Abstract
Background
The interaction between tumor size and the comparative prognosis of lobar and sublobar resection has been defined poorly.
Research Question
The purpose of this study was to characterize the relationship between tumor size and the receipt of segmentectomy or lobectomy in association with overall survival in patients with clinically node-negative non-small cell lung cancer (NSCLC).
Study Design and Methods
The 2004-2015 National Cancer Database (NCDB) was queried for patients with cT1-3N0M0 NSCLC who underwent segmentectomy or lobectomy without neoadjuvant therapy or missing survival data. The primary outcome was overall survival, which was evaluated using multivariate Cox proportional hazards including an interaction term between tumor size and type of surgery.
Results
A total of 143,040 patients were included: 135,446 (95%) underwent lobectomy and 7594 (5%) underwent segmentectomy. In multivariate Cox regression, a significant three-way interaction was found among tumor size, histologic results, and type of surgery (P < .001). When patients were stratified by histologic results, lobectomy was associated with significantly improved survival compared with segmentectomy beyond a tumor size of approximately 10 mm for adenocarcinoma and 15 mm for squamous cell carcinoma that was recapitulated in subgroup analyses. No interaction between tumor size and type of surgery was found for patients with neuroendocrine tumors.
Interpretation
In this NCDB study of patients with node-negative NSCLC, we found different tumor size thresholds, based on histologic results, that identified populations of patients who least and most benefitted from lobectomy compared with segmentectomy.
Key Words: lobectomy, non-small cell lung cancer, sublobar resection, surgery
Abbreviations: NCDB, National Cancer Database; NSCLC, non-small cell lung cancer; SEER, Surveillance, Epidemiology, and End Results
FOR EDITORIAL COMMENT, SEE PAGE 21
Although lobectomy is considered the standard-of-care operation for patients with resectable non-small cell lung cancer (NSCLC), anatomic segmentectomy may be associated with similar survival, particularly in patients with early stage disease.1, 2, 3, 4, 5, 6, 7, 8, 9, 10 The only prospective trial comparing the two methods and with complete outcomes is the Lung Cancer Study Group trial from the 1990s, which randomized 276 patients with clinical T1N0 NSCLC to either lobar or sublobar resection.11 The study reported that sublobar resection, compared with lobectomy, was associated with a 30% increase in overall mortality and a 50% increase in cancer-related mortality. However, the Lung Cancer Study Group trial had several limitations. It included only 82 patients (67% of the sublobar group) who underwent segmentectomy and reported no subgroup analysis of this population. Clinical staging was performed using chest radiography. The study also predated the routine use of video-assisted thoracoscopic surgery and other improvements in perioperative management of patients, which limits its applicability to contemporary clinical practice. Two contemporary trials comparing lobar and sublobar resection, the Cancer and Leukemia Group B/Alliance 140503 and Japan Clinical Oncology Group 0802 trials, have not reported mid-term or longer-term outcomes yet, although both trials have described similar perioperative outcomes between both groups of patients.12,13 Observational studies also have reported similar survival among patients undergoing lobar and sublobar resection,2,4, 5, 6, 7,14, 15, 16 suggesting that even patients with good pulmonary function may be candidates for a segmental resection, rather than a lobectomy, which would preserve lung parenchyma and also would offer patients a less invasive method of treatment. Further, segmental resection offers theoretical benefits compared with wedge resection because it typically involves a greater area of resection and a higher probability of local control by removal of the parenchymal region with common lymphatic drainage and vascular supply as the primary tumor.
Take-home Point.
The aim of this study was to examine if a threshold tumor size exists above which lobectomy is associated with improved survival compared with segmentectomy for early lung cancer. Lobectomy was associated with significantly improved survival compared with segmentectomy beyond a tumor size of approximately 10 mm for adenocarcinoma and 15 mm for squamous cell carcinoma, whereas no interaction between tumor size and extent of resection was found in neuroendocrine tumors. In this cohort study, we found that tumor size and histologic findings could be used to identify populations of patients who least and most benefit from lobectomy compared with segmentectomy.
One of the primary clinical dilemmas is the selection of patients for segmental resection, rather than lobectomy. In addition to comorbidities including lung function, malnutrition, and frailty, tumor-related variables like size, histologic features, and location also are likely to play a role in this calculus. Although contemporary trials comparing lobar and sublobar resection have enrolled patients with tumors of 2 cm or less, and observational studies largely have considered this patient population as well, no studies have examined rigorously the association between tumor size and the comparative prognosis of patients undergoing segmentectomy and lobectomy. The primary purpose of this study was to characterize the influence of the relationship between tumor size and extent of surgery on survival in patients with clinically node-negative NSCLC. The secondary aim was to understand if a range of tumor size exists at which lobectomy and segmentectomy are associated with similar survival.
Methods
Data Source and Cohort Identification
This study was deemed exempt by the Duke University Institutional Review Board. The National Cancer Database (NCDB) was the data source of this study. It is a prospectively maintained registry that collects data on 70% of cancers, including 80% of all lung cancers, diagnosed across 1,500 Commission on Cancer centers in the United States and Puerto Rico every year.17 Data are entered by certified, independent tumor registrars who use standardized coding guidelines. The NCDB is a collaborative effort of the American Cancer Society and the American College of Surgeons.
The 2004-2015 participant user files of the NCDB were used to identify patients with clinical T1-3N0M0 NSCLC tumors who underwent either segmentectomy or lobectomy, including bilobectomy (e-Fig 1). Patients were excluded for the following reasons: clinical T4 status (by the American Joint Committee on Cancer, seventh or sixth edition, staging system recorded in the NCDB), receipt of neoadjuvant chemotherapy or radiation, missing tumor size, or missing survival. Patients who underwent a bronchial sleeve resection or pneumonectomy also were excluded. Because patients who had synchronous primary tumors were coded as either T4 or M1 in prior American Joint Committee on Cancer systems, they were also excluded from analysis.
Analysis
The primary purpose of this study was to identify if a threshold tumor size exists beyond which lobectomy is associated with improved survival compared with segmentectomy. The primary outcome was overall survival, which was calculated from the time of surgery. Patients in the study cohort were stratified by type of surgery. Background characteristics between groups were compared using the Wilcoxon rank sum and Pearson χ2 tests for continuous and categorical measures, respectively. A multivariate Cox proportional hazards model was constructed using covariates determined a priori to be of prognostic importance in this patient population: age, sex, race or ethnicity, Charlson-Deyo comorbidity score, insurance status, treatment at an academic center, annualized center surgical volume, histologic findings, identity of pulmonary lobe with primary tumor, tumor size, and type of surgery. To determine if tumor size mediated the relationship between extent of surgery and survival, an interaction term between tumor size and extent of surgery was included in this model. This interaction term was found to be significant (P < .001). Other covariates in the model then were tested for three-way interactions, and tumor histologic results were found to have a significant three-way interaction with tumor size and type of surgery, suggesting a meaningful relationship among histologic findings, tumor size, and extent of surgery in association with survival (Table 1). The year of diagnosis was not found to have a significant interaction with tumor size and extent of surgery.
Table 1.
Cox Multivariate Regression of Variables Independently Associated With Survival in the Overall Cohort of Patients, Including a Three-Way Interaction Term of Histologic Findings, Tumor Size, and Type of Surgery
| Variable | Hazard Ratio | 95% CI |
P Value | |
|---|---|---|---|---|
| Lower | Upper | |||
| Age (per 5 y) | 1.15 | 1.14 | 1.16 | < .001 |
| Sex (female) | 0.72 | 0.70 | 0.73 | < .001 |
| Race (reference: White) | ||||
| Black | 1.05 | 1.01 | 1.08 | .008 |
| Other | 0.74 | 0.69 | 0.79 | < .001 |
| Year of diagnosis (per y) | 0.98 | 0.98 | 0.98 | < .001 |
| Charlson-Deyo score (reference: 0) | ||||
| 1 | 1.18 | 1.16 | 1.21 | < .001 |
| 2+ | 1.47 | 1.43 | 1.50 | < .001 |
| Insurance status (reference: private) | ||||
| Government | 1.19 | 1.16 | 1.22 | < .001 |
| None | 1.30 | 1.20 | 1.41 | < .001 |
| Facility type (reference: nonacademic) | ||||
| Academic/research program | 0.93 | 0.91 | 0.95 | < .001 |
| Annualized center surgical volume (per 5 cases/y) | 1.00 | 1.00 | 1.03 | < .001 |
| Histologic findings (reference: adenocarcinoma) | ||||
| Squamous cell carcinoma | 1.24 | 1.20 | 1.27 | < .001 |
| Adenosquamous carcinoma | 1.47 | 1.38 | 1.57 | < .001 |
| Large cell carcinoma | 1.54 | 1.43 | 1.65 | < .001 |
| Carcinoid | 0.45 | 0.41 | 0.50 | < .001 |
| Sarcomatoid | 1.57 | 1.32 | 1.86 | < .001 |
| Lobe (reference: right upper lobe) | ||||
| Right middle | 1.05 | 1.01 | 1.10 | .02 |
| Right lower | 1.10 | 1.07 | 1.13 | < .001 |
| Left upper | 1.02 | 1.00 | 1.05 | .04 |
| Left lower | 1.05 | 1.02 | 1.08 | < .001 |
| Tumor size (per 5 mm) | 1.00 | 1.00 | 1.03 | < .001 |
| Type of surgery (reference: lobectomy) | ||||
| Segmentectomy | 1.01 | 0.94 | 1.09 | .83 |
| Interaction of tumor size and type of surgery | .04 | |||
| Interaction of histologic findings, tumor size, and type of surgery | < .001 | |||
Complete cases: 132,318; events observed: 47,505.
For ease of modelling and interpretation, patients in the original cohort were divided into groups based on histologic findings for further analysis. Surveillance, Epidemiology, and End Results (SEER) International Classification of Diseases for Oncology Third Edition codes were used to subset patients by histologic findings. For each histologic group, a multivariate Cox model with identical covariates as above was developed, including a two-way interaction term between tumor size and type of surgery (Table 3). This interaction then was graphed to identify if an approximate tumor size exists above which adjusted survival is improved with lobectomy compared with segmentectomy and below which segmentectomy and lobectomy are associated with similar survival. Tumor size, age, and center volume were modelled using restricted cubic splines with five knots to obtain a more accurate visual representation.18 The region in tumor size where the survival curves of lobectomy and segmentectomy patients started to diverge and where the CIs were narrowest was determined to represent the approximate threshold. In some histologic groups, no clear threshold was identified.
Table 3.
Summary of Analyses in Patients Stratified by Histologic Findings
| Cohort | Cohort Size | No. of Patients Undergoing Lobectomy | No. of Patients Undergoing Segmentectomy | Overall P Value of Interaction Between Tumor Size and Type of Surgery |
|---|---|---|---|---|
| Adenocarcinoma | ||||
| Overall cohort | 86,573 | 81,926 | 4,647 | < .001 |
| Lepidic subgroup | 18,687 | 17,468 | 1,219 | .03 |
| Papillary subgroup | 2,099 | 1,990 | 109 | .61 |
| Mucinous subgroup | 7,739 | 7,273 | 466 | .21 |
| Acinar subgroup | 4,210 | 3,928 | 282 | .67 |
| pN0 subgroup | 66,303 | 62,784 | 3,519 | .007 |
| Squamous cell carcinoma | ||||
| Overall cohort | 36,613 | 34,747 | 1,866 | .03 |
| pN0 subgroup | 28,952 | 27,521 | 1,431 | < .001 |
| Neuroendocrine tumors | ||||
| Large cell | 3,240 | 3,076 | 164 | .27 |
| Carcinoid | 6,927 | 6,491 | 436 | .61 |
Each row represents a unique multivariate Cox proportional hazards regression. The models were adjusted for age, sex, race and ethnicity, year of diagnosis, Charlson-Deyo score, insurance status, treatment at an academic center, annualized center surgical volume, identity of lobe, tumor size, type of surgery, and an interaction term between tumor size and type of surgery.
Two subgroup analyses were performed to examine findings from the central analyses further. First, because the NCDB catalogs the first staging documented by a physician as the clinical stage, it is unlikely to include data from invasive nodal staging in the clinical stage. Although invasive nodal staging is not recommended routinely for patients with clinical stage I disease, we believed that it may have confounded the clinical nodal staging in the patient population, especially because of the significant disparity in pathologic nodal upstaging between patients undergoing a lobectomy and segmentectomy (Table 2). To mitigate this, we identified subgroups of patients with adenocarcinoma or squamous cell carcinoma who had both clinical and pathologic node-negative disease and repeated the multivariate Cox regression with interaction term modelling in these subgroups to identify if the survival curves changed substantially. In these analyses, three-way interactions among tumor size, type of treatment, and other covariates, including minimally invasive approach to surgery, number of lymph nodes assessed in surgery, and margin status, were assessed and found to be not significant. Second, in an effort to confirm if the visualized thresholds were accurate, we divided patients in each of the two major histologic groups, adenocarcinoma and squamous cell carcinoma, into two groups based on the identified threshold tumor size. We then performed multivariate Cox regression including the same covariates as above, but without an interaction term, to examine the effect estimate associated with type of surgery. For all analyses, a two-sided P value of 0.05 or less was determined to be significant. Missing data were handled with complete case analysis in regression. All analyses were performed with R version 3.5.1 software (R Foundation for Statistical Computing).
Table 2.
Background Characteristics of Study Patients Stratified by Extent of Surgery
| Variable | Lobectomy (n = 135,446) | Segmentectomy (n = 7,594) | P Value |
|---|---|---|---|
| Age, y | 68 (61-75) | 71 (64-77) | < .001 |
| Sex (female) | 72,253 (53) | 4,436 (58) | < .001 |
| Race/ethnicity | < .001 | ||
| White | 119,917 (89) | 6,838 (91) | |
| Black | 10,820 (8) | 505 (7) | |
| Other | 3,862 (3) | 192 (3) | |
| Year of diagnosis | 2011 (2008-2013) | 2011 (2009-2013) | < .001 |
| Charlson-Deyo score | < .001 | ||
| 0 | 70,407 (52) | 3,542 (47) | |
| 1 | 47,096 (35) | 2,784 (37) | |
| ≥2 | 17,943 (13) | 1,268 (17) | |
| Insurance | < .001 | ||
| Private | 41,501 (31) | 1,952 (26) | |
| Government | 90,104 (67) | 5,491 (73) | |
| None | 2,306 (2) | 75 (1) | |
| Academic center | 48,474 (36) | 3,368 (44) | < .001 |
| Center surgical volume (cases/y) | 46 (25-78) | 59 (29-105) | < .001 |
| Histologic findings | < .001 | ||
| Adenocarcinoma | 81,926 (63) | 4,647 (63) | |
| Squamous cell carcinoma | 34,747 (27) | 1,866 (26) | |
| Adenosquamous carcinoma | 3,293 (3) | 182 (3) | |
| Large cell carcinoma | 3,076 (2) | 164 (2) | |
| Carcinoid | 6,491 (5) | 436 (6) | |
| Sarcomatoid | 909 (1) | 34 (1) | |
| Lobe | < .001 | ||
| Right upper | 45,659 (35) | 1,725 (23) | |
| Right middle | 8,296 (6) | 136 (2) | |
| Right lower | 24,616 (19) | 1,630 (22) | |
| Left upper | 33,499 (25) | 2,442 (33) | |
| Left lower | 20,177 (15) | 1,489 (20) | |
| Tumor size (mm) | 25 (17-35) | 20 (14-26) | < .001 |
| Minimally invasive approach | 30,573 (23) | 3,581 (34) | < .001 |
| Number of nodes examined | 8 (5-14) | 4 (1-9) | < .001 |
| Positive surgical margins | 4,138 (3) | 293 (4) | < .001 |
| Pathologic nodal upstaging | 20,312 (15) | 1,774 (23) | < .001 |
| Clinicopathologic T-status concordance | 94,703 (70) | 5,449 (72) | .002 |
| Adjuvant chemotherapy | 22,970 (17) | 642 (9) | < .001 |
Categorical variables are expressed as No. (%) and continuous variables are expressed as median (interquartile range).
Results
Overall Cohort
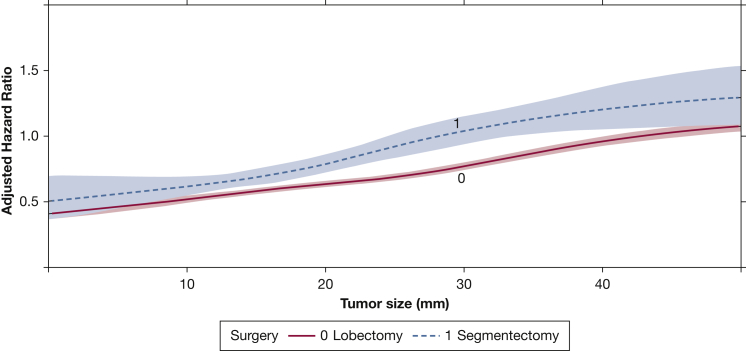
A total of 143,040 patients met criteria for the study, including 135,446 (95%) who underwent lobectomy and 7594 (5%) who underwent segmentectomy. Compared with those who underwent lobectomy, patients who underwent segmentectomy were more likely to be older, to be female, to have comorbidities, to have government insurance, to be treated at a higher-volume center and academic center, to have a left-sided primary lesion, and to have a smaller lesion (Table 2). In a multivariate Cox model, tumor size was found to have a significant two-way interaction with type of surgery as well as a three-way interaction with histologic findings and type of surgery (Table 1). After accounting for interactions, the receipt of segmentectomy was not associated with improved survival compared with lobectomy (hazard ratio, 1.01; 95% CI, 0.94-1.09). A graph of the interaction between tumor size and type of surgery showed that the survival curves of patients undergoing lobectomy or segmentectomy began to diverge beyond a tumor size of approximately 10 mm, suggesting that patients experienced a substantial survival benefit with lobectomy with increasing tumor size beyond 10 mm (Fig 1).
Figure 1.
Graph showing the interaction between tumor size and type of surgery as a function of adjusted hazard ratio of mortality from a multivariate Cox proportional hazards model including an interaction term among tumor size, histologic results, and type of surgery. The overall cohort, regardless of histologic findings, is modelled in this plot. The x-axis shows the tumor size in millimeters, whereas the y-axis demonstrates the adjusted hazard ratio from the Cox model. The survival curves for patients who underwent lobectomy or segmentectomy are depicted and modelled using restricted cubic splines. The shaded areas represent the bounds of the 95% CI.
Histologic Subgroups
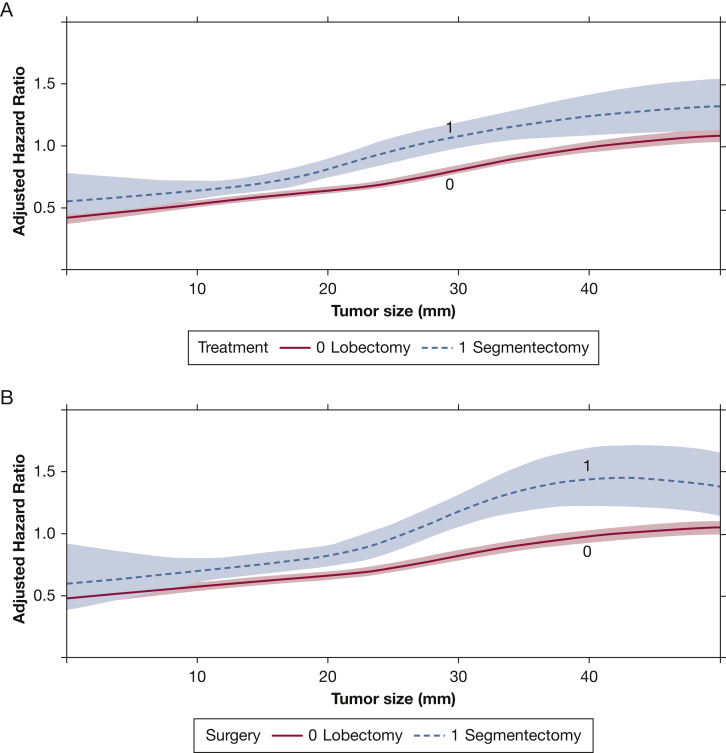
A total of 86,573 patients with adenocarcinoma were identified from the overall cohort. An interaction between tumor size and type of surgery persisted in this subgroup in a multivariate Cox model (Table 3). In a graph of this interaction, the survival curves of patients who underwent lobectomy and segmentectomy began to diverge beyond a tumor size of approximately 10 mm (Fig 2A). In a subgroup of patients with pathologic N0 disease, a similar divergence pattern was observed (Fig 2B). In a subset of 18,687 patients with lepidic histologic findings, no reliable difference between the survival curves could be identified as a function of tumor size, despite a significant interaction (Table 3, e-Fig 2A). In 2099 patients with papillary histologic results, no interaction, quantitative or visual, was observed (Table 3, e-Fig 2B). Interactions between tumor size and extent of surgery also were not found in patients with mucinous and acinar patterns (Table 3, e-Fig 2C, 2D). A sensitivity analysis then was performed in 3223 adenocarcinoma patients with a tumor size < 10 mm. In this group, segmentectomy was not associated with significantly worse survival compared with lobectomy in multivariate regression (e-Table 1). However, segmentectomy was associated with worse survival compared with lobectomy in 83,360 patients with tumor size ≥ 10 mm.
Figure 2.
A, B, Graphs showing the interaction between tumor size and type of surgery as a function of adjusted hazard ratio of mortality from a multivariate Cox proportional hazards model including an interaction term between tumor size and type of surgery. These graphs show the overall group of patients with adenocarcinoma (A) and the subgroup with pathologic N0 disease as well (B). The x-axis shows the tumor size in millimeters, whereas the y-axis demonstrates the adjusted hazard ratio from the Cox model. The survival curves for patients who underwent lobectomy or segmentectomy are depicted and modelled using restricted cubic splines. The shaded areas represent the bounds of the 95% CI.
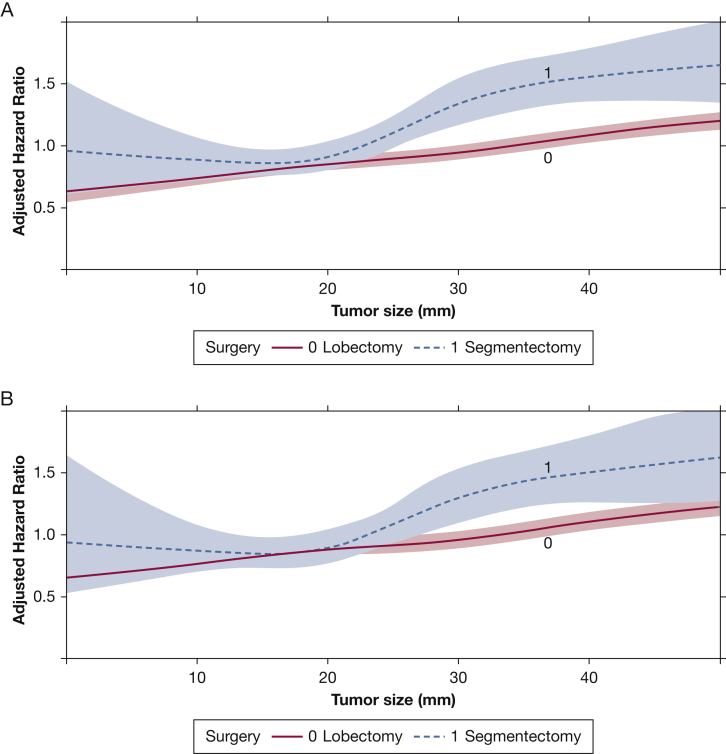
A total of 36,613 patients with squamous cell histologic findings were identified; once again, an interaction between tumor size and type of surgery was demonstrated in a multivariate Cox model in this subgroup (Table 3). A plot of this interaction showed convergence of the survival curves at a tumor size of approximately 15 mm, followed by divergence closer to 20 mm (Fig 3A). This finding was recapitulated in a subset of 28,952 patients with pathologic N0 disease (Fig 3B). In a subgroup of 3944 patients with tumors smaller than 15 mm, segmentectomy was not associated with significantly worse survival, whereas the survival difference reached significance in 32,676 patients with tumors ≥ 15 mm (e-Table 1).
Figure 3.
A, B, Graphs showing the interaction between tumor size and type of surgery as a function of adjusted hazard ratio of mortality from a multivariate Cox proportional hazards model including an interaction term between tumor size and type of surgery. These graphs show the overall group of patients with squamous cell carcinoma (A) and the subgroup with pathologic N0 disease as well (B). The x-axis shows the tumor size in millimeters, whereas the y-axis demonstrates the adjusted hazard ratio from the Cox model. The survival curves for patients who underwent lobectomy or segmentectomy are depicted and modelled using restricted cubic splines. The shaded areas represent the bounds of the 95% CI.
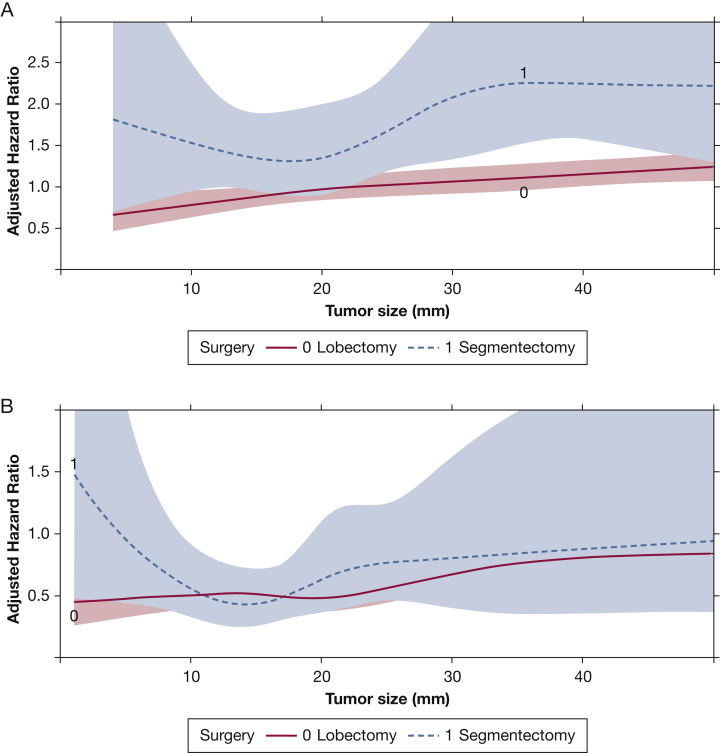
Among patients with neuroendocrine tumors, we identified 3240 patients with large cell lung cancer. No significant interaction was found between tumor size and type of surgery in this patient population (Table 3, Fig 4A). In this group, segmentectomy was associated with worse survival compared with lobectomy (hazard ratio, 1.62; 95% CI, 1.32-1.99) in multivariate regression. Similarly, no interaction was observed between tumor size and type of surgery in 6,927 patients with carcinoid tumors (Table 3, Fig 4B). Segmentectomy was not associated with worse survival compared with lobectomy in this population (hazard ratio, 1.09; 95% CI, 0.82-1.46).
Figure 4.
A, B, Graphs showing the interaction between tumor size and type of surgery as a function of adjusted hazard ratio of mortality from a multivariate Cox proportional hazards model including an interaction term between tumor size and type of surgery. These graphs show the overall group of patients with large cell lung cancer (A) and those with carcinoid histologic findings (B). The x-axis shows the tumor size in millimeters, whereas the y-axis demonstrates the adjusted hazard ratio from the Cox model. The survival curves for patients who underwent lobectomy or segmentectomy are depicted and modelled using restricted cubic splines. The shaded areas represent the bounds of the 95% CI.
Discussion
In this NCDB analysis of patients with clinically node-negative NSCLC, we found that tumor size, histologic results, and extent of surgery showed a meaningful relationship in association with overall survival. Further, we identified approximate threshold tumor sizes above which lobectomy was associated with improved survival compared with segmentectomy in patients with adenocarcinoma or squamous cell carcinoma. We also did not find a meaningful interaction between tumor size and survival associated with type of surgery with other histologic findings, including neuroendocrine tumors. Our study suggests that tumor size and histologic features may be considered in the decision to offer patients segmentectomy or lobectomy for early node-negative NSCLC.
Few studies have examined the relationship between tumor size and extent of surgery in patients with early NSCLC. Carr and coworkers19 stratified 429 patients at a single institution who underwent either lobectomy or segmentectomy by T stage and found no differences between the groups in unadjusted survival or recurrence for T1a or T1b disease. Whitson and coworkers20 examined 14,473 patients in the SEER registry with stage I NSCLC. After stratifying patients by tumor size, they found that lobectomy was associated with improved adjusted overall survival compared with segmentectomy for tumor size ≤ 2 cm and > 3 cm, but not for tumor size between 2 and 3 cm. In a more contemporary SEER analysis, Cao and coworkers7 studied 16,819 patients with clinical stage IA disease who underwent wedge resection, segmentectomy, or lobectomy. They stratified patients based on tumor size (< 1 cm, 1-2 cm, and 2-3 cm) and demonstrated that adjusted cancer-specific survival is similar between patients who underwent segmentectomy and lobectomy at a tumor size of up to 2 cm. However, these studies did not treat tumor size as a continuous variable, did not examine an interaction between tumor size and type of surgery in association with survival, did not consider histologic findings, and included far fewer patients than our study.
The results of our study generate the hypothesis that different tumor size thresholds, based on histologic results, may be used to allocate patients with early NSCLC to segmental or lobar resection. The thresholds identified in our study, 10 mm for adenocarcinoma and 15 mm for squamous cell carcinoma, are approximate, drawn from visual interpretation of interaction plots, and represent tumor sizes at which the survival benefit associated with lobectomy crosses significance. As a result, these thresholds should not be used as definitive cutoff values. Instead, the shape of the interaction curves proves more insightful. For patients with adenocarcinoma, the survival curves diverge substantially beyond a tumor size of approximately 15 mm and are far apart by a size of approximately 30 mm, suggesting that patients with tumors beyond 15 mm experience a great survival benefit with lobectomy compared with segmentectomy (Fig 2A). However, true separation of the survival curves occurs beyond approximately 20 mm and peaks at approximately 35 mm in patients with squamous cell histologic features (Fig 3), suggesting that the survival benefit associated with lobectomy is greatest in this range. The difference in tumor size thresholds between patients with adenocarcinoma and squamous cell carcinoma may be explained by study-related characteristics like cohort sizes or histologic-related features like invasiveness. However, tumor size does not seem to be related meaningfully to the survival associated with extent of surgery in patients with neuroendocrine histologic findings. In our study, patients with large cell lung cancer experienced a survival benefit with lobectomy over the range of tumor sizes. In contrast, patients with carcinoid tumors experienced similar survival with lobectomy compared with those undergoing segmentectomy. These trends have been demonstrated in previous studies.21, 22, 23
Our study is framed by important limitations. Selection bias almost certainly confounded the results of our analysis because the choice to offer patients segmentectomy or lobectomy often is predicated on factors like age and comorbidities, including preoperative lung function. Although we adjusted for age and the Charlson-Deyo comorbidity index score in our analyses, we were limited by the lack of availability of data in the NCDB about lung function and frailty. Adjustment for the likely higher preoperative risk for patients undergoing segmentectomy may have decreased the adjusted hazard of mortality in this group and may have led to convergence of the survival curves at higher tumor sizes. We also were unable to adjust for other potentially confounding variables in our model, including the specific anatomic features of the primary lesion (central or peripheral), solid component of nodules, major and minor histologic components, presence of spread through airspaces, targeted sequencing data like epidermal growth factor receptor status, and type of segmentectomy (identity of segments and whether the segmentectomy was single or composite). In addition, because the NCDB catalogs only the most definitive operation that a patient underwent, those who received a lobectomy and an additional segmental or wedge resection for a fissure-crossing lesion, for instance, would be cataloged as having undergone only a lobectomy. One of the important limitations of our study is that the NCDB catalogs tumor size primarily based on surgical pathology reports. The concordance between radiographic tumor size from axial imaging commonly used for clinical staging and tumor size measured in pathologic analysis remains to be demonstrated, although ≥ 70% of patients in our cohort showed clinicopathologic T-status concordance and studies of other malignancies have demonstrated radiographic-pathologic size concordance.24,25 As with other registry studies, we also are reliant on the accurate and consistent coding of variables, which we cannot ascertain. The NCDB also does not provide data about recurrence and disease-free survival, which would have provided a more accurate primary outcome measure and would be less likely to be confounded by the severity of patient comorbidities, which is not captured by the database. Although the SEER registry offers data about disease-free survival, it is comparatively limited by the absence of data about clinical stage and comorbidities and also represents a much smaller population sample than the NCDB. Finally, because this is the first study to use the methods described, the external validity of our findings is unknown and should be explored in independent data sources.
Conclusions
In this analysis of 143,040 patients with clinically node-negative NSCLC in the NCDB, we found that tumor size and histologic findings can be used to identify populations of patients who least and most benefit from lobectomy compared with segmentectomy.
Acknowledgements
Author contributions: V. R., C. J. Y., and B. C. T. designed the study. V. R. and O. K. J. performed the analysis. All authors analyzed the data and participated in writing the manuscript. All authors approved the final version of the manuscript.
Financial/nonfinancial disclosures: None declared.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Other contributions: The American College of Surgeons is in a Business Associate Agreement that includes a data use agreement with each of its Commission on Cancer-accredited hospitals. The data used in the study are derived from a de-identified National Cancer Data Base file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology used or the conclusions drawn from these data by the investigators.
Additional information: The e-Figures and e-Table can be found in the Supplemental Materials section of the online article.
Footnotes
Drs Yang and Tong contributed equally to this work.
FUNDING/SUPPORT: Dr Raman is supported by a National Institutes of Health T-32 grant [5T32CA093245] in surgical oncology. Dr Jawitz is supported by a National Institutes of Health T-32 grant [5T32HL069749] in clinical research.
Supplementary Data
References
- 1.Dai C., Shen J., Ren Y. Choice of surgical procedure for patients with non-small-cell lung cancer ≤ 1 cm or > 1 to 2 cm among lobectomy, segmentectomy, and wedge resection: a population-based study. J Clin Oncol. 2016;34(26):3175–3182. doi: 10.1200/JCO.2015.64.6729. [DOI] [PubMed] [Google Scholar]
- 2.Kamel M.K., Rahouma M., Lee B. Segmentectomy is equivalent to lobectomy in hypermetabolic clinical stage IA lung adenocarcinomas. Ann Thorac Surg. 2019;107(1):217–223. doi: 10.1016/j.athoracsur.2018.07.042. [DOI] [PubMed] [Google Scholar]
- 3.Zhao Z.-R., Situ D.-R., Lau R.W.H. Comparison of segmentectomy and lobectomy in stage IA adenocarcinomas. J Thorac Oncol. 2017;12(5):890–896. doi: 10.1016/j.jtho.2017.01.012. [DOI] [PubMed] [Google Scholar]
- 4.Dziedzic R., Zurek W., Marjanski T. Stage I non-small-cell lung cancer: long-term results of lobectomy versus sublobar resection from the Polish National Lung Cancer Registry. Eur J Cardiothorac Surg. 2017;52(2):363–369. doi: 10.1093/ejcts/ezx092. [DOI] [PubMed] [Google Scholar]
- 5.Bedetti B., Bertolaccini L., Rocco R., Schmidt J., Solli P., Scarci M. Segmentectomy versus lobectomy for stage I non-small cell lung cancer: a systematic review and meta-analysis. J Thorac Dis. 2017;9(6):1615–1623. doi: 10.21037/jtd.2017.05.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cao C., Tian D., Park J., Allan J., Pataky K.A., Yan T.D. A systematic review and meta-analysis of surgical treatments for malignant pleural mesothelioma. Lung Cancer. 2014;83(2):240–245. doi: 10.1016/j.lungcan.2013.11.026. [DOI] [PubMed] [Google Scholar]
- 7.Cao J., Yuan P., Wang Y. Survival rates after lobectomy, segmentectomy, and wedge resection for non-small cell lung cancer. Ann Thorac Surg. 2018;105(5):1483–1491. doi: 10.1016/j.athoracsur.2018.01.032. [DOI] [PubMed] [Google Scholar]
- 8.Khullar O.V., Liu Y., Gillespie T. Survival after sublobar resection versus lobectomy for clinical stage IA lung cancer: an analysis from the National Cancer Data Base. J Thorac Oncol. 2015;10(11):1625–1633. doi: 10.1097/JTO.0000000000000664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Subramanian M., McMurry T., Meyers B.F., Puri V., Kozower B.D. Long-term results for clinical stage IA lung cancer: comparing lobectomy and sublobar resection. Ann Thorac Surg. 2018;106(2):375–381. doi: 10.1016/j.athoracsur.2018.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhong C., Sakurai H., Wei S., Fang W., Asamura H. Sublobar resections for small-sized stage Ia lung adenocarcinoma: a Sino-Japanese multicenter study. J Thorac Dis. 2018;10(2):991–998. doi: 10.21037/jtd.2018.01.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ginsberg R.J., Rubinstein L.V. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Ann Thorac Surg. 1995;60(3):615–623. doi: 10.1016/0003-4975(95)00537-u. [DOI] [PubMed] [Google Scholar]
- 12.Suzuki K., Saji H., Aokage K. Comparison of pulmonary segmentectomy and lobectomy: safety results of a randomized trial. J Thorac Cardiovasc Surg. 2019;158(3):895–907. doi: 10.1016/j.jtcvs.2019.03.090. [DOI] [PubMed] [Google Scholar]
- 13.Altorki N.K., Wang X., Wigle D. Perioperative mortality and morbidity after sublobar versus lobar resection for early-stage non-small-cell lung cancer: post-hoc analysis of an international, randomised, phase 3 trial (CALGB/Alliance 140503) Lancet Respir Med. 2018;6(12):915–924. doi: 10.1016/S2213-2600(18)30411-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roman M., Labbouz S., Valtzoglou V. Lobectomy vs. segmentectomy. A propensity score matched comparison of outcomes. Eur J Surg Oncol. 2019;45(5):845–850. doi: 10.1016/j.ejso.2018.10.534. [DOI] [PubMed] [Google Scholar]
- 15.Cao C., Chandrakumar D., Gupta S., Yan T.D., Tian D.H. Could less be more?—A systematic review and meta-analysis of sublobar resections versus lobectomy for non-small cell lung cancer according to patient selection. Lung Cancer. 2015;89(2):121–132. doi: 10.1016/j.lungcan.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 16.Ijsseldijk M.A., Shoni M., Siegert C. Oncological outcomes of lobar resection, segmentectomy, and wedge resection for T1a non–small-cell lung carcinoma: a systematic review and meta-analysis. Semin Thorac Cardiovasc Surg. 2020;32(3):582–590. doi: 10.1053/j.semtcvs.2019.08.004. [DOI] [PubMed] [Google Scholar]
- 17.Bilimoria K.Y., Stewart A.K., Winchester D.P., Ko C.Y. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol. 2008;15(3):683–690. doi: 10.1245/s10434-007-9747-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harrell F.E. Multivariable modeling strategies. In: Harrell F.E. Jr., editor. Regression Modeling Strategies: With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis. Springer Series in Statistics. New York, NY:Springer International Publishing; 2015. pp. 63–102. [Google Scholar]
- 19.Carr S.R., Schuchert M.J., Pennathur A. Impact of tumor size on outcomes after anatomic lung resection for stage 1A non-small cell lung cancer based on the current staging system. J Thorac Cardiovasc Surg. 2012;143(2):390–397. doi: 10.1016/j.jtcvs.2011.10.023. [DOI] [PubMed] [Google Scholar]
- 20.Whitson B.A., Groth S.S., Andrade R.S., Maddaus M.A., Habermann E.B., D’Cunha J. Survival after lobectomy versus segmentectomy for stage I non-small cell lung cancer: a population-based analysis. Ann Thorac Surg. 2011;92(6):1943–1950. doi: 10.1016/j.athoracsur.2011.05.091. [DOI] [PubMed] [Google Scholar]
- 21.Raman V., Jawitz O.K., Yang C.-F.J. Outcomes for surgery in large cell lung neuroendocrine cancer. J Thorac Oncol. 2019;14(12):2143–2151. doi: 10.1016/j.jtho.2019.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cattoni M., Vallières E., Brown L.M. Sublobar resection in the treatment of peripheral typical carcinoid tumors of the lung. Ann Thorac Surg. 2019;108(3):859–865. doi: 10.1016/j.athoracsur.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 23.Yendamuri S., Gold D., Jayaprakash V., Dexter E., Nwogu C., Demmy T. Is sublobar resection sufficient for carcinoid tumors? Ann Thorac Surg. 2011;92(5):1774–1778. doi: 10.1016/j.athoracsur.2010.08.080. discussion 1778-1779. [DOI] [PubMed] [Google Scholar]
- 24.Chen W., Wang L., Yang Q. Comparison of radiographic and pathologic sizes of renal tumors. Int Braz J Urol. 2013;39(2):189–194. doi: 10.1590/S1677-5538.IBJU.2013.02.06. [DOI] [PubMed] [Google Scholar]
- 25.Lee S.E., Lee W.K., Kim D.S. Comparison of radiographic and pathologic sizes of renal tumors. World J Urol. 2010;28(3):263–267. doi: 10.1007/s00345-010-0511-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.