Abstract
Objective
The immediate signals that couple exercise to metabolic adaptations are incompletely understood. Nicotinamide adenine dinucleotide phosphate oxidase 4 (Nox4) produces reactive oxygen species (ROS) and plays a significant role in metabolic and vascular adaptation during stress conditions. Our objective was to determine the role of Nox4 in exercise-induced skeletal muscle metabolism.
Methods
Mice were subjected to acute exercise to assess their immediate responses. mRNA and protein expression responses to Nox4 and hydrogen peroxide (H2O2) were measured by qPCR and immunoblotting. Functional metabolic flux was measured via ex vivo fatty acid and glucose oxidation assays using 14C-labeled palmitate and glucose, respectively. A chronic exercise regimen was also utilized and the time to exhaustion along with key markers of exercise adaptation (skeletal muscle citrate synthase and beta-hydroxyacyl-coA-dehydrogenase activity) were measured. Endothelial-specific Nox4-deficient mice were then subjected to the same acute exercise regimen and their subsequent substrate oxidation was measured.
Results
We identified key exercise-responsive metabolic genes that depend on H2O2 and Nox4 using catalase and Nox4-deficient mice. Nox4 was required for the expression of uncoupling protein 3 (Ucp3), hexokinase 2 (Hk2), and pyruvate dehydrogenase kinase 4 (Pdk4), but not the expression of peroxisome proliferator-activated receptor gamma coactivator 1-alpha (Pgc-1α). Global Nox4 deletion resulted in decreased UCP3 protein expression and impaired glucose and fatty acid oxidization in response to acute exercise. Furthermore, Nox4-deficient mice demonstrated impaired adaptation to chronic exercise as measured by the time to exhaustion and activity of skeletal muscle citrate synthase and beta-hydroxyacyl-coA-dehydrogenase. Importantly, mice deficient in endothelial-Nox4 similarly demonstrated attenuated glucose and fatty acid oxidation following acute exercise.
Conclusions
We report that H2O2 and Nox4 promote immediate responses to exercise in skeletal muscle. Glucose and fatty acid oxidation were blunted in the Nox4-deficient mice post-exercise, potentially through regulation of UCP3 expression. Our data demonstrate that endothelial-Nox4 is required for glucose and fatty acid oxidation, suggesting inter-tissue cross-talk between the endothelium and skeletal muscle in response to exercise.
Keywords: Exercise, Nox4, ROS, Metabolic adaptation, Skeletal muscle metabolism, Endothelium
Highlights
-
•
Nox4 and hydrogen peroxide (H2O2) activate genes involved in skeletal muscle metabolism after acute exercise.
-
•
Global deletion of Nox4 attenuates muscle glucose and fatty acid oxidation post-acute exercise.
-
•
Endothelial-specific deletion of Nox4 blunts acute adaptation to exercise in muscle suggesting inter-tissue cross-talk.
-
•
Nox4 is required for chronic exercise adaptation.
1. Introduction
Skeletal muscle is an extraordinarily plastic tissue that quickly responds to repeated contractions in an effort to supply sufficient adenosine triphosphate (ATP) for working muscle. Exercise is a potent strategy to reduce muscle wasting and prevent many chronic vascular and metabolic diseases. A single bout of exercise elicits the immediate upregulation of mRNA expression associated with metabolic adaptation, stimulation of glucose and fatty acid transport, and increased substrate oxidation (glucose and fatty acid) [1]. Consistent, repeated bouts of exercise over weeks to months (chronic exercise) increases angiogenesis and mitochondrial biogenesis and enhances glucose and lipid metabolism [1]. These changes in skeletal muscle metabolism are critically linked to skeletal muscle health and systemic metabolism. Therefore, understanding the initiating stimuli that trigger exercise-mediated metabolic adaptation is important to characterize the underlying molecular signaling involved in the health benefits of exercise.
Skeletal muscle produces reactive oxygen species (ROS) during exercise [2]. ROS levels are also increased in subjects with muscular diseases, leading to fatigue and atrophy [3]. Therefore, ROS production during exercise was initially thought to be solely deleterious. However, multiple studies have demonstrated that, in healthy adults, ROS-responsive signaling pathways are important for improved glucose metabolism and increased efficiency of mitochondrial function in response to exercise [4]. Specifically, human studies have demonstrated that quenching the ROS signal with antioxidant supplementation attenuates increased insulin sensitivity and mitochondrial biogenesis [5]. While studies have documented that exercise-induced ROS promote muscle adaptation to exercise, the source(s) and target(s) of these ROS are largely unknown.
Several lines of evidence suggest that exercise promotes ROS production in the skeletal muscle from multiple sources (Nox2, Nox4, mitochondria, and xanthine oxidase) [[6], [7], [8], [9], [10], [11]]. However, NADPH oxidase (Nox) enzymes contribute to cytosolic ROS production both at rest and during contraction [6]. The Nox family of enzymes transfers electrons from NADPH to molecular oxygen, producing ROS. Inhibition of Nox enzymes blocks both basal and stretch/contraction-stimulated skeletal muscle ROS production [6,10]. Skeletal muscle is a mixed tissue; its parenchyma consists of myofibers while its stromal composition includes myocytes, endothelial cells, pericytes, and immune cells. Thus, Nox's contribution to skeletal muscle ROS production is likely significantly influenced by its expression patterns in these different cell types.
Of the multiple types of ROS, hydrogen peroxide (H2O2) is an adept signaling molecule. H2O2 is a relatively stable oxidant that is able to cross membranes and react with protein thiol moieties to produce post-translational modifications, altering protein function [12]. Nox4 is unique among the Nox enzymes (Nox 1–5) as it primarily produces H2O2 [13,14] due to a unique third extracytosolic loop (E-loop) [13]. Initially, Nox4 was thought to be constitutively active and regulated at the transcriptional level; however, other reports have demonstrated that post-translational modifications introduce an alternative level of regulation [15]. Recent data demonstrated that Nox4 can be regulated by ATP levels as it contains an ATP-binding motif [16]. ATP can directly bind and negatively regulate Nox4 activity, suggesting that Nox4 can serve as a metabolic sensor and become activated with decreased mitochondrial ATP levels, which may be important in the response to exercise. Thus, we hypothesized that Nox4 may be responsible for initiating metabolic adaptations to exercise. Our findings demonstrate that Nox4 is responsible for initiating transcriptional changes and mediating glucose and fatty acid oxidation in skeletal muscle in response to acute exercise.
2. Materials and methods
2.1. Animals
Nox4-floxed (Nox4fl/fl) mice were obtained from Jun Sadoshima [17] and bred with the CMV-Cre line (Jackson Laboratories, Bar Harbor, ME; Jax 006054) to produce a line with global Nox4 deletion (Nox4−/−). C57Bl/6J mice (Jax 000664) were used as controls for the Nox4−/− mice. To produce the endothelial-specific Nox4 deletion, the Nox4-floxed mice were bred with Ve-cadherin Cre (Jax 006137). All of these mouse lines were back crossed for a minimum of 10 generations to C57Bl/6J mice. Male mice were used in all of the groups. All of the groups were allowed to eat ad libitum throughout the duration of the study (except during the time periods of exercise/sedentary). The animals were housed on a 12:12-h light–dark cycle in a temperature-controlled room at 25 °C. The Virginia Tech and University of Massachusetts Medical School Institutional Animal Care and Use Committee approved all of the procedures.
2.2. Exercise intervention
Exercise was conducted on a motorized treadmill (Columbus Instruments Model #1055-SRM-D58, Columbus, OH, USA). All of the mice were acclimated to the treadmill for 3 days prior to the exercise regimens. On day 1, the mice were allowed to stand on the stationary treadmill for 15 min. On day 2, the mice walked on the treadmill at 5 m/min for 15 min. On day 3, the mice began walking at 5 m/min; the treadmill speed was gradually increased to 15 m/min and the mice ran at this speed for 15 min. To control for any non-exercise effects of treadmill running (handling, novel environment, noise, and vibrations), the non-exercised group of mice (SED) was placed on the top of the treadmill apparatus for an identical period of time. The mice were not subjected to electric shock during the treadmill sessions to avoid stress.
The chronic exercise training consisted of treadmill running for 60 min/day at 18 m/min 5 days/week for 4 weeks. Tissue was harvested >24 h following the last exercise bout.
The exhaustive exercise protocol [18] began at 5 m/min for 15 min followed by gradual increases in speed at 3 min intervals until the treadmill speed reached 24.25 m/min, at which point it was held at this speed for 30 min or until the mice reached exhaustion. The state of exhaustion was established by a mouse remaining in the lower quarter of the treadmill 3 cumulative times despite gentle encouragement.
The acute exercise protocol consisted of a 60 min run beginning with a 4 min warm-up period from which the mice progressed from 0 to 20 m/min. The mice were then exercised at 20 m/min (∼85% VO2 max) [19] for the remainder of the 60 min protocol. The mice were fasted for 4 h prior to exercise to eliminate any acute metabolic changes due to food and assigned to either a non-exercise control group or an exercise group. Previous studies showed that healthy young C57BL/6 mice participating in this exercise protocol completed the 60 min run close to 100% of the time [20].
2.3. Glucose tolerance test
The mice were fasted for 12 h and glucose (2 g/kg) was delivered by intraperitoneal injection. Blood samples were harvested from the tail vein at the indicated times, and glycemia was determined using a Bayer Breeze 2 glucometer. Data were plotted as milligrams (mg)/deciliters (dl) over time and the area under the curve (AUC).
2.4. RNA extraction and gene expression
Tissue was harvested and total RNA isolated using QIAzol (QIAGEN) and Qiagen RNeasy Mini Kit (RNeasy Mini Kit, QIAGEN, 74106). 1 ug of total RNA was used for one-step real-time reverse transcription PCR (iScript cDNA Synthesis Kit, 1708890, BioRad). For specific mRNA expression analysis, TaqMan and SYBR Green gene expresssion (Table 1 for SYBR Green primers) assays were used. The ΔΔcycle threshold method was used for relative mRNA quantification and the gene expression was normalized to the housekeeping gene (HPRT).
Table 1.
SYBR Green primer sequences.
| Gene | Forward Sequence | Reverse sequence |
|---|---|---|
| Hprt | GGACTAATTATGGACAGGACTG | GCTCTTCAGTCTGATAAAATCTAC |
| Sik1 | CTGTAGGCTACCCACCTCCT | GCGAGTCAGAAGGGTTGACA |
| Mt2 | TCACCACGACTTCAACGTCC | GTTGGGGTCCATTCCGAGAT |
| Nr4a3 | TGCTGCAAAGTGTAACCCAGA | ACATCTCAAGCCCTGTCACC |
| Slc25a25 | CACGTGTGTACCACTCTGCT | TGCCGTTCCCTCTGTTTCTG |
| Pdk4 | CCGAAGCTGATGACTGGTGT | CTTCTCCCGGGTCATCCAAC |
| Pgc-1α | GACAGGTGCCTTCAGTTCAC | CAACCAGAGCAGCACACTCTA |
| Hk2 | GCCACCAGACGAAACTGGAT | TGTCAAAGTCCCCTCTGCG |
| Ucp3 | CCATGATACGCCTGGGAACT | CTGGCGATGGTTCTGTAGGC |
2.5. Microarray analysis
RNA was harvested from gastrocnemius (GC) and isolated as described above. The RNA quality was assessed using an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA). Only samples with an RNA integrity number higher than 7.5 and normal 18-s and 28-s fractions on microfluidic electrophoresis were used. Second-strand cDNA was labeled with an Affymetrix WT terminal labeling kit, and the samples were hybridized to Affymetrix Mouse Gene 1.0 ST arrays (Affymetrix, Santa Clara, CA, USA). Pathway analysis of the transcriptomic data was conducted using Ingenuity Pathway Analysis (IPA, Qiagen). Heatmapper (http://www.heatmapper.ca) was used to create the heat maps [21].
2.6. Adenoviral constructs
An adenoviral vector expressing CMV-driven catalase (Ad-Cat) was a gift from Dr. Kai Chen [22]. An adenoviral vector expressing the control virus, adenoviral CMV GFP (Ad-GFP), was purchased from Vector Biolabs (Cat 1060). For the in vivo administration of adenovirus, 5 days before exercise, either Ad-GFP or Ad-Cat (2 x 108 plaque-forming units) was injected into the gastrocnemius (GC).
2.7. Protein expression
GC was collected and homogenized in lysis buffer (25 mM of HEPES, pH 7.0, and 0.4% CHAPS) with Calbiochem Protease Inhibitor Cocktail Set III (EMD Biosciences, La Jolla, CA, USA) for immunoblotting. Proteins (10 μg loaded) were separated by SDS-Page and transferred onto PVDF membranes. The membranes were blocked in 5% non-fat dry milk or bovine serum albumin (BSA), washed in tris-buffered saline containing 0.1% Tween-20 (TBST), and then placed in primary antibody: UCP3 (PA1-055, Invitrogen), green fluorescent protein (GFP, ab290, Abcam), and catalase (MAB3398, R&D Systems). The membranes were then incubated in secondary antibody (donkey anti-rabbit, 711-035-152, 1:5000, Jackson ImmunoResearch) or goat anti-mouse (LI-COR 1:10,000) and imaged on a Bio-Rad ChemiDoc MP or LI-COR Odyssey CLx. Blots were analyzed using ImageJ (https://imagej.nih.gov/ij/index.html) for densitometry and normalized to total protein (control) as quantified using No-Stain Protein-Labeling Reagent (A44449, Invitrogen), Ponceau S (59803S, Cell Signaling Technologies) or pan-actin (mAb 8456, Cell Signaling Technologies).
2.8. Substrate oxidation
GC were excised and washed in cold PBS. Approximately 15–50 mg of muscle was placed in 200 μL of modified sucrose EDTA medium (SET buffer) containing 250 mM of sucrose, 1 mM of EDTA, 10 mM of Tris–HCl, and 1 mM of ATP at a pH of 7.4. The muscle samples were minced with scissors followed by adding SET buffer to produce a final 20-fold dilution (wt:vol). The samples were then homogenized in a Potter-Elvehjem glass homogenizer for 10 passes over 30 s at 150 RPM with a motor-driven Teflon pestle.
Fatty acid oxidation was assessed using radiolabeled fatty acid ([1-14C]- palmitic acid, American Radiolabeled Chemicals, St. Louis, MO, USA) to quantify 14CO2 production and 14C-labeled acid-soluble metabolites. The samples were incubated in 0.5 μCi/mL of [1-14C]-palmitic acid for 1 h after which the media was acidified with 200 μL of 70% perchloric acid for 1 h to liberate 14CO2. The 14CO2 was trapped in a tube containing 1 M of NaOH, which was then placed into a scintillation vial with 5 mL of scintillation fluid. The 14C concentrations within the vials were measured on a 4500 Beckman Coulter scintillation counter. Acid soluble metabolites were determined by collecting the acidified media and measuring the 14C levels as previously described [23].
Glucose oxidation was measured utilizing a similar method to that of fatty acid oxidation with the exception of the substitution of [U–14C]-glucose for [1-14C]-palmitic acid. The total protein content in the skeletal muscle homogenates was measured via a bicinchoninic acid procedure (Thermo Fisher Scientific, Waltham, MA, USA) and used to normalize the oxidation values.
2.9. Metabolic enzyme assays
The activity of citrate synthase (CS), a biochemical marker of mitochondrial density and oxidative capacity [24], and beta-hydroxyacyl-coA-dehydrogenase (BHAD), a key regulatory enzyme in the beta oxidation of fatty acids to acetyl-CoA, were determined spectrophotometrically in muscle homogenates [25]. Briefly, CS activity was measured at 37 °C in 0.1 M of Tris–HCl (pH 8.3) assay buffer containing 0.12 mM of 5,5′-dithio-bis (2-nitrobenzoic acid) and 0.6 mM of oxaloacetate. After an initial 2-min absorbance reading taken at 412 nm, the reaction was initiated by adding 3.0 mM of acetyl-CoA, and the change in absorbance was measured every 10 s for 7 min. BHAD activity was measured at 37 °C in assay buffer containing 0.1 M of triethanolamine-HCl, 5 mM of EDTA, and 0.45 mM of NADH (pH 7.0). After an initial 2-min absorbance reading at 340 nm, the reaction was initiated by adding 0.1 mM of acetoacetyl-CoA, and the change in absorbance was measured every 10 s for 5 min. Maximal enzymatic activity is presented in μM/mg of protein/min.
2.10. Cell culture
Mouse lung endothelial cells were prepared by immunoselection with anti-ICAM-2 antibody as previously described [26] and used for experiments during passages 2–6. The mouse skeletal muscle cell line C2C12 was purchased from ATCC and differentiated after reaching confluence by switching the medium to 2% horse serum and 1 μM of insulin. Differentiation media was changed every 24 h for the next 3 days.
2.11. Statistics
The results are expressed as means ± SEM. Data were analyzed by GraphPad Prism 8.0 software (GraphPad Software, La Jolla, CA, USA) using Student's t-test, one-way analysis of variance (ANOVA), or two-way ANOVA with the Newman–Keuls post hoc test as appropriate. Differences were considered significant when p < 0.05∗.
3. Results
3.1. Acute exercise transcriptional responses were influenced by hydrogen peroxide signaling
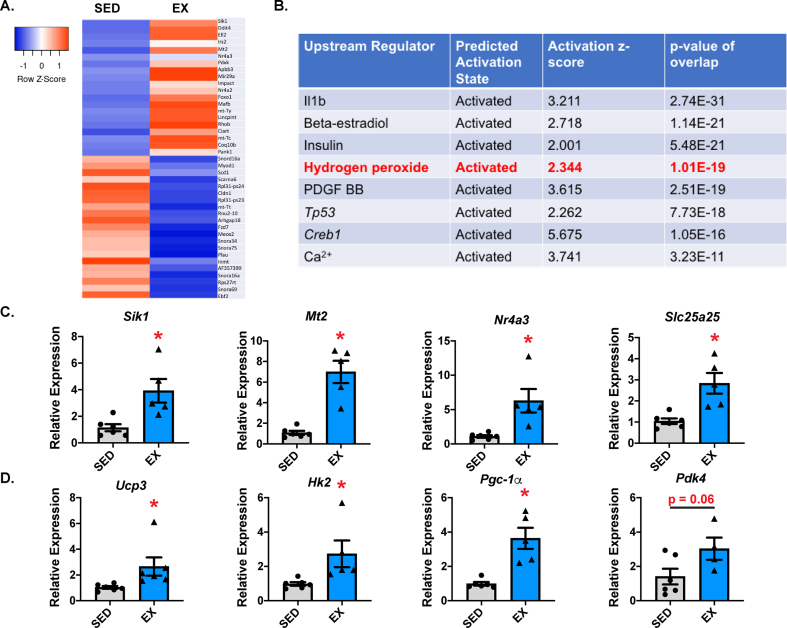
After an acute bout of exhaustive exercise, multiple metabolic changes occur in the skeletal muscle, such as increased fatty acid and glucose metabolism [1]. To gain insight into the immediate signals that initiate the change in metabolism after exercise, we utilized a single bout of treadmill exercise on C57Bl/6J wild-type (WT) mice and examined the gastrocnemius (GC) transcriptomic profiles of the exercised (EX) mice compared to sedentary (SED) controls (Figure 1A). To elucidate the upstream pathways mediating these early gene changes, we utilized Ingenuity Pathway Analysis (IPA, Qiagen) (Figure 1B). Prominent among these activated pathways was hydrogen peroxide (H2O2) signaling. We confirmed our transcriptomic analysis using qPCR. Figure 1C shows the top genes from our microarray. Figure 1D confirms expression of genes involved in metabolic adaptation to acute exercise [27,28]. These genes include uncoupling protein 3 (Ucp3), known to be important for mediating substrate oxidation; hexokinase 2 (Hk2), which is involved in glycolysis; peroxisome proliferator-activated receptor gamma coactivator 1-alpha (Pgc-1α), which mediates multiple adaptations including mitochondrial biogenesis; and pyruvate dehydrogenase kinase 4 (Pdk4), which is involved in metabolic flexibility [[29], [30], [31], [32], [33]]. Together, these data demonstrated that acute exercise increased skeletal muscle expression of several genes involved in substrate oxidation, suggesting potential regulation by H2O2.
Figure 1.
Acute exercise mediated early transcriptional responses influenced by hydrogen peroxide. (A) Microarrays were performed on pooled (N = 3/group) gastrocnemius (GC) muscle from the WT mice after a bout of exhaustive exercise. A heat map of the top upregulated and downregulated genes is shown. (B) Ingenuity Pathway Analysis (IPA, Qiagen) identified the upstream regulators of the expression patterns after exercise. (C) qPCR confirmed top exercise-responsive genes and (D) exercise-responsive metabolic genes (N = 5–7/group; ∗p < 0.05 compared to SED). Data are presented as mean ± SEM.
3.2. Hydrogen peroxide was responsible for mediating a subset of genes involved in substrate metabolism after acute exercise
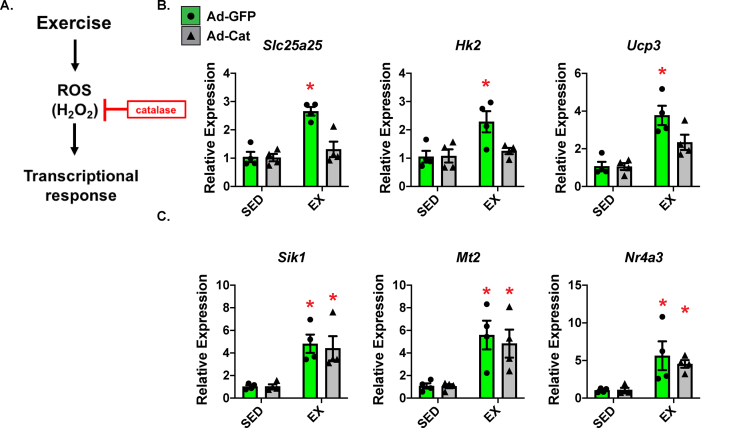
H2O2 is responsible for mediating multiple signaling pathways [34]. Our IPA results identified H2O2 signaling as an upstream pathway responsible for initiating transcriptional responses to exercise. Thus, we tested whether H2O2 signaling was important in the acute exercise response using catalase, which is a potent enzyme that reduces H2O2 to water (Figure 2A). For this experiment, we injected one hind leg (GC) with adenoviral catalase (Ad-Cat; Supplementary Figure 1) in the WT mice. The other hind leg was used as a control and injected with adenoviral GFP (Ad-GFP). We observed robust protein expression of both GFP and catalase (Supplementary Figures 1B and C). Gene expression of GFP and catalase was not different due to treatment (SED vs EX; Supplementary Figures 1B and C). We then examined the exercise-responsive genes by comparing the Ad-Cat injected leg to the control Ad-GFP injected leg. We found that a subset of genes were specifically modified by H2O2 after acute exercise (Slc25a25, Hk2, and Ucp3; Figure 2B), whereas other genes (Sik1, Mt2, and Nr4a3), were not altered in expression by adding catalase (Figure 2C).
Figure 2.
Hydrogen peroxide was responsible for mediating a subset of genes involved in metabolism after acute exercise. (A) We utilized catalase, which is a potent enzyme that reduces H2O2 to water to investigate the influence of H2O2 (shown in the schematic). Adenoviral catalase (Ad-Cat) was injected into one leg and control virus (adenoviral GFP; Ad-GFP) was injected into the other leg. Five days post-injection, the treadmill exercised mice were examined by qPCR for gene expression of (B) H2O2-responsive and (C) H2O2-unresponsive transcripts (N = 4/group; ∗p < 0.05 compared to SED or indicated control leg (paired comparison)). Data are presented as mean ± SEM.
3.3. Metabolic adaptation to exercise required Nox4
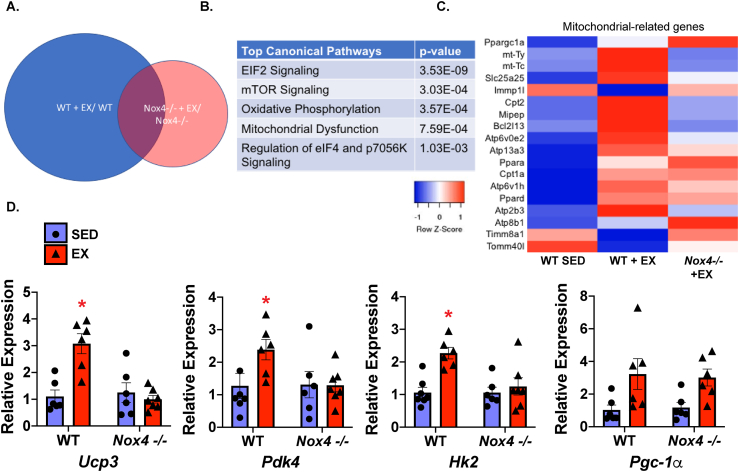
As Nox4 is a prominent H2O2 producer in the skeletal muscle, we utilized mice with global Nox4 deletion (Nox4−/−; Supplementary Figure 2) and investigated the genes that changed with exercise. To identify pathways that were specifically influenced by Nox4 with exercise, we compared the Nox4−/− EX/SED gene profile with the control WT EX/SED gene profile and used IPA to determine the top canonical pathways modified by exercise in the WT mice that were not activated in the Nox4−/− mice. These data demonstrated that genes involved in oxidative phosphorylation and mitochondrial metabolism were not changed with exercise in the mice lacking Nox4 (Figures 3A–C). To further investigate the transcriptome profile of the Nox4−/− mice after exercise, we examined their gene expression using qPCR (Figure 3D). We found that Nox4 expression was important for the exercise response of Ucp3, Pdk4, and Hk2, but not Pgc-1α.
Figure 3.
Nox4 mediated early transcriptional responses after acute exercise. (A) Microarrays were performed on pooled (N = 3/group) gastrocnemius (GC) muscle in each group and pathways activated in the WT + EX mice that were not activated by Nox4−/− + EX were identified. (B) Using IPA (Qiagen), the top canonical pathways driven by Nox4 (activated only in the WT + EX group and not in the Nox4−/− + EX) were identified. (C) Mitochondrial-related genes are shown in the heat map (from left to right: WT SED; WT + EX; Nox4-/-+ EX). (D) qPCR confirmed changes in metabolism-related genes: uncoupling protein 3 (Ucp3), hexokinase 2 (Hk2), pyruvate dehydrogenase kinase 4 (Pdk4), and peroxisome proliferator-activated receptor gamma coactivator 1-alpha (Pgc-1α) (N = 6–7/group; ∗p < 0.05 compared to SED). Data are presented as mean ± SEM.
As our data suggested Nox4 plays an important role in metabolic adaptation to acute exercise, we determined if the Nox4-deficient mice had a similarly blunted response to chronic exercise. Indeed, the WT mice significantly increased the distance and time to exhaustion after chronic exercise training, but this response was significantly blunted in the Nox4-deficient mice (Supplementary Figures 3A and B). We measured enzymes important for metabolic adaptation to exercise. Citrate synthase (CS) is the first enzyme in the tri-carboxylic acid (TCA) cycle and a known marker of adaptation to chronic exercise [35]. In the red GC, we found that CS enzyme activity was significantly increased in the WT but not in the Nox4-deficient mice (Supplementary Figures 3C and D). We similarly examined beta-hydroxyacyl-coA-dehydrogenase (BHAD) activity as this enzyme is important for fatty acid oxidation and its enzyme activity increases with exercise [36]. The WT-exercised red GC had a significant increase in BHAD activity that was not seen in the Nox4−/− mice (Supplementary Figures 3E and F). Together, these data suggest the impairment of the immediate responses to exercise in the mice lacking Nox4 resulted in defects in chronic adaptation to exercise.
3.4. Endothelial Nox4 was required for substrate oxidation following acute exercise
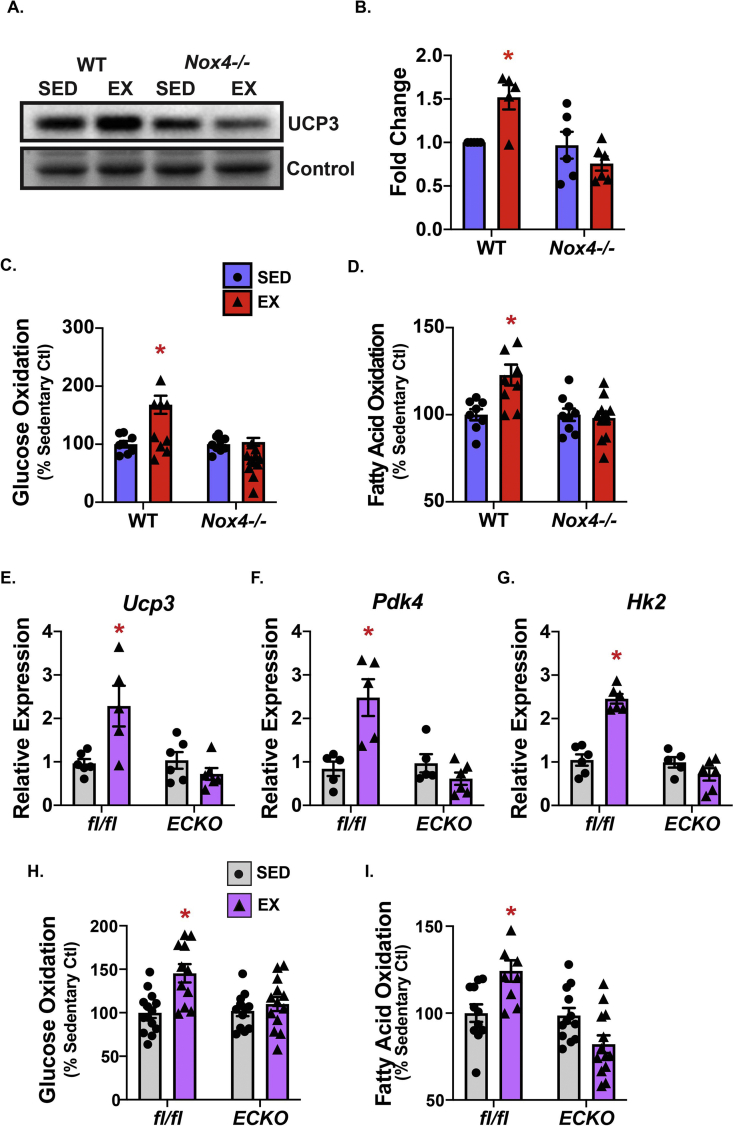
Recovery from exercise is a metabolic state involving increased reliance on fatty acid metabolism and the elevated expression of uncoupling protein 3 (UCP3) [37]. H2O2 (Figure 2B) and Nox4 (Figure 3D) are important for the expression of Ucp3 following exercise. Therefore, we examined UCP3 protein expression and found that the WT mice had a significant increase in UCP3 after exercise, which was significantly blunted in the mice lacking Nox4 (Figures 4A and B).
Figure 4.
Endothelial Nox4 was required for substrate oxidation in response to acute exercise. The mice were subjected to an acute bout of exercise. Red gastrocnemius (GC) was harvested 3 h after exercise and immediately analyzed for (A) UCP3 expression shown by immunoblotting and (B) quantified using ImageJ (N = 5–6; ∗p < 0.05 compared to SED). (C) Glucose oxidation and (D) fatty acid oxidation (N = 8–11/group; ∗p < 0.05 compared to SED). We crossed our Nox4-floxed (Nox4fl/fl) mice with our Ve-cadherin Cre mouse line, resulting in endothelial-specific Nox4 deletion (Nox4ECKO). The mice were subjected to acute exercise and GC was harvested for (E) relative expression changes in metabolism-related genes: mitochondrial uncoupling protein 3 (Ucp3), (F) pyruvate dehydrogenase 4 (Pdk4), (G) hexokinase 2 (Hk2), (H) glucose oxidation, and (I) fatty acid oxidation (N = 11–14/group; ∗p < 0.05 compared to SED). Data are presented as mean ± SEM.
Changes in UCP3 expression are known to modulate substrate oxidation [38]. Therefore, we investigated both glucose and fatty acid oxidation following a single acute bout of exercise. In the WT mice, we found a significant increase in glucose oxidation that was blunted in the Nox4−/− mice (Figure 4C). Similarly, we found that fatty acid oxidation significantly increased in the WT mice after acute exercise and this response was blunted in the Nox4-deficient mice (Figure 4D).
Given the heterogenous cell populations within skeletal muscle, we wanted to understand the cell type-specific mediation of the changes in substrate oxidation. Nox4 is highly expressed in the endothelium (Supplementary Figure 4A). Therefore, we hypothesized that it may be endothelial Nox4 that principally mediates the response to acute exercise. To investigate this, we utilized Ve-cadherin-driven Cre mice and crossed them with our Nox4-floxed mice to generate endothelial cell (EC)-specific Nox4 deletion (Nox4ECKO). With Nox4 deleted in the endothelium alone, the mixed skeletal muscle expression of Nox4 decreased by ∼80%, indicating that Nox4 was more highly expressed in the endothelial cells than myocytes in vivo (Supplementary Figure 4B). We also found that in the red gastrocnemius, which is highly vascularized compared to the white GC, Nox4 was more highly expressed (Supplementary Figure 4C), which also aligns with UCP3 expression [39]. To determine whether endothelial Nox4 deletion drives gene expression similar to global deletion, we examined the expression of Ucp3, Pdk4, and Hk2. Following exercise, expression of these genes was blunted in Nox4ECKO (Figures 4E–G). Next, to assess the effect of endothelial Nox4 deletion on functional metabolism, we measured glucose and fatty acid oxidation. We found that the mice deficient in endothelial Nox4 phenocopied the global Nox4 deletion as both glucose and fatty acid oxidation were similarly blunted after exercise (Figures 4H and I), suggesting that endothelial Nox4 may be important for driving the acute increase in substrate oxidation in skeletal muscle after exercise.
4. Discussion
In this study, we investigated the metabolic pathways initiated by exercise and uncovered a novel role of Nox4 in the regulation of skeletal muscle metabolism. Our primary observations demonstrated that Nox4 influenced the acute expression of key metabolic transcripts following exercise. Loss of Nox4 blunted UCP3 expression and impaired glucose and fatty acid oxidation post-exercise. Endothelial Nox4 deletion similarly blunted glucose and fatty acid oxidation post-exercise, suggesting an important role of the endothelium in mediating the early metabolic responses to exercise.
By analyzing the transcriptomic profile of the WT mice, we identified H2O2 as an important upstream regulator of the skeletal muscle response to exercise. We then confirmed these observations using single-leg injections of catalase (an H2O2 scavenger). Previous studies supported our data by showing that the formation of H2O2 occurs in contracting skeletal muscle [40] and during acute exercise [41]. In addition, there are multiple H2O2-responsive transcription factors such as Ap-1, Nrf2, Creb, Hif-1, and Nf-kB [42] that are essential for the metabolic response to exercise and subsequent adaptation. We found that Nox4 deletion mediated similar gene expression profiles to catalase expression, indicating that Nox4 may be responsible, in part, for the exercise-induced H2O2 initiation of metabolic gene expression. Nox4 predominantly produces H2O2 [13,14] and may serve as a metabolic sensor during acute exercise. Nox4 was previously thought to be constitutively active; however, a recent publication found that Nox4 directly binds ATP [16], and with decreased mitochondrial ATP, Nox4 activity increases. In terms of acute exercise, this mechanism of Nox4 activation would make sense when Nox4-dependent ROS production may be driven by decreased mitochondrial ATP.
UCP3 is a major regulator of ROS homeostasis and mitochondrial metabolism [43]. The UCP3 gene is highly responsive to changes in the metabolic environment, including diet and exercise, and in both humans and rodents, expression increases following exercise [29,44]. ROS are known to promote the expression of Ucp3 [45] and specifically H2O2 increases UCP3 expression [46]. This is supported by our data, which demonstrated that exercise-induced Ucp3 expression was blunted in the presence of catalase or with deletion of Nox4 following exercise. It has been proposed that UCP3 abundance correlates with the degree of fatty acid oxidation [37] which occurs during the recovery phase following an acute bout of exercise [38]. We observed diminished UCP3 expression and blunted substrate oxidation in Nox4-deficient mice after acute exercise. Taken together, these observations suggest that the induction of UCP3 may be in response to the increased production of ROS, which drives post-exercise increases in substrate oxidation [47].
Our data represent a new avenue of investigation in which Nox4 is responsible for initiating immediate metabolic changes in glucose and fatty acid oxidation in skeletal muscle. A previous study demonstrated the importance of Nox4 in acute exercise adaptation in the heart in which Nox4-deficient mice exhibit reduced mitochondrial antioxidant capacity as shown through the decreased activation of the ROS-regulated transcription factor nuclear factor erythroid 2 related factor (Nrf2) [48]. While Nrf2 was not identified as an upstream regulator in our studies, we cannot rule out the possibility that Nox4 activation of Nrf2 occurs in the skeletal muscle, which may be important for some of the downstream metabolic effects in our models. Our results regarding the requirement for Nox4 to drive fatty acid oxidation resonate with findings demonstrating that overexpression of Nox4 promotes fatty acid oxidation in heart failure [49]. Interestingly, in contrast to our loss-of-function data, Nox4 overexpression in the heart did not impact glucose oxidation.
Chronic exercise adaptation is the result of multiple acute bouts of exercise; we found that Nox4 was necessary for adaptive responses to chronic exercise. Given ROS are short-acting molecules; our hypothesis was that Nox4 initiated immediate metabolic changes that ultimately resulted in chronic adaptation. With chronic exercise, previous studies have documented that Nox4 is required for increased vascularization [50], which is consistent with studies from our lab and others demonstrating that Nox4 increases angiogenesis in a mouse model of peripheral artery disease [26,51]. Importantly, one study concluded that Nox4 is dispensable for exercise-induced muscle fiber type switching [52]. This observation may appear to contradict the current findings, which demonstrated significant adaptations in enzymatic activity (CS and BHAD) to chronic endurance-type exercise in the WT mice that was blunted in the Nox4−/− mice. However, it is important to note that the pathways that drive exercise-induced fiber-type switching may differ from those driving metabolic adaptation [53]. In support of our findings with chronic exercise, a recent publication similarly reported that Nox4 promoted exercise adaptation in obese mice, demonstrating increased muscle citrate synthase activity in response to chronic exercise [54]. Future studies will be important to address the interactions between physiologic and pathologic ROS production in skeletal muscle with exercise.
To meet increases in energy demand with exercise, multiple intracellular processes need to occur at different subcellular locations (plasma membrane, cytosol, and mitochondria) in parallel [1]. This suggests that localized and/or compartmentalized ROS production may be important for the spatial and temporal resolution of metabolic adaptation to different modes of exercise (duration, intensity, and type). In this study, our data demonstrated the role of Nox4 in oxidative metabolism, which suggests a deficit in mitochondrial function, although this speculation will require further study. Recent reports have demonstrated skeletal muscle Nox2 as a major producer of cytosolic ROS in acute exercise, which is important for acute glucose uptake [11] and metabolic stress-responsive gene expression [55]. One study examining loss of Nox2 in both acute and chronic high-intensity exercise [56] suggested that there was crosstalk between Nox2 and mitochondrial ROS, emphasizing the potential coordination between different sites of ROS production in skeletal muscle responses to exercise. It is likely that Nox2 and Nox4 are responsible for mediating specific signaling pathways via ROS production in different subcellular microdomains, leading to localized metabolic signaling responses [57]. In the current study, we did not find any differences in Nox2 expression in any of our models; however, as Nox2 is mainly regulated by complex assembly [58], we cannot rule out that a subset of these metabolic effects was due to Nox2. Given the potential crosstalk between Nox4 and Nox2, it will be interesting to further delineate the relative contributions of these Nox members in different modes of exercise and specific subcellular microdomains.
An additional factor that may be important for defining the metabolic role of Nox in exercise adaptation is the relative expression and activity of the Nox members in skeletal muscle. We primarily examined the GC, which is composed of both red (oxidative) and white (glycolytic) muscle. Nox4 mRNA expression (Supplementary Figure 4) and activity is higher in red GC than white GC [39]. Furthermore, mRNA expression patterns of genes involved in energy metabolism, such as Ucp3, follow a similar pattern to Nox4 expression in red GC compared to white GC [39,59]. This was further supported by our findings that glucose and fatty acid oxidation were blunted after acute exercise in red GC in both the Nox4−/− and Nox4ECKO mice. Interestingly, our chronic exercise studies similarly demonstrated a robust influence of Nox4 expression on enzyme activity in red GC but not in white GC (Supplementary Figure 3). It is likely that there are multiple sources of ROS production both during and post-exercise that mediate discrete signaling niches based on their localization and expression profiles.
Importantly, we found that endothelial Nox4 may initiate substrate oxidation with acute exercise in muscle. Skeletal muscle is a mixed tissue composed of myocytes, endothelial cells, pericytes, and immune cells. Therefore, the Nox contribution to skeletal muscle ROS production could reflect expression patterns in these other cell types. Utilizing endothelial-specific Nox4 deletion, the total Nox4 mRNA level in the skeletal muscle significantly decreased (∼80%), suggesting that the endothelium expresses Nox4 much more highly than the myocytes. Indeed, we found that in vitro, Nox4 was expressed much more significantly in the microvascular endothelial cells than the myocytes. We found a comparable impact on gene expression and substrate oxidation between the endothelial-specific Nox4 and global Nox4 deletion. These data demonstrated that endothelial Nox4 is important for influencing exercise-induced immediate changes to skeletal muscle gene expression and substrate oxidation. Indeed, a significant relationship between the endothelium and skeletal muscle in regard to metabolism has been demonstrated as multiple studies showed that the oxidative capacity of muscles correlates with capillary density [60,61]. This is in line with the fact that patients with severe reductions in blood flow reportedly have decreased oxidative capacity and impaired TCA enzyme activity, which is likely involved in the development of muscle atrophy [62,63]. While no basal differences in skeletal muscle capillarization have been observed in mice lacking Nox4 [50], there may be paracrine signaling between the two tissues that is responsible for the endothelial Nox4-dependent metabolic adaptation observed herein. Studies have demonstrated that the skeletal muscle mitochondria closely line the vasculature [64]. This strategically places the skeletal muscle mitochondria adjacent to the endothelium to receive paracrine signals. Additional studies are needed to further define the inter-tissue crosstalk between the endothelium and muscle.
5. Conclusions
Our findings support the hypothesis that Nox4 is responsible for initiating important immediate metabolic signaling in the skeletal muscle response to exercise. Taken together, our results demonstrated that Nox4 serves as a catalyst for altered gene expression and subsequent glucose and fatty acid oxidation after acute exercise. Furthermore, we showed that endothelial Nox4 is required for the response in skeletal muscle, suggesting that inter-tissue crosstalk between the endothelium and muscle is important for metabolic responses to exercise.
Acknowledgments
This study was supported by K01AR073332 (to SMC) from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, HL098407 and HL151626 (to JFK) from the National Heart, Lung, and Blood Institute, and 16SDG29660007 (to SK) from the American Heart Association.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.molmet.2020.101160.
Conflict of interest
None declared.
Abbreviations
- Nox4
nicotinamide dinucleotide phosphate (NADPH) oxidase 4
- Nox4−/−
global Nox4 deletion
- Nox4fl/fl
Nox4 floxed
- Nox4ECKO
endothelial-specific deletion of Nox4
- Ad-Cat
adenoviral catalase
- Ad-GFP
adenoviral green fluorescent protein
- H2O2
hydrogen peroxide
- ROS
reactive oxygen species
- TCA cycle
tri-carboxylic acid cycle
- CS
citrate synthase
- BHAD
beta-hydroxyacyl-coA-dehydrogenase
- GC
gastrocnemius
- UCP3
uncoupling protein 3
- Hk2
hexokinase 2
- Pgc-1α
peroxisome proliferator-activated receptor gamma coactivator 1-alpha
- Pdk4
pyruvate dehydrogenase kinase 4
- Slc25a25
calcium-binding mitochondrial carrier protein
- Sik1
salt-inducible kinase 1
- Mt2
metallothionein 1
- Nr4a3
nuclear receptor subfamily 4 group A member 3
- Ap-1
activator protein 1
- Nrf2
nuclear factor erythroid 2-related factor 2
- Creb
CAMP-responsive element-binding protein 1
- Hif-1
hypoxia-inducible factor-1
- Nf-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Supplementary Figure 1: Ad-cat expression was not different between sedentary and exercise groups. (A) Adenoviral green fluorescent protein (Ad-GFP) was injected into the left leg (L) and adenoviral catalase (Ad-Cat) to inhibit H2O2 signaling was injected into the right leg (R). (B,C) Five days post-injection, treadmill exercised mice were examined by qPCR and immunoblotting for gene and protein expression. Data shown for qPCR are the DCt (dCt). Catalase protein expression was quantified by ImageJ densitometry in comparison to the Ad-GFP control leg for each mouse (N = 4/group). Data are presented as mean ± SEM. Supplementary Figure 2: Confirmation of Nox4 deletion. (A) Exon expression in WT vs. Nox4-/- mice. (B) mRNA expression of Nox4 and Nox2 in WT and Nox4-/-. (C) Protein expression of Nox4 in WT and Nox4-/- mice. No difference in (D) weight or (E) glucose tolerance test (GTT) or (F) GTT area under the curve (AUC) was observed in Nox4-/-mice (∗p < 0.05). Data are presented as mean ± SEM. Supplementary Figure 3: Enzymatic adaptation to chronic exercise was blunted inNox4-/-mice. We examined the influence of loss of Nox4 on adaptation to chronic exercise (4 weeks treadmill). (A) Distance and (B) time run to exhaustion was measured. (C,D) Red and white GC was assessed for activity of citrate synthase (E,F) and beta-hydroxyacyl-coA-dehydrogenase (BHAD) (N = 6-8/group; ∗p < 0.05 compared to WT SED; &p < 0.05 compared to WT + EX). Data are presented as mean ± SEM. Supplementary Figure 4: Nox4 expression in the endothelium.(A) Cells were harvested and qPCR of Nox4 expression measured. (N = 3; ∗p < 0.05). (B) mRNA expression of Nox4 from GC. (C) Nox4 expression in red vs. white GC. Data are presented as mean ± SEM.
References
- 1.Egan B., Zierath J.R. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metabolism. 2013;17(2):162–184. doi: 10.1016/j.cmet.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 2.Davies K.J., Quintanilha A.T., Brooks G.A., Packer L. Free radicals and tissue damage produced by exercise. Biochemical and Biophysical Research Communications. 1982;107(4):1198–1205. doi: 10.1016/s0006-291x(82)80124-1. [DOI] [PubMed] [Google Scholar]
- 3.Powers S.K., Morton A.B., Ahn B., Smuder A.J. Redox control of skeletal muscle atrophy. Free Radical Biology & Medicine. 2016;98:208–217. doi: 10.1016/j.freeradbiomed.2016.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thirupathi A., de Souza C.T. Multi-regulatory network of ROS: the interconnection of ROS, PGC-1 alpha, and AMPK-SIRT1 during exercise. Journal of Physiology & Biochemistry. 2017;73(4):487–494. doi: 10.1007/s13105-017-0576-y. [DOI] [PubMed] [Google Scholar]
- 5.Ristow M., Zarse K., Oberbach A., Klöting N., Birringer M., Kiehntopf M. Antioxidants prevent health-promoting effects of physical exercise in humans. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(21):8665–8670. doi: 10.1073/pnas.0903485106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sakellariou G.K., Vasilaki A., Palomero J., Kayani A., Zibrik L., McArdle A. Studies of mitochondrial and nonmitochondrial sources implicate nicotinamide adenine dinucleotide phosphate oxidase(s) in the increased skeletal muscle superoxide generation that occurs during contractile activity. Antioxidants and Redox Signaling. 2013;18(6):603–621. doi: 10.1089/ars.2012.4623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xia R., Webb J.A., Gnall L.L.M., Cutler K., Abramson J.J. Skeletal muscle sarcoplasmic reticulum contains a NADH-dependent oxidase that generates superoxide. American Journal of Physiology - Cell Physiology. 2003;285(1):C215–C221. doi: 10.1152/ajpcell.00034.2002. [DOI] [PubMed] [Google Scholar]
- 8.Kang C., O'Moore K.M., Dickman J.R., Ji L.L. Exercise activation of muscle peroxisome proliferator-activated receptor-gamma coactivator-1alpha signaling is redox sensitive. Free Radical Biology & Medicine. 2009;47(10):1394–1400. doi: 10.1016/j.freeradbiomed.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 9.Pattwell D.M., Patwell D.M., McArdle A., Morgan J.E., Patridge T.A., Jackson M.J. Release of reactive oxygen and nitrogen species from contracting skeletal muscle cells. Free Radical Biology & Medicine. 2004;37(7):1064–1072. doi: 10.1016/j.freeradbiomed.2004.06.026. [DOI] [PubMed] [Google Scholar]
- 10.Javeshghani D., Javesghani D., Magder S.A., Barreiro E., Quinn M.T., Hussain S.N.A. Molecular characterization of a superoxide-generating NAD(P)H oxidase in the ventilatory muscles. American Journal of Respiratory and Critical Care Medicine. 2002;165(3):412–418. doi: 10.1164/ajrccm.165.3.2103028. [DOI] [PubMed] [Google Scholar]
- 11.Henríquez-Olguín C., Knudsen J.R., Raun S.H., Li Z., Dalbram E., Treebak J.T. Cytosolic ROS production by NADPH oxidase 2 regulates muscle glucose uptake during exercise. Nature Communications. 2019;10(1) doi: 10.1038/s41467-019-12523-9. 4623–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sies H., Jones D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nature Reviews Molecular Cell Biology. 2020;294 doi: 10.1038/s41580-020-0230-3. 19683–21. [DOI] [PubMed] [Google Scholar]
- 13.Takac I., Schröder K., Zhang L., Lardy B., Anilkumar N., Lambeth J.D. The E-loop is involved in hydrogen peroxide formation by the NADPH oxidase Nox4. Journal of Biological Chemistry. 2011;286(15):13304–13313. doi: 10.1074/jbc.M110.192138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nisimoto Y., Diebold B.A., Constentino-Gomes D., Lambeth J.D. Nox4: a hydrogen peroxide-generating oxygen sensor. Biochemistry. 2014;53(31):5111–5120. doi: 10.1021/bi500331y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen F., Haigh S., Barman S., Fulton D.J.R. From form to function: the role of Nox4 in the cardiovascular system. Frontiers in Physiology. 2012;3:412. doi: 10.3389/fphys.2012.00412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shanmugasundaram K., Nayak B.K., Friedrichs W.E., Kaushik D., Rodriguez R., Block K. NOX4 functions as a mitochondrial energetic sensor coupling cancer metabolic reprogramming to drug resistance. Nature Communications. 2017;8(1):997. doi: 10.1038/s41467-017-01106-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuroda J., Ago T., Matsushima S., Zhai P., Schneider M.D., Sadoshima J. NADPH oxidase 4 (Nox4) is a major source of oxidative stress in the failing heart. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(35):15565–15570. doi: 10.1073/pnas.1002178107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Min S.Y., Learnard H., Kant S., Gealikman O., Rojas-Rodriguez R., DeSouza T. Exercise rescues gene pathways involved in vascular expansion and promotes functional angiogenesis in subcutaneous white adipose tissue. International Journal of Molecular Sciences. 2019;20(8) doi: 10.3390/ijms20082046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schefer V., Talan M.I. Oxygen consumption in adult and AGED C57BL/6J mice during acute treadmill exercise of different intensity. Experimental Gerontology. 1996;31(3):387–392. doi: 10.1016/0531-5565(95)02032-2. [DOI] [PubMed] [Google Scholar]
- 20.Hamada T., Arias E.B., Cartee G.D. Increased submaximal insulin-stimulated glucose uptake in mouse skeletal muscle after treadmill exercise. Journal of Applied Physiology (Bethesda, Md: 1985) 2006;101(5):1368–1376. doi: 10.1152/japplphysiol.00416.2006. [DOI] [PubMed] [Google Scholar]
- 21.Babicki S., Arndt D., Marcu A., Liang Y., Grant J.R., Maciejewski A. Heatmapper: web-enabled heat mapping for all. Nucleic Acids Research. 2016;44(W1):W147–W153. doi: 10.1093/nar/gkw419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen K., Kirber M.T., Xiao H., Yang Y., Keaney J.F. Regulation of ROS signal transduction by NADPH oxidase 4 localization. The Journal of Cell Biology. 2008;181(7):1129–1139. doi: 10.1083/jcb.200709049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frisard M.I., McMillan R.P., Marchand J., Wahlberg K.A., Wu Y., Voelker K.A. Toll-like receptor 4 modulates skeletal muscle substrate metabolism. The Australian Journal of Pharmacy: Endocrinology and Metabolism. 2010;298(5):E988–E998. doi: 10.1152/ajpendo.00307.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Larsen S., Nielsen J., Hansen C.N., Nielsen L.B., Wibrand F., Stride N. Biomarkers of mitochondrial content in skeletal muscle of healthy young human subjects. The Journal of Physiology. 2012;590(14):3349–3360. doi: 10.1113/jphysiol.2012.230185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heilbronn L.K., Civitarese A.E., Bogacka I., Smith S.R., Hulver M., Ravussin E. Glucose tolerance and skeletal muscle gene expression in response to alternate day fasting. Obesity Research. 2005;13(3):574–581. doi: 10.1038/oby.2005.61. [DOI] [PubMed] [Google Scholar]
- 26.Craige S.M., Chen K., Pei Y., Li C., Huang X., Chen C. NADPH oxidase 4 promotes endothelial angiogenesis through endothelial nitric oxide synthase activation. Circulation. 2011;124(6):731–740. doi: 10.1161/CIRCULATIONAHA.111.030775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Silveira L.R., Pilegaard H., Kusuhara K., Curi R., Hellsten Y. The contraction induced increase in gene expression of peroxisome proliferator-activated receptor (PPAR)-gamma coactivator 1alpha (PGC-1alpha), mitochondrial uncoupling protein 3 (UCP3) and hexokinase II (HKII) in primary rat skeletal muscle cells is dependent on reactive oxygen species. Biochimica et Biophysica Acta. 2006;1763(9):969–976. doi: 10.1016/j.bbamcr.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 28.Miotto P.M., Holloway G.P. Exercise-induced reductions in mitochondrial ADP sensitivity contribute to the induction of gene expression and mitochondrial biogenesis through enhanced mitochondrial H2O2 emission. Mitochondrion. 2019;46:116–122. doi: 10.1016/j.mito.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 29.Pilegaard H., Ordway G.A., Saltin B., Neufer P.D. Transcriptional regulation of gene expression in human skeletal muscle during recovery from exercise. American Journal of Physiology Endocrinology and Metabolism. 2000;279(4):E806–E814. doi: 10.1152/ajpendo.2000.279.4.E806. [DOI] [PubMed] [Google Scholar]
- 30.O'Doherty R.M., Bracy D.P., Granner D.K., Wasserman D.H. Transcription of the rat skeletal muscle hexokinase II gene is increased by acute exercise. Journal of Applied Physiology (Bethesda, Md: 1985) 1996;81(2):789–793. doi: 10.1152/jappl.1996.81.2.789. [DOI] [PubMed] [Google Scholar]
- 31.Koval J.A., DeFronzo R.A., O'Doherty R.M., Printz R., Ardehali H., Granner D.K. Regulation of hexokinase II activity and expression in human muscle by moderate exercise. American Journal of Physiology. 1998;274(2 Pt 1):E304–E308. doi: 10.1152/ajpendo.1998.274.2.E304. [DOI] [PubMed] [Google Scholar]
- 32.Zhang S., Hulver M.W., McMillan R.P., Cline M.A., Gilbert E.R. The pivotal role of pyruvate dehydrogenase kinases in metabolic flexibility. Nutrition and Metabolism. 2014;11(1):10. doi: 10.1186/1743-7075-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pilegaard H., Neufer P.D. Transcriptional regulation of pyruvate dehydrogenase kinase 4 in skeletal muscle during and after exercise. Proceedings of the Nutrition Society. 2004;63(2):221–226. doi: 10.1079/pns2004345. [DOI] [PubMed] [Google Scholar]
- 34.Sies H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: oxidative eustress. Redox Biology. 2017;11:613–619. doi: 10.1016/j.redox.2016.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holloszy J.O. Biochemical adaptations in muscle. Effects of exercise on mitochondrial oxygen uptake and respiratory enzyme activity in skeletal muscle. Journal of Biological Chemistry. 1967;242(9):2278–2282. [PubMed] [Google Scholar]
- 36.Schultz R.L., Kullman E.L., Waters R.P., Huang H., Kirwan J.P., Gerdes A.M. Metabolic adaptations of skeletal muscle to voluntary wheel running exercise in hypertensive heart failure rats. Physiological Research. 2013;62(4):361–369. doi: 10.33549/physiolres.932330. [DOI] [PubMed] [Google Scholar]
- 37.Pohl E.E., Rupprecht A., Macher G., Hilse K.E. Important trends in UCP3 investigation. Frontiers in Physiology. 2019;10:470. doi: 10.3389/fphys.2019.00470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anderson E.J., Yamazaki H., Neufer P.D. Induction of endogenous uncoupling protein 3 suppresses mitochondrial oxidant emission during fatty acid-supported respiration. Journal of Biological Chemistry. 2007;282(43):31257–31266. doi: 10.1074/jbc.M706129200. [DOI] [PubMed] [Google Scholar]
- 39.Loureiro A.C.C., do Rêgo-Monteiro I.C., Louzada R.A., Ortenzi V.H., de Aguiar A.P., de Abreu E.S. Differential expression of NADPH oxidases depends on skeletal muscle fiber type in rats. Oxidative Medicine & Cellular Longevity. 2016;2016(1):6738701–6738710. doi: 10.1155/2016/6738701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Silveira L.R., Pereira-Da-Silva L., Juel C., Hellsten Y. Formation of hydrogen peroxide and nitric oxide in rat skeletal muscle cells during contractions. Free Radical Biology & Medicine. 2003;35(5):455–464. doi: 10.1016/s0891-5849(03)00271-5. [DOI] [PubMed] [Google Scholar]
- 41.Wang P., Li C.G., Qi Z., Cui D., Ding S. Acute exercise induced mitochondrial H₂O₂ production in mouse skeletal muscle: association with p(66Shc) and FOXO3a signaling and antioxidant enzymes. Oxidative Medicine & Cellular Longevity. 2015;2015(1) doi: 10.1155/2015/536456. 536456–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marinho H.S., Real C., Cyrne L., Soares H., Antunes F. Hydrogen peroxide sensing, signaling and regulation of transcription factors. Redox Biology. 2014;2:535–562. doi: 10.1016/j.redox.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Azzu V., Mookerjee S.A., Brand M.D. Rapid turnover of mitochondrial uncoupling protein 3. Biochemical Journal. 2010;426(1):13–17. doi: 10.1042/BJ20091321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jones T.E., Baar K., Ojuka E., Chen M., Holloszy J.O. Exercise induces an increase in muscle UCP3 as a component of the increase in mitochondrial biogenesis. American Journal of Physiology Endocrinology and Metabolism. 2003;284(1):E96–E101. doi: 10.1152/ajpendo.00316.2002. [DOI] [PubMed] [Google Scholar]
- 45.Mailloux R.J., Harper M.-E. Uncoupling proteins and the control of mitochondrial reactive oxygen species production. Free Radical Biology & Medicine. 2011;51(6):1106–1115. doi: 10.1016/j.freeradbiomed.2011.06.022. [DOI] [PubMed] [Google Scholar]
- 46.Anedda A., López-Bernardo E., Acosta-Iborra B., Saadeh Suleiman M., Landázuri M.O., Cadenas S. The transcription factor Nrf2 promotes survival by enhancing the expression of uncoupling protein 3 under conditions of oxidative stress. Free Radical Biology & Medicine. 2013;61:395–407. doi: 10.1016/j.freeradbiomed.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 47.Jiang N., Zhang G., Bo H., Qu J., Ma G., Cao D. Upregulation of uncoupling protein-3 in skeletal muscle during exercise: a potential antioxidant function. Free Radical Biology & Medicine. 2009;46(2):138–145. doi: 10.1016/j.freeradbiomed.2008.09.026. [DOI] [PubMed] [Google Scholar]
- 48.Hancock M., Hafstad A.D., Nabeebaccus A.A., Catibog N., Logan A., Smyrnias I. Myocardial NADPH oxidase-4 regulates the physiological response to acute exercise. eLife. 2018;7:1347. doi: 10.7554/eLife.41044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nabeebaccus A.A., Zoccarato A., Hafstad A.D., Santos C.X., Aasum E., Brewer A.C. Nox4 reprograms cardiac substrate metabolism via protein O-GlcNAcylation to enhance stress adaptation. JCI Insight. 2017;2(24) doi: 10.1172/jci.insight.96184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vogel J., Kruse C., Zhang M., Schröder K. Nox4 supports proper capillary growth in exercise and retina neo-vascularization. The Journal of Physiology. 2015 doi: 10.1113/jphysiol.2014.284901. n/a–n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schröder K., Zhang M., Benkhoff S., Mieth A., Pliquett R., Kosowski J. Nox4 is a protective reactive oxygen species generating vascular NADPH oxidase. Circulation Research. 2012;110(9):1217–1225. doi: 10.1161/CIRCRESAHA.112.267054. [DOI] [PubMed] [Google Scholar]
- 52.Vogel J., Figueiredo de Rezende F., Rohrbach S., Zhang M., Schröder K. Nox4 is dispensable for exercise induced muscle fibre switch. PloS One. 2015;10(6) doi: 10.1371/journal.pone.0130769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rowe G.C., Patten I.S., Zsengeller Z.K., El-Khoury R., Okutsu M., Bampoh S. Disconnecting mitochondrial content from respiratory chain capacity in PGC-1-deficient skeletal muscle. Cell Reports. 2013;3(5):1449–1456. doi: 10.1016/j.celrep.2013.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brendel H., Shahid A., Hofmann A., Mittag J., Bornstein S.R., Morawietz H. NADPH oxidase 4 mediates the protective effects of physical activity against obesity-induced vascular dysfunction. Cardiovascular Research. 2020;116(10):1767–1778. doi: 10.1093/cvr/cvz322. [DOI] [PubMed] [Google Scholar]
- 55.Henríquez-Olguín C., Díaz-Vegas A., Utreras-Mendoza Y., Campos C., Arias-Calderón M., Llanos P. NOX2 inhibition impairs early muscle gene expression induced by a single exercise bout. Frontiers in Physiology. 2016;7:19587. doi: 10.1016/j.freeradbiomed.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Henríquez-Olguín C., Renani L.B., Arab-Ceschia L., Raun S.H., Bhatia A., Li Z. Adaptations to high-intensity interval training in skeletal muscle require NADPH oxidase 2. Redox Biology. 2019;24:101188. doi: 10.1016/j.redox.2019.101188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wojtovich A.P., Berry B.J., Galkin A. Redox signaling through compartmentalization of reactive oxygen species: implications for health and disease. Antioxidants and Redox Signaling. 2019;31(9):591–593. doi: 10.1089/ars.2019.7804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Buvelot H., Jaquet V., Krause K.-H. Mammalian NADPH oxidases. Methods in Molecular Biology (Clifton, N.J.) 2019;1982(Pt 1):17–36. doi: 10.1007/978-1-4939-9424-3_2. [DOI] [PubMed] [Google Scholar]
- 59.Osório Alves J., Matta Pereira L., Cabral Coutinho do Rêgo Monteiro I., Pontes dos Santos L.H., Soares Marreiros Ferraz A., Carneiro Loureiro A.C. Strenuous acute exercise induces slow and fast twitch-dependent NADPH oxidase expression in rat skeletal muscle. Antioxidants (Basel, Switzerland) 2020;9(1) doi: 10.3390/antiox9010057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hoppeler H., Hudlicka O., Uhlmann E. Relationship between mitochondria and oxygen consumption in isolated cat muscles. The Journal of Physiology. 1987;385(1):661–675. doi: 10.1113/jphysiol.1987.sp016513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kayar S.R., Hoppeler H., Mermod L., Weibel E.R. Mitochondrial size and shape in equine skeletal muscle: a three-dimensional reconstruction study. The Anatomical Record. 1988;222(4):333–339. doi: 10.1002/ar.1092220405. [DOI] [PubMed] [Google Scholar]
- 62.Clyne C.A., Mears H., Weller R.O., O'Donnell T.F. Calf muscle adaptation to peripheral vascular disease. Cardiovascular Research. 1985;19(8):507–512. doi: 10.1093/cvr/19.8.507. [DOI] [PubMed] [Google Scholar]
- 63.Brass E.P., Wang H., Hiatt W.R. Multiple skeletal muscle mitochondrial DNA deletions in patients with unilateral peripheral arterial disease. Vascular Medicine (London, England) 2000;5(4):225–230. [PubMed] [Google Scholar]
- 64.Glancy B., Hsu L.-Y., Dao L., Bakalar M., French S., Chess D.J. In VivoMicroscopy reveals extensive embedding of capillaries within the sarcolemma of skeletal muscle fibers. Microcirculation (New York, N.Y: 1994) 2014;21(2):131–147. doi: 10.1111/micc.12098. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: Ad-cat expression was not different between sedentary and exercise groups. (A) Adenoviral green fluorescent protein (Ad-GFP) was injected into the left leg (L) and adenoviral catalase (Ad-Cat) to inhibit H2O2 signaling was injected into the right leg (R). (B,C) Five days post-injection, treadmill exercised mice were examined by qPCR and immunoblotting for gene and protein expression. Data shown for qPCR are the DCt (dCt). Catalase protein expression was quantified by ImageJ densitometry in comparison to the Ad-GFP control leg for each mouse (N = 4/group). Data are presented as mean ± SEM. Supplementary Figure 2: Confirmation of Nox4 deletion. (A) Exon expression in WT vs. Nox4-/- mice. (B) mRNA expression of Nox4 and Nox2 in WT and Nox4-/-. (C) Protein expression of Nox4 in WT and Nox4-/- mice. No difference in (D) weight or (E) glucose tolerance test (GTT) or (F) GTT area under the curve (AUC) was observed in Nox4-/-mice (∗p < 0.05). Data are presented as mean ± SEM. Supplementary Figure 3: Enzymatic adaptation to chronic exercise was blunted inNox4-/-mice. We examined the influence of loss of Nox4 on adaptation to chronic exercise (4 weeks treadmill). (A) Distance and (B) time run to exhaustion was measured. (C,D) Red and white GC was assessed for activity of citrate synthase (E,F) and beta-hydroxyacyl-coA-dehydrogenase (BHAD) (N = 6-8/group; ∗p < 0.05 compared to WT SED; &p < 0.05 compared to WT + EX). Data are presented as mean ± SEM. Supplementary Figure 4: Nox4 expression in the endothelium.(A) Cells were harvested and qPCR of Nox4 expression measured. (N = 3; ∗p < 0.05). (B) mRNA expression of Nox4 from GC. (C) Nox4 expression in red vs. white GC. Data are presented as mean ± SEM.