Abstract
Background
Coronavirus disease 2019 (COVID-19) presents a unique challenge to United States Navy hospital ships. The aim of this study was to determine the prevalence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection among US Navy personnel deployed on the USNS COMFORT to augment the inpatient health care capacity in New York City.
Methods
This was a cross-sectional study conducted on USNS COMFORT crewmembers returning to Norfolk, Virginia, following deployment. Participants completed an electronic questionnaire and provided a serum sample at Day 14 post-deployment. Polymerase chain reaction (PCR) results from testing of symptomatic crewmembers during deployment and Day 0 and Day 14 post-deployment screening swabs conducted on all crewmembers, per military order, were abstracted. SARS-CoV-2 infection was defined as a positive SARS-CoV-2 spike glycoprotein immunoglobulin G antibody or PCR result.
Results
Of the ship’s total complement of 1200 crewmembers, 450 were enrolled: 432 (96.0%) completed the questionnaire and provided a serum sample. The median age of participants (interquartile range) was 30 (24–39) years, 50.8% were female, 58.6% were White, and 14.0% were Black; 80.1% had a clinical role during deployment. The cumulative prevalence of SARS-CoV-2 infection was 3.01% (13/432; 95% CI, 1.61%–5.09%). Twelve of 13 infections occurred in health care providers, and 8 of 13 were asymptomatic. The antibody profile of infected crewmembers varied by suspected timing of infection.
Conclusions
We observed a low prevalence of SARS-CoV-2 infection among USNS COMFORT crewmembers despite the inherent risk of a shipboard deployment to an area with high rates of community transmission. Our findings suggest that early infection control measures mitigated the spread of SARS-CoV-2 among crewmembers.
Keywords: epidemiology, health care workers, SARS-CoV-2, shipboard
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) presents a unique challenge to United States Navy hospital ships due to the provision of health care within the close confines of a shipboard setting. Coronavirus disease 2019 (COVID-19) outbreaks on the aircraft carrier USS THEODORE ROOSEVELT [1] and the destroyer USS KIDD occurred while the ships were underway and involved ~30% of the crew [2]. The high proportion of asymptomatic and mildly symptomatic infections in young, healthy active duty crewmembers led to delayed detection and rapid spread of the virus [3]. Outbreaks on ships are also propagated by the inability to enforce physical distancing in congregate living quarters with shared toilets, crowded galleys (ie, cafeteria), tight workspaces, and the utilization of recirculated air [4, 5]. Implementing infection control measures to prevent nosocomial transmission to health care workers (HCWs) on a hospital ship is especially challenging due to open bay wards, limited negative pressure rooms, and the close proximity and open access between hospital wards and berthing spaces.
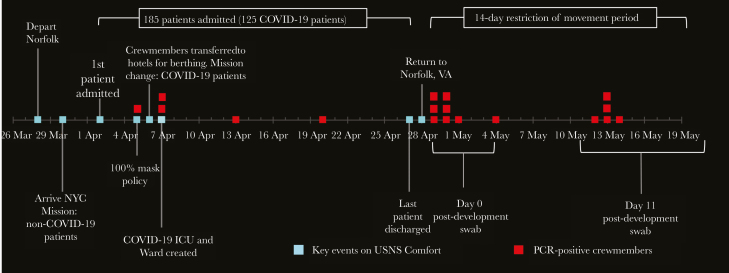
The USNS COMFORT is a rapidly deployable, 1000-bed hospital ship that has deployed 12 times for humanitarian missions and disaster relief since 1994. The ship was designed to support warfighters, so it has open bay wards of ~30 beds, excellent operating rooms, and post-anesthesia care units for a trauma hospital, but lacks enhancements relevant to infection control such as private rooms and negative pressure rooms. From March 28 to April 30, 2020, a crew of ~1000 US Navy personnel were deployed on the USNS COMFORT from Norfolk, Virginia, to New York City (NYC) to assist with the COVID-19 medical response. An additional 200 military health care workers joined the crew in NYC. Crewmembers were screened for ILI symptoms and a temperature check but were not quarantined or screened for SARS-CoV-2 by polymerase chain reaction (PCR) before embarkation. The initial mission was to reduce the patient burden on NYC hospitals by providing medical care for non-COVID-19 patients. Crewmembers did not observe 100% masking and eye protection use during the first week. The mission was shifted to the care of COVID-19 patients within 1 week of arrival due to the relative paucity of non-COVID-19 patients. The timeline of events and COVID-19 cases among crewmembers is summarized in Figure 1. Infection control measures were instituted in the first week of deployment in response to the change in mission, the diagnosis of COVID-19 in a symptomatic crewmember, and exposure of HCWs caring for a “non-COVID” patient who was found to be COVID positive after 3 preceding negative tests. These measures included 100% masking (ie, surgical masks for all personnel and N95 respirators, eye protection, and gowns in the COVID-19 unit), strict hand hygiene, frequent cleaning and disinfection of surfaces, isolation of symptomatic crewmembers for at least 7 days, separation of clinical and nonclinical spaces, and monitoring of PPE compliance. Intensive care unit (ICU) bays were converted into COVID ICUs, and a dedicated negative pressure isolation room was designed with return air passed through high-efficiency particulate air (HEPA) filters [6]. Most of the clinical crew was moved from the ship to single-occupancy hotel rooms in the city; they were only permitted to travel between the hotel and ship in order to minimize community exposures and reduce the risk of shipboard transmission in living spaces. During deployment, 39 crewmembers reported respiratory symptoms and were tested and isolated, and 5 (13%) were SARS-CoV-2 PCR positive. Following completion of the mission, 1159 personnel returned to Norfolk, Virginia, for a 14-day quarantine (restriction of movement [ROM]) and underwent nasopharyngeal (NP) swabs for SARS-CoV-2 PCR testing on Day 0 and Day 14 post-deployment, per military order. Fourteen (1.5%) were PCR positive during ROM (1 of whom was also positive during mission), resulting in 18 total PCR positives during and after deployment for >1200 personnel (1.5%).
Figure 1.
Timeline of USNS COMFORT mission and confirmed cases of COVID-19 in crewmembers. The figure represents all crewmembers (ie, not limited to study participants) and illustrates significant events and the first positive PCR test for each positive crewmember. Crewmembers were screened for influenza-like illness symptoms and a temperature check but were not quarantined or screened for SARS-CoV-2 by PCR before embarkation. Universal COVID-19 precautions (ie, 100% masks, eye protection, contact precautions) were not used during the first week when the mission was caring for non-COVID-19 patients. Three symptomatic crewmembers were diagnosed with COVID-19 during the first 10 days of deployment. The first was a non-HCW who likely acquired infection before embarkation. The second, also a non-HCW, was a close contact of the first positive crewmember. The third was a provider who performed an AGP on a “non-COVID-19” admission who was later found to be SARS-CoV-2 positive on repeat testing. Ten patients with an unknown COVID-19 status were admitted during the first week. These patients were given surgical masks, and all were screened by PCR; 3 were positive and kept in isolation. However, the staff had a high index of suspicion for SARS-CoV-2 infection in some of the PCR-negative patients based on symptoms and CT findings. In the first week of deployment, an 11-bed COVID-19 ICU was created along with a negative pressure room used to perform AGPs. However, some AGPs such as an emergent re-intubation and management of a bleeding tracheostomy site could not be conducted in the negative pressure room. Presumed COVID-19-negative patients who underwent bronchoscopy or induced sputum for bacterial culture were later found to be COVID-19 positive. Seventy-eight health care–related COVID-19 exposures among crewmembers were reported during the first week, and these members were placed in a working quarantine for 7 days (ie, symptom monitoring, appropriate masking and PPE during work hours, and quarantine in separate area of the ship during meals and off-work hours). Any crewmembers who became symptomatic were placed in isolation and tested by PCR. The mission was shifted to both COVID-19 and non-COVID-19 care a week after arrival in NYC, and the following infection control measures were instituted: 100% masking of crewmembers with surgical masks, conversion of several ICU bays into COVID ICUs with strict enforcement of PPE (N95 respirator, eye protection, gloves and gown), strict hand hygiene and frequent disinfection of surfaces, isolation of symptomatic crewmembers for at least 7 days, and separation of clinical and nonclinical spaces. There was an adequate supply of PPE during the mission, and adequate patient/staff ratios were maintained. In the ICUs, the nursing-to-patient ratio was ~1:2 for ICU-experienced nurses and 1:1 for the ER. In addition, most of the clinical crew was moved from the ship to single-occupancy hotel rooms and only permitted to travel between the hotel and ship in order to minimize exposure from the community. Two HCWs were diagnosed with symptomatic COVID-19 during the second week of deployment, presumably from health care–related exposures during the first week of deployment. Of the 5 crewmembers with COVID-19 during deployment, 1 enrolled in the study. A total of 1159 crewmembers returned to Norfolk, Virginia, by air or on the ship over a period of 6 days. Upon return, all crewmembers were required to stay in quarantine for 14 days (ROM) and undergo collection of NPs for SARS-CoV-2 PCR testing at Day 0 and Day 14, per military order. Fourteen of 1159 personnel (1.5%) tested after deployment were PCR positive during ROM (1 of whom was also positive during mission), resulting in 18 total PCR positives during and after deployment. Eight of these 18 PCR-positive personnel enrolled in this study. Abbreviations: AGP, aerosol-generating procedures; COVID-19, coronavirus disease 2019; CT, computed tomography; ER, emergency room; ICU, intensive care unit; NPs, nasopharyngeal swabs; PCR, polymerase chain reaction; PPE, personal protective equipment; ROM, restriction of movement; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Knowing the heightened risk of SARS-CoV-2 transmission in the shipboard environment, we conducted a cross-sectional, post-deployment study using serologic and PCR data to determine the proportion of crewmembers on the USNS COMFORT with COVID-19.
METHODS
All 1159 crewmembers who returned to Norfolk, Virginia, for ROM were eligible for the study, and no specific groups among the crew were targeted for enrollment. Study personnel contacted eligible crewmembers by phone and administered an electronic informed consent form and questionnaire using REDCap [7]. The questionnaire collected demographic data, living quarters and workspace, potential exposures to SARS-CoV-2 in the 2 weeks before and during deployment, and the occurrence of symptoms (ie, headache, fever, sore throat, shortness of breath, cough, loss of taste, loss of smell, fatigue, muscle aches, decreased appetite, nasal congestion/runny nose, nausea/vomiting, diarrhea), and/or testing during deployment. Serum was collected from participants when they presented to the testing site for their Day 14 NP swab. PCR results from testing of symptomatic crewmembers during deployment and Day 0 and Day 14 post-deployment screening swabs were abstracted from medical records. SARS-CoV-2 PCR testing on NP swabs was performed using the BioFire COVID-19 Test during deployment, and Day 0 and Day 14 post-deployment swabs were tested using LabCorp 2019 Novel Coronavirus (COVID-19) nucleic acid amplification test [8, 9]. All enrollments and study procedures were conducted between May 8 and May 16, 2020. The planned sample size was 450 enrollees, based on feasibility of enrolling enough participants in a limited time period to capture a 5% (precision ±2%) seroprevalence of SARS-CoV-2 IgG antibodies in the study population.
Serum specimens were tested using a multiplex microsphere–based immunoassay (MMIA) that included the envelope spike (S) glycoproteins from 5 medically relevant betacoronaviruses (SARS-CoV-2, SARS-CoV-1, MERS-CoV, HCoV-HKU1, and HCoV-OC43) and the receptor binding domain (RBD) of the SARS-CoV-2 surface glycoprotein. Prefusion stabilized SARS-CoV-2 S-2P glycoprotein ectodomain trimers (LakePharma, Inc., Hopkinton, MA, USA) were used. Samples were screened for SARS-CoV-2 spike protein IgG in duplicate plates at a dilution of 1:400 and analyzed on a Bio-Plex 200 multiplexing system (Bio-Rad, Hercules, CA, USA), based on Luminex xMAP technology. The phosphate-buffered saline–adjusted median fluorescence intensity (MFI) values were reported. SARS-CoV-2 spike protein IgG-positive samples were further evaluated for an end point dilution titer and RBD IgG antibodies to evaluate the potential neutralizing activity of antibodies. Sera from subjects who were SARS-CoV-2 spike protein IgG or SARS-CoV-2 PCR positive were also tested for the presence of SARS-CoV-2 spike protein IgM antibodies to estimate the timing of the infection. The performance characteristics of the SARS-CoV-2 spike protein IgG assay on sera from SARS-CoV-2 PCR-positive patients collected ≥10 days post–symptom onset and negative controls are as follows: sensitivity 0.980 (95% CI, 0.931–0.998), specificity 1.00 (95% CI, 0.968–1.00), positive predictive value at 5% seroprevalence 1.00 (95% CI, 0.95–1.00), negative predictive value 0.983 (95% CI, 0.933–0.997) [10].
The main end point was SARS-CoV-2 infection, defined as a positive SARS-CoV-2 spike protein IgG or PCR result. Descriptive statistics were used to determine the prevalence and 95% confidence interval and to present the survey and laboratory data. Data analysis was performed using R software (version 4.0.2; The R Foundation).
All participants provided informed consent using an electronic consent platform (REDCap) before participation. The study was approved by the Naval Medical Center, Portsmouth, Virginia Institutional Review Board (protocol number USUID.2020.0093).
RESULTS
Study enrollment was capped at 450 subjects: 449 (99.7%) completed the online questionnaire, and 432 (96.0%) completed the Day 14 post-deployment blood draw. Seventeen subjects completed the questionnaire but did not provide a blood sample and were PCR negative. One subject was unable to provide an NP swab on Day 0 and had it collected on Day 7 instead. All other subjects completed the Day 0 and Day 14 NP swabs. The median age of participants (interquartile range) was 30 (24–39) years, 50.8% were female, 58.6% were white, and 14.0% were black (Table 1). Seventy-nine percent had a clinical role during deployment (43.9% corpsmen, 26.3% nurses, and 9.1% physicians or medical assistants), and 20.7% had a nonclinical role. Thirteen of the 432 participants (3.01%) tested SARS-CoV-2 PCR or IgG antibody positive (Table 1). Twelve of 13 SARS-CoV-2 infections occurred in HCWs: 6 nurses, 5 corpsmen, and 1 physician. Eight of the 13 (61.5%) were asymptomatic. Of the 5 symptomatic crewmembers, 3 sought medical care during deployment and were tested for SARS-CoV-2 by PCR: 2 were negative, and 1 was positive. All symptomatic crewmembers were isolated for at least 7 days.
Table 1.
Characteristics of the Overall Cohort and by SARS-CoV-2 infectiona
| Overall (n = 432), No. (%) | SARS-CoV-2 Negative (n = 419), No. (%) | SARS-CoV-2 Positive (n = 13), No. (%) | |
|---|---|---|---|
| (n = 432) | |||
| Age, y | |||
| 18–29 | 209 (49.4) | 201 (48.0) | 8 (61.5) |
| 30–39 | 120 (28.4) | 118 (28.2) | 2 (15.4) |
| 40+ | 103 (24.4) | 100 (23.9) | 3 (23.1) |
| Female gender | 215 (50.8) | 207 (49.4) | 8 (61.5) |
| Race | |||
| White | 248 (58.6) | 242 (57.8) | 6 (46.2) |
| Black | 59 (14) | 57 (13.6) | 2 (15.4) |
| Hispanic | 40 (9.5) | 39 (9.3) | 1 (7.7) |
| Other | 85 (20) | 81 (19.3) | 4 (30.8) |
| Any direct interaction with COVID-19 patients or individuals in the 2 wk before deployment? | |||
| No | 168 (39.7) | 167 (39.9) | 1 (7.7) |
| Don’t know | 66 (15.6) | 63 (15.0) | 3 (23.1) |
| Yes | 198 (46.8) | 189 (45.1) | 9 (69.2) |
| Primary workspace during deployment: ICU or Wardb | 286 (67.6) | 274 (65.4) | 12 (92.3) |
| Spent two-thirds or more of their time in direct patient care during deployment | 252 (59.6) | 241 (57.5) | 11 (84.6) |
| Performed aerosol-generating procedures | 96 (22.2) | 91 (21.7) | 5 (38.4) |
| Clinical role during deployment | |||
| Nonclinicalc | 86 (20.3) | 85 (20.3) | 1 (7.7) |
| Corpsman | 193 (45.6) | 188 (44.9) | 5 (38.5) |
| Nurse | 114 (26.9) | 108 (25.8) | 6 (46.2) |
| Physician/medical assistant | 39 (9.2) | 38 (9.1) | 1 (7.7) |
| Direct care of COVID-19 patients during deployment | 303 (71.6) | 291 (69.5) | 12 (92.3) |
| Anyone in workspace/berthing/social circle placed in isolation/quarantine | |||
| No | 88 (20.8) | 87 (20.8) | 1 (7.7) |
| Don’t know | 65 (15.3) | 62 (14.8) | 3 (23.1) |
| Yes | 279 (66) | 270 (64.4) | 9 (69.2) |
| Symptoms reported during deployment | 48 (11.1) | 43 (10.3) | 5 (38.5) |
| Berthing during deployment | |||
| Enlisted berthing | 77 (18.2) | 77 (18.4) | 0 (0.0) |
| Nongovernment organization berthing | 17 (4) | 16 (3.8) | 1 (7.7) |
| Officer berthing | 59 (14) | 58 (13.8) | 1 (7.7) |
| Private hotel room | 279 (66) | 268 (64.0) | 11 (84.6) |
| Place where meals were consumed | |||
| Galley | 138 (32.6) | 136 (32.5) | 2 (15.4) |
| Hotel room | 212 (50.2) | 204 (48.7) | 8 (61.5) |
| Other | 19 (4.5) | 18 (4.3) | 1 (7.7) |
| Workspace | 63 (14.9) | 61 (14.6) | 2 (15.4) |
Abbreviations: COVID-19, coronavirus disease 2019; ICU, intensive care unit; PCR, polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
aSubjects who completed a survey and had serology and/or PCR data (n = 432).
bICU/Ward included Patient/Casualty Receiving, Sick Bay, and Dental. Non-ICU/Ward setting included Operating Room, Laboratory, Post-Anesthesia Care Unit, Sick Call Pharmacy, Radiology, Galley, Administrative.
cNonclinical roles were those without direct patient care such as ashore Liason Officer, public affairs office, quarterdeck or radio operators, pharmacy staff, oxygen plant operators, flight deck, and administrative staff in patient care areas.
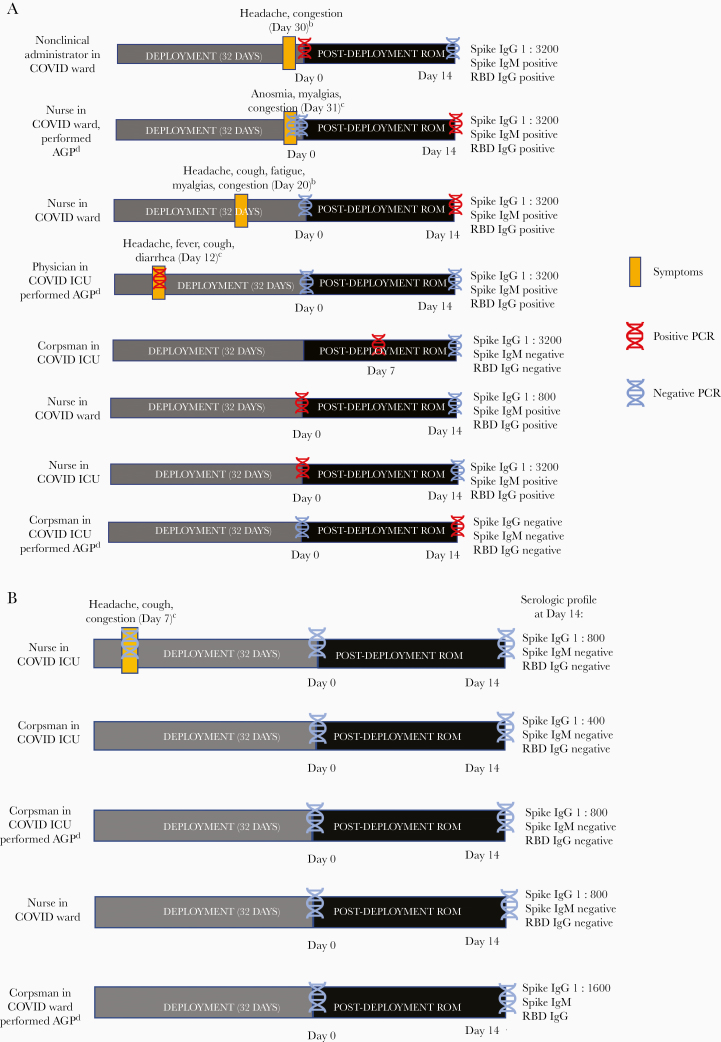
The serologic profiles of subjects expectedly varied, dependent on PCR status and presence of symptoms, and provided an additional immunological window to narrow the suspected timing of infection (Figure 2). Crewmembers who were symptomatic and PCR positive during deployment or on postdeployment screening (n = 4) had high SARS-CoV-2 spike protein IgG end point dilution titers (≥1:3200) and were additionally spike protein IgM and RBD IgG positive. Asymptomatic, PCR-positive crewmembers with a 14-day interval between the positive PCR screen and serologic testing (n = 2) had a similar antibody profile (spike protein IgG/IgM and RBD IgG positive) to symptomatic crewmembers, while a crewmember with a 7-day interval between the positive PCR screen and serologic testing was spike protein IgM positive only. An asymptomatic crewmember who was PCR positive on Day 14 was seronegative. Four of the 5 PCR-negative, asymptomatic subjects who had detectable spike protein IgG antibodies were IgM- and RBD IgG–negative, suggesting that they either had a remote SARS-CoV-2 infection before or early during deployment with waning convalescent antibodies or a less robust antibody response.
Figure 2.
A, Serologic characteristics of PCR-positive crewmembers.a B, Serologic profile of PCR-negative and spike protein IgG–positive crewmembers. aOnly positive PCR and/or serologic results are shown in the figure. All subjects underwent Day 0 and Day 14 post-deployment swabs (except the fifth subject, who had a Day 7 instead of Day 0 swab) and Day 14 serologic testing with SARS-CoV-2 spike protein IgG with end point dilution titer, IgM, and RBD IgG. bDid not report symptoms to health care staff during deployment and was not tested by PCR or isolated. cReported symptoms to health care staff during deployment and was tested by PCR and isolated. Abbreviations: AGP, aerosol-generating procedures; IgG, immunoglobulin G; IgM, immunoglobulin M; PCR, polymerase chain reaction; ROM, restriction of movement; RBD, receptor binding domain; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
DISCUSSION
Among a shipboard population of US Navy personnel deployed to NYC on the USNS COMFORT, we observed a low prevalence of SARS-CoV-2 infections (3.01%; 95% CI, 1.61%–5.09%), likely due to sporadic transmission from patients to health care workers without extensive transmission among the ship’s crew. At the time of deployment in April, the estimated seroprevalence among NYC residents was 22.7% (95% CI, 21.5%–24.0%) [11]. Our finding suggests that infection control measures instituted during the first week of deployment were effective in preventing nosocomial and “community” (ie, outside the clinical space on the ship) transmission among crewmembers, 80% of whom were HCWs with a significant risk of work-related exposures. This complements a growing body of observational data demonstrating the effectiveness of infection control measures and PPE in preventing nosocomial transmission of SARS-CoV-2 to HCWs [12–14]. Seroprevalence studies conducted in HCWs during the peak of the epidemic in Long Island, New York [15], Spain [16], and China [17] found a significantly lower prevalence of SARS-CoV-2 antibodies compared with the general public.
In addition to standard infection control measures and a 100% face mask policy, innovative measures were used to overcome the challenges of physical distancing on the ship. The interior of the USNS COMFORT was restructured to separate clinical and nonclinical spaces, and most HCWs were transferred to single-occupancy hotel rooms following their work shift. This strategy was likely more effective than a traditional symptom- or exposure-based approach to testing and isolation, as the cases of SARS-CoV-2 among crewmembers were asymptomatic or mild and were not always reported to medical providers. In contrast to the USNS COMFORT, the USS THEODORE ROOSEVELT was underway when the first cases of COVID-19 occurred among crewmembers, and implementing physical distancing, masking, or quarantines was difficult. A sharp rise in the number of cases (from 8 cases to 46) was observed as transfer of crewmembers to single-occupancy hotel rooms was delayed for a period of 2–3 days [18]. An outbreak investigation report found that 62% of sampled crewmembers on the USS THEODORE ROOSEVELT were positive for SARS-CoV-2 by PCR or serology. Behaviors associated with lower risk of infection included avoidance of common areas and use of face coverings [5]. The impact of social distancing and quarantines in a shipboard setting was also demonstrated in a study of the Diamond Princess Cruise Ship that reported a marked reduction in the transmission potential of SARS-CoV-2 (from a mean reproduction number [Rt] ~11 to Rt ~ 0.35) following the implementation of a 14-day quarantine period during which passengers and crew were restricted to their cabins [19]. Although the USNS COMFORT did not experience a COVID-19 outbreak or enforce a stringent quarantine, infection control measures likely had a similar impact on transmission potential, as crewmembers were living in single-occupancy hotel rooms and observed 100% masking, strict hand hygiene, and appropriate use of PPE while working on the ship.
The evaluation of antibody responses in the context of PCR and survey data provided several insights into the humoral immune response to SARS-CoV-2, despite the limitations of a single blood draw. Subjects who were symptomatic during deployment and were PCR positive either during or after deployment had robust antibody responses with high titers (1:3200) of spike protein IgG and detection of spike protein IgM and RBD IgG antibodies. In contrast, 4 of 5 subjects who were positive by serology but negative by PCR had lower titers of spike protein IgG (1:800–1:1600) and were spike protein IgM and RBD IgG negative, suggesting a longer duration between infection and serologic testing and/or a less robust immune response with the lack of RBD-reactive IgG antibodies. It is possible that these HCWs were infected before deployment. Crewmembers did not undergo predeployment testing for SARS-CoV-2, as testing capabilities were limited and Center for Disease Control recommendations limited testing to symptomatic individuals who were hospitalized, had comorbid conditions associated with poor outcomes, or had been in close contact with a known or suspected COVID-19 case in the preceding 2 weeks. Most study participants, including the 4 who were only positive for spike protein IgG, were front-line HCWs stationed in Hampton Roads, Virginia, before deployment, where the COVID-19 prevalence in the community was low. At the time of deployment, Naval Medical Center Portsmouth, Virginia, had a total of 6 COVID-19 inpatients and 10 outpatients since the start of the pandemic and had appropriate infection control measures and provision of PPE to minimize risk of transmission to HCWs. The total number of COVID-19-positive cases in Norfolk, Virginia, at the end of March was ~100, and the prevalence in New York was roughly 15 times that of Virginia (28.25/100 000 population; 95% CI, 22.89–34.55; vs 462.24/100 000 population; 95% CI, 388.72–539.3), indicating that the risk of community-acquired COVID-19 before deployment was low [20]. Although 46% of crewmembers reported interacting with COVID-19 cases in the 2 weeks before deployment, we think this may be an overestimation of exposure risk by crewmembers in part due to limited availability of testing in March 2020 to inform risk perception. Nonetheless, predeployment screening with SARS-CoV2 PCR would have prevented asymptomatic or presymptomatic individuals with COVID-19 from embarking and would assist with ascertaining the timing of infection, especially in cases that were PCR negative but serology positive. In a study of hospitalized COVID-19 patients, IgM seroreversion was observed as early as 24 days (median, 46.9 days), but the longevity of IgM responses in mildly symptomatic or asymptomatic patients has not been completely elucidated [21]. Thus, the timing of infection in these individuals is difficult to determine and is a limitation of the cross-sectional study design. It is also possible that these were false-positive IgG results, although this is less likely given the high specificity and PPV of the assay even at a low prevalence and as all IgG-positive serum samples were independently tested a second time at 1:400 serum dilution, and then a third MMIA test was performed to determine end point titers. Luminex-based multiplexing systems have an inherently greater dynamic range and can detect end point titers to specific antigen 2–4-fold higher than enzyme-linked immunosorbent assays (in this case, the prefusion stabilized spike glycoprotein trimer) and are therefore able to detect early seroconversion in these asymptomatic individuals [9].
To meet the challenges associated with shipboard deployment during a pandemic, the US Navy has published Operational Guidelines to prevent and, when necessary, contain COVID-19 infections while remaining on mission [22]. These include the concept of creating a COVID-free “bubble” or unit before deployment with the institution of a 14-day ROM Sequester during which interactions with outside individuals is minimized and Sailors undergo frequent symptom and temperature checks and a screening PCR (depending on the deploying unit and mission). This COVID-free bubble is maintained during deployment by minimizing risk of COVID-19 exposure. Other measures instituted on naval vessels include universal masking, frequent hand washing or sanitizer use, eliminating self-service options in the galley, and maintaining social distancing when possible. Testing capabilities on naval vessels are being expanded, and the Navy COVID-19 Surveillance Testing Program conducts periodic screening of asymptomatic individuals in high-risk settings (HCWs, personnel on Naval vessels that are in port for maintenance, security personnel, etc.) [23].
Our study has several limitations. Due to the rapid deployment of the USNS COMFORT, we were unable to collect predeployment serum samples and were only able to access crewmembers who returned to Norfolk, Virginia, following deployment. Collection of serum samples at multiple time points and increasing the sampled population would have further refined our prevalence estimate for COVID-19 infections in this population. The distribution of clinical and nonclinical crewmembers in the sampled population was representative of the ship’s complement, but it is possible that we did not sample an adequate number of nonclinical crewmembers to ascertain the risk of COVID-19 among workers in other settings such as the galley. The symptom and risk factor data were based on self-report and could be affected by recall bias. We were unable to ascertain whether transmission was due to nosocomial or “community” spread, as this was not an outbreak investigation with contact tracing, and the limited number of SARS-CoV-2 cases precluded a multivariate risk factor analysis.
The findings of this study provide important insights into the epidemiology of COVID-19 on a military hospital ship providing COVID-19 patient care and the antibody responses of infected crewmembers. The risk of a large outbreak in the crew of the USNS COMFORT as they provided medical care to COVID-19 patients was significant. The shore-based berthing, along with the robust and extensive infection control measures, instituted early and universally applied, allowed the military crew to safely assist overwhelmed NYC hospitals while minimizing the risk of SARS-CoV-2 transmission.
Acknowledgments
The authors would like the thank the USNS COMFORT crewmembers for participating in the study and the team of clinical research coordinators and laboratory personnel from the Naval Medical Center Portsmouth, Virginia, for their support of and dedication to the project.
Author contributions. Concept and design: T.La., T.Le., K.C.K., E.V.M., T.B., T.E.W., E.D.L., C.C.B.; acquisition, analysis, or interpretation of data: T.La., T.Le., K.C.K., E.D.L., C.C.B., S.A.R., N.E.; drafting of the manuscript: T.La., T.Le., A.R.; critical revision of the manuscript for important intellectual content: all authors; statistical analysis: S.A.R., N.E., E.V.M.; administrative, technical, or laboratory support: E.D.L., A.R., E.C., M.L., M.B., T.B., T.M., S.P., E.S., H.N., C.C.B., T.E.W., T.B., K.C.K.
Declarations. The investigators have adhered to the policies for protection of human subjects as prescribed in 45CRF46. Research data were derived from an approved Naval Medical Center Portsmouth IRB (NMCP.2020.0093) protocol. The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Disclaimer. The contents of this publication are the sole responsibility of the author(s) and do not necessarily reflect the views, opinions, or policies of the Uniformed Services University of the Health Sciences (USU), The Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., the National Institute of Health or the Department of Health and Human Services, the US Department of Defense (DoD), the Departments of the Army, Navy, or Air Force, or the US Government. Mention of trade names, commercial products, or organization does not imply endorsement by the US Government. A number of the co-authors are military service members or employees of the US Government. This work was prepared as part of their official duties. Title 17 U.S.C. §105 provides that “Copyright protection under this title is not available for any work of the United States Government.” Title 17 U.S.C. §101 defines a US Government work as a work prepared by a military service member or employee of the US Government as part of that person’s official duties.
Financial support. This study was conducted by the Infectious Disease Clinical Research Program (IDCRP), a Department of Defense (DoD) program executed by the Uniformed Services University of the Health Sciences (USU) through a cooperative agreement with The Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc. (H.J.F.). This project has been funded in whole or in part with federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, under Inter-Agency Agreement Y1-AI-5072, and the Defense Health Program, US DoD, under award HU0001190002.
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. National Broadcasting Company 7 San Diego. USS Theodore Roosevelt COVID-19 Cases Exceed 1,100, Navy to Decrease Reporting. Available at: https://www.nbcsandiego.com/news/local/uss-theodore-roosevelt-covid-19-cases-exceed-1100-navy-to-decrease-reporting/2316749/. Accessed 19 August 2020.
- 2. Cable News Network. Number of coronavirus cases from second warship outbreak nears 100 as Navy restricts information on pandemic.Available at: https://www.cnn.com/2020/05/01/politics/uss-kidd-coronavirus-cases/index.html. Accessed 23 August 2020.
- 3. Alvarado GR, Pierson BC, Teemer ES, et al. Symptom characterization and outcomes of sailors in isolation after a COVID-19 outbreak on a US Aircraft Carrier. JAMA Netw Open 2020; 3:e2020981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Malone JD USS Theodore Roosevelt, COVID-19, and ships: lessons learned. JAMA Netw Open 2020; 3:e2022095. [DOI] [PubMed] [Google Scholar]
- 5. Payne DC, Smith-Jeffcoat SE, Nowak G, et al. ; CDC COVID-19 Surge Laboratory Group SARS-CoV-2 infections and serologic responses from a sample of U.S. Navy service members—USS Theodore Roosevelt, April 2020. MMWR Morb Mortal Wkly Rep 2020; 69:714–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hill CJ, Capra GG, McDonald TP, et al. Misconceptions about negative pressure rooms and their impact aboard USNS Comfort in New York City. Otolaryngol Head Neck Surg 2020; 163:1134–6. [DOI] [PubMed] [Google Scholar]
- 7. (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Labcorp. 2019 Novel Coronavirus (COVID-19), NAA. Available at: https://www.labcorp.com/tests/139900/2019-novel-coronavirus-covid-19-naa. Accessed 3 August 2020.
- 9. BioFire. BioFire’s Respiratory Solutions with SARS-CoV-2. Available at: https://www.biofiredx.com/covid-19/. Accessed 3August 2020.
- 10. Laing ED, Sterling SL, Richard SA, et al. A betacoronavirus multiplex microsphere immunoassay detects early SARS-CoV-2 seroconversion and controls for pre-existing seasonal human coronavirus antibody cross-reactivity. medRxiv 2020.10.14.20207050 [Preprint]. 16 October 2020 Available at: https://wwwmedrxivorg/content/101101/2020101420207050v1. Accessed 30 October 2020.
- 11. Rosenberg ES, Tesoriero JM, Rosenthal EM, et al. Cumulative incidence and diagnosis of SARS-CoV-2 infection in New York. Ann Epidemiol 2020; 48:23–29.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gomez-Ochoa SA, Franco OH, Rojas LZ, et al. COVID-19 in healthcare workers: a living systematic review and meta-analysis of prevalence, risk factors, clinical characteristics, and outcomes. Am J Epidemiol. 2021; 190:161–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wong SCY, Kwong RT, Wu TC, et al. Risk of nosocomial transmission of coronavirus disease 2019: an experience in a general ward setting in Hong Kong. J Hosp Infect 2020; 105:119–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ng K, Poon BH, Kiat Puar TH, et al. COVID-19 and the risk to health care workers: a case report. Ann Intern Med 2020; 172:766–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jeremias A, Nguyen J, Levine J, et al. Prevalence of SARS-CoV-2 infection among health care workers in a tertiary community hospital. JAMA Intern Med. 2020; 180:1707–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Garcia-Basteiro AL, Moncunill G, Tortajada M, et al. Seroprevalence of antibodies against SARS-CoV-2 among health care workers in a large Spanish reference hospital. Nat Commun 2020; 11:3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lai X, Wang M, Qin C, et al. Coronavirus disease 2019 (COVID-2019) infection among health care workers and implications for prevention measures in a tertiary hospital in Wuhan, China. JAMA Netw Open 2020; 3:e209666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. United States Naval Institute News. Timeline: Theodore Roosevelt COVID-19 Outbreak Investigation. Available at: https://news.usni.org/2020/06/23/timeline-theodore-roosevelt-covid-19-outbreak-investigation. Accessed 7 August 2020.
- 19. Mizumoto K, Chowell G. Transmission potential of the novel coronavirus (COVID-19) onboard the diamond Princess Cruises Ship, 2020. Infect Dis Model 2020; 5:264–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Institute for Health Metrics and Evaluation. COVID-19 Projections. Available at: https://covid19.healthdata.org/united-states-of-america/virginia. Accessed 30 October 2020.
- 21. Iyer AS, Jones FK, Nodoushani A, et al. Dynamics and significance of the antibody response to SARS-CoV-2 infection. medRxiv 2020.07.18.20155374 [Preprint]. 20 July 2020. Available at: 10.1101/2020.07.18.20155374. Accessed 30 October 2020. [DOI] [Google Scholar]
- 22. Navy Personnel Command. U.S. Navy COVID-19 Standardized Operational Guidance Version 2.0. Available at: https://www.public.navy.mil/bupers-npc/reference/messages/Documents/NAVADMINS/NAV2020/NAV20173.txt. Accessed 11 September 2020.
- 23. United States Navy. Navy Establishes COVID-19 Surveillance Testing Program. Available at: https://www.navy.mil/Press-Office/Press-Releases/display-pressreleases/Article/2284554/navy-establishes-covid-19-surveillance-testing-program/. Accessed 11 September 2020.