symptoms and receive a second injection compared to placebo.
2. Materials and methods
2.1. Participants
The study was approved by the Institutional Review Board (IRB) of the New York State Psychiatric Institute. Participants were recruited through IRB approved advertisements placed in local newspapers, public transportation, radio stations and by clinical referrals, through liaison to other local clinical services and word of mouth in the New York City area. All participants gave informed written consent. We enrolled 60 participants who met Diagnostic and Statistical Manual of Mental Disorders Fifth Edition (American Psychiatric Association (APA), 2013) for OUD, supported by positive urine toxicology for opioids. Subsequent to the initiation of the study, after 7 participants were enrolled, urine samples were also tested for fentanyl. Urines were tested for fentanyl because it became commonplace in NYC during the trial and we thought that it might be possibly impacting outcomes.
The MINI-International Neuropsychiatric Interview was performed as part of a comprehensive psychiatric and medical evaluation (Sheehan et al., 1998). Participants were between the ages of 18–60, seeking treatment for OUD, capable of giving informed consent and complying with study procedures, and not underweight; defined as Body Mass Index (BMI) ≥ 18.5. Participants were excluded if they: 1) had a lifetime history of DSM-5 diagnosis of schizophrenia, schizoaffective disorder, or bipolar disorder; 2) had current DSM-5 criteria for any other psychiatric disorder that in the investigator’s judgment was unstable, would be disrupted by the study medication, or was likely to require pharmacotherapy or psychotherapy during the study; 3) were on concurrent treatment with other psychotropic medication; 4) met DSM-5 criteria for any substance use disorders - severe, other than opioid and nicotine use disorder; physiological dependence on alcohol or sedative-hypnotics was exclusionary; 5) had a recent history of binge-use of alcohol or sedative-hypnotics (using large amounts in a short time to severe intoxication or blackouts); 6) were pregnant, lactating or failing to use adequate contraceptive method in female patients who were currently engaging in sexual activity with men; 7) had unstable medical conditions, such as AIDS, cancer, uncontrolled hypertension (blood pressure > 140/90), uncontrolled diabetes, pulmonary hypertension or heart disease; 8) were legally mandated to participate in a substance use disorder treatment program; 9) had a current or recent history of significant violent or suicidal behavior, risk for suicide or homicide; 10) had a current DSM-5 diagnosis for an eating disorder with low body weight (BMI < 20); 11) had elevated liver function tests (AST and ALT > 3 times the upper limit of normal) or impaired renal function (GFR < 60 ml/min); 12) had a known history of allergy, intolerance, or hypersensitivity to lorcaserin, naltrexone or any other study medications; or 13) were concurrently using migraine medications containing ergotamine (Cafergot, Ergomar) or dihydroergotamine (Migranal), 5HT2B receptor agonists like cabergoline, or medications metabolized by CYP2D6 (thioridazine, tamoxifen, metoprolol, aripiprazole, codeine).
2.2. Treatment
2.2.1. Overall design
A 2-arm, randomized, double-blind, placebo-controlled 8-week long trial was conducted to obtain initial data regarding the efficacy of lorcaserin for increasing successful initiation onto XR-NTX and assess whether this approach is both feasible and well-tolerated. Sixty treatment-seeking adults with moderate-to-severe OUD were offered outpatient detoxification followed by treatment with XR-NTX. Participants were offered two injections of XR-NTX and twice weekly psychotherapy. We selected adherence with naltrexone injections as the primary outcome because it is the most clinically meaningful outcome of this treatment. Forty-nine randomized participants received either lorcaserin or placebo in a 2:1 allocation for up to 6 weeks followed by 2 additional weeks off of medication.
2.2.2. Detoxification and induction onto injectable naltrexone
The detoxification-naltrexone induction was performed at the STARS outpatient research clinic, which is outfitted with a detoxification suite. Following the consent procedure, participants were sent home with ancillary medications, instructed to abstain from opioids for 12–24 hours prior to Study Day 1, and directed to use ancillary medication if needed. The medications provided were: clonidine to alleviate withdrawal (Maximum Daily Dose (MDD) = 0.3 mg), prochlorperazine for nausea (MDD = 30 mg), clonazepam to reduce anxiety and dysphoria (MDD = 1.5 mg), and zolpidem for insomnia (MDD = 10 mg). Participants were seen daily for the first 5 days of the induction period and provided take-home doses of ancillary medications in small doses and on a tapering schedule for two weeks (including the detoxification week) to alleviate any protracted opiate withdrawal. Additional doses were offered as clinically determined for participants experiencing continued withdrawal symptoms.
On Days 1–5 Clinical Opiate Withdrawal Scale (COWS)/Subjective Opiate Withdrawal Scale (SOWS) assessments were completed prior to administration of daily medications, 60 minutes after administration of oral naltrexone, and at the end of the study visit. The COWS is an 11-item rating with a score ranging from 0 to 48 (Wesson and Ling, 2003). The SOWS is a 16-item rating scale with a score ranging from 0 to 64 (Handelsman et al., 1987). Buprenorphine, was initiated on Day 1 after an overnight abstinence based on a COWS score of 6 or greater, given first as a test 2 mg dose followed by additional 4 mg dose in the clinic and 2 mg dose to take at night if discernible withdrawal symptoms were present. On Day 3 oral naltrexone was started at 1 mg dose, with an additional 3 mg dose administered 3 hours later; on Day 4, 12.5 mg of oral naltrexone was administered; and on Day 5 the target 25 mg of oral naltrexone was given at which point it was safe to administer the injection of naltrexone (see Table 1 for schedule of drug administration during induction week). This induction approach of initially giving buprenorphine followed by ascending doses of naltrexone was based on several studies that suggest that this approach assists induction onto XR-NTX (Bisaga et al., 2014, 2015; Mannelli et al., 2014; Sullivan et al., 2017); therefore this approach was utilized for this trial.
Table 1.
Schedule of drug administration during the Induction week.
| Medication | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 |
|---|---|---|---|---|---|
| Randomization | |||||
| Buprenorphine | 2+2+2 mg +(2hs) | ||||
| Lorcaserin or Placebo | 1 mg (b.i.d) or matched placebo | ||||
| Naltrexone | 1+3 mg | 12.5 mg | 25 mg+ XR-Naltrexone 380mg i.m. | ||
| Ancillary Medications | clonidine [Maximum Daily Dose (MDD) = 0.3mg], prochlorperazine (MDD= 30mg), clonazepam (MDD= 1.5mg), and zolpidem (MDD=10mg) were prescribed. | ||||
All participants received naltrexone injections (Vivitrol 380 mg i.m.) under open label condition. If opioid use occurred in combination with missed doses of oral naltrexone, a naloxone challenge was offered to confirm that oral naltrexone would be tolerated; if necessary, an additional day of adjuvant medication was provided.
Study medications (lorcaserin or placebo) were administered under double-blind conditions. Starting with Day 1 of the induction period (which lasted 5–7 days) and lasting for a total of up to 6 weeks all participants received two oral capsules daily (lorcaserin 10 mg or matched placebo administered b.i.d.). Participants received a 1-week supply of lorcaserin or placebo at a time. Adherence was assessed using a Timeline Followback (TLFB) pill count interview and medication bottle return. The results of the TLFB pill count and medication bottle return were discussed with participants by the research psychiatrist to enhance adherence. After initiating the study, the study procedures were amended where all capsules also contained 12.5 mg of riboflavin in order for adherence to be additionally assessed using a quantitative fluorometric detection of urinary riboflavin concentration (Herron et al., 2013). As previously mentioned, urine testing for fentanyl analogues was started after the enrollment of 7 participants (6 randomizations) after conjecturing that baseline urines for fentanyl might moderate treatment response.
2.2.3. Study procedures after induction onto extended-release naltrexone
Participants were seen in the clinic twice per week during weeks 2–8. At each visit, the patient met with medical staff to complete research ratings, vital signs including BMI, and urine samples, along with inquiries about side effects (using the Systematic Assessment for Treatment Emergent Effects (SAFTEE); Johnson et al., 2005). Side effects were reviewed at least weekly during the visit with a psychiatrist who adjusted medication dose if necessary. Participant’s opioid withdrawal was assessed weekly using both the COWS and SOWS. Similar to medication adherence, the Timeline Followback (Litten and Allen, 1992) was completed each week to document opioid and other drug use using a calendar procedure. Serum pregnancy tests were collected on female participants during screening and then urine pregnancy tests were collected monthly during the study.
All participants received manualized, twice weekly therapy that was derived from counseling aspects of Behavioral Naltrexone Therapy (BNT), emphasizing relapse prevention and adherence to medication (Rothenberg et al., 2002).
2.3. Data analysis
All participants were randomized 2:1 lorcaserin to placebo and stratified by severity of use (Low: ≤ 200 mg or ≤ 5 bags vs. High: > 200 mg or > 5 bags). The randomization scheme was skewed toward the experimental group in order to maximize the amount and precision of information on the feasibility of lorcaserin treatment. All participants were block randomized with randomly selected blocks of size three, six, and nine (Efrid, 2011). The randomization sequence was designed by an independent statistician and utilized by the research pharmacist. Participants and all other study staff were blind to treatment assignment.
The total number of 60 subjects was chosen to minimize cost, to reach the study end within two years, and to estimate the potential effect size and 95% confidence interval with reasonable precision. With 40 subjects in the lorcaserin group and 20 subjects in the placebo group, and assuming that the observed proportion meeting the primary outcome in the placebo group is 45% (a conservative estimate consistent with our prior trials), provides 80% power, at two-tailed level of significance 5%, to detect a significant treatment effect of 45% vs 81% on placebo vs lorcaserin, with a margin of error of 26% for 95% confidence interval estimates.
The primary outcome was successful induction onto XR-NTX. Secondary outcomes were: (a) severity of acute withdrawal during detoxification and induction prior to the first injection, and (b) and receiving the second injection of XR-NTX.
The outcomes of successful induction onto XR-NTX and receiving 2nd injection were analyzed in SAS® using logistic regression with predictors: treatment (lorcaserin vs. placebo), age, baseline severity of use (high vs. low), and opioid type. A 4-level categorical predictor was created to represent opioid type (heroin vs. opioid pills) and whether subjects were fentanyl positive at baseline. The categories were: (1) fentanyl-positive heroin, (2) fentanyl-negative heroin, (3) fentanyl-unknown heroin (fentanyl testing was initiated later in the study, so the first 6 randomized participants were not tested), and (4) fentanyl-negative opioid pills (reference group). There were no participants who were opioid pill users and were fentanyl-positive when tested.
Sensitivity analyses were also performed to account for the 6 subjects missing fentanyl status. The analyses were performed under four alternative scenarios where the 6 subjects with missing fentanyl status were assumed to be: 1) fentanyl-positive heroin users, 2) fentanyl-negative heroin users, 3) fentanyl-positive heroin users if randomized to the treatment group and fentanyl-negative heroin users if randomized to the placebo group, and 4) fentanyl-positive heroin users if randomized to the placebo group and fentanyl-negative heroin users if randomized to the treatment group.
Withdrawal during the induction period, as measured by COWS and SOWS, was analyzed using longitudinal mixed effect models with a random intercept for participant and an autoregressive correlation structure (AR1) to account for the within subject correlation. Predictors included: treatment, time, and time-by-treatment two-way interaction, adjusted by the baseline withdrawal score, age, baseline severity of use, and opioid type.
Retention to study week 8 and time to drop out was compared using Kaplan-Meier curves and Cox proportional hazard models.
For each outcome, the interaction of treatment-by-opioid type was also included in the model to examine moderator effects. If the interaction was not significant, then the interaction was removed from the final model. All analyses described in the preceding sections were conducted on the intent-to-treat sample of all randomized participants with 2-tailed tests and a significance level of 5%.
A Data and Safety Monitoring Board met yearly during the trial to review study enrollment, overall medication tolerability, adverse and serious adverse events. The DSMB made no recommendations to alter the protocol or cease study enrollment.
3. Results
3.1. Participant progress in study and demographics
Three hundred participants were screened, 60 participants entered the trial (10 were lost to follow-up after consent and 1 was no longer interested in treatment), and a total of 49 participants (see CONSORT diagram, Figure 1) were randomized to either lorcaserin (n = 33; 67.4%) or placebo (n = 16; 32.7%). Recruitment began in June 2017 and all follow-up was completed in June 2019. The most common reason for screen failure for this trial was not meeting eligibility criteria. Characteristics of the randomized participants are shown in Table 2. Based on 43 participants having fentanyl testing results available, 55% of the lorcaserin group and 63% of the placebo group were fentanyl positive. The majority of the sample were fentanyl-positive heroin users (57%; lorcaserin: 64%; placebo: 36%), followed by: fentanyl-negative opioid pill users (20%; lorcaserin: 60%; placebo: 40%), fentanyl-unknown heroin users (12%; lorcaserin: 67%; placebo: 33%), and fentanyl-negative heroin users (10%; lorcaserin: 100%; placebo: 0%).
Figure 1.
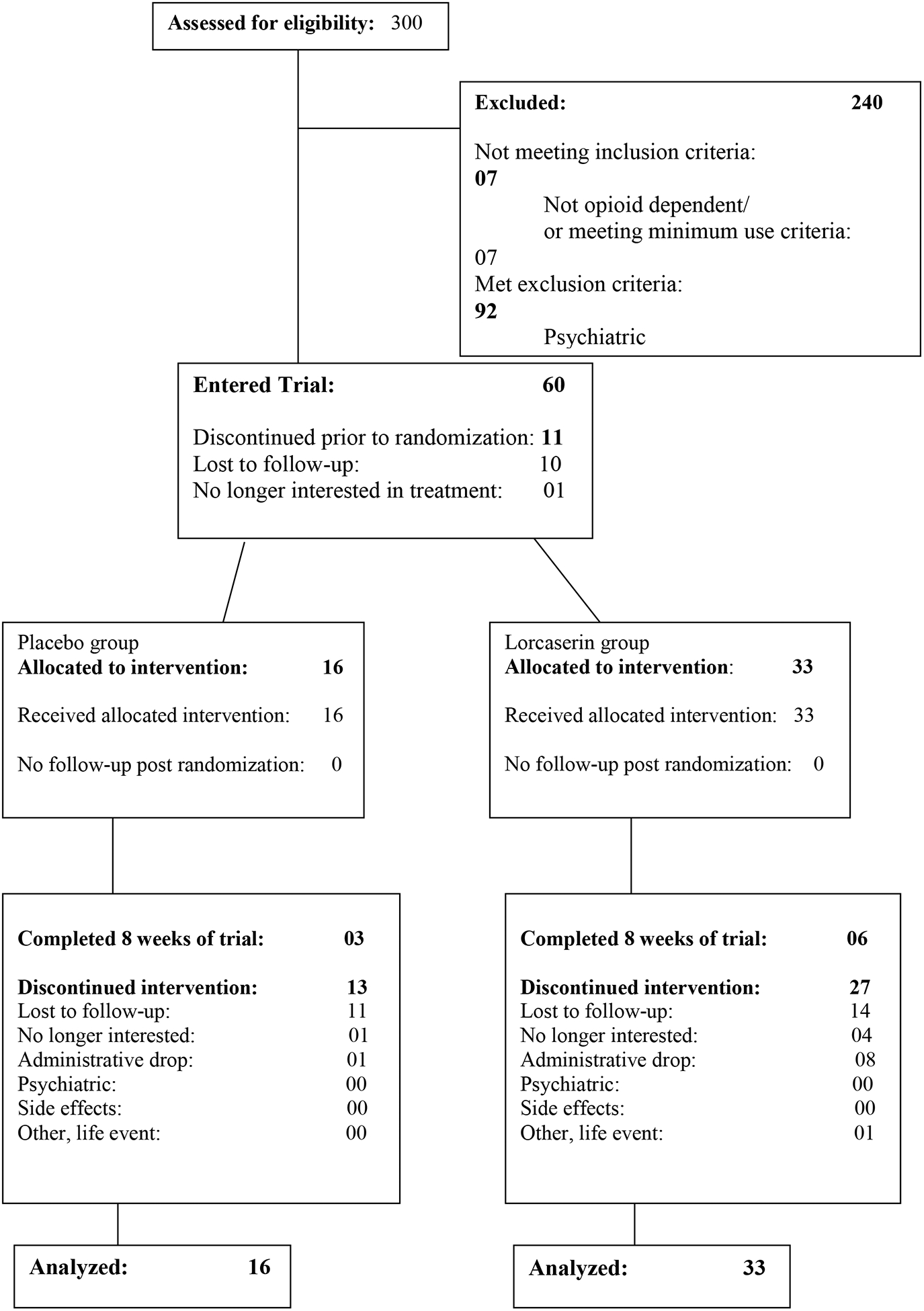
CONSORT Diagram.
Table 2.
Demographic and clinical characteristics of randomized sample (N=49).
| Total (N=49) | Placebo (n=16) | Lorcaserin (n=33) | ||||
|---|---|---|---|---|---|---|
| Variables | n | Mean (SD) or % | n | Mean (SD) or % | n | Mean (SD) or % |
| Demographics | ||||||
| Age | 49 | 37.0 (10.9) | 16 | 39.5 (12.6) | 33 | 35.8 (9.9) |
| Gender | ||||||
| Male | 38 | 77.6% | 13 | 81.3% | 25 | 75.8% |
| Female | 11 | 22.4% | 3 | 18.8% | 8 | 24.2% |
| Race | ||||||
| White | 32 | 65.3% | 10 | 62.5% | 22 | 66.7% |
| Black | 9 | 18.4% | 4 | 25.0% | 5 | 15.2% |
| Asian | 1 | 2.0% | 1 | 6.3% | 0 | 0.0% |
| Other or More than 1 race | 3 | 6.1% | 0 | 0.0% | 3 | 9.1% |
| American Indian | 1 | 2.0% | 1 | 6.3% | 0 | 0.0% |
| Unknown | 3 | 6.1% | 0 | 0.0% | 3 | 9.1% |
| Ethnicity | ||||||
| Hispanic/Latino | 9 | 18.4% | 2 | 12.5% | 7 | 21.2% |
| Not Hispanic/Latino | 40 | 81.6% | 14 | 87.5% | 26 | 78.8% |
| Years of Education | 46 | 13.7 (2.2) | 14 | 14.3 (2) | 32 | 13.4 (2.3) |
| Marital Statusa | ||||||
| Single | 37 | 78.7% | 15 | 100.0% | 22 | 68.8% |
| Married | 6 | 12.8% | 0 | 0.0% | 6 | 18.8% |
| Separated | 1 | 2.1% | 0 | 0.0% | 1 | 3.1% |
| Divorced | 2 | 4.3% | 0 | 0.0% | 2 | 6.3% |
| Widowed | 1 | 2.1% | 0 | 0.0% | 1 | 3.1% |
| Employmenta | ||||||
| Full-Time | 14 | 29.8% | 5 | 33.3% | 9 | 28.1% |
| Part-Time | 4 | 8.5% | 2 | 13.3% | 2 | 6.3% |
| Unemployed | 26 | 55.3% | 7 | 46.7% | 19 | 59.4% |
| Disabled | 1 | 2.1% | 0 | 0.0% | 1 | 3.1% |
| Other | 2 | 4.3% | 1 | 6.7% | 1 | 3.1% |
| Opiate use | ||||||
| Severity of Use | ||||||
| Low (≤200mg or ≤5 bags) | 13 | 26.5% | 5 | 31.3% | 8 | 24.2% |
| High (>200mg or >5 bags) | 36 | 73.5% | 11 | 68.8% | 25 | 75.8% |
| Type of Opiate at Screening | ||||||
| Heroin | 39 | 79.6% | 12 | 75.0% | 27 | 81.8% |
| Opioid pills | 10 | 20.4% | 4 | 25.0% | 6 | 18.2% |
| Type of Opiate at Screening with Fentanyl Status | ||||||
| Fentanyl-negative opioid pills | 10 | 20.4% | 4 | 25.0% | 6 | 18.2% |
| Fentanyl-negative heroin | 5 | 10.2% | 0 | 0.0% | 5 | 15.2% |
| Fentanyl-positive heroin | 28 | 57.1% | 10 | 62.5% | 18 | 54.5% |
| Fentanyl-unknown heroin | 6 | 12.2% | 2 | 12.5% | 4 | 12.1% |
| Route of Use | ||||||
| IN | 28 | 57.1% | 9 | 56.’% | 19 | 57.6% |
| IV | 10 | 20.4% | 2 | 12.5% | 8 | 24.2% |
| PO | 8 | 16.3% | 4 | 25.0% | 4 | 12.1% |
| smoked | 3 | 6.1% | 1 | 6.3% | 2 | 6.1% |
| Dropped before 8 weeks | ||||||
| No | 9 | 18.4% | 3 | 18.8% | 6 | 18.2% |
| Yes | 40 | 81.6% | 13 | 81.3% | 27 | 81.8% |
2 subjects were missing employment and marital status info, Total N=47, Placebo n=15, Lorcaserin n=32.
3.2. Primary outcome
The proportion of participants who were successfully inducted onto XR-NTX was 36.4% (12/33) for those receiving lorcaserin and 43.8% (7/16) for those receiving placebo. The estimated odds ratio (OR) was 0.73 (95% confidence interval (CI):0.17, 3.14; p = .67) suggesting that the likelihood of successful induction onto XR-NTX was not significantly different between those receiving treatment and those receiving placebo, while adjusting for age (OR=0.95, 95% CI:0.89, 1.02; p = .15), severity of use (High vs. Low OR=0.44, 95% CI:0.06, 03.44; p = .44), and opioid type (compared to the reference fentanyl-negative opioid pills group; fentanyl-positive heroin OR=0.12, 95% CI:0.01, 1.03; fentanyl-negative heroin OR=0.13, 95% CI:0.005, 3.10; and fentanyl-unknown heroin OR=0.48, 95% CI:0.03, 7.63; p = .14). When a two-way interaction between opioid type and treatment was modeled, it was not significant (p = .85). Figure 2 shows the proportions of those successfully inducted onto first injection by study arm and by opioid type and study arm. For those tested, 28/33 (85%) of the heroin using group had a fentanyl-positive urine at baseline. Because only 5 heroin using individuals were fentanyl-negative, the sample was too small to adequately compare fentanyl positive versus fentanyl negative heroin users for the primary outcome.
Figure 2.
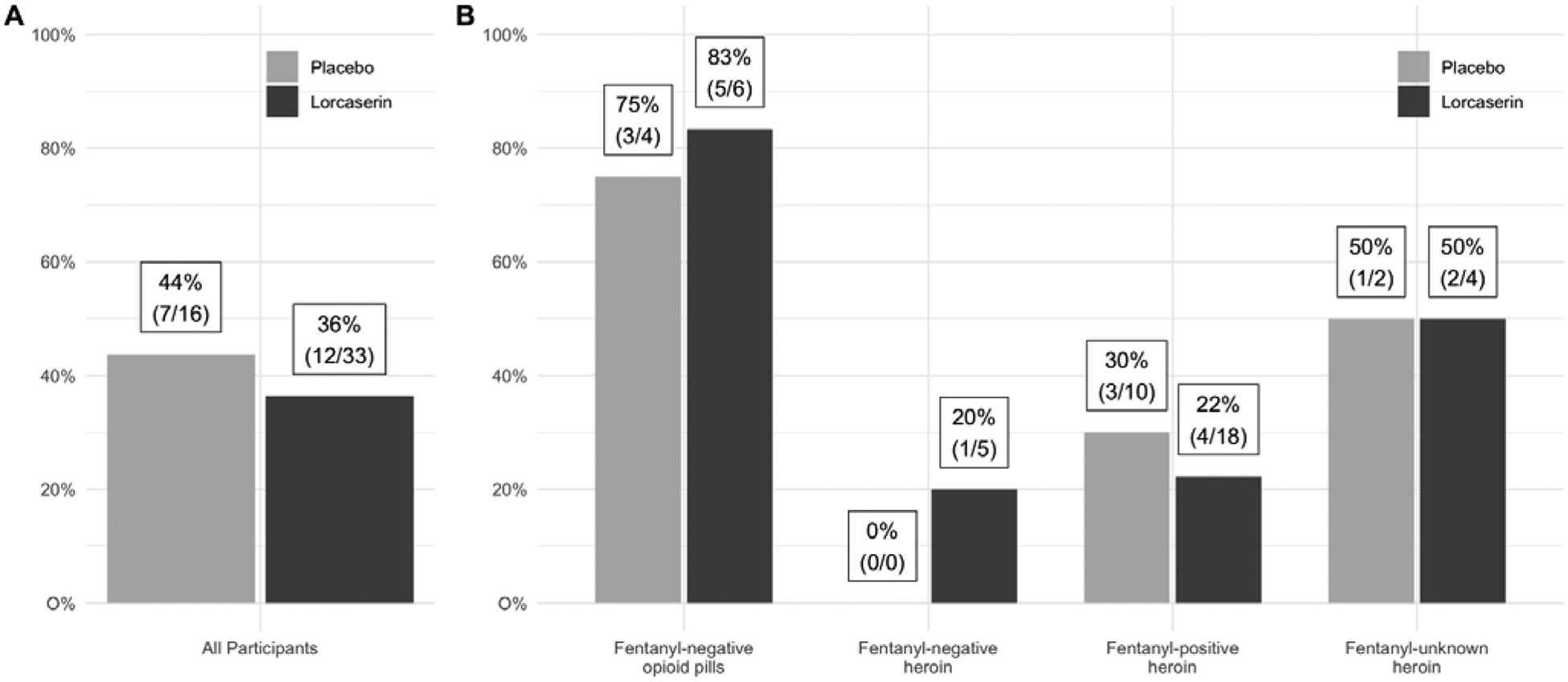
(A) Proportion of all participants who received 1st injection by study arm (B) Proportion of heroin or opioid pill users (with fentanyl status) who received the 1st injection by study arm.
Overall, there was a notable difference observed among pill users and heroin users in receiving the first injection. Prescription pill users (80%, 8/10) were significantly associated with receiving first dose of XR-NTX (Fisher’s exact test p = .008) compared to heroin users (28%, 11/39). After adjusting for age (OR=0.96, 95% CI:0.90, 1.02; p = .19), severity of use (High vs. Low OR=0.59, 95% CI:0.09, 4.13; p = .60), and study arm (Lorcaserin vs. Placebo OR=0.72, 95% CI:0.18, 2.87; p = .64), the odds of receiving first injection was not significantly different for pill users (OR=7.53, 95% CI:0.89, 64.0; p = .06) compared to heroin users. Of note, two patients initiated abstinence several days earlier than instructed and induction was accelerated. As both were fentanyl-positive heroin users and evenly distributed between the lorcaserin and placebo groups, their inclusion in the study was unlikely to alter the interpretation of the results.
Sensitivity analyses of all four scenarios of missingness, as described in the methods section, did not show any differences in results for the first injection.
3.3. Secondary outcomes
3.3.1. Withdrawal prior to first injection
Severity of withdrawal, measured by COWS, did not significantly change over time between lorcaserin and placebo groups (time*treatment interaction, p=.11; see figure 3A) while adjusting for baseline COWS (p = .71), age (p = .36), severity of use (p = .94), and opioid type (p = .03). Similar results were found when using SOWS withdrawal scores. There was no significant treatment by time two-way interaction (p = .39; see Figure 3B), while adjusting for baseline SOWS (p = .03), age (p = .56), severity of use (p = .60), and opioid type (p = .05). When a three-way interaction between treatment, opioid type, and time was modeled, the interaction was not significant, suggesting no moderating effect of opioid type on treatment on either COWS (p = .38) or SOWS (p = .27) over time. Withdrawal scores at baseline were not significantly (COWS p = .33 and SOWS p = .06) associated with receiving the first injection, after controlling for the same covariates used in the primary aim. However, because of the persistent opioid use (see below) it may have compromised our ability to detect differences in withdrawal using the COWS or the SOWS and limited the utility of these instruments among ongoing opioid users.
Figure 3.
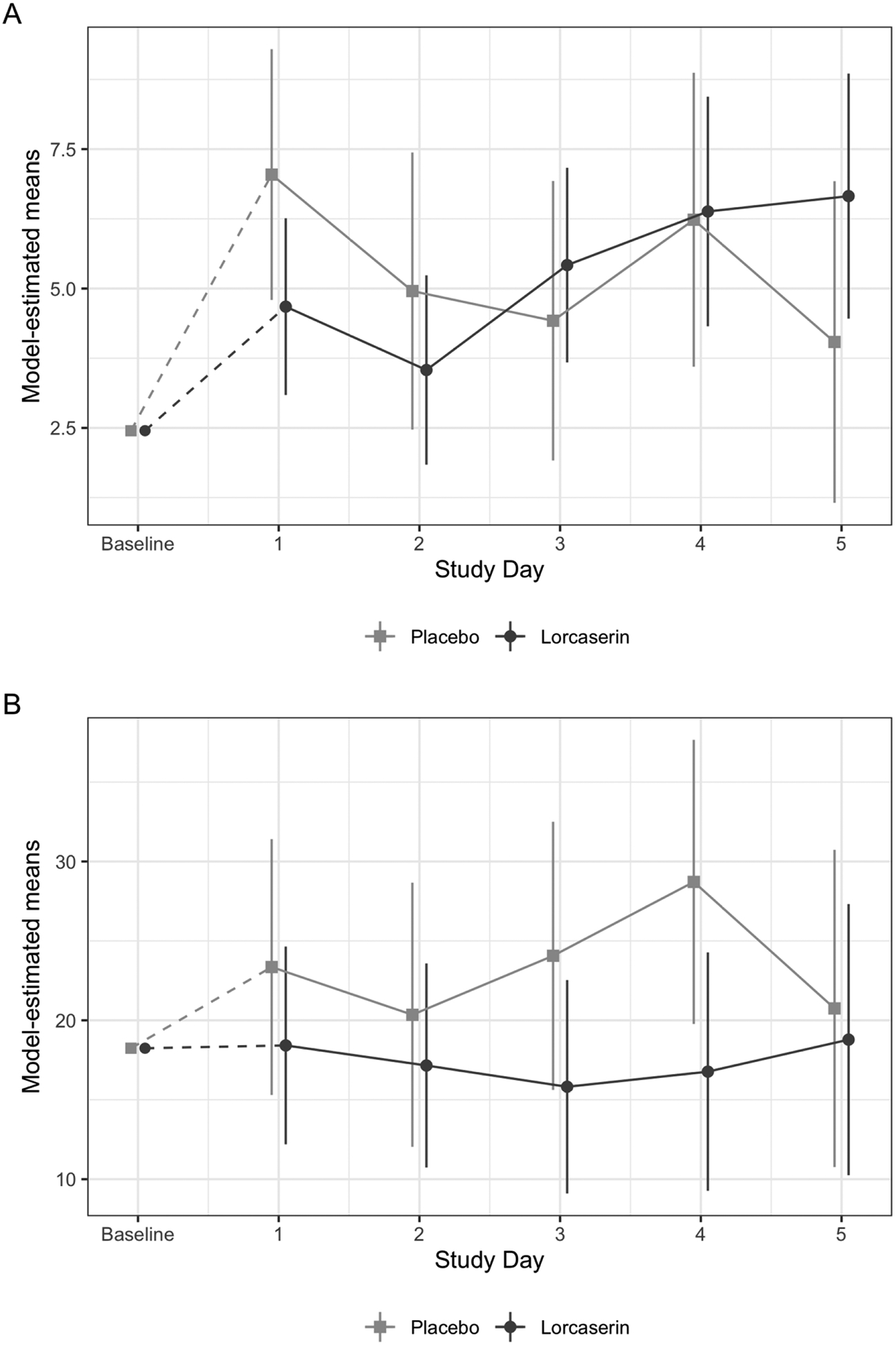
Model-estimated (adjusted by baseline withdrawal, age, opioid type, and severity of use) means and 95% confidence intervals of withdrawal for (A) COWS and (B) SOWS, before first injection.
3.3.2. Proportion of participants receiving second injection
Of the 19 participants who were successfully inducted onto XR-NTX, a total of 14 participants (73.7%) received the second injection. The proportion of participants who received the 2nd injection was 27.3% (9/33) for those receiving lorcaserin and 31.3% (5/16) for those receiving placebo. The estimated odds ratio was 0.80 (95% CI: 0.17, 3.67; p = .77) suggesting that the likelihood of receiving the 2nd injection was not significantly different between those receiving lorcaserin and those receiving placebo, while adjusting for covariates. Among the covariates, baseline severity of use was significant (OR=0.12, 95% CI:0.02, 0.89; p = .04), which suggests that high users were less likely to receive the 2nd injection compared to low users. The other covariates were not significant: age (OR=0.98, 95% CI: 0.91, 1.04; p = .47) and opioid type (compared to the reference fentanyl-negative opioid pills group; fentanyl-positive heroin OR=0.74, 95% CI:0.10, 5.42; fentanyl-negative heroin OR=1.68, 95% CI:0.07, 41.3; fentanyl-unknown heroin OR=6.23, 95% CI:0.40, 97.8; p = .24).
When a two-way interaction between treatment and opioid type (4 subgroups listed above) was modeled, the interaction was not significant, suggesting no moderating effect of opioid type on treatment (p = .97).
Sensitivity analyses of all four scenarios of missingness, as described in the methods section, did not show any differences in results for the second injection.
3.3.3. Retention
The proportion of subjects who completed all 8 weeks of the study in the lorcaserin group was 18% (6/33) and 19% (3/16) in the placebo group. Time to dropout throughout the 8 week trial was not significantly different between the lorcaserin and placebo groups (Hazard Ratio (placebo compared to lorcaserin)=1.10; 95% CI=0.55–2.22; p = .78), while controlling for age (p = .60), severity of use (p = .08), and opioid type (p = .55). When a two-way interaction between treatment and opioid type was modeled, the interaction was not significant, suggesting opioid type does not moderate the effect of treatment (p = .84) on time to dropout.
3.4. Opioid use during the induction period
Of the 49 participants who were randomized to the study, self-reported opioid use reports were collected on or after day one of induction for 39 participants (10 dropped out such that we did not have data on opioid use). Almost half (46%, 18/39) of these participants reported opioid use at some time during the induction. The proportion of participants who progressed through the induction period and reported using illicit opioids during the induction/detoxification week, were 28% (11/39) on Day 1, 22% (8/36) on Day 2, 13% (4/30) on Day 3, 24% (7/29) on Day 4, and 5% (1/21) on Day 5. Of the 19 participants who received XR-NTX, 4/19 (21%) reported using opioids at some point during the induction time but they were able to tolerate increasing doses of naltrexone and received XR-NTX (as compared to 70% or 14/20 who did not receive the injection and reported opioid use during the detoxification period). Fisher’s exact test showed a significant association, suggesting that participants with self-reported opioid use during the induction were less likely to receive the injection (p = .004). Of the participants who received XR-NTX, 63% (12/19) had a urine toxicology test positive for opioids (morphine or buprenorphine) on the day of injection. Of these 12 participants, 25% (3/12) reported using illicit opioids at least once since Day 1. Notably, self-reported opioid use during induction did not differ by treatment group (lorcaserin 13/28 (46%) vs. placebo 5/11 (45%), Fisher’s exact p = 1.00), or opioid type (prescription pills 3/10 (30%) vs. Heroin 15/29 (52%), Fisher’s exact p = .29).
3.5. Ancillary medications
Observed means and 95% confidence intervals of actual administered daily doses (mg) of ancillary medications are shown in Appendix A Supplement Figure 1. There were no substantial clinically meaningful dosing differences between treatment arms. There was no indication that clonazepam or zolpidem, given daily in the doses determined by the protocol to take at home during the induction, or after the XR-NTX injection, caused excessive sedation or withdrawal with the need for a taper, based on self-report of participants.
3.6. Safety, tolerability and medication adherence
Adverse events reported as moderate or severe are described in Table 3. Due to the small cell size, Fisher’s exact tests were used to identify treatment differences in the proportion of adverse events. There were no adverse events that were reported significantly more in either group. The most common adverse event was insomnia (12%, 6/49). Two Serious Adverse Events (SAEs) occurred during the trial, both involving participants in the lorcaserin group. One participant voluntarily entered an inpatient rehabilitation program after receiving the first XR-NTX injection. He returned and continued in the trial until he decided to pursue alternative treatments just prior to the second injection time point. A second participant voluntarily entered an inpatient rehabilitation program during the detoxification week prior to completing the induction onto XR-NTX and was discontinued from the trial.
Table 3.
Moderate to severe adverse events.
| Adverse Event | Total (N=49) n (%) | Placebo (n=16) n (%) | Lorcaserin (n=33) n (%) | Fisher’s exact test p-value |
|---|---|---|---|---|
| Insomnia | 6 (12.2) | 3 (18.8) | 3 (9.1) | 0.377 |
| GI Upset | 5 (10.2) | 1 (6.3) | 4 (12.1) | 1.000 |
| Anorexia | 4 (8.2) | 2 (12.5) | 2 (6.1) | 0.588 |
| Vomiting | 4 (8.2) | 1 (6.3) | 3 (9.1) | 1.000 |
| Anxiety | 3 (6.1) | 0 (0.0) | 3 (9.1) | 0.541 |
| Chills | 2 (4.1) | 0 (0.0) | 2 (6.1) | 1.000 |
| Diarrhea | 2 (4.1) | 1 (6.3) | 1 (3.0) | 1.000 |
| Hot Flashes | 2 (4.1) | 0 (0.0) | 2 (6.1) | 1.000 |
| Precipitated Withdrawal | 2 (4.1) | 1 (6.3) | 1 (3.0) | 1.000 |
| Backache | 1 (2.0) | 0 (0.0) | 1 (3.0) | 1.000 |
| Blackout | 1 (2.0) | 1 (6.3) | 0 (0.0) | 0.3’7 |
| Constipation | 1 (2.0) | 1 (6.3) | 0 (0.0) | 0.327 |
| Dehydration | 1 (2.0) | 0 (0.0) | 1 (3.0) | 1.000 |
| Fatigue | 1 (2.0) | 1 (6.3) | 0 (0.0) | 0.327 |
| Fainting | 1 (2.0) | 0 (0.0) | 1 (3.0) | 1.000 |
| Gout | 1 (2.0) | 0 (0.0) | 1 (3.0) | 1.000 |
| Headache | 1 (2.0) | 0 (0.0) | 1 (3.0) | 1.000 |
| Head Sensation | 1 (2.0) | 0 (0.0) | 1 (3.0) | 1.000 |
| Irritable | 1 (2.0) | 0 (0.0) | 1 (3.0) | 1.000 |
| Leg Cramps | 1 (2.0) | 0 (0.0) | 1 (3.0) | 1.000 |
| Loss of Libido | 1 (2.0) | 0 (0.0) | 1 (3.0) | 1.000 |
| Muscle Aches | 1 (2.0) | 1 (6.3) | 0 (0.0) | 0.327 |
| Nausea | 1 (2.0) | 1 (6.3) | 0 (0.0) | 0.327 |
| Sexual Dysfunction | 1 (2.0) | 0 (0.0) | 1 (3.0) | 1.000 |
| Suicidal Ideation | 1 (2.0) | 0 (0.0) | 1 (3.0) | 1.000 |
| Ulcer GI | 1 (2.0) | 1 (6.3) | 0 (0.0) | 0.327 |
Treatment groups also did not significantly differ by the proportion of study medication capsules taken, or samples that fluoresced for riboflavin (using quantitative assessment). For each subject, the proportion of pills taken divided by the number of pills prescribed was computed for each day with available compliance data, and the daily average was computed. A total of 45 subjects (placebo n=15, lorcaserin n=30) had medication compliance data (data unavailable for 4 of the patients who did not continue through induction period). The median (interquartile range (IQR)) average proportion of pills taken was 64.3% (50.0–98.3) in the placebo group compared to 83.3% (50.0–100.0) in the lorcaserin group. These proportions were not significantly different (Wilcoxon rank sum test, p = .55).
Thirty subjects (placebo n = 10, lorcaserin n = 20) had urine samples tested for riboflavin as testing was not initiated at the onset of the trial and not available for the full sample. For each subject, the proportion of urine samples with riboflavin > 900 was computed. This cut-off was used based on a prior study demonstrating high detection and low false positive rates with 0 mg and varying doses of riboflavin (Herron et al., 2013). The median (IQR) proportion of riboflavin samples that fluoresced was 84.6% (40.0–100.0) in the placebo group compared to 86.7% (25.0–100.0) in the lorcaserin group. These proportions were not significantly different (Wilcoxon rank sum test, p = .83).
4. Discussion
This outpatient pilot study was designed to test whether lorcaserin was more likely than placebo to increase the likelihood of induction onto XR-NTX among those with OUD. Secondary aims were to determine whether lorcaserin was superior to placebo in reducing withdrawal after opioid cessation as well as increased likelihood of receiving a second injection of XR-NTX a month after the first injection. To maximize exposure to the active medication in this pilot study, individuals were randomized in a 2:1 ratio. While there were no significant differences in adverse events between the 2 treatment arms there were no clear advantages associated with using lorcaserin to facilitate induction onto XR-NTX or mitigate withdrawal symptoms.
This study was conducted concurrently to an inpatient study that evaluated lorcaserin as a potential treatment for opioid withdrawal in a controlled human laboratory setting (Brandt et al., 2020). In that study, lorcaserin did not alter oxycodone self-administration, even though the study was adequately powered to detect meaningful differences in self-administration and desire for heroin when oxycodone was available. In sum these studies do not support the utility of lorcaserin as an intervention for opioid use disorder. Moreover, a recently published trial in non-human primates found that lorcaserin did not reduce opioid self-administration (Townsend et al., 2020) in contrast to an earlier study (Kohut and Bergman, 2018).
Based on our prior work (Bisaga et al., 2018; Sullivan et al., 2017), we expected that approximately 50% of the heroin-using individuals in the placebo arm of this study would receive the first XR-NTX injection, as compared to those using prescription opioids (60–70%). Notably, in a much larger 3-arm comparison of various outpatient induction methods onto XR-NTX, the treatment arm that received buprenorphine and ascending doses of oral naltrexone (similar to our study), the rate of outpatient induction onto the first XR-NTX injection was 47% (Bisaga et al., 2018), a rate only slightly higher than the placebo arm of our trial (44%), suggesting that there was not a floor effect masking a potential difference between lorcaserin and placebo. Further, we expected that these rates would remain the same using a slightly shortened induction period based on our recent experience (Sibai et al., 2020). In the study conducted by Sibai et al. (2020) 54% of the heroin users and 78% of the prescription pill users received their first dose of XR-NTX. In our study, we noted that less heroin using individuals were being inducted onto XR-NTX. Because the study was blinded we did not know if this was due to the addition of lorcaserin or some other factor. The decision was made by the team to start testing baseline urines for fentanyl because it was becoming clear in our clinical practice experience that with the increased adulteration of highly potent synthetic opioids, such as fentanyl, it was harder to induct heroin users onto XR-NTX or for that matter, buprenorphine. Notably, we found that approximately 85% or 28 out of our 33 heroin users who were tested for fentanyl at baseline were positive for fentanyl consistent with the rise of fentanyl in NYC (Colon-Berezin et al., 2019; Nolan et al., 2018). Whereas we did not have any reduction in rates of induction for our prescription pill opioid group who were all fentanyl negative (80%, 8/10 received the first injection) compared to the Sibai et al. (2020) study, the rate of induction for our heroin group overall was considerably less at 28%, with similar rates for those that were fentanyl positive (25%, 7/28) and those that were fentanyl negative at baseline (20%, 1/5). Because these percentages are based on a small number of patients, we cannot draw conclusions regarding the moderating effect of fentanyl. Further, we only collected a single fentanyl test at baseline. It is possible for a heroin user to have had a negative fentanyl test at baseline but have used heroin with fentanyl before or after the baseline fentanyl test. Given that earlier induction studies were conducted prior to the upswing in widespread fentanyl use, this may be an important factor to consider and perhaps monitor throughout a clinical trial that is assessing new methods of induction.
Notably, 46% of those that we had self-reported use data for during the detoxification period, reported opioid use during detoxification. Not surprisingly, patients who used opioids during the induction period were less likely to be inducted onto XR-NTX. Sibai et al. (2020) also found that rates of induction were lower among those who continued to use opioids versus those that did not. Conducting a XR-NTX induction in the inpatient setting mitigates this problem, but then restricts the utilization of XR-NTX.
There are several limitations to this study that reduce our ability to draw firm conclusions. First, our sample size was modest; the total number of 60 participants was chosen to minimize cost and to successfully reach study end within two years. It was also chosen to estimate the potential treatment effect size and 95% confidence intervals, providing a range of plausible estimates with a margin of error of 26%. While it remains conjecture, individuals using highly potent synthetic opioids, such as fentanyl, may require medications that powerfully reduce withdrawal symptoms, rather than a medication that may act more modestly. Second, we chose a lorcaserin dose of 10 mg twice a day. This is the standard dose for treatment of obesity but we do not know if this is an adequate dose in treating acute or protracted opioid withdrawal. Third, while adherence was fairly good with 83% (IQR=50–100) of the lorcaserin group self-reporting compliance, a higher percentage would have been preferable. Fourth, a large percentage of the participants enrolled in the study (46%) continued to use opioids during the induction period. Despite this, some were able to be inducted onto XR-NTX, but most were not. A successful outpatient induction requires a discontinuation of opioids or at least a substantial reduction in use allowing the administration of naltrexone. Fifth, a complicated induction approach was used starting with a single dose of buprenorphine followed by ascending doses of naltrexone that may not have been necessary. A recent large, randomized clinical trial suggests that this approach does not perform better than using ancillary medications alone (Bisaga et al., 2018), therefore, this approach may be unduly complicated and hamper generalizability in community settings. Sixth, there was a high drop-out rate. Less than twenty percent of participants in either group completed the full 8-week trial. Although this would not affect the primary and secondary outcomes analyzed, it may have impaired our ability to detect a clinically meaningful effect.
Another development that occurred after the study was completed was the finding from a longitudinal study that long-term lorcaserin use was associated with a higher cancer risk (Federal Drug Administration (FDA), 2020). This led to the manufacturer voluntarily taking the medication off the market. The consequence of this is that it may preclude investigation of other medications with this mechanism of action for opioid or other substance use disorders.
When this study was initiated we had not been prepared for the changing pattern of opioid use among those seeking treatment for OUD. In our earlier patient samples (Bisaga et al., 2018; Sullivan et al., 2017), the percentages of prescription opioid users were higher than the rate in this trial. Moreover, when we initiated the study, we did not test for fentanyl. However, after having increased difficulties in inducting individuals onto XR-NTX, we began testing for fentanyl suspecting this might be confounding the results of this pilot study. However, our study was not powered to assess the impact of fentanyl on induction success. Future trials might need to take this factor into account or chose samples that are/are not current users of highly potent synthetic opioids.
5. Conclusion
The current trial does not support the use of lorcaserin for facilitating induction onto XR-NTX. While it was hoped that lorcaserin might have clinical utility, confounders such as enrollment of primarily heroin users with/without concurrent fentanyl use may have led to a more refractory group that is less likely to respond to an outpatient induction. Moreover, whether one is a prescription opioid or heroin user, continued opioid use is also associated with less XR-NTX induction success. Future studies that focus on behavioral and pharmacologic approaches that reduce the likelihood of opioid use during the induction period is needed if outpatient XR-NTX inductions are to be widely adopted.
Supplementary Material
Highlights.
Trial studied improving outpatient extended-release naltrexone (XR-NTX) induction.
Participants with OUD were randomized to lorcaserin or placebo.
Lorcaserin did not improve outpatient XR-NTX induction rates or mitigate withdrawal.
85% of the heroin users tested positive for fentanyl at baseline.
Participants using opioids during induction were less likely to receive XR-NTX.
Acknowledgments
We would like to thank the staff of the Substance Treatment and Research Service (STARS) of the Columbia University Medical Center/New York State Psychiatric Institute for their clinical support.
Role of Funding Source
Funding for this research was provided by the National Institute on Drug Abuse (NIDA). NIDA grant U54DA037842 (Dr. Levin). NIDA program staff contributed to the study design but had no involvement in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Trial Registration: Clinicaltrials.gov Identifier: NCT03169816
Conflict of Interest
Drs. Mariani, Pavlicova, and Naqvi reported no biomedical financial interests or potential conflicts of interest. Also, Ms. Choi, Mr. Brooks, Mr. Basaraba and Ms. Mahony reported no biomedical financial interests or potential conflicts of interest. Dr. Levin has acted as an unpaid consultant for Alkermes, Novartis, and US WorldMeds. She receives research support from US WorldMeds. Dr. Bisaga has received research support from Alkermes.
REFERENCES
- American Psychiatric Association (APA), 2013. Diagnostic and Statistical Manual of Mental Disorders 5th Edition Arlington, VA, American Psychiatric Association. [Google Scholar]
- Bisaga A, Sullivan MA, Glass A, Mishlen K, Carpenter KM, Mariani JJ, Levin FR, Nunes EV, 2014. A placebo-controlled trial of memantine as an adjunct to injectable extended-release naltrexone for opioid dependence. J. Subst. Abuse Treat 46, 546–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisaga A, Sullivan MA, Glass A, Mishlen K, Pavlicova M, Haney M, Raby WN, Levin FR, Carpenter KM, Mariani JJ, Nunes EV, 2015. The effects of dronabinol during detoxification and the initiation of treatment with extended release naltrexone. Drug Alcohol Depend 154, 38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisaga A, Mannelli P, Yu M, Nangia N, Graham CE, Tompkins DA, Kosten TR, Akerman SC, Silverman BL, Sullivan MA, 2018. Outpatient transition to extended-release injectable naltrexone for patients with opioid use disorder: A phase 3 randomized trial. Drug Alcohol Depend 187, 171–178. [DOI] [PubMed] [Google Scholar]
- Brandt L, Jones JD, Martinez S, Manubay JM, Mogali S, Ramey T, Levin FR, Comer SD, 2020. Effects of lorcaserin on oxycodone self-administration and subjective responses in participants with opioid use disorder. Drug Alcohol Depend 208, 107859 10.1016/j.drugalcdep.2020.107859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC), 2020. Drug overdose deaths https://www.cdc.gov/drugoverdose/data/statedeaths.html (Accessed 23 June, 2020).
- Colon-Berezin C, Nolan ML, Blachman-Forshay J, Paone D, 2019. Overdose Deaths Involving Fentanyl and Fentanyl Analogs - New York City, 2000–2017. MMWR Morb. Mortal. Wkly 68, 37–40. 10.15585/mmwr.mm6802a3 (Accessed 23 June, 2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham KA, Anastasio NC, 2014. Serotonin at the nexus of impulsivity and cue reactivity in cocaine addiction. Neuropharmacology 76, 460–478. doi: 10.1016/j.neuropharm.2013.06.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efrid J, 2011. Blocked randomization with randomly selected block sizes. Int. J. Environ. Res. Public Health 8, 15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federal Drug Administration (FDA). (2020, February 13). FDA requests the withdrawal of the weight-loss drug belviq, belviq-xr (lorcaserin) from the market FDA.gov; https://www.fda.gov/drugs/drug-safety-and-availability/fda-requests-withdrawal-weight-loss-drug-belviq-belviq-xr-lorcaserin-market (Accessed 3 June 2020). [Google Scholar]
- Handelsman L, Cochrane KJ, Aronson MJ, Ness R, Rubinstein KJ, Kanof PD, 1987. Two new rating scales for opiate withdrawal. Am. J. Drug Alcohol Abuse 13, 293–308. doi: 10.3109/00952998709001515. [DOI] [PubMed] [Google Scholar]
- Herron AJ, Mariani JJ, Pavlicova M,. Parrinello CM, Bold KW, Levin FR, Nunes EV, Sullivan MA, Raby WN, Bisaga A, 2013. Assessment of riboflavin as a tracer substance: comparison of a qualitative to a quantitative method of riboflavin measurement Drug Alcohol Depend 128, 77–82. 10.1016/j.drugalcdep.2012.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hser YI, Saxon AJ, Huang D, Hasson A, Thomas C, Hillhouse M, Jacobs P, Teruya C, McLaughlin P, Wiest K, Cohen A, Ling W, 2014. Treatment retention among patients randomized to buprenorphine/naloxone compared to methadone in a multi-site trial. Addiction 109, 79–87. doi: 10.1111/add.12333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BA, Ait-Daoud N, Roache JD, 2005. The COMBINE SAFTEE: a structured instrument for collecting adverse events adapted for clinical studies in the alcoholism field. J. Stud. Alcohol Suppl 15, 157–167; discussion 140. [DOI] [PubMed] [Google Scholar]
- Jones CM, Campopiano M, Baldwin G, McCance-Katz E, 2015. National and state treatment need and capacity for opioid agonist medication-assisted treatment. Am. J. Public Health 105, e55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakko J, Svanborg KD, Kreek MJ, Heilig M, 2003. 1-year retention and social function after buprenorphine-assisted relapse prevention treatment for heroin dependence in Sweden: a randomised, placebo-controlled trial. Lancet 361, 662–668. [DOI] [PubMed] [Google Scholar]
- Koehl JL, Zimmerman DE, Bridgeman PJ, 2019. Medications for management of opioid use disorder. Am. J. of Health Syst. Pharm 76, 1097–1103. 10.1093/ajhp/zxz105 [DOI] [PubMed] [Google Scholar]
- Kohut SJ, Bergman J, 2018. Lorcaserin decreases the reinforcing effects of heroin, but not food, in rhesus monkeys. Eur. J. Pharmacol 840, 28–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JD, Nunes EV Jr, Novo P, Bachrach K, Bailey GL, Bhatt S, Farkas S, Fishman M, Gauthier P, Hodgkins CC, King J, Lindblad R, Liu D, Matthews AG, May J, Peavy KM, Ross S, Salazar D, Schkolnik P, Shmueli-Blumberg D, Stablein D, Subramaniam G, Rotrosen J, 2018. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet 391, 309–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litten R, Allen J, 1992. Measuring Alcohol Consumption: Psychosocial and Biochemical Methods Humana Press Inc., Totowa, NJ. [Google Scholar]
- Mannelli P, Wu L, Peindl KS, Swartz MS, Woody GE, 2014. Extended release naltrexone injection is performed in the majority of opioid dependent patients receiving outpatient induction: a very low dose naltrexone and buprenorphine open label trial. Drug Alcohol Depend 138, 83–88. doi: 10.1016/j.drugalcdep.2014.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino LA, Campbell AN, Nunes EV, Sederer LI, Dixon LB, 2019. Factors influencing buprenorphine prescribing among physicians in New York State. J. Addict 2019, 7832752. doi: 10.1155/2019/7832752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JR, Schackman BR, Leff JA, Linas BP, Walley AY, 2018. Injectable naltrexone, oral naltrexone, and buprenorphine utilization and discontinuation among individuals treated for opioid use disorder in a United States commercially insured population. J. Subst. Abuse Treat 85, 90–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JR, Schackman BR, Weinstein ZM, Walley AY, Linas BP, 2019. Overdose following initiation of naltrexone and buprenorphine medication treatment for opioid use disorder in a United States commercially insured cohort. Drug Alcohol Depend 200, 34–39. doi: 10.1016/j.drugalcdep.2019.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neelakantan H, Holliday ED, Fox RG, Stutz SJ, Comer SD, Haney M, Anastasio NC, Moeller FG, Cunningham KA, 2017. Lorcaserin suppresses oxycodone self-administration and relapse vulnerability in rats. ACS Chem. Neurosci 8, 1065–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan ML, Tuazon E, Blachman-Forshay J, Paone D, 2018. Unintentional drug poisoning (overdose) deaths in New York City, 2000 to 2017 New York, NY: New York City Department of Health and Mental Hygiene; https://www1.nyc.gov/assets/doh/downloads/pdf/epi/databrief104.pdf (Accessed 22 June 2020). [Google Scholar]
- Rothenberg JL, Sullivan MA, Church SH, Seracini A, Collins E, Kleber HD, Nunes EV, 2002. Behavioral naltrexone therapy: An integrated treatment for opiate dependence. J. Subst. Abuse Treat 23, 351–360. 10.1016/S0740-5472(02)00301-X [DOI] [PubMed] [Google Scholar]
- Shanahan WR, Rose JE, Glicklich A, Stubbe S, Sanchez-Kam M, 2017. Lorcaserin for smoking cessation and associated weight gain: a randomized 12-Week clinical trial. Nicotine Tob. Res 19, 944–951. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC, 1998. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry 59 Suppl 20, 22–33; quiz 34–57. [PubMed] [Google Scholar]
- Shram MJ, Schoedel KA, Bartlett C, Shazer RL, Anderson CM, Sellers EM, 2011. Evaluation of the abuse potential of lorcaserin, a serotonin 2C (5-HT2C) receptor agonist, in recreational polydrug users. Clin. Pharmacol. Ther 89, 683–692. [DOI] [PubMed] [Google Scholar]
- Sibai M, Mishlen K, Nunes EV, Levin FR, Mariani JJ, Bisaga A, 2020. A week-long outpatient induction onto XR-naltrexone in patients with opioid use disorder. Am. J. Drug Alcohol Abuse 46, 289–296. DOI: 10.1080/00952990.2019.1700265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sordo L, Barrio G, Bravo MJ, Indave BI, Degenhardt L, Wiessing L, Ferri M, Pastor-Barriuso R, 2017. Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies. BMJ 357, j1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA), 2019a. Key substance use and mental health indicators in the United States: Results from the 2018 National Survey on Drug Use and Health (HHS Publication No. PEP19–5068, NSDUH Series H-54) Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration; Retrieved from https://www.samhsa.gov/data/ (accessed on May 27, 2020). [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA), (2019b, August 20). SAMHSA’s 2018 National Survey on Drug Use and Health (NSDUH) YouTube; https://www.youtube.com/watch?v=Hb7pPHuRNMA (accessed on May 27, 2020). [Google Scholar]
- Sullivan M, Bisaga A, Pavlicova M, Choi CJ, Mishlen K, Carpenter KM, Levin FR, Dakwar E, Mariani JJ, Nunes EV, 2017. Long-acting injectable naltrexone induction: a randomized trial of outpatient opioid detoxification with naltrexone versus buprenorphine. Am. J. Psychiatry 174, 459–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend EA,, Negus SS, Poklis JL, Banks ML, 2020. Lorcaserin maintenance fails to attenuate heroin vs. food choice in rhesus monkeys. Drug Alcohol Depend 208:107848. doi: 10.1016/j.drugalcdep.2020.107848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesson DR, Ling W, 2003. The Clinical Opiate Withdrawal Scale (COWS). J. Psychoactive Drugs 35, 253–259. [DOI] [PubMed] [Google Scholar]
- Wilson N, Kariisa M, Seth P, Smith H, Davis NL, 2020. Drug and Opioid-Involved Overdose Deaths—United States, 2017–2018. MMWR Morb. Mortal. Wkly 69, 290–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Pang G, Zhang Y-M, Li G, Xu S, Dong L, Stackman RW, Zhang G, 2015. Activation of serotonin 5-HT2C receptor suppresses behavioral sensitization and naloxone-precipitated withdrawal symptoms in heroin-treated mice. Neurosci. Lett 607, 23–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


