Abstract
Increasing rates of antimicrobial-resistant organisms have focused attention on sink drainage systems as reservoirs for hospital-acquired Gammaproteobacteria colonization and infection. We aimed to assess the quality of evidence for transmission from this reservoir. We searched 8 databases and identified 52 studies implicating sink drainage systems in acute care hospitals as a reservoir for Gammaproteobacterial colonization/infection. We used a causality tool to summarize the quality of evidence. Included studies provided evidence of co-occurrence of contaminated sink drainage systems and colonization/infection, temporal sequencing compatible with sink drainage reservoirs, some steps in potential causal pathways, and relatedness between bacteria from sink drainage systems and patients. Some studies provided convincing evidence of reduced risk of organism acquisition following interventions. No single study provided convincing evidence across all causality domains, and the attributable fraction of infections related to sink drainage systems remains unknown. These results may help to guide conduct and reporting in future studies.
Keywords: Gammaproteobacteria, gram-negative bacteria, sink drains, waste water, infection control
Gammaproteobacteria are members of a bacterial class that includes the Enterobacteriaceae, Pseudomonas species, and other nonfermenting gram-negative bacilli [1]. These organisms thrive in moist or wet environments and are an important cause of health care–associated infections [2–5].
Guidelines for health care facility design mandate that sinks be placed in acute care facilities to promote hand hygiene and protect patients from hospital-acquired infections [6–8].
It has become accepted that Legionella species in potable water and other Gammaproteobacteria contaminating sink faucets in hospitals may pose risk to patients [9–12]. There are also decades of studies that document an association between Gammaproteobacteria from sink drainage systems (including from sink drains, traps, drainpipes, and/or air samples above sink drainage outflow) and hospital-acquired colonization or infection of patients or health care workers [13–21]. The first suggestions to heat sink traps or modify sink construction to reduce splashing were made more than 40 years ago [22]. Despite this, it is only the increasing transmission of carbapenem-resistant organisms in hospitals that has refocused attention on the possible role of sink drainage systems as a reservoir [20, 21, 23, 24]. Some hospitals have become convinced of the importance of sinks as a reservoir for hospital-acquired Gammaproteobacterial infections and have removed them entirely from their intensive care units [25]. However, much of the evidence suggesting that sink drainage systems are reservoirs is circumstantial, and directionality of contamination of sinks and colonization or infection of patients is challenging to establish [25–28].
RATIONALE
The quality of evidence for causality of sink drainage systems as reservoirs for hospital-acquired Gammaproteobacterial colonization or infection has not been objectively evaluated.
OBJECTIVES
The objective of this study was to systematically assess the quality of evidence that Gammaproteobacteria in sink drainage systems represent a reservoir for colonization or infection of patients or health care workers in acute care hospitals.
METHODS
Protocol and Registration
This systematic review was prospectively registered with PROSPERO (CRD42015027811). The original protocol can be accessed at https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=27811.
Eligibility Criteria
Studies of any design were included if the authors presented data that led them to conclude that sink drainage systems in patient care areas of acute care hospitals were a reservoir for hospital-acquired Gammaproteobacteria colonization or infection of patients or health care workers. Studies that reported only on fungi, mycobacteria, or Legionella species were excluded, because the ecology of these organisms differs from that of other Gammaproteobacteria [27, 29]. Studies that focused only on water sources other than sink drainage systems were also excluded.
Search Strategies
A professional librarian (E.U.) ran searches in MEDLINE and EMBASE (OvidSP), CINAHL (EBSCOHost), Cochrane (Wiley), Aqualine (ProQuest), Scopus (ScienceDirect), BIOSIS, and Web of Science (Thomson Reuters) on October 6, 2016. Reference lists were reviewed to identify relevant citations. We used both subject headings and text words to search for articles on (bacteria or infection terms) AND (hospital departments) AND (sink or plumbing or hand hygiene terms). The results were not limited by language or publication year. Search strategies are listed in the Supplementary Data.
Study Selection
One reviewer assessed eligibility by title, and 2 independent reviewers screened English, French, German, and Spanish abstracts and full-text articles for eligibility (see the Supplementary Data for screening questions). Abstracts and full-text articles in other languages were read by 1 reviewer and discussed with another investigator (C.V.). A third reviewer was used if consensus was not achieved among the first 2 reviewers. Reviewers were not blinded to author, institution, or journal.
Data Collection Process
Data were extracted independently from included articles by 2 authors. For studies published between 2007 and 2016 where it was unclear which sink structure was cultured or implicated as causal in patient or health care worker colonization or infection, authors were contacted to try to obtain this information; studies were included if it was confirmed that criteria were met.
Data Items
Data were collected on study and population characteristics, sink design, sampling of sinks and other potential reservoirs, microbiologic methods, patient or health care worker colonization or infection, sink drainage system interventions and co-interventions, and potential causal pathways for transmission of Gammaproteobacteria from sink drainage systems to patients and/or health care workers.
Quality Assessment
During data extraction, 2 reviewers applied the Modified CADDIS Tool for Causality Assessment of Sink Drains as a Reservoir for Hospital-Acquired Gammaproteobacterial Infection or Colonization (Supplementary Table 1) to assess the quality of evidence for causality of sink drainage systems as reservoirs. This tool was modified from a US Environmental Protection Agency online causality application using a modified Delphi process with experts in infection control and hospital epidemiology [30, 31].
The Modified CADDIS tool includes 6 domains of evidence to evaluate the likelihood of a causal relationship: Spatial/Temporal Co-occurrence, Temporal Sequence, Stressor–Response Relationship, Causal Pathway, Evidence of Exposure and Biological Specificity, and Manipulation of Exposure. Within each domain, the scoring system contains phrasing that describes how evidence/findings influence the likelihood of causality of sink drainage systems in hospital-acquired infection or colonization (organism acquisition), with corresponding scores rated as (+++)/convincingly supports, (++)/strongly supports, (+)/somewhat supports, (0)/neither supports nor weakens, (-)/somewhat weakens, (--)/strongly weakens, (---)/convincingly weakens, or (R)/refutes causality.
Synthesis of Results
Characteristics of studies were entered in duplicate, cleaned, and analyzed in Microsoft Excel using descriptive statistics. The quality of evidence to determine causality was summarized using the Modified CADDIS tool scores and narrative description. Investigators’ hypotheses regarding potential causal pathways and the success of attempts to manipulate exposure to sink drainage systems and reduce organism acquisition were summarized.
RESULTS
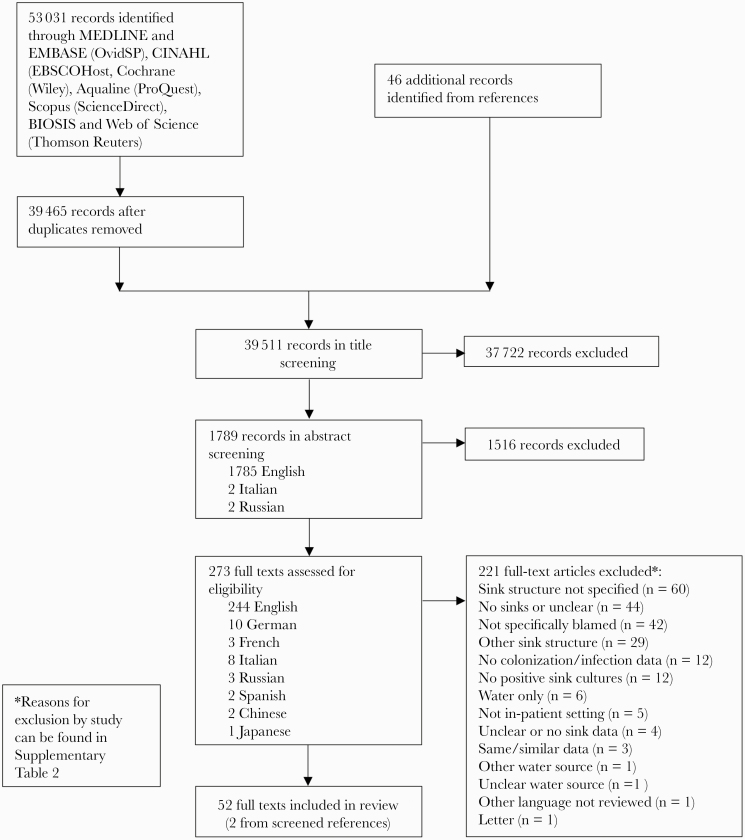
We screened 39511 records and identified 52 studies that met the inclusion criteria (Figure 1) [13–23, 32–72]. The characteristics of these studies are summarized in Table 1. All studies were conducted in acute care hospitals; 35/48 (73%) [13, 15, 17–23, 33, 36–39, 41, 43, 45, 48, 49, 51, 52, 54–58, 60–62, 64, 65, 69–72] were identified as tertiary care or university/teaching hospitals. Over half of studies (35/52, 67%) included intensive care units (ICUs) [14–19, 21–23, 32–34, 36, 38, 39, 41, 43, 45, 48, 49, 51, 52, 54, 56–58, 60, 63, 64, 67–72].
Figure 1.
Flow diagram results of literature search.
Table 1.
Characteristics of Included Studies (n = 52)
| Author, Country | Study Dates | Hospital Unit(s) | Population Characteristics | Bacteria | Typing Methodsa |
|---|---|---|---|---|---|
| Cabrera [13], USA | 1959–1960 | Ward | Neonatal | F. meningosepticum | Biotyping, serotyping |
| Kohn [50], England | 1963–1964 | Ward | Medical-surgical, burn | P. pyocyanea | Serotyping, pyocin typing |
| Thomas [66], England | 1970 | OR | Surgical | P. aeruginosa, other gram-negative bacteria | Serotyping, phage typing |
| Teres [22], USA | 1970–1972 | ICU | Medical-surgical | P. aeruginosa | Pyocin typing |
| Edmonds [14], USA | 1971-1971 | ICU | Medical-surgical, burn | P. aeruginosa | Serotyping, pyocin typing, phage typing |
| Riser [59], England | 1971–1976 | Ward | Medical-surgical | K. aerogenes | Serotyping |
| Breitfellner [35], Austria | 1973–1974 | Ward | Neonatal, obstetric | E. coli, P. aeruginosa | Antibiotic susceptibility, serotyping |
| Brown [36], USA | NS (pre-1977) | ICU | Neonatal | P. aeruginosa | Pyocin typing |
| Cooke [40], England | NS (pre-1979) | Ward | Medical-surgical, neonatal | K. aerogenes | Serotyping |
| Gunther [47], Germany | NS (pre-1980) | Ward | Medical, pediatric | P. aeruginosa | Pyocin typing |
| Levin [15], USA | NS (pre-1984) | ICU | Medical-surgical | P. aeruginosa | Serotyping |
| Döring [43], Germany | 1988–1989 | ICU | Medical-surgical | P. aeruginosa | Exotoxin A probing |
| Döring [42], Germany | 1989–1989 | Ward | Medical, pediatric, immunocompromised | P. aeruginosa | Exotoxin A probing |
| Döring [44], Germany | 1992–1992 | Ward | Medical | P. aeruginosa, B. cepacia | PFGE |
| Kerr [16], Ireland | 1993-1993 | ICU | Medical | P. aeruginosa | RAPD PCR |
| Bert [34], France | 1995–1997 | ICU | Surgical | P. aeruginosa | PFGE |
| Berthelot [17], France | 1995–1996 | ICU | Medical-surgical, mechanically ventilated | P. aeruginosa | PFGE |
| Pitten [58], Germany | 1997–1998 | ICU, ward | Medical-surgical | P. aeruginosa | PFGE |
| Gillespie [46], Scotland | 1997–1998 | Ward | Medical, immunocompromised | P. aeruginosa | PFGE |
| Lowe [19], Canada | 1997–2011 | ICU, ward | Medical-surgical | K. oxytoca | PFGE |
| Orrett [57], Trinadad | 1998–1998 | ICU | Surgical, neonatal | P. aeruginosa | Antibiotic susceptibility |
| Ahmad [32], Pakistan | 1998–2002 | ICU, ward | Medical, pediatric | B. cepacia | Antibiotic susceptibility |
| Sissoko [63], Germany | 2002–2004 | ICU | Medical | Gram-negative bacteria | Typing not reported |
| Hota [18], Canada | 2004–2006 | ICU | Medical-surgical, immunocompromised | P. aeruginosa | PFGE |
| La Forgia [51], USA | 2004–2008 | ICU | Medical-surgical | A. baumanii | Restriction endonuclease of genomic DNA |
| Johansson [49], Sweden | 2004–2009 | ICU, ward | Medical-surgical, immunocompromised | P. aeruginosa | PFGE, MLVA |
| Schneider [61], Germany | 2004–2010 | Ward | Medical, pediatric, immunocompromised | P. aeruginosa | RAPD PCR |
| Cholley [38], France | 2006–2006 | ICU | Medical-surgical | P. aeruginosa | PFGE |
| Longtin [54], Switzerland | 2006–2008 | ICU | Medical-surgical | P. aeruginosa | PFGE |
| Inglis [48], Australia | 2006–2008 | ICU, ward | Medical-surgical | P. aeruginosa | PFGE |
| Tofteland [67], Norway | 2007–2010 | ICU | Medical-surgical | K. pneumoniae | PFGE |
| Maltezou [56], Greece | 2007–2010 | ICU | Neonatal | S. marscecens | PFGE |
| Salimi [60], Iran | 2008–2008 | ICU | Medical-surgical, burn | P. aeruginosa | PFGE |
| Landelle [52], France | 2008–2009 | ICU, ward | Medical-surgical | A. baumanii | PFGE |
| Stjarne Aspelund [65], Sweden | 2008–2015 | Ward | Medical | P. aeruginosa | PFGE |
| Ling [53], Singapore | 2009–2009 | Ward | Medical, immunocompromised | P. aeruginosa | PFGE |
| Vergara Lopez [68], Spain | 2009–2011 | ICU | Medical-surgical | K. oxytoca | PFGE |
| Kotsanas [23], Australia | 2009–2012 | ICU | Medical-surgical | S. marscecens, K. pneumoniae, E.cloacae, E. coli | PFGE |
| Willmann [70], Germany | 2009–2013 | ICU, ward | Medical, immunocompromised | P. aeruginosa | WGS |
| Starlander [64], Sweden | 2010 | ICU | Surgical | K. pneumoniae | PFGE |
| Zhou [72], China | 2011–2011 | ICU | Surgical | P. aeruginosa | PFGE |
| Amoureux [33], France | 2011–2012 | ICU, ward | Medical-surgical, pediatric | A. xylosoxidans | PFGE |
| Wolf [71], the Netherlands | 2011–2012 | ICU | NS | ESBL-producing gram-negative bacilli | AFLP |
| Lowe [55], Canada | 2011–2012 | Ward | Medical-surgical | K. pneumoniae | PFGE |
| Chapuis [37], France | 2011–2013 | Ward | Medical, immunocompromised | E. cloacae | PFGE |
| Leitner [20], Austria | 2011–2013 | Ward | Medical | K. oxytoca | MLST, rep-PCR |
| Fusch [45], Canada | 2011–2013 | ICU | Neonatal | P. aeruginosa | Typing not reported |
| Wendel [69], Germany | 2011–2014 | ICU, ward | Medical-surgical | P. aeruginosa | PFGE |
| Clarivet [39], France | 2012–2014 | ICU | Medical-surgical | K. pneumoniae | PFGE, rep-PCR |
| Pantel [21], France | 2012–2014 | ICU, OR | NS | K. pneumoniae | MLST, rep-PCR |
| Seara [62], Spain | 2013–2014 | NS | Medical-surgical | K. pneumoniae | PFGE |
| Davis [41], Australia | 2013–2014 | ICU | Neonatal | P. aeruginosa | WGS |
Abbreviations: ESBL, extended-spectrum beta lactamase; ICU, intensive care unit; NS, not specified; OR, operating room.
aTyping method examples and their relative discriminatory power based on consensus evaluation by microbiologists and infection control practitioners: Adequately discriminatory: PFGE, pulsed field gel electrophoresis; RAPD PCR, random amplified polymorphic DNA polymerase chain reaction; RFLP, restriction fragment length polymorphism; WGS, whole-genome sequencing. Less discriminatory: AFLP, amplified fragment length polymorphism; ERIC PCR, enterobacterial repetitive intergenic consensus polymerase chain reaction; MLEE, multilocus enzyme electrophoresis; MLST, multilocus sequence typing; MLVA, multiple locus variable number tandem repeat analysis; rep-PCR, repetitive element palindromic polymerase chain reaction; VNTR, variable number tandem repeat. Inadequate: phage typing, biotyping, antibiotic susceptibility pattern, pyocin typing.
The most common organisms involved were Pseudomonas aeruginosa (pyocyanea; 31/52, 60%) [14–18, 22, 34–36, 38, 41–50, 53, 54, 57, 58, 60, 61, 65, 66, 69, 70, 72] and Klebsiella pneumoniae (7/52, 13%) [21, 23, 39, 55, 62, 64, 67]. Reduced susceptibility or resistance of implicated bacteria to at least 1 carbapenem antibiotic was reported in 21/52 (40%) studies [18, 20, 21, 23, 33, 34, 39, 48, 51–53, 55, 58, 60, 62, 65, 67–70, 72], and carbapenemase production was reported in 12/52 (23%) [20, 21, 23, 33, 39, 55, 62, 65, 67–70].
The number of patients colonized or infected with Gammaproteobacteria that were also cultured from sink drainage systems was not consistently reported. Patients underwent some screening for carriage, most commonly from rectal (19/52 studies, 37%) [15, 19, 21, 23, 36, 38, 39, 43–45, 50, 52, 55, 56, 62, 68, 70–72], throat (14/52, 27%) [13, 15, 17, 22, 36, 37, 43, 44, 50, 52, 56, 68, 70, 71], feces (8/52, 15%) [17, 35, 37, 40, 42, 46, 67, 70], nasal (8/52, 15%) [13, 38, 39, 41, 42, 44, 50, 72], wound (7/52, 13%) [14, 36, 43, 47, 55, 60, 65], or sputum (7/52, 13%) [17, 22, 37, 44, 49, 65, 71] specimens. In the 16 studies that reported numerator and denominator data for screening, 411/5932 (6.9%) [14, 15, 17, 22, 23, 35, 37, 38, 40, 42–44, 55, 67, 68, 72] patients were colonized with the same Gammaproteobacterial species found in sink drainage systems, with a median (range) of 13.5% (0.01%–68.8%). However, not all cases were attributed to sink drainage systems. The mean or median time from admission or exposure to colonization or infection was reported by only 8 studies and ranged from 8 to 53 days (median, 17 days) [14, 23, 34, 50, 53, 55, 62, 68].
Health care workers were screened for carriage of Gammaproteobacteria from hands in 11 (21%) [13, 14, 22, 36, 40, 42, 44, 48, 57, 58, 72], throat in 5 (10%) [13, 50, 56, 66, 68], nose in 4 (8%) [13, 42, 50, 66], rectum in 2 (4%) [56, 68], feces in 2 (4%) [35, 42], hair in 1 (2%) [14], and nasopharynx in 1 (2%) [14] of 52 studies. Where the anatomical site was specified, cultures were positive for the same Gammaproteobacteria species found in sink drainage systems from hands in 5/11 (45%) [22, 36, 40, 42, 44], stool in 1/2 (50%) [42], and 1 nasopharyngeal swab in a single study [14]. However, methods of sampling and timing relative to handwashing varied, and only 1 study used adequately discriminatory typing (pulsed field gel electrophoresis) to determine that the strain of P. aeruginosa cultured from health care worker hands matched that sampled from sink drainage systems [44]. These cultures were obtained after hand disinfection and then washing in sinks known to be contaminated [44].
Most studies implicated more than 1 sink in transmission, though a single sink was deemed the culprit in 12 of 52 (23%) studies [13, 21, 32, 39, 41, 51, 55, 57, 60, 62, 64, 68]. Bacterial cultures were performed of drains in 37 (71%) [14–16, 18–21, 23, 32, 33, 36, 37, 40–44, 46–49, 51, 53–56, 58–61, 64–69, 71], traps in 21 (40%) [13, 17, 18, 22, 34, 35, 38, 39, 47, 48, 50–52, 57, 61–63, 66, 68, 70, 72], drainpipes in 2 (4%) [65, 68], and adjacent air in 4 (8%) [22, 43, 45, 63] of 52 studies. In 32 (62%) [14–20, 23, 32, 35, 36, 39–41, 46–48, 50–56, 58, 59, 65, 66, 68–70, 72] studies, at least 1 other sink structure was sampled, including (with number and percentage of studies with at least 1 positive culture) the adjacent rim or counter (3/3, 100%) [19, 59, 69], splash-backs (1/1, 100%) [41], sink basins (6/7, 86%) [14, 19, 35, 50, 51, 68, 70], overflows (3/4, 75%) [17, 20, 47, 68], faucets (6/20, 30%) [14, 18, 23, 32, 39, 46–48, 50–56, 58, 65, 66, 68, 72], and/or faucet aerators (2/6, 33%) [15, 19, 39, 41, 48, 65]. Water was sampled from the faucet in 19/52 (37%) studies and was culture positive in 26% (5/19) [17–19, 34, 37–39, 45, 49, 52, 54, 56–58, 60, 65, 68, 69, 72].
Environmental sampling of potential sources other than sinks was reported in 45/52 (87%) studies [13–23, 32–37, 39–44, 46, 47, 49–62, 66–70, 72], and 1 or more of these were found to be positive in 26 (58%) studies [13–16, 18, 20, 22, 33–35, 37, 40, 42, 46, 47, 49, 50, 52, 58, 59, 62, 66, 68–70, 72]. The following nonsink sites were sampled in at least 5 studies, and their percent positivity for implicated bacteria was as follows: showers (9/12, 75%) [18, 20, 33, 37, 42, 44, 46, 49, 53, 58, 62, 70], air or settle plates not described in relation to sinks (3/6, 50%) [13, 14, 36, 40, 50, 52], toilets (3/9, 33%) [22, 33, 35, 37, 42–44, 46, 70], trolleys/carts, table/countertops, or desks (3/10, 30%) [14, 17, 18, 21, 32, 37, 52, 55, 69, 72], disinfectants or their dispensers (2/8, 25%) [19, 32, 35, 54, 56, 60, 61, 66], ventilatory apparatus (3/11, 27%) [16, 18, 21, 22, 51, 52, 54, 55, 57, 58, 68], other respiratory equipment (3/15, 20%) [14–19, 32, 36, 40, 52, 54, 56–58, 68], beds or bedrails (2/10, 20%) [14, 15, 17, 21, 22, 32, 37, 52, 55, 72], and intravenous monitors or poles (0/5, 0%) [15, 18, 52, 55, 72].
There were limited data on sink design features considered to influence transmission of Gammaproteobacteria from sink drainage systems to patients. Six studies reported that water outflow from faucets was directed onto the drain [18, 20, 23, 37, 61, 65], 2 studies reported shallow sink basins [18, 23], and 6 studies described the use of aerators in faucets [15, 19, 39, 41, 48, 65].
Causality Assessment
Spatial/Temporal Co-occurrence
All 52 studies provided some evidence in support of a spatial or temporal co-occurrence of contaminated sink drainage systems and organism acquisition (Table 2). Thirty-six (69%) studies reported on specific sink locations [13, 16–21, 33–39, 42–47, 51, 53, 55, 58, 59, 61, 63–72], which in 32 (89%) included sinks located directly within the patient room [16–21, 33–39, 42–47, 51, 53, 55, 58, 63–65, 67–72].
Table 2.
Summary Table of Scores for 2 Raters Applying the Modified CADDIS to 52 Articles Included in a Systematic Review
| Study | Spatial/Temporal Co-occurrence | Temporal Sequence | Stressor–Response Relationship | Causal Pathwaya | Evidence of Exposure and Biological Specificityb | Manipulation of Exposure |
|---|---|---|---|---|---|---|
| Hota [18] | + | 0 | 0 | + | ++ | +++ |
| Bert [34] | + | 0 | 0 | + | ++ | +++ |
| Schneider [61] | + | 0 | 0 | 0 | ++ | +++ |
| Stjarne Aspelund [65] | + | 0 | 0 | 0 | ++ | +++ |
| Tofteland [67] | + | 0 | 0 | 0 | ++ | +++ |
| Vergara Lopez [68] | + | 0 | 0 | 0 | ++ | +++ |
| Wolf [71] | + | ++ | 0 | 0 | 0 | +++ |
| Sissoko [63] | + | 0 | 0 | + | 0 | +++ |
| Cabrera [13] | + | ++ | 0 | + | 0 | +++ |
| Chapuis [37] | + | ++ | 0 | 0 | ++ | + |
| Clarivet [39] | + | ++ | 0 | 0 | ++ | + |
| Longtin [54] | + | ++ | 0 | 0 | ++ | + |
| Lowe [19] | + | ++ | 0 | 0 | ++ | + |
| Lowe [55] | + | ++ | 0 | 0 | ++ | + |
| Davis [41] | + | 0 | 0 | + | ++ | + |
| Landelle [52] | + | 0 | 0 | + | ++ | + |
| Starlander [64] | + | 0 | 0 | + | ++ | + |
| Wendel [69] | + | 0 | 0 | + | ++ | + |
| Gillespie [46] | + | 0 | 0 | 0 | ++ | + |
| Johansson [49] | + | 0 | 0 | 0 | ++ | + |
| La Forgia [51] | + | 0 | 0 | 0 | ++ | + |
| Ling [53] | + | 0 | 0 | 0 | ++ | + |
| Maltezou [56] | + | 0 | 0 | 0 | ++ | + |
| Pitten [58] | + | 0 | 0 | 0 | ++ | + |
| Seara [62] | + | 0 | 0 | 0 | ++ | + |
| Willmann [70] | + | 0 | 0 | 0 | ++ | + |
| Leitner [20] | + | 0 | 0 | 0 | + | + |
| Pantel [21] | + | 0 | 0 | 0 | + | + |
| Riser [59] | + | 0 | 0 | + | 0 | + |
| Thomas [66] | + | 0 | 0 | + | 0 | + |
| Ahmad [32] | + | 0 | 0 | 0 | 0 | + |
| Breitfellner [35] | + | 0 | 0 | 0 | 0 | + |
| Fusch [45] | + | 0 | 0 | + | 0 | + |
| Orrett [57] | + | 0 | 0 | 0 | 0 | + |
| Döring [44] | + | ++ | 0 | + | ++ | 0 |
| Berthelot [17] | + | ++ | 0 | 0 | ++ | + |
| Zhou [72] | + | ++ | 0 | 0 | ++ | 0 |
| Cholley [38] | + | ++ | 0 | 0 | ++ | 0 |
| Amoureux [33] | + | 0 | 0 | 0 | ++ | 0 |
| Inglis [48] | + | 0 | 0 | 0 | ++ | 0 |
| Kerr [16] | + | 0 | 0 | 0 | ++ | 0 |
| Kotsanas [23] | + | 0 | 0 | 0 | ++ | 0 |
| Salimi [60] | + | 0 | 0 | 0 | ++ | 0 |
| Döring [42] | + | ++ | 0 | + | + | 0 |
| Brown [36] | + | ++ | 0 | + | 0 | 0 |
| Cooke [40] | + | ++ | 0 | + | 0 | 0 |
| Döring [43] | + | ++ | 0 | + | + | 0 |
| Teres [22] | + | ++ | 0 | + | 0 | 0 |
| Edmonds [14] | + | ++ | 0 | 0 | 0 | 0 |
| Gunther [47] | + | ++ | 0 | 0 | 0 | 0 |
| Levin [15] | + | ++ | 0 | 0 | 0 | 0 |
| Kohn [50] | + | ++ | 0 | 0 | 0 | 0 |
*The original Causal Analysis/Diagnosis Decision Information System (CADDIS) Summary Table of Scores can be found at https://www.epa.gov/caddis-vol1/summary-tables-scores[31].
+++ convincingly supports, ++ strongly supports, + somewhat supports, 0 neither supports nor weakens, - somewhat weakens, -- strongly weakens, --- convincingly weakens, or (R)/refutes causality.
aCausal pathway example: bacterial transmission from sinks splashing onto health care workers’ hands, and then to patients. See Döring et al. [44].
bEvidence of Exposure and Biological Specificity is based on adequacy of typing methods employed (Supplementary Table 1).
Temporal Sequence
Twenty (38%) studies provided some evidence that patient or health care worker exposure to contaminated sink drainage systems was present before they were found to be colonized or infected with the implicated organism. Most studies did not collect or report adequate data to establish a temporal sequence.
Stressor–Response Relationship
No study presented clear evidence that the likelihood of organism acquisition differs in relation to duration of exposure or degree of sink drainage system contamination, with the exception of those where differences appeared to be due to a direct effect of manipulation of exposure (see below).
Causal Pathway
Hypothetical causal pathways were mentioned in 36 of 52 (69%) studies, most commonly involving splash-back of drain contents to health care worker hands, fomites, or surroundings (Table 3). Seventeen (47%) of these studies demonstrated 1 or more potential steps in corresponding causal pathway(s) [13, 18, 22, 34, 36, 40–45, 52, 59, 63, 64, 66, 69]. These included positive cultures from health care worker hands (n = 5) [36, 40, 42–44], positive water cultures from contaminated sinks used for patient care (n = 1) [34], or findings that suggest splash, aerosolization, or leak of bacteria from contaminated sink drainage systems onto taps, surroundings, or fomites (n = 12) [13, 18, 22, 40, 41, 45, 52, 59, 63, 64, 66, 69].
Table 3. .
Hypothetical Causal Pathways Between Sink Drainage and Patient or Health Care Worker Colonization or Infection
| Hypothetical Causal Pathways | Number of Studies That Mention This Causal Pathway (References) | Number of Studies That Demonstrate 1 or More Potential Steps in This Causal Pathway (References) |
|---|---|---|
| Direct patient use of sinks with contaminated drains | 6 [20, 33, 37, 42, 53, 61] | |
| Water from sinks with contaminated drains used in relation to patient care activity | 1 [34] | 1 [34] |
| Contamination of health care personnel hands or gowns during use of sinks with contaminated drains, and subsequent transmission to patients | 24 [13, 15, 17, 18, 22, 23, 32, 33, 35–37, 39, 40, 42–44, 50, 51, 63, 66, 67, 71, 72] | 5 [36, 40, 42–44] |
| Splash or aerosolization of bacteria from contaminated sink drains into taps | 1 [66] | 1 [66] |
| Splash, aerosolization, or leak of bacteria from contaminated sink drains onto surroundings/fomites | 19 [13, 14, 17, 18, 23, 32, 37, 40, 41, 45, 51, 52, 59, 62–64, 67–69, 71, 72] | 11 [13, 18, 22, 40, 41, 45, 52, 59, 63, 64, 69] |
| Splash or aerosolization of bacteria from contaminated sink drains directly onto patients | 3 [18, 35, 39] |
Evidence of Exposure and Biological Specificity
Thirty-two (62%) studies utilized adequately discriminatory typing methods (based on consensus evaluation by microbiologists and infection control practitioners) (Supplementary Table 1) to identify that the organisms cultured from sink drainage systems and colonized or infected patients or health care workers were the same or closely related [16–19, 23, 33, 34, 37–39, 41, 44, 46, 48, 49, 51–56, 58, 60–62, 64, 65, 67–70, 72]. Among these, 2 used whole-genome sequencing [41, 70]. None of the 10 studies published before 1990 used adequately discriminatory typing methods.
There were 319 colonized or infected patients with isolates reported as matching those recovered from sink drainage systems in the 35 (67%) studies that provided these data. In the 13 studies that used adequately discriminatory typing methods to determine the proportion of colonization or infection related to sink drainage systems, a median (range) of 75% (7%–100%) of colonized or infected patients had isolates matched to bacteria from contaminated sink drainage.
Manipulation of Exposure
Attempts to manipulate exposure to sink drainage systems were made in 40 (77%) studies (Table 4; Supplementary Table 3), most commonly by cleaning or replacing some or all parts of the sink or its drainage system [13, 17–23, 32, 34, 35, 37, 39, 41, 42, 45, 46, 48–59, 61–71]. In 9 (23%) of these studies, organism acquisition appeared to be affected by the manipulation, and there were no reported co-interventions (“+++” under Manipulation of Exposure in Table 2) [13, 18, 34, 61, 63, 65, 67, 68, 71]. In 26 (65%) of these studies, 1 or more sink drainage system interventions were reported to be successful, but there were co-interventions rendering assessment of effect challenging, or the evidence was not convincing because too few data were presented (“+” under Manipulation of Exposure in Table 2) [17, 19–21, 32, 35, 37, 39, 41, 45, 46, 49, 51–59, 62, 64, 66, 69, 70]. In 1 study, all trialed interventions were unsuccessful [23], and in another the effect of sink drainage system manipulation was unclear [48]. In 3 other studies the authors reported some success in reducing drain contamination, but data to support a reduction in organism acquisition were not provided [22, 42, 50]. In addition to frequent co-interventions, short or unclear duration of follow-up in many studies limited interpretation of findings.
Table 4.
Manipulationsa of Exposure to Sink Drainage Systems in Effort to Reduce Patient or Health Care Worker Colonization or Infection
| Intervention Type | No. of Studies | No. (%) of Studies With Elimination or Reduction in Organism Acquisition | Proportion (%) of Successful Studies Reporting Co-intervention(s) | Durabilityb of Success in Studies Reporting Elimination or Reduction in Organism Acquisition, Duration, No. (%) of Studies | No. (%) of Studies With Reduction in Sink Drain Contamination but no Data on Impact on Organism Acquisition Before Other Interventions | No. (%) of Studies Reporting Unsuccessful Attempts to Reduce Sink Drain Contamination or Organism Acquisition |
|---|---|---|---|---|---|---|
| Additional sink cleaningc | 17 | 8 (47)d | 6 (75) | >6 mo: 4 (50%) <6 mo: 1 (13%) Uncertain: 3 (38%) |
3 (18)e | 5 (29)f |
| Repair or replacement of all or part of sink drainage | 10 | 8 (80) | 7 (88) | <6 mo: 1 (13%) >6 mo: 2 (25%) Uncertain: 5 (63%) |
2 (20) | |
| Installation of heater–vibrator trap devices | 6 | 4 (67) | 1 (25) | >6 mo: 3 (75%) Uncertain: 1 (25%) |
2 (33) | |
| Replacement, cleaning, or disinfection of taps thought contaminated from drain | 1 | 1 (100) | 1 (100) | >6 mo: 1 (100%) | ||
| Sink removal, relocation, or closure of unit not otherwise detailed | 3 | 3 (100) | 2 (67) | >6 mo: 1 (33%) Uncertain: 2 (67%) |
||
| Additional sink cleaning, repair, or replacement of all or part of sink drainage | 8 | 8 (100) | 5 (63) | <6 mo: 1 (13%) >6 mo: 3 38%) Uncertain: 4 (50%) |
||
| Installation of heater–vibrator trap devices, taps redirected away from drain | 1 | 1 (100) | 0 | >6 mo: 1 (100%) | ||
| Replacement of traps, distance and barrier between drain and workspace or patient, taps redirected away from drain, water pressure reduced | 1 | 1 (100) | 0 | >6 mo: 1 (100%) | ||
| Exchange and disinfection of traps, use of sink for patient care or placement of materials adjacent to sink forbidden, use of reusable hair wash basins stopped | 1 | 1 (100) | 1 (100) | >6 mo: 1 (100%) |
aAdditional details and references provided in Supplementary Table 3.
bDurability of successful reduction in organism acquisition by patients or health care workers determined based on reported periods with significantly reduced or no new cases of colonization or infection attributed to sinks, or time to manuscript submission/receipt in those who had reported sustained effect.
cUnclear effect of manipulation of exposure for 1 study with additional cleaning (disinfectant poured down drains).
dAdditional sink drain cleaning that was reported to result in some success without structural sink intervention included the use of chlorine or bleach products (n = 4), formalin (n = 1), or acetic acid (n = 1) with or without dismantling and physical cleaning, sink disinfection or cleaning not further specified (n = 2).
eAdditional sink drain cleaning that without structural sink intervention was reported to result in some reduction in drain contamination but not patient acquisition: accelerated H2O2 gel poured into drains and weekly cleaning with a sodium hypochlorite solution (n = 1), use of phenolics or paracetic acid (n = 1), use of bleach or chlorine and sodium hydroxide and alkyldimethyl amine oxide with steam cleaning (n = 1).
fAdditional sink drain cleaning that without structural sink intervention was reported unsuccessful included the use of bleach products with mechanical brushing (n = 1) or with phenolic flushing (n = 1), or with steam cleaning (n = 1), or the use of hydrogen peroxide (n = 1), or sink cleaning not further specified (n = 1).
DISCUSSION
Summary of Evidence
When applying the Modified CADDIS tool to the 52 included studies, for each domain other than the stressor response domain there was >1 study with evidence supporting causality of sink drainage systems in hospital-acquired Gammaproteobacterial colonization or infection. However, even after excluding the stressor response domain, no single study provided supporting evidence for all remaining Modified CADDIS domains. As each domain is necessary but not sufficient to assess causality, this limits the overall causality assessment.
It is not surprising that there was evidence of a spatial and/or temporal co-occurrence of contaminated sink drainage systems and acquisition of Gammaproteobacteria in studies that implicate sink drainage systems, although many studies did not report the specific sink location. While some studies have documented splash distance from sinks, the risk to patients in relation to sink proximity is not known and presumably depends on the causal pathway responsible for transmission. Among the other domains, changes in rates of organism acquisition with manipulation of sink drainage systems, typing to ensure organisms in sink drainage and patients are the same or closely related, and demonstration of a temporal sequence of exposure to contaminated sink drainage systems before organism acquisition would seem likely to provide the most support for causality when present. However, no single study provided evidence that strongly or convincingly supported causality of sink drainage systems across all of these important domains.
Evidence for a temporal sequence of sink drainage system contamination before organism acquisition by a patient or health care worker may be lacking in many studies because most institutions do not perform routine screening for bacteria in sink drainage systems, and once they are a suspected reservoir, investigators are unwilling to allow persistent risk of transmission from sink drainage systems to patients while better evidence is collected. Stressor response data are difficult to collect because measures of increased exposure, which might for instance include concentration in drains and the number of drains that are contaminated, have not been established or validated. Measurement of causal pathways is fraught with challenges in measurement (eg, lack of standard methods to culture sink surroundings or fomites), an inherent temporal component, and the fact that more than 1 causal pathway for transmission of Gammaproteobacteria between contaminated sink drainage systems and patients or health care workers may exist.
While more studies found support for the Evidence of Exposure and Biological Specificity domain than for other domains, early studies were limited by the lack of specificity in typing systems. When adequate typing methods are employed, directionality is difficult to determine, and few studies were able to convincingly document acquisition of organisms from sink drainage as compared with other sources. While some studies provided data on the proportion of cases of acquired Gammaproteobacteria that were matched, or in some cases attributed, to sink drainage system contamination, the methods used to ascertain this were inconsistent.
Evidence for successful interventions on contaminated sink drainage systems was often confounded by co-intervention and unclear or short duration of follow-up measurement of clinical cultures. Few studies reported a sustained decrease in organism acquisition attributed to additional cleaning of sink drainage systems, but heterogeneity of agents and regimens limits interpretation.
Installation of self-cleaning traps that use a combination of heat and vibration appeared to have a beneficial and durable effect, but the limited number and size of studies suggest that further data are needed before broad implementation of this intervention [45, 61, 63, 70, 71]. However, a recent 2-armed nonrandomized intervention trial of traps with similar properties compared with a new polyvinylchloride trap showed a significant reduction in sink drain and patient colonization with multidrug-resistant (MDR) Pseudomonas aeruginosa as compared with baseline rates [73].
It was noted during the review process that there was variation in sink structures sampled and in how authors determined the most likely source of acquired Gammaproteobacteria. Bert et al. blamed tap water from contaminated faucets for transmission of Gammaproteobacteria to patients, but they also implicated bacterial persistence in sink drains/traps, and an outbreak was only controlled after sinks were replaced and sink trap disinfection commenced [34]. An article we excluded identified faucet aerators as a common source reservoir in an outbreak of MDR P. aeruginosa [74]. However, in a recently published follow-up study, investigators identified the sink drainage system as the reservoir and reported a significant reduction in patient colonization with installation of self-disinfecting traps [73].
The data reported in studies included in this review were heterogenous, and we were unable to perform a meta-analysis. The strengths of this review lie in the broad search of 8 databases without date or language restrictions and in the use of a standardized causality tool. While there are no absolute criteria that can be used to determine causality, the Modified CADDIS is based on the fundamental principles of causal analysis, and use of the tool provided a more objective and transparent means to assess the quality of evidence for acquisition of Gammaproteobacteria from sink drainage systems in acute care settings.
Selection of only studies that implicate sink drainage systems in organism acquisition may be interpreted to introduce bias toward favoring these as causal. However, bacteria are acquired from multiple sources in hospitals. Inclusion of studies in which sink drainage systems were not blamed would be unlikely to change the assessment of the quality of published evidence for causality of sink drainage systems.
In summary, the studies included in this review provide evidence that sink drainage systems are a reservoir for hospital-acquired Gammaproteobacterial colonization or infection. However, these data do not assist in quantifying the attributable fraction of hospital-acquired Gammaproteobacterial infections acquired from sink drainage systems and are of limited value in understanding the causal pathways for infection or optimal mitigation strategies. Ideally more studies will be performed outside of outbreak settings and include prospective screening of patients and the environment with consideration of potential causal pathways, documentation of patient proximity and duration of stay relative to sinks with contaminated drainage systems, attempts to standardize methods and quantify environmental cultures, use of whole-genome sequencing, and before–after or cluster randomization studies of interventions.
Context
The literature regarding waste water drainage systems as reservoirs for Gammaproteobacterial infection is expanding rapidly. However, both individual publications and reviews have usually focused on outbreaks and carbapenem-resistant organisms and have taken at face value author conclusions about whether sinks and/or drains were the relevant reservoir for the outbreak [75–79]. In these reviews, the findings regarding organism and species distribution and difficulties in sustaining drain decolonization with different interventions were similar to ours. However, as might be expected, outbreaks of carbapenem-resistant organisms were found more commonly in ICUs and immunocompromised patients, while our review suggests that acquisition of infection from sink drains may be more widespread in in-patients [75, 77]. Other studies have either failed to detect or did not report health care worker hand colonization, which was identified in 10% of our reports, supporting the possibility of this route of sink-to-patient transmission in some cases. Our review was also able to describe presumed modes of sink-to-patient transmission, as well as time from patient admission to colonization/infection.
More recently, the introduction of whole-genome sequencing and a systematic approach to culturing and sequencing in waste water drainage systems resulted in the ability to define sink-to-patient directionality in a single infection due to E. coli, though even here the spatial association was not clear (the patient was housed in the same ward where the sink drain isolate was recovered) [80]. More experimental work is also being done to investigate how bacteria from sink drainage systems may make their way into the environment where they pose risk to patients, as well as sink features that may be associated with this dispersal [81–85]. Further work is needed to define the burden of infection associated with endemic hospital-acquired infections from sink drain reservoirs.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We are grateful to the University of Toronto libraries for assistance with retrieval of the articles and to Agron Plevneshi, Angel Li, and Tamara Vikulova for translation of non-English studies.
Author contribution. Cheryl Volling: development of modified causality tool, literature review, data extraction, analysis, initial drafting and review/revision of manuscript. Narges Ahangari: literature review, data extraction, analysis, and review/revision of manuscript. Jessica J. Bartoszko: conception and review/revision of manuscript. Brenda L. Coleman: conception and review/revision of manuscript. Felipe Garcia Jeldes: literature review and review/revision of manuscript. Alainna Jamal: development of modified causality tool, literature review, and review/revision of manuscript. Jennie Johnstone: development of modified causality tool and review/revision of manuscript. Chris Kandel: development of modified causality tool, literature review, and review/revision of manuscript. Philipp Kohler: literature review and review/revision of manuscript. Helena C. Maltezou: development of modified causality tool and review/revision of manuscript. Lorraine Maze dit Mieusement: literature review and review/revision of manuscript. Nneka McKenzie: literature review and review/revision of manuscript. Dominik Mertz: development of modified causality tool and review/revision of manuscript. Adam Monod: literature review and review/revision of manuscript. Salman Saeed: conception and review/revision of manuscript. Barbara Shea: literature review and review/revision of manuscript. Rhonda Stuart: development of modified causality tool and review/revision of manuscript. Sera Thomas: conception and review/revision of manuscript. Elizabeth Uleryk: literature search and review/revision of manuscript. Allison McGeer: conception, development of modified causality tool, literature review, and review/revision of manuscript.
Financial support. This study was funded in part by an Industrial Research Assistance Program Grant from the National Research Council of Canada.
Potential conflicts of interest. Dr. Allison McGeer has received funding for research evaluating the impact of a novel copper sink drain on sink drain contamination and associated patient infections. No other authors have conflicts of interest to declare in relation to this manuscript. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Patient consent. This review did not include factors necessitating patient consent.
References
- 1. Gao B, Mohan R, Gupta RS. Phylogenomics and protein signatures elucidating the evolutionary relationships among the Gammaproteobacteria. Int J Syst Evol Microbiol 2009; 59:234–47. [DOI] [PubMed] [Google Scholar]
- 2. Gaynes R, Edwards JR; National Nosocomial Infections Surveillance System Overview of nosocomial infections caused by gram-negative bacilli. Clin Infect Dis 2005; 41:848–54. [DOI] [PubMed] [Google Scholar]
- 3. Peleg AY, Hooper DC. Hospital-acquired infections due to gram-negative bacteria. N Engl J Med 2010; 362:1804–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Decker BK, Palmore TN. The role of water in healthcare-associated infections. Curr Opin Infect Dis 2013; 26:345–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Muscarella LF Contribution of tap water and environmental surfaces to nosocomial transmission of antibiotic-resistant Pseudomonas aeruginosa. Infect Control Hosp Epidemiol 2004; 25:342–5. [DOI] [PubMed] [Google Scholar]
- 6. Roux D, Aubier B, Cochard H, et al. ; HAI Prevention Group of the Réseau des Hygiénistes du Centre Contaminated sinks in intensive care units: an underestimated source of extended-spectrum beta-lactamase-producing Enterobacteriaceae in the patient environment. J Hosp Infect 2013; 85:106–11. [DOI] [PubMed] [Google Scholar]
- 7. CSA. Canadian Health Care Facilities. CSA Z8000 Ottawa, Ontario, Canada: Canadian Standards Association, 2016. [Google Scholar]
- 8. PIDAC. Best Practices for Hand Hygiene in All Health Care Settings. 4th ed Toronto: PIDAC; 2014. [Google Scholar]
- 9. Exner M, Kramer A, Lajoie L, et al. Prevention and control of health care-associated waterborne infections in health care facilities. Am J Infect Control 2005; 33:S26–40. [DOI] [PubMed] [Google Scholar]
- 10. Exner M, Hartemann P, Lee J, et al. The international forum for water hygiene in buildings: recommendations for water quality in healthcare premises. J Hosp Infect 2010; 76:S40. [Google Scholar]
- 11. WHO. Water Safety in Buildings. Geneva: World Health Organization; 2011. [Google Scholar]
- 12. Williams MM, Armbruster CR, Arduino MJ. Plumbing of hospital premises is a reservoir for opportunistically pathogenic microorganisms: a review. Biofouling 2013; 29:147–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cabrera HA, Davis GH. Epidemic meningitis of the newborn caused by flavobacteria. I. Epidemiology and bacteriology. Am J Dis Child 1961; 101:289–95. [DOI] [PubMed] [Google Scholar]
- 14. Edmonds P, Suskind RR, Macmillan BG, Holder IA. Epidemiology of Pseudomonas aeruginosa in a burns hospital: surveillance by a combined typing system. Appl Microbiol 1972; 24:219–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Levin MH, Olson B, Nathan C, et al. Pseudomonas in the sinks in an intensive care unit: relation to patients. J Clin Pathol 1984; 37:424–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kerr JR, Moore JE, Curran MD, et al. Investigation of a nosocomial outbreak of Pseudomonas aeruginosa pneumonia in an intensive care unit by random amplification of polymorphic DNA assay. J Hosp Infect 1995; 30:125–31. [DOI] [PubMed] [Google Scholar]
- 17. Berthelot P, Grattard F, Mahul P, et al. Prospective study of nosocomial colonization and infection due to Pseudomonas aeruginosa in mechanically ventilated patients. Intensive Care Med 2001; 27:503–12. [DOI] [PubMed] [Google Scholar]
- 18. Hota S, Hirji Z, Stockton K, et al. Outbreak of multidrug-resistant Pseudomonas aeruginosa colonization and infection secondary to imperfect intensive care unit room design. Infect Control Hosp Epidemiol 2009; 30:25–33. [DOI] [PubMed] [Google Scholar]
- 19. Lowe C, Willey B, O’Shaughnessy A, et al. ; Mount Sinai Hospital Infection Control Team Outbreak of extended-spectrum β-lactamase-producing Klebsiella oxytoca infections associated with contaminated handwashing sinks. Emerg Infect Dis 2012; 18:1242–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Leitner E, Zarfel G, Luxner J, et al. Contaminated handwashing sinks as the source of a clonal outbreak of KPC-2-producing Klebsiella oxytoca on a hematology ward. Antimicrob Agents Chemother 2015; 59:714–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pantel A, Richaud-Morel B, Cazaban M, et al. Environmental persistence of OXA-48-producing Klebsiella pneumoniae in a French intensive care unit. Am J Infect Control 2016; 44:366–8. [DOI] [PubMed] [Google Scholar]
- 22. Teres D, Schweers P, Bushnell LS, et al. Sources of Pseudomonas aeruginosa infection in a respiratory-surgical intensive-therapy unit. Lancet 1973; 1:415–7. [DOI] [PubMed] [Google Scholar]
- 23. Kotsanas D, Wijesooriya WR, Korman TM, et al. “Down the drain”: carbapenem-resistant bacteria in intensive care unit patients and handwashing sinks. Med J Aust 2013; 198:267–9. [DOI] [PubMed] [Google Scholar]
- 24. van Loon K, Voor In “t Holt AF, Vos MC. A systematic review and meta-analyses of the clinical epidemiology of carbapenem-resistant Enterobacteriaceae. Antimicrob Agents Chemother 2018; 62:e01730–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hopman J, Tostmann A, Wertheim H, et al. Reduced rate of intensive care unit acquired gram-negative bacilli after removal of sinks and introduction of ‘water-free’ patient care. Antimicrob Resist Infect Control 2017; 6:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shaw E, Gavaldà L, Càmara J, et al. Control of endemic multidrug-resistant gram-negative bacteria after removal of sinks and implementing a new water-safe policy in an intensive care unit. J Hosp Infect 2018; 98:275–81. [DOI] [PubMed] [Google Scholar]
- 27. Decker BK, Palmore TN. Hospital water and opportunities for infection prevention. Curr Infect Dis Rep 2014; 16:432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Snitkin ES Contamination of hospital plumbing: a source or a sink for antibiotic-resistant organisms? JAMA Netw Open 2019; 2:e187660. [DOI] [PubMed] [Google Scholar]
- 29. Falkinham JO 3rd, Hilborn ED, Arduino MJ, et al. Epidemiology and ecology of opportunistic premise plumbing pathogens: Legionella pneumophila, Mycobacterium avium, and Pseudomonas aeruginosa. Environ Health Perspect 2015; 123:749–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.US Environmental Protection Agency, Office of Research and Development. Causal Analysis/Diagnosis Decision Information System (CADDIS). Washington, DC: US Environmental Protection Agency, Office of Research and Development; 2017. Available at: https://www.epa.gov/caddis. Accessed 23 August 2018. [Google Scholar]
- 31.US Environmental Protection Agency, Office of Research and Development. Causal Analysis/Diagnosis Decision Information System (CADDIS) - Summary Table of Scores. Washington, DC: US Environmental Protection Agency, Office of Research and Development; 2017. Available at: https://www.epa.gov/caddis-vol1/summary-tables-scores. Accessed 23 August 2018. [Google Scholar]
- 32. Ahmad K, Khan UF, Hafeez A. Control of Burkholderia (Pseudomonas) bacteraemia in the intensive care and paediatric units. J Coll Physicians Surg Pak 2004; 14:102–4. [PubMed] [Google Scholar]
- 33. Amoureux L, Bador J, Fardeheb S, et al. Detection of Achromobacter xylosoxidans in hospital, domestic, and outdoor environmental samples and comparison with human clinical isolates. Appl Environ Microbiol 2013; 79:7142–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bert F, Maubec E, Bruneau B, et al. Multi-resistant Pseudomonas aeruginosa outbreak associated with contaminated tap water in a neurosurgery intensive care unit. J Hosp Infect 1998; 39:53–62. [DOI] [PubMed] [Google Scholar]
- 35. Breitfellner G, Meschkat AA. Hospital infection by E coli 0111: b4 and Pseudomonas aeruginosa (author’s transl). Wien Klin Wochenschr 1975; 87:424–7. [PubMed] [Google Scholar]
- 36. Brown DG, Baublis J. Reservoirs of pseudomonas in an intensive care unit for newborn infants: mechanisms of control. J Pediatr 1977; 90:453–7. [DOI] [PubMed] [Google Scholar]
- 37. Chapuis A, Amoureux L, Bador J, et al. Outbreak of extended-spectrum beta-lactamase producing Enterobacter cloacae with high MICs of quaternary ammonium compounds in a hematology ward associated with contaminated sinks. Front Microbiol 2016; 7:1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cholley P, Thouverez M, Floret N, et al. The role of water fittings in intensive care rooms as reservoirs for the colonization of patients with Pseudomonas aeruginosa. Intensive Care Med 2008; 34:1428–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Clarivet B, Grau D, Jumas-Bilak E, et al. Persisting transmission of carbapenemase-producing Klebsiella pneumoniae due to an environmental reservoir in a university hospital, France, 2012 to 2014. Eurosurveillance 2016; 21. doi: 10.2807/1560-7917.ES.2016.21.17.30213. [DOI] [PubMed] [Google Scholar]
- 40. Cooke EM, Pool R, Brayson JC, et al. Further studies on the sources of Klebsiella aerogenes in hospital patients. J Hyg (Lond) 1979; 83:391–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Davis RJ, Jensen SO, Van Hal S, et al. Whole genome sequencing in real-time investigation and management of a Pseudomonas aeruginosa outbreak on a neonatal intensive care unit. Infect Control Hosp Epidemiol 2015; 36:1058–64. [DOI] [PubMed] [Google Scholar]
- 42. Döring G, Ulrich M, Müller W, et al. Generation of Pseudomonas aeruginosa aerosols during handwashing from contaminated sink drains, transmission to hands of hospital personnel, and its prevention by use of a new heating device. Zentralbl Hyg Umweltmed 1991; 191:494–505. [PubMed] [Google Scholar]
- 43. Döring G, Hörz M, Ortelt J, et al. Molecular epidemiology of Pseudomonas aeruginosa in an intensive care unit. Epidemiol Infect 1993; 110:427–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Döring G, Jansen S, Noll H, et al. Distribution and transmission of Pseudomonas aeruginosa and Burkholderia cepacia in a hospital ward. Pediatr Pulmonol 1996; 21:90–100. [DOI] [PubMed] [Google Scholar]
- 45. Fusch C, Pogorzelski D, Main C, et al. Self-disinfecting sink drains reduce the Pseudomonas aeruginosa bioburden in a neonatal intensive care unit. Acta Paediatr 2015; 104:e344–9. [DOI] [PubMed] [Google Scholar]
- 46. Gillespie TA, Johnson PR, Notman AW, et al. Eradication of a resistant Pseudomonas aeruginosa strain after a cluster of infections in a hematology/oncology unit. Clin Microbiol Infect 2000; 6:125–30. [DOI] [PubMed] [Google Scholar]
- 47. Günther A, Horn H. [Pseudomonas aeruginosa in washing and bathing facilities of a pediatric hospital]. Kinderarztl Prax 1980; 48:33–9. [PubMed] [Google Scholar]
- 48. Inglis TJ, Benson KA, O’Reilly L, et al. Emergence of multi-resistant Pseudomonas aeruginosa in a Western Australian hospital. J Hosp Infect 2010; 76:60–5. [DOI] [PubMed] [Google Scholar]
- 49. Johansson E, Welinder-Olsson C, Gilljam M. Genotyping of Pseudomonas aeruginosa isolates from lung transplant recipients and aquatic environment-detected in-hospital transmission. APMIS 2014; 122:85–91. [DOI] [PubMed] [Google Scholar]
- 50. Kohn J. A Study of Ps. pyocyanea Cross Infection in a Burns Unit. Alexander BW, Andrew WW , eds. Edinburgh: E. & S. Livingstone; 1966. [Google Scholar]
- 51. La Forgia C, Franke J, Hacek DM, et al. Management of a multidrug-resistant Acinetobacter baumannii outbreak in an intensive care unit using novel environmental disinfection: a 38-month report. Am J Infect Control 2010; 38:259–63. [DOI] [PubMed] [Google Scholar]
- 52. Landelle C, Legrand P, Lesprit P, et al. Protracted outbreak of multidrug-resistant Acinetobacter baumannii after intercontinental transfer of colonized patients. Infect Control Hosp Epidemiol 2013; 34:119–24. [DOI] [PubMed] [Google Scholar]
- 53. Ling ML, How KB. Pseudomonas aeruginosa outbreak linked to sink drainage design. Healthcare Infect 2013; 18:143–6. [Google Scholar]
- 54. Longtin Y, Troillet N, Touveneau S, et al. Pseudomonas aeruginosa outbreak in a pediatric intensive care unit linked to a humanitarian organization residential center. Pediatr Infect Dis J 2010; 29:233–7. [DOI] [PubMed] [Google Scholar]
- 55. Lowe CF, Kus JV, Salt N, et al. Nosocomial transmission of New Delhi metallo-β-lactamase-1-producing Klebsiella pneumoniae in Toronto, Canada. Infect Control Hosp Epidemiol 2013; 34:49–55. [DOI] [PubMed] [Google Scholar]
- 56. Maltezou HC, Tryfinopoulou K, Katerelos P, et al. Consecutive Serratia marcescens multiclone outbreaks in a neonatal intensive care unit. Am J Infect Control 2012; 40:637–42. [DOI] [PubMed] [Google Scholar]
- 57. Orrett FA Fatal multi-resistant Pseudomonas aeruginosa septicemia outbreak in a neonatal intensive care unit in Trinidad. Ethiop Med J 2000; 38:85–91. [PubMed] [Google Scholar]
- 58. Pitten FA, Panzig B, Schröder G, et al. Transmission of a multiresistant Pseudomonas aeruginosa strain at a German university hospital. J Hosp Infect 2001; 47:125–30. [DOI] [PubMed] [Google Scholar]
- 59. Riser E, Noone P, Thompson RE. The use of a fluorescence typing method in an epidemiological study of Klebsiella infection in a London hospital. J Hyg (Lond) 1978; 80:43–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Salimi H, Yakhchali B, Owlia P, Lari AR. Molecular epidemiology and drug susceptibility of Pseudomonas aeruginosa strains isolated from burn patients. Lab Med 2010; 41:540–4. [Google Scholar]
- 61. Schneider H, Geginat G, Hogardt M, et al. Pseudomonas aeruginosa outbreak in a pediatric oncology care unit caused by an errant water jet into contaminated siphons. Pediatr Infect Dis J 2012; 31:648–50. [DOI] [PubMed] [Google Scholar]
- 62. Seara N, Oteo J, Carrillo R, et al. Interhospital spread of NDM-7-producing Klebsiella pneumoniae belonging to ST437 in Spain. Int J Antimicrob Agents 2015; 46:169–73. [DOI] [PubMed] [Google Scholar]
- 63. Sissoko B, Sutterlin R, Blaschke M, et al. Sink drains as a source of pathogens and infections: prevention of nosocomial infections [in German]. Hygiene Medizin 2004; 29:451–5. [Google Scholar]
- 64. Starlander G, Melhus Å. Minor outbreak of extended-spectrum β-lactamase-producing Klebsiella pneumoniae in an intensive care unit due to a contaminated sink. J Hosp Infect 2012; 82:122–4. [DOI] [PubMed] [Google Scholar]
- 65. Stjärne Aspelund A, Sjöström K, Olsson Liljequist B, et al. Acetic acid as a decontamination method for sink drains in a nosocomial outbreak of metallo-β-lactamase-producing Pseudomonas aeruginosa. J Hosp Infect 2016; 94:13–20. [DOI] [PubMed] [Google Scholar]
- 66. Thomas ME, Piper E, Maurer IM. Contamination of an operating theatre by gram-negative bacteria. Examination of water supplies, cleaning methods and wound infections. J Hyg (Lond) 1972; 70:63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Tofteland S, Naseer U, Lislevand JH, et al. A long-term low-frequency hospital outbreak of KPC-producing Klebsiella pneumoniae involving Intergenus plasmid diffusion and a persisting environmental reservoir. PLoS One 2013; 8:e59015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Vergara-López S, Domínguez MC, Conejo MC, et al. Wastewater drainage system as an occult reservoir in a protracted clonal outbreak due to metallo-β-lactamase-producing Klebsiella oxytoca. Clin Microbiol Infect 2013; 19:E490–8. [DOI] [PubMed] [Google Scholar]
- 69. Wendel AF, Kolbe-Busch S, Ressina S, et al. Detection and termination of an extended low-frequency hospital outbreak of GIM-1-producing Pseudomonas aeruginosa ST111 in Germany. Am J Infect Control 2015; 43:635–9. [DOI] [PubMed] [Google Scholar]
- 70. Willmann M, Bezdan D, Zapata L, et al. Analysis of a long-term outbreak of XDR Pseudomonas aeruginosa: a molecular epidemiological study. J Antimicrob Chemother 2015; 70:1322–30. [DOI] [PubMed] [Google Scholar]
- 71. Wolf I, Bergervoet PW, Sebens FW, et al. The sink as a correctable source of extended-spectrum β-lactamase contamination for patients in the intensive care unit. J Hosp Infect 2014; 87:126–30. [DOI] [PubMed] [Google Scholar]
- 72. Zhou Z, Hu B, Gao X, et al. Sources of sporadic Pseudomonas aeruginosa colonizations/infections in surgical ICUs: association with contaminated sink trap. J Infect Chemother 2016; 22:450–5. [DOI] [PubMed] [Google Scholar]
- 73. de Jonge E, de Boer MGJ, van Essen EHR, et al. Effects of a disinfection device on colonization of sink drains and patients during a prolonged outbreak of multidrug-resistant Pseudomonas aeruginosa in an intensive care unit. J Hosp Infect 2019; 102:70–4. [DOI] [PubMed] [Google Scholar]
- 74. Knoester M, de Boer MG, Maarleveld JJ, et al. An integrated approach to control a prolonged outbreak of multidrug-resistant Pseudomonas aeruginosa in an intensive care unit. Clin Microbiol Infect 2014; 20:O207–15. [DOI] [PubMed] [Google Scholar]
- 75. Kizny Gordon AE, Mathers AJ, Cheong EYL, et al. The hospital water environment as a reservoir for carbapenem-resistant organisms causing hospital-acquired infections—a systematic review of the literature. Clin Infect Dis 2017; 64:1435–44. [DOI] [PubMed] [Google Scholar]
- 76. Parkes LO, Hota SS. Sink-related outbreaks and mitigation strategies in healthcare facilities. Curr Infect Dis Rep 2018; 20:42. [DOI] [PubMed] [Google Scholar]
- 77. Carling PC Wastewater drains: epidemiology and interventions in 23 carbapenem-resistant organism outbreaks. Infect Control Hosp Epidemiol 2018; 39:972–9. [DOI] [PubMed] [Google Scholar]
- 78. Facciolà A, Pellicanò GF, Visalli G, et al. The role of the hospital environment in the healthcare-associated infections: a general review of the literature. Eur Rev Med Pharmacol Sci 2019; 23:1266–78. [DOI] [PubMed] [Google Scholar]
- 79. Chia PY, Sengupta S, Kukreja A, et al. The role of hospital environment in transmissions of multidrug-resistant gram-negative organisms. Antimicrob Resist Infect Control 2020; 9:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Constantinides B, Chau KK, Quan TP, et al. Genomic surveillance of Escherichia coli and Klebsiella spp. in hospital sink drains and patients. Microb Genom 2020; 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Kotay S, Chai W, Guilford W, et al. Spread from the sink to the patient: in situ study using green fluorescent protein (GFP)-expressing Escherichia coli to model bacterial dispersion from hand-washing sink-trap reservoirs. Appl Environ Microbiol 2017; 83:e03327–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Aranega-Bou P, George RP, Verlander NQ, et al. ; TRACE Investigators’ Group Carbapenem-resistant Enterobacteriaceae dispersal from sinks is linked to drain position and drainage rates in a laboratory model system. J Hosp Infect 2019; 102:63–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Gestrich SA, Jencson AL, Cadnum JL, et al. A multicenter investigation to characterize the risk for pathogen transmission from healthcare facility sinks. Infect Control Hosp Epidemiol 2018; 39:1467–9. [DOI] [PubMed] [Google Scholar]
- 84. Hajar Z, Mana TSC, Cadnum JL, Donskey CJ. Dispersal of gram-negative bacilli from contaminated sink drains to cover gowns and hands during hand washing. Infect Control Hosp Epidemiol 2019; 40:460–2. [DOI] [PubMed] [Google Scholar]
- 85. Livingston SH, Cadnum JL, Gestrich S, et al. A novel sink drain cover prevents dispersal of microorganisms from contaminated sink drains. Infect Control Hosp Epidemiol 2018; 39:1254–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.