Summary
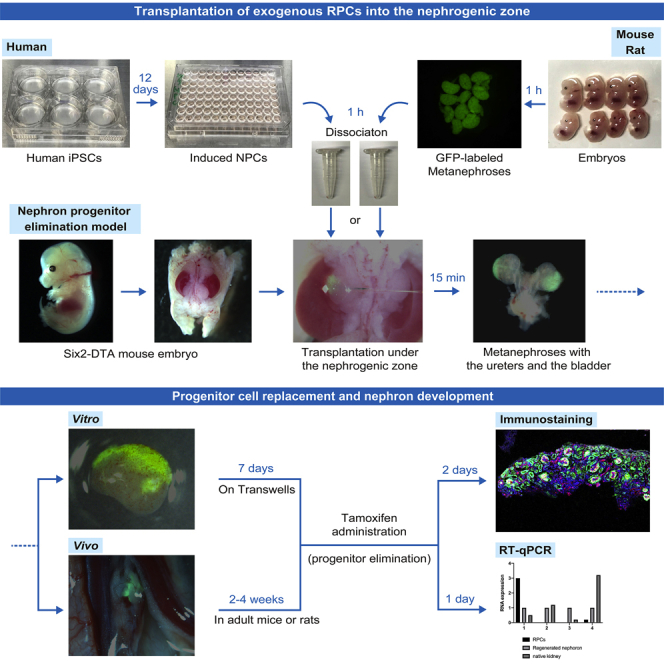
Renal progenitor cells induced from pluripotent stem cells have attracted attention as a cell source for organ regeneration. Here, we report an in vivo protocol for the regeneration of urine-producing nephrons, i.e., neo-nephrons, in mice. We outline steps to transplant exogenous renal progenitor cells into the nephrogenic zone of transgenic mice and subsequently analyze these neo-nephrons.
For complete details on the use and execution of this protocol, please refer to Fujimoto et al. (2020).
Subject areas: Cell isolation, Model organisms, Stem cells, Organoids
Graphical abstract
Highlights
-
•
Enzymatic dissociation of rodent MNs and induction of NPCs from hiPSCs
-
•
Injection of exogenous RPCs into the nephrogenic zone with the elimination of host NPCs
-
•
In vitro and in vivo regeneration of neo-nephrons within the mouse organ niche
-
•
Morphological, functional, and transcriptomic analyses of neo-nephrons
Renal progenitor cells induced from pluripotent stem cells have attracted attention as a cell source for organ regeneration. Here, we report an in vivo protocol for the regeneration of urine-producing nephrons, i.e., neo-nephrons, in mice. We outline steps to transplant exogenous renal progenitor cells into the nephrogenic zone of transgenic mice and subsequently analyze these neo-nephrons.
Before you begin
Preparation of media
Base media for metanephroses (MNs)
Prepare media for culturing MNs by supplementing MEM α with 20% fetal bovine serum (FBS) and 1% penicillin/streptomycin. The media should be stored at 4°C for up to 1 month.
Base media for human nephron progenitor cells (NPCs)
Prepare media for culturing human NPCs. The media comprises DMEM/F12 supplemented with 2% (vol/vol) B-27 minus vitamin A, 2 mM GlutaMAX, 1% (vol/vol) insulin-transferrin-selenium solution, 1% (vol/vol) non-essential amino acids, 90 μM 2-mercaptoethanol, and 0.5% (vol/vol) penicillin/streptomycin. The media should be stored at 4°C for up to 1 month.
Preparation of glass needles
Prepare glass needles for injections of exogenous renal progenitor cells (RPCs) into the nephrogenic zone. Sharpen thin-walled glass capillaries using a pipette puller, and then cut the end with micro-tweezers. Sterilize the prepared glass needles using heat (Figure 1A).
Figure 1.
Glass needles used to inject exogenous renal progenitor cells (RPCs) into the nephrogenic zone
(A) A glass needle connected to the aspirator tube assembly. The thin tip is filled with pellets.
(B) A mouth pipette, which comprises a glass needle, an aspirator tube assembly, and one 1-mL syringe.
(C) A glass needle connected to a motor-driven pen-type syringe pump.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Polyclonal rabbit anti-Six2 antibody | Proteintech | Cat# 11562-1-AP; RRID: AB_2189084 |
| Monoclonal rat anti-CK8 (Cytokeratin 8) antibody | DSHB | Cat# TROMA-I; RRID: AB_531826 |
| Polyclonal rabbit anti-WT1 antibody | Santa Cruz Biotechnology | Cat# sc-68880; RRID: AB_2241672 |
| Polyclonal goat anti-Nephrin antibody | Santa Cruz Biotechnology | Cat# sc-19000; RRID: AB_650006 |
| Polyclonal rabbit anti-CDH6 antibody | Sigma-Aldrich | Cat# HPA007456; RRID: AB_1078373 |
| Polyclonal rabbit anti-AQP1 (Aquaporin 1) antibody | Millipore | Cat# AB2219; RRID: AB_1163380 |
| Polyclonal goat anti-Megalin antibody | Santa Cruz Biotechnology | Cat# sc-16478; RRID: AB_2234897 |
| Monoclonal mouse anti-ECAD (E-Cadherin) antibody | BD | Cat# 610181; RRID: AB_397580 |
| Polyclonal rabbit anti-CD31 antibody | Abcam | Cat# ab28364; RRID: AB_726362 |
| Monoclonal rabbit anti-Pax8 antibody | Abcam | Cat# ab189249; RRID: AB_2801268 |
| Polyclonal rabbit anti-GFP (green fluorescent protein) antibody | MBL | Cat# MBL598; RRID: AB_591819 |
| Polyclonal chicken anti-GFP antibody | Abcam | Cat# ab13970; RRID: AB_300798 |
| Monoclonal mouse anti-Nuclei antibody | Millipore | Cat# MAB1281; RRID: AB_94090 |
| Monoclonal mouse anti-Vimentin [V9] antibody | Abcam | Cat# ab8069; RRID: AB_306239 |
| Biotinylated polyclonal goat anti-mouse ITGA8 antibody | R&D | Cat# BAF4076; RRID: AB_2128449 |
| APC-conjugated streptavidin | BioLegend | Cat# 405207 |
| PE-conjugated monoclonal mouse anti-human PDGFRα antibody | BioLegend | Cat# 323506; RRID: AB_2268113 |
| Chemicals, peptides, and recombinant proteins | ||
| MEM α | Gibco | Cat# 12561056 |
| Accutase | Innovative Cell Technologies | Cat# AT104-500 |
| Fetal bovine serum | GE Healthcare Life Science | Cat# SH3007.03 |
| iMatrix-511 | Nippi | Cat# 892012 |
| Stemfit AK02N | Ajinomoto | Cat# RCAK02N |
| Y27632 | Wako | Cat# 257-00511 |
| Activin A | R&D | Cat# 338-AC |
| FGF-basic | R&D | Cat# 233-FB |
| CHIR | Axon | Cat# AXN1386-00 |
| Bmp4 | R&D | Cat# 314-BP |
| Retinoic acid | Sigma-Aldrich | Cat# R-2625-50MG |
| FGF-9 | R&D | Cat# 273-F9-02 |
| Neuron Dissociation Solutions kit | Wako | Cat# 291-78001 |
| DMEM/F12 | Gibco | Cat# 11320033 |
| B-27 minus vitamin A | Gibco | Cat# 12587010 |
| GlutaMAX | Gibco | Cat# 35050061 |
| Insulin-transferrin-selenium solution | Gibco | Cat# 41400045 |
| MEM non-essential amino acids solution (100×) | Gibco | Cat# 11140050 |
| 2-Mercaptoethanol | Gibco | Cat# 21985023 |
| Penicillin-streptomycin (5,000 U/mL) | Gibco | Cat# 15070063 |
| 4-Hydroxytamoxifen | Sigma-Aldrich | Cat# H7904-5MG |
| Tamoxifen | Sigma-Aldrich | Cat# T5648-1G |
| Corn oil | Wako | Cat# 032-17016 |
| Isoflurane | Pfizer | N/A |
| Pentobarbital | Kyoritsu Seiyaku | N/A |
| Tacrolimus | Astellas Pharma | N/A |
| Methylprednisolone | Pfizer | N/A |
| Tetramethylrhodamine-labeled dextran | Invitrogen | Cat# D1817 |
| TWEEN 20 | Sigma-Aldrich | Cat# P1379-100ML |
| Donkey serum | Jackson ImmunoResearch | Cat# 017-000-001 |
| Skim milk | Wako | Cat# 190-12865 |
| Triton X-100 | Nacalai Tesque | Cat# 12967-32 |
| HistoVT One | Nacalai Tesque | Cat# 06380-05 |
| Blocking One Histo | Nacalai Tesque | Cat# 06349-64 |
| Signal Enhancer HIKARI for Immunostain Solution A | Nacalai Tesque | Cat# 02373-54 |
| Critical commercial assays | ||
| RNeasy Plus Micro kit | QIAGEN | Cat# 74034 |
| PrimeScript RT reagent Kit with gDNA Eraser (Perfect Real Time) | TAKARA | Cat# RR047A |
| TaqMan Gene Expression Master Mix | Applied Biosystems | Cat# 4369016 |
| Six2 (Rn01769531_m1) TaqMan Assay | Applied Biosystems | N/A |
| Nphs1 (Rn00674268_m1) TaqMan Assay | Applied Biosystems | N/A |
| Nphs2 (Rn00709834_m1) TaqMan Assay | Applied Biosystems | N/A |
| Lrp2 (Rn00578067_m1) TaqMan Assay | Applied Biosystems | N/A |
| Aqp1 (Rn00562834_m1) TaqMan Assay | Applied Biosystems | N/A |
| Slc12a1 (Rn00692576_m1) TaqMan Assay | Applied Biosystems | N/A |
| Scnn1a (Rn00580652_m1) TaqMan Assay | Applied Biosystems | N/A |
| Gapdh (Rn01775763_g1) TaqMan Assay | Applied Biosystems | N/A |
| Experimental models: cell lines | ||
| 201B7 human iPSC line | RIKEN BRC | Cat# HPS0063 |
| Experimental models: organisms/strains | ||
| Mouse: B6;129-Gt(ROSA)26Sor[tm1(DTA) Mrc]/J (Rosa26-DTA176) | Jackson Laboratory | Cat# 010527 |
| Mouse: B6;129-Six2[tm3(EGFP/cre/ERT2) Amc]/J (Six2-GCE) | Jackson Laboratory | Cat# 009600 |
| Mouse: C57BL/6-Tg (CAG-EGFP) | SLC Japan | N/A |
| Mouse: NOD/Shi-scid, IL-2RγKO Jic (NOG) | CLEA Japan | N/A |
| Rat: Sprague-Dawley-Tg (CAG-EGFP) | SLC Japan | N/A |
| Rat: LEW/SsNSlc (Lewis) | SLC Japan | N/A |
| Software and algorithms | ||
| Prism7 | GraphPad | https://www.graphpad.com/scientific-software/prism/ |
| Fiji | Schindelin et al., 2012 | https://imagej.net/Fiji |
| Other | ||
| Fluorescence stereo microscope | Leica Microsystems | Cat# M205FA |
| Greenough stereo microscope | Leica Microsystems | Cat# S9D |
| MACSQuantify | Miltenyi Biotec | Cat# 130-094-556 |
| LSM 880 with Airyscan | ZEISS | Cat# LSM880 |
| All-in-one fluorescence microscope | KEYENCE | Cat# BZ-X800 |
| 40-μm cell strainer | Corning | Cat# 352340 |
| 12 mm Transwell | Corning | Cat# 3401 |
| Microplate for tissue culture (for adhesion cell) 6-well | IWAKI | Cat# 3810-006 |
| V-bottom 96-well low-cell-binding plates | Sumitomo Bakelite | Cat# MS-9096V |
| U-bottom 96-well low-cell-binding plates | Thermo Scientific | Cat# 174929 |
| SlowFade Diamond Antifade Mountant | Invitrogen | Cat# S36972 |
| Prolong Gold Antifade Mountant with DAPI | Invitrogen | Cat# P36931 |
| Tissue-Tek OCT compound | Sakura Finetechnical | Cat# 4583 |
| PLATINUM PRO glass slide | Matsunami Glass Ind., Ltd. | Cat# SPRO-04 |
| Thin-walled glass capillary | NARISHIGE | Cat# G-100 |
| Puller | NARISHIGE | Cat# PC-10 |
| Aspirator tube assembly | Drummond Scientific Company | Cat# 2-040-000 |
| Pen-type syringe pump | Takasago Fluidic Systems | Cat# SBP-100G-LL |
Step-by-step method details
Mouse mating
Timing: 2 weeks
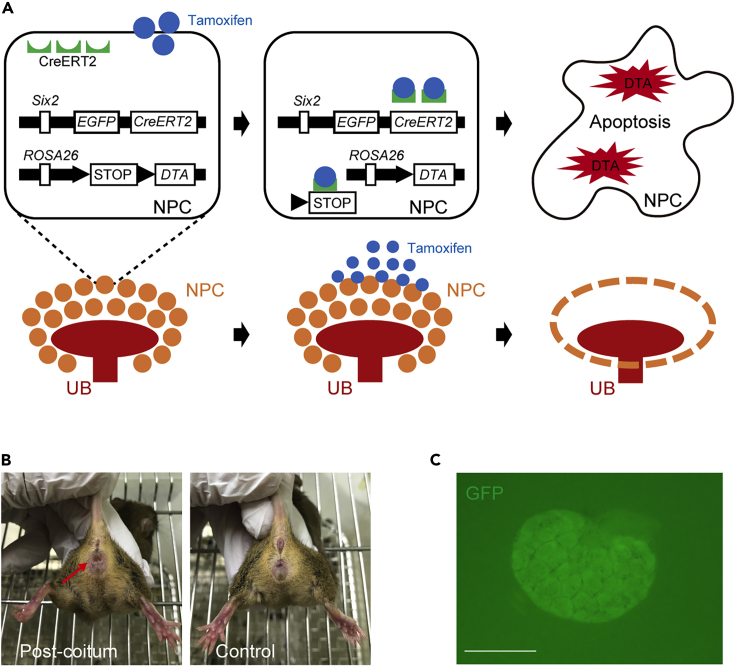
Create Six2-GCE+/DTA+ (Six2-DTA) mice that allow specific ablation of Six2-expressing NPCs after exposure to tamoxifen, without damaging exogenous RPCs including human induced pluripotent stem cell (hiPSC)-derived NPCs (Figure 2A).
-
1.
Mate female/male B6;129-Six2[tm3(EGFP/cre/ERT2) Amc]/J (Six2-GCE) mice (Kobayashi et al., 2008) with male/female B6;129-Gt(ROSA)26Sor[tm1(DTA) Mrc]/J (Rosa26-DTA176) mice (Wu et al., 2006) to obtain offspring Six2-DTA mice. For breeding, use timed mating: consider 12 pm of the day that the vaginal plug was detected as 0.5 days post-coitum (Figure 2B).
-
2.
Six2-GCE mice are heterozygous, while Rosa26-DTA176 mice are homozygous. Therefore, 50% of the embryos are Six2-DTA, which can be identified based on the expression of Six2-green fluorescent protein (GFP) by NPCs in MNs under a fluorescent stereomicroscope (M205FA, Leica Microsystems) (Figure 2C).
Note: MNs contain RPCs, which include NPCs, stromal progenitor cells (SPCs), and ureteric buds (UBs). In contrast, the NPCs derived from hiPSCs according to our protocol do not include SPCs or UBs.
Note:
Figure 2.
Representative images of Six2-DTA mouse embryos
(A) Schematic of the Six2-CreERT2-diphtheria toxin fragment A (DTA) system. Nephron progenitor cells (NPCs) consist cap mesenchyme surrounding ureteric buds (UBs). With tamoxifen, CreERT2 in Six2-expressing NPCs accesses the nuclear compartment, removing the loxP-flanked stop sequence. Accordingly, DTA is expressed, and NPCs are ablated while UBs remain unperturbed. Figure reprinted with permission from Fujimoto et al., 2020.
(B) A vaginal plug of a female Rosa26-DTA176 mouse 0.5 days post-coitum (red arrow) and a control.
(C) A metanephros (MN) of a Six2-DTA mouse embryo on embryonic day 13.5 (E13.5). Six2-green fluorescent protein (GFP) expression by NPCs is observed in the MN. Scale bar, 500 μm.
The Six2-GCE allele expresses an eGFPCreERT2 (EGFP and creERT2) fusion protein from the Six2 promoter/enhancer elements. CreERT2 is restricted to the cytoplasm, and it can only gain access to the nuclear compartment after exposure to tamoxifen. While homozygous Six2-GCE mice die shortly after birth due to the loss of Six2 gene function, heterozygous mice are viable and fertile with no reported abnormalities.
The ROSA26-DTA176 mice carry a loxP-flanked stop cassette followed by the coding sequence of an attenuated diphtheria toxin A chain (DTA176) in the Rosa26 locus. The DTA176 coding sequence is only expressed after the loxP-flanked stop sequence is removed via Cre-mediated recombination, leading to cell apoptosis. Homozygous ROSA26-DTA176 mice are viable and fertile, with no reported abnormalities.
In the resulting Six2-DTA mice, tamoxifen activates CreERT2 and induces specific DTA expression in Six2-expressing NPCs, leading to apoptosis of NPCs.
Note: Use both Six2-GCE mice and Rosa26-DTA176 mice at 10–25 weeks of age for mating. It does not matter which of the pair is male or female. House all mice in a temperature- and humidity-controlled environment with a 12-h light-dark cycle, and provide all mice with standard laboratory chow and water.
Optional: To ascertain that NPCs of Six2-DTA mice are eliminated using tamoxifen, culture the MNs of those mice on embryonic day 13.5 (E13.5) with 4-hydroxytamoxifen (4OHT). For the organ culture protocol, please refer to steps 16–19. Subsequent immunostaining reveals atrophy of the MNs and almost complete elimination of Six2-expressing NPCs. Furthermore, 4OHT at this concentration does not damage the MNs of nontransgenic mice (Fujimoto et al., 2020).
Enzymatic dissociation of the metanephroses isolated from GFP-labeled mice and rats
Timing: 2 h
Prepare the dissociated RPCs from the MNs of GFP-labeled mice or rats for subsequent injections into the nephrogenic zone (see Troubleshooting 1). The preferred age of pregnant animals is 13.5–14.5 days for mice and 15.5–16.5 days for rats.
-
3.Extract embryos from GFP-mice.
-
a.Euthanize the GFP-mouse at 13.5–14.5 days of pregnancy by dislocation of the cervical vertebrae.
-
b.Remove the uteruses through a median incision and place them in a 10-cm dish containing Hanks' Balanced Salt Solution (HBSS) with calcium and magnesium but without phenol red.
-
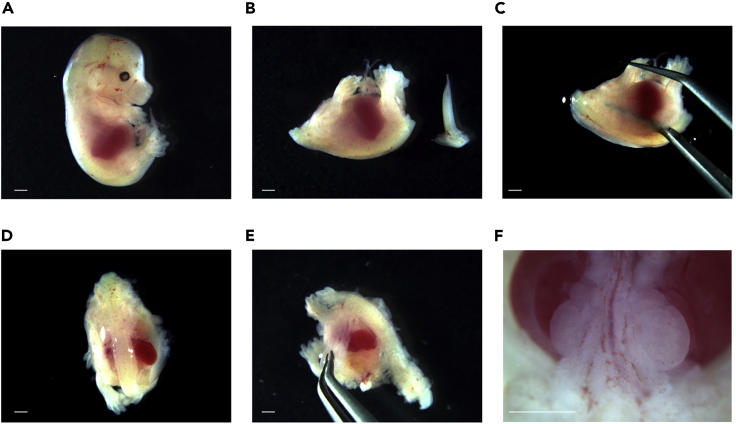
c.Extract the embryos from the isolated uteruses and euthanize them by decapitation (Figures 3A and 3B).
-
a.
-
4.Collect pairs of MNs from embryos using micro-tweezers under a stereoscopic microscope.
-
a.Remove the tail (Figure 3B).
-
b.Dissect both sides along the vertebral column from the caudal end to the neck. Both sides of the rib cage should be cut so that the vertebral column can be easily separated (Figures 3C and 3D).
-
c.Remove the vertebral column from the body and lay the embryo in a prone position (Figures 3E and 3F).
-
d.Dissect the MNs and collect them into a 1.5-mL tube containing 1 mL of minimum essential medium α (MEM α).
-
a.
-
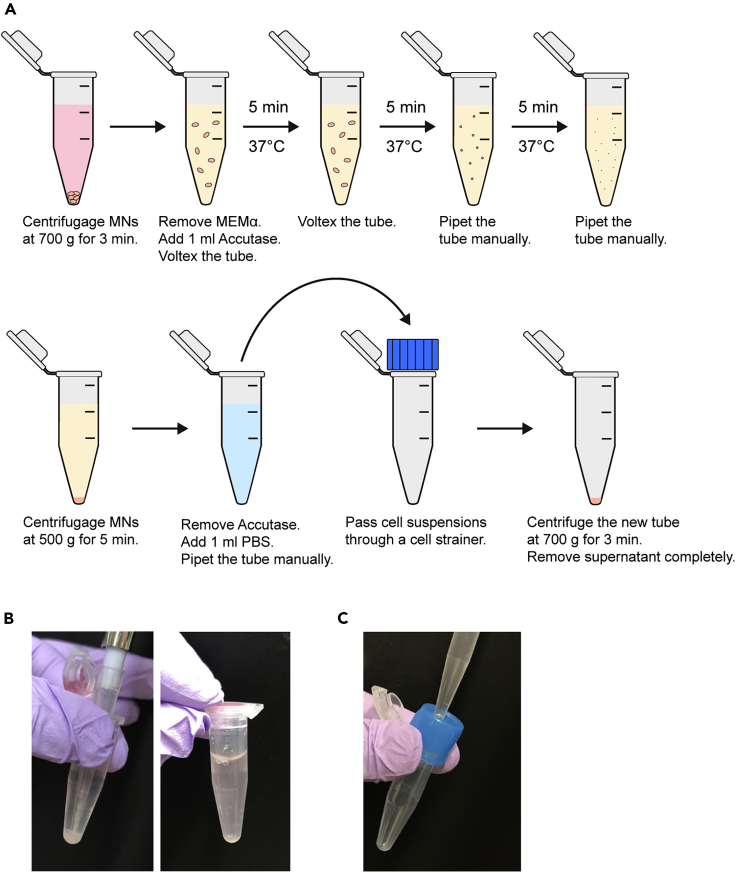
5.Enzymatically dissociate MNs using a modified version of the protocol described by Davies et al. (2012) (Figure 4A).
-
a.Centrifuge MNs at 700 × g for 3 min.
-
b.Remove the supernatants and add 1 mL of Accutase (15°C–25°C) into the tube. Vortex to aid digestion.
-
c.Incubate MNs at 37°C for 15 min, vortex after 5 min, and manually pipette using a 200-μL pipette tip after 10 and 15 min (Figure 4B).
-
d.Centrifuge MNs at 300 × g for 5 min.
-
e.Remove the supernatants and resuspend the pellet with 1 mL of phosphate-buffered saline (PBS). Dissociate MNs by manual pipetting with a 200-μL pipette tip.
-
f.Filter the cell suspension using a 40-μm cell strainer to obtain a single-cell suspension (Figure 4C). Centrifuge the suspension at 700 × g for 3 min.
-
g.Completely remove the supernatants. Tap and mix the pellet.
-
a.
Pause Point: The pellet can be incubated on ice until its use within the same day, though incubation for more than 4 h leads to a decline in cell viability.
CRITICAL: Manual pipetting of MNs should be carefully performed. Too much pipetting is harmful to the cells, while too little is not enough to dissociate the cells.
Note: When extracting embryos from GFP-rats, anesthetize the pregnant rats by isoflurane inhalation, unlike GFP-mice. After removing the uteruses, euthanize the mother immediately by pentobarbital (120 mg/kg) infusion. Subsequent steps to isolate MNs from GFP-rat embryos are the same as those from GFP-mouse embryos.
Note: According to a study that used flow cytometry (Yamanaka et al., 2019), NPCs account for approximately 30% of dissociated single cells extracted from the MNs of E13.5 mouse embryos. The remaining cells include SPCs and UBs.
Note: There is no need to detect the sex of embryos because MNs from both sexes can be used.
Optional: In step 5f, before centrifugation, stain the sample with Trypan Blue and observe under a light microscope to determine the cell number and viability and ensure that a single-cell suspension is obtained.
Figure 3.
Extraction of metanephroses (MNs) under a stereoscopic microscope
(A) An isolated mouse embryo on embryonic day 14.5 (E14.5).
(B) The tail is removed from the decapitated embryo.
(C and D) Both sides along the vertebral column are dissected using micro-tweezers.
(E) The vertebral column is carefully removed.
(F) Exposed MNs. Scale bar, 1 mm.
Figure 4.
Enzymatic dissociation of metanephroses (MNs)
(A) Schematic of the procedure.
(B) MNs in Accutase before (left) and after (right) manual pipetting with a 200-μL pipette tip.
(C) Cell suspension passing through a 40-μm cell strainer.
Maintenance of hiPSCs
Timing: 6 days
Human NPCs for subsequent injections into the nephrogenic zone are induced from hiPSCs, which are maintained according to the following step.
-
6.
Maintain 201B7 cells, a hiPSC line, on iMatrix-511 in StemFit AK02N. Culture the cells in 6-well plates in a humidified atmosphere of 5% CO2 at 37°C, and passage them every 6 days. After the cells attain 70%–80% confluence, treat them with Accutase. Seed the cells into another well with a diameter of 35 mm at a concentration of 1.5 × 104 cells per well.
Induction and dissociation of NPCs from hiPSCs
Timing: 12 days
Induce NPCs from iPSCs as follows, according to the previously established method (Taguchi et al., 2014; Taguchi and Nishinakamura, 2017).
-
7.
Reaggregate hiPSCs at 10 000 cells/aggregate in V-bottom 96-well low cell-binding plates in the presence of base media for human NPCs supplemented with 10 μM Y27632, 1 ng/mL human Activin A, and 20 ng/mL human basic fibroblast growth factor (FGF-basic) (100 μL/well).
-
8.
After 24 h, i.e., on day 1, transfer the spheres to U-bottom 96-well low-cell-binding plates containing base media for human NPCs supplemented with 10 μM CHIR and 10 μM Y27632 (200 μL/well). Subsequently, replace half of the culture media with new media every other day, i.e., on days 3 and 5.
-
9.
On day 7, transfer the spheres to new U-bottom plates containing the base media for human NPCs supplemented with 10 ng/mL human Activin A, 3 ng/mL human bone morphogenetic protein 4 (Bmp4), 3 μM CHIR, 0.1 μM retinoic acid, and 10 μM Y27632 (200 μL/well).
-
10.
On day 9, transfer the spheres to new U-bottom plates containing the base media for human NPCs supplemented with 1 μM CHIR, 5 ng/mL fibroblast growth factor-9 (FGF-9), and 10 μM Y27632 (200 μL/well).
-
11.On day 12, create the NPC spheres (NPSs).
-
a.Incubate NPCs in 5 mL of Enzyme Solution (a component of the Neuron Dissociation Solutions kit) at 37°C for 30 min.
-
b.Pellet the NPCs by centrifugation at 190 × g for 4 min.
-
c.Resuspend the pellets in 5 mL of Dispersion solution (a component of the Neuron Dissociation Solutions kit). Add 5 mL of Isolation solution (a component of Neuron Dissociation Solutions kit).
-
d.Pellet the NPCs by centrifugation at 160 × g for 5 min.
-
e.Resuspend the pellets in 1 mL of PBS and filter them through a 40-μm cell strainer.
-
f.Pellet again them by centrifugation at 160 × g for 5 min.
-
g.Completely remove the supernatant. Tap the tube to mix the pellet and incubate on ice until its use within the same day.
-
a.
Note: Neuron Dissociation Solutions kit is a product by Wako that comprises an Enzyme Solution, a Dispersion solution, and an Isolation solution.
Flow cytometry
Timing: 1 h
Perform flow cytometry to confirm the purity of NPCs in NPSs before the injections into the nephrogenic zone. NPCs are characterized by the presence of integrin α8 (ITGA8) and the absence of platelet-derived growth factor receptor α (PDGFRα) (Kaku et al., 2017).
-
12.
Use about 5 × 105 cells from the single-cell solution obtained in step 11e. Treat the solution with biotinylated anti-ITGA8, allophycocyanin (APC)-conjugated streptavidin, and phycoerythrin (PE)-conjugated anti-PDGFRα for cell staining. Acquire and analyze the data using the MACSQuantify analysis software.
Note: In flow cytometry, to evaluate the purity of the induced NPSs, only a part of the suspension is used. The procedure itself does not affect the following steps, given that the fraction of suspension used is discarded.
The injection of exogenous RPCs into the nephrogenic zone
Timing: 1–2 h
Using a sharp glass needle, inject RPCs (dissociated from MNs of GFP-mice and GFP-rats) and human NPCs (induced from hiPSCs) into the nephrogenic zone under the MN capsule of Six2-DTA mice. The MNs that underwent injections are subsequently cultured in vitro and in vivo. Please also refer to Yamanaka et al. (2017) regarding these steps.
-
13.
Use E13.5–14.5 Six2-DTA mouse embryos. Extract the embryos as described in step 3.
-
14.
Expose the MN pairs in embryos with micro-tweezers, as described in step 4.
-
15.Inject exogenous RPCs into the nephrogenic zone. Use RPCs from GFP-mice or GFP-rats, or NPCs from hiPSCs in each experiment.
-
a.Fix the embryo with micro-tweezers.
-
b.Fill the glass needle with the dissociated exogenous RPCs.
- c.
-
d.After injecting the indicated number of cells (4–8 × 104 cells in 0.2–0.4 μL) into the nephrogenic zone, pull out the needle carefully not to rupture the capsule (Figure 5B). (See Troubleshooting 2)
-
a.
CRITICAL: Exogenous RPCs should be injected into the subcapsular area and not the renal parenchyma to ensure that the injected cells spread widely to interact with the host cap mesenchyme as much as possible. Several injections (up to 2–4) are needed to cover this area.
CRITICAL: Carefully put forward the glass needle immediately before the capsule to spread the RPCs in the nephrogenic zone.
CRITICAL: The supernatants of centrifuged pellets should be removed entirely because moist pellets tend to tear the capsule by being released from the needle in large droplets.
CRITICAL: It is challenging to perform RPCs injections in MNs from Six2-DTA mouse embryos older than 15.5 days, since their capsule becomes tightly attached to the nephrogenic zone.
Note: The outer diameter of the sharpened glass needle tip is approximately 0.1 mm, while that of the original needle is 1.0 mm. The length of the sharp tip is approximately 1.0 mm. Cell pellets will be contained in the tip whose volume is approximately 0.1 μL (2 × 104 cells).
Note: The injections require about 15 min for both MNs of one embryo. The total time depends on the number of target embryos.
Optional: In step 15c, a pen-type syringe pump (a motorized injector drive) can be used instead of mouth pipetting (Figure 1C).
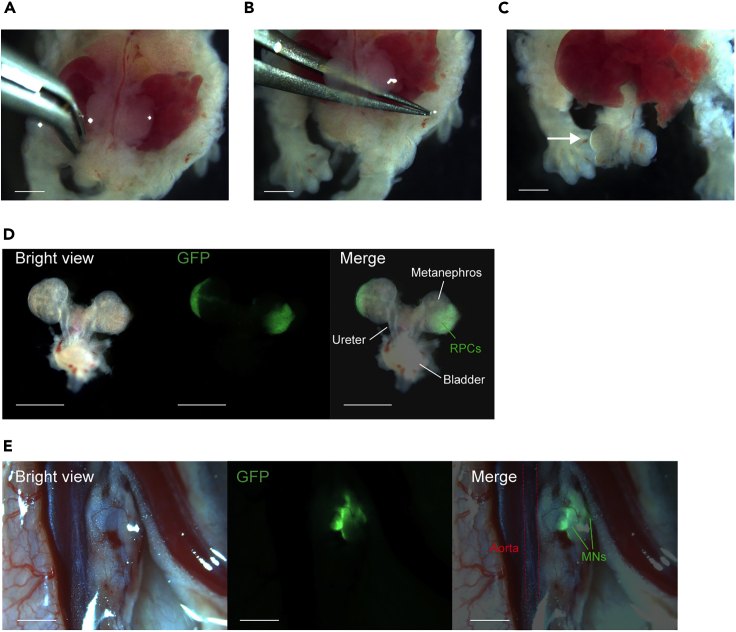
Figure 5.
The injection of exogenous renal progenitor cells (RPCs) into the nephrogenic zone
(A) The embryo in a prone position is held with micro-tweezers. A glass needle is inserted under the renal capsule. Green fluorescent protein (GFP)-RPCs are injected.
(B) Metanephroses (MNs) after three injections of GFP-RPCs into each MN. Scale bar, 1 mm.
Organ culture of isolated metanephroses
Timing: 7 days
In vitro regeneration of nephrons derived from exogenous RPCs in the nephrogenic zone of Six2-DTA mouse MNs: Culture Six2-DTA mouse MNs which were injected with exogenous RPCs on Transwell plates with tamoxifen.
-
16.
Detach the MNs from the embryos of Six2-DTA mice containing exogenous RPCs.
-
17.
Place the isolated MNs on the air-fluid interface of polycarbonate filters with an average pore size of 0.4 μm (12-mm Transwell plates).
-
18.
Soak the plates with base media for MNs (400 μL/well) supplemented with 4OHT (1 μg/well; 2.5 μg/mL).
-
19.
Culture MNs for 7 days at 37°C in a 5% CO2 atmosphere. Change the media and add 4OHT (1 μg/well; 2.5 μg/mL) daily.
Transplantation of metanephroses with the ureters and bladder into adult mice or rats
Timing: 2–4 weeks
In vivo regeneration of nephrons derived from exogenous RPCs in the nephrogenic zone of Six2-DTA mouse MNs: Transplant MNs with the ureters and bladder (MNBs) into the retroperitoneum of adult NOD/Shi-scid, IL-2RγKO Jic (NOG) mice or LEW/SsNSlc (Lewis) rats with tamoxifen. Lewis rats need to be treated with immunosuppressants to prevent acute rejection. Thereafter, the neo-nephrons will produce urine, which will be excreted to the embryonic bladder. The MNs can grow without hydronephrosis for a certain period of time.
-
20.After injections of exogenous RPCs into the bilateral MNs as explained in steps 13–15, detach the MNBs from the embryos of Six2-DTA mice.
-
a.Carefully remove the bilateral mesonephroses and the intestine connected to the MNs.
-
b.Dissect both groins vertically to reveal the bladder (Figure 6A).
-
c.Insert micro-tweezers under the MNs to peel the connective tissue and cut the cranial side of the MNs (Figure 6B).
-
d.Lift the MNs and remove the remaining intestine and the spleen (Figure 6C).
-
e.Detach the MNBs by cutting the root of the bladder and trim the MNBs (Figure 6D).
-
a.
Pause Point: The MNBs can be incubated in HBSS on ice for less than 4 h, until their use in the next step.
-
21.
Anesthetize host animals by isoflurane inhalation and reveal the descending aorta via a median incision. Cut the retroperitoneum in the vicinity of the descending aorta approximately 1–2 mm horizontally, and make a pocket using micro-tweezers, under a surgical microscope (S9D, Leica Microsystems). Transplant isolated MNBs into the pocket (Figure 6E).
-
22.
Dissolve tamoxifen in corn oil at a concentration of 40 mg/mL. Treat host animals with tamoxifen by daily oral gavage for six days, starting the day before transplantation (NOG mice: 200 mg/kg/day; Lewis rats: 60 mg/kg/day).
-
23.
To prevent acute rejection in Lewis rats, administer 2 mg/kg tacrolimus subcutaneously and 5 mg/kg methylprednisolone intraperitoneally daily starting the day before transplantation.
-
24.
To evaluate urine production, administer intravenous tetramethylrhodamine-labeled dextran (10 000 MW, 200 μL of 5 mg/mL) daily to a few NOG mice, starting 5 days prior to the collection of MNBs.
-
25.
Two or four weeks later, euthanize host animals by intraperitoneal pentobarbital infusion and remove transplanted MNBs for histological analysis.
CRITICAL: If the capsule is peeled off during the detachment of MNBs, the injected RPCs can be lost. In addition, care should be taken not to cut the ureters to avoid hydronephrosis. Some connective tissue remnants around the MNBs are permitted.
Note: Differentiation into mature nephrons can be confirmed after 2 weeks, but urine production cannot be ascertained until 4 weeks after the injections of exogenous RPCs.
Figure 6.
Transplantation of metanephroses with ureters and bladder (MNBs) containing exogenous renal progenitor cells (RPCs) to host animals
(A–C) Detachment of MNBs.
(A) Both groins are dissected vertically.
(B) Micro-tweezers are inserted under the metanephroses (MNs) to peel off the connective tissue.
(C) The MNs are lifted, and the bladder remains connected to the body (arrow).
(D) The MNB is detached and trimmed. Injected Green fluorescent protein-tagged renal progenitor cells (GFP-RPCs) are confirmed.
(E) The MNB is transplanted into the pocket of the retroperitoneum in the vicinity of the descending aorta in a NOD/Shi-scid, IL-2RγKO Jic (NOG) mouse. Scale bar, 1 mm.
Whole-mount immunostaining
Timing: 2 days
Morphological analysis for the in vitro regeneration and differentiation of nephrons from exogenous RPCs within the nephrogenic zone of MNs with concurrent elimination of host NPCs.
-
26.
Fix the MNs on Transwell plates with 4% paraformaldehyde in PBS at 4°C for 15 min.
-
27.
Wash the samples with 0.1% TWEEN 20/PBS three times at 15°C–25°C for 5 min.
-
28.
Block the samples using PBS with 1% donkey serum, 0.2% skimmed milk, and 0.3% Triton X at 15°C–25°C for 1 h.
-
29.
Wash the samples with 0.1% TWEEN 20/PBS three times at 15°C–25°C for 5 min and incubate them at 4°C for 12–18 h with primary antibodies diluted in PBS with 1% donkey serum, 0.2% skimmed milk, and 0.3% Triton X (1:100). The list of primary antibodies is provided in the Key Resources Table.
-
30.
Wash the samples with 0.1% TWEEN 20/PBS three times at 15°C–25°C for 1 h and incubate them with secondary antibodies conjugated with Alexa Fluor 488, Alexa Fluor 546, or Alexa Fluor 647 (1:200), together with 4′,6-diamidino-2-phenylindole (DAPI, 1:2,000), diluted in PBS with 1% donkey serum, 0.2% skimmed milk, and 0.3% Triton X, at 15°C–25°C for 1 h.
-
31.
Wash the samples with 0.1% TWEEN 20/PBS three times at 15°C–25°C for 30 min and mount them using the SlowFade Diamond Antifade Mountant.
-
32.
Examine the samples under a confocal laser scanning microscope (LSM880, Zeiss).
Hematoxylin-eosin staining and immunostaining of frozen sections
Timing: 3 days
Create frozen sections of neo-nephrons regenerated in vivo. Analyze their differentiation morphologically by Hematoxylin-eosin staining and immunostaining, and their urine-producing ability by immunostaining for dextran.
Fix the MNs in 4% paraformaldehyde for 3 h and dehydrate them by incubation with 20% sucrose in PBS at 4°C for 12–18 h.
-
33.
Embed the MNs with optimal cutting temperature (OCT) compound and cryosection them to obtain 8-μm-thick sections.
-
34.
Perform Hematoxylin-eosin staining according to standard protocols for histological analysis. Examine the samples under a microscope (BZ-X800, KEYENCE).
-
35.Perform the immunostaining as detailed below, according to the manufacturer’s protocol.
-
a.Wash the sections with PBS three times at 15°C–25°C for 5 min.
-
b.Perform antigen retrieval by incubating the sections in 10% HistoVT One/PBS at 70°C for 20 min. (See Troubleshooting 3)
-
c.Wash the sections with 0.1% TWEEN 20/PBS twice at 15°C–25°C for 5 min.
-
d.For blocking, add the Blocking One Histo buffer directly to the sections and incubate them at 15°C–25°C for 10 min.
-
e.Wash the sections once with 0.1% TWEEN 20/PBS at 15°C–25°C for 5 min.
-
f.Incubate the sections with primary antibodies diluted in Signal Enhancer HIKARI for Immunostain Solution A (1:100) at 4°C for 12–18 h.
-
g.Wash the sections with 0.1% TWEEN 20/PBS three times at 15°C–25°C for 5 min.
-
h.Incubate the sections with secondary antibodies diluted in Signal Enhancer HIKARI for Immunostain Solution A (1:200) at 15°C–25°C for 1 h.
-
i.Wash the sections once with 0.1% TWEEN 20/PBS at 15°C–25°C for 5 min.
-
j.Mount the sections with Prolong Gold Antifade Mountant with DAPI.
-
k.Examine the samples under a confocal laser scanning microscope (LSM880, Zeiss) or a fluorescence microscope (BZ-X800, KEYENCE).
-
a.
Note: Antigen retrieval is required for some antibodies to bind to the specific antigens. In immunostaining, MNs from E13.5–14.5 mouse embryos can be used as positive controls, while sections treated without primary antibodies can be used as negative controls.
Note: The primary antibodies listed in the key resources table correspond to specific structures: Six2 for NPCs; CK8 for UBs; WT1 and Nephrin for glomeruli; CDH6, AQP1, and Megalin for proximal tubules; ECAD for distal tubules; and CD31 for vascular endothelial cells. Pax8 staining is positive in distal nephrons connecting with the ureteric tip. Anti-GFP antibodies enable the detection of structures derived from exogenous RPCs from GFP-mice or rats. Anti-human nuclei antibodies are used to detect human cells because hiPSCs used in this protocol are not labeled with fluorescent proteins. Anti-vimentin antibodies are used for the species-specific detection of rat cells. Do not mix primary antibodies from the same species in a given sample. For generating immunostaining figures using these antibodies, please refer to Fujimoto et al. (2020).
RNA extraction, reverse transcription, and real-time quantitative polymerase chain reaction (RT-qPCR)
Timing: 1 day
Perform RT-qPCR to analyze the expression of nephron markers in regenerated nephrons and compare it with that of RPCs and native MNs.
-
36.
Using the RNeasy Micro Kit and the PrimeScript RT Reagent Kit with gDNA Eraser, isolate total RNA from the collected samples and synthesize cDNA using 50 ng of RNA template for each sample, according to the manufacturer's protocol.
-
37.
Conduct a RT-qPCR analysis of the cDNAs using the TaqMan Master Mix, the TaqMan assay, and a thermocycler (Rotor-Gene Q, Qiagen). Normalize the expression levels of target markers with those of the glyceraldehyde 3-phosphate dehydrogenase (Gaphd) gene.
Pause Point: Isolated total RNA can be stored at –80°C while synthesized cDNA can be stored at −20°C.
Note: TaqMan Assays listed in the Key Resources Table correspond to the genes expressed in specific cell types: Six2 for NPCs, Nphs1 and Nphs2 for terminally differentiated podocytes, Lrp2 and Aqp1 for proximal tubules, Slc12A1 for the loop of Henle, and Scnn1a for distal tubules.
Expected outcomes
Using our method, MNs from Six2-DTA mice serve as niches for the differentiation of exogenous RPCs. In these MNs, Six2-expressing NPCs are specifically ablated by tamoxifen without inducing the apoptosis of human cells.
In contrast, human cell apoptosis is inevitable in the previously reported system (Yamanaka et al., 2017). This system uses transgenic mice with NPCs expressing the diphtheria toxin receptor (DTR), which are specifically ablated by the administration of diphtheria toxin (DT). DT also leads to apoptosis of human cells due to their endogenous expression of active DTR.
By injecting exogenous mouse (allogeneic) or rat (xenogeneic) RPCs into the nephrogenic zone of MNs with the concurrent elimination of host NPCs, the transplanted NPCs in the exogenous RPCs connect to the host UBs and differentiate into mature nephrons in vitro and in vivo (Figure 7A).
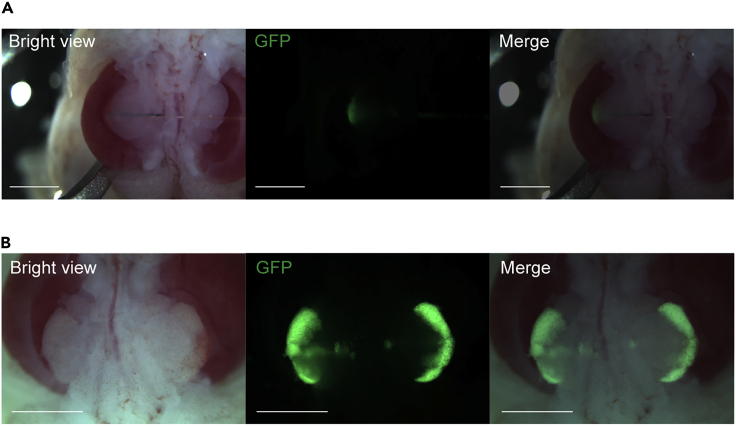
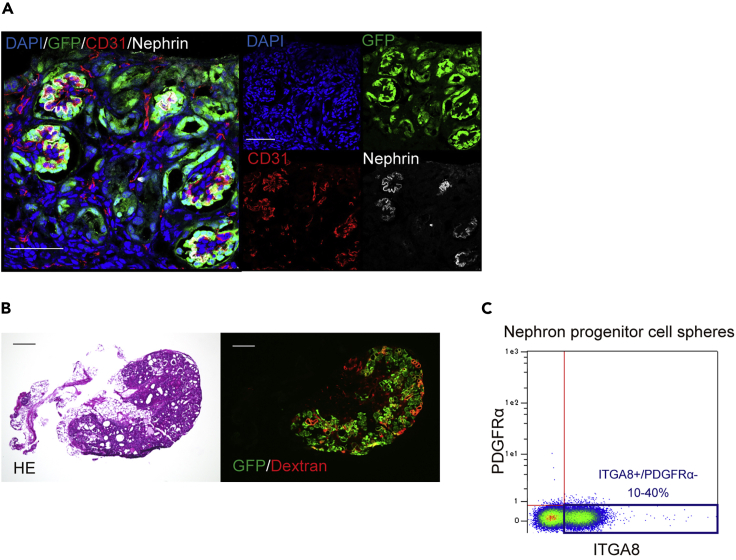
Figure 7.
Representative images of expected outcomes
(A) Immunostaining of a frozen section of metanephroses with ureters and bladder (MNBs) that contain exogenous mouse renal progenitor cells (RPCs) 2 weeks after transplantation (in vivo). Green fluorescent protein (GFP)-positive neo-glomeruli originating from the transplanted RPCs are confirmed. Scale bar, 50 μm.
(B) Hematoxylin and eosin (HE) staining and immunostaining of a frozen section of MNBs containing exogenous rat RPCs 4 weeks after transplantation (in vivo). Fluorescence microscope analysis revealed that dextran is filtrated by GFP-positive glomerular and tubular structures of neo-nephrons. Scale bar, 500 μm.
(C) According to flow cytometry, 10%–40% of the nephron progenitor cell spheres (NPSs) induced from human induced pluripotent stem cells (hiPSCs) are ITGA8+/PDGFRA− pure nephron progenitor cells (NPCs).
According to RT-qPCR, the expression of nephron-specific markers of nephrons regenerated in vitro and in vivo from rat RPCs was higher than that of rat RPCs before the injections and was comparable with that of age-matched native rat MNs (Fujimoto et al., 2020).
The regenerated nephrons grow with vasculature supplied by the adult recipients and produce urine in vivo, as confirmed by dextran filtration (Figure 7B). We have not evaluated the recovery of kidney function in the recipient animals. However, in vitro, the nephrons do not produce urine without vasculature from the recipients.
According to flow cytometry, ITGA8+/PDGFRA− NPCs represent approximately 10%–40% of all NPSs induced from hiPSCs (Figure 7C).
This NPC replacement system can be applied to NPCs derived from hiPSC in vitro, although they can currently differentiate only into immature renal vesicles (Fujimoto et al., 2020).
We did not succeed in creating well-organized nephrons from hiPSC-derived NPCs in MNs in vivo; thus, urine production from human-cell-derived neo-nephrons has not been attained.
Limitations
We have established a method for the enzymatic dissociation of MNs and the injection of exogenous RPCs below the MN capsule of transgenic mice. However, our method, and especially the RPCs injection, requires practice. Injections by mouth pipetting should be performed gently to avoid rupturing the capsule. Additionally, if RPCs are not injected into the nephrogenic zone, sufficient interaction with the host cap mesenchyme does not occur, leading to insufficient differentiation.
Another limitation lies in the transplantation of MNBs. Transplanted MNBs with RPCs are sometimes lost from the retroperitoneal pocket. RPCs are also easily lost from MNs if the capsule is torn. Furthermore, MNBs transplanted to Lewis rats can be rejected even under immunosuppressive treatment.
Our protocol used organ-specific progenitor cells that have lost pluripotency, avoiding unintended chimeras in the nervous and reproductive systems. We do not have enough long-term data on tumorigenesis because we did not transplant MNs with hiPSC-derived NPCs into adult animals. However, at the examined time-point, no tumor formation from exogenous RPCs was observed.
Troubleshooting
Problem 1
MNs are lost or damaged during their extraction from embryos.
Potential solution
MNs can easily be damaged during the dissection along the vertebral column in the back of embryos. Create shallow slits in the caudal end and deep cuts in the cranial end.
MNs can be peeled together during the removal of the vertebral column. The aorta runs as deep as the MNs, indicating how deep the vertebral column can be peeled.
Problem 2
The rupture of the capsule during RPCs injections leads to a leakage of the injected cells outside the MNs.
Potential solution
First, excessively sharp glass needles are inappropriate, as they can easily tear the capsule. Do not polish glass needles during preparation.
Second, completely remove the supernatants from the pellets because moist pellets are released from the needle in large droplets.
Finally, while mouth pipetting, blow through the syringe very gently and in short intervals.
Problem 3
Samples of the frozen sections are sometimes lost while washing or during antigen retrieval.
Potential solution
First, use PLATINUM PRO glass slides (SPRO-04, Matsunami Glass Ind., Ltd.) with a special coating that allows strong adhesion.
Second, before starting the immunostaining, dry the sections with warm air to allow samples to adhere to the glass slides.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Shuichiro Yamanaka (shu.yamanaka@jikei.ac.jp).
Materials availability
This study did not generate any new unique reagents.
Data and code availability
This study did not generate any datasets or code.
Acknowledgments
We would like to thank Enago (https://www.enago.jp/) for the English language review. This research was supported by the Japan Agency for Medical Research and Development (AMED; grants 19bk0104094h0001 and 20bm0704049h0001) and the Japan Society for the Promotion of Science (JSPS-KAKENHI; grants 19K17756).
Author contributions
Conceptualization, S.Y. and T.Y.; investigation, K. Matsui, Y.S., T.T., N.M., T.F., and S.Y.; writing, K. Matsui, T.T., and S.Y.; funding acquisition, S.Y. and T.Y.; supervision, S.T., K. Matsumoto, S.Y., and T.Y.
Declaration of interests
The authors declare no competing interests.
References
- Davies J.A., Unbekandt M., Ineson J., Lusis M., Little M.H. Dissociation of embryonic kidney followed by re-aggregation as a method for chimeric analysis. Methods Mol. Biol. 2012;886:135–146. doi: 10.1007/978-1-61779-851-1_12. [DOI] [PubMed] [Google Scholar]
- Fujimoto T., Yamanaka S., Tajiri S., Takamura T., Saito Y., Matsumoto N., Matsumoto K., Tachibana T., Okano H.J., Yokoo T. Generation of human renal vesicles in mouse organ niche using nephron progenitor cell replacement system. Cell Rep. 2020;32:108130. doi: 10.1016/j.celrep.2020.108130. [DOI] [PubMed] [Google Scholar]
- Kaku Y., Taguchi A., Tanigawa S., Haque F., Sakuma T., Yamamoto T., Nishinakamura R. PAX2 is dispensable for in vitro nephron formation from human induced pluripotent stem cells. Sci. Rep. 2017;7:4554. doi: 10.1038/s41598-017-04813-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi A., Valerius M.T., Mugford J.W., Carroll T.J., Self M., Oliver G., McMahon A.P. Six2 defines and regulates a multipotent self-renewing nephron progenitor population throughout mammalian kidney development. Cell Stem Cell. 2008;3:169–181. doi: 10.1016/j.stem.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., Preibisch S., Rueden C., Saalfeld S., Schmid B. Fiji: an open-source platform for biological-image analysis. Nat. Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi A., Nishinakamura R. Higher-order kidney organogenesis from pluripotent stem cells. Cell Stem Cell. 2017;21:730–746.e6. doi: 10.1016/j.stem.2017.10.011. [DOI] [PubMed] [Google Scholar]
- Taguchi A., Kaku Y., Ohmori T., Sharmin S., Ogawa M., Sasaki H., Nishinakamura R. Redefining the in vivo origin of metanephric nephron progenitors enables generation of complex kidney structures from pluripotent stem cells. Cell Stem Cell. 2014;14:53–67. doi: 10.1016/j.stem.2013.11.010. [DOI] [PubMed] [Google Scholar]
- Wu S., Wu Y., Capecchi M.R. Motoneurons and oligodendrocytes are sequentially generated from neural stem cells but do not appear to share common lineage-restricted progenitors in vivo. Development. 2006;133:581–590. doi: 10.1242/dev.02236. [DOI] [PubMed] [Google Scholar]
- Yamanaka S., Tajiri S., Fujimoto T., Matsumoto K., Fukunaga S., Kim B.S., Okano H.J., Yokoo T. Generation of interspecies limited chimeric nephrons using a conditional nephron progenitor cell replacement system. Nat. Commun. 2017;8:1719. doi: 10.1038/s41467-017-01922-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka S., Saito Y., Fujimoto T., Takamura T., Tajiri S., Matsumoto K., Yokoo T. Kidney regeneration in later-stage mouse embryos via transplanted renal progenitor cells. J. Am. Soc. Nephrol. 2019;30:2293–2305. doi: 10.1681/ASN.2019020148. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This study did not generate any datasets or code.