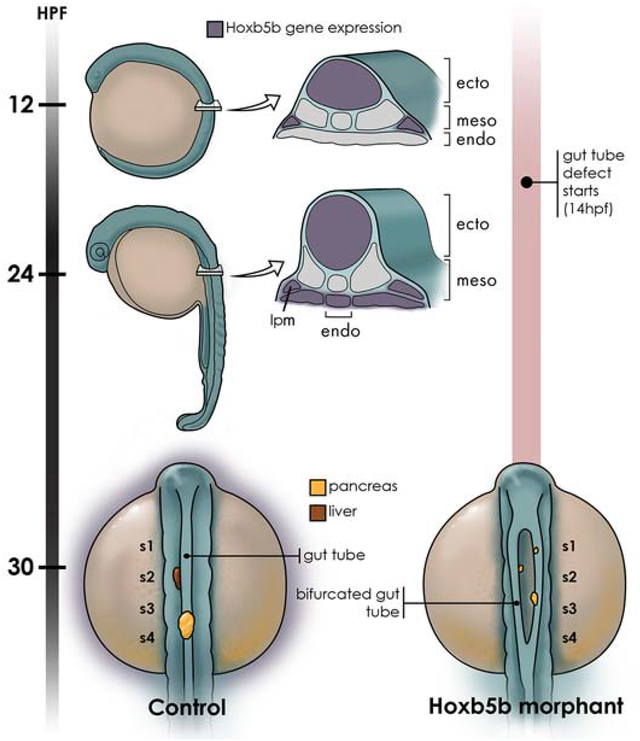
Abstract
During vertebrate embryonic development complex morphogenetic events drive the formation of internal organs associated with the developing digestive tract. The foregut organs derive from hepatopancreatic precursor cells that originate bilaterally within the endoderm monolayer, and subsequently converge toward the midline where they coalesce to produce the gut tube from which the liver and pancreas form. The progenitor cells of these internal organs are influenced by the lateral plate mesoderm (LPM), which helps direct them towards their specific fates. However, it is not completely understood how the bilateral organ precursors move toward the embryonic midline and ultimately coalesce to form functional organs. Here we demonstrate that the zebrafish homeobox gene hoxb5b regulates morphogenesis of the foregut endoderm at the midline. At early segmentation stages, hoxb5b is expressed in the LPM adjacent to the developing foregut endoderm. By 24 hpf hoxb5b is expressed directly in the endoderm cells of the developing gut tube. When Hoxb5b function is disrupted, either by morpholino knockdown or sgRNA/Cas9 somatic disruption, the process of foregut morphogenesis is disrupted, resulting in a bifurcated foregut. By contrast, knockdown of the paralogous hoxb5a gene does not alter gut morphology. Further analysis has indicated that Hoxb5b knockdown specimens produce endocrine pancreas cell types, but liver cells are absent. Finally, cell transplantation experiments revealed that Hoxb5b function in the endoderm is not needed for proper coalescence of the foregut at the midline. Together, our findings imply that midline morphogenesis of foregut endoderm is guided by a hoxb5b-mediated mechanism that functions extrinsically, likely within the LPM. Loss of hoxb5b function prevents normal coalescence of endoderm cells at the midline and thus disrupts gut morphogenesis.
Keywords: endoderm, foregut, hoxb5, pancreas, liver, CRISPR/Cas9
Graphical Abstract
Introduction
Establishment of the body plan depends upon interactions between germ layers during embryogenesis. For example, development of endoderm-derived internal organs requires signals from neighboring tissues such as the lateral plate mesoderm (LPM) (Grapin-Botton, 2005). At the onset of zebrafish gastrulation the endoderm cells are the first to involute at the blastoderm margin, rapidly becoming dispersed over the yolk via a ‘random walk’ (Pézeron et al., 2008) to form a sparse monolayer. Beginning at mid-gastrulation, endoderm cells migrate in a more directed manner, converging towards the dorsal midline of the developing embryo where they form an endodermal sheet along the anteroposterior axis (Ober et al., 2003; Pézeron et al., 2008; Warga and Nüsslein-Volhard, 1999). As gastrulation concludes and somitogenesis proceeds, the endoderm cells continue to converge towards the midline, coalescing into a gut rod at the midline by 20 hours post fertilization (hpf) (Ober et al., 2003). The gut rod, which is surrounded by mesoderm, then cavitates to produce the gut tube, which is regionalized along its anteroposterior (AP) axis into foregut, midgut and hindgut. The foregut gives rise to multiple internal organs, including liver and pancreas, whereas the midgut and hindgut produce the small and large intestine, respectively. Precisely how the nascent foregut endoderm is patterned to produce different organs, and the role of mesodermal cues in this process, remain incompletely understood.
Both extrinsic and intrinsic signals play key roles in zebrafish endoderm development. Early in development the coordinated gastrulation movements of endoderm and mesoderm cells are under the control of Sdf1/Cxcr4 chemokine signaling, with absence of either ligand or receptor resulting in bifurcation of the foregut endoderm (Mizoguchi et al., 2008; Nair and Schilling, 2008). Gastrulation movements in turn function to bring both endoderm and mesoderm into the range of influence of secreted signaling molecules, including Bmps, Fgfs and Wnts (der Hardt et al., 2007; Solnica-Krezel, 2006; Warga and Kimmel, 1990). During segmentation stages (~14 hpf), cell fate choices of the foregut endoderm—at the level of the first three somites— are influenced by the medio-lateral positions of the cells. The more lateral endoderm cells, closest to LPM-derived BMP signals, produce liver, with more medial cells producing pancreatic fates; BMP signals further influence exocrine versus endocrine pancreas fates (Chung et al., 2008). At subsequent pharyngula stages (24–30 hpf) asymmetric migration of the zebrafish LPM, and extracellular matrix remodeling, regulate the looping of the gut tube (Horne-Badovinac, 2003; Yin et al., 2010). Intrinsic signals are equally critical to the establishment of endodermal cell fates. For example, in zebrafish the transcription factors Hhex, Sox2 and FoxA2 demarcate regional identity of the foregut endoderm, and the Cdx Parahox factors play a key role in positioning the foregut-hindgut boundary (Kinkel et al., 2008). Importantly, the evolutionarily conserved Hox transcription factors also play roles in endoderm regionalization (Grapin-Botton and Melton, 2000).
Hox genes are arrayed in clusters within the genome, and while mammals have four clusters comprising 39 Hox genes, the zebrafish has 7 clusters comprising 48 Hox genes as the consequence of an ancestral whole-genome duplication event in the lineage leading to teleost fishes (Hurley et al., 2007). The Hox genes are further arranged in 13 paralog groups, with those Hox genes localized most 3’ in the genome having the most anterior expression domains along the primary body axis. Importantly, the Hox genes are expressed in nested “colinear” domains along the anteroposterior axis and play roles in regionalizing all three germ layers. Here, we specifically address the function of the zebrafish paralog group 5 gene hoxb5b in endoderm regionalization.
We demonstrate that hoxb5b knockdown causes defects in foregut morphogenesis. While hoxb5b is expressed in the endoderm by one day of development, it is also expressed in the LPM at early segmentation stages. Using cell transplantation to generate chimeric embryos, we demonstrate that hoxb5b function is not required in the endoderm for proper midline morphogenesis of the foregut, revealing a non-autonomous role, likely in the adjacent LPM. Interestingly, lack of hoxb5b function abrogates the differentiation of liver cells, but not endocrine pancreas cells. Our study highlights the relevance of transcription factor function in tissues that non-autonomously influence the development of adjacent endoderm.
Results
Zebrafish hoxb5b is expressed in all three germ layers during early development
Expression of zebrafish hoxb5b has been reported in the neural tube, notochord and somites (Bruce et al., 2001), as well as in the LPM (Waxman et al., 2008). However, expression in the endoderm has not been described in detail. To explore a potential role for hoxb5b in the development and patterning of the foregut endoderm, we analyzed hoxb5b expression in the developing zebrafish embryo using whole mount in situ hybridization at 12 and 24 hpf. At 12 hpf (Fig. 1A), when the endoderm is still a converging monolayer of cells overlying the yolk, we detected robust expression of hoxb5b in the neural tube and noted expression in the lateral plate mesoderm (LPM) at the level of somites 2 and 3, adjacent to the developing foregut (yellow arrowhead), consistent with previous reports. We next analyzed transverse sections taken from this same mid-trunk region and confirmed hoxb5b expression in neural tube and LPM (yellow arrowheads; Fig. 1A’). At this stage, we could not reliably detect hoxb5b expression in the endoderm (ventral to the somites and above the yolk), although very low-level expression, at or near the limits of detection, was occasionally detected in a small number of endoderm cells (black arrowheads; Fig. 1A’). At 24 hpf (Fig. 1B, B’), when the gut rod has formed, expression of hoxb5b was sustained in the neural tube and LPM (yellow arrowheads), and we additionally detected robust hoxb5b expression in the endoderm (bracket) and adjacent mesenchyme (yellow arrows). The most lateral expression (asterisks) is likely localized to the fin buds. Having detected expression in the foregut endoderm, as well as in earlier LPM lying adjacent to the endoderm, we hypothesized that hoxb5b might play a role in endoderm patterning.
Fig. 1. hoxb5b is expressed in multiple tissues.
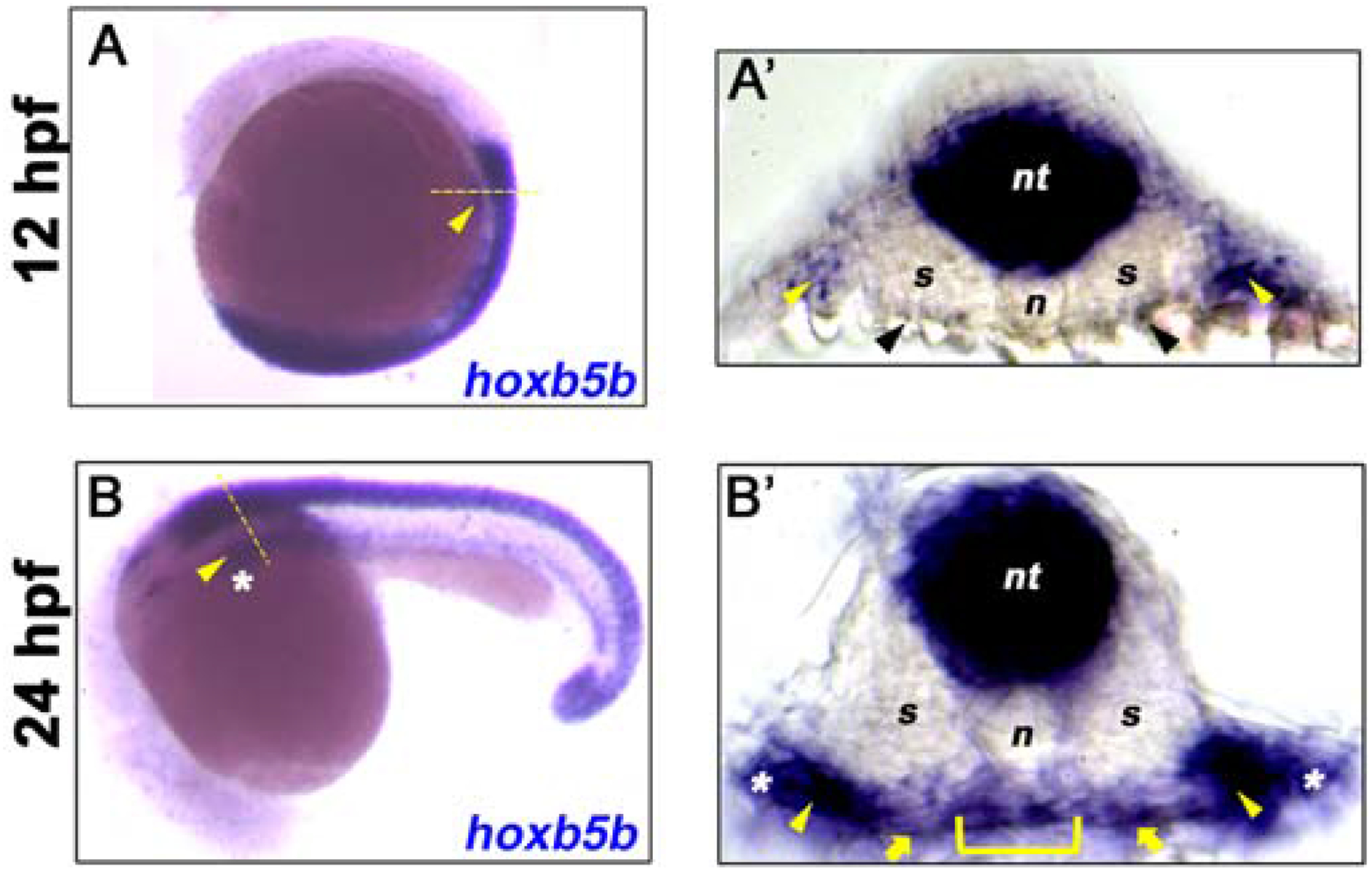
(A-B) in situ hybridization for hoxb5b at 12 hpf and 24 hpf shows lateral plate mesoderm (LPM, yellow arrowhead) expression of hoxb5b transcripts (blue). (A’) Transverse section taken from the mid-trunk region of 12 hpf embryo in A (dashed yellow line) shows hoxb5b expression in neural tube (nt) and LPM regions (yellow arrowheads), low level sparse endoderm expression at or close to the limit of detection is indicated (black arrowheads). (B’) Transverse section taken from the mid-trunk region of 24 hpf embryo in B (dashed yellow line) shows hoxb5b expression in neural tube (nt), foregut endoderm (yellow bracket), adjacent mesenchyme (yellow arrows), and LPM (yellow arrowheads). The most lateral expression (white asterisks) is likely within the developing fin buds. Anterior to top (A) and left (B).
Knockdown of Hoxb5b function causes bifurcated gut tubes
To investigate the function of hoxb5b in the development of foregut endoderm we used a morpholino (MO) knockdown approach. We used a translation blocking Hoxb5b MO (as previously described, Waxman et al., 2008) and compared to results with a standard control MO. Injection of Hoxb5b MO did not cause any significant gross morphological defects (data not shown). To analyze foregut endoderm development, we first examined the expression of the pancreatic and anterior intestinal primordium marker pancreatic and duodenal homeobox 1 (pdx1) by whole mount in situ hybridization. In 30 hpf control specimens, pdx1 was expressed in endoderm located at the midline, consistent with previous reports (Argenton et al., 1999). By contrast, specimens injected with Hoxb5b MO (Hoxb5b morphants) showed bilateral pdx1 expression (n=39, 86.6%) (Fig. 2A, B). This altered expression suggests that Hoxb5b morphants have a deficit in the morphogenesis processes that bring the pancreatic and anterior intestinal primordia to the midline. To investigate other regions of the gut epithelium we examined the expression of endoderm marker foxa3 and pancreatic beta-cell marker insulin by whole mount double in situ hybridization at 30 hpf. Similar to pdx1 expression, the foxa3 and insulin expression domains were bifurcated in Hoxb5b morphants (n=59, 77.6%), rather that showing midline expression as in controls (Fig. 2C, D). Injection of a second—splice blocking—MO (Waxman et al. 2008) caused similar defects, although at higher MO concentrations (Material and Methods, data not shown). We also examined whether the duplicate zebrafish hoxb5 gene, hoxb5a, might play a similar function in endoderm morphogenesis. We found that injection of Hoxb5a MO (as described; Waxman et al., 2008) did not cause bifurcation of the gut epithelium (n=48, 100%) (Fig. 2E). In summary, the zebrafish hoxb5b gene, but not the duplicate hoxb5a gene, is required for normal development of a single pancreatic organ at the midline.
Fig. 2. Hoxb5b morphants have bifurcated foregut endoderm.
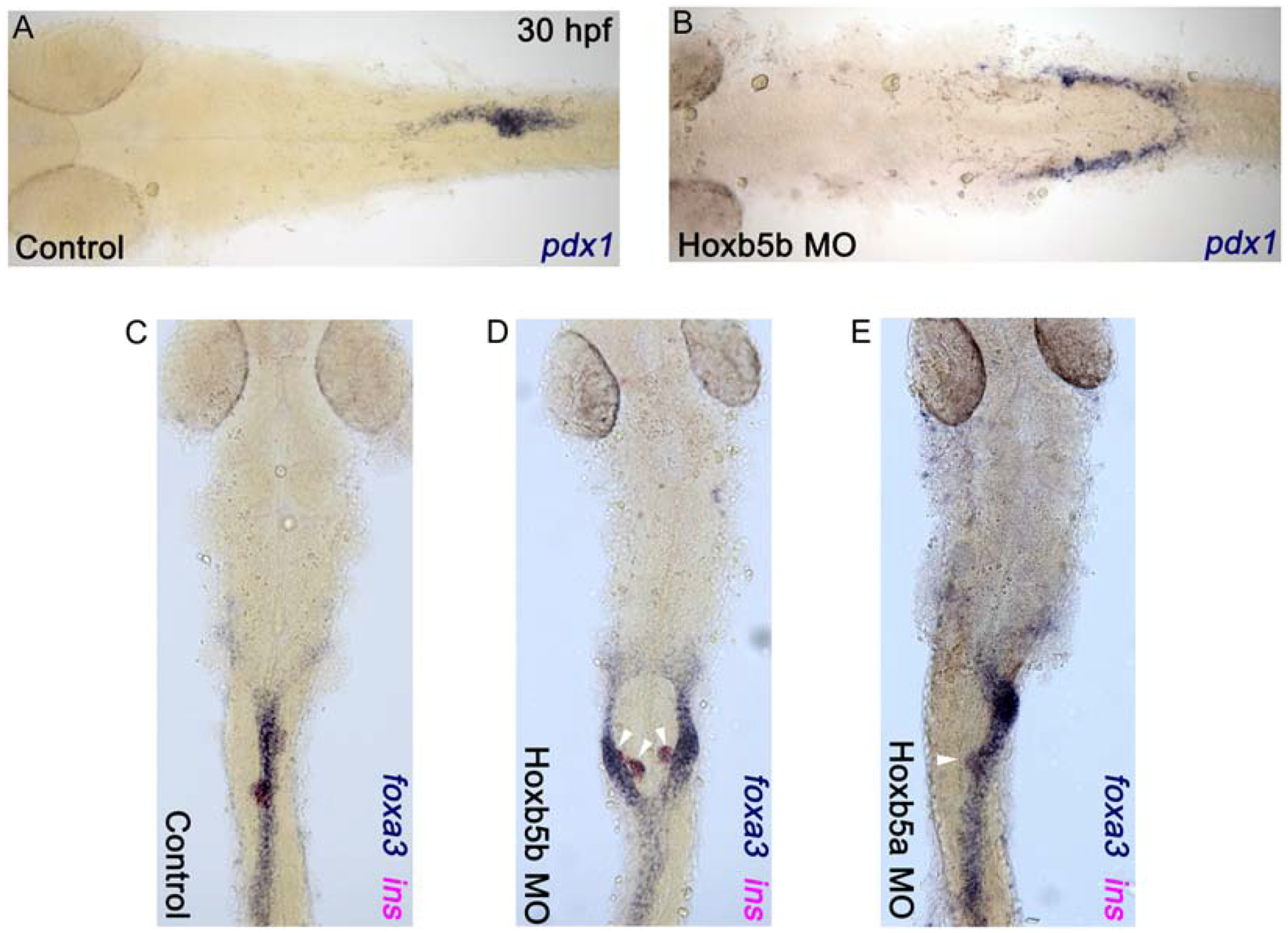
(A and C) Control embryos; (B and D) Hoxb5b morphants; (E) Hoxb5a morphant. (A and B) in situ hybridization for pdx1 and (C-E) double in situ hybridization for foxa3 (blue) and insulin (magenta) for control and morphant embryos at 30 hpf. Anterior to the left (A and B) and to the top (C-E), results are from 3 independent experiments with a minimum of 45 embryos for hoxb5b morphants, and from 2 independent experiments with a minimum of 20 embryos for hoxb5a morphants.
Pancreatic endocrine, but not liver, cells develop in Hoxb5b-morphant embryos
Our data show that although foregut endoderm is bifurcated, pancreatic beta cells nevertheless develop in Hoxb5b morphants. We observed expression of both the pancreatic and anterior intestinal primordium marker pdx1 and the beta-cell marker insulin, suggesting that pancreatic endocrine cells are specified bilaterally in the bifurcated gut of Hoxb5b-morphant embryos. To explore this possibility further, we used the Tg(sox17:EGFP) line, in which GFP labels all endoderm cells (Mizoguchi et al., 2008). We found that in 30 hpf Hoxb5b morphants, the endoderm is bifurcated at anteroposterior (AP) levels extending from the posterior pharynx to anterior of the intestine (n=23, 76.6%) (compare Figs 3A & D). We used anti-Islet1 antibody to label the endocrine pancreas and defined its AP location by using anti-Myosin antibody to mark the somites. In controls, Islet1-positive endocrine cells lie within the endoderm adjacent to somites 3 and 4 (Fig. 3A). By contrast, in Hoxb5b morphants, bifurcated Islet1-positive endocrine cells are dispersed adjacent to somites 1–4 (Fig. 3D). These data confirm that endocrine cells are specified in Hoxb5b morphants, and consistent with this result we also found bilateral expression of the alpha-cell marker Glucagon (gcga) and the delta-cell marker Somatostatin (sst1) in Hoxb5b morphants (Figs 3B & E and data not shown).
Fig. 3. Hoxb5b morphants form endocrine pancreas cell types but fail to form liver cells.

(A-C) Control embryos; (D-F) Hoxb5b morphants. (A and D) Confocal images of representative 30 hpf Tg(sox17:EGFP) embryos. Whole mount immunolabeling for GFP (green), Islet1 (red) and Myosin to label somites (blue) of control and Hoxb5b morphant embryos. (B and E) in situ hybridization for glucagon (gcga, blue); (C and F) double in situ hybridization for ceruloplasmin (cp, blue) and insulin (ins, magenta) for control and Hoxb5b morphant embryos at 30 hpf. (C’ and F’) high magnification views of foregut region. Anterior to the left, pancreatic cells (white arrowhead) and liver cells (black arrowhead). Results are from 2 independent experiments with a minimum of 25 embryos per group. Scale bar = 100 μm.
We next examined whether another nearby endodermal organ, the liver, is affected by knockdown of Hoxb5b. The expression of liver marker ceruloplasmin (cp) and beta-cell marker insulin were compared in controls and Hoxb5b morphants by whole mount double in situ hybridization at 30 hpf. In control specimens, cp expressing cells lie anterior to insulin expressing cells (Fig. 3C, C’). By contrast, Hoxb5b morphants fail to express cp, while insulin transcripts are found in scattered bilateral cells (Fig. 3F, F’). We conclude that hoxb5b function is required for the differentiation of liver cells.
Disruption of the endogenous hoxb5b locus by sgRNA/Cas9 phenocopies foregut defects found in Hoxb5b morphants
To confirm the role of Hoxb5b in migration of foregut endoderm we used CRISPR genome editing to disrupt the endogenous hoxb5b locus. We selected two different hoxb5b genomic target sites (Fig. 4A) and tested sgRNA efficiencies using a T7 endonuclease assay (T7EI). Individual hoxb5b sgRNA/Cas9 injected embryos were randomly selected for the T7 endonuclease I (T7EI) assay, which revealed that both sgRNAs are able to induce insertion-deletion (indel) mutations in hoxb5b (Fig. 4B). Using Tg(sox17:EGFP) embryos, we confirmed that hoxb5b sgRNA1 or 2/Cas9 injections also produce bifurcated foregut phenotypes (n=19, 38% and n=12, 25% for sgRNA1/Cas9 and sgRNA2/Cas9 injected specimens, respectively) (Fig. 4C–E). We noted that the penetrance of the bifurcated foregut phenotype in the sgRNA/Cas9-injected specimens was significantly lower than in the Hoxb5b morphants, likely because hoxb5b sgRNA/Cas9-generated transient mutants have incomplete penetrance in the F0 generation. Nonetheless, these results confirmed that Hoxb5b function is necessary for normal foregut endoderm morphogenesis.
Fig. 4. Hoxb5b sgRNA/Cas9 mediated somatic mutagenesis phenocopies the bifurcated foregut defects found in Hoxb5b morphants.
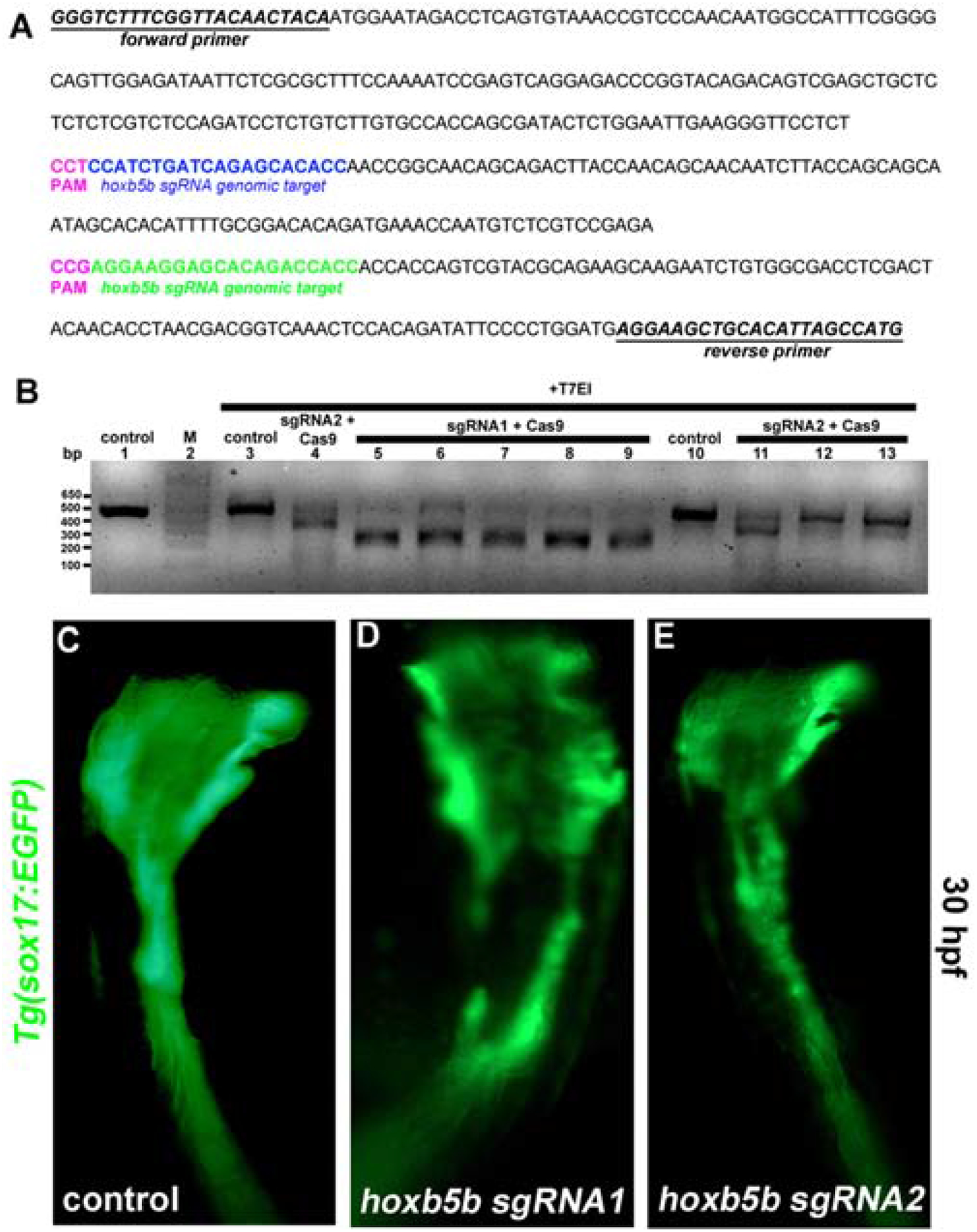
(A) Forward and reverse primer sequences are shown (underlined). Hoxb5b sgRNA1 (blue) and sgRNA2 (green) genomic target sequences are shown. Protospacer adjacent motif-PAM sequence (pink). (B) T7 endonuclease I (T7EI) assay. (Lane 2) marker. (Lane 1) untreated and (lane 3,10) T7EI treated amplicons from control embryos. (Lane 5–9) amplicons from embryos injected with hoxb5b sgRNA1/Cas9 or (Lane 4,11–13) hoxb5b sgRNA2/Cas9 were digested by T7EI. Live images of (C) Control, and (D, E) sgRNA1/Cas9 and sgRNA2/Cas9 injected Tg(sox17:EGFP) at 30 hpf. Somatic disruption of the Hoxb5b locus by two separate sgRNAs phenocopies foregut defects in Hoxb5b morphants. Anterior to the top, results are from 2 independent experiments.
Gut tube convergence defects begin early in somitogenesis
Our analysis of fixed specimens established that Hoxb5b morphants have bifurcated foregut endoderm at 30 hpf. To investigate the developmental stage at which these defects initiated we used the Tg(sox17:EGFP) line to observe the location and movements of all endoderm cells. We found that localization and behavior of GFP-labeled endoderm cells was indistinguishable between control and Hoxb5b morphants throughout gastrulation stages, with differences first becoming detectable at 14 hpf, during segmentation stages. As shown in Fig. 5, we documented these differences through the capture of image series of developing Tg(sox17:EGFP) embryos. In control specimens, 14 hpf GFP-labeled endoderm cells formed a bilateral sheet of cells over the yolk and beneath the somites, which converged towards the midline and coalesced to produce a gut rod by 24 hpf (Fig. 5A–D). By contrast, at 14 hpf endoderm cells of Hoxb5b morphants already began to show morphogenesis defects anterior to the level of somite 4, with small cell-free patches visible at the midline (asterisk, Fig. 5E). One hour later, these cell-free patches had become more obvious (Fig. 5F). While Hoxb5b-morphant endoderm morphogenesis proceeded as normal in anterior and posterior locations, the foregut endoderm cells failed to move towards and coalesce at the midline, but nevertheless aggregated into two bilateral rods to form a bifurcated foregut by 24 hpf (Fig. 5F–H). We conclude that foregut endoderm morphogenesis defects caused by knockdown of Hoxb5b have initiated by the 14 hpf stage.
Fig. 5. Midline defects in the foregut endoderm first become apparent at 14 hpf in Hoxb5b morphants.
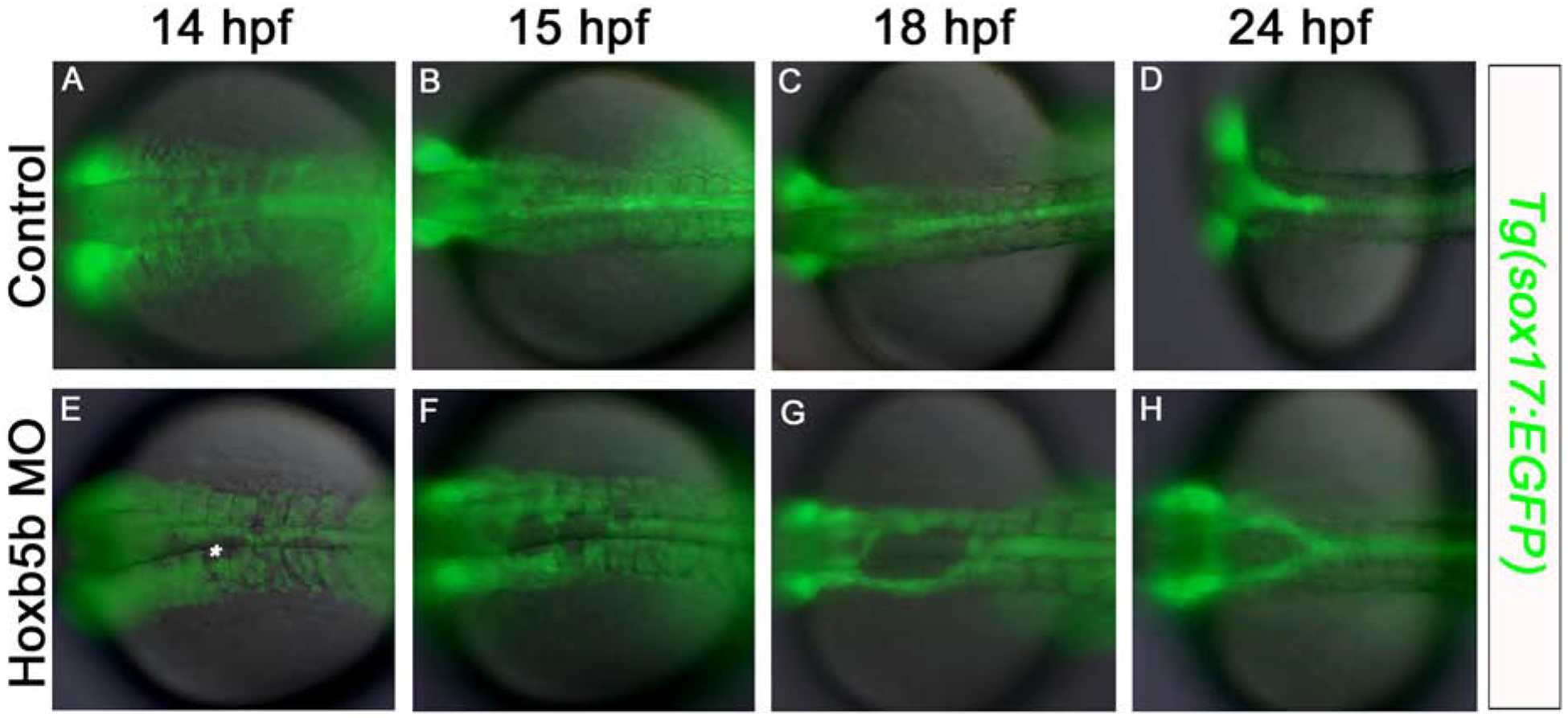
Fluorescent imaging of live Tg(sox17:EGFP) embryos (A-D) control, and (E-H) Hoxb5b morphant, at (A,E) 14 hpf; (B,F) 15 hpf; (C,G) 18 hpf; (D,H) 24 hpf. At 14 hpf endoderm cell-free patches are apparent at the midline in Hoxb5b-deficent specimens (asterisk). Anterior to the left, results are from 2 independent experiments and minimum of 8 embryos per group.
Hoxb5b function is not required in the endoderm for midline coalescence of foregut cells
Our expression analysis detected robust hoxb5b expression in LPM, but not endoderm, at 12 hpf (Fig. 1A, A’), shortly before we first observed deficits in morphogenesis of the foregut endoderm (Fig. 5A & E). Together, these findings suggest that hoxb5b may function within the LPM, rather than the endoderm, to promote midline coalescence of foregut endoderm cells. To test this hypothesis we used a cell transplantation approach to generate chimeric embryos in which the endoderm germ layer derives from a donor embryo, but the other germ layers derive from a host. This was achieved by using sox32 mRNA injection to promote endoderm fates in donor embryos and Sox32 morpholino injection to block endoderm fates in host embryos (Stafford et al., 2006). As previously established, the Sox32 MO injected Tg(sox17:EGFP) specimens were completely devoid of endoderm (n=30, Fig. 6A). We again utilized the Tg(sox17:EGFP) line to follow the fate of GFP-positive endoderm cells (schematized in Fig. 6). Control chimeric embryos, in which all germ layers expressed hoxb5b, were generated by injecting sox32 mRNA into Tg(sox17:EGFP) donor embryos, to convert mesendoderm to an endoderm fate, and transplanting donor cells into Sox32 morphant hosts at blastula stage. In successful transplants (n=5, 62.5%) the endoderm of 30 hpf host embryos was fully reconstituted with donor cells and showed normal morphology (Fig. 6B). Using a similar approach we analyzed the fate of Hoxb5b morphant Tg(sox17:EGFP) donor cells in an otherwise wild-type host environment. In chimeric embryos, Hoxb5b-morphant donor cells reconstituted normal gut morphology and did not produce a bifurcated gut tube (n=4, 57%; Fig. 6C). The ability of Hoxb5b-morphant endoderm cells to produce a normal gut tube supports our hypothesis that hoxb5b function is not necessary in the endoderm, but rather in the adjacent LPM, to allow proper midline coalescence of foregut endoderm.
Fig. 6. Hoxb5b function in the endoderm is not necessary for midline coalescence of the foregut endoderm.
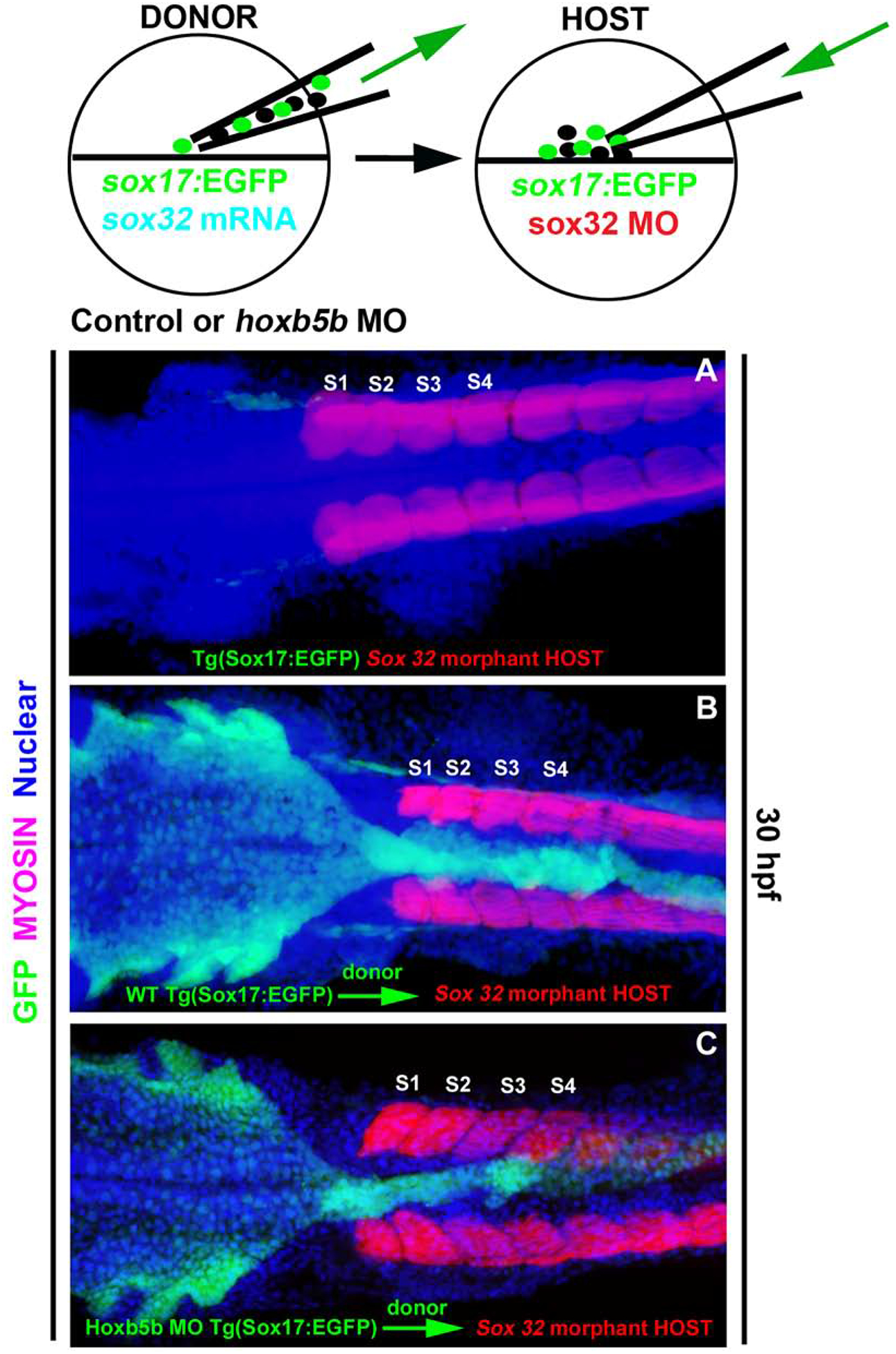
Cell transplantation approach: Tg(sox17:EGFP) embryos were used to follow the fate of endodermal cells in chimeric embryos. (A) Confocal image of a Sox32 morphant embryo; these lack endoderm and were used as hosts. (B) Tg(sox17:EGFP) donor embryos were injected with sox32 mRNA, or (C) together with Hoxb5b morpholino. Donor cells were transplanted into endoderm-deficient Sox32 morphant hosts. Whole mount immunolabeling for GFP (green), Myosin to label somites (magenta) and nuclear marker (blue). Both wild-type (WT) (B) and Hoxb5b morphant (C) donor cells reconstitute a normal gut in Sox32 morphant hosts (A). Anterior to the left, results are from 2 independent experiments with a minimum of 4 embryos per group.
Discussion
Hox genes play crucial roles in organizing the body plan of all animals. In this study we have taken advantage of the zebrafish model to show that the Hox gene hoxb5b regulates midline morphogenesis of the foregut endoderm. Embryos with abrogated Hoxb5b function showed defects in the morphogenesis of endoderm cells at the midline by 14 hpf, with a bifurcated gut tube established by 24 hpf. Marker analysis showed that pancreatic endocrine cells differentiated in the dysmorphic foregut whereas liver cells were unable to form. Further, using a cell transplantation approach, we demonstrated that Hoxb5b function is not needed in the endoderm for normal foregut coalescence to occur. Our data are consistent with a model in which Hoxb5b-mediated signaling from the LPM—a tissue which lies in close proximity to the endoderm—is necessary for midline morphogenesis of the foregut endoderm.
To date, multiple signaling pathways have been implicated in the regulation of zebrafish endoderm migration and in the later establishment of hepatopancreatic fates, and below we consider how these pathways may interact with Hoxb5b function. Sdf1/Cxcr4 signaling controls the migration of endodermal cells during gastrulation, and, interestingly, anterior foregut endoderm is bifurcated in Sdf1/Cxcr4-knockdown specimens (Mizoguchi et al., 2008; Nair and Schilling, 2008), showing some similarities with the phenotype we describe in this study. One important difference from the Hoxb5b knockdown phenotype is that specimens lacking normal Sdf1/Cxcr4 function begin to show deficits in endoderm cell movements earlier in development, during gastrulation stages (Mizoguchi et al., 2008; Nair and Schilling, 2008). The temporal differences between the phenotypes are consistent with our understanding of the timing of hoxb5b expression. Waxman et al. (2008) reported LPM expression of hoxb5b at 10 hpf, at the end of gastrulation, and we were unable to reliably detect expression before this stage. Nevertheless, similar to Hoxb5b morphants, Sdf1/Cxcr4 morphants specimens lack later liver derivatives, as indicated by absence of ceruloplasmin expression (Mizoguchi et al., 2008). As Sdf1 is expressed in the mesoderm overlying the Cxcr4a-expressing endodermal cells, it is possible that Sdf1/Cxcr4 could have a downstream influence on Hoxb5b-regulated signaling from the LPM.
Wnt signaling initiates a variety of morphogenetic behaviors that lead to specification and positioning of organs (Petersen and Reddien, 2009). More specifically, the noncanonical (nc) Wnt signaling pathway is critical for convergence and extension movements during gastrulation (Roszko et al., 2009). Matsui et al. showed that the nc-Wnt/PCP pathway is required for the midline convergence of foregut endoderm cells (Matsui, 2005). In contrast, canonical Wnt signaling (Wnt2 and Wnt2bb) controls the later specification of hepatopancreas cell differentiation towards the liver fate (Lancman et al., 2013; Ober et al., 2006; Poulain and Ober, 2011). By 24 hpf Wnt2 and Wnt2bb are expressed in the LPM region surrounding the liver primordium (Lancman et al., 2013; Ober et al., 2006) and genetic manipulation of Wnt2/Wnt2bb suggests Wnt signaling in the LPM controls hepatoblast differentiation and proliferation, but not pancreas formation. We suggest that Hoxb5b is likely acting downstream of nc-Wnt action—as we did not observe gastrulation defects in Tg(sox17:EGFP) Hoxb5b morphant specimens—but potentially upstream of canonical Wnt signaling in the LPM. Consistent with such a model, Hox5 paralog genes in mouse have been shown to regulate Wnt2/2b expression in the distal lung mesenchyme (Hrycaj et al., 2015).
Liver and pancreas cells originate from a common progenitor pool (Chung et al., 2008; Deutsch et al., 2001; Rossi, 2001), yet, in Hoxb5b-morphant bifurcated endoderm the liver cells fail to differentiate while pancreatic cell differentiation remains unaffected. There are several potential mechanisms that could explain the observed loss of liver fates: (1) the mislocalized endoderm cells may be too distant from a necessary signal to respond; (2) the signal itself may be missing; or (3) the endoderm cells may lack molecular competence to receive and interpret a required signal. While we cannot completely rule out any of these possibilities, previously published work provides some insights. Specifically, Vegfc (Vascular endothelial growth factor C) signaling is required for early formation of the most dorsal endoderm and also for coalescence of the anterior endoderm at the midline (Ober et al., 2004). Similar to our findings with Hoxb5b morphants, Vegfc morphants show bifurcation of the anterior gut tube and duplication of the pancreatic buds. However, in contrast to Hoxb5b morphants, Vegfc morphants also have duplicated liver buds, which express normal levels of ceruloplasmin. These findings demonstrate that liver cells are able to differentiate even when the relative positions of endoderm and mesoderm are significantly disrupted. We therefore suggest that the lack of liver fates in Hoxb5b-deficent specimens is unlikely to reflect solely a physical separation of the relevant tissues. Rather, we favor models based on loss of a signal or an inability to respond to that signal. These two options would in turn reflect either an extrinsic change—for example in the mesoderm from which signals derive—or an intrinsic change in the responding endoderm. As discussed above, the canonical Wnt signaling pathway might represent a fruitful avenue for future experimentation. Whether the disruption is extrinsic or intrinsic, Hoxb5b-morphant presumptive liver cells could in principle switch fate to become pancreatic. Formally evaluating the fate of liver progenitors would require single cell lineage tracing. Although in situ hybridization analysis does not allow precise assessment of the number of pancreatic cells, our Hoxb5b morphants do not show the obvious increase in cell numbers that might be expected to accompany a switch in fate. However, it is important to note that our analyses were performed at developmental stages when only dorsal pancreatic bud derivatives are present, whereas previous single cell lineage studies (Chung et al. 2018) have established that it is the later-developing ventral pancreatic bud derivatives that share common progenitors with the liver. Thus, future studies would also need to extend over more protracted stages of development.
During gastrulation, Bone morphogenetic proteins (Bmps) regulate AP patterning of zebrafish endoderm (Tiso et al., 2002). Later, during segmentation stages, Bmp2b is expressed in LPM, and functional analyses have shown that high levels of Bmp2b induce endodermal cells to become liver (Chung et al., 2008). However, misregulation of Bmp signaling did not disrupt endoderm morphogenesis in these studies, suggesting that multiple factors are necessary to (1) pattern foregut endoderm cells along the AP axis, and (2) direct the morphogenetic movements of hepatopancreatic progenitors to allow proper liver and pancreas development. While Hox genes are well known to influence AP pattern, here we have established a non-autonomous role for Hoxb5b in the second—but potentially related—step of foregut endoderm morphogenesis. Consistent with the model of posterior prevalence (Duboule and Morata, 1994), which explains how Hox genes tend to function close to their anterior limit of expression, the endoderm defect in Hoxb5b morphants is highly localized, affecting only the foregut. Our transplantation experiments demonstrated that endoderm expression of Hoxb5b is not necessary for proper foregut morphogenesis to occur, indicating a non-autonomous role. As the adjacent LPM shows high levels of Hoxb5b expression, this tissue is the most likely source of a relevant secreted signal. Interestingly, this is not the first non-autonomous role described for Hoxb5b: Waxman et al. (2008) showed that Hoxb5b expression in the forelimb field functions to limit the number of atrial progenitors in the adjacent heart field, structures that form at the same AP level as the foregut.
Despite the major defects in foregut morphogenesis of Hoxb5b-deficent embryos, both the more anterior pharyngeal endoderm and the more posterior gut endoderm undergo normal morphogenesis. Whether expression domains of other Hox genes are altered in response to disruption of Hoxb5b function, and how this might potentially alter an endodermal “Hox code”, remains a topic for future study. Similarly, potential roles for Hox genes expressed at other AP levels in the morphogenesis of the more anterior or posterior endoderm remain to be evaluated.
Material and Methods
Zebrafish husbandry
Zebrafish (Danio rerio) were maintained as described (Westerfield, 1995). Embryos were obtained from wild-type AB and Tg (−5.0sox17:EGFP)zf99 [hereafter Tg(sox17:EGFP)] (Mizoguchi et al., 2008) lines, raised and staged as described (Kimmel et al., 1995).
Microinjections of morpholino antisense oligonucleotides
Morpholino (MO) antisense oligonucleotides for Hoxb5a MO: 5’-AGCTCATTTAGGTTGTTTTAGAGGG, Hoxb5b MO: 5’-GATCTTGGTCGTTAAAATCCAGCG and Hoxb5b splice blocking MO: 5’ -AGATGTTTATACCATGGCTAATGTG (Waxman et al., 2008) were purchased from Gene Tools, LLC. Sox32 MOs were used as described (Dalgin et al., 2011). Wild-type AB and Tg(sox17:EGFP) embryos were microinjected at the one to two-cell stage with 1 nl of 2–3 μg/μl Hoxb5b or Hoxb5a MO and 1 nl of 10 μg/μl of Hoxb5b splice blocking MO.
Whole mount in situ hybridization, immunohistochemistry and imaging
Whole mount in situ hybridization, immunohistochemistry and vibratome sections were performed as described and antisense riboprobes synthesized for the following transcripts; ceruloplasmin (cp), foxa3, glucagon (glu;gcga), hoxb5b, insulin (ins;insa), pdx1, somatostatin (sst1) (Dalgin et al., 2011; Dalgın and Prince, 2015). The following antibodies were used: rabbit anti-GFP488 (1:500; Molecular Probes A21311), monoclonal mouse anti-Islet1 [1:50; Developmental Studies Hybridoma Bank (DSHB), clone 39.4D5], and monoclonal mouse anti-Myosin (1:100; DSHB, clone A4.1025). To obtain bright-field images embryos were deyolked, flat-mounted, and photographed under a Zeiss Axioskop microscope. To obtain fluorescent images embryos were flat-mounted and imaged using a Zeiss LSM 710 confocal microscope. Live imaging of transgenic embryos was performed on a Zeiss Axioskop fluorescent microscope.
Isolation of genomic DNA and T7 endonuclease I assay
Experiments were performed as described (Dalgin and Prince, 2015). Briefly, genomic DNA was isolated from dechorionated single embryos and used to PCR amplify a 457 bp genomic region flanking the hoxb5b target site. The PCR amplicon was denatured and reannealed to facilitate heteroduplex formation, and reannealed amplicons were digested with T7 endonuclease I (NEB M0302S). Reaction products were resolved by electrophoresis through a 2% agarose gel.
Microinjections of sgRNA and Cas9
The sgRNA design and microinjections of sgRNA/Cas9 were performed as described (Dalgin et al., 2015). Briefly, the CHOPCHOP website (Montague et al., 2014) was used to select genomic target sites on the hoxb5b locus. The gBlock (Integrated DNA Technologies) containing an optimized sgRNA scaffold (Chen et al., 2013) was used as a template for transcribing sgRNA (Dalgin and Prince, 2015). The sgRNA and capped-polyadenylated Cas9 mRNA (Addgene 51307, Guo et al., 2014) were transcribed using Ambion MEGAscript SP6 kit (AM 1330) and RNA was isolated according to manufacturer’s instruction with modifications (Dalgin and Prince, 2015).
Tg(sox17:EGFP) embryos were microinjected at the one-cell stage with 60 pg sgRNA and 500 pg Cas9 mRNA per embryo.
Cell transplantation
Transplantation was performed as previously described (Ho and Kane, 1990; Stafford et al., 2006). The Tg(sox17:EGFP) host embryos were injected with Sox32 MO at the one-cell stage to block endoderm development. The Tg(sox17:EGFP) donor embryos were injected at the one-cell stage with synthetic capped sox32 mRNA (Ambion MEGAscript kit), or together with Hoxb5b MO. At 4 hpf, ~40 cells from a donor embryo were transplanted into the blastoderm margin of each host embryo. Transplanted embryos with a reconstituted endoderm of normal morphology (derived exclusively from the donor) were assayed by immunohistochemistry at 30 hpf.
Highlights.
Morpholino knockdown of zebrafish Hoxb5b causes foregut bifurcation
At segmentation stages, foregut endoderm fails to coalesce in Hoxb5b morphants
Liver cells are absent but endocrine pancreas cells form in Hoxb5b morphants
The role of Hoxb5b in foregut morphogenesis is non-autonomous
sgRNA/Cas9 mediated disruption of hoxb5b phenocopies Hoxb5b morphants
Acknowledgements
We thank members of the V.E.P. lab, for helpful advice, discussion and expert fish care, and we are grateful to Alex Frederking, M.S. for assistance with the graphical abstract. This work benefitted from the resources freely available at Zfin.org.
Funding
This work was supported by the National Institutes of Health [grant DK064973 to V.E.P]; a Juvenile Diabetes Research Foundation (JDRF) fellowship and a University of Chicago Diabetes Research Center P&F [grant P30 DK020595] to G.D.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests
The authors declare no competing financial interests.
References
- Argenton F, Zecchin E, Bortolussi M, 1999. Early appearance of pancreatic hormone-expressing cells in the zebrafish embryo. Mechanisms of Development 87, 217–221. [DOI] [PubMed] [Google Scholar]
- Bruce AE, Oates AC, Prince VE, Ho RK, 2001. Additional hox clusters in the zebrafish: divergent expression patterns belie equivalent activities of duplicate hoxB5 genes. Evolution & Development 3, 127–144. [DOI] [PubMed] [Google Scholar]
- Chen B, Gilbert LA, Cimini BA, Schnitzbauer J, Zhang W, Li G-W, Park J, Blackburn EH, Weissman JS, Qi LS, Huang B, 2013. Dynamic Imaging of Genomic Lociin Living Human Cells by an Optimized CRISPR/Cas System. Cell 155, 1479–1491. doi: 10.1016/j.cell.2013.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung W-S, Shin CH, Stainier DYR, 2008. Bmp2 Signaling Regulates the Hepatic versus Pancreatic Fate Decision. Developmental Cell 15, 738–748. doi: 10.1016/j.devcel.2008.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalgin G, Ward AB, Hao LT, Beattie CE, Nechiporuk A, Prince VE, 2011. Zebrafish mnx1 controls cell fate choice in the developing endocrine pancreas. Development 138, 4597–4608. doi: 10.1242/dev.067736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalgın G, Prince VE, 2015. Differential levels of Neurod establish zebrafish endocrine pancreas cell fates. Dev Biol. doi: 10.1016/j.ydbio.2015.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- der Hardt, von S, Bakkers J, Inbal A, Carvalho L, Solnica-Krezel L, Heisenberg C-P, Hammerschmidt M, 2007. The Bmp gradient of the zebrafish gastrula guides migrating lateral cells by regulating cell-cell adhesion. Current Biology 17, 475–87. doi: 10.1016/j.cub.2007.02.013 [DOI] [PubMed] [Google Scholar]
- Deutsch G, Jung J, Zheng M, Lóra J, Zaret KS, 2001. A bipotential precursor population for pancreas and liver within the embryonic endoderm. Development 128, 871–881. [DOI] [PubMed] [Google Scholar]
- Duboule D, Morata G, 1994. Colinearity and functional hierarchy among genes of the homeotic complexes. Trends Genet 10, 358–364. [DOI] [PubMed] [Google Scholar]
- Grapin-Botton A, 2005. Antero-posterior patterning of the vertebrate digestive tract: 40 years after Nicole Le Douarin’s PhD thesis. Int J Dev Biol 49, 335–347. doi: 10.1387/ijdb.041946ag [DOI] [PubMed] [Google Scholar]
- Grapin-Botton A, Melton DA, 2000. Endoderm development: from patterning to organogenesis. Trends Genet 16, 124–130. doi: 10.1016/s0168-9525(99)01957-5 [DOI] [PubMed] [Google Scholar]
- Guo X, Zhang T, Hu Z, Zhang Y, Shi Z, Wang Q, Cui Y, Wang F, Zhao H, Chen Y, 2014. Efficient RNA/Cas9-mediated genome editing in Xenopus tropicalis. Development 141, 707–714. doi: 10.1242/dev.099853 [DOI] [PubMed] [Google Scholar]
- Ho RK, Kane DA, 1990. Cell-autonomous action of zebrafish spt-1 mutation in specific mesodermal precursors. Nature 348, 728–730. doi: 10.1038/348728a0 [DOI] [PubMed] [Google Scholar]
- Horne-Badovinac S, 2003. A Cellular Framework for Gut-Looping Morphogenesis in Zebrafish. Science (New York, NY) 302, 662–665. doi: 10.1126/science.1085397 [DOI] [PubMed] [Google Scholar]
- Hrycaj SM, Dye BR, Baker NC, Larsen BM, Burke AC, Spence JR, Wellik DM, 2015. Hox5 Genes Regulate the Wnt2/2b-Bmp4-Signaling Axis during Lung Development. CellReports 1–25. doi: 10.1016/j.celrep.2015.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley IA, Scemama AJ-L, Prince VE, 2007. Consequences of Hoxb1 dublication in teleost fish. Evolution & Development 9, 540–554. [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF, 1995. Stages of embryonic development of the zebrafish. Dev Dyn 203, 253–310. doi: 10.1002/aja.1002030302 [DOI] [PubMed] [Google Scholar]
- Kinkel MD, Eames SC, Alonzo MR, Prince VE, 2008. Cdx4 is required in the endoderm to localize the pancreas and limit beta-cell number. Development 135, 919–929. doi: 10.1242/dev.010660 [DOI] [PubMed] [Google Scholar]
- Lancman JJ, Zvenigorodsky N, Gates KP, Zhang D, Solomon K, Humphrey RK, Kuo T, Setiawan L, Verkade H, Chi Y-I, Jhala US, Wright CVE, Stainier DYR, Dong PDS, 2013. Specification of hepatopancreas progenitors in zebrafish by hnf1ba and wnt2bb. Development 140, 2669–2679. doi: 10.1242/dev.090993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui T, 2005. Noncanonical Wnt signaling regulates midline convergence of organ primordia during zebrafish development. Genes Dev 19, 164–175. doi: 10.1101/gad.1253605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizoguchi T, Verkade H, Heath J, Kuroiwa A, Kikuchi Y, 2008. Sdf1/Cxcr4 signaling controls the dorsal migration of endodermal cells during zebrafish gastrulation. Development 2521–2529. doi: 10.1242/dev.020107 [DOI] [PubMed] [Google Scholar]
- Montague TG, Cruz JM, Gagnon JA, Church GM, Valen E, 2014. CHOPCHOP: a CRISPR/Cas9 and TALEN web tool for genome editing. Nucleic Acids Res 42, W401–7. doi: 10.1093/nar/gku410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair S, Schilling TF, 2008. Chemokine Signaling Controls Endodermal Migration During Zebrafish Gastrulation. Science (New York, NY) 322, 89–92. doi: 10.1126/science.1160038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ober EA, Field HA, Stainier DYR, 2003. From endoderm formation to liver and pancreas development in zebrafish. Mechanisms of Development 120, 5–18. [DOI] [PubMed] [Google Scholar]
- Ober EA, Olofsson B, Mäkinen T, Jin S-W, Shoji W, Koh GY, Alitalo K, Stainier DYR, 2004. Vegfc is required for vascular development and endoderm morphogenesis in zebrafish. EMBO Rep 5, 78–84. doi: 10.1038/sj.embor.7400047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ober EA, Verkade H, Field HA, Stainier DYR, 2006. Mesodermal Wnt2b signalling positively regulates liver specification. Nature 442, 688–691. doi: 10.1038/nature04888 [DOI] [PubMed] [Google Scholar]
- Petersen CP, Reddien PW, 2009. Wnt Signaling and the Polarity of the Primary Body Axis. Cell 139, 1056–1068. doi: 10.1016/j.cell.2009.11.035 [DOI] [PubMed] [Google Scholar]
- Pézeron G, Mourrain P, Courty S, Ghislain J, Becker TS, Rosa FM, David NB, 2008. Live analysis of endodermal layer formation identifies random walk as a novel gastrulation movement. Current Biology 18, 276–281. doi: 10.1016/j.cub.2008.01.028 [DOI] [PubMed] [Google Scholar]
- Poulain M, Ober EA, 2011. Interplay between Wnt2 and Wnt2bb controls multiple steps of early foregut-derived organ development. Development 138, 3557–3568. doi: 10.1242/dev.055921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi JM, 2001. Distinct mesodermal signals, including BMPs from the septum transversum mesenchyme, are required in combination for hepatogenesis from the endoderm. Genes Dev 15, 1998–2009. doi: 10.1101/gad.904601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roszko I, Sawada A, Solnica-Krezel L, 2009. Regulation of convergence and extension movements during vertebrate gastrulation by the Wnt/PCP pathway. Semin. Cell Dev. Biol 20, 986–997. doi: 10.1016/j.semcdb.2009.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solnica-Krezel L, 2006. Gastrulation in zebrafish — all just about adhesion? Current Opinion in Genetics & Development 16, 433–441. doi: 10.1016/j.gde.2006.06.009 [DOI] [PubMed] [Google Scholar]
- Stafford D, White RJ, Kinkel MD, Linville A, Schilling TF, Prince VE, 2006. Retinoids signal directly to zebrafish endoderm to specify insulin-expressing beta-cells. Development 133, 949–956. doi: 10.1242/dev.02263 [DOI] [PubMed] [Google Scholar]
- Tiso N, Filippi A, Pauls S, Bortolussi M, Argenton F, 2002. BMP signalling regulates anteroposterior endoderm patterning in zebrafish. Mechanisms of Development 118, 29–37. [DOI] [PubMed] [Google Scholar]
- Warga RM, Kimmel CB, 1990. Cell movements during epiboly and gastrulation in zebrafish. Development 108, 569–580. [DOI] [PubMed] [Google Scholar]
- Warga RM, Nüsslein-Volhard C, 1999. Origin and development of the zebrafish endoderm. Development 126, 827–838. [DOI] [PubMed] [Google Scholar]
- Waxman JS, Keegan BR, Roberts RW, Poss KD, Yelon D, 2008. Hoxb5b Acts Downstream of Retinoic Acid Signaling in the Forelimb Field to Restrict Heart Field Potential in Zebrafish. Developmental Cell 15, 923–934. doi: 10.1016/j.devcel.2008.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerfield M, 1995. The Zebrafish Book: A guide for the laboratory use of zebrafish (Danio rerio), 3rd ed University of Oregon, Eugene, OR. [Google Scholar]
- Yin C, Kikuchi K, Hochgreb T, Poss KD, Stainier DYR, 2010. Hand2 Regulates Extracellular Matrix Remodeling Essential for Gut-Looping Morphogenesis in Zebrafish. Developmental Cell 18, 973–984. doi: 10.1016/j.devcel.2010.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]


