Abstract
One of the significant challenges in the field of drug delivery remains to be insufficient targeting of diseased tissues or cells. While efforts to perform targeted drug delivery by engineered nanoparticles have shown some success, there are underlying targeting, toxicity and immunogenicity challenges. On the other hand, live cells usually have innate targeting mechanisms, and can be used as drug delivery vehicles to increase the efficiency with which a drug accumulates to act on the intended tissue. In some cases, when no native cell types exhibit the desired therapeutic phenotype, preferred outcomes can be achieved by genetically modifying and reprogramming cells with gene circuits. This review highlights recent advances in the use of cells to deliver therapeutics. Specifically, we discuss how red blood cells (RBCs), platelets, neutrophils, mesenchymal stem cells (MSCs), and bacteria have been utilized to advance drug delivery.
Keywords: therapeutic cells, drug delivery, synthetic biology
Cells as Delivery Devices
Nanoparticles (see Glossary) have been used extensively to enhance the therapeutic efficacy of drugs, however, there is an emerging opinion that these may not be adequate for universal drug delivery [1]. This is based on the observations that only about 1% of injected doses of nanoparticles reach the intended sites, there are toxic impacts of cell-nanoparticle interactions, there is difficulty of cell selectivity in heterogenous diseased tissues such as chronic wounds and cancer, phagocytic clearance causes nanoparticle depletion, and there has been limited clinical success [2, 3]. While future efforts will likely not exclude this technology, these challenges have led to exploring alternative approaches for drug delivery.
One of these alternative approaches is using cells as drug delivery vehicles. Cells have been touted as “living drugs” due to their inherent compatibility that enables them to persist in the body and elicit a desired effect for a duration much longer than that of natural or synthetic pharmaceutical free drugs since the injection of free proteins and therapeutics into the body is usually followed by the immune system’s response and their rapid removal from the bloodstream [4]. Cells have the natural ability to sense, integrate, and respond to dynamic environments in vivo, making them an attractive vehicle for the delivery of various therapeutic agents. Therefore, the use of live cells to treat or cure a disease has become a significant focus in the drug delivery field as they can be highly biocompatible, have a natural capacity to target tissues, have an inherent biodegradability, have high drug loading capacity, and have a natural ability to cross biological barriers [5]. Combining cellular half-life and tissue specificity with the delivery of active drugs accentuates the advantages of both systems to create unique, mosaic therapies to better treat disease. Below we discuss the use of biological cells as delivery vehicles to deliver therapeutic molecules and bioactive proteins, in addition to discussing their advantages and disadvantages (Figure 1).
Figure 1: Biological cells as therapeutic delivery vehicles.
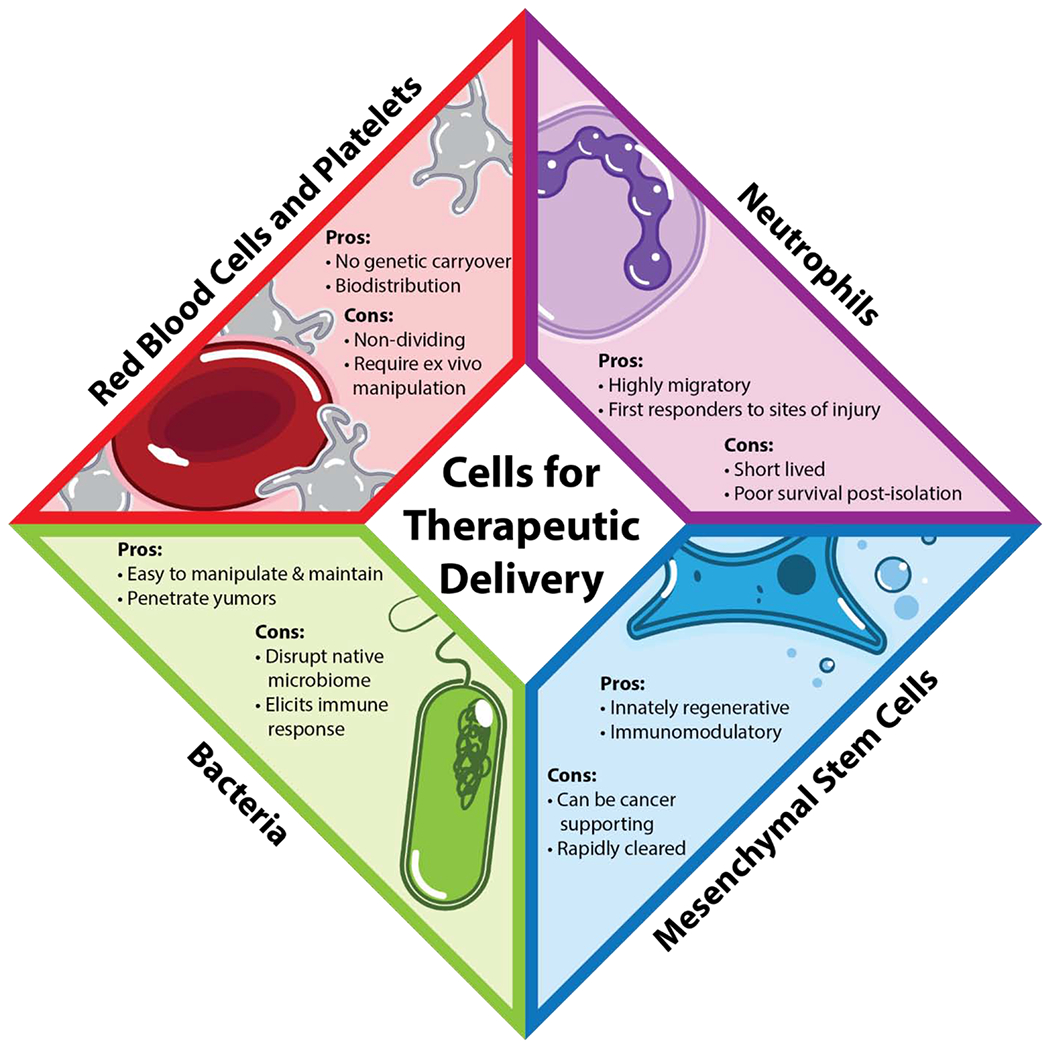
Various cell types can be used for therapeutic delivery. The choice of cell type is important for different applications. Anucleate cells (red) include red blood cells and platelets. These cells have no nucleus and a wide biodistribution (pro), however, they are non-dividing cells which makes them incapable of expanding in vitro without manipulation (con). Mesenchymal stem cells (MSCs) (blue) are innately regenerative, immunomodulatory, and can target injured tissue (pros), however, when MSCs are injected into the body, they are rapidly cleared and cancer cells have been shown to hijack MSCs for their own benefit (cons). Bacteria (green) are easy to manipulate, they double every 45 minutes, and they can penetrate tumors (pros), however, they can also disrupt the native microbiome and elicit immune responses (cons). Neutrophils (purple) are natural responders to sites of inflammation and are usually the first cells on site for repair (pros), however neutrophils are short-lived and they have poor survival after isolation, preventing their expansion in vitro (cons).
Anucleate Cells as Delivery Systems
Red blood cells (RBCs) and platelets originate from hematopoietic stem cells (Box 1) and are unique components of the blood circulatory system since they are devoid of nuclei and therefore contain no hereditary genomic information; hence they have no inherent risk of abnormal growth or tumorigenic transformation. They also circulate unimpeded in the blood stream for days, which becomes a distinct advantage for drug delivery, especially when considering that the injection of free proteins into the body is usually followed by a swift immune response resulting in the rapid removal of the protein from the blood stream. To improve the delivery of therapeutics, efforts have been made to engineer anucleate cells to be custom drug delivery vehicles for molecules and proteins to target specific locations in the body and to carry designer payloads for treating injury and disease [6]. This can be done by loading the therapeutic molecules inside of the cells (intracellular encapsulation) or by attaching them to the cell surface (surface modification) to be transported throughout the circulation. In this section we discuss approaches for modifying and engineering anucleate cells for improved drug delivery.
Box 1. Hematopoietic Stem Cells.
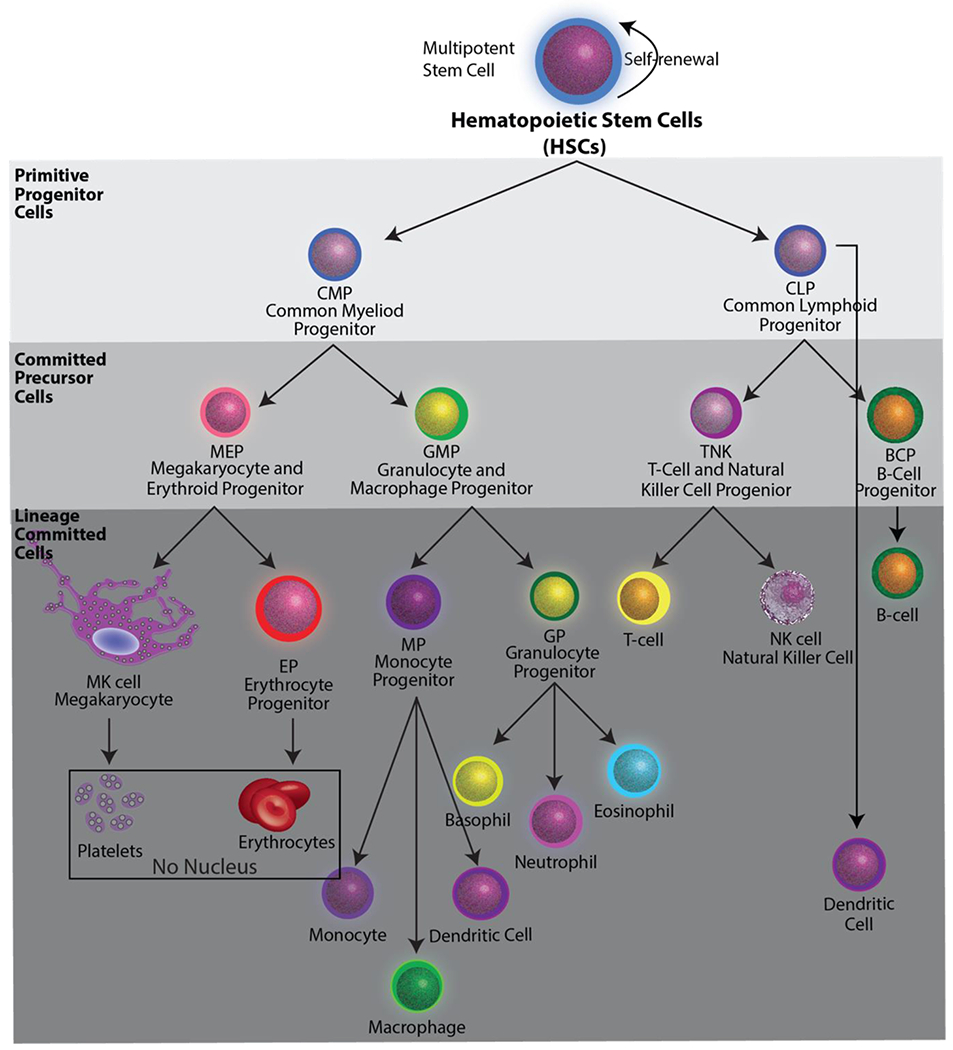
Hematopoietic stem cells (HSCs) reside in the bone marrow and have the potential to become cells of the immune system or anucleate red blood cells and platelets (Box 1, Figure I). RBCs and platelets originate from CD34+ HSCs, which sense and respond to the bone marrow microenvironment for signals to either self-renew to maintain their multipotent potential, or to commit to a more specialized cell type by differentiating into a common progenitor called a megakaryocyte-erythroid progenitor cell (Box 1, Figure I) [86]. The mechanisms by which RBCs and platelets develop from megakaryocyte-erythroid progenitor cells are drastically different. Immature erythrocytes exit the cell cycle, decrease in size, condense their nucleus, and prepare for nuclear expulsion in a process similar to cell division, to produce RBCs [87]. By contrast, megakaryocytes (MKs) prepare for platelet production by completely remodeling cytoskeletal components to extend proplatelets into the vascular flow, where they fragment to form platelets that mature in circulation [88]. Methods that recapitulate the in vivo conditions to produce RBCs and platelets ex vivo and in vitro can be clinically scalable under select conditions, requiring CD34+ hematopoietic stem cell expansion and differentiation, large-scale induced pluripotent stem cell (iPSC) cultures, immortalized progenitors, and/or custom bioreactor systems [89–91].
Box 1, Figure I: Hematopoiesis.
Hematopoietic stem cells have the potential to become all of the cells of the blood system. They can become all of the cells of the immune system, or anucleate cells red blood cells and platelets (black box).
Engineering RBCs for drug delivery
Intracellular encapsulation
Incorporating active drug molecules in the intracellular space of RBCs provides an immune-privileged environment for encapsulated drugs that increases their circulation time. One approach to achieve intracellular encapsulation is to place RBCs in a hypotonic solution to induce the opening of resealable pores within the cell membrane so molecules can enter into the cell, and then restoring the isotonicity of the solution to reseal pores thereby encapsulating molecules inside of the cells [7]. This method has been utilized for loading prodrugs into the RBC cytoplasmic space that are unable to diffuse through the RBC membrane [8]. Native RBC machinery converts the prodrug into an active form over time, which diffuses through the membrane to elicit its effect. To demonstrate this, the ant-inflammatory and immunosuppressive prodrug molecule, dexamethasone sodium phosphate, the prodrug of dexamethasone, was encapsulated inside of RBCs, which has had success in the clinic for treatment of inflammatory bowel disease, cystic fibrosis, and ataxia telangiectasia [7]. While inside of RBCs, the prodrug gets dephosphorylated by resident enzymes and converted into its active and diffusible form, dexamethasone. In its active form dexamethasone can cause significant side effects such as osteoporosis, glaucoma, or skin atrophy with prolonged and high dosage levels in vivo [9]; however, by loading its prodrug into RBCs, a slow release of the active molecule is diffused into the blood stream through the RBC membrane. This has shown to provide concentrations of the active drug within therapeutic values for several days. Similarly, enzymatic proteins like L-Asparaginase have also been loaded into RBCs to reduce aberrant levels of the amino acid asparagine that have been shown to block the growth of tumor cells that need asparagine to grow (Figure 2, left panel) [10, 11].
Figure 2: Anucleate Cells as Delivery Systems.
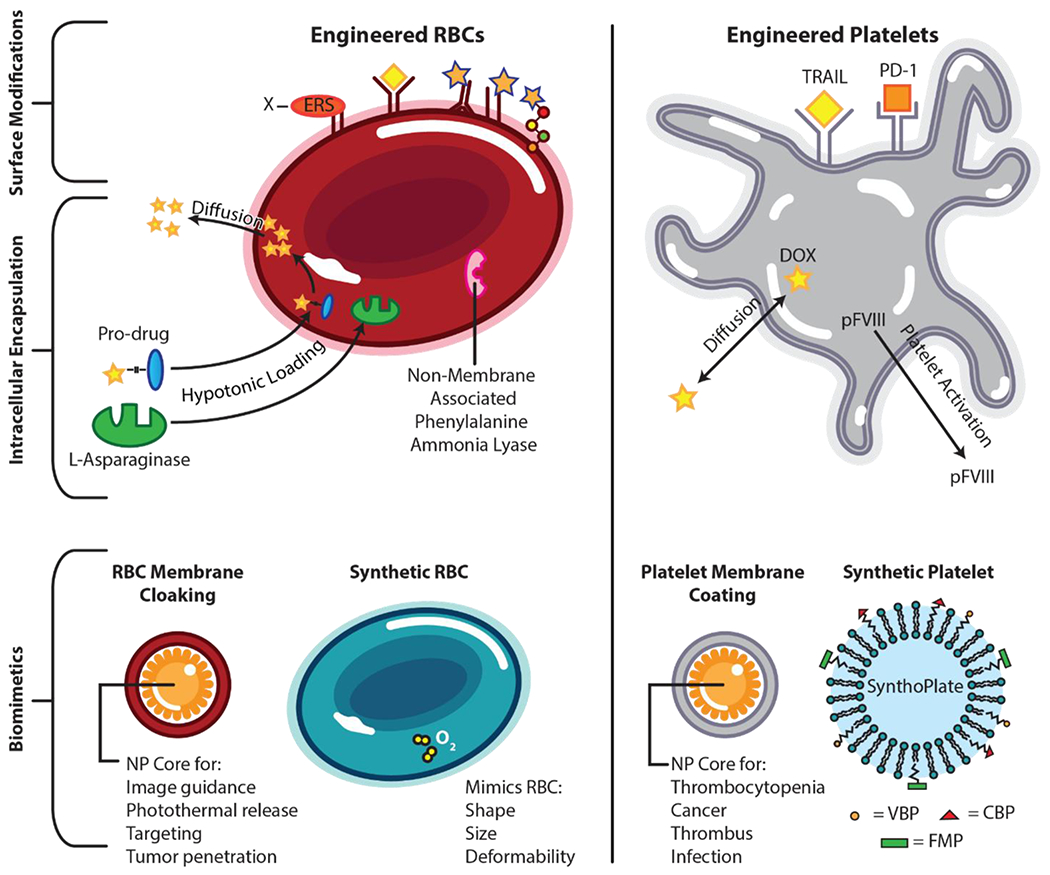
Engineered RBCs are surface modified (upper) by expressing an enzyme recognition site (ERS) on the RBC surface, enabling the covalent tethering of an array of functional molecules to the RBC surface. This endows RBCs with various therapeutic molecules, denoted by the yellow diamond attached to an RBC receptor. Drug molecules can also be conjugated to antibodies, antibody fragments, and peptides, respectively, to native RBC surface molecules. Intracellular encapsulation of therapeutics in RBCs is achieved through hypotonic loading of pro-drugs into the RBC. Once inside, the native RBC machinery converts prodrugs into active forms that freely diffuses out of the membrane. Enzymatic proteins like L- Asparaginase are also loaded into RBCs using this method to reduce aberrant levels of substrate throughout the body. Additionally, non-membrane associated enzymatic proteins like phenylalanine ammonia lyase are expressed in RBCs where they act in concert as a miniature bioreactor to breakdown toxic substrates in circulation. Biomimetic design of RBCs (lower left) includes using native RBC membranes to cloak nanoparticle (NP) cores (left) and synthetic RBCs that mimic native RBC shape, size, and deformability used to transport oxygen or drug molecules (right). Engineered platelets are surface modified (upper) to express TRAIL and PD-1 as anti-cancer therapeutics. Intracellular encapsulation (middle) of therapeutics in platelets is achieved via diffusion of free doxorubicin (DOX) into the intracellular platelet compartment. Additionally, platelets have been engineered to express intracellular platelet factor VIII (pFVIII) that gets released upon platelet activation. Biomimetic design of platelets (lower) includes coating NP cores with native platelet membrane (left) and use of heteromultivalent, surface-decorated liposomes marketed as SynthoPlate (right). VBP = von Willebrand binding peptide; CBP = collagen binding peptide; FMP = fibrinogen mimetic peptide.
Surface modification
RBCs are concave discs that enable maximum oxygen uptake and the flexibility to pass through capillaries to release oxygen molecules. They also naturally express surface molecules such as receptors, proteins, and functional groups on the surface of their membrane that provide binding sites for antibodies and drugs [12]. Scientists have successfully used these membrane surface molecules to functionalize the RBC membrane with a variety of antibodies and drugs for the improved delivery efficiency of therapeutic molecules at locations of injury and disease [5]. In almost all cases, such tethering strategies improves the pharmacokinetics and biodistribution of the drug compared to the free drug and, in select cases, localization to the RBC surface improves the specificity of the drug, leading to functional aspects that could not be achieved with the free drug alone. For example, it was shown that RBCs can be genetically engineered in vitro to introduce non-native genes that encode for enzyme recognition sites (ERS) on the surface of the RBCs to enable therapeutic proteins or drugs to be covalently tethered to the RBC membrane while still preserving the integrity of the membrane [13]. This study initially used biotin to demonstrate that their modified RBCs could circulate in vivo for at least 28 days. Next, antibodies were attached to the RBCs to enable the engineered cells to bind specifically to target cells that express the antibody target protein demonstrating that RBCs can be engineered to target specific locations in the body. This same tethering technique was also used to display disease associated autoantigens on the surface of RBCs for inducing antigen-specific tolerance for autoimmune diseases [14].
RBC biomimetics
Efforts to design RBC biomimetics utilize design approaches to integrate at least one native RBC element to enhance nanoparticle performance. For example, coating nanoparticles with red blood cell membranes extends their circulation time by evading the immune system from macrophage uptake to avoid their rapid clearance [15, 16]. Furthermore, RBC membrane components such as lipids and other associated membrane proteins accompany the membrane coating to endow the nanoparticles with the same bound proteins as the RBCs (Figure 2, left panel). For example, recently iron oxide magnetic clusters were coated with an RBC membrane coating to improve their blood retention time when injected into mice, which allows them to accumulate in tumors. Shining near-infrared light on the magnetic clusters within the tumor led to enhanced tumor cell killing [17]. This approach to coat various nanoparticles with RBC membranes has also found applications in pathogen targeting [18] and solid tumor penetration [19].
Efforts have also been made to create artificial RBCs that mimic one or more of the rudimentary functions possessed by the natural cell that include size, shape, and deformability to allow passage through vasculature, the ability to carrying oxygen to be released in tissues, and the presence of multiple biomarkers on the cell membrane including self-recognition markers to prevent immune attack and rapid clearance from circulation (Figure 2, left panel) [20–22]. More recently, biomimetic synthetic rebuilt RBCs (RRBCs) were engineered out of polymers with etched silica cores that possess all of the combined features of natural RBCs, in addition to endowing the RRBCs with non-native functionalities including drug delivery, magnetism for imaging, and toxin sensing and sequestration [23]. The utility of these synthetic cells was demonstrated to function similarly to natural RBC, in addition to their loading with MRI contrast for imaging capabilities and various anticancer drugs with varying release profiles to be used for treating cancer patients.
Platelets as delivery vehicles
Intracellular encapsulation
Platelet activation causes drastic morphological changes and leads to the release of intracellular content. Sites of natural platelet aggregation and activation have been leveraged for the targeted release of therapeutics upon activation. For instance, free doxorubicin (DOX), an anti-cancer therapeutic, suffers from poor circulation times and cardiotoxic effects [24]. Platelets passively loaded with DOX via diffusion home to tumor cells and subsequently activate the release DOX (Figure 2, right panel). Loading DOX into platelets eliminates the detrimental effects of free DOX and leads to preferential killing of the tumor cells in both in vitro and in vivo models of lymphoma [25]. Another approach is to genetically engineer platelet progenitor stem cells to eventually express therapeutic proteins in platelets. This strategy has been used as a treatment for hemophilia A where platelet factor VIII (pFVIII) is expressed in induced pluripotent stem (iPS) cell-derived MKs (Box 1). Platelets derived from such MKs survive in circulation and release pFVIII upon activation for improved hemostatic function in mouse models of hemophilia A (Figure 2, right panel) [26].
Surface modification
Akin to RBCs, the ability of platelets to circulate unimpeded in the blood stream is a distinct advantage for drug delivery. Moreover, platelets naturally aggregate with circulating tumor cells (CTCs) via specific interactions of surface ligands to promote metastasis [27]. To take advantage of this, hematopoietic stem cells were genetically engineered to express the tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), a ligand that induces apoptosis in tumor cells. In this study, when the genetically engineered HSCs were differentiated into platelets (Box 1), the TRAIL-expressing platelets were shown to kill cancer cells and significantly reduce metastasis in a prostate cancer mouse model [28]. In another example, the progenitor cells of platelets, megakaryocytes (MKs), were genetically engineered to express the programmed cell death protein 1 (PD-1) on their cell membrane. Tumor cells expressing PD-L1 suppress the immune system by exhausting the T cells that would normally attack and clear the tumor. In this study, the platelets expressing the PD-1 protein on their membrane accumulated in tumors and were shown to reinvigorate the exhausted T cells enabling the T cells to significantly shrink the tumor in mice [29].
Platelet biomimetics
Similar to approaches with RBCs, platelet membrane components have been used to coat nanoparticles that suffer from poor circulation time due to interactions with the blood circulation. Currently, platelet transfusions are the most common way to improve a patient’s platelet count. However, due to their short storage life and potential contamination risk because of room temperature storage requirements, there is a clinical need for synthetic biomimetic platelets. Initial efforts to create synthetic platelets included sonification of platelets in the presence of nanoparticles that resulted in complete platelet membrane coverage of the nanoparticle surface without affecting nanoparticle composition (Figure 3, right panel). This approach has been used to coat nanoparticles for the treatment of thrombocytopenia [30], multiple myeloma and thrombus [31]. In addition to coating nanoparticles with platelet cell membranes, more recent efforts to engineer synthetic platelets from various biomaterials (e.g. liposomes, albumin microparticles, and latex particles) are underway to match platelet shape and mechanics to enable similar clot capabilities as of natural platelets [32]. These synthetic platelets are being designed to target wounds and upon arrival, facilitate healing interactions with the surrounding cells and tissue to augment the body’s natural healing capacity. Synthetic platelets can also be functionalized with various molecules including peptides, antibodies, and growth factors to confer targeting and therapeutic capabilities. For example, adding the Von Willebrand factor (VWF-A1) domain to synthetic platelets demonstrated their adherence to injured endothelial vascular walls under high shear stress, and the formation of an aggregate similar to a natural blood clot at the injury site in addition to maintaining hemostasis [33].
Figure 3: Harnessing the innate properties of mesenchymal stem cells for drug delivery.
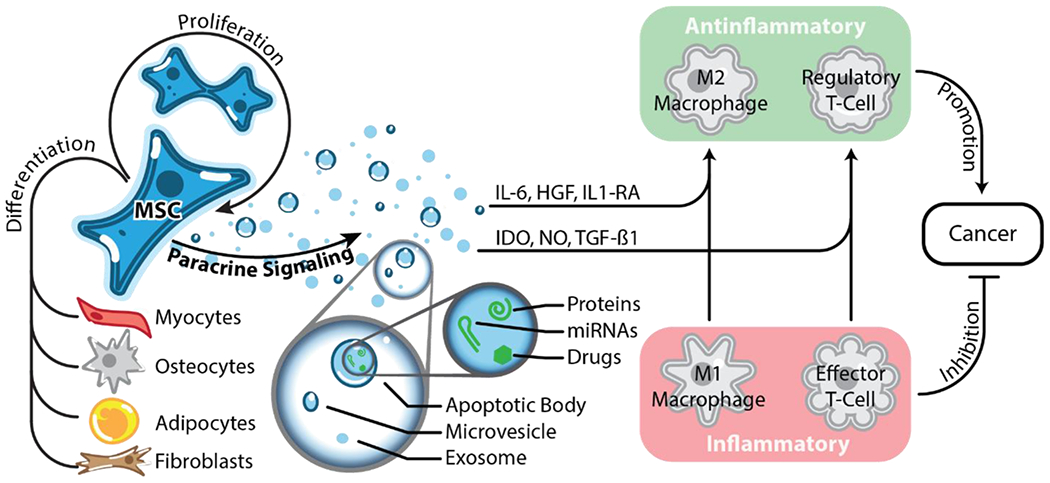
Mesenchymal stem cells (MSCs) are multipotent adult stem cells capable of self-renewal and differentiation into myocytes, osteocytes, adipocytes, chondrocytes, and fibroblasts. The paracrine function of MSCs them their primary therapeutic functions that can be leveraged for the delivery of therapeutics. Using paracrine cues, MSCs are able to modulate the immune system and generally favor anti-inflammatory phenotypes. This innate property of MSCs is beneficial during wound healing and treating cancer.
Mesenchymal Stem Cells as Delivery Systems
Mesenchymal stem cells (MSCs) are adult multipotent stem cells that can be found in the bone marrow and possess the ability to self-renew and to differentiate into more specialized tissues like bone, fat, and cartilage [34, 35]. MSCs are also known to promote tissue regeneration and wound healing via the secretion of paracrine factors (Figure 3) [36].
Paracrine function of MSCs
In addition to their tissue repair properties, MSCs show anti-apoptotic, anti-inflammatory, immunomodulatory, and pro-angiogenic properties that are mediated via paracrine signaling and make MSCs desirable delivery systems [37] (Figure 3). Since MSCs are inherently immunomodulatory, they can have both pro-inflammatory and anti-inflammatory effects, and most therapies using MSCs focus on their anti-inflammatory and immunomodulatory properties. For example, osteoarthritis (OA) is an inflammatory condition within joints that lacks effective treatments. Preclinical data indicate that injection of MSCs into the affected joints decreases pain and inflammation, which is believed to be the result of MSCs secreting anti-inflammatory cytokines including interleukin (IL)-6 and IL-10 [38]. Moreover, accumulating evidence indicates that MSCs also play an important immunomodulatory role in regulating the immune system by strongly influencing immune cell function and proliferation [39]. Studies suggest that MSCs can influence the function of macrophages in tissues to transition them from an inflammatory (M1) to an anti-inflammatory (M2) phenotype [40]. For example, in a myocardial infarction mouse model, the macrophages that infiltrated the cardiac tissue and were primarily in the M1 phenotype. However, when MSCs were injected into the injured myocardium, the macrophages adjacent to MSCs showed strong a preference for the M2 phenotype and the tissue had accelerated repair and cardiac remodeling properties [41]. Similarly, MSCs have been shown to secrete other immunomodulatory agents such as indolamine 2, 3-dioxygenase (IDO), nitric oxide (NO), programmed death ligand 1 (PD-L1), Galectin-1, and transforming growth factor β1 (TGF-β1), which convert inflammatory effector T-cells to anti-inflammatory regulatory T cells [38]. Importantly, the phenotype of these T cells have been implicated in playing an important role in cancer (Figure 3).
Engineering MSCs for targeting brain cancers
MSCs have become an attractive candidate for cell-based therapies to treat brain cancers (such as glioblastomas (GBMs)) as they can cross the blood brain barrier (BBB), naturally home to tumor cells, have paracrine functions, and low immunogenicity [42]. For these reasons, the use of MSCs to deliver therapeutic agents has been widely investigated for cancer treatments by genetically engineering them to secrete cytokines directly into the tumor upon arrival to decrease tumor growth [43]. In these studies, MSCs have been modified with tumor-targeting carriers of drugs with cytokines and proteins such as the anti-inflammatory cytokine interferon (IFN)-β [44]. TRAIL, and bone morphogenetic factor 4 (BMP4), a growth factor known to decrease the tumorigenicity of brain tumor initiating cells [45]. Altogether, these efforts have shown promising results for improved survival of patients with GBMs and other cancers [46].
Neutrophils as Delivery Systems
Neutrophils are the most abundant white blood cells in the human circulatory system, playing a critical role in the immune response against pathogens as well as recruitment to sites of injury [47, 48]. Neutrophils migrate toward sites of inflammation by following chemical gradients of interleukin-8 (IL-8), complement component a (C5a), leukotriene B4 (LTB4), platelet activating factor, as well as N-formyl peptides that are generated by invading bacteria [49]. Neutrophils have a number of characteristics that make them attractive delivery vehicles: they are highly motile; they can traverse biological barriers like the BBB where other cells or molecules cannot; they are highly biocompatible; they chemotax toward invading pathogens; and they target inflamed tissues for site-specific delivery [50]. As such, many groups have recently explored using neutrophils for the delivering of small molecules, as well as polymeric and metallic nanoparticle formulations [51]. However, the use of neutrophils as carriers of peptides, proteins, and nucleic acids remains largely uninvestigated, making this is an area ripe for exploration.
Neutrophils as drug carriers
A common challenge with many delivery approaches is the ability to penetrate the BBB. Neutrophils have the native ability to traverse the BBB and enter glioma and glioblastoma sites [52]. In a recent study, this property was exploited by loading neutrophils with liposomes filled with the chemotherapy drug, paclitaxel. It was demonstrated that these neutrophils loaded with liposomes filled with paclitaxel could carry the drug across the BBB and deliver it to gliomas [53]. Moreover, after tumor resection, neutrophil-mediated delivery of paclitaxel allowed for the recognition of postoperative inflammatory signals such as IL-8 and C-X-C motif chemokine ligand (CXCL1) that resulted in an overall slowing of recurrent tumor growth and improved survival. Additionally, neutrophils can be used to track the fate of drug-loaded neutrophils. For example, neutrophils loaded with DOX and magnetic mesoporous silica nanoparticles (ND-MMSNs) can be tracked to gliomas by magnetic resonance imaging when injected systemically into inflamed mouse models to validate proper delivery of the drug to the intended tumor site [54].
There are also several notable studies involving nanoparticle formulations that have utilized the cell surface of neutrophils to effectively “hitch-hike” to targeted sites in vivo [55]. During injury and disease, the vascular endothelium becomes inflamed and recruits neutrophils. This natural targeting of neutrophils to the injured endothelium has been exploited to deliver nanoparticles across blood vessel barriers, an otherwise significant challenge for nanoparticle delivery. To accomplish this, drug-loaded nanoparticles were internalized by the neutrophils and these neutrophils were shown to carry the nanoparticles across blood vessel barriers and enter into sites of inflammation or infected tissue for the deliver anti-inflammatory or antibacterial therapeutics [56]. Similarly, neutrophils have also been modified for the targeted therapeutic delivery to tumors. Albumin nanoparticles were loaded with pyropheophorbide-a (Ppa), a drug used for tumor therapy, and bound to activated neutrophils for the in vivo delivery to tumors. It was demonstrated that these Ppa-loaded nanoparticles attached to activated neutrophils, were delivered directly to tumor sites, improving outcomes by suppressing tumor growth and increasing the survival rates [57]. In a similar study, a poly(sialic acid) (PSA) biopolymer, which naturally binds to receptors expressed on neutrophils, was conjugated to an octadecylamine (ODA) compound, forming a PSA-ODA conjugate. This conjugate was anchored onto the surface of liposomes filled with pixantrone, a chemotherapy drug. These PSA-ODA-filled liposomes were then bound to neutrophil membranes to enhance the neutrophil-targeting of in vivo tumor mouse models [58]. This study showed considerably higher cellular uptake of the loaded neutrophils that led to tumor growth inhibition.
Another approach that takes advantage of neutrophil’s natural ability to home to infections and their ability to recognize and phagocytose bacteria, was to load neutrophils with deactivated nonpathogenic bacteria reagents coupled to antimicrobial agents [59]. In this study, deactivated bacteria were incubated in a solution with chlorhexidine (CHX), an antimicrobial that naturally attaches to the bacterial cell wall. Once CHX was attached to the bacterial cell wall, the bacteria were washed and incubated with neutrophils to be engulfed and therefore loading the neutrophils with antibacterial drugs. These loaded neutrophils were injected into a liver abscess mouse model, which demonstrated that the loaded neutrophils maintained their ability to home to sites of infection and released the antibacterial therapies upon reaching the site of infection.
Bacteria as Delivery Systems
Bacteria innately inhabit many different microbiomes within the human body, including the nose, mouth, breast, and gut. This human microbiota is important in maintaining immune and metabolic homeostasis over the course of a life time [60]. Because certain bacteria inhabit niches specific to certain areas of the body, and can preferentially target and survive in tumor microenvironments, bacterial-based therapeutic delivery systems are an attractive option for the delivery of drugs and other molecules [61]. Below we discuss approaches where bacterial systems have been used as therapeutic delivery vehicles.
Targeting cancer
Defective vascular architecture, elevated interstitial pressures, and increased permeation distances in the tumor microenvironment are significant barriers for delivering therapeutics into the tumor site [62]. In recent years, bacteria have been modified to deliver drug-containing nanoliposomes [63], nanobody antagonists [64], and functional nucleic acid molecules [65] to cells within tumor microenvironments. Similarly, it was demonstrated that the magnetotactic bacteria, Magnetococcus marinus strain MC-1, was able to deliver nanoliposomes loaded with the anti-tumor drug, 7-ethyl-10-hydroxycamptothecin (SN38), to hypoxic areas of tumors [63]. The drug-loaded liposomes were attached to the surface of MC-1 bacteria cells by chemical conjugation and the modified bacteria cells were injected near tumors in mice and guided to the center of the tumor using an external magnetic field (via magnetotaxis). The magnetic field was then turned off and the modified MC-1 cells preferentially migrated deep into the tumor, toward areas of lower oxygen concentration. Up to 55% of the injected MC-1 cells were able to enter into the hypoxic region of the tumor, demonstrating the ability of these bacterial cells to penetrate into solid organ tumors and regions lacking vascularization.
Bacteria have also been utilized to deliver functional nucleic acids into solid tumors in humans [65]. Recently a study found that the reporters green fluorescence protein (GFP), firefly luciferase, and secreted alkaline phosphatase (SEAP) could be successfully expressed in mouse tumor models by delivering them using nanoparticles attached to the surface of bacteria [65]. After phagocytosis by the tumor cells, these bacteria release the encapsulated DNA into the tumor cells and the genes were successfully expressed. These findings highlight the potential of using bacteria as a possible alternative for the non-viral delivery of genes to tumor cells that can possibly reprogram tumor cells for cell death.
Synthetic biology towards improved delivery devices
While using cells as drug delivery vehicles has greatly expanded the landscape of drug delivery to provide exciting approaches for targeting various diseased states, there are some applications where native cells do not exhibit the required phenotype to implement the desired therapeutic outcomes. These desired phenotypes can be engineered into cells by genetically modifying and reprogramming them with gene circuits [66]. Thus, the field of synthetic biology has the potential to improve current strategies for using cells to deliver therapeutic molecules in vivo. A common goal in synthetic biology is to create programmed therapeutic cells that function as living drugs for autonomously treating injury and disease [67, 68]. Over the last two decades, synthetic biologists have focused on building genetic tools to enabled the reprogramming of cells with new biological functions using genetic circuits that have endowed cells with biosensing capabilities, oscillatory behavior, logic gates, and switches [69–74] all of which have shown efficacy in many therapeutic applications. Combining the latest advances in cell delivery and targeting with tools in synthetic biology has the potential to create in vivo delivery systems that can autonomously sense and respond to diverse environments and deliver payloads at controlled levels in response to the local microenvironment dynamics and/or the degree of disease or injury. One such example is a recent study where E. coli were engineered with a genetic circuit that displays quorum sensing, the ability to regulate gene expression in response to the E. coli population density within the tumor [75]. Once the engineered bacteria reached high cell numbers, they underwent lysis to locally release immunotherapeutic agents that promoted tumor regression.
Furthermore, synthetic biology tools that can dynamically control gene expression in a spatiotemporal way [76, 77] can also be used to reprogram stem cells to efficiently drive stem cell differentiation for the production of large numbers of needed adult cells to be loaded with desired therapeutic agents. The spatial and temporal control of gene expression using genetic circuits during stem cell differentiation enables the creation of custom receptors on cell surfaces, and the loading of cells with therapeutic proteins at specific stages during cell fate [78]. Altogether, genetic circuits can reprogram stem cells for custom loading of therapeutic proteins, in addition to engineering custom receptors as designer triggers for the activation and release of payloads at desired locations [79–81].
Conclusions and Future Perspectives
Many biological cells typically have inherent targeting mechanisms, are biocompatible, have a longer circulation lifespan, and a natural ability to cross biological barriers, making them ideal for drug delivery vehicles. Here we reviewed the potential of using RBCs, platelets, neutrophils, MSCs and bacteria in delivering therapeutics. When deciding which cell type to use for targeted delivery, it is important to consider each cells’ innate biological function, the application for which the cells will be used, and the potential challenges with each cell type.
Red blood cells and platelets circulate throughout the body, making them ideal for delivering therapeutics systemically or at a targeted location that can be controlled via cell surface modifications and intracellular encapsulation. However, there is not currently a single “best” way to produce anucleate cells or their associated cellular mimetics, limiting the use of these cells for drug delivery to small-scale therapies (See Outstanding Questions). Until good manufacturing practice (GMP) grade scale-up of anucleate cells can be obtained, it will be difficult to realize the enormous potential of this technology in the clinic. MSCs have inherent paracrine and targeting functions that have made them ideal for therapeutic strategies. Augmenting their natural targeting functions, MSCs can be modified as carriers for delivering various therapeutics at specific locations. MSCs convey potential therapeutic responses in a variety of diseases, and yet they have not been shown to exhibit long-term engraftment [82]. Moreover, while MSCs have cancer inhibiting paracrine properties, it has been suggested that cancer cells can hijack MSCs to promote their own survival [83]. A better understanding of how cancer cells take advantage of MSCs is needed before widespread clinical use (See Outstanding Questions). One possible suggestion is that MSCs can used as delivery vehicles to target the desired site, release their payload before being cleared from the system, an approach that would not require long-term engraftment [84]. Neutrophils are the most abundant leukocyte in the body and one of the first responders to sites of injury, making them ideal for carrying therapeutics to areas of inflammation. However, because they are non-dividing cells they have poor survival after isolation, making it challenging to manipulate them for targeted therapies.
Outstanding Questions.
What manufacturing practices need to be done to scale-up the production of GMP grade anucleate cells?
How do cancer cells take advantage of MSCs in tumor microenvironments?
How can bacteria be engineered as delivery vehicles to not interfere with natural bacterial microbiomes already in the body?
Bacteria have become a popular vehicle for drug delivery because they can inhabit many different microbiomes throughout the body, and can preferentially target tumors. Nonetheless, caution should be taken when designing bacteria drug delivery vehicles because native bacteria possess an important symbiosis within their microbiomes and disturbances in this balance can cause a multitude of diseases [85] (see Outstanding Questions). Additionally, for lysis-driven systems when bacteria lyse to deliver their payload, the byproducts of bacterial cell lysate might build up and be absorbed into the bloodstream causing toxic systemic effects. Furthermore, drug delivery with living cells raises concerns related to immunogenicity and safety. The use of autologous cells minimizes this risk because minimal immunogenic responses are expected since the immune cells will recognize them as “self”. A future alternative approach to harvesting adult cells for manipulation is patient-specific induced pluripotent stem (iPS) cells. These cells can be made from individual patients and they have limitless expansion potential that can be differentiated into the desired cell types for targeted delivery.
Highlights.
Cells have innate targeting mechanisms that can improve drug delivery efficacy and decrease off-target effects
Multiple cell types, such as red blood cells (RBCs), platelets, neutrophils, mesenchymal stem cells (MSCs), and bacteria can be used as delivery vehicles
Cells can be engineered to have desired surface markers for improved tissue and cell targeting
Synthetic biology can be used to engineer cells to implement desired therapeutic outcomes when native cells do not exhibit the required phenotype
Acknowledgements
We thank the members of the Deans Laboratory for helpful discussions on the manuscript. We also thank the generous support from the following programs: the National Science Foundation CAREER Program [CBET-1554017], the Office of Naval Research Young Investigator Program [N00014-16-1-3012], the National Institute of Health Trailblazer Award [1R21EB025413-01], and the National Institutes of Health Director New Innovator Award [1DP2CA250006-01].
GLOSSARY
- Ataxia telangiectasia
a disease that causes enlarged blood vessels and uncoordinated movements
- Biomimetics
the synthesis of materials, synthetic systems, or machines that are inspired by nature
- Biotin
a water-soluble vitamin that can be used to for labeling target molecules
- Cystic fibrosis
a disease that affects the lungs and limits the ability to breathe over time.
- Glioblastoma
an aggressive type of cancer that occurs in the brain and/or spinal cord
- Hemophilia A
a bleeding disorder from missing the clotting factor VIII protein
- Hemostasis
a process to prevent and stop bleeding
- Induced pluripotent stem (iPS) cells
cells derived from adult cells and reprogrammed to become pluripotent stem cells
- Magnetotaxis
directional movement of a cell in response to a magnetic field
- Multiple myeloma
a cancer that forms in the bone marrow
- Myocardial infarction
a heart attack
- Nanoparticle
a microscopic particle that is commonly used to transport small molecules for therapeutic applications.
- Pluripotent stem cells
stem cells that have the ability to self-renew and to give rise to all of the cells of the tissues in the body.
- Prodrug
a biologically inactive compound that can be metabolized in the body to produce an active drug
- Progenitor cell
a multipotent cell that is programmed to become a specific type of adult cell.
- T cell exhaustion
T cells that have a reduced capacity to secrete cytokines and an increased expression of inhibitory receptors
- Thrombocytopenia
abnormally low platelet counts in the blood
- Thrombus
a blood clot in circulation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimer Statement
There are no conflicts to declare.
References:
- 1.Park K (2019) The beginning of the end of the nanomedicine hype. J Control Release 305, 221–222. [DOI] [PubMed] [Google Scholar]
- 2.Wilhelm S et al. (2016) Analysis of nanoparticle delivery to tumors. Nature Reviews Materials 1. [Google Scholar]
- 3.Park K (2013) Facing the truth about nanotechnology in drug delivery. ACS Nano 7 (9), 7442–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fliervoet LAL and Mastrobattista E (2016) Drug delivery with living cells. Adv Drug Deliv Rev 106 (Pt A), 63–72. [DOI] [PubMed] [Google Scholar]
- 5.Agrahari V et al. (2017) Next generation drug delivery: circulatory cells-mediated nanotherapeutic approaches. Expert Opin Drug Deliv 14 (3), 285–289. [DOI] [PubMed] [Google Scholar]
- 6.Sun D et al. (2019) Advances in refunctionalization of erythrocyte-based nanomedicine for enhancing cancer-targeted drug delivery. Theranostics 9 (23), 6885–6900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coker SA et al. (2018) A Study of the Pharmacokinetic Properties and the In Vivo Kinetics of Erythrocytes Loaded With Dexamethasone Sodium Phosphate in Healthy Volunteers. Transfus Med Rev 32 (2), 102–110. [DOI] [PubMed] [Google Scholar]
- 8.Biagiotti S et al. (2011) Drug delivery by red blood cells. IUBMB Life 63 (8), 621–31. [DOI] [PubMed] [Google Scholar]
- 9.Santiago T and da Silva JA (2014) Safety of low- to medium-dose glucocorticoid treatment in rheumatoid arthritis: myths and reality over the years. Ann N Y Acad Sci 1318, 41–9. [DOI] [PubMed] [Google Scholar]
- 10.Hammel P et al. (2020) Erythrocyte-encapsulated asparaginase (eryaspase) combined with chemotherapy in second-line treatment of advanced pancreatic cancer: An open-label, randomized Phase 11 b trial. Eur J Cancer 124, 91–101. [DOI] [PubMed] [Google Scholar]
- 11.Hunault-Berger M et al. (2015) A Phase 2 study of L-asparaginase encapsulated in erythrocytes in elderly patients with Philadelphia chromosome negative acute lymphoblastic leukemia: The GRASPALL/GRAALL-SA2-2008 study. Am J Hematol 90 (9), 811–8. [DOI] [PubMed] [Google Scholar]
- 12.Du Y and Chen B (2019) Combination of drugs and carriers in drug delivery technology and its development. Drug Des Devel Ther 13, 1401–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shi J et al. (2014) Engineered red blood cells as carriers for systemic delivery of a wide array of functional probes. Proc Natl Acad Sci U S A 111 (28), 10131–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pishesha N et al. (2017) Engineered erythrocytes covalently linked to antigenic peptides can protect against autoimmune disease. Proc Natl Acad Sci U S A 114 (12), 3157–3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu CM et al. (2013) ‘Marker-of-self’ functionalization of nanoscale particles through a top-down cellular membrane coating approach. Nanoscale 5 (7), 2664–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu CM et al. (2011) Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc Natl Acad Sci U S A 108 (27), 10980–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ren X et al. (2016) Red blood cell membrane camouflaged magnetic nanoclusters for imaging-guided photothermal therapy. Biomaterials 92, 13–24. [DOI] [PubMed] [Google Scholar]
- 18.Escajadillo T et al. (2017) A Red Blood Cell Membrane-Camouflaged Nanoparticle Counteracts Streptolysin O-Mediated Virulence Phenotypes of Invasive Group A Streptococcus. Front Pharmacol 8, 477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luk BT and Zhang L (2015) Cell membrane-camouflaged nanoparticles for drug delivery. J Control Release 220 (Pt B), 600–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doshi N et al. (2009) Red blood cell-mimicking synthetic biomaterial particles. Proc Natl Acad Sci U S A 106 (51), 21495–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Merkel TJ et al. (2011) Using mechanobiological mimicry of red blood cells to extend circulation times of hydrogel microparticles. Proc Natl Acad Sci U S A 108 (2), 586–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun J et al. (2009) Oxygen carrier based on hemoglobin/poly(L-lysine)-block-poly(L-phenylalanine) vesicles. Langmuir 25 (24), 13726–9. [DOI] [PubMed] [Google Scholar]
- 23.Guo J et al. (2020) Biomimetic Rebuilding of Multifunctional Red Blood Cells: Modular Design Using Functional Components. ACS Nano 14 (7), 7847–7859. [DOI] [PubMed] [Google Scholar]
- 24.Shi Y et al. (2011) Mechanisms and management of doxorubicin cardiotoxicity. Herz 36 (4), 296–305. [DOI] [PubMed] [Google Scholar]
- 25.Xu P et al. (2017) Doxorubicin-loaded platelets as a smart drug delivery system: An improved therapy for lymphoma. Sci Rep 7, 42632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lyde RB et al. (2019) Infused factor VIII-expressing platelets or megakaryocytes as a novel therapeutic strategy for hemophilia A. Blood Adv 3 (9), 1368–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mammadova-Bach E et al. (2020) Platelet glycoprotein VI promotes metastasis through interaction with cancer cell-derived galectin-3. Blood 135 (14), 1146–1160. [DOI] [PubMed] [Google Scholar]
- 28.Li J et al. (2016) Genetic engineering of platelets to neutralize circulating tumor cells. J Control Release 228, 38–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang X et al. (2018) Engineering PD-1-Presenting Platelets for Cancer Immunotherapy. Nano Lett 18 (9), 5716–5725. [DOI] [PubMed] [Google Scholar]
- 30.Wei X et al. (2016) Nanoparticles camouflaged in platelet membrane coating as an antibody decoy for the treatment of immune thrombocytopenia. Biomaterials 111, 116–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu Q et al. (2016) Engineered Nanoplatelets for Enhanced Treatment of Multiple Myeloma and Thrombus. Adv Mater 28 (43), 9573–9580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nandi S and Brown AC (2016) Platelet-mimetic strategies for modulating the wound environment and inflammatory responses. Exp Biol Med (Maywood) 241 (10), 1138–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Doshi N et al. (2012) Platelet mimetic particles for targeting thrombi in flowing blood. Adv Mater 24 (28), 3864–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chamberlain G et al. (2007) Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells 25 (11), 2739–49. [DOI] [PubMed] [Google Scholar]
- 35.Deans TL and Elisseeff JH (2009) Stem cells in musculoskeletal engineered tissue. Curr Opin Biotechnol 20 (5), 537–44. [DOI] [PubMed] [Google Scholar]
- 36.Berglund AK et al. (2017) Immunoprivileged no more: measuring the immunogenicity of allogeneic adult mesenchymal stem cells. Stem Cell Res Ther 8 (1), 288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baraniak PR and McDevitt TC (2010) Stem cell paracrine actions and tissue regeneration. Regen Med 5 (1), 121–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou Y et al. (2019) The Immunomodulatory Functions of Mesenchymal Stromal/Stem Cells Mediated via Paracrine Activity. J Clin Med 8 (7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fan XL et al. (2020) Mechanisms underlying the protective effects of mesenchymal stem cell-based therapy. Cell Mol Life Sci 77 (14), 2771–2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cho DI et al. (2014) Mesenchymal stem cells reciprocally regulate the M1/M2 balance in mouse bone marrow-derived macrophages. Exp Mol Med 46, e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jin L et al. (2019) Mesenchymal stem cells promote type 2 macrophage polarization to ameliorate the myocardial injury caused by diabetic cardiomyopathy. J Transl Med 17 (1), 251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mangraviti A et al. (2016) Non-virally engineered human adipose mesenchymal stem cells produce BMP4, target brain tumors, and extend survival. Biomaterials 100, 53–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reagan MR and Kaplan DL (2011) Concise review: Mesenchymal stem cell tumor homing: detection methods in disease model systems. Stem Cells 29 (6), 920–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Altaner C et al. (2014) Complete regression of glioblastoma by mesenchymal stem cells mediated prodrug gene therapy simulating clinical therapeutic scenario. Int J Cancer 134 (6), 1458–65. [DOI] [PubMed] [Google Scholar]
- 45.Li Q et al. (2014) Mesenchymal stem cells from human fat engineered to secrete BMP4 are nononcogenic, suppress brain cancer, and prolong survival. Clin Cancer Res 20 (9), 2375–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang Q et al. (2018) Current status and potential challenges of mesenchymal stem cell-based therapy for malignant gliomas. Stem Cell Res Ther 9 (1), 228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mayadas TN et al. (2014) The multifaceted functions of neutrophils. Annu Rev Pathol 9, 181–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mocsai A (2013) Diverse novel functions of neutrophils in immunity, inflammation, and beyond. J Exp Med 210 (7), 1283–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Harvath L (1991) Neutrophil chemotactic factors. EXS 59, 35–52. [DOI] [PubMed] [Google Scholar]
- 50.Chu D et al. (2018) Neutrophil-Based Drug Delivery Systems. Adv Mater 30 (22), e1706245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lin MH et al. (2018) The Interplay Between Nanoparticles and Neutrophils. J Biomed Nanotechnol 14 (1), 66–85. [DOI] [PubMed] [Google Scholar]
- 52.Fossati G et al. (1999) Neutrophil infiltration into human gliomas. Acta Neuropathol 98 (4), 349–54. [DOI] [PubMed] [Google Scholar]
- 53.Xue J et al. (2017) Neutrophil-mediated anticancer drug delivery for suppression of postoperative malignant glioma recurrence. Nat Nanotechnol 12 (7), 692–700. [DOI] [PubMed] [Google Scholar]
- 54.Wu M et al. (2018) MR imaging tracking of inflammation-activatable engineered neutrophils for targeted therapy of surgically treated glioma. Nat Commun 9 (1), 4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang C et al. (2017) Direct Macromolecular Drug Delivery to Cerebral Ischemia Area using Neutrophil-Mediated Nanoparticles. Theranostics 7 (13), 3260–3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chu D et al. (2015) Neutrophil-Mediated Delivery of Therapeutic Nanoparticles across Blood Vessel Barrier for Treatment of Inflammation and Infection. ACS Nano 9 (12), 11800–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chu D et al. (2017) Photosensitization Priming of Tumor Microenvironments Improves Delivery of Nanotherapeutics via Neutrophil Infiltration. Adv Mater 29 (27). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Luo X et al. (2018) Neutrophil-mediated delivery of pixantrone-loaded liposomes decorated with poly(sialic acid)-octadecylamine conjugate for lung cancer treatment. Drug Deliv 25 (1), 1200–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wendel SO et al. (2015) Cell Based Drug Delivery: Micrococcus luteus Loaded Neutrophils as Chlorhexidine Delivery Vehicles in a Mouse Model of Liver Abscesses in Cattle. PLoS One 10 (5), e0128144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thursby E and Juge N (2017) Introduction to the human gut microbiota. Biochem J 474 (11), 1823–1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nejman D et al. (2020) The human tumor microbiome is composed of tumor type-specific intracellular bacteria. Science 368 (6494), 973–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Maeda H et al. (2000) Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Release 65 (1-2), 271–84. [DOI] [PubMed] [Google Scholar]
- 63.Felfoul O et al. (2016) Magneto-aerotactic bacteria deliver drug-containing nanoliposomes to tumour hypoxic regions. Nat Nanotechnol 11 (11), 941–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gurbatri CR et al. (2020) Engineered probiotics for local tumor delivery of checkpoint blockade nanobodies. Sci Transl Med 12 (530). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Akin D et al. (2007) Bacteria-mediated delivery of nanoparticles and cargo into cells. Nat Nanotechnol 2 (7), 441–9. [DOI] [PubMed] [Google Scholar]
- 66.Xie M and Fussenegger M (2018) Designing cell function: assembly of synthetic gene circuits for cell biology applications. Nat Rev Mol Cell Biol 19 (8), 507–525. [DOI] [PubMed] [Google Scholar]
- 67.Krawczyk K et al. (2020) Rewiring of endogenous signaling pathways to genomic targets for therapeutic cell reprogramming. Nat Commun 11 (1), 608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tolle F et al. (2019) Genetic circuitry for personalized human cell therapy. Curr Opin Biotechnol 59, 31–38. [DOI] [PubMed] [Google Scholar]
- 69.Callura JM et al. (2012) Genetic switchboard for synthetic biology applications. Proc Natl Acad Sci U S A 109 (15), 5850–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schwarz KA et al. (2017) Rewiring human cellular input-output using modular extracellular sensors. Nat Chem Biol 13 (2), 202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Deans TL (2014) Parallel Networks: Synthetic Biology and Artificial Intelligence. ACM Journal on Emerging Technologies in computing systems (JETC) 11 (3), 21:1–21:11. [Google Scholar]
- 72.Khalil AS and Collins JJ (2010) Synthetic biology: applications come of age. Nat Rev Genet 11 (5), 367–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.MacDonald IC and Deans TL (2016) Tools and applications in synthetic biology. Adv Drug Deliv Rev 105 (Pt A), 20–34. [DOI] [PubMed] [Google Scholar]
- 74.Scheller L and Fussenegger M (2019) From synthetic biology to human therapy: engineered mammalian cells. Curr Opin Biotechnol 58, 108–116. [DOI] [PubMed] [Google Scholar]
- 75.Chowdhury S et al. (2019) Programmable bacteria induce durable tumor regression and systemic antitumor immunity. Nat Med 25 (7), 1057–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Deans TL et al. (2007) A tunable genetic switch based on RNAi and repressor proteins for regulating gene expression in mammalian cells. Cell 130 (2), 363–72. [DOI] [PubMed] [Google Scholar]
- 77.Fitzgerald M et al. (2017) Adoption of the Q Transcriptional System for Regulating Gene Expression in Stem Cells. ACS Synth Biol 6 (11), 2014–2020. [DOI] [PubMed] [Google Scholar]
- 78.Guye P et al. (2016) Genetically engineering self-organization of human pluripotent stem cells into a liver bud-like tissue using Gata6. Nat Commun 7, 10243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vogel AM et al. (2019) Synthetic biology for improving cell fate decisions and tissue engineering outcomes. Emerging Topics in Life Sciences. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Healy CP and Deans TL (2019) Genetic circuits to engineer tissues with alternative functions. J Biol Eng 13, 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bush LM et al. (2019) Synthetic biology: paving the way with novel drug delivery. The Biochemist 41 (3), 24. [Google Scholar]
- 82.Parekkadan B and Milwid JM (2010) Mesenchymal stem cells as therapeutics. Annu Rev Biomed Eng 12, 87–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee HY and Hong IS (2017) Double-edged sword of mesenchymal stem cells: Cancer-promoting versus therapeutic potential. Cancer Sci 108 (10), 1939–1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kean TJ et al. (2013) MSCs: Delivery Routes and Engraftment, Cell-Targeting Strategies, and Immune Modulation. Stem Cells Int 2013, 732742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Claesen J and Fischbach MA (2015) Synthetic microbes as drug delivery systems. ACS Synth Biol 4 (4), 358–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wei Q and Frenette PS (2018) Niches for Hematopoietic Stem Cells and Their Progeny. Immunity 48 (4), 632–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liang R et al. (2015) A Systems Approach Identifies Essential FOXO3 Functions at Key Steps of Terminal Erythropoiesis. PLoS Genet 11 (10), e1005526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Eckly A et al. (2014) Biogenesis of the demarcation membrane system (DMS) in megakaryocytes. Blood 123 (6), 921–30. [DOI] [PubMed] [Google Scholar]
- 89.Ito Y et al. (2018) Turbulence Activates Platelet Biogenesis to Enable Clinical Scale Ex Vivo Production. Cell 174 (3), 636–648 e18. [DOI] [PubMed] [Google Scholar]
- 90.Moreau T et al. (2016) Large-scale production of megakaryocytes from human pluripotent stem cells by chemically defined forward programming. Nat Commun 7, 11208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Trakarnsanga K et al. (2017) An immortalized adult human erythroid line facilitates sustainable and scalable generation of functional red cells. Nat Commun 8, 14750. [DOI] [PMC free article] [PubMed] [Google Scholar]


