Abstract
Background
Positive airway pressure (PAP) is a standard therapy for the treatment of OSA in children, but objective data on the effectiveness of PAP in infants are sparse. The aim of this study was to compare the effectiveness of PAP in infants younger than 6 months of age with that in school-aged children.
Research Question
Compared with PAP in school-aged children, can PAP be titrated as successfully in infants, and is adherence to PAP similar in both age groups?
Study Design and Methods
Single-center retrospective study. For consecutive infants younger than 6 months of age and school-aged children 5 to 10 years of age with OSA treated with PAP, baseline and titration polysomnography data, PAP adherence data, and parent-reported barriers to adherence were compared between groups.
Results
Forty-one infants and 109 school-aged children were included. Median obstructive apnea hypopnea index (OAHI) in infants was 25.7/h (interquartile range [IQR], 17.8-35.9/h) and was greater than that in school-aged children (12.1/hr; IQR, 7.6-21.5/h; P < .0001). After PAP titration, OAHI was reduced by a median of 92.1% in infants, similar to the median 93.4% reduction in school-aged children (P = .67). PAP was used in infants on 94.7% of nights, which was more than the 83% in school-aged children (P = .003). No differences were found in barriers to adherence between infants and school-aged children, with behavioral barriers being most common in both groups.
Interpretation
Objective data demonstrate that PAP is both highly effective at treating OSA and well-tolerated in infants. Like older patients, PAP should be considered along with other therapies for the treatment of OSA in even the youngest children.
Key Words: CPAP, OSA, pediatrics
Abbreviations: IQR, interquartile range; OAHI, obstructive apnea hypopnea index; PAP, positive airway pressure
OSA increasingly is being recognized in infants, especially in those with congenital craniofacial or airway conditions and neuromuscular abnormalities.1,2 In infants, OSA has been associated with reduced quality of life, failure to thrive, developmental delays, and sudden death.3, 4, 5 In addition, untreated OSA may reduce the arousal threshold in infants.6
Positive airway pressure (PAP) is effective in treating OSA in children and has been shown to improve sleepiness and quality of life in pediatric patients.7, 8, 9 Efficacy of PAP can be limited by poor adherence to therapy in children,7,10 but a multidisciplinary team can improve PAP use, even in children with medical and developmental comorbidities.11, 12, 13 The medical management of OSA in infants is not standardized because of unique challenges in this patient population, including lack of infant-specific polysomnographic rules, a paucity of available devices to treat OSA, and anatomic challenges, including small facial structure, irregular sleep patterns, and a high degree of medical complexity.14 Parents dealing with the recent addition to their family of a medically complex child also may undergo significant stress, adding an additional challenge. Although several case series have reported successfully using PAP in infants with OSA,15,16 little objective evidence is available to assess the effectiveness of PAP and barriers to its use in this age groups. Using objective data from PAP machines and polysomnography provides stronger evidence than subjective assessment used in previous studies and could improve guidelines for PAP use in infants.
The aims of this study were to compare the effectiveness and barriers to adherence of PAP treatment in infants and school-aged children with OSA. We hypothesized that PAP would be able to be titrated effectively to treat OSA in infants, that infants would have similar adherence to school-aged children, and that mask fit would be the most common barrier to PAP use in infants.
Methods
Study Design and Participants
This was a single-center retrospective study of children who received PAP for OSA between March 2013 and August 2019. Included were consecutive infants who began PAP treatment before 6 months of age and school-aged children who began PAP treatment between 5 and 10 years of age. To be included, participants in both groups underwent a diagnostic polysomnography that demonstrated OSA, began PAP for OSA, underwent a PAP titration polysomnography examination, and underwent a follow-up clinic visit, where they received a standardized multidisciplinary evaluation that included assessment of barriers to adherence. In all patients, the initial diagnostic polysomnography and first titration study were used for analysis. No repeated measures were used in this study. All patients were managed by our multidisciplinary CPAP program, which includes nursing, respiratory therapy, and behavioral sleep medicine personnel.13 All patients treated with PAP received an individualized treatment plan determined collaboratively between the family and CPAP team. Although some infants used PAP for regular daytime naps, other infants with more irregular daytime sleep or less consolidated sleep used PAP primarily for overnight sleep. Patients receiving noninvasive ventilation for respiratory failure or hypoventilation and those who required positive pressure via tracheostomy were excluded. Successful PAP titration was defined as having a residual OAHI of < 5/h on a pressure studied on overnight polysomnography. All polysomnography examinations were conducted and scored according to American Academy of Sleep Medicine pediatric specifications.17 This study was approved by the institutional review board at The Children’s Hospital of Philadelphia, which waived consent for this retrospective study (Identifier: 17-14434).
Data Collection
Data were collected at clinical visits to the Sleep Center and Sleep Laboratory at Children’s Hospital of Philadelphia and entered into an institutional review board-approved database in real time (research electronic data capture18). Data extracted for this study included demographics (age, past medical, and surgical history), diagnostic polysomnography results (OAHI, saturation nadir, and end-tidal CO2), titration polysomnography results (optimal pressure, residual OAHI at optimal pressure, and saturation nadir at optimal pressure), PAP download (set pressure and usage data), and barriers to adherence reported at clinic visits, typically every three to six months. PAP downloads were carried out at sleep center clinic visits when barriers to adherence were being assessed. All PAP downloads were carried out from Respironics CPAP machines or Respironics Trilogy ventilators (Philips Respironics).
Data Analysis
The most recent download within one year of PAP initiation was used, allowing time for PAP desensitization for both infants and school-aged children. PAP downloads included adherence data for the period since the previous sleep center visit. Barriers to PAP included child behavior (child refusing PAP), caregiver factors (caregiver responsible for PAP not present at bedtime, caregiver not attempting to place PAP), and technical or medical barriers, including poor mask fit, skin irritation, nasal congestion or dryness, and the machine not functioning properly.
Statistical Analysis
Data were summarized as mean ± SD if normally distributed or median (range). Differences between infant and school-aged groups were analyzed using the χ 2 test to compare proportions and the Wilcoxon rank-sum test to compare continuous variables between groups. Statistical analysis was performed using Stata version 13.1 software (StataCorp).
Results
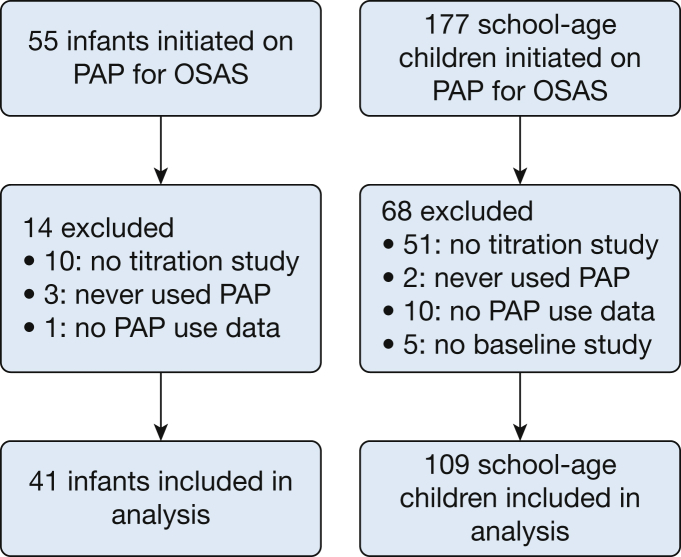
Fifty-five infants and 177 school-aged children began PAP therapy for OSA during the study period (Fig 1). Fourteen infants and 68 school-aged children were excluded from the final cohort, most commonly because they never underwent a titration study. Forty-one infants and 109 school-aged children were included in the final analysis. Compared with those included from both the infant and school-aged cohorts, no differences were found in those excluded in terms of age at PAP initiation, OAHI, oxygen saturation as measured by pulse oximetry nadir, or the proportion who were male, obese, or had genetic, neurologic, or craniofacial syndromes. Median age at PAP initiation was 2 months for infants and 7.6 years for the school-aged group (Table 1). Both age groups were about two-thirds male. Twenty infants (48.8%) had an underlying craniofacial abnormality compared with 10 school-aged children (9.2%; P < .0001). Genetic syndromes were common in both groups, including 19 infants (46.3%) and 32 of school-aged children (29.4%). Most school-aged children underwent adenotonsillectomy before PAP initiation, whereas less than 25% of infants underwent surgery before starting PAP, most commonly mandibular distraction osteogenesis for infants with micrognathia.
Figure 1.
Flow diagram showing participants included in the final analysis. PAP = positive airway pressure.
Table 1.
Demographic Data
| Variable | Infants (n = 41) | School-Aged Children (n = 109) | P Value |
|---|---|---|---|
| Age at initiation, median (interquartile range), mo | 2.1 (1.0, 4.7) | 91 (75,107) | < .0001 |
| Male sex | 28 (68.3) | 67 (61.5) | .44 |
| Obesity | 1 (2.4) | 39 (35.8) | < .0001 |
| Craniofacial abnormality | 20 (48.8) | 10 (9.2) | < .0001 |
| Neurologic abnormality | 5 (12.2) | 11 (10.1) | .71 |
| Any genetic syndrome | 19 (46.3) | 32 (29.4) | .052 |
| Trisomy 21 | 4 (9.8) | 20 (18.4) | .20 |
| Surgery for OSA before PAP | 10 (24.4) | 92 (84.4) | < .0001 |
| Adenotonsillectomy | 0 (0) | 88 (80.7) | < .0001 |
| Mandibular distraction | 6 (14.6) | 1 (0.9) | .0004 |
| Other surgery | 4 (9.8) | 3 (2.8) | .07 |
Data are presented as No. (%) unless otherwise specified. Differences between infant and school-aged groups were analyzed using the χ 2 test to compare proportions and the Wilcoxon rank sum test to compare continuous variables between groups. PAP = positive airway pressure.
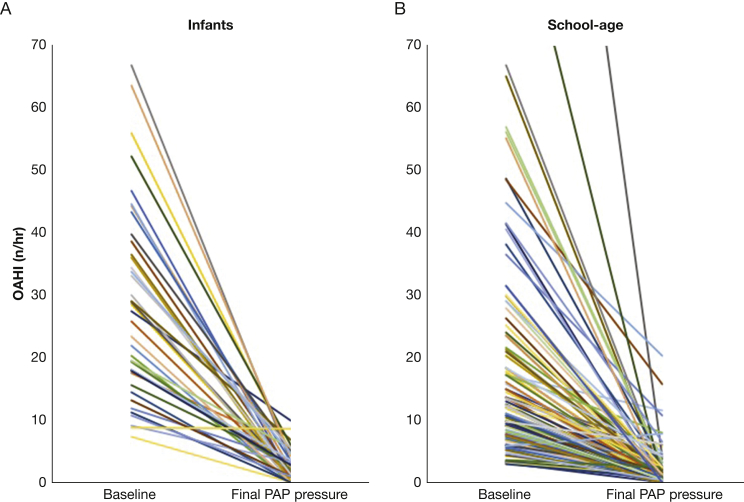
OSA was more substantial in infants who began PAP treatment compared with school-aged children who did, both in terms of OAHI and saturation nadir; a wide range of OSA severity was found for both age groups (Table 2). On diagnostic polysomnography, peak end-tidal CO2 was significantly greater in the school-aged group, perhaps because of a lack of end-tidal plateau in many infant studies. Median PAP titration was performed after 13 days (interquartile range [IQR], 6-91 days) in infants and 151 days (IQR, 94-215 days) in school-aged children. Most patients in both age groups achieved successful PAP titration: effective titration (residual OAHI < 5/h) was achieved in 82% of infants and 92% of school-aged children (P = .07). After titration, the residual OAHI and saturation nadir were somewhat better in the school-aged group compared with the infants, but substantial improvement was found in both groups (Fig 2). The percent reduction in baseline OAHI was the same between the two groups. No infants in this cohort were treated with autotitrating PAP or bilevel PAP, but about 10% of school-aged patients were treated with each of these methods. In those treated with CPAP, pressure was similar between the two groups. Supplemental oxygen was not used for any patient in either group during either the diagnostic or titration polysomnography.
Table 2.
Polysomnography and PAP Data
| Variable | Infants (n = 41) | School-Aged Children (n = 109) | P Value |
|---|---|---|---|
| Diagnostic polysomnography | ... | ... | ... |
| Baseline OAHI, /h | 25.7 (17.8-35.9) | 12.1 (7.6-21.5) | < .0001 |
| Baseline obstructive apnea index, /h | 11.1 (4.3-14.4) | 2.6 (0.6-6.9) | < .001 |
| Baseline SpO2 nadir, % | 81 (75-86) | 87 (79-89) | .004 |
| Peak end-tidal CO2, mm Hg | 49 (43-51) | 54 (51-58) | < .001 |
| PAP titration polysomnography | ... | ... | ... |
| OAHI < 5 on titrated pressure, % | 34 (82.9) | 101 (92.7) | .07 |
| Residual OAHI on final pressure, /h | 2.3 (0.9-4.1) | 0.8 (0.2-2.0) | .009 |
| Residual obstructive apnea index on final pressure, /h | 0.4 (0-1.3) | 0 (0-0.5) | .04 |
| Reduction in OAHI from baseline, % | 92.1 (83.8-98.1) | 93.4 (84.1-99.6) | .67 |
| SpO2 nadir on final pressure, % | 91 (88-93) | 93 (92-95) | .0001 |
| Final CPAP titration level, cm H2O | 7 (5-8) | 8 (6-10) | .053 |
| Bilevel PAP used | 0 (0) | 11 (10.1) | .03 |
| Autotitrating PAP used | 0 (0) | 12 (11.1) | .03 |
| PAP download data | ... | ... | ... |
| Duration of PAP download, d | 63 (30-90) | 110 (55-182) | .0001 |
| Nights with PAP use, % | 94.7 (21.2-100) | 83 (0.7-100) | .003 |
| Nights with PAP use > 4 h, % | 76.5 (40.1-89.3) | 61.2 (21.9-82.9) | .062 |
| Duration of PAP on nights used, min | 430 (255-531) | 378 (236-485) | .20 |
| Residual AHI, /h | 0.5 (0.2-1.2) | 3.5 (2.3-5.9) | .0005 |
| Reported barriers to adherence | ... | ... | ... |
| Child behavior | 12 (29.3) | 38 (34.9) | .52 |
| Caregiver factors | 4 (9.8) | 23 (21.1) | .11 |
| Poor mask fit | 5 (12.2) | 11 (10.1) | .71 |
| Skin irritation | 2 (4.9) | 4 (3.7) | .74 |
| Nasal congestion or dryness | 1 (2.4) | 3 (2.8) | .89 |
| Machine not working properly | 0 (0) | 8 (7.3) | .08 |
Data are presented as median (interquartile range) or No. (%) unless otherwise specified. Differences between infant and school-aged groups were analyzed using the χ 2 test to compare proportions and the Wilcoxon rank sum test to compare continuous variables between groups. OAHI = obstructive apnea hypopnea index; SpO2 = oxygen saturation as measured by pulse oximetry. See Table 1 legend for expansion of other abbreviation.
Figure 2.
A, B, Graphs showing change in OAHI in infants and school-aged children treated with PAP. For infants (A), median reduction in OAHI was 92.1% on the final PAP setting. For school-aged children (B), median reduction in OAHI was 93.4%. OAHI = obstructive apnea hypopnea index. See Figure 1 legend for expansion of other abbreviation.
PAP downloads included 63 days (IQR, 30-90 days) of data in infants and 110 days (IQR, 55-182 days) of data in school-aged children. PAP adherence data demonstrated significantly more nights with PAP use in infants compared with school-aged children (P = .003), with a trend toward more nights with more than 4 h of total use (median, 76.5% of nights compared with 61.2%; P = .062) (Table 2). PAP duration on nights used was highly variable in both groups; median use was 7.2 h/night in the infant group and 6.3 h/night in the school-aged group (P = .20). In many cases for both age groups, the percentage of the night that PAP was used and duration of use per night continued to improve throughout the first year of use. Residual apnea hypopnea index reported from the PAP download was significantly lower in the infant group compared with the school-aged group, but data were limited to 8 infants (19.5%) and 80 school-aged children (73.4%) because some PAP reports, especially in infants treated with ventilators, did not include this result. The reliability of residual apnea hypopnea index in these age groups has not been validated.
Reported barriers to PAP use were similar between the infant and school-aged groups despite the substantial difference in age. Child behavior, including the child crying or refusing to wear the PAP device, was the most common barrier in both age groups. No difference was found in reported problems related to mask fit, which was a barrier in 5 infants (12.2%) and 11 school-aged children (10.1%). A trend was found toward school-aged children having more caregiver barriers and broken equipment, but these did not reach statistical significance. No differences were found in barriers related to skin or nasal irritation, which were uncommon in both age groups.
For the infant group, PAP was discontinued because of resolution of OSA in 23 patients (56.1%). Eleven infants in this cohort continue PAP therapy, although only five have been treated with PAP for more than two years. Six participants are no longer cared for by our center, and one infant died of cardiac arrest related to septic shock while still being treated with PAP. Including those still being treated with PAP, duration of use was 563 ± 430 days. Of the five infants treated with PAP for more than two years, all had genetic syndromes, underlying complex neurologic conditions—including myopathy, significant hypotonia, and chest wall deformity—or both. In this cohort, 38 patients used a nasal mask (35 of whom used 1 of 2 different models), and 3 were treated with a cannula interface. Thirty of 41 infants in this cohort received PAP through a ventilator, whereas the others used a CPAP machine.
Discussion
To our knowledge, this is the largest study to assess the effectiveness of PAP for the treatment of OSA in infants using objective data. Despite a greater awareness of OSA in a variety of infant populations, few studies have assessed the effectiveness of treatment in this age group.2,19,20 Our data showed that PAP is effective in treating OSA in most infants with a similar pressure to that of school-aged children. In most patients with a residual OAHI of > 5/h with PAP, very severe OSA was present at baseline that still improved substantially with PAP. Although using PAP for the treatment of OSA in infants has been reported since the 1990s,21, 22, 23, 24 limited objective data exist regarding its efficacy in these very young patients. McNamara and Sullivan15 reported near-complete resolution of obstructive apnea in their cohort of 21 infants who underwent a CPAP titration study, but hypopneas were not recorded during this early study. The baseline obstructive apnea index in their cohort (14.6 ± 3.9/h) was similar to that seen in our cohort (11.1/h [IQR, 4.3-14.4/h]), and the residual obstructive apnea index on final PAP pressure in our cohort was also very low (0.4/h [IQR, 0-1.3/h]). Other retrospective studies have used reported resolution of symptoms to assess PAP efficacy in infants.21,22
Also, data are limited regarding adherence and consequences of PAP use in infants. To our knowledge, ours is the first study to provide systematic objective adherence and parent-reported barriers to adherence in infants. McNamara and Sullivan15 reported that for the 18 infants in their cohort treated with PAP at home for more than one month, no problems were reported by parents concerning the PAP interface or tolerating the pressure, nor did parents report any skin breakdown or skeletal changes. In a series of 42 children treated with PAP by Massa and colleagues23 that included 18 infants, no serious complications were reported, but frequent nasal symptoms or skin or eye irritation resulting from poor mask fit were noted. In that study, good compliance was noted by two-thirds of parents, but specific adherence data in the infants were not provided. Of note, both of these studies reported on the use of nasal CPAP in the 1990s using older interfaces before objective machine-based adherence data were available. Although resources for assisting children with PAP adherence and clinical practice vary across institutions, our data showed that when using the same clinical team, PAP adherence in infants was at least as good as in school-aged children. Although infants experienced significantly more nights with PAP use compared with school-aged children, the duration of use in a 24-h period was not greater in infants. Because appropriate sleep duration for infants is 12 to 16 h/day compared with 9 to 12 h/day for school-aged children,25 the percent of sleep time when PAP was used actually may have been less in infants. This finding may be related to the less consolidated nature of sleep in infants compared with older children. Because the family is responsible for managing CPAP in the home environment, it is critical to incorporate caregivers as team members at every stage in the process of initiating and maintaining CPAP therapy (Fig 3).
Figure 3.
Infant and father sleeping with CPAP at home. Photograph used with permission.
Compared with older children, for whom evidence-based guidelines direct management,26 infants with OSA pose unique challenges because treatment for OSA in this age group is not well standardized. Current treatment approaches include watchful waiting or positioning, supplemental oxygen or airflow via nasal cannula, or surgery. As was recommended in a recent European Respiratory Society statement, a multidisciplinary, stepwise, individualized treatment approach including CPAP should be taken in the management of infants with OSA.27 At our center, the approach to initiating and titrating PAP in infants is similar to that in older children in many respects. We start CPAP at a low set pressure to assist with desensitization and then increase the pressure in the sleep laboratory as needed to treat OSA effectively. Because some infants may be susceptible to PAP-induced central apnea, particularly at higher pressures, complete resolution of all obstructive events is not always achieved. At our center, autotitrating PAP is not used because these algorithms have not been validated in infants. Because infants growth and mature rapidly, polysomnography is repeated regularly to re-evaluate OSA severity and optimal PAP settings.
Although PAP may be effective in improving OSA in infants, its consequences also must be considered. In addition to the risk of skin breakdown28 and nasal trauma,29 evidence also exists to suggest that prolonged CPAP use may contribute to retrusion of the midface, particularly in patients with underlying craniofacial conditions.28,30,31 With particularly immature skulls, infants may be at particularly increased risk and should be monitored closely with continued PAP use. In addition to the barriers discussed above, obtaining insurance approval for PAP use in infants is another challenge many providers face, because masks, headgear, and machines often are not approved for use in infants or young children.32 We were able to work with local durable medical equipment companies to ensure that PAP devices were made available to all patients, but a number of patients were treated using home ventilators instead of CPAP machines. Addressing disparities in equipment availability nationally and worldwide is a challenge that in some cases is the limiting factor for treatment of these young patients.
This study had several limitations. In this retrospective analysis, a significant number of patients prescribed PAP did not meet inclusion criteria. Because the primary outcome was effectiveness of PAP, those who never underwent a titration study or did not have PAP use data available were excluded because this could not be assessed. This could bias results toward those successfully undergoing a successful PAP titration and with greater PAP use. Also, because the data described here were clinically derived, the data collection time points were not strictly consistent for all patients. A school-aged cohort was chosen to provide a distinct pediatric comparison without the biases of young noninfants or nearly-adult adolescents. A more comprehensive assessment of the efficacy of and barriers to PAP across the pediatric age spectrum or health-related benefits to PAP in infants was beyond the scope of this study. Our hospital is a large tertiary care center, so infants treated with PAP may have more severe OSA than in the general population.
Conclusions
PAP is an effective treatment for OSA in infants, even in patients with underlying craniofacial conditions or genetic syndromes. If pediatric-specific resources are available to support patients with OSA, PAP should be considered along with other treatment options, as it is in older children. Additional infant-specific PAP interfaces and size-appropriate headgear are needed to provide options to the smallest infants and those with craniofacial differences like midface hypoplasia. Studies comparing the efficacy of PAP with other treatments of OSA in infants, like surgery and nasal cannula systems, are needed, including prospective clinical trials assessing impact on the consequences of OSA. In addition, future qualitative research is needed to describe better the family experience with both ventilators and CPAP machines for OSA in infants and children. Finally, the natural history of OSA in infants is poorly understood, and studies assessing the optimal frequency for reassessment of OSA severity and retitration of PAP in infants are needed.
Acknowledgments
Author contributions: C. M. C., M. S. X., S. E. B., and I. E. T. contributed to the conception of the study design. C. M. C., M. S.X., P. H., and A. M. C. contributed to the acquisition and analysis of the data. All authors contributed to the interpretation of the data and the writing of the manuscript. C. M.C. takes responsibility for the integrity of the data and the accuracy of the data analysis.
Financial/nonfinancial disclosures: None declared.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Footnotes
FUNDING/SUPPORT: C. M. C. is supported by a National Institutes of Health grant [K23 HL135346]. I. E. T. is supported by a National Institutes of Health grant [K01 HL130719].
References
- 1.Qubty W.F., Mrelashvili A., Kotagal S., Lloyd R.M. Comorbidities in infants with obstructive sleep apnea. J Clin Sleep Med. 2014;10(11):1213–1216. doi: 10.5664/jcsm.4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maclean J.E., Fitzsimons D., Fitzgerald D.A., Waters K.A. The spectrum of sleep-disordered breathing symptoms and respiratory events in infants with cleft lip and/or palate. Arch Dis Child. 2012;97(12):1058–1063. doi: 10.1136/archdischild-2012-302104. [DOI] [PubMed] [Google Scholar]
- 3.Smith C.B., Walker K., Badawi N., Waters K.A., MacLean J.E. Impact of sleep and breathing in infancy on outcomes at three years of age for children with cleft lip and/or palate. Sleep. 2014;37(5):919–925. doi: 10.5665/sleep.3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leiberman A., Tal A., Brama I., Sofer S. Obstructive sleep apnea in young infants. Int J Pediatr Otorhinolaryngol. 1988;16(1):39–44. doi: 10.1016/0165-5876(88)90098-5. [DOI] [PubMed] [Google Scholar]
- 5.Logjes R.J.H., Haasnoot M., Lemmers P.M.A. Mortality in Robin sequence: identification of risk factors. Eur J Pediatr. 2018;177(5):781–789. doi: 10.1007/s00431-018-3111-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McNamara F., Sullivan C.E. Effects of nasal CPAP therapy on respiratory and spontaneous arousals in infants with OSA. J Appl Physiol. 1999;87(3):889–896. doi: 10.1152/jappl.1999.87.3.889. [DOI] [PubMed] [Google Scholar]
- 7.Marcus C.L., Rosen G., Ward S.L. Adherence to and effectiveness of positive airway pressure therapy in children with obstructive sleep apnea. Pediatrics. 2006;117(3):e442–e451. doi: 10.1542/peds.2005-1634. [DOI] [PubMed] [Google Scholar]
- 8.Marcus C.L., Radcliffe J., Konstantinopoulou S. Effects of positive airway pressure therapy on neurobehavioral outcomes in children with obstructive sleep apnea. Am J Respir Crit Care Med. 2012;185(9):998–1003. doi: 10.1164/rccm.201112-2167OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uong E.C., Epperson M., Bathon S.A., Jeffe D.B. Adherence to nasal positive airway pressure therapy among school-aged children and adolescents with obstructive sleep apnea syndrome. Pediatrics. 2007;120(5):e1203–e1211. doi: 10.1542/peds.2006-2731. [DOI] [PubMed] [Google Scholar]
- 10.Nixon G.M., Mihai R., Verginis N., Davey M.J. Patterns of continuous positive airway pressure adherence during the first 3 months of treatment in children. J Pediatr. 2011;159(5):802–807. doi: 10.1016/j.jpeds.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 11.Koontz K.L., Slifer K.J., Cataldo M.D., Marcus C.L. Improving pediatric compliance with positive airway pressure therapy: the impact of behavioral intervention. Sleep. 2003;26(8):1010–1015. doi: 10.1093/sleep/26.8.1010. [DOI] [PubMed] [Google Scholar]
- 12.King M.S., Xanthopoulos M.S., Marcus C.L. Improving positive airway pressure adherence in children. Sleep Med Clin. 2014;9(2):219–234. doi: 10.1016/j.jsmc.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Riley E.B., Fieldston E.S., Xanthopoulos M.S. Financial analysis of an intensive pediatric continuous positive airway pressure program. Sleep. 2017;40(2):1–8. doi: 10.1093/sleep/zsw051. [DOI] [PubMed] [Google Scholar]
- 14.Katz E.S., Mitchell R.B., D’Ambrosio C.M. Obstructive sleep apnea in infants. Am J Respir Crit Care Med. 2012;185(8):805–816. doi: 10.1164/rccm.201108-1455CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McNamara F., Sullivan C.E. Obstructive sleep apnea in infants and its management with nasal continuous positive airway pressure. Chest. 1999;116(1):10–16. doi: 10.1378/chest.116.1.10. [DOI] [PubMed] [Google Scholar]
- 16.Guilleminault C., Pelayo R., Clerk A., Leger D., Bocian R.C. Home nasal continuous positive airway pressure in infants with sleep-disordered breathing. J Pediatr. 1995;127(6):905–912. doi: 10.1016/s0022-3476(95)70026-9. [DOI] [PubMed] [Google Scholar]
- 17.Berry R.B., Albertario C.L., Harding S.M., for the American Academy of Sleep Medicine . American Academy of Sleep Medicine; Darien, IL: 2018. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology, and Technical Specifications, Version 2.5. [Google Scholar]
- 18.Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cielo C.M., Duffy K.A., Taylor J.A., Marcus C.L., Kalish J.M. Obstructive sleep apnea in children with Beckwith-Wiedemann syndrome. J Clin Sleep Med. 2019;15(3):375–381. doi: 10.5664/jcsm.7656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Driessen C., Joosten K.F., Bannink N. How does obstructive sleep apnoea evolve in syndromic craniosynostosis? A prospective cohort study. Arch Dis Child. 2013;98(7):538–543. doi: 10.1136/archdischild-2012-302745. [DOI] [PubMed] [Google Scholar]
- 21.Ramgopal S., Kothare S.V., Rana M., Singh K., Khatwa U. Obstructive sleep apnea in infancy: a 7-year experience at a pediatric sleep center. Pediatr Pulmonol. 2014;49(6):554–560. doi: 10.1002/ppul.22867. [DOI] [PubMed] [Google Scholar]
- 22.Leonardis R.L., Robison J.G., Otteson T.D. Evaluating the management of obstructive sleep apnea in neonates and infants. JAMA Otolaryngol Head Neck Surg. 2013;139(2):139–146. doi: 10.1001/jamaoto.2013.1331. [DOI] [PubMed] [Google Scholar]
- 23.Massa F., Gonsalez S., Laverty A., Wallis C., Lane R. The use of nasal continuous positive airway pressure to treat obstructive sleep apnoea. Arch Dis Child. 2002;87(5):438–443. doi: 10.1136/adc.87.5.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Amaddeo A., Moreau J., Frapin A. Long term continuous positive airway pressure (CPAP) and noninvasive ventilation (NIV) in children: initiation criteria in real life. Pediatr Pulmonol. 2016;51(9):968–974. doi: 10.1002/ppul.23416. [DOI] [PubMed] [Google Scholar]
- 25.Paruthi S., Brooks L.J., D’Ambrosio C. Recommended amount of sleep for pediatric populations: a consensus statement of the American Academy of Sleep Medicine. J Clin Sleep Med. 2016;12(6):785–786. doi: 10.5664/jcsm.5866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marcus C.L., Brooks L.J., Draper K.A. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012;130(3):576–584. doi: 10.1542/peds.2012-1671. [DOI] [PubMed] [Google Scholar]
- 27.Kaditis A.G., Alonso Alvarez M.L., Boudewyns A. ERS statement on obstructive sleep disordered breathing in 1- to 23-month-old children. Eur Respir J. 2017;50(6) doi: 10.1183/13993003.00985-2017. :1700985. [DOI] [PubMed] [Google Scholar]
- 28.Fauroux B., Lavis J.F., Nicot F. Facial side effects during noninvasive positive pressure ventilation in children. Intens Care Med. 2005;31(7):1700985. doi: 10.1007/s00134-005-2669-2. [DOI] [PubMed] [Google Scholar]
- 29.Hong H., Li X.X., Li J., Zhang Z.Q. High-flow nasal cannula versus nasal continuous positive airway pressure for respiratory support in preterm infants: a meta-analysis of randomized controlled trials. J Matern Fetal Neonatal Med. 2019:1–8. doi: 10.1080/14767058.2019.1606193. [DOI] [PubMed] [Google Scholar]
- 30.Roberts S.D., Kapadia H., Greenlee G., Chen M.L. Midfacial and dental changes associated with nasal positive airway pressure in children with obstructive sleep apnea and craniofacial conditions. J Clin Sleep Med. 2016;12(4):469–475. doi: 10.5664/jcsm.5668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Villa M.P., Pagani J., Ambrosio R., Ronchetti R., Bernkopf E. Mid-face hypoplasia after long-term nasal ventilation. Am J Respir Crit Care Med. 2002;166(8):1142–1143. doi: 10.1164/ajrccm.166.8.257c. [DOI] [PubMed] [Google Scholar]
- 32.Bedi P.K., Castro-Codesal M.L., Featherstone R. Long-term non-invasive ventilation in infants: a systematic review and meta-analysis. Front Pediatr. 2018;6:13. doi: 10.3389/fped.2018.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]