Abstract
BACKGROUND:
Low airway surface pH is associated with many airway diseases, impairs antimicrobial host defense, and worsens airway inflammation. Inhaled Optate is designed to safely raise airway surface pH and is well tolerated in humans. Raising intracellular pH partially prevents activation of SARS-CoV-2 in primary normal human airway epithelial (NHAE) cells, decreasing viral replication by several mechanisms.
METHODS:
We grew primary NHAE cells from healthy subjects, infected them with SARS-CoV-2 (isolate USA-WA1/2020), and used clinical Optate at concentrations used in humans in vivo to determine whether Optate would prevent viral infection and replication. Cells were pretreated with Optate or placebo prior to infection (multiplicity of infection = 1), and viral replication was determined with plaque assay and nucleocapsid (N) protein levels. Healthy human subjects also inhaled Optate as part of a Phase 2a safety trial.
RESULTS:
Optate almost completely prevented viral replication at each time point between 24 h and 120 h, relative to placebo, on both plaque assay and N protein expression (P < .001). Mechanistically, Optate inhibited expression of major endosomal trafficking genes and raised NHAE intracellular pH. Optate had no effect on NHAE cell viability at any time point. Inhaled Optate was well tolerated in 10 normal subjects, with no change in lung function, vital signs, or oxygenation.
CONCLUSIONS:
Inhaled Optate may be well suited for a clinical trial in patients with pulmonary SARS-CoV-2 infection. However, it is vitally important for patient safety that formulations designed for inhalation with regard to pH, isotonicity, and osmolality be used. An inhalational treatment that safely prevents SARS-CoV-2 viral replication could be helpful for treating patients with pulmonary SARS-CoV-2 infection.
Keywords: airway pH, COVID-19, SARS-CoV-2
Introduction
Airway surface lining fluid (ALF) coats the extracellular space of the epithelial cells that line the airways. ALF acidification contributes to the pathophysiology of asthma and other respiratory diseases.1–3 Specifically, ALF acidification is known to increase cough and bronchospasm, facilitate bacterial growth, increase mucus viscosity, decrease ciliary beat frequency, and cause general epithelial dysfunction.2,4–10 ALF acidification occurs during inflammatory and infectious airway disease exacerbations.2,6,8–12 Therefore, we have been investigating the effects of raising human ALF pH using an inhalational treatment, Optate (Airbase Breathing Company, Indianapolis, Indiana). Optate is an inhaled isotonic, isosmotic, alkaline medication designed to safely modify airway pH without irritating the airway epithelium. We previously demonstrated in several safety trials (Phase 1 and Phase 2) that Optate inhalation is well tolerated in healthy humans and those with stable asthma and COPD.9 We also demonstrated that a single inhalation of Optate effectively raises ALF pH, as indicated by a decrease in exhaled nitric oxide and an increase in exhaled breath condensate pH.9
Pandemic infection with SARS-CoV-2 (COVID-19) has cost hundreds of thousands of lives within the last year (https://www.who.int, Accessed August 9, 2020). In vitro, raising intracellular pH partially prevents activation of SARS-CoV-2 in primary normal human airway epithelial (NHAE) cells; indeed, intracellular alkalinization decreases viral replication by several mechanisms.13–16 Because Optate is effective at raising extracellular pH in the human airway, we studied whether it would prevent SARS-CoV-2 replication in NHAE cells. Specifically, we hypothesized that Optate would increase intracellular pH and decrease SARS-CoV-2 replication in NHAE cells compared to those treated with a placebo with the hope that it could serve as a treatment for this disease.
QUICK LOOK.
Current knowledge
Low airway epithelial surface pH impairs antimicrobial host defense and worsens airway inflammation. Low airway epithelial intracellular pH facilitates SARS-CoV-2 cell entry and replication. Inhaled Optate is designed to raise airway epithelial surface pH.
What this paper contributes to our knowledge
At the dose inhaled in human trials, Optate raises airway epithelial intracellular pH and almost completely prevents SARS-CoV-2 replication in NHAE cells, ablating both viral plaque formation and nucleocapsid protein expression. Optate is thus an effective antiviral in vitro that was safe for human inhalation in healthy subjects.
Methods
Cell Culture
Primary NHAE cells were grown as previously described from healthy, non-smoking donors.17,18
Optate (120 mM) was prepared and assayed for purity, potency, osmolality (target ∼ 330 mOsm), pH (target 9.8), and sterility prior to all experiments (IND #139144; Arena District Pharmacy, Columbus, Ohio). For the in vivo study, 10 healthy subjects > 18 y old with no history of lung disease were recruited under our approved protocol (Case Western Reserve University Institutional Review Board #03-18-28). FEV1, FVC, heart rate, , and adverse events were monitored before and after a single nebulization of 2.5 mL Optate (120 mM) as previously described9 and reviewed by our data safety monitoring board. No change was noted in any vital signs of any subject before and after treatment.
For the in vitro studies, cells were exposed to the same batch of 120-mM Optate described above in a 1:1 ratio with cell culture media to mimic the expected dilution effects of airway lining fluid in vivo. Phosphate-buffered saline (PBS) was used as the in vitro placebo. Airway cells were acquired by brush biopsy with informed consent (Indiana University Institutional Review Board Protocol #1408855616).
Intracellular pH Assays
Intracellular pH was evaluated with 2 fluorescent dyes: 1.25-μM 2',7'-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein, acetoxymethyl ester (BCECF-AM, Invitrogen, Carlsbad, California), which increases in green fluorescent intensity (E:E 500:531 nm) as pH increases19; and 20-µM pHrodo Red (Invitrogen), which decreases in red fluorescent intensity (E:E 560:585 nm) as pH increases.20 After washing with PBS, cells were treated with Optate (clinical solution, 1:1 dilution in medium) or PBS. As a negative control, cells were treated with ZnCl2 (100 µM), which acidifies the intracellular space by inhibiting Hv1.21
Viral Growth and Plaque Assays
SARS-CoV-2 Isolate USA-WA1/2020 was provided by Biodefense and Emerging Infection Resources (Manassas, Virginia). In our Biosafety Level 3 facility, viral stocks were prepared in Vero E6 cells (African green monkey kidney cell line, ATCC, Manassas, Virginia) at 37°C for 2–4 d until cytopathic effect was observed. Media from the cells were collected and centrifuged (1,000 g; 5 min). Virus was quantified with plaque assay (see the supplementary materials at http://www.rcjournal.com).
Vero E6 or NHAE cells were plated the day prior to the experiment. Cells were pretreated with Optate or PBS for 5–10 min prior to SARS-CoV-2 infection (multiplicity of infection = 1). After 1 h, cells were washed with PBS, and medium with or without Optate was added. For viral titers, the media was not changed prior to harvesting supernatant for viral titers at 24 h after infection. Supernatant was harvested every 24 h, and viral replication was determined with plaque assay. Data are from 3 experiments, each done in duplicate. NHAE infection studies used 2 different donors to reduce donor effect on results.
Immunoblots
Using the same protocol as that for plaque assay, cells were lysed in radioimmunoprecipitation assay buffer. Capillary electrophoresis was performed on the automated JESS system (ProteinSimple, San Jose, California). Briefly, 0.5 μg/μL lysate was plated and run according to the manufacturer's recommendations. Antibodies are listed in the supplementary materials (available at http://www.rcjournal.com). Compass software (ProteinSimple) generated digitally rendered bands on the basis of chemiluminescence electrophoretogram.
RNA Processing
RNA was extracted from control and Optate-treated NHAE cells (48 h) using RNeasy Plus kit (Qiagen, Hilden, Germany) following the manufacturer-recommended protocol. Total RNA was first evaluated for its quantity and quality (Bioanalyzer 2100, Agilent Technologies, Santa Clara, California); all samples had an RNA integrity number of 9 or higher. Total RNA (100 ng) was used for cDNA library preparation and quantitation, (supplementary material related to this paper is available at http://www.rcjournal.com). More than 95% of the sequencing reads reached Q30 (ie, 99.9% base call accuracy).
Sequenced libraries were mapped to the human genome (UCSC hg38) using STAR RNA-seq aligner 2.5 with the following parameter: -outSAMmapqUnique 60. Read distribution across the genome was assessed using bamutils (from ngsutils 5.9). Uniquely mapped sequencing reads were assigned to hg38 refGene genes using featureCounts (subread 1.5.1) with the following parameters: -s 2 -Q 10. Reads with count per million < 0.5 in more than 3 samples were removed.
Cytotoxicity Assays
Cytotoxicity of Optate was measured with both lactate dehydrogenase (LDH) concentration (Cayman Chemical assay, Ann Arbor, Michigan) and Trypan Blue exclusion (TC20 automated cell counter, Bio-Rad Laboratories, Hercules, California) according to the manufacturers' specifications as previously described.22
Data Analysis
The Student unpaired, 2-tailed t test was used to evaluate cytotoxicity, pH, viral plaque, and protein levels comparing control groups and treatment groups. The Wilcoxon rank-sum test was used for non-Gaussian distributed data as determined with the Shapiro-Wilk test. A mixed-effects analysis of variance model was used for repeated measures, with a Tukey post hoc correction for multiple comparisons. For RNA sequencing, gene symbols were converted to entrez ids using biomaRt and analyzed with the clusterProfiler package in R (R Foundation for Statistical Computing, Vienna, Austria).23,24 Data were normalized using trimmed mean of M values. Differential expression analysis was performed using edgeR 3.12.1. Family error rate was controlled for using Bonferroni.
Results
Optate Raises Intracellular pH in NHAE Cells
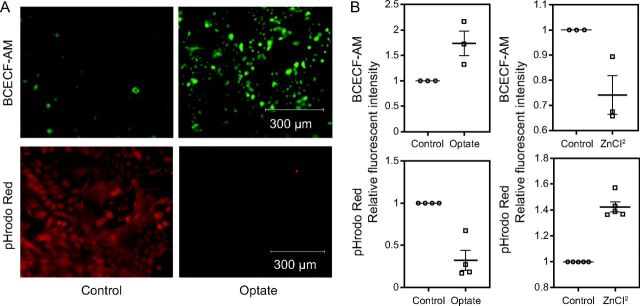
After loading with BCECF-AM (green) or pHrodo Red (red) dye, cells were treated with Optate or placebo and imaged for intracellular fluorescent intensity. Cells treated with Optate showed a significant increase in green fluorescent intensity (n = 4, P = .039) and decrease in red fluorescent intensity (n = 4, P = .001) compared to those treated with placebo, indicating an increase in intracellular pH (Fig. 1). ZnCl2, which is known to acidify the intracellular space,21 had an effect opposite compared to Optate (n = 3 and 5, P = .036 and .001, respectively).
Fig. 1.
Optate raises intracellular pH of NHAE cells. (A) Optate raised intracellular pH. NHAE cells were loaded with BCECF-AM, which increases in fluorescence intensity as pH increases across the physiological range (top), or pHrodo Red, which decreases in fluorescence intensity as pH increases (bottom). Cells were imaged before or after treatment. (B) Fluorescence intensity increased in cells loaded with BCECF-AM and decreased in cells loaded with pHrodo Red (4 each, P = .039 and P = .001, respectively). As a control, we treated cells with ZnCl2, which acidifies the intracellular space by inhibiting voltage-gated proton channel Hv121. Zinc had an effect opposite Optate (3 and 5, P = .036 and P = .001, respectively). Center lines denote means and whiskers show standard error. NHAE = normal human airway epithelial; BCECF-AM = 2',7'-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein, acetoxymethyl ester.
Optate Decreases SARS-CoV-2 Viral Replication
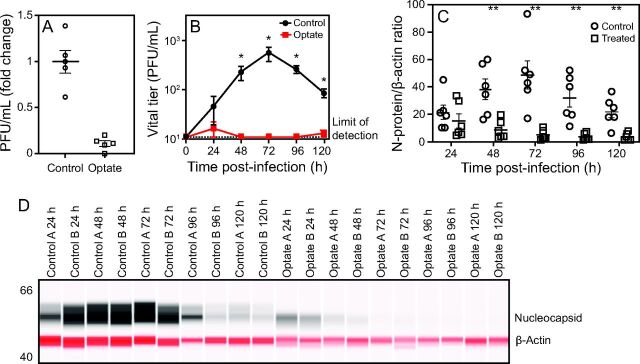
SARS-CoV-2 plaque-forming units (PFUs) were measured in culture media from Optate- and placebo-treated Vero E6 cells (the cells used to expand viral stock) infected with SARS-CoV-2. All cells were treated once, immediately after infection. Optate ablated viral PFUs in the Vero E6 cells (n = 3 experiments, 2 replicates each, P < .001) (Fig. 2). Under similar conditions, NHAE cells were then infected with SARS-CoV-2 and treated with Optate or placebo; then SARS-CoV-2 PFUs and N protein levels were measured daily for 5 d. After infection was established (24 h), both viral PFUs and N protein levels were ablated in the Optate-treated cells, unlike the cells treated with placebo (3 experiments using cells from 2 subjects, 2 replicates each, P < .001) (Fig. 2).
Fig. 2.
Optate decreases SARS-CoV-2 viral replication in Vero E6 and NHAE cells. (A) PFUs were measured in culture media from control and Optate-treated Vero E6 cells infected with SARS-CoV-2. Optate ablated viral infection in the Vero E6 cells (3 experiments, 2 replicates each, P < .001). (B) Control and Optate-treated primary NHAE cell cultures were then infected with SARS-CoV-2, and PFUs in culture media were analyzed under similar conditions for 120 h, starting from 24 h after infection. After infection was established (24 h), viral infection was ablated in the Optate-treated cells (3 experiments using cells from 2 subjects, 2 replicates each, P < .001). (C, D) N protein expression was then studied in cell lysates from NHAE cells described in (B). SARS-CoV-2 N protein expression, normalized to β-actin, was almost completely suppressed at time points after 24 h (P < .001). Center lines or points denote means, and whiskers show standard error. *P < .01, **P < .001. NHAE = normal human airway epithelial; PFU = plaque-forming units.
Optate Alters Endosomal Trafficking in NHAE Cells
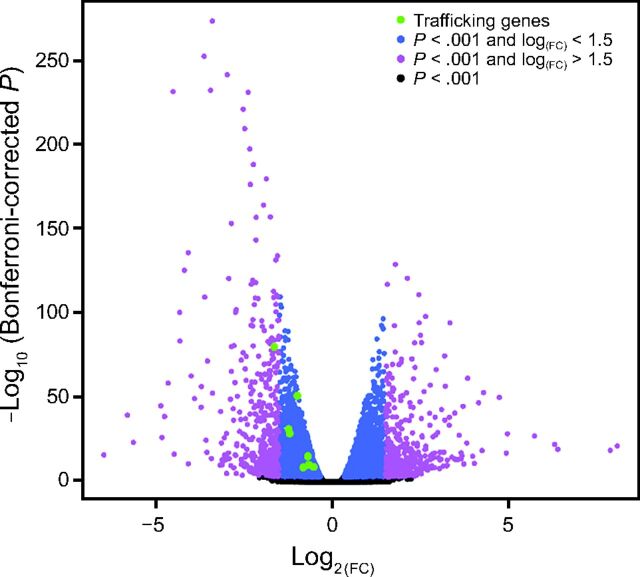
RNA sequencing results were compared between control and Optate-treated NHAE cells. Genes with significant differences between the groups are presented in a volcano plot (Fig. 3). Genes decreased in the Optate-treated group are negative (left) and those that increased are positive (right). Downregulated genes for endocytosis and endosomal trafficking are labeled in green. A list of downregulated genes is provided in the supplementary materials (available at http://www.rcjournal.com). Genes in blue have a Bonferroni-corrected P value < .001 and the absolute log(FC) < 1.5; genes in magenta have a Bonferroni-corrected P value < .001 and the absolute log(FC) > 1.5. Genes in black are below the significance threshold of .001.
Fig. 3.
Optate alters endocytosis and endosomal trafficking genes in NHAE cells. RNA sequencing results were compared between control and Optate-treated NHAE cells. Genes with significant differences between the groups are presented in a volcano plot. Genes decreased in the Optate-treated group are negative (to the left), and those that increase are positive (to the right). Downregulated genes for endocytosis and endosomal trafficking are labeled in green. Genes in blue have a Bonferroni-corrected P < .001 and the absolute log(FC) < 1.5; genes in magenta have a Bonferroni-corrected P < .001 and the absolute log(FC) > 1.5. Genes in black are below the significance threshold of .001. NHAE = normal human airway epithelial.
Optate Is Safe and Well Tolerated
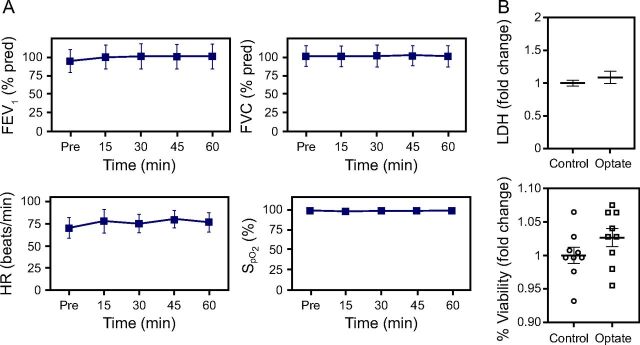
Human subjects (n = 10 normal volunteers) who inhaled Optate (120 mM) had no significant change in FEV1 (P = .84), FVC (P = .99), heart rate (P = .42), or (P = .99) after inhalation, consistent with previous data (Fig. 4).9 To validate that the observed antiviral effects were not due to Optate killing the host cells, we evaluated cytotoxicity in the control cells and the Optate-treated cells. Primary NHAE cells exposed to 120-mM Optate did not differ from the control group in cytotoxicity as determined with Trypan Blue exclusion or LDH levels (Fig. 4).
Fig. 4.
Optate safely is well tolerated in the human airway in vitro and in vivo. (A) Human subjects had no significant change in FEV1 (P = .84), FVC (P = .99), heart rate (P = .42), or (P = .99) after Optate inhalation (n = 10 normal volunteers). (B) NHAE cells exposed to Optate for 5 d did not differ from the control group in cytotoxicity evaluated with lactate dehydrogenase (LDH) levels (top) or percentage viability determined with Trypan Blue exclusion (bottom). Center lines or points denote means, and whiskers show standard error. NHAE = normal human airway epithelial.
Discussion
Our group previously reported that inhaled compounds can alter airway pH in vivo.1,9 Alterations of airway pH for therapeutic purposes have been proposed for chlorine gas inhalation, asthma, and cystic fibrosis.1,25,26 These applications have all focused on altering extracellular pH, likely due to the fact that most commonly known toxicities of ALF pH disturbance are extracellular. However, most respiratory viruses require an intracellular acidic event within airway epithelial cells to enter and to replicate.13,16 Our findings indicate that Optate, which was previously shown to increase ALF (extracellular) pH,9 indeed raises the intracellular pH of NHAE cells and ablates SARS-CoV-2 viral replication after infection. A safe, inhaled medication for raising intracellular pH could be of benefit for treating respiratory viral diseases even beyond SARS-CoV-2.
This work has several limitations. As with any in vitro experiment, the cell culture models used here are not necessarily representative of the in vivo systems and disease processes, and our observations may not translate. Moreover, COVID-19 involves many physiologic processes downstream from SARS-CoV-2 infection and replication. It is possible that different therapeutic targets rather than viral replication will be necessary to benefit patients. However, the fact that Optate inhalation does alkalinize the extracellular milieu in vivo suggests the observed effects may occur in patients with SARS-CoV-2. Thus, we are preparing to conduct a randomized, placebo-controlled, multicenter trial to better evaluate the therapeutic value of Optate. There were also technical limitations regarding the experiments used. For instance, viral infection of NHAE cells took > 24 h and resulted in lower titers than that seen with Vero E6 cells. This was previously observed in NHAE infection models of SARS-CoV-127,28 and was therefore unsurprising; however, it did warrant validation in several replicates and required the use of multiple cell lines. Further studies are needed to optimize this model, including evaluation of different time courses, the ability for viral replication to occur after treatment cessation, and the duration of effects. Finally, although our results suggest that our mechanism of action results from intracellular alkalinization leading to altered endocytic pathways and endosomal trafficking, there could be other mechanisms by which Optate ablates SARS-CoV-2 infection in Vero E6 and NHAE cells.
Inhalation of pH-altering compounds should be done with caution. Exposures to pH > 10 and < 6.5 are known to adversely affect NHAE cell function.7,22 Moreover, inhalation of hypotonic and hyposmotic solutions, such as many intravenous medications, causes airway irritation, bronchial hyperreactivity, and coughing.29 Our 1:1 PBS/cell culture media placebo group had a pH of 8 and still had significant viral infection, suggesting a fairly narrow therapeutic window for this purpose. Therefore, a specific formulation designed for inhalation with regard to isotonicity and osmolality that is buffered to an ideal pH within the therapeutic window needed to inhibit viral replication is required.
Conclusions
Optate raises intracellular pH and prevents SARS-CoV-2 replication in NHAE cells. Inhaled Optate raises ALF pH in human subjects, and it is non-toxic in vitro and in vivo, suggesting that a clinical trial to prevent treat life-threatening SARS-CoV-2 respiratory infection could be contemplated.
Supplementary Material
Acknowledgments
We thank Ms Kenzie Mahan for her administrative support.
Footnotes
Supplementary material related to this paper is available at http://www.rcjournal.com.
Drs Davis and Gaston are partially supported by NIH P01 HL128192-01A1, and P01 HL158507 are patent holders of Optate, and are co-founders of Airbase Breathing Company. Drs Gilk and Robinson are also patent holders of Optate. The remaining authors have disclosed no conflicts of interest.
References
- 1. Gaston B, Kelly R, Urban P, Liu L, Henderson EM, Doctor A, et al. Buffering airway acid decreases exhaled nitric oxide in asthma. J Allergy Clin Immunol 2006;118(4):817–822. [DOI] [PubMed] [Google Scholar]
- 2. Hunt JF, Fang K, Malik R, Snyder A, Malhotra N, Platts-Mills TA, et al. Endogenous airway acidification: implications for asthma pathophysiology. Am J Respir Crit Care Med 2000;161(3):694–699. [DOI] [PubMed] [Google Scholar]
- 3. Pezzulo AA, Tang XX, Hoegger MJ, Abou Alaiwa MH, Ramachandran S, Moninger TO, et al. Reduced airway surface pH impairs bacterial killing in the porcine cystic fibrosis lung. Nature 2012;487(7405):109–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Abou Alaiwa MH, Reznikov LR, Gansemer ND, Sheets KA, Horswill AR, Stoltz DA, et al. pH modulates the activity and synergism of the airway surface liquid antimicrobials β-defensin-3 and LL-37. Proc Natl Acad Sci U S A 2014;111(52):18703–18708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Berkebile AR, McCray PB, Jr. Effects of airway surface liquid pH on host defense in cystic fibrosis. Int J Biochem Cell Biol 2014;52:124–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Coakley RD, Grubb BR, Paradiso AM, Gatzy JT, Johnson LG, Kreda SM, et al. Abnormal surface liquid pH regulation by cultured cystic fibrosis bronchial epithelium. Proc Natl Acad Sci U S A 2003;100(26):16083–16088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Davis MD, Donn SM, Ward RM. Administration of inhaled pulmonary vasodilators to the mechanically ventilated neonatal patient. Paediatr Drugs 2017;19(3):183–192. [DOI] [PubMed] [Google Scholar]
- 8. Davis MD, Hunt J. Exhaled breath condensate pH assays. Immunol Allergy Clin North Am 2012;32(3):377–386. [DOI] [PubMed] [Google Scholar]
- 9. Davis MD, Walsh BK, Dwyer ST, Combs C, Vehse N, Paget-Brown A, et al. Safety of an alkalinizing buffer designed for inhaled medications in humans. Respir Care 2013;58(7):1226–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ricciardolo FL, Gaston B, Hunt J. Acid stress in the pathology of asthma. J Allergy Clin Immunol 2004;113(4):610–619. [DOI] [PubMed] [Google Scholar]
- 11. Ngamtrakulpanit L, Yu Y, Adjei A, Amoah G, Gaston B, Hunt J. Identification of intrinsic airway acidification in pulmonary tuberculosis. Glob J Health Sci 2010;2(1):106–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Walsh BK, Davis MD, Hunt JF, Kheir JN, Smallwood CD, Arnold JH. The effects of lung recruitment maneuvers on exhaled breath condensate pH. J Breath Res 2015;9(3):036009. [DOI] [PubMed] [Google Scholar]
- 13. Helenius A. Virus entry: what has pH got to do with it? Nat Cell Biol 2013;15(2):125. [DOI] [PubMed] [Google Scholar]
- 14. Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020;181(2):271–280.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Poon LLM, Peiris M. Emergence of a novel human coronavirus threatening human health. Nat Med 2020;26(3):317–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Smith AE, Helenius A. How viruses enter animal cells. Science 2004;304(5668):237–242. [DOI] [PubMed] [Google Scholar]
- 17. Davis MD, Suzaki I, Kawano S, Komiya K, Cai Q, Oh Y, Rubin BK. Tissue factor facilitates wound healing in human airway epithelial cells. Chest 2019;155(3):534–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fulcher ML, Randell SH. Human nasal and tracheo-bronchial respiratory epithelial cell culture. Methods Mol Biol 2013;945:109–121. [DOI] [PubMed] [Google Scholar]
- 19. Lanz E, Slavík J, Kotyk A. 2',7'-bis-(2-carboxyethyl)-5(6)-carboxyfluorescein as a dual-emission fluorescent indicator of intracellular pH suitable for argon laser confocal microscopy. Folia Microbiol (Praha) 1999;44(4):429–434. [DOI] [PubMed] [Google Scholar]
- 20. Benjamin D, Robay D, Hindupur SK, Pohlmann J, Colombi M, El-Shemerly MY, et al. Dual inhibition of the lactate transporters MCT1 and MCT4 is synthetic lethal with metformin due to NAD+ depletion in cancer cells. Cell Rep 2018;25(11):3047–3058.e3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. De La Rosa V, Bennett AL, Ramsey IS. Coupling between an electrostatic network and the Zn(2+) binding site modulates Hv1 activation. J Gen Physiol 2018;150(6):863–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kuch BA, Linssen R, Yoshikawa H, Smallwood CD, Davis MD. Local effects of two intravenous formulations of pulmonary vasodilators on airway epithelium. Respir Care 2020;65(10):1427–1432. [DOI] [PubMed] [Google Scholar]
- 23. Durinck S, Spellman PT, Birney E, Huber W. Mapping identifiers for the integration of genomic datasets with the R/Bioconductor package biomaRt. Nat Protoc 2009;4(8):1184–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yu G, Wang LG, Han Y, He QY. ClusterProfiler: an R package for comparing biological themes among gene clusters. OMICS 2012;16(5):284–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Douidar SM. Nebulized sodium bicarbonate in acute chlorine inhalation. Pediatr Emerg Care 1997;13(6):406–407. [DOI] [PubMed] [Google Scholar]
- 26. Gomez CCS, Parazzi PLF, Clinckspoor KJ, Mauch RM, Pessine FBT, Levy CE, et al. Safety, tolerability, and effects of sodium bicarbonate inhalation in cystic fibrosis. Clin Drug Investig 2020;40(2):105–117. [DOI] [PubMed] [Google Scholar]
- 27. Sims AC, Burkett SE, Yount B, Pickles RJ. SARS-CoV replication and pathogenesis in an in vitro model of the human conducting airway epithelium. Virus Res 2008;133(1):33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sims AC, Yount B, Burkett SE, Baric RS, Pickles RJ. SARS CoV replication and pathogenesis in human airway epithelial cultures. Adv Exp Med Biol 2006;581:535–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schoeffel RE, Anderson SD, Altounyan RE. Bronchial hyperreactivity in response to inhalation of ultrasonically nebulised solutions of distilled water and saline. Br Med J (Clin Res Ed) 1981;283(6302):1285–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.