Abstract
Background:
Intracranial atherosclerotic disease (ICAD) is a common cause of ischemic stroke with a high risk of clinical stroke recurrence. Multiple mechanisms may underlie cerebral ischemia in this condition. The study’s objective is to discern the mechanisms of recurrent ischemia in ICAD through imaging biomarkers of impaired antegrade flow, poor distal perfusion, abnormal vasoreactivity, and artery-to-artery embolism.
Methods:
This prospective multicenter observational study enrolled patients with recent (≤21 days) ischemic stroke or transient ischemic attack (TIA) caused by ICAD with 50–99% stenosis treated medically. We obtained baseline quantitative MRA (QMRA), perfusion MRI (PWI), transcranial Doppler vasoreactivity (VMR), and emboli detection studies (EDS). The primary outcome was ischemic stroke in the territory of the stenotic artery within 1 year of follow-up; secondary outcomes were TIA at 1 year and new infarcts in the territory on MRI at 6–8 weeks.
Results:
Amongst 102 of 105 participants with clinical follow-up (mean 253±131 days), the primary outcome occurred in 8.8% (12.7/100 patient-years), while 5.9% (8.5/100 patient-years) had a TIA. A new infarct in the territory of the symptomatic artery was noted in 24.7% at 6–8 weeks. A low flow state on QMRA was noted in 25.5%, poor distal perfusion on PWI in 43.5%, impaired vasoreactivity on VMR in 67.5%, and microemboli on EDS in 39.0%. No significant association was identified between these imaging biomarkers and primary or secondary outcomes.
Conclusions:
Despite intensive medical management in ICAD, there is a high risk of clinical cerebrovascular events at 1 year and an even higher risk of new imaging-evident infarcts in the subacute period after index stroke. Hemodynamic and plaque instability biomarkers did not identify a higher risk group. Further work is needed to identify mechanisms of ischemic stroke and infarct recurrence and their consequence on long-term physical and cognitive outcomes.
Trial Registration:
Keywords: stroke, cerebral infarction, intracranial arterial disease, biomarkers
Introduction
Intracranial atherosclerotic disease (ICAD) accounts for about 8% of strokes in the US and up to 50% of strokes in China and Southeastern Asia;1 as such, ICAD is probably the most common global cause of ischemic stroke. It also carries the highest risk of stroke recurrence, estimated at 12% at 1 year despite aggressive medical therapy.2,3 The lack of benefit of stenting, a primary flow restoration strategy, in clinical trials completed to date4,5 suggests that different mechanisms of ischemic injury may be responsible for stroke in patients with ICAD. We hypothesized that hemodynamic factors and plaque instability, assessed through imaging biomarkers, would identify those at highest risk of stroke recurrence, that the occurrence of subclinical infarcts would be greater than clinical stroke events, and that the early period after the index event was particularly vulnerable.
The Mechanisms of Early Recurrence in Intracranial Atherosclerotic Disease (MyRIAD) Study is an investigator-initiated prospective observational study funded by the NIH/NINDS with the overall objective of discerning the mechanisms of ischemic stroke in ICAD through imaging biomarkers for specific pathophysiological processes that underlie stroke recurrence, namely impaired antegrade flow, poor distal perfusion, abnormal vasoreactivity and artery-to-artery embolism.6
Flow quantification of antegrade flow, evaluated by phase contrast MR angiography (QMRA)7 is associated with increased risk of recurrent stroke in ICAD.8 Distal tissue perfusion, assessed by perfusion MR imaging (PWI), identifies those with ICAD at risk for future cerebral ischemia.9 Vasomotor reactivity (VMR) measures the response of arterioles to vasodilatory challenges. Transcranial Doppler with breath-holding (TCD BHI) has shown association with increased risk in large vessel stenosis.10 Microemboli detected by transcranial Doppler (emboli detection study or TCD EDS), a marker of plaque instability11 is also associated with greater risk of recurrent stroke in ICAD.12
Methods
The design of MyRIAD has been previously described.6 Ten recruiting sites participated in MyRIAD; all patients signed informed consent, and the participating institutions’ ethics committees approved the study. Eligible patients had a recent (≤21 days) ischemic stroke or a transient ischemic attack (TIA) caused by ICAD of the intracranial carotid artery, middle cerebral artery M1 segment, basilar artery, or vertebral artery V4 segment, with 50–99% stenosis, in the absence of proximal cervical arterial stenosis >50% or a cardioembolic source. Ischemic stroke required symptoms lasting >24 hours and imaging consistent with an ischemic event; TIA had symptoms lasting <24 hours and either diffusion weighted imaging (DWI) abnormality, or 2 or more stereotypical events with unequivocally ischemic symptoms. The degree of s tenosis was calculated by established methods13 on digital substraction angiography (n=23), CT angiography (n=64) or MR angiography (n=10); a flow gap on MR angiography (n=8) was considered eligible. Vascular imaging for eligibility was reviewed centrally. Eligible patients were ≥30 years of age, but those of age 30–49 years had either established atherosclerosis in another vascular bed or 2 or more vascular risk factors. We excluded those with contraindications to MRI, MR contrast agents, including allergy, creatinine >1.5 or GFR <30 mL/min/1.73 m2, and those with planned endovascular treatment for ICAD. All enrolled patients were treated with aggressive medical therapy based on the Stenting and Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis (SAMMPRIS) trial medical regimen.14
We recorded demographic and clinical characteristics, medications, laboratory tests at the time of the index event, and collected all eligibility brain parenchymal and vascular imaging. Consented patients who did not undergo baseline hemodynamic imaging were considered screen failures. Enrolled patients underwent initial study-related imaging including QMRA, PWI, TCD VMR, and TCD EDS within 21 days of index stroke. A brain MRI with FLAIR and DWI/ADC sequences was obtained at 6–8 weeks. Clinical follow-up occurred at 6–8 weeks, 3 months ± 15 days, 6 months ± 15 days and 12 months ± 21 days to determine if an endpoint had occurred and to record treatment adherence.
The baseline study imaging was evaluated by central readers. Decreased antegrade flow in the stenotic vessel was considered when volumetric flow was ≥20% lower than the vessel and age specific normal values.7,15 Abnormal tissue perfusion on PWI was defined by a time to peak (TTP) >4 sec involving ≥10 cc brain tissue volume. Low vasomotor reactivity was defined as a TCD BHI <0.69.10 Any microembolic signal on TCD EDS during 30 minutes of monitoring was considered abnormal.12
The primary outcome was ischemic stroke in the territory of the stenotic artery, based on clinical finding of new focal symptoms lasting >24 hours with confirmatory imaging of new infarct in the territory. The secondary endpoints were: a) TIA in the territory of the stenotic artery; and b) new infarct detected on MRI at 6–8 weeks in the territory of the stenotic artery. A new infarct at 6–8 weeks was considered when new DWI/ADC or FLAIR sequence lesion developed compared to the baseline MRI; when a baseline MRI was not available (n=9), only new DWI/ADC lesions were counted. Primary and secondary outcomes were ascertained by 2 independent experienced vascular neurologists who reviewed clinical data and imaging studies, including that obtained at baseline, 6–8 weeks, and at the time of a clinical endpoint. In case of disagreement, they reviewed case to reach consensus.
We estimated a prevalence of 30% of abnormal imaging states and a relative risk of 3.3 for the primary endpoint in the presence of an abnormal imaging biomarker.12,15,16,17 We calculated a sample size of 110 participants, assuming 10% lost to follow-up, to have 80% power (5% significance level) to detect an association between the primary endpoint and impaired antegrade flow, poor distal perfusion, and abnormal vasoreactivity, and 60% power for artery-to-artery embolism.
Planned analyses for the primary and secondary endpoints included Kaplan-Meier curve comparisons using log-rank tests to compare the abnormal and normal groups, defined by the marker of primary interest measured at baseline. In addition, Fisher’s Exact Tests or, where feasible, asymptotic chi-square tests were used to compare outcome risk differences in the abnormal and normal groups, and confidence intervals for risk differences were computed. Analyses were performed using SAS software, version 9.4 and R version 3.6.1. A significance level of 5% (confidence level of 95%) was used for inference-making; all inferences were two-sided.
Results
Between 04/2015 and 05/2019, 141 patients met eligibility criteria and 36 (25.5%) were screen failures; the main reasons for screen failure were the inability to complete study imaging in time (11), withdrawal of consent (11), and inability to complete MRI due to claustrophobia, movement, or metallic implant verification (6), ineligible after consent (2), endovascular procedure performed prior to study imaging (1). A total of 105 patients were enrolled at 14.9 ± 8.8 (mean ± SD) days from the qualifying event. Baseline and qualifying event characteristics are included in Table 1. As noted, the cohort had a large burden of vascular risk factors, including a very high proportion with diabetes mellitus (54.3%). Overall, 73.7% had anterior circulation disease at index stroke or TIA. Most patients were enrolled after a stroke; these were relatively mild (NIHSS 2.4 ± 3.4 [mean ± SD], median 1.0 [IQR 0–3]) and amongst those with an available baseline eligibility MRI (n=96), the median infarct volume on DWI was 5 cc (n=71, IQR 5–10). The degree of stenosis was 78.6 ± 10.4% (mean ± SD; n=97) and 85.7% had stenosis in the 70–100% range. Flow gap was observed, and no stenosis measurement recorded, in 8 patients.
Table 1.
Baseline and qualifying event characteristics.
| Demographic characteristics | |
| Age (mean±SD) | 63.7±11.8 |
| Female sex | 43% |
| Hispanic ethnicity | 21% |
| Race White | 56% |
| African American | 37% |
| Asian | 2% |
| Other | 5% |
| Risk factors | |
| Hypertension | 85.7% |
| Diabetes mellitus | 54.3% |
| Hyperlipidemia | 67.7% |
| Body mass index (mean±SD) | 30.2±7.5 |
| Tobacco current use | 20% |
| Tobacco cessation <2 years | 6.7% |
| No regular exercise | 61.9% |
| Prior Stroke (>30 days) | 20% |
| Prior TIA (>30 days) | 11.4% |
| Coronary artery disease | 18.1% |
| Baseline laboratory tests | |
| Cholesterol mg/dL (mean±SD) | 182.4 ± 51 |
| LDL mg/dL (mean±SD) | 108.8 ± 43.9 |
| HDL mg/dL (mean±SD) | 41.3 ± 11.5 |
| Triglycerides mg/dL (mean±SD) | 166.6 ± 102.4 |
| HbA1C % (mean±SD) | 7.4 ± 2.3 |
| Qualifying event | |
| Ischemic stroke | 77.4% |
| TIA | 22.6% |
| Symptomatic vessel | |
| Intracranial carotid artery | 16.9% |
| Middle cerebral artery | 55.8% |
| Vertebral artery | 4.2% |
| Basilar artery | 23.2% |
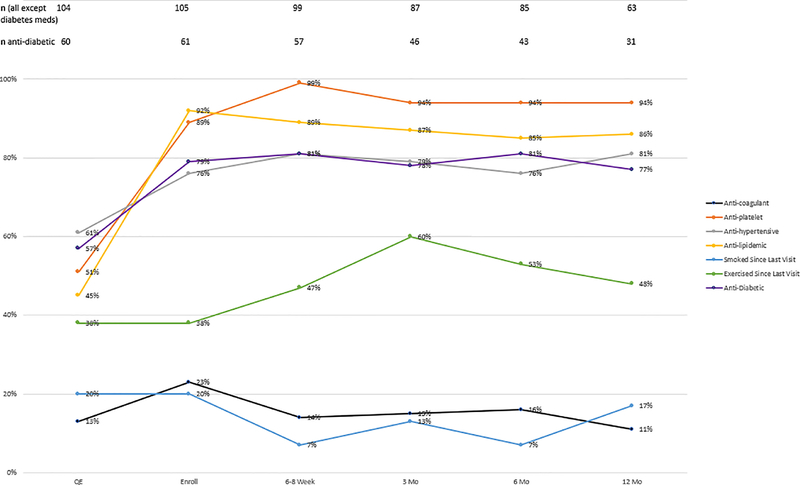
Treatment adherence is depicted in Figure 1. At the time of the qualifying event, 64% were on antithrombotic agents (51% on antiplatelet agents and 13% on an anticoagulant), 61% on antihypertensive agents, 45% on antilipidemic agents, and 57% of diabetic patients on antidiabetic treatment. Intensive medical treatment was instituted by the time of enrollment, which was highly sustained during the course of the study: stroke prevention medications were taken by over 80% at 6–8 weeks, and by over 75% at 3, 6 and 12 months. Lifestyle modification was modestly successful. Although 62% did not exercise regularly at baseline, 47–60% reported exercising regularly during follow-up. Active tobacco use was reported in 20% at baseline; there was an early reduction but 17% reported smoking at last follow-up.
Figure 1.
Treatment adherence at baseline, enrollment and during follow-up visits.
Clinical follow-up beyond baseline was available in 102 patients. During follow up of 253 ± 131 days (mean ± SD), an ischemic stroke in the territory of the stenotic artery developed in 9 participants (8.8%, 12.7/100 patient-years), including 5 before the first 6–8 week follow-up visit, while 6 (5.9%, 8.5/100 patient-years) had a TIA attributed to the symptomatic artery. There were 2 deaths, one from pneumonia/sepsis 8 weeks after the qualifying event, and one from cardiac arrest at 37 weeks.
A study MRI at 6–8 weeks was available for 89 patients; the time from the qualifying event to imaging was 51.3 ± 16.6 days (mean ± SD). A new infarct in the territory of the target vessel was found in 24.7%. Amongst the 22 patients with new infarcts at 6–8 weeks, a DWI abnormality was noted in 14 while the rest had only new FLAIR lesions.
Overall, low flow state on QMRA was noted in 25.5%, poor distal perfusion on PWI in 43.5%, impaired vasoreactivity on TCD VMR with BHI in 67.5%, and presence of microemboli on TCD EDS in 39.0%. The association of MyRIAD imaging biomarkers with outcomes is illustrated in Table 2 and Figure 2 (A–D). At the predefined cut points, we did not identify a significant association between an abnormal imaging biomarker and recurrent stroke, TIA, or new infarcts at 6–8 weeks. We also performed pre-planned analysis for combination of abnormal biomarkers, including PWI with QMRA and TCD VMR with TCD EDS, as described in Table 2 and Figure 3, without a clear synergistic effect. Recurrent infarct was noted in 33% percent of 42 patients for whom two or more imaging biomarker abnormalities were noted, vs. 17% of 47 patients with one or fewer abnormalities (P=0.07).
Table 2.
Association between hemodynamic and plaque instability imaging biomarkers and outcomes.
| Baseline Abnormality | Stroke | TIA | Infarct (6–8 week) | |||
|---|---|---|---|---|---|---|
| n (%) | p* | n (%) | p | n (%) | p | |
| QMRA (VFR >20% lower) | ||||||
| Yes | 3/24 (13%) | 0.7 | 2/24 (8%) | 0.6 | 7/23 (30%) | 0.4† |
| No | 6/71 (8%) | 3/71 (4%) | 13/60 (22%) | |||
| 95% CI for Risk Difference‡ | (−19%, 27%) | (−19%, 27%) | (−13%, 30%) | |||
| PWI (Tmax >4 sec in >10 cc) | ||||||
| Yes | 3/39 (8%) | 1.0 | 2/39 (5%) | 1.0 | 9/35 (26%) | 0.9† |
| No | 5/51 (10%) | 3/51 (6%) | 11/45 (24%) | |||
| 95% CI for Risk Difference‡ | (−23%, 19%) | (−21%, 20%) | (−18%, 20%) | |||
| TCD VMR (BHI < 0.69) | ||||||
| Yes | 5/50 (10%) | 1.0 | 3/50 (6%) | 1.0 | 12/43 (28%) | 0.7† |
| No | 2/25 (8%) | 1/25 (4%) | 5/22 (23%) | |||
| 95% CI for Risk Difference‡ | (−23%, 27%) | (−23%, 27%) | (−17%, 27%) | |||
| TCD EDS (any microemboli) | ||||||
| Yes | 1/29 (3%) | 0.2 | 3/29 (10%) | 0.3 | 8/26 (31%) | 0.7† |
| No | 6/46 (13%) | 1/46 (2%) | 10/39 (26%) | |||
| 95% CI for Risk Difference‡ | (−32%, 14%) | (−15%, 31%) | (−17%, 28%) | |||
| Abnormal TCD VMR plus TCD EDS | ||||||
| Yes | 1/19 (5%) | 0.7 | 2/19 (11%) | 0.3 | 6/17 (35%) | 0.5 |
| No | 6/57 (11%) | 2/57 (4%) | 12/49(24%) | |||
| 95% CI for Risk Difference‡ | (−31%, 21%) | (−20%, 33%) | (−17%, 38%) | |||
| Abnormal PWI and QMRA | ||||||
| Yes | 2/16 (13%) | 0.6 | 1/16 (6%) | 1.0 | 5/15 (33%) | 0.5 |
| No | 7/81 (9%) | 4/81 (5%) | 16/70 (23%) | |||
| 95% CI for Risk Difference‡ | (−23%, 30%) | (−25%, 28%) | (−18%, 38%) | |||
p = p-value for test of differences, between the group that had the baseline abnormality and the group that did not have the baseline abnormality, in risk (i.e. in proportions having the event).
Asymptotic chi-square test p-value. All other p-values are for Fisher’s Exact test.
95% confidence interval for the risk difference (risk in group with the abnormality minus risk in group without the abnormality). Intervals were computed using exact methods where exact tests were performed, and using the Wald method where asymptotic chi-square tests were performed.
Figure 2.
Kaplan-Meier curves for ischemic stroke in the territory showing the cumulative probability of stroke versus follow-up time, stratified by the presence/absence of baseline biomarker abnormality. Log-rank test p-values comparing the strata: 2A (PWI abnormality) p=0.77; 2B (QMRA abnormality) p=0.60; 2C (TCD VMR abnormality) p=0.79; 2D (TCD EDS abnormality) p=0.18.
Figure 3.
Kaplan-Meier curves for ischemic stroke in the territory showing the cumulative probability of stroke versus follow-up time, stratified by the presence/absence of prespecified combination of baseline biomarker abnormalities. Log-rank test p-values comparing the strata: 3A (TCD VMR plus EDS abnormality) p=0.47; 3B (PWI plus QMRA abnormality) p=0.60.
Discussion
In a multi-center recently symptomatic ICAD cohort, we observed a high risk of recurrent cerebral ischemia. The rate of clinical stroke recurrence on contemporary medical therapy (12.7/100 patient-years) was similar to that reported by recent ICAD clinical trials: in the medical treatment arm of the SAMMPRIS trial, the 1-year rate of stroke or death within 30 days or recurrent stroke in the territory beyond 30 days was 12.2%,3 while in the Vitesse Stent Ischemic Therapy (VISSIT) trial, the 1 year risk of stroke in the territory was 9.4%.5 In MyRIAD, 5% of patients had a clinical stroke by 6–8 weeks, which is similar to recent clinical trials4 and much lower than previous reports before intensive medical management was instituted.18
The risk of new imaging infarcts at 6–8 weeks (24.7%) was much higher that of clinical events. This risk of early infarct recurrence has not been well described before. In a small retrospective series of 25 patients with moderate to severe non-occlusive ICAD, 36% had a recurrent infarct within 1 week.19 The impact of early small infarct recurrence is unknown. However, previous cerebral ischemia in ICAD is a risk factor for stroke recurrence,20 and baseline infarct patterns such as subcortical location, multiple infarcts21 and borderzone distribution22 have been associated with greater risk of recurrence. There are also potential cognitive consequences to silent infarcts. In the Rotterdam study, baseline silent infarcts doubled the risk of developing dementia and the cognitive decline was greater in those with infarcts on follow up imaging.23 The cause of dementia in this population is likely vascular: the presence of ICAD is associated with risk of dementia in population-based studies,24,25 and ICAD is not associated with amyloid PET deposition,26 indicating non-AD pathology of dementia in ICAD.
There was a high prevalence of imaging biomarker abnormalities. A low flow state on QMRA of 25.5% is very similar to that found in the Vertebrobasilar Flow Evaluation and Risk of Transient Ischemic Attack and Stroke (VERiTAS) study.8 However, since we applied different definitions to accommodate the anterior circulation in our study, it is difficult to compare the 2 studies directly. We also found a high prevalence of poor distal perfusion. Others have described similar finding; prolonged mean transit time on perfusion imaging was reported in 31% of patients with symptomatic ICAD.9 Impaired vasoreactivity has been well described in ICAD with greater decrements at higher degrees of stenosis.27 We found decreased VMR in two-thirds of patients, in line with other reports.27 Although the 39% rate of microembolic signals in MyRIAD is in a similar range to previous reports,12,28 the true incidence may be underestimated as we found a significant amount of turbulence, particularly in distal middle cerebral artery M1 segment stenosis, that precluded reliable identification of microembolic signals.
Based on the predefined definition of abnormal hemodynamic and plaque instability biomarkers, we were unable to define a particularly vulnerable subgroup of recently symptomatic ICAD at risk for recurrent clinical or subclinical events. It is possible that the study was underpowered as there was a directional indication, although not statistically significant, that the combination of abnormal biomarkers correlate with infarct recurrence. Other biomarkers of high risk such as vessel wall imaging and plaque load hold promise as novel predictors to identify a particularly vulnerable group29,30 though they remain investigational to date.
This study has limitations. First, we relied on non-invasive estimation of stenosis in most (n=82) patients, and it is possible that we included some patients with <50% stenosis; however, non-invasive techniques, particularly CTA, have excellent accuracy,31 and we had independent adjudication for imaging eligibility. Second, treatment was not mandated or funded by the study, but sites indicated in a pre-study survey their intent to follow guidelines-based intensive medical management,32 and we identified relatively good adherence to prescribed treatment, except for lifestyle changes. The suboptimal adherence to tobacco cessation and exercise are an opportunity to improve secondary prevention strategies in this population. There are also important strengths: the characterization of a high-risk ICAD cohort in the earlier period after an index event, a particularly vulnerable period for recurrence, than previous clinical trials;2,4,5 and the determination of infarcts on MRI as a surrogate secondary outcome in the subacute timeline with independent adjudication.
Conclusions
In this contemporary cohort of recently symptomatic ICAD with intensive medical management, we confirmed the high risk of recurrent clinical stroke described in clinical trials, and found a much greater risk of early asymptomatic infarct recurrence which developed in nearly 1 in 4 individuals. We were unable to define a higher risk group based on abnormal hemodynamic and plaque instability imaging biomarkers. Novel technologies and biomarkers including plaque burden and genetic risk profile may help identify baseline characteristics of higher risk for early recurrence and aid in individualized management.29,30,33,34 This study reinforces the need to develop better strategies to treat this highly prevalent and high-risk condition.
Supplementary Material
Acknowledgements
The authors would like to acknowledge Lauren Ostergren from VasSol, Inc. for her assistance with the performance of the QMRA studies
Grant Support: NIH/NINDS (R01 NS084288)
Source of Funding
MyRIAD is supported through a grant by the NIH/NINDS (R01 NS084288). QMRA was performed on the NOVA platform, provided by VasSol Inc (River Forest, IL) at no cost to recruiting sites. The institutional review board/ethics committee at each participating institution approved this study, which is registered at ClinicalTrials.gov (NCT02121028).
This study was supported by an NIH/NINDS grant (R01 NS084288). JGR, ICB, DSL, SP, AN, GC, EF, SK, TR received salary support for their work on this grant.
Footnotes
Conflicting interests/Disclosures
• JG Romano reports no conflicts of interest.
• I Campo-Bustillo reports no conflicts of interest.
• DS Liebeskind reports no conflicts of interest.
• S Prabhakaran reports no conflicts of interest.
• A Nizam reports no conflicts of interest.
• G Cotsonis reports no conflicts of interest.
• R Sangha reports no conflicts of interest.
• E Feldmann reports no conflicts of interest.
• S Koch reports no conflicts of interest.
• T Rundek reports no conflicts of interest.
• MI Chimowitz reports no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jose G. Romano, University of Miami, Miami, FL, USA.
Shyam Prabhakaran, The University of Chicago, Chicago, IL, USA.
Azhar Nizam, Emory University, Atlanta, GA, USA.
Edward Feldmann, The University of Massachusetts Medical School-Baystate, Springfield, MA, USA.
Rajbeer Sangha, University of Alabama at Birmingham, Birmingham, AL, USA.
George Cotsonis, Emory University, Atlanta, GA, USA.
Iszet Campo-Bustillo, University of Miami, Miami, FL, USA.
Sebastian Koch, University of Miami, Miami, FL, USA.
Tatjana Rundek, University of Miami, Miami, FL, USA.
Marc I. Chimowitz, Medical University of South Carolina, Charleston, SC, USA.
David S. Liebeskind, University of California at Los Angeles, Los Angeles, CA, USA.
References
- 1.Gorelick PB, Wong KS, Bae H-J, et al. Large artery intracranial occlusive disease: a large worldwide burden but a relatively neglected frontier. Stroke 2008,39:2396–2399. [DOI] [PubMed] [Google Scholar]
- 2.Chimowitz MI, Lynn MJ, Howlett-Smith H, et al. Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis. N Engl J Med. 2005;352:1305–1316. [DOI] [PubMed] [Google Scholar]
- 3.Derdeyn CP, Chimowitz MI, Lynn MJ, et al. Aggressive medical treatment with or without stenting in high-risk patients with intracranial artery stenosis (SAMMPRIS): the final results of a randomised trial. Lancet. 2014;383:333–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chimowitz MI, Lynn MJ, Derdeyn CP, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med. 2011;365:993–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zaidat OO, Fitzsimmons BF, Woodward BK, et al. Effect of a balloon-expandable intracranial stent vs medical therapy on risk of stroke in patients with symptomatic intracranial stenosis: the VISSIT randomized clinical trial. JAMA. 2015;313:1240–1248. [DOI] [PubMed] [Google Scholar]
- 6.Liebeskind DS, Prabhakaran S, Azhar N, et al. Mechanisms of Early Recurrence in Intracranial Atherosclerotic Disease (MyRIAD): Rationale and Design. J Stroke Cerebrovasc Dis 2020;29 Published online 10.1016/j.jstrokecerebrovasdis.2020.105051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao M, Amin-Hanjani S, Ruland S, et al. Regional cerebral blood flow using quantitative MR angiography. AJNR Am J Neuroradiol. 2007;28:1470–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amin-Hanjani S, Pandey DK, Rose-Finnell L, et al. Effect of Hemodynamics on Stroke Risk in Symptomatic Atherosclerotic Vertebrobasilar Occlusive Disease. JAMA Neurol. 2016;73:178–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lan L, Leng X, Ip V, et al. Prolonged Perfusion Predicts Recurrent Ischemic Stroke but not Transient Ischemic Attack in Patients with Symptomatic Intracranial Stenosis. Curr Neurovasc Res. 2017;14:149–157. [DOI] [PubMed] [Google Scholar]
- 10.Silvestrini M, Vernieri F, Pasqualetti P, et al. Impaired cerebral vasoreactivity and risk of stroke in patients with asymptomatic carotid artery stenosis. JAMA. 2000;283:2122–2127. [DOI] [PubMed] [Google Scholar]
- 11.Altaf N, Kandiyil N, Hosseini A, et al. Risk factors associated with cerebrovascular recurrence in symptomatic carotid disease: a comparative study of carotid plaque morphology, microemboli assessment and the European Carotid Surgery Trial risk model. J Am Heart Assoc. 2014;3:e000173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao S, Wong KS, Hansberg T, et al. Microembolic signal predicts recurrent cerebral ischemic events in acute stroke patients with middle cerebral artery stenosis. Stroke. 2004;35:2832–2836. [DOI] [PubMed] [Google Scholar]
- 13.Samuels OB, Joseph GJ, Lynn MJ, et al. A standardized method for measuring intracranial arterial stenosis. AJNR Am J Neuroradiol. 2000;21:643–646. [PMC free article] [PubMed] [Google Scholar]
- 14.Turan TN, Lynn MJ, Nizam A, et al. Rationale, design, and implementation of aggressive risk factor management in the Stenting and Aggressive Medical Management for Prevention of Recurrent Stroke in Intracranial Stenosis (SAMMPRIS) trial. Circ Cardiovasc Qual Outcomes. 2012;5:e51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amin-Hanjani S, Du X, Zhao M, et al. Use of quantitative magnetic resonance angiography to stratify stroke risk in symptomatic vertebrobasilar disease. Stroke. 2005;36:1140–1145. [DOI] [PubMed] [Google Scholar]
- 16.Ogasawara K, Ogawa A, Yoshimoto T. Cerebrovascular reactivity to acetazolamide and outcome in patients with symptomatic internal carotid or middle cerebral artery occlusion: a xenon-133 single-photon emission computed tomography study. Stroke. 2002;33:1857–1862. [DOI] [PubMed] [Google Scholar]
- 17.Liebeskind DL, Derdeyn CP, Sanossian N, et al. Perfusion imaging of intracranial atherosclerotic disease in SAMMPRIS. Stroke. 2015;46:A138 (Abstract). [Google Scholar]
- 18.Lovett JK, Coull AJ, Rothwell PM. Early risk of recurrence by subtype of ischemic stroke in population-based incidence studies. Neurology. 2004;62:569–573. [DOI] [PubMed] [Google Scholar]
- 19.Kang DW, Kwon SU, Yoo SH, et al. Early recurrent ischemic lesions on diffusion-weighted imaging in symptomatic intracranial atherosclerosis. Arch Neurol. 2007;64:50–54. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y, Zhao X, Liu L, et al. Prevalence and outcomes of symptomatic intracranial large artery stenoses and occlusions in China: the Chinese Intracranial Atherosclerosis (CICAS) Study. Stroke. 2014;45:663–669. [DOI] [PubMed] [Google Scholar]
- 21.Jung JM, Kang DW, Yu KH, et al. Predictors of recurrent stroke in patients with symptomatic intracranial arterial stenosis. Stroke. 2012;43:2785–2787. [DOI] [PubMed] [Google Scholar]
- 22.Wabnitz AM, Derdeyn CP, Fiorella DJ, et al. Hemodynamic Markers in the Anterior Circulation as Predictors of Recurrent Stroke in Patients With Intracranial Stenosis. Stroke. 2019;50:143–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vermeer SE, Prins ND, den Heijer T, et al. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med. 2003;348:1215–1222. [DOI] [PubMed] [Google Scholar]
- 24.Bos D, Vernooij MW, de Bruijn RF, et al. Atherosclerotic calcification is related to a higher risk of dementia and cognitive decline. Alzheimers Dement. 2015;11:639–647. [DOI] [PubMed] [Google Scholar]
- 25.Dearborn JL, Zhang Y, Qiao Y, et al. Intracranial atherosclerosis and dementia: The Atherosclerosis Risk in Communities (ARIC) Study. Neurology. 2017;88:1556–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gottesman RF, Mosley TH, Knopman DS, et al. Association of Intracranial Atherosclerotic Disease With Brain β-Amyloid Deposition: Secondary Analysis of the ARIC Study. JAMA Neurol. 2019;77:350–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee JY, Lee YS. Vasomotor reactivity in middle cerebral artery stenosis. J Neurol Sci. 2011;301:35–37. [DOI] [PubMed] [Google Scholar]
- 28.Wong KS, Chen C, Fu J, et al. Clopidogrel plus aspirin versus aspirin alone for reducing embolisation in patients with acute symptomatic cerebral or carotid artery stenosis (CLAIR study): a randomised, open-label, blinded-endpoint trial. Lancet Neurol. 2010;9:489–497. [DOI] [PubMed] [Google Scholar]
- 29.Turan TN, Rumboldt Z, Granholm AC, et al. Intracranial atherosclerosis: correlation between in-vivo 3T high resolution MRI and pathology. Atherosclerosis. 2014;237:460–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim BS, Chung PW, Park KY, et al. Burden of Intracranial Atherosclerosis Is Associated With Long-Term Vascular Outcome in Patients With Ischemic Stroke. Stroke. 2017;48:2819–2826 [DOI] [PubMed] [Google Scholar]
- 31.Duffis EJ, Jethwa P, Gupta G, et al. Accuracy of computed tomographic angiography compared to digital subtraction angiography in the diagnosis of intracranial stenosis and its impact on clinical decision-making. J Stroke Cerebrovasc Dis. 2013;22:1013–1017. [DOI] [PubMed] [Google Scholar]
- 32.Kernan WN, Ovbiagele B, Black HR, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:2160–2236. [DOI] [PubMed] [Google Scholar]
- 33.Traylor M, Farrall M, Holliday EG, et al. Genetic risk factors for ischaemic stroke and its subtypes (the METASTROKE collaboration): a meta-analysis of genome-wide association studies. Lancet Neurol. 2012;11:951–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fernández-Cadenas I, Mendióroz M, Giralt D, et al. GRECOS Project (Genotyping Recurrence Risk of Stroke): The Use of Genetics to Predict the Vascular Recurrence After Stroke. Stroke. 2017;48:1147–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.