Abstract
Deep partial thickness burns are clinically prevalent and difficult to diagnose. In order to develop methods to assess burn depth and therapies to treat deep partial thickness burns, reliable, accurate animal models are needed. The variety of animal models in the literature and the lack of precise details reported for the experimental procedures make comparison of research between investigators challenging and ultimately affect translation to patients. They sought to compare deep partial thickness porcine burn models from five well-established laboratories. In doing so, they uncovered a lack of consistency in approaches to the evaluation of burn injury depth that was present within and among various models. They then used an iterative process to develop a scoring rubric with an educational component to facilitate burn injury depth evaluation that improved reliability of the scoring. Using the developed rubric to re-score the five burn models, they found that all models created a deep partial thickness injury and that agreement about specific characteristics identified on histological staining was improved. Finally, they present consensus statements on the evaluation and interpretation of the microanatomy of deep partial thickness burns in pigs.
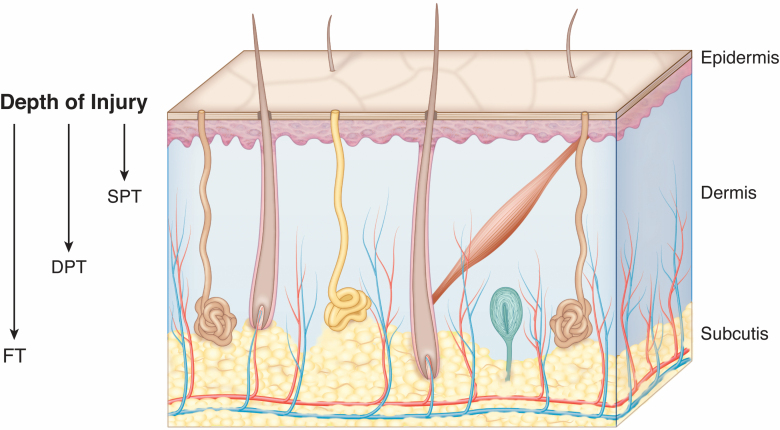
The assessment of burn depth is key to the clinical management of patients with burn injury. Cutaneous burns are classified as superficial partial thickness (SPT), deep partial thickness (DPT), or full thickness (FT), and often have heterogeneous depths throughout the surface area of the wound, complicating the assessment (Figure 1). SPT and FT burns are straightforward in their management, requiring wound care, or excision and grafting, respectively.1 Burns classified as DPT are often of indeterminate depth early after injury and present a unique clinical challenge.2 These DPT burns may initially look the same, but can be divided into two separate categories. The first category includes burns that heal normally within 2 to 3 weeks without surgical intervention.3,4 The second category includes those DPT burns that, without surgical intervention, take over 3 weeks to heal and therefore have the propensity towards hypertrophic scarring with sub-optimal outcomes.5,6 An ability to distinguish between these two DPT burn categories is needed to reduce unnecessary surgeries and thus shorten length of stay, reduce costs, and improve outcomes related to donor site morbidities. However, the accurate and timely clinical estimation of burn depth is highly variable amongst providers and is often inadequate.7,8
Figure 1.
Skin anatomy demonstrating various depths of thermal injury. Depth of clinically relevant thermal injury is defined according to depth into the dermal tissue. Superficial partial thickness (SPT) burns are limited to the upper dermis. Deep partial thickness (DPT) burns extend deep into the lower dermis. Full thickness (FT) burns extend all the way through the dermis into the underlying subcutaneous tissue or beyond.
ANIMAL MODEL CONSIDERATIONS FOR STUDIES ON BURN DEPTH
Preclinical research has relied on animal models to study burn wound characteristics and the degree of tissue damage where the burn stimulus, severity, timing, and measurement can be controlled. These preclinical models are also essential for the evaluation and translation of novel burn wound treatments. The species most commonly reported are mouse, rat, and pig, which vary with respect to skin anatomy, wound healing processes, and cost.9 Pigs are well accepted as an appropriate animal model as their skin is most anatomically and physically similar to human skin; 10–13 therefore, porcine burn models were chosen for these studies.
Published porcine burn models developed by numerous research groups vary with respect to mechanism of burn (eg, contact, scald, or flame), size of burn, materials used to create the burn (eg, brass, aluminum, and water through a plastic membrane), burn conditions (eg, temperature, pressure, and duration of exposure), sex, age, and breed.14 Burn studies are performed on pigs of varying breed including Large White or Yorkshire, Landrace, red Duroc, Yucatan minipig, and Gottingen minipig. The Large White breed, and the Yorkshire breed from which it was derived, are among the most commonly utilized breeds. These pigs have little pigmentation and are white with areas of pale pink skin. They are championed in wound healing research as they heal in a manner that approximates the normal wound healing process in humans.15,16 These pigs are large in size allowing for multiple treatment/study sites on the same animal. Similarly, the red Duroc breed provides substantial skin surface area for study with significantly more pigmentation in their red to brown colored skin. Red Durocs, in particular, have been championed for scar research due to the human-like, hypertrophic scars that form following injury.17
Adding to the potential bias in interpretation of wound healing in these models, there is no uniform protocol for assessment of the extent of tissue injury. The differences in published models present challenges when making comparisons of outcomes in research occurring across various laboratories. In this report, important topics to consider when evaluating pig models of DPT burn injury are reviewed. Published burn models are then compared with respect to the methods used to create and evaluate the injury.
HISTOLOGICAL CONSIDERATIONS FOR BURN DEPTH ASSESSMENT
FT punch biopsies with subsequent histological assessment are considered the “gold standard” for burn depth assessment, particularly for animal models.18,19 Examination of the microanatomy allows for more objective and quantitative evaluation of the model’s characteristics. However, there is no published concensus within the burn research community of a standard protocol for the collection, evaluation, and assessment of depth in burn wound biopsies. Importantly, the location of the biopsy, at the edge of the wound versus the center, can affect the interpretation of burn depth and healing potential. Additionally, the zone of stasis in a burn wound represents a region adjacent to the necrotic tissue (zone of necrosis) where cells are in limbo between reversible and irreversible injury. In a process known as burn wound conversion, the cells in the zone of stasis become irreversibly injured in the days to weeks after a burn injury, ultimately leading to a deeper burn.20–22 A previously published systematic review of porcine burn models identified that a variety of time points, from 0 hours to 8 days postburn, were utilized to measure depth of injury, further complicating the interpretation of burn depth.14 These authors reported that the most common mechanism of injury in these porcine burn models was contact (81%), followed by scald mechanism (10%), and the most consistently reported time points where biopsies have been collected for burn wound histological analyses were 1, 24, and 48 hours postburn.14
Multiple approaches have been utilized to detect tissue damage from burn injury in biopsy samples that have been formalin-fixed and paraffin embedded (FFPE). Stains include hematoxylin and eosin (H&E),23–34 hematoxylin phloxine saffron (HPS),34 Masson’s Trichrome,29,34–40 and Elastin Van Gieson.34,41 Immunohistochemical probes for vimentin,24,34,42 caspase 3a,28,34,43,44 Ki-67,34,45 and high mobility box 1 (HMGB1)34,43,44 proteins have also been proposed. Lastly, terminal deoxynucleotide transferase dUTP nick end labeling (TUNEL) has also been used as a surrogate for apoptosis detection.30,46,47 For samples that have been snap frozen and freshly sectioned, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)48,49 and lactate dehydrogenase (LDH) assays50,51 have been used to differentiate live versus dead tissue. Collectively, these histological methods detect various aspects of dermal cellular and tissue injury, including collagen alteration or discoloration,18,34,35,52,53 vascular patency, endothelial,10,25,34,36,54,55 and epithelial cell damage.10,34,54 To a lesser extent, damage to mesenchymal cells and smooth muscle have also been reported as injury markers.25,27,34
Histologically, burn depth can be calculated by measuring depth in millimeters between the epidermal basement membrane and the most deeply irreparably injured (dead/necrotic) dermal component based on the parameter of interest.25,36,40,56 Various scoring systems are composed of any number of the components described above. A certain depth of injury is often given a numerical score to indicate severity. As an alternative to categorical, semi-quantitative scoring, measurements of the actual depth in millimeters of thermal damage can be provided instead of a score. However, this method suffers from propagation of error related to biopsy collection, embedding, and sectioning and requires the usage of multiple sections per experimental condition and normalization to uninjured tissue.
There remains no universally agreed upon set of criteria for the measurement of histological burn depth. The reproducibility of the depth measurements depends upon the expertise and training of the person evaluating the sample. Dermatopathologists are specialists in evaluating human skin samples; however, their experience with burn tissue is limited given that human burn tissue is rarely, if ever, submitted for dermatopathological evaluation.57
The authors of this paper have previously published several different DPT porcine contact burn models, which have used a variety of different histological assessments of depth of injury in burn wound punch biopsies to assess burn severity. The aims of this paper are twofold: 1) to combine multiple, disparate assessment methods into a single scoring rubric that can assist researchers (eg, not pathologists/dermatologists) to evaluate histological burn depth and damage in pig contact burn models, and 2) to assess the variability of burn depth in several well-characterized and self-reported “DPT burn injury” pig contact burn models.
METHODS
Study Design
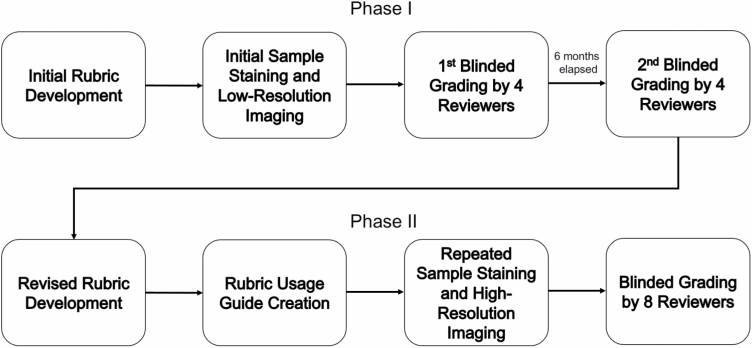
This study was conducted as an iterative process in two phases with input from laboratories with extensive experience utilizing porcine burn wound models (Figure 2). The data from the initial phase of model comparisons were presented at the American Burn Association (ABA) 51st Annual Meeting, and iterations were developed based upon comments and discussions within the ABA Research Committee Subgroup on Cutaneous Thermal Injury.58
Figure 2.
Schematic of study design.
Porcine Burn Models
The chosen models are published and reported as DPT burn injuries in pigs (Sus scrofa domesticus). All tissue samples were provided from individual labs and Table 1 provides the details and references for each model. Four of five models had samples from early and late time points after injury. One model only had samples obtained early after injury.
Table 1.
Deep partial thickness burn wound porcine models
| Model A5 | Model B32 | Model C64 | Model D28 | Model E65 | |
|---|---|---|---|---|---|
| Method | Hot water enclosed in cling wrap | Aluminum rod contact | Brass rod contact | Aluminum rod contact | Brass rod contact |
| Temperature (°C) | 92 | 100 | 100 | 80 | 100 |
| Thermocouple | Yes | No | Yes | No | No |
| Contact time (second) | 15 | 12 | 15 | 20 | 6 |
| Pressure | Weight of water (300 g) | Weight of rod (486 g) | 0.089 kg/cm2 | Weight of rod (150 g) + spring loaded device applied pressure (2 kg) |
Weight of rod (358 g) |
| Dry or wet bath | N/A | Wet bath, rod wiped dry | Dry | Wet bath, rod wiped dry | Wet bath, rod wiped dry |
| Wound size | 7.5 cm diameter circle | 1 cm by 1 cm square | 6 cm diameter circle | 2.5 by 2.5 cm square | 0.85 cm diameter circle |
| Breed | Large white | Duroc | First generation Red Duroc cross with Yorkshire | Yorkshire | Specific pathogen-free Yorkshire |
| Sex | Female | Castrated male | Female | Female | Female |
Histology and Imaging
Existing tissue samples from five laboratories experienced in conducting and publishing research using DPT pig burn models were included for analyses in this iterative two-phase study. For each burn model, new tissue sections from the same tissue blocks were cut fresh for staining, image capture, and scoring in both phases. All samples within each phase were stained at the same time in one lab. Standard H&E staining and EVG staining (Miilipore Sigma, Burlington MA. Cat #HT25A-1KT) were performed per manufacturers instruction.
Phase I
Nine porcine samples identified as DPT burns were included in this analysis. Of the porcine samples, five labs contributed an early sample (0 hours after injury: n = 1, and 1–2.5 hours after injury: n = 4), and four labs contributed a late sample (24 hours after injury: n = 4). The central region of the H&E-stained tissue sections was imaged with 4× and 20× objectives to capture the entire depth of the tissue using a Nikon Ti-S inverted microscope. Digital images were acquired with a Nikon DS Ri2 cooled color camera, X-Cite 120LED BOOST System lamp from Excelitas, and Nikon Imaging Software (NIS Elements, Nikon, Tokyo, Japan). The 4× and 20× images of H&E-stained tissues were placed into Microsoft PowerPoint slides for blinded review by four independent researchers familiar with H&E-stained burned skin, using the initial scoring rubric (Supplementary Appendix A).
Phase II
Three uninjured normal porcine skin samples and 13 porcine skin samples previously identified as DPT burns were included in this analysis. Five labs contributed an early sample (0 hours after injury: n = 3 and 1 to 2 hours after injury: n = 4), and four labs contributed a late sample (24 hours after injury: n = 4, and 48 hours after injury: n = 2) and three labs contributed a normal, nonburned sample (n = 3). The entire slides of H&E stained tissue sections were imaged with the Aperio Digital Pathology Slide Scanner System (Leica Biosystems, Buffalo Grove, IL). The Aperio ImageScope pathology slide viewing software was used to visualize the entire high-resolution tissue section image file with magnification ranges of 2× to 40×. The EVG-stained slides were imaged with a 4× slide scanner to view the entire tissue sample using PathScan Enabler 5 (Meyer Instruments, Houston, TX). Image files were reviewed independently by eight authors in a blinded fashion, using the rubric usage guide (Supplementary Appendix B) and revised scoring rubric (Supplementary Appendix C).
Depth Assessment Scoring
Initial rubric development. phase I
An initial rubric was created by the authors by combining two previously established rubrics that evaluated depth of thermal injury in porcine samples. These previously established rubrics have been used to assess depth of thermal injury in published articles from individual labs, but the rubrics themselves have not been published in detail. Prior to scoring, authors responsible for slide imaging ensured that the stained slides would be sufficient for usage with the rubric. An iterative phase of discussion resulted in five versions of the rubric before a final version was used for scoring of samples in Phase I (Supplementary Appendix A). Four reviewers then used the rubric to score nine slides to assess interrater reliability. Six months after the initial scoring was completed, the same four reviewers scored the images again in a blinded fashion to assess intra-rater reliability.
Rubric refinement
After the initial scoring, the entire group submitted feedback on the rubric and scoring system. The investigators who performed the Phase I scoring review of slides raised several concerns. One concern focused on the format and low quality of the images for scoring which led to difficulty in assessing nuanced collagen discoloration differences. Additionally, consolidation of all dermal damage characteristics into a single question (Supplementary Appendix A, Question 5) was raised as an issue. Finally, the lack of examples of representative photomicrographs that illustrated the characteristics of the rubric was a main concern among users. After analysis of the Phase I scoring, it was determined through group consensus that a new rubric, focused on the importance of including depth and intensity, with prescoring education was required to facilitate more consistent scoring among raters.
Phase II
A revised version of the rubric was created to address the concerns noted with the initial version. The revised rubric split dermal damage into three separate components: collagen discoloration, vascular blockage, and dermal appendage damage. Depth of injury and intensity (severity) of the damage were included for each component. Inflammatory cell infiltrate, as a characteristic, was removed as this was not consistently observed for sections ≤ 48 hours postburn. The hair follicle examination was broadened to encompass all dermal appendages given the heterogeneity of the tissue sections. It was agreed that increasing the magnification to high-resolution 40× imaging with a slide scanner would provide the reviewers with enough detail to grade the slides sufficiently while allowing evaluation of the entire tissue section, and could be accomplished remotely. Lastly, to assist reviewers, a “Rubric Usage Guide” was created to provide example photomicrographs for each question (Supplementary Appendix B). Example images were collated from the Phase I stained samples with known severity of burn injury based on burn model experimental methods in addition to negative (uninjured skin) and positive (FT burns) controls that were stained at the same time as the Phase I samples. The samples that were used to generate the Rubric Usage Guide were not included as samples that were scored in Phase II. This version of the rubric contained substantially more detail than the Phase I rubric and was not intended to be simplistic. Extensive discussions resulted in nine iterative versions of the rubric before a final revised rubric (Supplementary Appendix C) was used for blinded scoring of samples in Phase II by eight reviewers. The reviewers all have had extensive experience with burn depth research and included PhD scientists and a burn surgeon scientist.
Statistical Analyses
Phase I
Scores ranging from 0 to 5, with 0 indicating no burn and 5 being a FT burn (100% of the tissue), were given by the four raters (R1–R4) for the depth of damage (Supplementary Appendix A, Question 5) of histological slides from each model (Models A–E). Raters scored each histological slide twice, with a 6-month gap in time between reviews. To evaluate the consistency of each rater, we examined the frequency of exact agreement with the mode between scores for review 1 and review 2 and the frequency when the two scores were within 1 point. In each of the first and second reviews, for the early time point (1–2 hours after burn injury), there were 20 comparisons. For the 24-hour time point after burn injury, there were 16 comparisons, as 24-hour time point samples for one model were not available (Model B). To examine the reliability of responses, an intra-class correlation coefficient (ICC) and its associated 95% confidence interval were calculated from each rater’s first score using a two-way random effects model based on absolute-agreement and single-measures. To evaluate interrater reliability for additional questions, percent agreement and percent within 1 point of the mode response for each sample were calculated. These percentages were then averaged across all time points.
Phase II
Scores were given by the eight raters for histological slides from Models A–E. Burn depth was assessed by three questions (Supplementary Appendix C, Questions 5, 7, and 8) ranging from 0 to 5, with 0 indicating no burn and 5 being a FT burn (100% of the tissue). Intensity of damage was assessed by three questions (Supplementary Appendix C, Questions 3, 6, and 9) ranging from 0 to 3, with 0 indicating normal and 3 being severe. For each sample within each question, the mode of the scores for eight raters was determined and percent exact agreement with the mode or percent within 1 point of the mode was calculated. The percent exact agreement or percent within 1 point agreement for each question was then calculated by averaging across all samples, including early and late time points. Percent agreement and percent within 1 point agreement for early and late time points were calculated by averaging across 0 to 2 hour burns and 24 to 48 hour burns, respectively. Summary statistics were used to evaluate all samples over the time points. The median values of the scores from eight raters for all samples at each time point were averaged with interquartile range displayed. To further assess interrater reliability, an ICC and 95% confidence interval were calculated for Question 8, which is directly comparable to Question 5 from phase 1, using a two-way random effects model based on absolute-agreement and single-measures.
RESULTS
Initial Porcine Burn Model Comparison (Phase I)
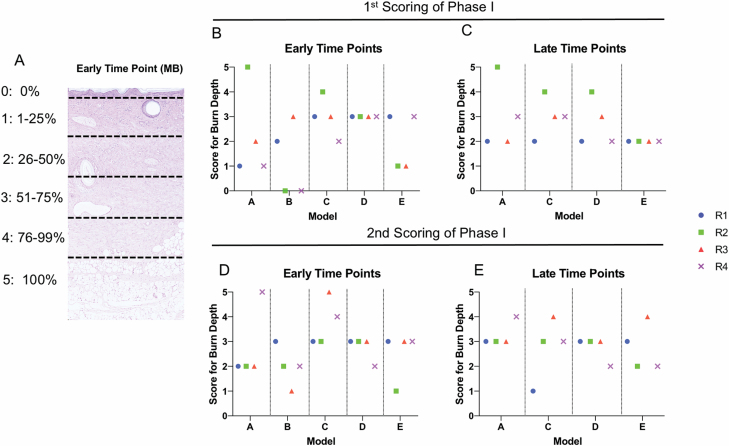
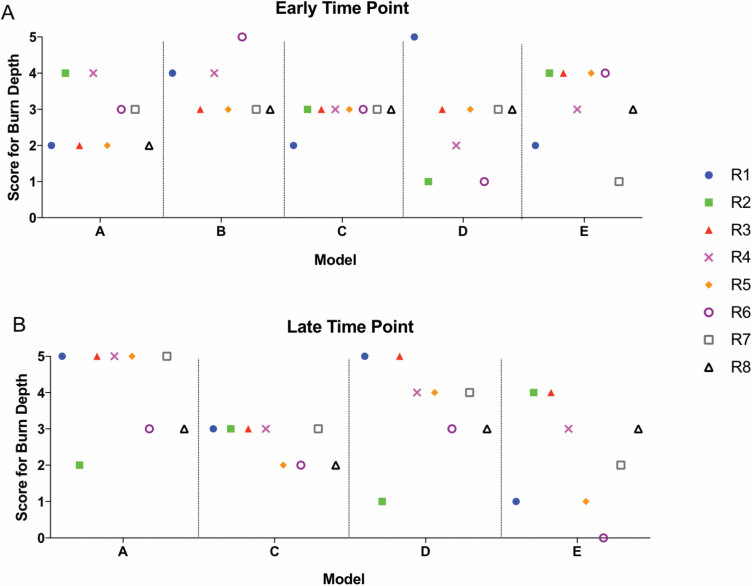
Clinically, a DPT wound represents injury in the mid to lower third of the dermis, correlating to a score of three (Supplementary Appendix A, Question 5) on the rubric (Figure 3A). Scores of injury depth in phase I were analyzed between raters and models, and repeated scoring within raters (Figure 3B–E). The scoring of damage to the dermis and hair follicles was variable between raters and models, at early and late time points, ranging from 1 to 5 (superficial to FT injury). Table 2 shows interrater reliability of all scores for all questions in the rubric, using only the initial score given by a rater for each sample. When averaged across all time points, the percent of the four raters scores in exact agreement ranged from 50% to 69.4%. The agreement increased to a range of 66.7% to 86.1% when considering scores within one point of the mode. Initial scores related to dermal appendage damage depth (Question 5) had an ICC = 0.097 with a 95% CI = −0.099–0.056. Given this poor agreement, we evaluated interrater reliability between a rater’s first and second score (performed 6 months apart) for one question (Supplementary Appendix A, Question 5). There was agreement on the depth of injury to the dermis and hair follicles only 36% of the time. The agreement increased to 72% when considering the scores that were within one point of the mode. Given the poor reliability in all raters and models, no further analyses were completed. When evaluating these phase I results, individual raters commented on the difficulty of using magnified partial images of the tissue section rather than having an entire slide to review. Furthermore, raters commented on the lack of representative examples given for individual questions within the rubric, which led to difficulty in choosing between two adjacent scores and resulted in poor intra-rater and interrater reliability of the scores.
Figure 3.
Variability of scoring is present within individual raters, between raters, and between models in phase I. (A) Representative 20× image of H&E-stained sections with score of depth correlated to percentage of total depth of tissue. Individual scores for each rater (R) and model (M) in samples early (B, D) or late (C, E) after injury. “1st” and “2nd” represent scoring of the same images by the same individuals 6 months apart.
Table 2.
Interrater reliability of phase I
| Phase 1—averaged across all time points—first review | ||
|---|---|---|
| Feature | Exact score | Within 1 point |
| Epidermal damage intensity | 69.4% | 86.1% |
| Dermal appendage damage depth | 58.3% | 75% |
| Dermal appendage damage intensity | 61.1% | 86.1% |
| Inflammatory cell infiltrate | 50% | 69.4% |
| Hair follicle damage severity | 52.7% | 66.7% |
Revised Porcine Burn Model Comparison (Phase II)
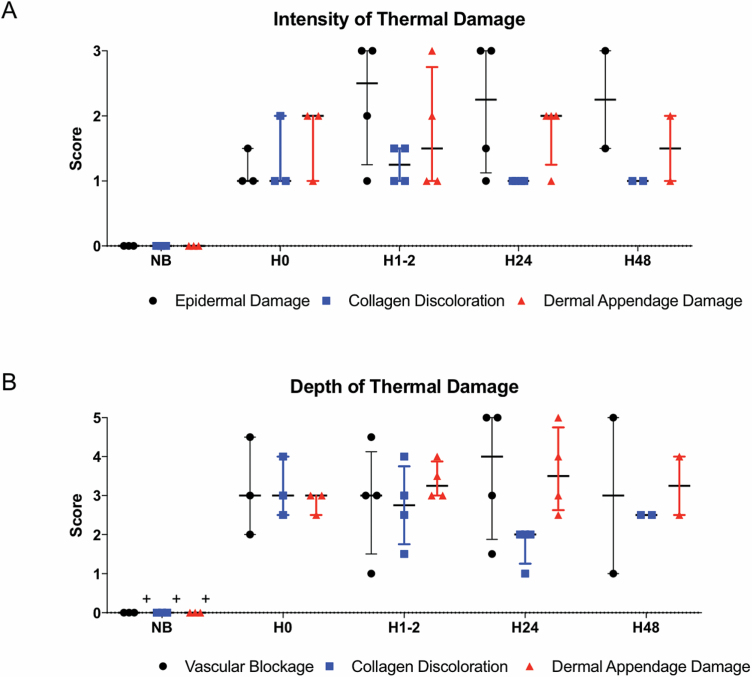
In this phase, questions were grouped into intensity (mild to severe damage) and depth categories (0–100% of tissue). Nonburned (NB) skin had a median score of 0, indicating the ability of this rubric to distinguish between nonburned and burned skin. Across all models, the median scores in questions related to intensity of thermal damage showed a mild to moderate intensity of damage to dermis, with more consistent scoring noted in samples immediately after the injury (Figures 4A and 5, and Supplementary Figures). Intensity of the epidermal damage immediately after injury was lower than at later time points, likely reflecting the fragile nature of the superficial component of skin in the environment over time.
Figure 4.
Intensity and depth of thermal damage is variable over time. A median score was obtained for each sample based on n = 8 raters. Samples were grouped by time point (H, hours) and scores for different models at each time point were grouped. Box whisker plot shows median, range, and quartiles; “0” indicates a score of 0. NB (n = 3), H0 (n = 3), H1–2 (n = 4), H24 (n = 4), H48 (n = 2). (A) Scores for questions related to intensity of epidermal damage, collagen discoloration, and dermal appendage damage. (B) Scores for questions related to depth of epidermal damage, collagen discoloration, and dermal appendage damage.
Figure 5.
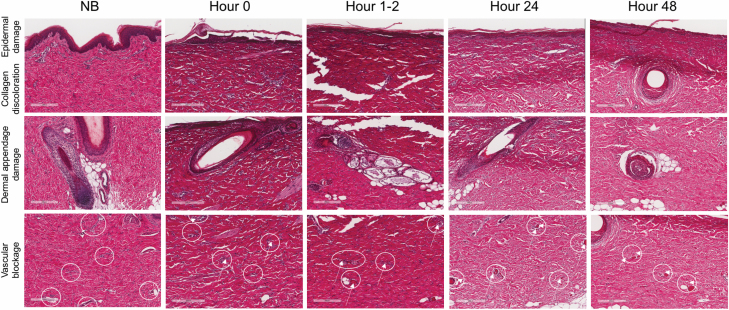
Histological changes in burn microanatomy over time. Shown are tissue sections from a single porcine model (Model A) over multiple time points stained with H&E. NB = not burned, H = hours postburn. Scale bar = 200 μm. White circle = blood vessel. White arrow = vascular occlusion.
DPT burns are important clinically because they represent injury deep into the dermis where the density of regenerative cells is lower, as is the chance of healing without surgery. The questions relating to the depth of injury focused on collagen discoloration, vascular blockage, and dermal appendage damage. Over time, median scores for vascular blockage and dermal appendage damage reflected DPT injury (Figures 4B and 5, and Supplementary Figures). However, the range of scores for vascular blockage increased over time, suggesting that this measure may not be helpful at late time points. The depth of dermal appendage damage was lowest at 0 hours after injury (H0) and increased slightly over time suggesting progression of injury depth. Nevertheless, consistency in scoring of this question decreased over time, making the confirmation of burn wound conversion challenging. Over time, intensity and depth of collagen discoloration decreased (Figure 4A and B). This finding correlated with decreased EVG staining of the tissue sections visualized at 24 and 48 hours (Figure 6).
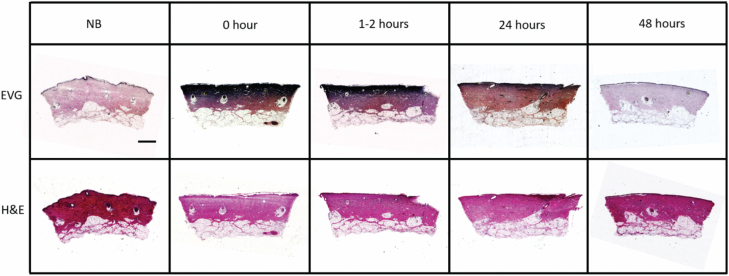
Figure 6.
EVG staining reveals decreased intensity and depth of collagen damage over time. Shown are tissue sections from a single porcine model over multiple time points. Top panel: EVG-stained tissue sections. Dark purple in the dermis represents damaged collagen. Bottom panel: Corresponding H&E-stained sections. Scale bar = 1 mm.
Table 3 shows interrater reliability of all scores for all questions in the revised rubric. Among the eight raters, averaged across all time points, the agreement for intensity scores that were identical ranged from 67.2% to 77.3%. This agreement increased (96.9%–97.7%) when considering scores within one point of the mode. When scores were averaged across early or late time points, the agreement for scores that were identical ranged from 62.5% to 80.4% (early) or 62.5% to 81.3% (late). The agreement increased (92.9%–96.4% for early and 95.8%–100% for late) when considering scores within one point of the mode. Overall, the agreement for depth scores that were identical ranged from 53.1% to 59.4%. This agreement increased to a range of 82% to 84.4% when considering scores within one point of the mode. When scores were averaged across early or late time points, the agreement for scores that were identical ranged from 46.4% to 55.4% (early) or 43.8% to 62.5% (late). The agreement increased to a range of 71.4% to 89.3% (early) or 66.7% to 89.6% (late) when considering scores within one point of the mode. Overall, there was lower agreement in questions related to depth compared with intensity; however, this could be affected by the different scales for intensity (0–3) vs depth questions (0–5). Question 8 scores, which related to dermal appendage damage depth, had an ICC = 0.67 with a 95% CI = 0.483–0.849, representing moderate reliability59 and an improvement over a similar question in phase I which yielded poor reliability (ICC = 0.097).
Table 3.
Interrater reliability of phase II
| Phase 2—averaged across all time points | |||
|---|---|---|---|
| Feature | Exact score | Within 1 point | |
| Intensity of damage | Epidermal damage | 77.3% | 96.9% |
| Collagen discoloration | 76.6% | 97.7% | |
| Dermal appendages | 67.2% | 96.9% | |
| Depth of damage | Collagen discoloration | 53.1% | 82% |
| Vascular blockage | 59.4% | 83.6% | |
| Dermal appendages | 57% | 84.4% | |
| Phase 2 – averaged across early time points (H0-2) | |||
| Feature | Exact Score | Within 1 point | |
| Intensity of Damage | Epidermal damage | 80.4% | 96.4% |
| Collagen discoloration | 67.9% | 96.4% | |
| Dermal appendages | 62.5% | 92.9% | |
| Depth of Damage |
Collagen discoloration | 46.4% | 89.3% |
| Vascular blockage | 46.4% | 71.4% | |
| Dermal appendages | 55.4% | 82.1% | |
| Phase 2—averaged across late time points (H24–48) | |||
| Feature | Exact score | Within 1 point | |
| Intensity of damage | Epidermal damage | 68.8% | 95.8% |
| Collagen discoloration | 81.3% | 97.9% | |
| Dermal appendages | 62.5% | 100% | |
| Depth of damage | Collagen discoloration | 43.8% | 66.7% |
| Vascular blockage | 62.5% | 89.6% | |
| Dermal appendages | 43.8% | 79.2% |
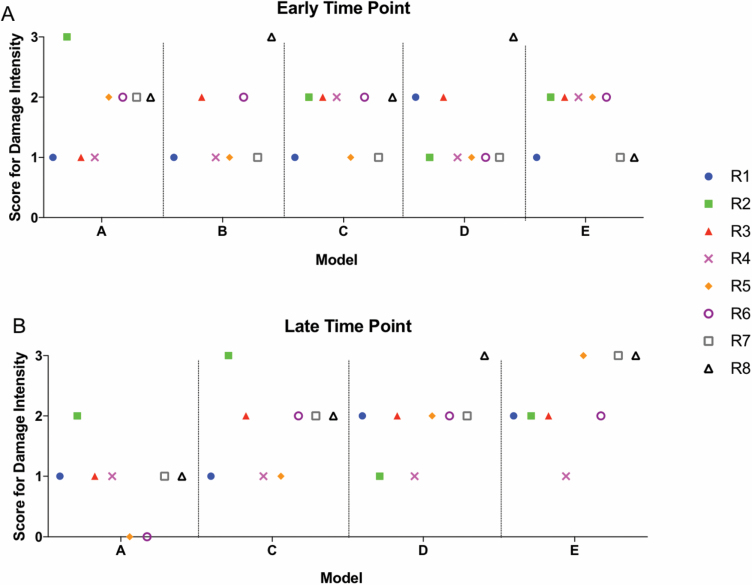
In addition to potential variability resulting from different raters scoring the sample and different time points from injury, variability in the damage observed in the samples can occur as a function of the pig model used to generate the injury. Individual scores obtained for samples in each model for two questions on dermal appendage damage depth (Figure 7) and intensity (Figure 8) demonstrate the model variability that was encountered. No clearly superior model with respect to consistency was identified, with the exception of the early time point in Model C (Figure 7A) where 7 of 8 raters scored the sample as DPT (score 3). This variability within and among all of the models was present for all questions (Supplementary Figures).
Figure 7.
Variability of scoring of dermal appendage depth of injury is present within individual raters, between raters, and between models in phase II. Individual scores for each rater (R1–R8) and model (A–E) in samples early (A) or late (B) time points after injury. Data represent Question 8 from phase II rubric which evaluated depth of thermal injury by damage to dermal appendages.
Figure 8.
Variability of scoring of dermal appendage intensity of injury is present within individual raters, between raters, and between models in phase II. Individual scores for each rater (R1–R8) and model (A–E) in samples early (A) or late (B) after injury. Data represent Question 8 from phase II rubric which evaluated intensity of thermal injury by damage to dermal appendages.
Overall, positive comments from the raters about the revised rubric included the improved ease of scoring with examples of images for each question in the Rubric Usage Guide corresponding to a specific score, the enhanced image quality, and the ability to scan the entire slide to determine the score for each question.
DISCUSSION
The advancement of burn depth research depends upon the reproducibility and comparability of findings from different research teams. A working group on cutaneous thermal injury sponsored by the American Burn Association and Underwriters Laboratory brought together experts on burn injury and burn wound modeling to identify biologic standards.58 The gap in knowledge identified at this conference prompted the work presented here. The initial goal of the study was to compare models of DPT burns from various published models through histological evaluation of H&E-stained tissue. However, the previously unreported complexity of this type of evaluation was uncovered during the study and prompted the addition of an educational component and expert opinion consensus on how to evaluate DPT burn models. The results from phase I of this study highlight the challenges that we anticipated in attempting to compare porcine burn models from various well-established labs. Most importantly, the low intra-rater reliability and poor ICC meant that all other analyses in phase I were unreliable. Therefore, through an iterative process, phase II focused on the development and implementation of an educational process and guidelines for burn depth interpretation for the burn research community to enhance the reproducibility and translatability of future research. Unfortunately, despite lengthy discussions and iterations the reliability of the improved scoring rubric only improved modestly, putting into question the ability to compare pig burn models of DPT burns using H&E alone.
The low agreement of injury depth scoring seen in the first phase was likely multifactorial, including ambiguity in the descriptors for the scoring rubric, low-quality images, lack of prescoring education to define the characteristics, and potential variability in depth created by each model. However, without a reproducible method to score the different models, the conclusion about variability between models was premature. Upon revising our scoring rubric and providing educational materials, we found an increase in interrater agreement and improved ICC on comparable questions (phase I question 5 and phase II question 8) allowing us to use this rubric to compare models more consistently. The decision to split the sample into five distinct regions of depth into the tissue for scoring was made to allow more discrimination between the samples. A score of 3 was chosen to represent a DPT burn because losing more than 75% of the viability in the tissue (maximum depth with a score of 3) is clinically considered a FT injury that requires grafting to avoid complications associated with prolonged wound healing. The use of the measurement of percent agreement within 1 point allowed us to include those samples that were on the border between two scores in the assessment of reliability in scoring, as the clinical difference in those samples is likely negligible.
When analyzing the dermal component of skin, it is challenging to differentiate between structural and functional cell death, and reversible and irreversible injury.18 Many of the stains highlighted in the introduction provide information about the structure of certain cell or tissue components, but provide no information on whether these cells will recover from damage and be able to function normally (reversible damage), or whether the damage acquired will result in cell death (irreversible damage). Cellular (eg, endothelial cells) and extracellular matrix (eg, collagen) have different heat capacities resulting in differences in the maximum temperatures reached by each component and their subsequent ability to recover from this damage.60 These properties of skin contribute to the challenge of choosing various stains to evaluate depth as they may indicate different burn depths for the same injury.34 The use of EVG in these studies allowed a clear colorimetric readout (ie, dark purple), whereas determining collagen changes through H&E (ie, discriminating changes in fiber thickness and organization) can be challenging and can also be altered by tissue processing and staining.57,61 It was reassuring that collagen changes scored on H&E-stained samples correlated with those seen on EVG-stained samples and suggest that the effects of thermal injury to collagen can be determined from H&E. However, the decrease in collagen damage seen in our studies over time likely represents a remodeling that is occurring in the extracellular matrix of the wound after infiltration with inflammatory cells, and it suggests that using collagen damage at later time points may not represent true depth of injury. It also supports different timelines of collagen and cellular recovery. In addition, different burn etiologies such as low temperature, prolonged scald, or contact burns may result in different tissue injury profiles compared with high temperature scalds, chemical, or flame burns.34,62 Although not evaluated in this study, these challenges in characterizing depth highlight the importance of establishing time to healing in each model as a marker for initial depth of injury.
The phenomenon of burn wound conversion,21 in which the vertical and horizontal depth of the damage increases over the first few days after injury, occurs as the cells in the zone of stasis convert from at-risk cells (ie, reversible injury) to irreversible cell death. This process supports the need to evaluate the depth of injury at multiple time points because H&E staining does not discriminate between reversible and irreversible injury. Evidence of burn wound conversion in the samples was not consistently observed when the samples were evaluated using the rubric. However, the fact that these data failed to demonstrate a clear increase in depth of injury in all variables considered over time may be a function of the definition of depth as a score rather than an absolute measurement.
An additional consideration for histological burn depth assessment is determining who is considered qualified to perform such an assessment. Although it has been acknowledged by those in the burn research field that dermatopathologists are not called upon to assess burn depth, many researchers rely on dermatopathologists to assess burn depth in a research setting.34,57 A previous study comparing a dermatopathologist, a burn surgeon and a novice surgeon reported an improvement in scoring of H&E for burn depth in human burn patient samples by a dermatopathologist; 61 however, this was only after training of the pathologist on the samples collaboratively with the burn surgeon to put context into the samples based on patients outcomes and visual wound characteristics, and yet H&E remained an inconsistent method to measure cellular viability. We show that if rubric education is provided, burn researchers could provide consistent/accurate scoring of sections and a specialized dermatopathologist may not be needed.
Overall, we found that all models included in this study produced burn wounds that are considered DPT; however, there were noticeable differences in each model related to the thickness of the skin, timing of evaluation, and processing of the tissue. It is important to be cognizant of the many variables that may contribute to bias in the interpretation of wound healing data. For example, porcine breeds are chosen for wound healing phenotypes such as hypertrophic scarring in the red Duroc pig.63 Furthermore, selection of biopsy site (edge vs central wound), processing of tissue biopsy, and the selection of staining to identify cellular death or extracellular matrix changes all contribute to variability in the results. Future studies are necessary to compare the models using the same time points, ideally performed in a single setting to minimize these variables as much as possible.
To aid the burn research community in generating burn depth data that can be compared across models, the authors collaboratively developed guiding principles from this study based on the collective expert opinion in the group.
Expert opinion consensus statements:
-
•
For accurate comparison of DPT burn injury in pig models between labs, a consistent rubric is necessary for scoring depth and intensity of injury.
-
•
While H&E is considered the “gold standard” for analysis of burn depth, there are challenges associated with the nuances of evaluating cell death and tissue damage. More detailed descriptors of the depth of injury do not improve the consistency of scoring.
-
•
H&E is unreliable to distinguish between reversible and nonreversible damage. Therefore, time to wound closure with standard wound care should be established and reported for each model.
-
•
When evaluating collagen discoloration through H&E or EVG stains, time from injury must be considered. Earlier time points show distinct damage that is not visible at later time points.
-
•
Dermal appendage evaluation is critical, and therefore, when evaluating histological burn depth, sections should include dermal appendages.
-
•
Given changes in depth of injury that may occur over time, early (within 2 hours) and late (>24 hours) time points should be included in the study.
-
•
A dermatopathologist without specialized burn experience for reviewing and interpretation of H&E histology of burn injured tissue is comparable to a burn researcher trained in reviewing burn tissue histology.
-
•
Caution should be given to extrapolation of findings from porcine models to human clinical care, given the differences in pig versus human wound healing. However, the challenges noted in the evaluation of thermally injured pig skin are the same that occur with human skin and should be considered in the development of automated intelligence algorithms for human burn depth assessment.
-
•
In future studies using burn depth as an outcome measure, the authors recommend use of the scoring rubric and education (Supplementary Appendices B and C) to evaluate depth of thermal injury in research studies. Scores for each question in the rubric should be provided in the supplementary methods section of articles submitted for publication. For the intensity questions, scores of ~2 in the dermal region indicating that moderate intensity should be considered DPT. For the depth questions, scores of ~3 indicating that 50% to 75% injury should be considered DPT. Although we did not specifically evaluate our rubric on tissue that was identified as SPT or FT injury by the investigators, it was felt that depth scores of 1 and 2 represent SPT burns and those in the range of 4 to 5 represent FT. It would follow that a severity score of 1 is more likely to be associated with a SPT, whereas a score of 3 is consistent with a FT injury. Ideally, the scoring should be performed by at least one blinded reviewer and should include nonburned skin and FT burn as negative and positive controls.
Supplementary Material
ACKNOWLEDGMENTS
The authors acknowledge Stephen C. Davis, PhD and Robert J. Christy, PhD for providing samples from their respective porcine burn models. The authors acknowledge USAISR Comparative Pathology Department, in particular LTC Brian Smith, MAJ Nathan Wienandt, and COL (Ret) J Scot Estep for their guidance in contributing to an initial phase I rubric and Glen Leverson, PhD for his assistance with statistical analyses. Additionally, the authors acknowledge the University of Wisconsin Translational Research Initiatives in Pathology laboratory (TRIP), supported by the UW Department of Pathology and Laboratory Medicine, UWCCC (P30 CA014520) and the Office of The Director- NIH (S10OD023526) for use of its facilities and services. Finally, the authors acknowledge Lori Palfalvi for her assistance in facilitating group meetings, and the American Burn Association and Underwriters Laboratories for their financial support of the initial workshop on thermal injury. R.S. and C.K. are government employees and the views expressed in this article (book, speech, etc.) are those of the author(s) and do not reflect the official policy or position of the U.S. Army Medical Department, Department of the Army, DoD, or the U.S. Government.
Funding
The workshop serving as the basis for this article was funded by the Underwriters Laboratories and supported by the American Burn Association. This research was supported by the UW Department of Pathology and Laboratory Medicine, UWCCC (P30 CA014520) and the Office of The Director-NIH (S10OD023526).
REFERENCES
- 1. Committee IPG, Steering S, Advisory S. ISBI practice guidelines for burn care. Burns 2016; 42:953–1021. [DOI] [PubMed] [Google Scholar]
- 2. Karim AS, Shaum K, Gibson ALF. Indeterminate-depth burn injury-exploring the uncertainty. J Surg Res 2020;245:183–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cubison TC, Pape SA, Parkhouse N. Evidence for the link between healing time and the development of hypertrophic scars (HTS) in paediatric burns due to scald injury. Burns 2006;32:992–9. [DOI] [PubMed] [Google Scholar]
- 4. Deitch EA, Wheelahan TM, Rose MP, Clothier J, Cotter J. Hypertrophic burn scars: analysis of variables. J Trauma 1983;23:895–8. [PubMed] [Google Scholar]
- 5. Cuttle L, Kempf M, Phillips GE, et al. A porcine deep dermal partial thickness burn model with hypertrophic scarring. Burns 2006;32:806–20. [DOI] [PubMed] [Google Scholar]
- 6. Chipp E, Charles L, Thomas C, Whiting K, Moiemen N, Wilson Y. A prospective study of time to healing and hypertrophic scarring in paediatric burns: every day counts. Burns Trauma 2017;5:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jan SN, Khan FA, Bashir MM, et al. Comparison of Laser Doppler Imaging (LDI) and clinical assessment in differentiating between superficial and deep partial thickness burn wounds. Burns 2018;44:405–13. [DOI] [PubMed] [Google Scholar]
- 8. Karim AS, Yan A, Ocotl E, et al. Discordance between histologic and visual assessment of tissue viability in excised burn wound tissue. Wound Repair Regen 2019;27:150–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Abdullahi A, Amini-Nik S, Jeschke MG. Animal models in burn research. Cell Mol Life Sci 2014;71:3241–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moritz AR, Henriques FC. Studies of thermal injury: II. The relative importance of time and surface temperature in the causation of cutaneous burns. Am J Pathol 1947;23:695–720. [PMC free article] [PubMed] [Google Scholar]
- 11. Sullivan TP, Eaglstein WH, Davis SC, Mertz P. The pig as a model for human wound healing. Wound Repair Regen 2001;9:66–76. [DOI] [PubMed] [Google Scholar]
- 12. Meyer W, Schwarz R, Neurand K. The skin of domestic mammals as a model for the human skin, with special reference to the domestic pig. Curr Probl Dermatol 1978;7:39–52. [DOI] [PubMed] [Google Scholar]
- 13. Montagna W, Yun JS. The skin of the domestic pig. J Invest Dermatol 1964;42:11–21. [PubMed] [Google Scholar]
- 14. Andrews CJ, Cuttle L. Comparing the reported burn conditions for different severity burns in porcine models: a systematic review. Int Wound J 2017;14:1199–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang JF, Olson ME, Reno CR, Kulyk W, Wright JB, Hart DA. Molecular and cell biology of skin wound healing in a pig model. Connect Tissue Res 2000;41:195–211. [DOI] [PubMed] [Google Scholar]
- 16. Wang JF, Olson ME, Reno CR, Wright JB, Hart DA. The pig as a model for excisional skin wound healing: characterization of the molecular and cellular biology, and bacteriology of the healing process. Comp Med 2001;51:341–8. [PubMed] [Google Scholar]
- 17. Gallant CL, Olson ME, Hart DA. Molecular, histologic, and gross phenotype of skin wound healing in red Duroc pigs reveals an abnormal healing phenotype of hypercontracted, hyperpigmented scarring. Wound Repair Regen 2004;12:305–19. [DOI] [PubMed] [Google Scholar]
- 18. Monstrey S, Hoeksema H, Verbelen J, Pirayesh A, Blondeel P. Assessment of burn depth and burn wound healing potential. Burns 2008;34:761–9. [DOI] [PubMed] [Google Scholar]
- 19. Jaskille AD, Shupp JW, Jordan MH, Jeng JC. Critical review of burn depth assessment techniques: Part I. Historical review. J Burn Care Res 2009;30:937–47. [DOI] [PubMed] [Google Scholar]
- 20. Singh V, Devgan L, Bhat S, Milner SM. The pathogenesis of burn wound conversion. Ann Plast Surg 2007;59:109–15. [DOI] [PubMed] [Google Scholar]
- 21. Shupp JW, Nasabzadeh TJ, Rosenthal DS, Jordan MH, Fidler P, Jeng JC. A review of the local pathophysiologic bases of burn wound progression. J Burn Care Res 2010;31:849–73. [DOI] [PubMed] [Google Scholar]
- 22. Schmauss D, Rezaeian F, Finck T, Machens HG, Wettstein R, Harder Y. Treatment of secondary burn wound progression in contact burns-a systematic review of experimental approaches. J Burn Care Res 2015;36:e176–89. [DOI] [PubMed] [Google Scholar]
- 23. Knabl JS, Bayer GS, Bauer WA, et al. Controlled partial skin thickness burns: an animal model for studies of burnwound progression. Burns 1999;25:229–35. [DOI] [PubMed] [Google Scholar]
- 24. Ho-Asjoe M, Chronnell CM, Frame JD, Leigh IM, Carver N. Immunohistochemical analysis of burn depth. J Burn Care Rehabil 1999;20:207–11. [DOI] [PubMed] [Google Scholar]
- 25. Singer AJ, Berruti L, Thode HC Jr, McClain SA. Standardized burn model using a multiparametric histologic analysis of burn depth. Acad Emerg Med 2000;7:1–6. [DOI] [PubMed] [Google Scholar]
- 26. Papp A, Kiraly K, Härmä M, Lahtinen T, Uusaro A, Alhava E. The progression of burn depth in experimental burns: a histological and methodological study. Burns 2004;30:684–90. [DOI] [PubMed] [Google Scholar]
- 27. Meyerholz DK, Piester TL, Sokolich JC, Zamba GK, Light TD. Morphological parameters for assessment of burn severity in an acute burn injury rat model. Int J Exp Pathol 2009;90:26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Singer AJ, Hirth D, McClain SA, Crawford L, Lin F, Clark RA. Validation of a vertical progression porcine burn model. J Burn Care Res 2011;32:638–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sheu SY, Wang WL, Fu YT, et al. The pig as an experimental model for mid-dermal burns research. Burns 2014;40:1679–88. [DOI] [PubMed] [Google Scholar]
- 30. Medina JL, Fourcaudot AB, Sebastian EA, Shankar R, Brown AW, Leung KP. Standardization of deep partial-thickness scald burns in C57BL/6 mice. Int J Burns Trauma 2018;8:26–33. [PMC free article] [PubMed] [Google Scholar]
- 31. Bingöl UA, Öksüz S, Demiröz A, Arslan H. A practical noncontact model to create standardized experimental burn wounds of any thickness: blue beam laser pointer for burn induction. J Burn Care Res 2019;40:805–8. [DOI] [PubMed] [Google Scholar]
- 32. Prindeze NJ, Fathi P, Mino MJ, et al. Examination of the early diagnostic applicability of active dynamic thermography for burn wound depth assessment and concept analysis. J Burn Care Res 2015;36:626–35. [DOI] [PubMed] [Google Scholar]
- 33. Davis SC, Li J, Gil J, et al. Preclinical evaluation of a novel silver gelling fiber dressing on Pseudomonas aeruginosa in a porcine wound infection model. Wound Repair Regen 2019;27:360–5. [DOI] [PubMed] [Google Scholar]
- 34. Hirth DA, Singer AJ, Clark RA, McClain SA. Histopathologic staining of low temperature cutaneous burns: comparing biomarkers of epithelial and vascular injury reveals utility of HMGB1 and hematoxylin phloxine saffron. Wound Repair Regen 2012;20:918–27. [DOI] [PubMed] [Google Scholar]
- 35. Chvapil M, Speer DP, Owen JA, Chvapil TA. Identification of the depth of burn injury by collagen stainability. Plast Reconstr Surg 1984;73:438–41. [DOI] [PubMed] [Google Scholar]
- 36. Watts AM, Tyler MP, Perry ME, Roberts AH, McGrouther DA. Burn depth and its histological measurement. Burns 2001;27:154–60. [DOI] [PubMed] [Google Scholar]
- 37. Prindeze NJ, Hoffman HA, Ardanuy JG, et al. Active dynamic thermography is a sensitive method for distinguishing burn wound conversion. J Burn Care Res 2016;37:e559–68. [DOI] [PubMed] [Google Scholar]
- 38. Ponticorvo A, Burmeister DM, Yang B, Choi B, Christy RJ, Durkin AJ. Quantitative assessment of graded burn wounds in a porcine model using spatial frequency domain imaging (SFDI) and laser speckle imaging (LSI). Biomed Opt Express 2014;5:3467–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ponticorvo A, Rowland R, Baldado M, et al. Evaluating clinical observation versus Spatial Frequency Domain Imaging (SFDI), Laser Speckle Imaging (LSI) and thermal imaging for the assessment of burn depth. Burns 2019;45:450–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kim JY, Dunham DM, Supp DM, Sen CK, Powell HM. Novel burn device for rapid, reproducible burn wound generation. Burns 2016;42:384–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hoekstra MJ, Hupkens P, Dutrieux RP, Bosch MM, Brans TA, Kreis RW. A comparative burn wound model in the New Yorkshire pig for the histopathological evaluation of local therapeutic regimens: silver sulfadiazine cream as a standard. Br J Plast Surg 1993;46:585–9. [DOI] [PubMed] [Google Scholar]
- 42. Nanney LB, Wenczak BA, Lynch JB. Progressive burn injury documented with vimentin immunostaining. J Burn Care Rehabil 1996;17:191–8. [DOI] [PubMed] [Google Scholar]
- 43. Hirth D, McClain SA, Singer AJ, Clark RA. Endothelial necrosis at 1 hour postburn predicts progression of tissue injury. Wound Repair Regen 2013;21:563–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Singer AJ, McClain SA, Taira BR, Guerriero JL, Zong W. Apoptosis and necrosis in the ischemic zone adjacent to third degree burns. Acad Emerg Med 2008;15:549–54. [DOI] [PubMed] [Google Scholar]
- 45. Farhangkhoee H, Cross KM, Koljonen V, Ghazarian D, Fish JS. Evaluation of Ki-67 as a histological index of burn damage in a swine model. J Burn Care Res 2012;33:e55–e62. [DOI] [PubMed] [Google Scholar]
- 46. Gravante G, Palmieri MB, Esposito G, et al. Apoptotic death in deep partial thickness burns vs. normal skin of burned patients. J Surg Res 2007;141:141–5. [DOI] [PubMed] [Google Scholar]
- 47. McNamara AR, Zamba KD, Sokolich JC, et al. Apoptosis is differentially regulated by burn severity and dermal location. J Surg Res 2010;162:258–63. [DOI] [PubMed] [Google Scholar]
- 48. Henze U, Lennartz A, Hafemann B, Goldmann C, Kirkpatrick CJ, Klosterhalfen B. The influence of the C1-inhibitor BERINERT and the protein-free haemodialysate ACTIHAEMYL20% on the evolution of the depth of scald burns in a porcine model. Burns 1997;23:473–7. [DOI] [PubMed] [Google Scholar]
- 49. Radosevich JA, Haines GK, Elseth KM, Shambaugh GE 3rd, Maker VK. A new method for the detection of viable cells in tissue sections using 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT): an application in the assessment of tissue damage by surgical instruments. Virchows Arch B Cell Pathol Incl Mol Pathol 1993;63:345–50. [DOI] [PubMed] [Google Scholar]
- 50. Gibson ALF, Shatadal S. A simple and improved method to determine cell viability in burn-injured tissue. J Surg Res 2017;215:83–7. [DOI] [PubMed] [Google Scholar]
- 51. Sherwood ME, Flotte TJ. Improved staining method for determining the extent of thermal damage to cells. Lasers Surg Med 2007;39:128–31. [DOI] [PubMed] [Google Scholar]
- 52. Gaines C, Poranki D, Du W, Clark RA, Van Dyke M. Development of a porcine deep partial thickness burn model. Burns 2013;39:311–19. [DOI] [PubMed] [Google Scholar]
- 53. Heimbach D, Engrav L, Grube B, Marvin J. Burn depth: a review. World J Surg 1992;16:10–15. [DOI] [PubMed] [Google Scholar]
- 54. Koenig PA Diagnosis of depth of burning. Br Med J 1965;1:1622–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kahn AM, McCrady VL, Rosen VJ. Burn wound biopsy. Multiple uses in patient management. Scand J Plast Reconstr Surg 1979;13:53–6. [DOI] [PubMed] [Google Scholar]
- 56. Andrews CJ, Kimble RM, Kempf M, Cuttle L. Evidence-based injury prediction data for the water temperature and duration of exposure for clinically relevant deep dermal scald injuries. Wound Repair Regen 2017;25:792–804. [DOI] [PubMed] [Google Scholar]
- 57. Rosenberg AS Reconsidering the H&E stain as the gold standard in assessing the depth of burn wounds. J Cutan Pathol 2017;44:1049–050. [DOI] [PubMed] [Google Scholar]
- 58. Moffatt LT, Madrzykowski D, Gibson ALF, et al. ; Standards in Biologic Lesions Working Group Standards in biologic lesions: cutaneous thermal injury and inhalation injury working group 2018 meeting proceedings. J Burn Care Res 2020;41:604–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Koo TK, Li MY. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med 2016;15:155–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Despa F, Orgill DP, Neuwalder J, Lee RC. The relative thermal stability of tissue macromolecules and cellular structure in burn injury. Burns 2005;31:568–77. [DOI] [PubMed] [Google Scholar]
- 61. Gibson ALF, Bennett DD, Taylor LJ. Improving the histologic characterization of burn depth. J Cutan Pathol 2017;44:998–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Brans TA, Dutrieux RP, Hoekstra MJ, Kreis RW, du Pont JS. Histopathological evaluation of scalds and contact burns in the pig model. Burns 1994;20(Suppl 1):S48–51. [DOI] [PubMed] [Google Scholar]
- 63. Gallant-Behm CL, Tsao H, Reno C, Olson ME, Hart DA. Skin wound healing in the first generation (F1) offspring of Yorkshire and red Duroc pigs: evidence for genetic inheritance of wound phenotype. Burns 2006;32:180–93. [DOI] [PubMed] [Google Scholar]
- 64. Carlsson AH, Rose LF, Fletcher JL, Wu JC, Leung KP, Chan RK. Antecedent thermal injury worsens split-thickness skin graft quality: a clinically relevant porcine model of full-thickness burn, excision and grafting. Burns 2017;43:223–31. [DOI] [PubMed] [Google Scholar]
- 65. Davis SC, Mertz PM, Bilevich ED, Cazzaniga AL, Eaglstein WH. Early debridement of second-degree burn wounds enhances the rate of epithelization–an animal model to evaluate burn wound therapies. J Burn Care Rehabil 1996;17(6 Pt 1):558–61. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.