Abstract
Objective(s):
To elucidate relationships in antiretroviral (ARV) resistance between HIV-1 infected mother-infant pairs by defining the resistance profiles in the mothers and infants and quantifying drug resistance prevalence in the pairs post-Option B+ implementation.
Design:
Collection of dried blood spots (DBS) from mother-infant pairs during routine HIV-1 screens in Lusaka, Zambia from 2015 to 2018.
Methods:
DNA was extracted from the DBS, the HIV-1 pol region was amplified, and the purified proviral DNA was sequenced using Sanger sequencing. Drug resistance mutations (DRM) were identified in sequenced DNA using the Stanford HIVdb (https://hivdb.stanford.edu/).
Results:
DRM were detected in 45% (44/97) of samples, and these samples were found to harbor resistance to at least two ARVs. The prevalence of non-nucleoside reverse transcriptase inhibitor (NNRTI) resistance was significantly higher than that of other ARV classes. DRM were detected disproportionately in infants (67%; 33/49) compared to mothers (23%; 11/48), but the magnitude of resistance did not differ when resistance was detected. The disparity in drug resistance profiles was reinforced in pairwise comparison of resistance profiles in mother-infant pairs.
Conclusions:
While Option B+ is effective in reducing mother to child transmission (MTCT), in cases where this regimen fails, high level NNRTI resistance is frequently detected in infants. This underscores the importance of pre-treatment drug resistance screening in both mothers and infants and emphasizes the necessary change to protease inhibitor (PI)-based and integrase inhibitor (INI)-based regimens for treatment of HIV-1 infected infants and mothers.
Keywords: Zambia, dried blood spots, HIV-1, HIVDR, PMTCT, mother-infant pairs
Introduction
In 2013, the World Health Organization (WHO) recommended Option B+ for prevention of mother to child transmission (PMTCT) of human immunodeficiency virus (HIV), and in 2015, it was implemented in Sub-Saharan Africa (SSA) [1]. Option B+ starts all HIV-infected pregnant and lactating women on antiretroviral therapy (ART) and continues this regimen for life. The combination recommended was Atripla™, containing tenofovir (TDF), emtricitabine (FTC) or lamivudine (3TC), and efavirenz (EFV). More recently, the protease inhibitor (PI), ritonavir boosted lopinavir (LPV/r), replaced EFV for pregnant women and the integrase inhibitor (INI), Dolutegravir (DTG), was suggested for non-pregnant women. Despite the scale-up of PMTCT and HIV treatment, economic and social barriers impede the maintenance of the PMTCT program leaving individuals in low-income, rural communities susceptible to developing HIV drug resistance (HIVDR) [2]. Option B+ has resulted in reduction of incident HIV infection in children (aged 0 to 14) from 300,000 in 2010 to 160,000 in 2016 [3]. In Zambia, this accumulative reduction in the MTCT rate has been quantified from 21% in 2009 [4] to 12% in 2010 to 3% in 2014 [1] and 2% in 2016 and 2017 [4]. Despite Option B+ successes, transmission still occurs in underserved communities; therefore, it was important to investigate whether infected infants are at increased risk for harboring viruses with high level HIVDR.
It is accepted that a minor variant of HIV typically gets transmitted and persists as the founder virus in the newly-infected individual [5]. Debate continues as to whether variants containing drug resistance mutations (DRM) are transmitted MTC or whether DRM develop de novo in the infected infant. Transmitted resistance to non-nucleoside reverse transcriptase inhibitors (NNRTIs) has been documented in SSA [6], but analysis of mother-infant pairs in the Swiss HIV Cohort Study suggested that children develop DRM de novo since new DRM emerged in the infant after birth [7]. Some DRM affect HIV fitness, whereby they might conceivably influence transmissibility or early phase replication. Nucleoside reverse transcriptase inhibitor (NRTI) DRM generally reduce replication fitness; whereas, NNRTI mutations are neutral [2]. Consistent with effects on fitness, a 2019 study using samples from the German HIV-1 Sero-converter Study Cohort quantified DRM persistence using non-parametric Kaplan-Meier approximations and showed those that impart resistance to NNRTIs persist longer than those for either NRTIs or PIs [8]. Similarly, in infants experiencing virologic failure NNRTI DRM did not disappear in the absence of selective pressure, but instead persisted until the infant was 3 years old [9]. Whether the transmitted variant contains DRM or the infant develops DRM independently, HIVDR may persist for years in the child, leading to future virologic failure if the treatment plan does not account for the resistance.
DRM are readily detected in infected infants. We previously showed 21% of HIV-1+ Zambian infants had DRM in 2007. This increased to 40% in 2014, along with increased resistance magnitude [10]. Although the frequency of DRM was higher in infants exposed to maternal or infant prophylaxis, PMTCT-unexposed infants also exhibited DRM [11]. While maternal viral load remains the greatest determinant of risk of transmission [12,13], if the mother had DRM, her infant was more likely to acquire or develop DRM [14]. To further elucidate the potential impact of Option B+ on maternal DRM and the likelihood of DRM transmission or development in infected infants, we have defined the resistance profiles in mother-infant pairs from Lusaka, Zambia. Our results highlight widespread NNRTI resistance in infants who become HIV-1 infected despite Option B+ PMTCT and suggest that maternal viral genotypes cannot be used to dictate infant treatment plans. We emphasize that pretreatment resistance screening in both infants and mothers will be needed to design appropriate treatment regimens. Our results also affirm the modification of Option B+ treatment practices to institute a PI- or INI-based regimen rather than an NNRTI-based regimen in HIV-1+ Zambian mothers and infants.
Materials and Methods
Patient Recruitment
University Teaching Hospital in Lusaka, Zambia procured dried blood spots (DBS) from infants during routine screening for HIV-1 and collected maternal DBS at recruitment into the study (median interval between infant and mother DBS collection was 29.5 days) [15]. The mother-infant pairs were recruited from the hospital where the mother was seeking health services for the infant. Children 2 years or younger were targeted since they would have been exposed to PMTCT option B+ protocol. Children were tested when presented to the hospital to aid in treatment decisions. Positive DBS were subjected to a second test, and after a second positive test, the patient began treatment. The maternal DBS were collected at the time infant was confirmed HIV+ and recruited into the study. The DBS were sent to University of Nebraska-Lincoln for DNA extraction and analysis.
Sample Processing
DBS were processed using lysis buffer and proteinase K, followed by DNA extraction with phenol-chloroform. The pol region (2002-3495 relative to HXB2) was amplified from proviral DNA using nested PCR (1st round: (946f) gCAAgAgTTTTggCTgAAgCAATgAg, (947r) CCTTgCCCCTgCTTCTgTATTTCTgC; 2nd round: (948f) TgCAgggCCCCTAggAAAAAgggCTg, (949r) CATgTACCggTTCTTTTAgAATCTCTCTgTT). Product size (≈1500bp) was validated on agarose gels, and the pol amplicon was extracted from agarose gel using E.Z.N.A. Gel Extraction Kit (Omega Bio-Tek, Norcross, Georgia, USA) or ZR-96 Zymoclean Gel DNA Recovery Kit (Zymo Research, Irvine, California, USA) following manufacturer’s instructions. The purified proviral DNA was sent to Eurofins MWG operon for Sanger sequencing with seven separate primer reactions (Eurofins MWG Operon LLC) (forward: (934f) ACCTACACCTgTCAACATAATTgg, (1091f) gTACTgAgAgACAggCTAATTTTTTAgggAA, (1264f) AGGGATGGAAAGGATCACCAGCAA; reverse: (1278r) TTAAgggTTCCCTgTCTTTCggCT, (1279r) TTgCTggTgATCCTTTCCATCCCT, (1281r) AATCCCTgCATAAATCTgACTTgCCCA, (1294r) gggCCATCCATTCCTggCTTTAAT). The resulting seven sequences were assembled into a single contig (≈1500bp) using BioEdit v7.2.5 [16]. HIV DRM were analyzed using Stanford’s HIVdb (https://hivdb.stanford.edu/) [17,18]. The total resistance score of each individual was calculated by summing the individual antiretroviral (ARV) resistance scores reported by HIVdb. All statistical analyses and figures were generated in GraphPad Prism v8.4.1. The data used for analysis is available as supplementary material (see Table, Supplemental Digital Content 1, which contains all the data used for analysis).
Sequence Quality
We were able to PCR amplify and detect HIV-1 pol for 96/143 infant DBS and 66/144 mother DBS, resulting in 49 mother-infant pairs. Non-amplifiable samples were associated with small blood spots and/or poor DNA quality; no other associated characteristics could be identified. Sequence quality was assessed by Stanford’s HIVdb [17,18]. The assembled contigs covered amino acids 1-100 of the PR, 97% of sequences covered amino acids 1-300 of the RT, and none of our sequences covered the integrase. The five contigs that didn’t reach 300 amino acids of the RT covered at least amino acids 1-254. All of the RT DRM identified in our contigs were between amino acids 62 and 236, therefore no samples were excluded due to length. A few contigs contained unusual characteristics: frameshifts-2, insertions-2, deletions-2, stop codons-1, APOBEC mutations-5. Since the raw data supported these anomalies, we did not account for them further in our analysis.
Results
Cohort Characteristics
Overall, we had a total of 162 amplified samples—96 infants and 66 mothers—containing 49 mother-infant pairs. Table 1 summarizes the characteristics of the final mother-infant pairs broken down by collection year. The median infant age at time of collection was 5 months which remained consistent from 2016 to 2018. Likewise, infant gender and maternal ART regimen remained evenly distributed from 2016 to 2018. Stanford’s HIVdb [17,18] assigned 98% of our contigs to subtype C, with CRF49_cpx and A accounting for the remaining 2 samples.
Table 1. Study population characteristics.
The table outlines the known characteristics of the mother-infant pairs.
| Mothers | Infants | |||||||
|---|---|---|---|---|---|---|---|---|
| All | 2016 | 2017 | 2018 | All | 2016 | 2017 | 2018 | |
| Number | 48 | 10 | 25 | 13 | 49 | 10* | 26 | 13 |
| Age (months) | ---- | 5 (0-15) | 4 (1-15) | 4 (1-12) | 5 (0-12) | |||
| Gender | ---- | |||||||
| Male | 24 | 7 | 12 | 5 | ||||
| Female | 25 | 3 | 14 | 8 | ||||
| Subtype | ||||||||
| C | 46 | 10 | 25 | 11 | 49 | 10 | 26 | 13 |
| A | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| CRF49_cpx | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| DRM | ||||||||
| Total | 11 | 1 | 5 | 5 | 33 | 7 | 18 | 8 |
| NNRTI | 11 | 1 | 5 | 5 | 32 | 7 | 17 | 8 |
| NRTI | 4 | 1 | 2 | 1 | 6 | 1 | 2 | 3 |
| PI | 1 | 0 | 1 | 0 | 2 | 0 | 1 | 1 |
| ART Regimen | ---- | |||||||
| Atripla™ | 39 | 8 | 19 | 12 | ||||
| Other | 3 | 0 | 2 | 1 | ||||
| None | 4 | 0 | 4 | 0 | ||||
| Missing | 2 | 2 | 0 | 0 | ||||
| Infant Prophylaxis | ---- | |||||||
| NVP | 28 | 8 | 14 | 6 | ||||
| Other | 7 | 1 | 2 | 4 | ||||
| None | 13 | 1 | 10 | 2 | ||||
| Missing | 1 | 0 | 0 | 1 | ||||
includes one infant DBS collected in 2015.
PI-Protease Inhibitor; NRTI-Nucleotide Reverse Transcriptase Inhibitor; NNRTI-Non Nucleoside Reverse Transcriptase Inhibitor; NVP- Nevirapine
Estimated Prevalence of HIV DRM and HIV Drug Resistance (HIVDR)
DRM were more prevalent in infants compared to mothers throughout our study period. Overall, DRM were identified in 45% of the individuals included in the study (23% of mothers and 67% of infants, Fig. 1A). The proportion of mothers with DRM is significantly different than the proportion of infants with DRM (Fig. 1B). Following the same trend, 44% of the study population was resistant to at least two ARVs (23% of mothers and 65% of infants, Fig. 1C). Of the 43 samples resistant to at least two ARVs, 25% were resistant to five or more ARVs and 26% were resistant to multiple classes of ARVs (most commonly NNRTI + NRTI, Fig. 1D).
Figure 1. Study population resistance profile.
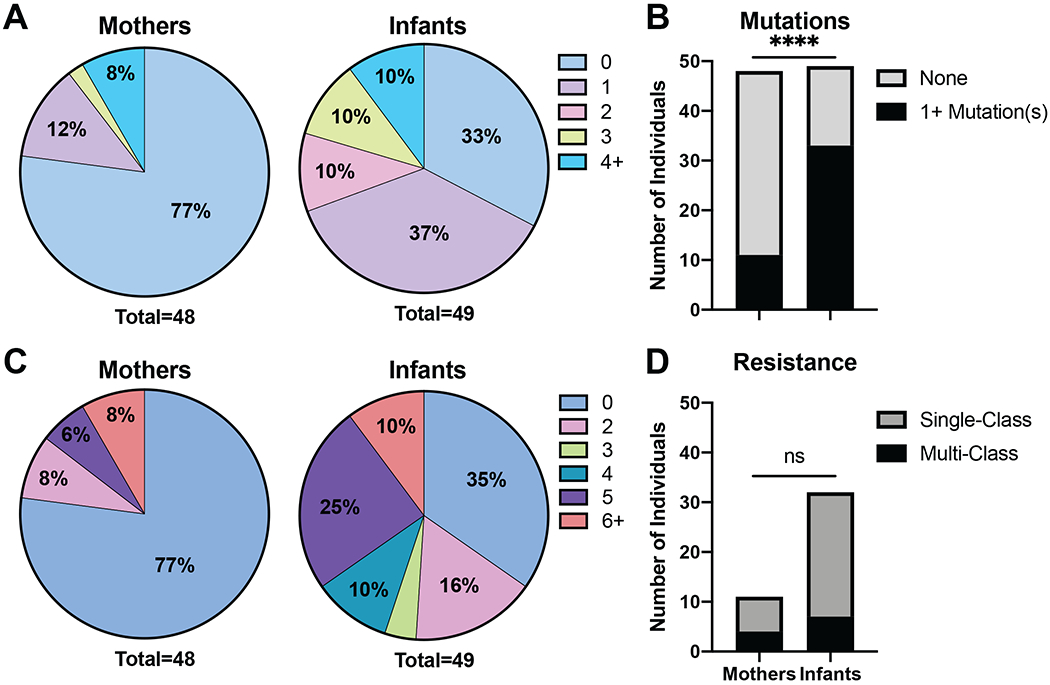
The pie charts represent the proportion of individuals respective to each group. (A) The proportion of individuals that have 0, 1, 2, 3, or 4+ drug resistance mutations (DRM) in pol. (B) The proportion of mothers and infants with or without DRM. The proportions were determined to be significantly different by Fisher’s exact test. (C) The proportion of individuals that are resistant to the respective number of antiretrovirals (ARVs). (D) The proportion of mothers and infants resistant to a single-class of ARVs or multiple classes of ARVs. Statistical significance was determined by Fisher’s Exact Tests.
(ns=not significant, *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001)
DRM-Drug Resistance Mutation; ARV-antiretroviral
NNRTI DRM were more frequently detected in both infants and mothers than other ARV classes: 44% (43/97) of samples were resistant to at least one NNRTI, where 10% (10/97) were resistant to at least one NRTI and 2% (2/97) were resistant to at least one PI. The estimated prevalence of each identified DRM and ARV are presented in Figure 2. In the NNRTI region, four DRM were highly prevalent: K103N/S, V106M, E138A/K, and Y181C/V (Fig. 2B). K103N/S is induced by EFV- and nevirapine (NVP)-containing regimens and leads to high level resistance [17–19]. V106M also causes high level resistance to EFV and NVP [17–19]. E138A/K is selected by rilpivirine (RPV) and leads to reduced susceptibility to RPV, especially in combination with M184I [17,18,20]. Additionally, E138A is more common in subtype C than subtype B [21]. Y181C/V gives rise to high level resistance to NVP and intermediate level to etravirine (ETR) and RVP after induction by NVP-, ETR-, and RPV-containing regimens [17,18,22]. The estimated prevalence of NNRTI resistance is presented in Figure 2D.
Figure 2. DRM and ARV resistance in the sample population.

The bar charts represent the proportion of individuals in each group (mothers or infants) that have the DRM or are resistant to the ARV. Statistical significance was determined by Fisher’s Exact Tests. (A) NRTI DRM, (B) NNRTI DRM, (C) NRTI Resistance, and (D) NNRTI Resistance.
(*p<0.05, **p<0.01, ***p<0.001, ****p<0.0001)
DRM-Drug Resistance Mutation; ARV-antiretroviral; PI-Protease Inhibitor; NRTI-Nucleotide Reverse Transcriptase Inhibitor; NNRTI-Non Nucleoside Reverse Transcriptase Inhibitor; ABC-Abacavir; AZT-Zidovudine; D4T-Stavudine; DDI-Didanosine; FTC-Emtricitabine; 3TC-Lamivudine; TDF-Tenofovir Disoproxil Fumarate; DOR-Doravirine; EFV-Efavirenz; ETR-Etravirine; NVP-Nevirapine; RPV-Rilpivirine
NRTI resistance proportionately affected both mothers and infants. The most common NRTI mutation was M184V/I (Fig. 2A), which is induced by 3TC/FTC and increases the fidelity of the reverse transcriptase, reducing the amount of mutations, and inherently, reducing the rate at which DRM develop [17,18,23]. Most mothers were treated with 3TC/FTC as part of the Option B+ regimen; however, M184V/I was only detected in 6% of mothers and 6% of infants (Fig. 2A). The estimated prevalence of NRTI resistance is presented in Figure 2C.
In summary, the DRM with the most marked differential in estimated prevalence between maternal and infant paired samples were: K103N/S, V106M, V179D/T/E, and Y181C/V. These DRM are associated with high level EFV and NVP resistance and account for the high level NNRTI resistance in infants; while NRTI resistance levels were low in both mothers and infants, and PI resistance was only in two samples—likely a consequence of PIs not being used in SSA until recently.
Magnitude of HIVDR
High level resistance is associated with reduced susceptibility to ARVs. The total resistance score is the sum of each ARV resistance score calculated by Stanford’s HIVdb [17,18]. Thus, an individual with a high total resistance score denotes the individual has high level resistance and decreased susceptibility to ARVs. Figure 3 displays the resistance scores for mothers and infants with respect to ARV class, infant gender, infant age, infant prophylaxis, and maternal treatment duration. Importantly, we have only included samples with detected resistance. By removing the samples without resistance from the analysis, magnitude of resistance can be explored without interference of proportion differences. For example, the total resistance scores between mothers and infants are not significantly different, demonstrating that while HIVDR is more frequently detected in infants (Fig. 1B), the magnitude of resistance is not different between mothers and infants when resistance is present (Fig. 3A). While NNRTI resistance is detected more frequently than NRTI/PI resistance in both mothers and infants (Fig. 2), the magnitude of resistance scores does not differ between classes or mothers/infants (Fig. 3B). Infant gender and age at time of collection did not have an influence on resistance score (Fig. 3C–D). Likewise, infants who were treated with prophylaxis post-delivery did not have significantly different resistance scores than infants who were not (Fig. 3E), and the proportion of infants with resistance did not significantly differ between prophylaxis treated and untreated (p=0.0504, Fisher’s exact test). The prophylaxis received may select for the drug resistant variant transmitted from the mother or select de novo DRM in the infant, but the infants do not accrue additional DRM as they age. However, prophylaxis is just one way for the infant to be exposed to ARVs. PMTCT exposure is defined as exposure to infant prophylaxis and/or maternal HIV treatment. Of the 49 infants, only 13 were not given prophylaxis; while 28 were given NVP alone (as recommended by Option B+); 7 were given NVP in combination with 3TC or zidovudine (AZT), AZT alone, or 3TC alone; 39 infants had mothers treated with Atripla™; 3 mothers were treated with other combination ARTs; and 4 mothers were not on HIV treatment. Only two infants remained PMTCT unexposed. DRM were detected in one of the two unexposed infants. The populations of non-breastfeeding infants and infants exposed to non-NNRTI containing regimens were too small to perform valid comparisons, but maternal treatment duration did correlate with infant resistance score (Fig. 3F, Spearman r = 0.4862). Interestingly, the maternal treatment duration did not correlate with mother resistance score (Fig. 3F, Spearman r = −0.06707). The median duration of maternal treatment was 7.5 months.
Figure 3. Resistance broken down by ARV class, infant gender, infant age, infant prophylaxis post-delivery, and maternal treatment duration.

The total resistance score is the sum of the resistance score of each ARV outputted by Stanford HIVdb for each individual. The samples with a resistance score of zero were excluded from this analysis in order to focus on magnitude of resistance without interference of prevalence. Statistical significance was determined by Mann Whitney Tests. (A) The median total resistance score, (B) the median total resistance score for each ARV class, (C) the median total resistance score for each gender of infant, (D) the infant total resistance scores versus infant age in months (Spearman r = −0.08659), (E) the median resistance score for each infant separated by prophylaxis regimen received post-delivery, (F) the mother and infant total resistance scores versus maternal treatment duration in months (Mothers-Spearman r = −0.06707; Infants-Spearman r = 0.4862).
(*p<0.05, **p<0.01, ***p<0.001, ****p<0.0001)
ARV-antiretroviral; PI-Protease Inhibitor; NRTI-Nucleotide Reverse Transcriptase Inhibitor; NNRTI-Non Nucleoside Reverse Transcriptase Inhibitor
Mother-Infant Pairs
The direct, pairwise comparisons of 49 mother-infant pairs reveal different resistance patterns between mothers and infants. In 69% of pairs, either the mother, infant, or both were resistant to one or more ARV. The heat-map in Figure 4A is subdivided by class of ARV and color-coded by resistance level. The individual breakdown of ARV resistance in the mother-infant pairs highlights the difference in HIVDR prevalence between the two groups, and more importantly from mother to paired infant (Fig. 4A). For example, in most of the pairs, the infant has resistance to 2-5 NNRTIs and the mother has no NNRTI resistance. Of note, there are cases where the infants have more NRTI resistance compared to their paired mothers (pairs 096, 094, and 115; Fig. 4A). Additionally, the total resistance scores of the matched mother-infant pairs are quantified and compared in Figure 4B. In over half (29/49) of the pairs, higher level resistance was detected in the infants than the paired mothers. Higher maternal resistance was only detected in 3/49 pairs (quantified in Fig. 4B). Pairs 078 and 143 are cases that demonstrate very high levels of resistance in the mothers, where their infants have no resistance. Pairs 012 and 033 exemplify the cases where mothers and infants have near equivalent resistance patterns; overall 17/49 pairs had near equivalent resistance scores (15 of which had no detectable resistance in either party and are not shown in Fig. 4).
Figure 4. Pairwise comparison of mothers and infants by ARV resistance and resistance scores.

Statistical significance was determined by Wilcoxon Matched-Pairs Signed Rank Tests. In 15/49 pairs, neither the mother nor the infant had any resistance and are not included in this figure. (A) The heat map is colored discreetly by level of resistance outputted by Stanford HIVdb. (B) The total resistance scores of the paired mother and infant are connected by a line.
(*p<0.05, **p<0.01, ***p<0.001, ****p<0.0001)
DRM-Drug Resistance Mutations; ARV-antiretroviral; PI-Protease Inhibitor; NRTI-Nucleotide Reverse Transcriptase Inhibitor; NNRTI-Non Nucleoside Reverse Transcriptase Inhibitor; ABC-Abacavir; AZT-Zidovudine; D4T-Stavudine; DDI-Didanosine; FTC-Emtricitabine; 3TC-Lamivudine; TDF-Tenofovir Disoproxil Fumarate; DOR-Doravirine; EFV-Efavirenz; ETR-Etravirine; NVP-Nevirapine; RPV-Rilpivirine
Discussion
In Zambia, the MTCT rate decreased from 20.1% without PMTCT to 4.2% with PMTCT [1], which is complementary to the decrease in MTCT rate over time from 21% in 2009 to 2% in 2017 [4]. However, we have reported potentially dangerous levels of DRM in infants increasing over this time period: 21% in 2007 to 40% in 2014 [10] and 70% in 2016, 69% in 2017, and 62% in 2018 (this study). In our study (2015-2018), we found that 67% of infants had at least one DRM, which is comparable to other recent studies in the region. For example, a study of Nigerian infants <18-months old between the years of 2014-2015 detected DRM in 48% (45% NNRTI, 22% NRTI, 20% NNRTI+NRTI, 2% PI) of the infants [24]. Globally, however, this is not consistent among all populations. A study of South African, Argentinian, and Brazilian mother-infant pairs found only 11% of infants with DRM (10% NNRTI, 2% NRTI, 2% PI), and DRM were found in greater than 10% of treatment-naïve pregnant women [14]. This points to the lack of resources and monitoring in low-income, rural communities leaving mothers and infants at risk for developing high level HIVDR. Additionally, a review of HIV-1 RT sequences submitted to GenBank from 2000-2013 found the proportion of individuals in SSA with at least one DRM has increased annually since ART scale-up [25]. In the end, Option B+ PMTCT is helping to decrease the transmission of HIV from mothers to infants globally and in SSA, but when transmission occurs, high level NNRTI resistance is frequently detected.
In our study, HIV-infected infants had higher prevalence of DRM than their paired mothers. NNRTI DRM were detected more often than other classes of ARVs in both mothers and infants, consistent with other studies. NNRTI DRM were detected at high frequencies among patients failing-first line treatments admitted to the AIDS clinical treatment center of Guangxi [26]. Additionally, in our previous work, we found high levels of Y181C mutants leading to NNRTI resistance in infants [10]. However, in this study NNRTI DRM K103N/S exceeds the prevalence of Y181C/V (Fig. 2B). NRTI DRM occur less frequently, and PI DRM are nominal in our population. Similarly, in a study of PI-naïve HIV-1 subtype C-infected individuals from South Africa, PI resistance occurred in less than 1% of individuals over a 13-year period [27].
It is currently unclear whether the mother is transmitting a drug resistant variant or if the infant is developing DRM de novo. Our study of mother-infant pairs suggests two possibilities: a minor variant is transmitted from mother to infant and/or the infant receives sub-optimal dosing of ART selecting for DRM-containing variants. We postulate the minor variant is transmitted rather than the major variant since the two major variants are different in the paired samples, and we see no correlation between infant age or time between mother and infant DBS collection dates and HIVDR score. Due to the limitations of our study, future studies are needed to investigate whether the transmitted minor variant contains DRM and/or if the infant is developing DRM de novo as a result of sub-optimal dosing of ART. First, we recognize that we used population-level sequencing rather than single-genome techniques limiting our ability to conclude whether the viruses with DRM in infants are acquired de novo or transmitted from the mother, since the minor variants will not appear in population level sequencing. Second, we amplified and analyzed proviral DNA which may not be representative of the circulating viral pool. Third, our study population consists of HIV-infected mothers who gave birth to HIV-infected infants, which is less than 2% of births to HIV-infected mothers on ART [4]. Of this 2%, about 67% of the infants harbor viruses with DRM based on the results of this study. This is a relatively small population in which we were expecting to find a larger prevalence of M184V mutants. We postulate that the high level NNRTI resistance and lack of M184V mutation may be due to low adherence to ART regimens or rare anomalies in the population. Nonetheless, this population should not be ignored since these HIV-infected infants will represent a large proportion of the next HIV-infected generation. In this next generation, the increased resistance will make controlling and treating the virus more difficult. Further, PMTCT has been shown to prevent transmission in most cases, but to accomplish the goal of eliminating new HIV infections we need to develop more efficient ways to prevent DRM development, monitor DRM, and treat infants with high level HIVDR.
In conclusion, NNRTI resistance is high in HIV-infected mother-infant pairs in Zambia; while NRTI resistance is moderate, and PI resistance is rare in our two studies conducted on HIV-infected infants in Zambia. Resistance was more prevalent in infants compared to mothers but when detected, there is no significant difference in HIVDR magnitude. Resistance testing can be used to design a treatment regimen for each HIV-infected mother and infant, and routine screening can aid in the prevention of treatment failure. In 2018, Zambia began switching treatment of HIV-1 infected mothers and infants to PI-based and INI-based regimens from the NNRTI-based regimens. The results of this study fully support the change and outline how severe the resulting DRM profiles can be if the change is not implemented. Future work should include surveillance of HIVDR with the phasing out of NNRTI and the introduction of PI-based regimens, investigation of the effects of pre-treatment HIVDR on HIV treatment, and use of single-genome sequencing strategies to better understand the relationship between mother and infant DRM to discover the origins and evolution of DRM in HIV-1 infected infants.
Supplementary Material
Supplemental Digital Content 1. Table that contains cohort characteristics and sequence/resistance summaries from the results of sequence analysis by Stanford’s HIVdb (https://hivdb.stanford.edu/). Xlsx
Acknowledgements
ST, data collection and analysis; CC, study design and sample collection; JTW and CW, study design and data analysis; KM, data collection; LP, data analysis; CC, sample collection. Writing, editing, and finalizing the manuscript was completed by all authors. We would like to thank Danielle Shea for training us in the laboratory techniques used in this study, Ruth Katuta for patient recruitment and clinical data collection, and Kaunda Lembalemba for data management. Finally, we would like to thank the study participants for their generosity in making this study possible. This research was supported by NIH/NCI U54CA221204 and NIH/Fogarty D43 TW010354.
Funding
NIH/NCI U54CA221204 and NIH/Fogarty D43 TW010354
Footnotes
Conflicts of Interest
The authors have no conflicts of interest.
References
- 1.Gumede-Moyo S, Filteau S, Munthali T, Todd J, Musonda P. Implementation effectiveness of revised (post-2010) World Health Organization guidelines on prevention of mother-to-child transmission of HIV using routinely collected data in sub-Saharan Africa. Medicine (Baltimore) 2017; 96:e8055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wertheim JO, Oster AM, Johnson JA, Switzer WM, Saduvala N, Hernandez AL, et al. Transmission fitness of drug-resistant HIV revealed in a surveillance system transmission network. Virus Evol 2017; 3:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chanda BC, Likwa RN, Zgambo J, Tembo L, Jacobs C. Acceptability of option B+ among HIV positive women receiving antenatal and postnatal care services in selected health centre’s in Lusaka. BMC Pregnancy Childbirth 2018; 18:510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mutanga JN, Mutembo S, Ezeamama AE, Fubisha RC, Sialondwe D, Simuchembu B, et al. Tracking Progress Toward Elimination of Mother to Child Transmission of HIV in Zambia: Findings from the Early Infant Diagnosis of HIV Program (2009–2017). J Trop Pediatr 2019; :1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shaw GM, Hunter E. HIV Transmission. Cold Spring Harb Perspect Med 2012; 2:a006965–a006965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chagomerana MB, Miller WC, Tang JH, Hoffman IF, Harrington BJ, DiPrete B, et al. Prevalence of antiretroviral therapy treatment failure among HIV-infected pregnant women at first antenatal care: PMTCT Option B+ in Malawi. PLoS One 2018; 13:e0209052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Compagno F, Naegele K, Kahlert CR, Hsli I, Aebi-Popp K, Martinez de Tejada B, et al. The rate of mother-to-child transmission of antiretroviral drug-resistant HIV strains is low in the Swiss Mother and Child HIV Cohort Study. Swiss Med Wkly 2019; :1–9. [DOI] [PubMed] [Google Scholar]
- 8.Machnowska P, Meixenberger K, Schmidt D, Jessen H, Hillenbrand H, Gunsenheimer-Bartmeyer B, et al. Prevalence and persistence of transmitted drug resistance mutations in the German HIV-1 Seroconverter Study Cohort. PLoS One 2019; 14:e0209605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kanthula R, Rossouw TM, Feucht UD, Van Dyk G, Beck IA, Silverman R, et al. Persistence of HIV drug resistance among South African children given nevirapine to prevent mother-to-child-transmission. Aids 2017; 31:1143–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poppe LK, Chunda-Liyoka C, Kwon EH, Gondwe C, West JT, Kankasa C, et al. HIV drug resistance in infants increases with changing prevention of mother-to-child transmission regimens. AIDS 2017; 31:1885–1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuhn L, Hunt G, Technau K-G, Coovadia A, Ledwaba J, Pickerill S, et al. Drug resistance among newly diagnosed HIV-infected children in the era of more efficacious antiretroviral prophylaxis. AIDS 2014; 28:1673–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fang G, Burger H, Grimson R, Tropper P, Nachman S, Mayers D, et al. Maternal plasma human immunodeficiency virus type 1 RNA level: a determinant and projected threshold for mother-to-child transmission. Proc Natl Acad Sci 1995; 92:12100–12104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Itiola AJ, Goga AE, Ramokolo V. Trends and predictors of mother-to-child transmission of HIV in an era of protocol changes: Findings from two large health facilities in North East Nigeria. PLoS One 2019; 14:e0224670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yeganeh N, Kerin T, Ank B, Watts DH, Camarca M, Joao EC, et al. Human Immunodeficiency Virus Antiretroviral Resistance and Transmission in Mother-Infant Pairs Enrolled in a Large Perinatal Study. Clin Infect Dis 2018; 66:1770–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salimo AT, Ledwaba J, Coovadia A, Abrams EJ, Technau K-G, Kuhn L, et al. The use of dried blood spot specimens for HIV-1 drug resistance genotyping in young children initiating antiretroviral therapy. J Virol Methods 2015; 223:30–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alzohairy A. BioEdit: An important software for molecular biology. ; 2011. [Google Scholar]
- 17.Liu TF, Shafer RW. Web Resources for HIV Type 1 Genotypic-Resistance Test Interpretation. Clin Infect Dis 2006; 42:1608–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rhee S-Y. Human immunodeficiency virus reverse transcriptase and protease sequence database. Nucleic Acids Res 2003; 31:298–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Melikian GL, Rhee SY, Varghese V, Porter D, White K, Taylor J, et al. Non-nucleoside reverse transcriptase inhibitor (NNRTI) cross-resistance: Implications for preclinical evaluation of novel NNRTIs and clinical genotypic resistance testing. J Antimicrob Chemother 2014; 69:12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu H-T, Colby-Germinario SP, Asahchop EL, Oliveira M, McCallum M, Schader SM, et al. Effect of Mutations at Position E138 in HIV-1 Reverse Transcriptase and Their Interactions with the M184I Mutation on Defining Patterns of Resistance to Nonnucleoside Reverse Transcriptase Inhibitors Rilpivirine and Etravirine. Antimicrob Agents Chemother 2013; 57:3100–3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sluis-Cremer N, Jordan MR, Huber K, Wallis CL, Bertagnolio S, Mellors JW, et al. E138A in HIV-1 reverse transcriptase is more common in subtype C than B: Implications for rilpivirine use in resource-limited settings. Antiviral Res 2014; 107:31–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Basson AE, Rhee SY, Parry CM, El-Khatib Z, Charalambous S, De Oliveira T, et al. Impact of drug resistance-associated amino acid changes in HIV-1 subtype C on susceptibility to newer nonnucleoside reverse transcriptase inhibitors. Antimicrob Agents Chemother 2015; 59:960–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turner D, Brenner B, Wainberg MA. Multiple Effects of the M184V Resistance Mutation in the Reverse Transcriptase of Human Immunodeficiency Virus Type 1. Clin Vaccine Immunol 2003; 10:979–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Inzaule SC, Osi SJ, Akinbiyi G, Emeka A, Khamofu H, Mpazanje R, et al. High prevalence of HIV drug resistance among newly diagnosed infants aged <18 months. J Acquir Immune Defic Syndr 2017; 77:1. [DOI] [PubMed] [Google Scholar]
- 25.Rhee S-Y, Blanco JL, Jordan MR, Taylor J, Lemey P, Varghese V, et al. Geographic and Temporal Trends in the Molecular Epidemiology and Genetic Mechanisms of Transmitted HIV-1 Drug Resistance: An Individual-Patient- and Sequence-Level Meta-Analysis. PLOS Med 2015; 12:e1001810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luo X, Mo L, Su G, Huang J, Wu J, Su H, et al. Incidence and types of HIV-1 drug resistance mutation among patients failing first-line antiretroviral therapy. J Pharmacol Sci 2019; 139:275–279. [DOI] [PubMed] [Google Scholar]
- 27.Ledwaba J, Sayed Y, Pillay V, Morris L, Hunt G. Low Frequency of Protease Inhibitor Resistance Mutations and Insertions in HIV-1 Subtype C Protease Inhibitor-Naïve Sequences. AIDS Res Hum Retroviruses 2019; 00:aid.2019.0012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content 1. Table that contains cohort characteristics and sequence/resistance summaries from the results of sequence analysis by Stanford’s HIVdb (https://hivdb.stanford.edu/). Xlsx


