Abstract
Introduction
The number of congenital syphilis (CS) cases in Arizona quadrupled from an average of 14 cases annually before 2017 to 61 cases in 2018, and a statewide outbreak was declared. The Arizona Department of Health Services (ADHS) analyzed statewide surveillance data to identify missed opportunities for prevention and collaborated with the Arizona Health Care Cost Containment System (AHCCCS) to inform response activities.
Methods
ADHS developed a metric to identify missed opportunities for CS prevention during pregnancy by using medical records, vital records, and case investigation notes for all mothers of infants born with CS from January 1, 2017, through June 30, 2018. AHCCCS conducted a cost-effectiveness analysis to calculate the effect of increasing perinatal syphilis screening.
Results
Arizona had 57 cases of CS during the study period, of which 17 (29.8%) could have been prevented through third-trimester screening for women who were in prenatal care but screened late (n = 9), were infected after their first prenatal visit screen (n = 7), or were reinfected after an initial reactive syphilis test and appropriate treatment and not rescreened (n = 1). The estimated net cost of combining the additional primary (screening) and secondary (treatment) costs of a third-trimester screen for all pregnant AHCCCS members and the estimated total per-year savings of all newborn hospitalizations was $527.
Practice Implications
Third-trimester syphilis screening could prevent CS in regions where syphilis transmission is high. Partnering with health insurance agencies to evaluate the cost effectiveness of screening recommendations may improve the accuracy of the estimate of the potential cost savings by using insurance agency–specific data for the population at risk for CS.
Keywords: policy, screening, syphilis, pregnant women
Congenital syphilis (CS) is a preventable disease that occurs when a woman transmits syphilis to her fetus during pregnancy.1 From 2011 through 2016, Arizona averaged 14 CS cases per year.2 That number rose to 31 in 2017 and 61 in 2018. Arizona had 35.5 CS cases per 100 000 live births in 2017, and the state was ranked sixth in the United States for incidence rate of CS.3 The number of CS deaths increased from approximately 1 stillbirth every other year from 2011 through 2016 to 10 CS deaths in 2018.2 Although most CS cases occur in Maricopa County, beginning in 2017, a higher proportion of cases began to appear in rural areas, leading to declaration of a statewide outbreak in September 2018 (Figure 1).2,4 The Arizona Department of Health Services (ADHS) defines an outbreak of sexually transmitted disease as a 100% increase in the number of cases using a 12-month historical mean in a defined population; a statewide outbreak requires multiple jurisdictions to observe this increase (unpublished report, ADHS, 2017).
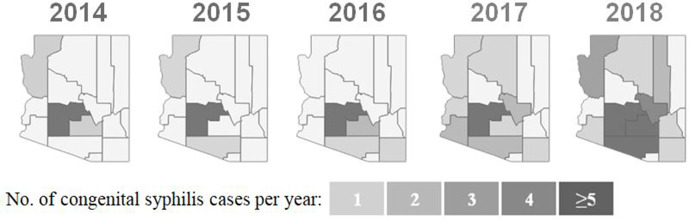
Figure 1.
Distribution of congenital syphilis (CS) incidence among newborns throughout Arizona counties from 2014 through 2018. Before 2017, CS primarily occurred in Maricopa County. The number of counties that reported CS increased substantially starting in 2017, leading to a declaration of a statewide outbreak of syphilis among women and infants in September 2018. By 2018, multiple counties were reporting >5 CS cases per year. Data source: Arizona Department of Health Services.2
Untreated syphilis during pregnancy can lead to stillbirth or newborn death in up to 40% of cases. Infants born with CS who survive may have deformed bones, cognitive impairments, and other severe problems.1 Although initiating appropriate treatment at least 30 days before delivery may help prevent adverse outcomes, several studies indicate a lower prevalence of adverse outcomes among infants of women who were treated in the first or second trimester vs the third trimester.5,6
The Centers for Disease Control and Prevention (CDC) recommends syphilis screening for all pregnant women at their first prenatal care visit and additional screening for pregnant women at increased risk for syphilis in the beginning of the third trimester (defined as 28-32 weeks of gestation) and at delivery.1 Women at increased risk for syphilis include uninsured women, women living in poverty, sex workers, women who use illicit drugs, and women in communities with high syphilis morbidity.7 Research on rescreening pregnant women for syphilis during the third trimester has found that this practice is not cost effective in most populations.8,9 In areas with an incidence of seroconversion during pregnancy >0.2% or with a high rate of primary, secondary, or early-stage syphilis among women, third-trimester screening could be cost saving.8,10 As of 2016, 43 states required that health care providers screen a pregnant woman at or shortly after her first prenatal care visit. Only 17 states required third-trimester syphilis screening: 12 specified that all pregnant women should be screened for syphilis in the third trimester, and 5 specified that only pregnant women at high risk for syphilis should be screened during the third trimester. These states have determined that syphilis screening during the third trimester reduces the incidence of CS in their jurisdictions.11 States such as Florida and Louisiana have been at the forefront of these efforts, with other states (eg, Arizona) following.10 Given the statewide outbreak of syphilis among women and infants, all pregnant women in Arizona are considered high risk, and third-trimester screening is now medically necessary.
Before 2018, Arizona statute required screening for syphilis only at the first prenatal care visit, although Maricopa County issued a Board Order in 2003 to add prenatal syphilis screening during the third trimester and at delivery.12 In 2018, the state administrative code was amended to include “serologic testing for syphilis at 28 to 32 weeks of gestation” among “pregnant syphilis case(s),” aligning with CDC screening recommendations.13 This requirement further aligned with other states that had a high morbidity of CS: 4 of 5 states with the highest CS rates have laws requiring third-trimester screening among all women, regardless of risk.14
Because of the phrasing of the administrative code, health care providers may interpret the additional screenings as required only for women who initially screen positive for syphilis. Studies have found that laws and regulations for syphilis screening are not regularly followed by health care providers.12,15 To clarify the recommendation, ADHS issued a press release,4 launched an outbreak page on the ADHS website,16 and sent an advisory via the Health Alert Network and the local infectious disease application, IDAZ, about the syphilis outbreak and new requirements for third-trimester screening on September 25, 2018 (unpublished report, ADHS, September 25, 2018).
CS rates continued to rise, which prompted ADHS concern for delayed uptake and possible misinterpretation of the new recommendations among health care providers. To evaluate adherence to the revised administrative code and promote adherence to third-trimester screening among subpopulations most affected by CS, ADHS conducted a missed opportunities analysis by using data from before and after the administrative code was updated in January 2018. The objectives of this project were to (1) identify missed opportunities for CS prevention through third-trimester screening and (2) assess the financial effect of increasing perinatal syphilis screening. Analyses identifying the gaps in prevention of CS in collaboration with health insurance providers have not been reported but are important, because policies and cost-effectiveness results are often not applicable to the entire population but may be applicable to subgroups at increased risk of CS, such as certain health insurance groups.
Materials and Methods
ADHS used surveillance data from January 1, 2017, through June 30, 2018, from 3 sources: (1) ADHS surveillance data on 7 variables (syphilis surveillance stage, age, pregnancy status, treatment dosage and date[s], treatment provider, all syphilis screening dates and results, and county of residence); (2) vital statistics data on 5 variables (number of prenatal care visits, date of first prenatal care visit, date of last prenatal care visit, method of payment for delivery [ie, health insurance provider], and delivery facility); and (3) medical records data on 3 variables (newborn symptoms from physical examination, newborn syphilis screening [eg, cerebral spinal fluid venereal disease research laboratory (CSF VDRL) test, long bone x-rays, rapid plasma reagin (RPR) test, Treponema pallidum particle agglutination test], and date of death of newborn [if applicable]) (unpublished data, ADHS, 2017-2018).
Data were collected on women who were infected with syphilis during their pregnancy and delivered an infant with CS. Infants who did not have serologic or physical signs or symptoms of syphilis and whose mothers were infected with syphilis but initiated adequate treatment >30 days before delivery were considered to have been prevented from acquiring CS.
Syphilis surveillance stage and RPR titer were reported for all mothers of infants with CS. Syphilis surveillance stage is reported in ADHS surveillance data after being determined according to the Council of State and Territorial Epidemiologists (CSTE) case definition, using a combination of symptomology and screening, and indicates how recently a person was infected with syphilis.17 Primary, secondary, or early-stage syphilis indicates infection during the previous 12 months. Late latent syphilis indicates infection >12 months ago and that the person is not currently infectious.17
Many people infected with syphilis do not notice their symptoms, leading to the importance of RPR titer results.18 Although an RPR titer result of any value indicates a positive syphilis test result, quantitative values determine the amount of syphilis antibody in the serum sample; a higher titer indicates a higher concentration of the syphilis antibody.19 Although titers cannot be used to determine the stage of syphilis, an early-stage syphilis case with a high titer could be misclassified as a late latent case under the CSTE case definition because of unrecognized symptoms.17-19 Regardless of syphilis stage or RPR titer result of the mother, syphilis can be spread from mother to child during pregnancy, although the highest risk of CS occurs when the mother has primary, secondary, or early-stage syphilis.5
We used surveillance data, medical records, and vital statistics data to identify circumstances in which intervention could have prevented CS, such as third-trimester screening, access to prenatal care, and timeliness of treatment, among mothers of infants with CS in Arizona. We arranged these circumstances into a cascade-of-care metric, which is an organizing framework used to codify quantifiable outcome measures at each stage in the cascade of care. ADHS used frequency tables to quantify the number of CS cases that met the criteria for each stage of the cascade of care and identify areas where health care services failed to prevent CS. We analyzed data by using SAS version 9.4.20
Within the cascade of care, ADHS first identified the number of women who did not receive prenatal care at least 45 days before delivery, to exclude infants born with CS that could not have been prevented through screening. Although the CSTE definition of CS requires initiation of treatment at least 30 days before delivery for a CS case to be considered prevented,21 based on Arizona surveillance data, the average time between specimen collection and initiation of treatment is 15 days (unpublished data, ADHS, 2017-2018). For this reason, we considered women who were screened >45 days before delivery to be preventable cases.
ADHS presented initial findings from the missed opportunities analysis during 3 meetings with Arizona Health Care Cost Containment System (AHCCCS) leadership from July through August 2018. These presentations led to a cost analysis to evaluate the cost of promoting third-trimester syphilis screening during pregnancy for all pregnant AHCCCS members.
AHCCCS used a combination of its pricing codes and literature on the topic to calculate the expected cost of an additional syphilis screen and treatment costs and compare it with the cost of treating an infant born with CS, to calculate the potential net savings for or net cost to AHCCCS. To calculate the expected cost of an additional syphilis screen during the third trimester for all pregnant women, AHCCCS used the average syphilis screening cost of $6.59 and adjusted for enrollment and reimbursement growth.
AHCCCS also considered a secondary effect of increasing screening: increased syphilis case detection leading to increased cost for treatment using penicillin. The average cost per use of penicillin by AHCCCS members was $12.53 in federal fiscal year 2017 (FFY 2017). AHCCCS multiplied this number by the number of CS cases that could have been averted through third-trimester syphilis screening, assuming all women would be treated with penicillin once.
CS treatment results in 10-14 days of hospitalization for neonates. An analysis of 2009 health insurance claims data found that hospital costs for an infant born with CS were $9969 higher than for an infant born without CS.22 AHCCCS calculated the current hospitalization fee per infant with CS by applying the inflation rate of 27%, calculated by using the US Consumer Price Index for Medicare Care Services from 2009 to 2017.23 Once inflation was applied, the cost in hospitalization fees increased to $12 660 in 2017. AHCCCS multiplied $12 660 by the number of CS cases that could have been averted through third-trimester screening to determine total annual cost savings of CS prevention. AHCCCS combined the calculated total cost of additional syphilis screening and treatment with the total annual cost savings of CS prevention to determine the total net cost or savings per year to AHCCCS.
We report the methodological practices for each analysis by using the Consolidated Health Economic Evaluation Reporting Standards checklist, a 24-item checklist that serves as a guide for key items to include when reporting on health economic evaluations (Table 1).24 The ADHS Human Subjects Review Board did not review and approve this study because it was considered non-research.
Table 1.
Components of analysis methodologies used to identify missed opportunities for preventing congenital syphilis (CS) through third-trimester screening and to evaluate its cost effect, Arizona, 2017-2018
| CHEERSa component | Missed opportunities analysis | Effect analysis |
|---|---|---|
| Target population | Pregnant Arizona women with syphilis | Pregnant women enrolled in AHCCCS residing in greater Arizona (ie, excluding Maricopa County). |
| Study perspective | Statewide surveillance data, vital statistics data, and medical records were used to determine missed opportunities for preventing CS through a cascade of care focused on screening and treatment opportunities that were missed throughout pregnancy. The results from the missed opportunities analysis led to further analyzing third-trimester screening as a potential missed opportunity for preventing CS. Case classification was determined by using the CSTE definition of CS.21 | AHCCCS data were used to determine the cost of covering an additional syphilis screening during pregnancy. The total number of pregnant AHCCCS members was estimated by using the AHCCCS Newborn Report for federal fiscal year (FFY) 2017 (unpublished report, AHCCCS, 2017). |
| Setting and location | Arizona | Greater Arizona (excluding Maricopa County) |
| Comparators | Once the cascade of care was complete and third-trimester screening was identified as a gap, Maricopa County data were used as a comparator for greater Arizona, and mothers whose health insurance agency was AHCCCS were used as a comparator with other mothers of infants with CS. | The analysis enumerated the additional screening and treatment costs after a change to the administrative code. |
| Time period | Mothers of infants with CS born from January 1, 2017, through June 30, 2018 | FFY 2017 |
| Choice of health outcomes | Evaluated health outcomes included the presence of prenatal care >45 days before delivery, syphilis screening >45 days before delivery, appropriate treatment initiated >30 days before delivery, and the existence of CS symptoms (despite appropriate screening and treatment). | The evaluated outcome was the cost of providing 1 additional screen per pregnant AHCCCS member in greater Arizona. |
| Estimating resources and costs | NA | The average cost per prenatal syphilis screen was estimated to be $6.59, using FFY 2017 reimbursement data for Current Procedural Terminology codes 86780, 86592, and 86593.25 The cost to treat most cases of adult syphilis, including pregnant women, was estimated to be $12.53 per case, using the average drug cost per AHCCCS penicillin claim during FFY 2017. |
| Assumptions | CSTE considers an infant born to a mother who initiated appropriate syphilis treatment >30 days before delivery to be ruled out as a CS case, unless the infant is symptomatic or has other criteria that meet the case definition for CS.21 A 15-day buffer was added to this threshold because surveillance data indicate that it can take an average of 2 weeks after the initial positive test result for Arizonan women to initiate treatment. Therefore, infants born to mothers with access to clinical services at least 45 days before delivery were considered to have preventable CS, and infants born to mothers who accessed care <45 days before delivery were considered to have CS that was not preventable. | The analysis adjusted for increased enrollment and reimbursement growth. An annual increase of 825 AHCCCS members per fiscal year was assumed. It was also assumed that all pregnant women insured by AHCCCS were insured throughout their pregnancy and would receive all recommended syphilis screenings. The model did not exclude women who were added to AHCCCS at delivery and missed their initial screening(s). In addition, because Maricopa County has had a Board Order recommending third-trimester screening since 2003, it was assumed that the expanded screening would affect only residents of greater Arizona, and Maricopa County residents were therefore excluded from the cost-effect analysis. |
| Analytic methods | Potential cases were classified by using the CSTE case definition.21 ADHS requested medical records for each infant with CS and the infant’s mother. Data from medical records and the vital records database were used to supplement missing information from the surveillance data and case investigation notes. Data were analyzed by using SAS version 9.4.20 | Screening and treatment costs were multiplied by the number of pregnant members residing in greater Arizona, adjusted for enrollment and reimbursement growth by using historical enrollment data. |
Abbreviations: ADHS, Arizona Department of Health Services; AHCCCS, Arizona Health Care Cost Containment System; CSTE, Council of State and Territorial Epidemiologists; NA, not applicable.
aThe Consolidated Health Economic Evaluation Reporting Standards checklist is a 24-item checklist that serves as a guide for key items to include when reporting on health economic evaluations.24
Results
Demographic Characteristics
From January 1, 2017, through June 30, 2018, ADHS surveillance data identified 981 syphilis cases among Arizona women, of which 844 (86.0%) were among women of reproductive age (13-45 years). Of these 844 women, 205 (24.3%) were pregnant. CS was prevented in 148 of 205 (72.2%) infants through testing and treatment at least 30 days before delivery. Fifty-seven (27.8%) infants were born with CS, 9 of whom died: 7 were stillborn and 2 died after birth.
Of 57 mothers of infants with CS, 35 (61.4%) had primary, secondary, or early-stage syphilis. An additional 15 (26.3%) mothers of infants with CS had late latent syphilis but had a high titer quantitative result in the nontreponemal test (>1:16). Six of the 9 mothers of infants who died had primary, secondary, or early-stage syphilis.
Of the 50 infants with CS who were born alive (excluding the 7 infants who were stillborn and did not have a physical examination or screening completed), 9 (18.0%) had a positive CSF VDRL test value, 11 (22.0%) had a positive long bone radiograph, 13 (26.0%) had an elevated white blood cell count in CSF, 25 (50.0%) had elevated protein in CSF, and 12 (24.0%) had other evidence of CS from a physical examination, including jaundice (n = 6), syphilitic skin rash (n = 3), hepatosplenomegaly (n = 3), and edema (n = 1).
Missed Opportunities
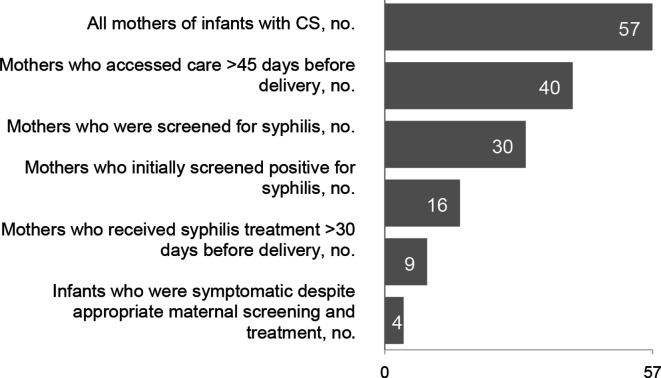
Among 57 women who gave birth to an infant with CS, 17 (29.8%) did not access prenatal care ≥45 days before delivery, 10 (17.5%) accessed prenatal care but were not screened ≥45 days before delivery, 7 (12.3%) were screened ≥45 days before delivery but were not treated 30 days before delivery, 14 (24.6%) tested negative for syphilis early in their pregnancy and then tested positive for syphilis at delivery, 5 (8.8%) were reinfected after treatment, and 4 (7.0%) had a symptomatic infant despite appropriate screening and treatment (Figure 2, Table 2). Among women who received timely testing and treatment, additional factors contributing to CS included delayed maternal treatment, seroconversion after an initial negative test, and symptomatic infants despite appropriate maternal testing and treatment.
Figure 2.
Stages along the cascade of syphilis screening and treatment during pregnancy among mothers of infants with congenital syphilis (CS) in Arizona, January 1, 2017, through June 30, 2018. Data sources: Arizona Department of Health Services surveillance data, vital statistics data, and medical records (unpublished data, ADHS, 2017-2018).
Table 2.
Factors contributing to congenital syphilis (CS) among 57 mothers of infants with CS in Arizona from January 1, 2017, through June 30, 2018a
| Prevention category | No. (%) | Missed opportunity | No. (%) |
|---|---|---|---|
| Mothers who accessed care >45 days before delivery | 40 (70.2) | Delayed access to care | 17 (29.8) |
| Mothers who were tested for syphilis | 30 (52.6) | Not tested in time | 10 (17.5) |
| Mothers who initially tested positive for syphilis | 16 (28.1) | Seroconverted after initial negative screen | 14 (24.6) |
| Mothers who received treatment >30 days before delivery | 9 (15.8) | Not treated in time | 7 (12.3) |
| Infants who were symptomatic despite appropriate maternal screening and treatment | 4 (7.0) | Reinfected after treatment | 5 (8.8) |
aArizona Department of Health Services (ADHS) surveillance data, vital statistics data, and medical records were used to calculate frequencies of pregnant women that fall within each gap of the cascade of care (unpublished data, ADHS, 2017-2018).
We identified third-trimester screening as a potential prevention gap for a high proportion of CS cases. Statewide, third-trimester screening may have prevented CS among 17 of 57 (29.8%) mothers of infants who (1) were in prenatal care but were not screened in time (9 statewide; 7 of 36 [19%] outside of Maricopa County), (2) seroconverted to positive during pregnancy after an initial negative test (7 statewide; 5 of 36 [14%] outside of Maricopa County), or (3) were reinfected during pregnancy (1 statewide; none outside of Maricopa County) (Table 3). The percentage of preventable CS cases from third-trimester screening was higher outside of Maricopa County (7 of 21; 33.3%) than in Maricopa County (10 of 36; 27.8%). In addition, AHCCCS insured 41 of 57 (71.9%) mothers of infants with CS statewide and 13 of 17 (76.5%) mothers of infants with CS statewide whose CS could have been prevented through third-trimester screening.
Table 3.
Factors in the cascade of care contributing to cases of congenital syphilis (CS) (n = 57), by whether CS was preventable through third-trimester screening, Arizona, 2017-2018
| Factors | Preventable with third-trimester screening (n = 17), no. (%)a | Not preventable with third-trimester screening (n = 40), no. (%)a |
|---|---|---|
| First prenatal care visit <45 days before delivery or no prenatal care | 0 | 17b (30) |
| In prenatal care, not screened in time | 9 (16) | 1c (2) |
| Not treated in time | 0 | 7d (12) |
| Mothers negative, later positive | 7 (12) | 7e (12) |
| Reinfected | 1 (2) | 4e (7) |
| Infant symptomatic | 0 | 4f (7) |
| Total | 17 (30) | 40 (70) |
aThe denominator for the percentages is the total number of cases of CS (n = 57).
bCS among infants born to mothers who had a first prenatal care visit <45 days before delivery or no prenatal care was not preventable with third-trimester screening because the mothers were not in prenatal care either at all or in time to receive third-trimester screening.
cThe infant was stillborn at 20 weeks of gestation, before third-trimester screening could have been performed.
dCS among infants born to mothers who were not treated in time was not preventable with third-trimester screening because they were not treated >30 days before delivery.
eMothers who seroconverted or were reinfected during pregnancy, whose disease was not preventable through third-trimester screening, became infected <45 days before delivery.
fCS among infants who were symptomatic was not preventable through third-trimester screening because the newborns were symptomatic despite proper care and prevention.
Effect Analysis
In FFY 2017, 14 716 pregnant AHCCCS members resided in greater Arizona. AHCCCS determined that a third-trimester screen for AHCCCS members in greater Arizona would cost AHCCCS an additional $97 000 annually, increasing to $113 300 annually when adjusted for enrollment and reimbursement growth. Through the results of the missed opportunities analysis, AHCCCS identified that 9 pregnant women insured with AHCCCS could have been identified with syphilis infection through third-trimester screening annually. As a result, the secondary effect of increasing screening estimated that an additional 9 pregnant women would require treatment for syphilis, costing AHCCCS an additional minimum of $113 annually.
With 9 infants born with CS per year insured by AHCCCS, AHCCCS estimated the potential savings in hospitalization fees resulting from screening averting CS to be $113 940 annually. The additional cost of a third-trimester screen estimated in this study, $113 300 in screening and $113 in treatment, combined with the potential newborn hospitalization savings, $113 940, would result in a net total savings of $527 per year to AHCCCS.
Discussion
Most mothers (61.4%) of infants with CS were in primary, secondary, or early-stage syphilis during their pregnancy. Pregnant women in the primary or secondary stage of syphilis have a higher risk of delivering an infant with CS than women in the latent stage of syphilis.5 Furthermore, women infected with syphilis in the 4 years before delivery have a 70% probability of transmitting the infection to their fetus and are at a higher risk for adverse outcomes than women infected with syphilis >4 years before delivery.26 This finding explains why 39 of 50 (78%) infants born alive with CS had symptoms, 2 were born alive and later died, and 7 were stillborn.
The cost-effectiveness analysis resulted in AHCCCS covering 3 syphilis screenings during pregnancy and requiring third-trimester screening among members of its health care provider network.27,28 Screening was expanded in part because of the increased prevalence of syphilis in rural Arizona and the number of infections that occurred after the initial prenatal care visit.
Limitations
This study had several limitations. First, detailed data on AHCCCS coverage throughout pregnancy were limited, making it difficult to ascertain whether mothers were covered by AHCCCS throughout their pregnancy or were provided emergency AHCCCS coverage when they delivered. Not knowing when AHCCCS coverage began prevented ADHS from determining if any of the women should have been excluded from the cost-effectiveness analysis due to lack of health insurance coverage during pregnancy before delivery. Second, the estimated annual hospitalization cost savings from CS prevention is likely an underestimate because it estimated only the hospitalization cost after delivery, not including the additional lifetime costs of CS beyond the initial hospitalization. The estimation of annual hospitalization cost saving additionally did not adjust for the increasing incidence of CS annually. Finally, the additional cost of treatment was likely underestimated because we assumed that all positive cases would need only 1 dose of penicillin. If a pregnant woman was diagnosed with latent syphilis, 3 doses of penicillin would be required. Some evidence even suggests that an additional dose of penicillin is beneficial for pregnant women with primary, secondary, or early-stage syphilis.5
Practice Implications
Given the nationwide increases in rates of CS among infants, jurisdictions should complete an assessment of gaps in their patient care cascades to identify areas in need of improved interventions. If third-trimester screening is one of the gaps, jurisdictions should consider expanding third-trimester screening recommendations through cost-effectiveness analyses. A gap analysis may reveal additional areas of intervention and opportunities to improve uptake of screening recommendations. Our analysis identified timeliness of treatment as a barrier to preventing CS for 7 infants diagnosed with CS, which prompted additional activities to improve this metric, such as health care provider education and standing orders for jurisdictions to expand field treatment of syphilis. Similarly, identifying strategies to improve partner elicitation and treatment may have prevented the reinfection of 5 pregnant women with syphilis during pregnancy.
Jurisdictions may also explore regional variations in the gaps identified in their care cascades, because third-trimester screening may not be cost effective in areas with low rates of disease transmission.29 Before approaching health insurance agencies to cover additional screenings, jurisdictions should consider evaluating the health insurers of mothers of infants with CS. Incidence rates of syphilis among pregnant women and CS among infants may differ among people covered by various health insurance providers, and the cost savings or additional cost may depend on the incidence in each population the health insurance provider covers. ADHS approached AHCCCS because most insured mothers of infants with CS were enrolled in AHCCCS. A cost-effectiveness analysis from a private health insurance provider may have shown higher cost to the provider, which would likely not have implemented third-trimester screening.
Few analyses identify the gaps in prevention of CS with health insurance coverage, and none explore collaboration with health insurance providers to determine the cost effectiveness of these interventions.30,31 Our analysis leveraged a partnership between ADHS and AHCCCS to prevent further incidence of CS through cost-effective approaches. As a result of our analysis, on November 20, 2018, AHCCCS revised its medical policy for maternal care to increase the number of covered syphilis screenings during pregnancy to 3, including a screening at the first prenatal care visit, during the third trimester, and at delivery.27,28 This policy applies to all members insured by AHCCCS to align with the new ADHS administrative code. This change in policy is in effect for the duration of the syphilis outbreak in Arizona, which was ongoing into 2020. This policy change should decrease the number of infants born with CS among women who initially test negative for syphilis or who are reinfected after their first-trimester screen.
Acknowledgments
The authors acknowledge Eric Tack, MD, JD, for his participation in the AHCCCS effect analysis.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This article was supported by the grant or cooperative agreement no. 6 NH25PS005157-01-01, funded by the Centers for Disease Control and Prevention (CDC). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of CDC or the US Department of Health and Human Services.
ORCID iD
Kaitlyn J. Sykes, MPH https://orcid.org/0000-0001-9540-0389
References
- 1.Centers for Disease Control and Prevention Congenital syphilis—CDC fact sheet. 2017. Accessed April 5, 2019 https://www.cdc.gov/std/syphilis/stdfact-congenital-syphilis.htm
- 2.Arizona Department of Health Services Sexually transmitted disease (STD): STD data. February 19, 2020. Accessed February 24, 2020 https://www.azdhs.gov/preparedness/epidemiology-disease-control/disease-integration-services/std-control/index.php#reports
- 3.Centers for Disease Control and Prevention. National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention Sexually transmitted disease surveillance 2017. Accessed April 4, 2019 https://www.cdc.gov/std/stats17/2017-STD-Surveillance-Report_CDC-clearance-9.10.18.pdf
- 4.Arizona Department of Health Services Syphilis outbreak impacts the health of women and babies. September 25, 2018 Accessed December 2, 2019 https://directorsblog.health.azdhs.gov/syphilis-outbreak-impacts-the-health-of-women-and-babies
- 5.Centers for Disease Control and Prevention Sexually transmitted diseases treatment guidelines—syphilis during pregnancy. Accessed April 1, 2019 https://www.cdc.gov/std/tg2015/syphilis-pregnancy.htm
- 6.Hawkes SJ., Gomez GB., Broutet N. Early antenatal care: does it make a difference to outcomes of pregnancy associated with syphilis? A systematic review and meta-analysis. PLoS One. 2013;8(2):e56713. 10.1371/journal.pone.0056713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. US Preventive Services Task Force Screening for syphilis infection in pregnancy: reaffirmation recommendation statement. Ann Intern Med. 2009;150(10):705-709. 10.7326/0003-4819-150-10-200905190-00008 [DOI] [PubMed] [Google Scholar]
- 8.Albright CM., Emerson JB., Werner EF., Hughes BL. Third-trimester prenatal syphilis screening: a cost-effectiveness analysis. Obstet Gynecol. 2015;126(3):479-485. 10.1097/AOG.0000000000000997 [DOI] [PubMed] [Google Scholar]
- 9.Edwards RK., Bennett M., Langstraat C., Greene D. Assessment of the value of rescreening for syphilis in the third trimester of pregnancy. Infect Dis Obstet Gynecol. 2006;2006:56504. 10.1155/IDOG/2006/56504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matthias JM., Rahman MM., Newman DR., Peterman TA. Effectiveness of prenatal screening and treatment to prevent congenital syphilis, Louisiana and Florida, 2013-2014. Sex Transm Dis. 2017;44(8):498-502. 10.1097/OLQ.0000000000000638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Warren HP., Cramer R., Kidd S., Leichliter JS. State requirements for prenatal syphilis screening in the United States, 2016. Matern Child Health J. 2018;22(9):1227-1232. 10.1007/s10995-018-2592-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collier MG., Taylor MM., Winscott MM., Mickey T., England B. Assessing compliance with a county board order for third trimester syphilis screening in Maricopa County, Arizona. Sex Reprod Healthc. 2011;2(3):125-128. 10.1016/j.srhc.2011.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arizona Administrative Code Title 9, Health Services, Ch 6. Accessed December 1, 2019 https://apps.azsos.gov/public_services/Title_09/9-06.pdf
- 14.Centers for Disease Control and Prevention State statutory and regulatory language regarding prenatal syphilis screenings in the United States, 2018. Prenatal syphilis screening laws. Accessed April 5, 2019 https://www.cdc.gov/std/treatment/syphilis-screenings-2018.htm
- 15.Trepka MJ., Bloom SA., Zhang G., Kim S., Nobles R. Inadequate syphilis screening among women with prenatal care in a community with a high syphilis incidence. Sex Transm Dis. 2006;33(11):670-674. 10.1097/01.olq.0000216032.52731.ea [DOI] [PubMed] [Google Scholar]
- 16.Arizona Department of Health Services Arizona syphilis outbreak: women and babies. Accessed December 2, 2019 https://azdhs.gov/preparedness/epidemiology-disease-control/disease-integration-services/std-control/congenital-syphilis/index.php
- 17.Centers for Disease Control and Prevention Syphilis (Treponema pallidum)—2018 case definition. Accessed April 5, 2019 https://wwwn.cdc.gov/nndss/conditions/syphilis/case-definition/2018
- 18.Centers for Disease Control and Prevention Sexually transmitted diseases (STDs): what is syphilis? Detailed fact sheet. Accessed April 5, 2019 https://www.cdc.gov/std/syphilis/stdfact-syphilis-detailed.htm
- 19.Henao-Martinez AF., Johnson SC. Diagnostic tests for syphilis: new tests and new algorithms. Neurol Clin Pract. 2014;4(2):114-122. 10.1212/01.CPJ.0000435752.17621.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. SAS [computer program] Version 9.4. SAS Institute Inc; 2014.
- 21.Centers for Disease Control and Prevention Congenital syphilis (Treponema pallidum)—2015 case definition. Accessed April 5, 2019 https://wwwn.cdc.gov/nndss/conditions/congenital-syphilis/case-definition/2015
- 22.Owusu-Edusei K., Introcaso CE., Chesson HW. Hospitalization cost of congenital syphilis diagnosis from insurance claims data in the United States. Sex Transm Dis. 2013;40(3):226-229. 10.1097/OLQ.0b013e31827c5b9f [DOI] [PubMed] [Google Scholar]
- 23.US Bureau of Labor Statistics Consumer Price Index—all urban consumers, medical care services (ID CUSR0000SAM2), 2009-2017 [time series]. Accessed January 15, 2019 https//www.bls.gov/data
- 24.Husereau D., Drummond M., Petrou Set al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. Value Health. 2013;16(2):e1-e5. 10.1016/j.jval.2013.02.010 [DOI] [PubMed] [Google Scholar]
- 25.American Medical Association CPT (Current Procedural Terminology). Accessed December 2, 2019 https://www.ama-assn.org/amaone/cpt-current-procedural-terminology
- 26.De Santis M., De Luca C., Mappa Iet al. Syphilis infection during pregnancy: fetal risks and clinical management. Infect Dis Obstet Gynecol. 2012;2012:430585. 10.1155/2012/430585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arizona Health Care Cost Containment System AHCCCS medical policy manual: chapter 400—medical policy for maternal and child health. Accessed December 1, 2019 https://www.azahcccs.gov/shared/Downloads/MedicalPolicyManual/400/410.pdf
- 28.Arizona Health Care Cost Containment System Addressing the increase of congenital syphilis in Arizona. February 7, 2019 Accessed December 1, 2019 https://www.azahcccs.gov/shared/Downloads/News/2019/syphilis_outbreak_info.pdf
- 29.Beltrami J., Berman S. Congenital syphilis: a persisting sentinel public health event. Sex Transm Dis. 2006;33(11):675-676. 10.1097/01.olq.0000225324.66955.61 [DOI] [PubMed] [Google Scholar]
- 30.Wilson EK., Gavin NI., Adams EK., Tao G., Chireau M. Patterns in prenatal syphilis screening among Florida Medicaid enrollees. Sex Transm Dis. 2007;34(6):378-383. 10.1097/01.olq.0000245908.23629.b8 [DOI] [PubMed] [Google Scholar]
- 31.Neblett Fanfair R., Tao G., Owusu-Edusei K., Gift TL., Bernstein KT. Suboptimal prenatal syphilis testing among commercially insured women in the United States, 2013. Sex Transm Dis. 2017;44(4):219-221. 10.1097/OLQ.0000000000000569 [DOI] [PubMed] [Google Scholar]