Abstract
Background
Comorbid conditions and adverse drug events are associated with poor treatment outcomes among patients with drug resistant tuberculosis (DR – TB). This study aimed at determining the treatment outcomes of DR – TB patients with poor prognostic indicators in Uganda.
Methods
We reviewed treatment records of DR – TB patients from 16 treatment sites in Uganda. Eligible patients had confirmed DR – TB, a treatment outcome in 2014–2019 and at least one of 15 pre-defined poor prognostic indicators at treatment initiation or during therapy. The pre-defined poor prognostic indicators were HIV co-infection, diabetes, heart failure, malignancy, psychiatric illness/symptoms, severe anaemia, alcohol use, cigarette smoking, low body mass index, elevated creatinine, hepatic dysfunction, hearing loss, resistance to fluoroquinolones and/or second-line aminoglycosides, previous exposure to second-line drugs (SLDs), and pregnancy. Tuberculosis treatment outcomes were treatment success, mortality, loss to follow up, and treatment failure as defined by the World Health Organisation. We used logistic and cox proportional hazards regression analysis to determine predictors of treatment success and mortality, respectively.
Results
Of 1122 DR – TB patients, 709 (63.2%) were male and the median (interquartile range, IQR) age was 36.0 (28.0–45.0) years. A total of 925 (82.4%) had ≥2 poor prognostic indicators. Treatment success and mortality occurred among 806 (71.8%) and 207 (18.4%) patients whereas treatment loss-to-follow-up and failure were observed among 96 (8.6%) and 13 (1.2%) patients, respectively. Mild (OR: 0.57, 95% CI 0.39–0.84, p = 0.004), moderate (OR: 0.18, 95% CI 0.12–0.26, p < 0.001) and severe anaemia (OR: 0.09, 95% CI 0.05–0.17, p < 0.001) and previous exposure to SLDs (OR: 0.19, 95% CI 0.08–0.48, p < 0.001) predicted lower odds of treatment success while the number of poor prognostic indicators (HR: 1.62, 95% CI 1.30–2.01, p < 0.001), for every additional poor prognostic indicator) predicted mortality.
Conclusion
Among DR – TB patients with multiple poor prognostic indicators, mortality was the most frequent unsuccessful outcomes. Every additional poor prognostic indicator increased the risk of mortality while anaemia and previous exposure to SLDs were associated with lower odds of treatment success. The management of anaemia among DR – TB patients needs to be evaluated by prospective studies. DR – TB programs should also optimise DR – TB treatment the first time it is initiated.
Keywords: Drug resistant, Tuberculosis, Outcomes, Treatment success, MDR-TB, Poor prognostic indicators
1. Background
Drug resistant tuberculosis (DR-TB) is emerging as a global threat to tuberculosis (TB) control with over 500,000 estimated cases of multi-drug resistant TB (MDR-TB)/rifampicin resistance in 2018 [1]. Worse still, the treatment success rate is low, with only 57% of patients on conventional treatment regimens achieving treatment success against a global target of 75% [1], [2]. This is partly because current DR-TB treatment is lengthy, complex, expensive, poorly tolerated and has low efficacy [3]. Poor treatment outcomes, that is, treatment failure, death and loss-to-follow-up were observed among 8%, 15% and 15% among DR – TB patients whose treatment outcome was reported in 2019 [1].
Predictors of poor treatment outcomes include HIV co-infection, diabetes, cancer, low albumin, anaemia, psychiatric disease, heart failure, resistance to fluoroquinolones or second-line aminoglycosides, cigarette smoking, alcohol use, cavitary disease, extensively drug resistant (XDR) TB, previous exposure to second line drugs (SLDs) and adverse drug effects (ADEs) [4], [5], [6], [7], [8], [9], [10], [11].
Traditionally, studies have focused on either identifying predictors of treatment outcomes among DR – TB patients in general [6], [7], [12] or among DR – TB patients with a single poor prognostic indicator such as diabetes [13], HIV co-infection [14], alcohol use [15], cigarette smoking [16] and ADEs [17]. Notwithstanding, DR – TB patients often have co-concurrent comorbid conditions and ADEs whose interaction could potentially affect treatment outcomes [18]. Over a quarter of DR- TB patients with at least 2 poor prognostic indicators are likely to have a poor treatment outcome [19]. It is therefore important to determine treatment outcomes and predictors of treatment success to inform treatment strategies for DR – TB patients with several poor prognostic indicators.
The aim of this study was to determine treatment outcomes of DR – TB patients with poor prognostic indicators in Uganda. As a secondary aim, we analysed for predictors of treatment success and mortality in this population.
2. Materials and Methods
2.1. Study population and setting
We conducted a retrospective review of patient records at 16 of 17 DR – TB treatment sites in Uganda. The inclusion criteria were: having confirmed DR – TB, a documented treatment outcome in 2014 to 2019 and any of 15 predefined poor prognostic indicators (see below) at baseline or during treatment. We excluded participants who had missing patient charts, those who were not initiated on DR – TB treatment due to pre-treatment loss to follow up or death, those who were returned to first line therapy, and those whose treatment outcome was not evaluated. Patients were followed from the date of treatment initiation to when a treatment outcome was documented in the DR – TB register. The 17 DR-TB treatment centres in Uganda comprised of 1 urban national referral hospital, 13 rural regional referral hospitals and 3 rural general district hospitals. We conducted a census across 16 DR – TB sites and the number of eligible participants enrolled from the hospitals were as follows: Mulago National Referral Hospital (321), Arua Regional Referral Hospital (104), Jinja Regional Referral Hospital (7), Iganga District Hospital (41), Mbale Regional Referral Hospital (66), Soroti Regional Referral Hospital (52), Lira Regional Referral Hospital (94), Matany District Hospital (28), Moroto District Hospital (2), Kitgum District Hospital (79), Mbarara Regional Referral Hospital (124), Kabale Regional Referral Hospital (44), Hoima Regional Referral Hospital (49), Masaka Regional Referral Hospital (51), Fortportal Regional Referral Hospital (50) and Mubende Regional Referral Hospital (10). For the one site (Gulu Regional Referral Hospital), data could not be collected due to travel restrictions following the coronavirus disease of 2019 pandemic.
An Xpert MTB/RIF® assay was the first line drug susceptibility test (DST) performed on all specimens of patients suspected to have TB. If the patient’s specimen sample yielded rifampicin resistance (RR) on the Xpert MTB/RIF® assay, a second sample was collected and transported to the national tuberculosis reference laboratory (NTRL) for culture based phenotypic DST. Additional resistance to fluroquinolones and second line injectable agents was tested for all patient with a positive culture at the NTRL.
2.1.1. Poor prognostic indicators
We defined poor prognostic factors as comorbid conditions or ADEs that are reported to be associated with poor DR – TB treatment outcomes from a systematic review, a meta-analysis and 4 large cohorts [10], [11], [17], [4], [5], [6]. We considered HIV co-infection, diabetes, heart failure, cancer, psychiatric illness/symptoms, severe anaemia, alcohol use, cigarette smoking, low BMI, elevated creatinine, hepatic dysfunction, hearing loss, resistance to fluoroquinolones and/or second-line aminoglycosides and previous exposure to SLDs. We also considered pregnancy as a possible poor prognostic indicator due to the limited number of reports of DR – TB outcomes among pregnant women, yet pregnancy requires regimen adjustments [20]. A baseline BMI of <18.5 kg/m2 was considered low. Anaemia was graded according to the following haemoglobin levels [21]: mild anaemia was 11.00–13 g per decilitre (g/dl) for a male individual and 11.00–12 g/dl for females; moderate anaemia was 8.00–11 g/dl (both sexes); and severe anaemia was <8 g/dl for both sexes. A serum creatinine level of >106.1 µmol per litre (µm/l) was considered elevated. We defined hepatic dysfunction as elevation in any of total bilirubin, aspartate aminotransferase (AST), gamma-glutamyl transferase, alanine aminotransferase (ALT) and alkaline phosphatase at the respective cut offs of: >2.5 mg/dl, >34.8 units per litre (U/L), >68.5 U/L, >42.8 U/L and >151 U/L. The cut offs were guided by normal range estimates among Ugandans [22]. A participant was considered to have diabetes if they had a baseline fasting blood sugar of >7 mmol per litre (mmol/l) or if they were on diabetes medication. The pregnancy status was determined from the result of the urine human chorionic gonadotrophic hormone test.
2.1.2. DR – TB treatment in Uganda
The programmatic management of DR – TB in Uganda began in 2012 although a local guideline was in place in 2011 [23]. The guidelines recommended a standardised regimen that consisted of a 6-months’ intensive phase with kanamycin (Km) (or capreomycin), levofloxacin (Lfx), ethionamide (Eto), cycloserine (Cs), and pyrazinamide (Z) followed by a continuation phase of 18 months without the aminoglycoside. Individualised regimens were recommended when informed by drug susceptibility testing and tolerance. The alternative agents were ethambutol (E), amikacin (Am) and p-amino salicylic acid (PAS). In 2016, the revised guidelines recommended an intensive phase of 6 months of Km + Lfx + Eto + Cs + Z or 4 months after culture conversion, whichever was longer. A continuation phase without the aminoglycoside for a duration of 14 months or at least 20-months post-culture conversion (whichever was longer), was recommended. Moxifloxacin (Mfx) was recommended as an alternative to Lfx, while Cfz and Lz were recommended as possible options to Eto. Bedaquiline and delamanid, newly discovered drugs for DR-TB treatment, were initially available on a compassionate basis until they were increasingly available in the country by 2018. A modification to the standard regimen was recommended for the treatment of XDR-TB and pre-XDR – TB (MDR – TB with additional resistance to either a fluroquinolone or second line injectable aminoglycoside) but the decision was to be made by the national DR – TB panel on a case-to-case basis. The national DR – TB panel also provides guidance on other difficult-to-treat patients. In 2017, an annex to the 2016 guidelines introduced the short term regimen (STR) which consisted of 4–6 months of Km + Mfx + Cfz + Z + E + Hhigh dose + Eto and 5 months of Mfx + Cfz + Z + E for patients with confirmed sensitivity to fluoroquinolones and an injectable aminoglycoside. Treatment was administered by health worker supervised directly observed therapy (DOT) using both facility and community-based models.
At baseline, patients had a baseline evaluation for hearing loss by pure tone audiometry (Interacoustics clinical audiometer AC33® at Mulago National Referral Hospital and Amplivox 116® at other treatment sites), HIV testing following national HIV testing guidelines [24], serum AST, GGT, creatinine and total bilirubin, fasting blood glucose and a full blood count. A baseline, assessment for the BMI, psychiatric symptoms, history of smoking and alcohol use and a pregnancy test (where applicable) using commercially available kits were also recommended. During treatment, evaluation of the creatinine, haemoglobin, liver enzymes and bilirubin was performed monthly in the first 6 months of therapy. Audiometry was performed monthly for patients on an injectable aminoglycoside. Treatment outcomes were documented in the patient charts and the DR – TB register at the treatment site.
2.2. Methods
Using a data abstraction form, we extracted data on age, sex, employment status, marital status, residence type (rural or urban), prior history of TB treatment, year of DR – TB treatment initiation and DR – TB site from patient charts. Any history of alcohol and/or cigarette use was obtained from the clinician’s notes or the socio-economic evaluation form in the patient chart. Baseline TB resistance patterns were extracted from the baseline molecular and/or phenotypic DST results. The diagnoses of heart failure, cancer, and psychiatric illness or symptoms were extracted from clinicians’ notes. The baseline BMI was calculated from the baseline height (in meters) and weight (in kilograms) using the formula BMI = weight/height2. Laboratory measurements were extracted from laboratory result reports. The occurrence of hearing loss was extracted from the monitoring audiograms. Hypertension was diagnosed if the baseline systolic and/or diastolic blood pressure were above 140 mm of mercury (mmHg) or 90 mmHg, respectively. Patients who were on anti-hypertension medication were also classified as hypertensive. From the patient charts, we further extracted the type and number of drugs in the patient treatment regimen and month of culture conversion. We determined whether the patient was reviewed by the national DR – TB expert panel from clinician notes. The duration from diagnosis to treatment initiation was calculated by subtracting the date of treatment initiation from the date of the first DST that showed resistance. The month of culture conversion was determined to be the first of two months with consecutive negative culture results following a positive baseline culture according to the WHO definition [25].
2.3. Data analysis
The study outcomes were the TB treatment outcomes as defined by WHO [25]. Treatment success was a composite of TB cure and treatment completion. Cure was defined as completion of DR – TB therapy without evidence of failure and three or more consecutive negative sputum cultures taken at least 30 days apart in the continuation phase of therapy. “Treatment completion” referred to patients who completed therapy according to the national policy but had no record of three or more consecutive negative culture results taken at least 30 days apart in the continuation phase of therapy. TB mortality was death from any cause during DR – TB treatment.
Data were entered in EpiData 4.4.0 and analysed with STATA 15.0 (STATA, College Station, Texas, USA). Continuous variables were summarised as medians or means with corresponding interquartile ranges (IQR) and standard deviations (SD) respectively, depending on the distribution of data. Categorical variables and treatment outcomes were summarised as proportions. We performed univariate logistic and cox proportional hazards regression analysis to determine crude odds and hazard ratios for predictors of treatment success and mortality, respectively. Variables with a crude odds/hazard ratio with p-value of ≤ 0.05 were selected for inclusion in the multivariable model. We then used stepwise backward regression method to select variables for the final multivariable regression model for which only variables with p < 0.05 were left in the model. We excluded heart failure because of the few observations. Also, diabetes was excluded because it induced collinearity. We tested for the goodness of fit of the logistic and cox proportional hazard models using Hosmer-Lemeshow test and Gronnesby and Borgan test, respectively. Predictors were considered statistically significant if p < 0.05 at the 95% confidence interval. For all modelling, we performed complete case analyses.
3. Results
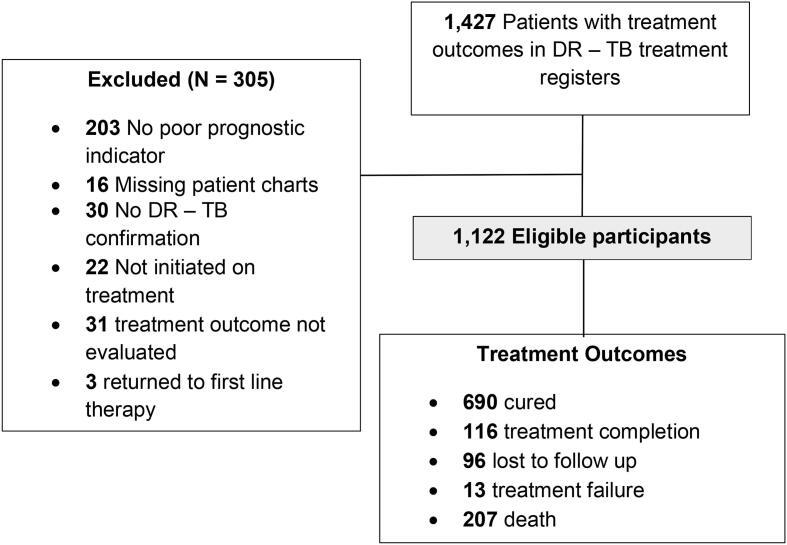
Between January and March 2020, a total of 1122 eligible patients were enrolled in the study. Fig. 1 shows the study flow diagram.
Fig. 1.
Study flow diagram.
3.1. Distribution of poor prognostic indicators among DR – TB patients
The median (IQR) number of poor prognostic factors was 3 (2–4) per patient and 925 (82.4%) had ≥ 2 poor prognostic indicators. Among patients with a known type of cancer, 10 (76.9%) had Kaposi’s sarcoma, 1 (7.7%) had lymphoma, 1 (7.7%) had stomach cancer and 1 (7.7%) had a CNS tumour. Table 1 shows the distribution of the poor prognostic indicators.
Table 1.
Distribution of poor prognostic indicators among DR – TB patients.
| Number (%)§ | Number with poor prognostic indicator at treatment initiation (%)§ | |
|---|---|---|
| Poor prognostic indicator | ||
| Hepatic dysfunction | 780 (84.1) | 151 (31.1) |
| HIV co-infection | 666 (59.4) | 489 (98.4) |
| Low baseline body mass index | 276 (58.3) | 276 (58.4) |
| Hearing loss | 359 (44.7) | 66 (24.8) |
| Alcohol use | 313 (38.4) | 313 (100.0) |
| Diabetes | 48 (31.8) | 48 (100.00 |
| Elevated creatinine | 279 (29.9) | 31 (18.1) |
| Cigarette smoking | 155 (19.0) | 155 (100.0) |
| Severe Anaemia | 115 (11.7) | 49 (59.8) |
| Psychiatric symptoms or mental illness | 69 (6.2) | 19 (32.2) |
| Pregnancy | 18 (4.4)† | 6 (54.6) |
| Previous exposure to second line drugs | 30 (2.7) | 30 (100.0) |
| Cancer | 17 (1.5) | 5 (55.6) |
| Resistance to fluoroquinolones and/or injectable aminoglycoside* | 14 (1.2) | 14 (100.0) |
| Heart failure | 5 (0.45) | 0 (0.0) |
| Number of poor prognostic indicators per patient | ||
| 1 | 197 (17.6) | |
| 2 | 329 (29.3) | |
| 3 | 280 (25.0) | |
| 4 | 174 (15.5) | |
| 5 | 88 (7.8) | |
| ≥6 | 54 (4.8) |
*Includes 2 patients with extensively drug resistant tuberculosis, §the denominator is the participants for whom data were available for a given poor prognostic indicator, †percentage is calculated out of the 413 females in the study.
3.2. Baseline characteristics of DR – TB patients with poor prognostic indicators
The baseline characteristics of study participants are shown in table 2. Of 1122 patients, 709 (63.2%) were male and the median (IQR) age was 36.0 (28.0–45.0) years. The mean (SD) baseline BMI was 18.0 (3.3) kg/m2. The median (IQR) number of drugs a participant was resistant to was 1 (1 – 3) and 25.5% were resistant to at least 3 drugs. The mean (SD) duration of culture conversion was 1.7 (3.1) months and 507 (78.5%) had culture converted by month 3. The median (IQR) number of drugs in the treatment regimen was 5 (5–6). The national DR – TB expert panel reviewed 189 (16.8%) of the study patients.
Table 2.
Characteristics of DR – TB patients with poor prognostic indicators.
| Characteristic | N = 1122 | Percentage |
|---|---|---|
| DR –TB treatment site | ||
| National referral hospital | 321 | 28.6 |
| Regional referral hospital | 653 | 58.2 |
| District hospital | 148 | 13.2 |
| Age (years), n = 1080 | ||
| <15 | 27 | 2.5 |
| 15-34 | 454 | 42.0 |
| 35-60 | 543 | 50.3 |
| >60 | 56 | 5.2 |
| Residence, n = 1080 | ||
| Rural | 696 | 64.7 |
| Urban | 380 | 35.3 |
| Married, n = 1069 | 535 | 50.0 |
| Employment status, n = 1060 | ||
| Unemployed | 352 | 33.2 |
| Self employed | 475 | 44.8 |
| Formal employment | 233 | 22.0 |
| History of tuberculosis treatment | 663 | 59.1 |
| Hypertensive, n = 479 | 24 | 5.0 |
| Year of treatment initiation | ||
| ≤2013 | 101 | 9.0 |
| 2014 | 111 | 10.0 |
| 2015 | 154 | 13.9 |
| 2016 | 219 | 19.7 |
| 2017 | 248 | 22.3 |
| 2018 | 203 | 18.3 |
| 2019 | 76 | 6.8 |
| Type of DR-TB at Baseline | ||
| Rifampicin resistanceµ | 662 | 59.0 |
| MDR-TB | 418 | 37.3 |
| Poly resistant tuberculosis (TB)† | 24 | 2.1 |
| Pre- XDR-TB* | 12 | 1.1 |
| XDR-TB | 3 | 0.3 |
| Isoniazid mono-resistance | 3 | 0.3 |
| Resistance at baseline | % of cases | |
| Rifampicin | 1119 | 99.7 |
| Isoniazid | 427 | 38.1 |
| Streptomycin | 243 | 21.7 |
| Ethambutol | 217 | 19.3 |
| Pyrazinamide | 17 | 1.5 |
| Aminoglycoside | 13 | 1.2 |
| Fluroquinolone | 3 | 0.3 |
| Drugs in the treatment regimen | % of cases | |
| Ethionamide | 1100 | 98.3 |
| Pyrazinamide | 1098 | 98.1 |
| Kanamycin | 973 | 87.0 |
| Levofloxacin | 925 | 82.7 |
| Ethambutol | 214 | 19.1 |
| Clofazimine | 208 | 18.6 |
| High dose Isoniazid | 197 | 17.6 |
| Moxifloxacin | 196 | 17.5 |
| Capreomycin | 158 | 14.1 |
| Bedaquiline | 41 | 3.7 |
| Linezolid | 20 | 1.8 |
| P-Amino salicylic acid | 17 | 1.5 |
| Amikacin | 12 | 1.1 |
| Time to treatment initiation (days), median (IQR), n = 1096 | 9 (4–23) | |
| Time to smear conversion (months), mean (SD) | 1.7 (3.1) | |
| Total treatment duration (months), median (IQR) | 20 (9–21) |
IQR: interquartile range, SD: standard deviation, DR – TB: drug resistant tuberculosis, MDR – TB: multi drug resistant TB, XDR – TB: extensively drug resistant tuberculosis, *Pre-XDR – TB indicates MDR – TB with additional resistance to either an injectable aminoglycoside or fluroquinolone, †all had rifampicin resistance as well except one participant, µpatients had GeneXpert showing rifampicin resistance but subsequent phenotypic drug susceptibility testing was not performed due to negative baseline culture or was unavailable.
3.3. Treatment outcomes and predictors of treatment success among DR – TB patients with poor prognostic indicators
Treatment was successful in 806 (71.8%) patients, of whom 690 (61.5%) were cured and 116 (10.3%) had treatment completion. Mortality occurred among 207 (18.4%) patients. Further, 96 (8.6%) were lost to follow up and 13 (1.2%) had treatment failure. Notably, the number of poor prognostic indicators predicted lower odds of treatment success at univariate analysis (odds ratio (OR): 0.83, 95% CI 0.76–0.91, p < 0.001, per additional poor prognostic indicator). However, at multivariate analysis, mild (OR: 0.57, 95% CI 0.39–0.84, p = 0.004), moderate (OR: 0.18, 95% CI 0.12 – 0.26, p < 0.001) and severe anaemia (OR: 0.09, 95% confidence interval, CI 0.05–0.17, p < 0.001) and previous exposure to SLDs (OR: 0.19, 95% CI 0.08 – 0.48, p < 0.001) were associated with lower odds of treatment success. Table 3 shows univariate and multivariate models for predictors of treatment success.
Table 3.
Predictors of treatment success among DR – TB patients with poor prognostic indicators.
| Univariate analysis |
Multivariable analysis |
|||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | OR | 95% CI | p-value | OR | 95% CI | p-value | ||
| Type of Treatment Site | ||||||||
| National Referral Hospital | 1 | |||||||
| Regional Referral Hospital | 1.55 | 1.16 | 2.06 | 0.003 | ||||
| District Hospital | 2.78 | 1.71 | 4.53 | <0.001 | ||||
| Residence | ||||||||
| Rural | 1 | |||||||
| Urban | 0.68 | 0.52 | 0.89 | 0.005 | ||||
| Age (years) | ||||||||
| <15 | 1 | |||||||
| 15–34 | 0.27 | 0.08 | 0.93 | 0.037 | ||||
| 35–60 | 0.34 | 0.10 | 1.13 | 0.079 | ||||
| >60 | 0.29 | 0.08 | 1.08 | 0.065 | ||||
| Alcohol use | ||||||||
| None | 1 | |||||||
| Yes | 0.64 | 0.46 | 0.88 | 0.007 | ||||
| Cigarette smoking | ||||||||
| None | 1 | |||||||
| Yes | 0.55 | 0.38 | 0.80 | 0.002 | ||||
| HIV status | ||||||||
| Positive | 1 | |||||||
| Negative | 2.22 | 1.70 | 2.95 | <0.001 | ||||
| Anaemia | ||||||||
| Normal haemoglobin level | 1 | 1 | ||||||
| Mild | 0.59 | 0.40 | 0.87 | 0.007 | 0.57 | 0.39 | 0.84 | 0.004 |
| Moderate | 0.18 | 0.12 | 0.27 | <0.001 | 0.18 | 0.12 | 0.26 | <0.001 |
| Severe | 0.09 | 0.05 | 0.17 | <0.001 | 0.09 | 0.05 | 0.17 | <0.001 |
| Baseline body mass index | 1.09 | 1.02 | 1.16 | 0.016 | ||||
| Cancer | ||||||||
| Yes | 1 | |||||||
| No | 2.92 | 1.12 | 7.65 | 0.029 | ||||
| Ethambutol resistance | ||||||||
| Yes | 1 | |||||||
| No | 0.69 | 0.48 | 0.98 | 0.038 | ||||
| Streptomycin resistance | ||||||||
| Yes | 1 | |||||||
| No | 0.70 | 0.50 | 0.99 | 0.042 | ||||
| No. of poor prognostic indicators | 0.83 | 0.76 | 0.91 | <0.001 | ||||
| Previous exposure to SLDs | ||||||||
| Yes | 0.22 | 0.10 | 0.46 | <0.001 | 0.19 | 0.08 | 0.48 | <0.001 |
| No | 1 | 1 | ||||||
| No. of drugs a patient was resistant to | 1.13 | 1.01 | 1.27 | 0.041 | ||||
| Drugs in the treatment regimen | ||||||||
| Levofloxacin | ||||||||
| No | 1 | |||||||
| Yes | 0.66 | 0.45 | 0.95 | 0.025 | ||||
| Moxifloxacin | ||||||||
| No | 1 | |||||||
| Yes | 1.54 | 1.06 | 2.22 | 0.022 | ||||
| Capreomycin | ||||||||
| No | 1 | |||||||
| Yes | 1.64 | 1.08 | 2.47 | 0.019 | ||||
| Clofazimine | ||||||||
| No | 1 | |||||||
| Yes | 1.57 | 1.09 | 2.25 | 0.015 | ||||
| High-dose Isoniazid | ||||||||
| No | 1 | |||||||
| Yes | 1.66 | 1.15 | 2.41 | 0.008 | ||||
| Ethambutol | ||||||||
| No | 1 | |||||||
| Yes | 1.64 | 1.15 | 2.36 | 0.007 | ||||
| Number of drugs in regimen | ||||||||
| <5 | 1 | |||||||
| ≥5 | 1.74 | 1.32 | 2.28 | <0.001 | ||||
OR – odds ratio, CI – confidence interval, SLDs – Second line drugs.
3.4. Predictors of mortality among DR – TB patients with poor prognostic indicators
The number of poor prognostic indicators (HR: 1.62, 95% CI 1.30 – 2.01, p < 0.001), for every additional poor prognostic indicator) independently predicted mortality as shown in Table 4.
Table 4.
Predictors of mortality among DR – TB patients with poor prognostic indicators.
| Univariate analysis |
Multivariable analysis |
|||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | HR | 95% CI | p-value | HR | 95% CI | p-value | ||
| Type of Treatment Site | ||||||||
| National Referral Hospital | 1 | |||||||
| Regional Referral Hospital | 0.89 | 0.66 | 1.20 | 0.444 | ||||
| District Hospital | 0.34 | 0.19 | 0.62 | <0.001 | ||||
| Year of enrolment | ||||||||
| ≤ 2013 | 1 | |||||||
| 2014 | 0.96 | 0.49 | 1.91 | 0.916 | ||||
| 2015 | 1.17 | 0.64 | 2.18 | 0.601 | ||||
| 2016 | 1.72 | 0.99 | 3.01 | 0.055 | ||||
| 2017 | 0.94 | 0.52 | 1.69 | 0.843 | ||||
| 2018 | 1.08 | 0.59 | 2.02 | 0.791 | ||||
| 2019 | 2.82 | 1.47 | 5.39 | 0.002 | ||||
| Alcohol use | ||||||||
| None | 1 | |||||||
| Yes | 1.75 | 1.24 | 2.46 | 0.001 | ||||
| No. of poor prognostic indicators | 1.28 | 1.18 | 1.40 | <0.001 | 1.62 | 1.30 | 2.01 | <0.001 |
| Cigarette smoking | ||||||||
| None | 1 | |||||||
| Yes | 2.15 | 1.49 | 3.09 | <0.001 | ||||
| HIV status | ||||||||
| Positive | 1 | |||||||
| Negative | 0.47 | 0.35 | 0.64 | <0.001 | ||||
| Anaemia | ||||||||
| Normal haemoglobin | 1 | |||||||
| Mild | 1.94 | 1.20 | 3.15 | <0.001 | ||||
| Moderate | 6.82 | 4.44 | 10.50 | <0.001 | ||||
| Severe | 13.19 | 7.97 | 21.84 | 0.007 | ||||
| Cancer | ||||||||
| No | 1 | |||||||
| Yes | 3.55 | 1.82 | 6.93 | <0.001 | ||||
| Streptomycin resistance | ||||||||
| No | 1 | |||||||
| Yes | 0.69 | 0.48 | 1.00 | 0.049 | ||||
| Body mass index | ||||||||
| <18.5 | 1 | |||||||
| 18.5–24.9 | 0.60 | 0.36 | 0.99 | 0.045 | ||||
| 25.0–29.9 | 0.31 | 0.04 | 2.27 | 0.251 | ||||
| No. of drugs a patient was resistant to | 0.87 | 0.76 | 0.99 | 0.033 | ||||
| Number of drugs in regimen | ||||||||
| <5 | 1 | |||||||
| ≥5 | 0.70 | 0.52 | 0.93 | 0.013 | ||||
| Month of culture conversion | 0.85 | 0.73 | 0.99 | 0.033 | ||||
HR – hazard ratio, CI – confidence interval.
4. Discussion
In this study, we determined the treatment outcomes of DR – TB patients with poor prognostic indicators. We further determined predictors of treatment success and mortality. We found treatment success to be 71.8% and mortality at 18.4%. The treatment loss-to-follow-up and failure were 8.6% and 1.2% respectively. Mild, moderate, and severe anaemia and previous exposure to SLDs were associated with 43%, 82%, 91% and 81% lower odds of treatment success, respectively. Every additional poor prognostic indicator increased the likelihood of mortality by 62%.
The treatment success we found is below the global target of ≥75%. However, it is comparable to 70% [9] and 69% [13] reported by Zhang, et al., and Latif, et al., in China and Pakistan; but lower than the 75.8% and 78.6% reported by Javaid, et al., [26] and Meressa, et al., [5] in Pakistan and Ethiopia respectively. Our estimate is higher than 41% [6] and 62% [7] reported by Tang et al. and Oliveira et al., in China and Portugal respectively, although these had more patients with XDR – TB than ours. Notably, none of these studies specifically selected patients with poor prognostic indicators. Further, compared to these studies, we report a higher mortality (cf. 17.4% [26], 14% [5], and 6.7% [9]). It is therefore apparent that the driver of poor treatment outcomes among patients with poor prognostic indicators is death rather than loss to follow up or treatment failure. In which case, emphasising treatment adherence or clinic attendance solely without addressing modifiable poor prognostic indicators may not be sufficient to improve treatment success.
In our cohort, poor prognostic indicators were prevalent. Notably, only 14% of potential participants were excluded because they did not have a poor prognostic indicator. This is consistent with a report from Nigeria where 70.3% of DR – TB patients had comorbidities at baseline [27]. These patients are prone to adverse drug events – which are additional poor prognostic indicators by themselves – upon initiation of treatment. It is therefore not surprising that our study which considered poor prognostic indicators at baseline and during therapy found over 82% of patients to have at least 2 poor prognostic indicators. Patients with comorbidities and organ dysfunction – such as hepatic dysfunction and elevated creatinine in our study – are likely to experience more drug toxicity, need substitution of drugs with less efficacious drugs, and death may arise from the comorbidity or adverse effect and not necessarily due to DR – TB [28]. These patients should benefit from review by a panel of experts, at the time of treatment initiation, to decide an appropriate regimen as recommended by WHO [29]. Unfortunately, less than a fifth of our study population had a documented review by the national DR – TB expert panel yet several poor prognostic indicators were identified at baseline. Similar to our findings, Alene, et al., [19] have shown that any additional poor prognostic indicator increases the risk of a poor treatment outcome. More comprehensive prognostic scores are needed to identify patients at risk of a poor outcome such that interventions can be instituted for modifiable factors at treatment initiation or during treatment.
Among the poor prognostic indicators, anaemia, a modifiable poor prognostic indicator, was identified as a risk factor for an unsuccessful treatment outcome in a dose-dependent fashion (the risk increased with severity of anaemia). In the general population, anaemia is a recognised independent predictor of all-cause mortality regardless of age, sex and cardiovascular disease (HR: 1.41) [30]. Similar to our finding, anaemia has been reported to predict poor treatment outcomes of DR – TB patients in Ethiopia [11]. Additionally, anaemia was the most reported comorbidity among DR – TB patients in Nigeria [27]. TB patients with anaemia are at risk of other infections and have impaired immune responses that could contribute to delayed sputum conversion, disease progression, and death [31], [32], [33]. There are no guideline recommendations regarding the target haemoglobin level among DR – TB patients during before treatment initiation or during therapy. Anaemia among DR – TB patients is likely due to inflammation and is thought to improve with TB treatment [34]. However, in our study population with a median BMI of 18.0 Kg/m2, nutritional factors may be contributory and DR – TB treatment alone may not correct the anaemia [35]. Linezolid is now considered among “group A” drugs that are preferred in constructing MDR – TB treatment regimens although it is associated with incident anaemia [36]. As its use becomes widespread, monitoring and characterising anaemia among DR – TB patients during routine clinical care will become very crucial in guiding specific interventions directed to the underlying causes, especially in low-income countries. In our study, there were very few patients initiated on linezolid and we could not further characterise the relationship between linezolid and anaemia.
The association of previous exposure to SLDs with lower odds of treatment success has also been reported in China [9], Latvia [37] and Peru [38]. Patients who have been exposed to SLDs often have fewer re-treatment options due to acquired additional resistance and long-term toxicities. Programs should therefore aim to optimise DR – TB treatment the first time it is initiated.
Contrary to our findings, treatment success was only 44% and loss to follow up was 30.5% among DR – TB patients with comorbidities in Georgia [39]. However, this cohort had very few HIV co-infected patients (<4%) yet HIV is a known risk factor for DR – TB mortality [40]. Further, Uganda has been implementing an incentive and enablers program for DR – TB patients since 2015 to foster treatment adherence. This could partly explain why loss to follow up was low in our study.
To our knowledge, our study is the first to evaluate treatment outcomes of DR – TB patients with multiple poor prognostic indicators. Our study population is large enough to give meaningful inferences. Further, we have used a multi-centre approach, making the results somewhat generalizable. However, our study has some limitations. We were unable to evaluate disease severity by chest imaging studies yet cavitary disease is a poor prognostic indicator. Chest imaging and documentation of imaging findings are not consistently done in our setting. Additionally, other data on disease severity, as measured by symptom burden, performance status, and bacillary load at baseline, were not consistently documented and thus available to us. Resistance profiles to fluoroquinolones and second-line injectable agents was available for only 460 patients because many patients had a negative baseline culture. Therefore, we may have missed other potential predictors of TB treatment success and mortality. Another important limitation is the use of pre-defined poor prognostic indicators. This approach assumes that prognostic indicators observed elsewhere apply to the population under study, which may not be true. However, there were no baseline data from our setting to suggest context-specific poor prognostic indicators. Further still, the pre-defined poor prognostic indicators used are reasonably established risk factors for poor outcomes from a systematic review, a meta-analysis and 4 large cohorts [10], [11], [17], [4], [5], [6].
5. Conclusion
In this large multi-centre retrospective study from a TB/HIV high burdened country, treatment success was below global targets. It was apparent that death is the major contributor to poor outcomes among DR –TB patients with multiple poor prognostic indicators rather than loos-to-follow-up or treatment failure. Every additional poor prognostic indicator increased the risk of mortality. Among poor prognostic indicators, anaemia (in a dose-dependent fashion) and previous exposure to SLDs also predicted lower odds of overall treatment success. The management of anaemia among DR – TB patients needs to be evaluated by prospective studies. DR – TB programs should also optimise DR – TB treatment the first time it is initiated. We also recommend development of more comprehensive prognostic scores to predict treatment success and mortality among patients with DR – TB and poor prognostic indicators.
CRediT authorship contribution statement
Joseph Baruch Baluku: Conceptualization, Investigation, Project administration, Supervision, Methodology, Funding acquisition, Data curation, Formal analysis, Writing - original draft, Writing - review & editing. Bridget Nakazibwe: Investigation, Data curation, Writing - review & editing. Joshua Naloka: Methodology, Data curation, Investigation, Writing - review & editing. Martin Nabwana: Methodology, Formal analysis, Writing - review & editing. Sarah Mwanja: Investigation, Data curation, Writing - review & editing, Rose Mulwana: Investigation, Data curation, Writing - review & editing. Mike Sempiira: Investigation, Data curation, Writing - review & editing. Sylvia Nassozi: Investigation, Data curation, Writing - review & editing. Febronius Babirye: Investigation, Data curation, Writing - review & editing, Methodology. Carol Namugenyi: Investigation, Data curation, Writing - review & editing. Samuel Ntambi: Investigation, Data curation, Writing - review & editing. Sharon Namiiro: Investigation, Data curation, Writing - review & editing. Felix Bongomin: Methodology, Investigation, Writing - review & editing. Richard Katuramu: Methodology, Investigation, Writing - review & editing. Irene Andia-Biraro: Methodology, Investigation, Writing - review & editing. William Worodria: Investigation, Supervision, Funding acquisition, Writing - review & editing.
Acknowledgments
Acknowledgment
We acknowledge the contribution of DR – TB focal persons at the DR – TB treatments sites for their role in data accrual.
Ethics approval and consent to participate.
The study was approved by the Mulago Hospital Research and Ethics Committee (MHREC) (MHREC 1679) and the Uganda National Council of Science and Technology (No. HS452ES). Waiver of participant consent was provided by the MHREC.
Availability of data and materials
Datasets used in this analysis are available from the corresponding author upon reasonable request.
Competing interests
IAB currently receives funding through the Makerere University/UVRI Infection and Immunity Research Training Programme (Wellcome Trust, grant number 084344). The other authors have no conflict of interest to declare.
Funding
Funding for this research was obtained from the East African Public Health Laboratory Networking (EAPHLN) Project, Uganda under the Ministry of Health, which was supported by the World Bank. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.World Health Organization, Global tuberculosis report 2019. In: Global tuberculosis report 2019; 2019.
- 2.Kibret K.T., Moges Y., Memiah P., Biadgilign S. Treatment outcomes for multidrug-resistant tuberculosis under DOTS-Plus: a systematic review and meta-analysis of published studies. Infect Dis Poverty. 2017;6(1):7. doi: 10.1186/s40249-016-0214-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brigden G., Nyang’wa B.-T., du Cros P., Varaine F., Hughes J., Rich M., Horsburgh C.R., Jr, Mitnick C.D., Nuermberger E., McIlleron H., Phillips P.PJ., Balasegaram M. Principles for designing future regimens for multidrug-resistant tuberculosis. Bull World Health Organ. 2014;92(1):68–74. doi: 10.2471/BLT.13.122028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Samuels J.P., Sood A., Campbell J.R., Ahmad Khan F., Johnston J.C. Comorbidities and treatment outcomes in multidrug resistant tuberculosis: a systematic review and meta-analysis. Sci Rep. 2018;8(1) doi: 10.1038/s41598-018-23344-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meressa D., Hurtado R.M., Andrews J.R., Diro E., Abato K., Daniel T., Prasad P., Prasad R., Fekade B., Tedla Y., Yusuf H., Tadesse M., Tefera D., Ashenafi A., Desta G., Aderaye G., Olson K., Thim S., Goldfeld A.E. Achieving high treatment success for multidrug-resistant TB in Africa: initiation and scale-up of MDR TB care in Ethiopia—an observational cohort study. Thorax. 2015;70(12):1181–1188. doi: 10.1136/thoraxjnl-2015-207374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang S. Risk factors for poor treatment outcomes in patients with MDR-TB and XDR-TB in China: retrospective multi-center investigation. PLoS ONE. 2013;8(12) doi: 10.1371/journal.pone.0082943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oliveira O., Gaio R., Villar M., Duarte R. Predictors of treatment outcome in multidrug-resistant tuberculosis in Portugal. Eur Respir J. 2013;42(6):1747–1749. doi: 10.1183/09031936.00197912. [DOI] [PubMed] [Google Scholar]
- 8.Johnston J.C., Shahidi N.C., Sadatsafavi M., Fitzgerald J.M. Treatment outcomes of multidrug-resistant tuberculosis: a systematic review and meta-analysis. PloS One. 2009;4(9) doi: 10.1371/journal.pone.0006914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang L., Meng Q., Chen S., Zhang M., Chen B., Wu B., Yan G., Wang X., Jia Z. Treatment outcomes of multidrug-resistant tuberculosis patients in Zhejiang, China, 2009–2013. Clin Microbiol Infect. 2018;24(4):381–388. doi: 10.1016/j.cmi.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 10.Cegielski J.P. Multidrug-resistant tuberculosis treatment outcomes in relation to treatment and initial versus acquired second-line drug resistance. Clin Infect Dis. 2016;62(4):418–430. doi: 10.1093/cid/civ910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ketema D.B., Muchie K.F., Andargie A.A. Time to poor treatment outcome and its predictors among drug-resistant tuberculosis patients on second-line anti-tuberculosis treatment in Amhara region, Ethiopia: retrospective cohort study. BMC Public Health. Nov. 2019;19(1):1481. doi: 10.1186/s12889-019-7838-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leveri T.H., Lekule I., Mollel E., Lyamuya F., Kilonzo K. Predictors of treatment outcomes among multidrug resistant tuberculosis patients in Tanzania. Tubercul. Res. Treatm. 2019;2019:1–10. doi: 10.1155/2019/3569018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Latif A., Ghafoor A., Wali A., Fatima R., ul-Haq M., Yaqoob A., Abdullah Z., Najmi H., Khan N.M. Did diabetes mellitus affect treatment outcome in drug-resistant tuberculosis patients in Pakistan from 2010 to 2014? Public Health Action. 2018;8(1):14–19. doi: 10.5588/pha.17.0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Magis-Escurra C., Günther G., Lange C., Alexandru S., Altet N., Avsar K., Bang D., Barbuta R., Bothamley G., Ciobanu A., Crudu V., Davilovits M., Dedicoat M., Duarte R., Gualano G., Kunst H., de Lange W., Leimane V., McLaughlin A.-M., Muylle I., Polcová V., Popa C., Rumetshofer R., Skrahina A., Solodovnikova V., Spinu V., Tiberi S., Viiklepp P., van Leth F. Treatment outcomes of MDR-TB and HIV co-infection in Europe. Eur Respir J. 2017;49(6):1602363. doi: 10.1183/13993003.02363-2016.Supp1. [DOI] [PubMed] [Google Scholar]
- 15.Duraisamy K. Does alcohol consumption during multidrug-resistant tuberculosis treatment affect outcome? Ann Am Thorac Soc. 2014;11(5):712–718. doi: 10.1513/AnnalsATS.201312-447OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pazarli P., Yetis Duman D., Yazicioglu Mocin O., Karagoz T. The effect of smoking on treatment outcome of multidrug-resistant tuberculosis. Tur Toraks Der. 2013 doi: 10.5152/ttd.2013.20. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y., Wu S., Xia Y., Wang N.i., Zhou L., Wang J., Fang R., Sun F., Chen M., Zhan S. Adverse events associated with treatment of multidrug-resistant tuberculosis in china: an ambispective cohort study. Med Sci Monit. 2017;23:2348–2356. doi: 10.12659/MSM.904682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Merid M.W., Gezie L.D., Kassa G.M., Muluneh A.G., Akalu T.Y., Yenit M.K. Incidence and predictors of major adverse drug events among drug-resistant tuberculosis patients on second-line anti-tuberculosis treatment in Amhara regional state public hospitals; Ethiopia: a retrospective cohort study. BMC Infect Dis. 2019;19(1):286. doi: 10.1186/s12879-019-3919-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alene K.A., Viney K., Gray D.J., McBryde E.S., Xu Z., Clements A.C.A. Development of a risk score for prediction of poor treatment outcomes among patients with multidrug-resistant tuberculosis. PLOS ONE. 2020;15(1) doi: 10.1371/journal.pone.0227100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dudnyk A., Pavel’chuk O. Multidrug-resistant tuberculosis in pregnant women: treatment and birth outcomes. Eur. Respir. J. 2016;48(suppl 60):PA1912. doi: 10.1183/13993003.congress-2016.PA1912. [DOI] [Google Scholar]
- 21.World Health Organization . World Health Organization; 2011. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. [Google Scholar]
- 22.Eller L.A. Reference intervals in healthy adult Ugandan blood donors and their impact on conducting international vaccine trials. PLOS ONE. 2008;3(12) doi: 10.1371/journal.pone.0003919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ministry of Health, Uganda national guidelines for the programmatic management of drug resistant tuberculosis; 2011.
- 24.Ministry of Health, National HIV Testing Services Policy and Implementation Guidelines. Ministry of Health of Uganda; 2016.
- 25.World Health Organization . World Health Organization; 2013. Definitions and reporting framework for tuberculosis–2013 revision. 9241505346. [Google Scholar]
- 26.Javaid A., Shaheen Z., Shafqat M., Khan A.H., Ahmad N. Risk factors for high death and loss-to-follow-up rates among patients with multidrug-resistant tuberculosis at a programmatic management unit. Am J Infect Control. 2017;45(2):190–193. doi: 10.1016/j.ajic.2016.07.026. [DOI] [PubMed] [Google Scholar]
- 27.Adejumo O.A. Trans R Soc Trop Med Hyg. 2020;114(6):415–423. doi: 10.1093/trstmh/trz126. [DOI] [PubMed] [Google Scholar]
- 28.Esmail A., Sabur N.F., Okpechi I., Dheda K. Management of drug-resistant tuberculosis in special sub-populations including those with HIV co-infection, pregnancy, diabetes, organ-specific dysfunction, and in the critically ill. J Thorac Dis. 2018;10(5):3102–3118. doi: 10.21037/jtd.2018.05.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.World Health Organization . World Health Organization; 2014. Policy implementation package for new TB drug introduction. [Google Scholar]
- 30.Liu Z., Sun R., Li J., Cheng W., Li L. Relations of anemia with the all-cause mortality and cardiovascular mortality in general population: a meta-analysis. Am J Med Sci. 2019;358(3):191–199. doi: 10.1016/j.amjms.2019.05.016. [DOI] [PubMed] [Google Scholar]
- 31.Hella J. Anemia in tuberculosis cases and household controls from Tanzania: contribution of disease, coinfections, and the role of hepcidin. PLoS ONE. 2018;13(4) doi: 10.1371/journal.pone.0195985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Isanaka S. Iron deficiency and anemia predict mortality in patients with tuberculosis. J. Nutr. 2012;142(2):350–357. doi: 10.3945/jn.111.144287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nagu T.J. Anemia at the initiation of tuberculosis therapy is associated with delayed sputum conversion among pulmonary tuberculosis patients in Dar-es-Salaam, Tanzania. PLOS ONE. 2014;9(3) doi: 10.1371/journal.pone.0091229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bahi G.A., Bidie A.P., Meite S., Djaman A.J. Exploring the iron metabolism in multidrug resistant tuberculosis (MDR-TB) patients in treatment. Int J Biol Chem Sci. 2017;11(3) doi: 10.4314/ijbcs.v11i3.9. [DOI] [Google Scholar]
- 35.Minchella P.A., Donkor S., Owolabi O., Sutherland J.S., McDermid J.M. Complex anemia in tuberculosis: the need to consider causes and timing when designing interventions. Clin Infect Dis. 2015;60(5):764–772. doi: 10.1093/cid/ciu945. [DOI] [PubMed] [Google Scholar]
- 36.Singh B., Cocker D., Ryan H., Sloan D.J. Linezolid for drug-resistant pulmonary tuberculosis. Cochrane Database Syst Rev. 2019;3:2019. doi: 10.1002/14651858.CD012836.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leimane V., Riekstina V., Holtz T.H., Zarovska E., Skripconoka V., Thorpe L.E., Laserson K.F., Wells C.D. Clinical outcome of individualised treatment of multidrug-resistant tuberculosis in Latvia: a retrospective cohort study. Lancet. 2005;365(9456):318–326. doi: 10.1016/S0140-6736(05)17786-1. [DOI] [PubMed] [Google Scholar]
- 38.Mitnick C.D. Aggressive regimens for multidrug-resistant tuberculosis decrease all-cause mortality. PLOS ONE. 2013;8(3) doi: 10.1371/journal.pone.0058664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kikvidze M., Ikiashvili L. Comorbidities and MDR-TB treatment outcomes in Georgia-2009-11 cohort. Eur Respir J. 2014;44(Suppl 58) [Google Scholar]
- 40.Bisson G.P., Bastos M., Campbell J.R., Bang D., Brust J.C., Isaakidis P., Lange C., Menzies D., Migliori G.B., Pape J.W., Palmero D., Baghaei P., Tabarsi P., Viiklepp P., Vilbrun S., Walsh J., Marks S.M. Mortality in adults with multidrug-resistant tuberculosis and HIV by antiretroviral therapy and tuberculosis drug use: an individual patient data meta-analysis. Lancet. 2020;396(10248):402–411. doi: 10.1016/S0140-6736(20)31316-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Datasets used in this analysis are available from the corresponding author upon reasonable request.