Key Points
Question
Is the dispatch of mobile stroke units in the out-of-hospital setting before arriving at the hospital associated with better functional outcomes among patients with acute ischemic stroke eligible for thrombolysis or thrombectomy?
Findings
In this prospective nonrandomized controlled intervention study involving 1543 patients in Berlin, Germany, the dispatch of mobile stroke units in addition to conventional ambulances vs conventional ambulances alone was significantly associated with lower levels of global disability at 3 months (common odds ratio for higher modified Rankin Scale scores [ie, worse outcome], 0.71).
Meaning
Among patients with acute ischemic stroke in Berlin, Germany, dispatch of a mobile stroke unit was associated with lower global disability at 3 months; further research in diverse settings is needed.
Abstract
Importance
Effects of thrombolysis in acute ischemic stroke are time-dependent. Ambulances that can administer thrombolysis (mobile stroke units [MSUs]) before arriving at the hospital have been shown to reduce time to treatment.
Objective
To determine whether dispatch of MSUs is associated with better clinical outcomes for patients with acute ischemic stroke.
Design, Setting, and Participants
This prospective, nonrandomized, controlled intervention study was conducted in Berlin, Germany, from February 1, 2017, to October 30, 2019. If an emergency call prompted suspicion of stroke, both a conventional ambulance and an MSU, when available, were dispatched. Functional outcomes of patients with final diagnosis of acute cerebral ischemia who were eligible for thrombolysis or thrombectomy were compared based on the initial dispatch (both MSU and conventional ambulance or conventional ambulance only).
Exposure
Simultaneous dispatch of an MSU (computed tomographic scanning with or without angiography, point-of-care laboratory testing, and thrombolysis capabilities on board) and a conventional ambulance (n = 749) vs conventional ambulance alone (n = 794).
Main Outcomes and Measures
The primary outcome was the distribution of modified Rankin Scale (mRS) scores (a disability score ranging from 0, no neurological deficits, to 6, death) at 3 months. The coprimary outcome was a 3-tier disability scale at 3 months (none to moderate disability; severe disability; death) with tier assignment based on mRS scores if available or place of residence if mRS scores were not available. Common odds ratios (ORs) were used to quantify the association between exposure and outcome; values less than 1.00 indicated a favorable shift in the mRS distribution and lower odds of higher levels of disability.
Results
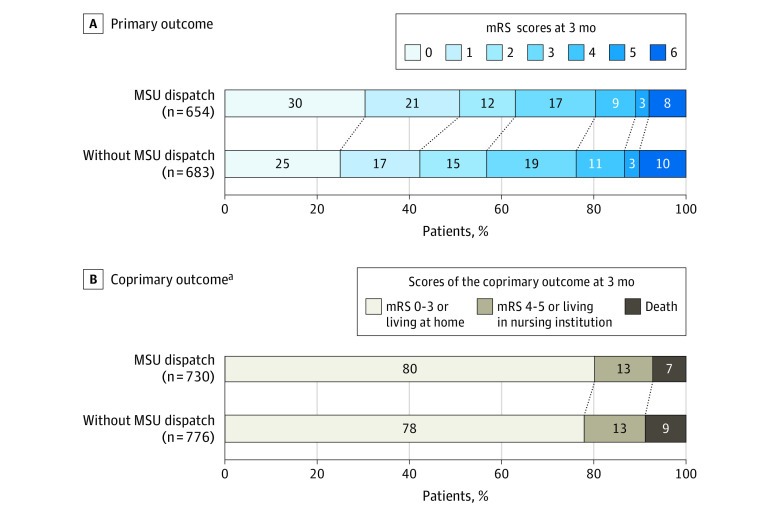
Of the 1543 patients (mean age, 74 years; 723 women [47%]) included in the adjusted primary analysis, 1337 (87%) had available mRS scores (primary outcome) and 1506 patients (98%) had available the 3-tier disability scale assessment (coprimary outcome). Patients with an MSU dispatched had lower median mRS scores at month 3 (1; interquartile range [IQR], 0-3) than did patients without an MSU dispatched (2; IQR, 0-3; common OR for worse mRS, 0.71; 95% CI, 0.58-0.86; P < .001). Similarly, patients with an MSU dispatched had lower 3-month coprimary disability scores: 586 patients (80.3%) had none to moderate disability; 92 (12.6%) had severe disability; and 52 (7.1%) had died vs patients without an MSU dispatched: 605 (78.0%) had none to moderate disability; 103 (13.3%) had severe disability; and 68 (8.8%) had died (common OR for worse functional outcome, 0.73, 95% CI, 0.54-0.99; P = .04).
Conclusions and Relevance
In this prospective, nonrandomized, controlled intervention study of patients with acute ischemic stroke in Berlin, Germany, the dispatch of mobile stroke units, compared with conventional ambulances alone, was significantly associated with lower global disability at 3 months. Clinical trials in other regions are warranted.
This cohort study compares global disability at 3 months among Berlin patients with out-of-hospital ischemic stroke brought to care via a mobile stroke unit ambulance (with prehospital CT scanning with or without angiography, point-of-care laboratory testing, prehospital thrombolysis) vs conventional ambulance alone.
Introduction
Current guidelines recommend thrombolysis as rapidly as possible for treating acute ischemic stroke.1 Mobile stroke units (MSUs) are ambulances equipped with a computed tomographic (CT) scanner designed to allow thrombolysis prior to hospital arrival.2,3,4 Mobile stroke units shorten the time to treatment, increase thrombolysis rates, and improve prehospital triage.5,6,7,8 However, potential effects of MSUs on functional outcomes after stroke are uncertain. Registry-based studies have suggested a potential for outcome improvement among patients who receive thrombolysis in MSUs compared with in-hospital thrombolysis.9,10 Most guidelines do not explicitly comment on the use of MSUs, in part due to sparse evidence.1,11 Currently, no randomized clinical trial has been completed.12
The objective of this study was to evaluate the association between the additional dispatch of MSUs vs conventional ambulance alone and functional outcomes at 3 months among patients with acute ischemic stroke.
Methods
In agreement with the data protection officers, an opt-out solution was chosen for informed consent. All enrolled patients with stroke were informed in writing 2 months after the qualifying event that their data including follow-up would be used for quality control and, if fulfilling the eligibility criteria for this study, they would be contacted for a telephone follow-up assessment 3 months after the index event. Patients had the option to complete a written questionnaire, decline follow-up (opt-out), or request deletion of their entire study documentation.
The study protocol was approved by the ethical review committee of the Charité–University Medicine Berlin (EA4/109/15) on September 2, 2015. The original Study Protocol, the Statistical Analysis Plan, and additional statistical evaluations performed during the manuscript revision are available in Supplement 1.
Study Design
This was an investigator-initiated, nonrandomized controlled intervention study with blinded outcome assessment, comparing outcomes from patients with acute cerebral ischemia in Berlin, Germany, for whom MSUs were or were not dispatched. Based on results of a preceding study6 with increased frequency of thrombolysis and shorter time to treatment, MSU service was integrated into the regular emergency medical services (EMS) in Berlin (so-called “provisional regular care”) under the condition of a parallel scientific evaluation of outcomes among patients to whom an MSU or a conventional ambulance was dispatched. In the previous study,13 the MSU was not available for 44% of calls because it was already in operation or in service or maintenance.6
Patients with an MSU dispatch code for whom an MSU was unavailable formed the comparison group in this current study. Dispatchers suspecting acute stroke assigned MSU dispatch codes that simultaneously sent both a conventional ambulance and an MSU to the scene.
Blinding of patients and medical personnel was not possible. Dispatchers used the routine acute stroke identification algorithm that leads to a code stroke, and if the emergency call was made within 4 hours of symptom onset or if onset was unknown, dispatchers were to send an MSU, if available. The use of the MSU dispatch code was made irrespective of MSU availability. If the MSU was not available, a conventional ambulance drove to the site. If the MSU was available, the code led to its simultaneous dispatch.
Data Collection
Data sources included the Berlin Specific Acute Treatment in Ischemic or Haemorrhagic Stroke With Long-Term Follow-up (B-SPATiAL, NCT03027453) registry for quality assessment of acute stroke management and outcomes in all 15 hospitals with stroke units in the city borders and documentation by the Berlin Fire Brigade, which runs the city’s EMS including MSUs. The registry included all patients with stroke and transient ischemic attack (TIA) who presented with neurological symptoms to EMS personnel or upon hospital arrival within 6 hours of symptom onset. Trained study nurses extracted data from the documentation systems and inpatient charts. They used the times as documented in the EMS database, in EMS protocols, and in electronic hospital records systems. The registry provided data on baseline and process parameters, final diagnosis, and follow-up information.
Each month, the Berlin Fire Brigade provided a list of all stroke alarms. Patients with an MSU dispatch code and who were transported to a hospital were included in the study. Patients who had symptoms when the EMS team arrived but whose symptoms had resolved by the time they arrived at the hospital remained in the study. A team of unblinded, trained, dedicated study nurses collected data; a clinical research associate monitored documentation completeness and quality by checking source data from all participating hospitals.
Patients
Patients with acute cerebral ischemia likely to be eligible for thrombolysis or thrombectomy were included (Figure 1). To be eligible, patients had to be at least 18 years old; their emergency call had to prompt an MSU dispatch code during the MSU’s operating hours (7 am to 11 pm, Monday-Sunday); onset of symptoms to dispatch had to be within 4 hours, allowing a minimum time of 30 minutes for travel to the emergency site and assessment within the 4.5-hour window for thrombolysis; patients had to be within the catchment area of a Berlin MSU; patients had to have been ambulatory before their stroke (a rough proxy of modified Rankin Scale scores [mRS] ≤3; range, 0, no neurological symptoms, to 6, death).
Figure 1. Participant Flowchart in the B_PROUD Study Population.
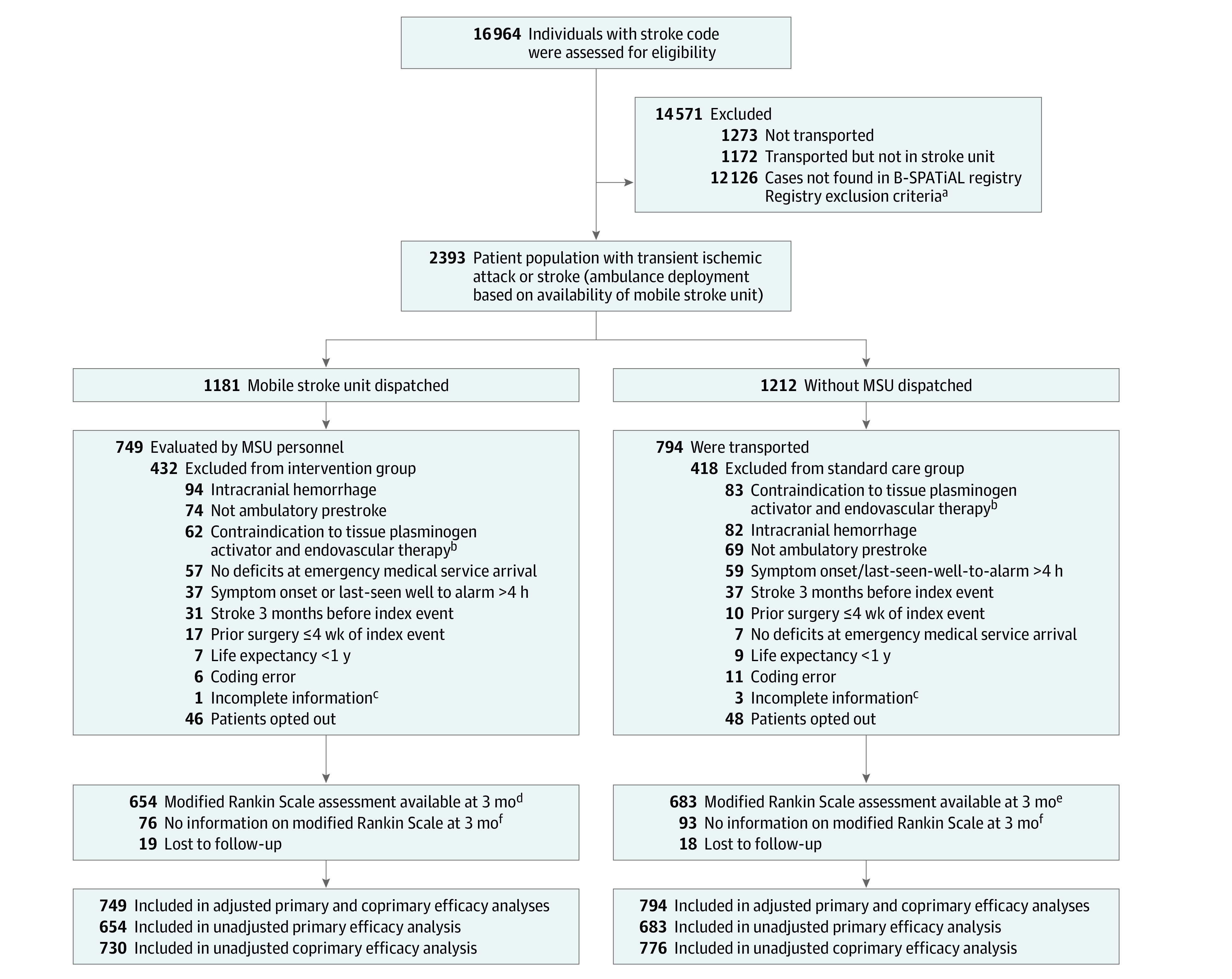
aThe list of the dispatch center does not provide reliable documentation of reasons for not matching with the Berlin Specific Acute Treatment in Ischemic and Hemorrhagic Stroke With Long-term (B-SPATiAL) registry except for when a patient is not transported to a hospital or is transported to a hospital that doesn’t have a stroke unit. Exclusion criteria included patients who were younger than 18 years, did not have a stroke or transient ischemic attack diagnosis, had no symptoms at the emergency department medical service or at hospital arrival, or arrived at the hospital more than 6 hours after symptom onset.
bPatients taking oral anticoagulation who had no large vessel occlusion.
cMissing information because of incomplete documentation in the B-SPATiAL registry because of expired access to electronic hospital records.
dThe majority had a blinded rating (381 recorded interviews, 181 written questionnaires, and 52 dead), while the remaining 40 had unblinded rating by study nurses.
eThe majority had a blinded rating (407 recorded modified Rankin Scale interviews, 165 written questionnaires, and 68 dead), while the remaining 43 had unblinded rating by study nurses.
fFor the coprimary outcome, the level of disability among patients without an mRS score was based on residential status—no to moderate disability was assigned to those living at home and severe disability was assigned to those living in a nursing care facility.
Study nurses determined eligibility based on MSU catchment area and operating hours and source data provided by the hospital and EMS. Because these study nurses could be blinded neither to mode of hospital transport (ambulance vs MSU) nor to short-term outcomes during the hospital stay, documentations of all uncertain cases were sent to a blinded adjudication committee for final decisions on participant inclusion.
Study enrollment was not restricted to patients receiving tissue plasminogen activator (tPA) but included patients with final hospital-based diagnosis of ischemic stroke (International Statistical Classification of Diseases and Related Health Problems, Tenth Revision [ICD-10], I63) or TIA (ICD-10, G45, except G45.4) without symptom resolution before arrival in either ambulance type.
A comparison restricted to patients treated with tPA potentially could have led to a bias favoring the MSU group because of earlier neurological assessment and possible spontaneous symptom resolution in the natural course of hyper–acute cerebral ischemia. Patients were excluded if they had absolute contraindications against both thrombolysis and thrombectomy (ie, patients who did not have large vessel occlusion taking oral anticoagulation or who had nonmedication induced hemophilia); malignancy or other severe primary disease with life expectancy of less than 1 year; major surgery within the past 4 weeks; or previous stroke within 3 months. Patients who only had a contraindication for thrombolysis but not for thrombectomy were considered eligible for treatment so were included. In uncertain cases (eg, patients who had smaller surgical interventions within 4 weeks before symptom onset or concomitant malignancy with uncertain life expectancy), the adjudication board reviewed cases blinded to group allocation status and ultimately determined whether the patient met eligibility criteria.
Exposures
In Berlin, MSUs are equipped with a CT scanner allowing for angiography, telemedicine-enabled connections to radiologists, and a point-of-care laboratory capable of measuring international normalized ratio; blood cell count; and blood glucose, creatinine, and electrolyte levels. They are staffed with a paramedic, a radiology technician with emergency training, and a neurologist with training in emergency medicine.
Conventional ambulances are staffed with a paramedic and an ambulance technician, both trained to stabilize and transport patients to hospitals. When the dispatcher suspected an acute stroke within 4 hours between symptom onset and emergency call, an MSU dispatch code was activated. If the emergency site was within an MSU catchment area and the MSU was available, it was deployed at the same time as the nearest conventional ambulance. If the conventional ambulance arrived first, its paramedics provided first aid until the MSU arrived. The MSU team then took over and dismissed the conventional ambulance paramedics whenever specific medical treatment was indicated.
If the paramedics of the conventional ambulance arrived first and concluded that the emergency was not a treatable stroke or if the waiting time for MSU was anticipated to be longer than the transport time to the next stroke-ready hospital, the MSU could be canceled. If the MSU was not available, only a conventional ambulance was deployed (in case of impaired consciousness or life-threatening conditions, conventional ambulances are dispatched with an emergency medicine physician, in accordance with Berlin EMS rules). By default, MSUs and conventional ambulances had to take patients with a suspected stroke to the nearest hospital with a stroke unit. Patients with a large artery occlusion detected by a CT angiographic scan were taken to the nearest hospital with thrombectomy capacity. When a stroke was suspected within 6 hours of onset, emergency departments were notified en route.
The study started with 1 MSU, and after 5-month run-in phases, a second MSU was added on September, 1, 2017, and a third one on September, 1, 2018, ultimately achieving 94% coverage of the Berlin population on this date (for geographic distribution, see Figure 2). On September 1, 2018, Global Positioning System tracking was added. Since then, the geographically closest MSU available was sent to the emergency scene.
Figure 2. Map of Berlin, Germany, Showing the 3 Catchment Areas for the Mobile Stroke Units.
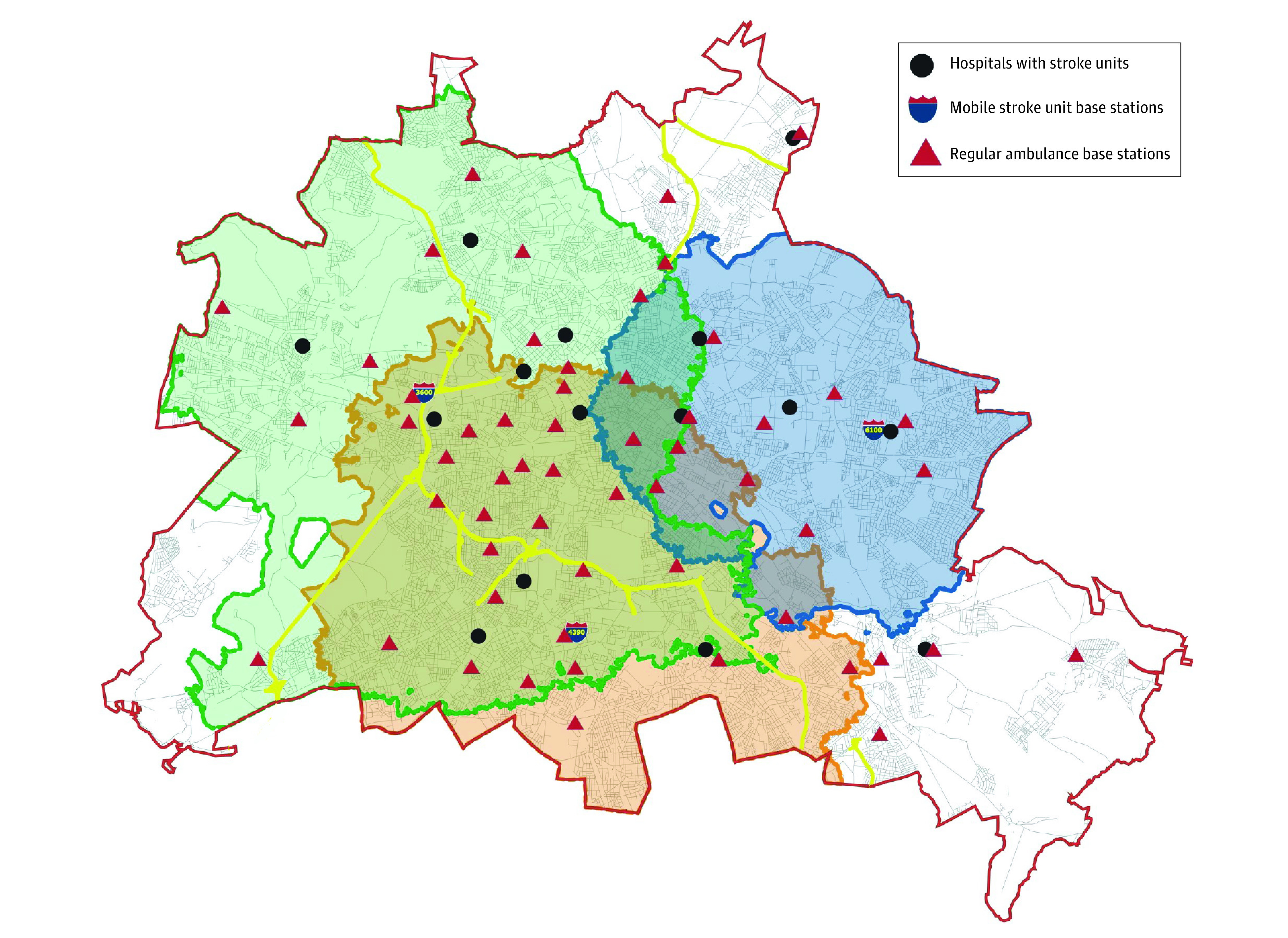
The red line indicates the outer border of the city. The 3 overlapping mobile stroke unit catchments are indicated by green, blue, and orange boundary lines with corresponding shading. The yellow lines show highways (“Autobahnen”). Mobile stroke unit (MSU) 3600 started in February 2011 and has operated at the shown base station since February 2018. The MSU 6100 started in April 2017 and MSU 4390 in April 2018. The map shows the base stations of the 3 MSUs as well as the 56 base stations for the normal ambulances and the locations of the 15 hospital Stroke Units. The approximate population of Berlin is 3.8 million over 344 square miles.
This map is modified from the EL Berliner Feuerwehr [Berlin Fire Brigade].
Outcomes
The primary outcome was the distribution of mRS ratings at 3 months. The mRS (range, 0, no neurological symptoms, to 6, death), especially when assessed through a structured interview, is the outcome of choice for clinical stroke research and has good to excellent interobserver agreement and reliability.14 We hypothesized that patients for whom an MSU was dispatched would have lower mRS scores than patients transported by conventional ambulance.
Standardized, blinded, recorded telephone interviews were used to assess the primary outcome and patient-centered secondary outcomes. Information indicating group allocation was deleted from recordings before uploading them to a central database. Three independent neurologists certified in mRS assessment rated outcomes using uploaded recordings. Unblinded ratings by certified study nurses were used only when the recording’s sound quality was insufficient or when patients had declined being recorded. Patients had the option of completing an mRS questionnaire that was returned by mail. If patients did not respond, their vital and residential status was requested via the state registry office after 4 months.
The coprimary outcome was introduced during the course of the study on February 26, 2018, to ensure that a reasonable number of patients had measurements for a surrogate measure of functional outcome if too many patients (>9%) were missing 3-month mRS information. The coprimary outcome was a 3-tier disability scale (no to moderate disability, severe disability, or dead) with tier assignment based on mRS scores if available or place of residence if the mRS scores were not available. Patients were considered to have no to moderate disability if their mRS score ranged from 0 to 3 or if they were living at home when no mRS score was available. Severe disability was considered present if the mRS score was 4 or 5 or if they were hospitalized or residing in a nursing care facility if the mRS score was not available.
We also report the following secondary outcomes: (1) rates of thrombolysis and mechanical thrombectomy; (2) times from onset to treatment; (3) times from dispatch to treatment; (4) times from dispatch to imaging; (5) times from imaging to treatment; (6) mRS with collapsed moderately severe or severe disability (mRS 4 and 5); (7) symptomatic secondary intracranial hemorrhage; (8) hospital discharge outcomes; (9) mortality within 7 days after admission for stroke; and (10) favorable outcomes at 3 months, defined as an mRS score of 0 or 1 for patients 80 years or younger living at home without disability before their stroke and an mRS score between 0 and 2 for patients older than 80 years or for patients who were living either at home with help or in a nursing care facility before their index stroke.
Additionally, quality of life as measured by the European Quality of Life 5-Dimensions (EQ-5D) was assessed. EQ-5Q is a descriptive measure of mobility, self-care, usual activities, pain or discomfort, and anxiety or depression with 3 levels for each dimension: no problems, some problems, and extreme problems. The most appropriate statement in each of the 5 dimensions results in a 1-digit number that is used for calculation of a total score with country-specific weights. Both this total score and the EQ-visual analog scale, a measure of generic health status, range from 0 (the worst health you can imagine) to 100 (the best health you can imagine).15 A 3-point difference is widely accepted as a minimal clinically important difference.16 Symptomatic intracranial hemorrhage was defined as any worsening of symptoms with evidence of hemorrhage shown on a brain imaging scan described in medical documentation.
Statistical Analysis
This analysis includes the clinical results of the primary study population, ie, patients fulfilling the inclusion criteria. Analysis results of the companion study population (patients with spontaneous intracranial hemorrhage or contraindications against intravenous thrombolysis and mechanical thrombectomy) as well as a formal cost-effectiveness analysis will be reported in subsequent articles.
The sample size calculation was based on pilot data from 808 consecutive patients who received thrombolysis (193 patients transported by MSU and 615 by conventional ambulance) of the Charité thrombolysis registry. For the primary outcome, mRS scores 3 months after their stroke, we observed the following differences in the pilot data (MSU vs conventional ambulance): mRS score of 0, 21% vs 21%; 1, 21% vs 15%; 2, 7% vs 9%; 3, 20% vs 12%; 4, 11% vs 14%; 5, 5% vs 9%; and 6, 15% vs 20%. This resulted in a probability of 55% for a worse outcome among those receiving conventional care than among those treated by the MSU team. The Mann-Whitney test with a 2-sided significance level of .05 has 80% power to detect such a group difference with at least 686 patients per group. Inclusion of 1500 patients was deemed necessary, anticipating a potential 9% being lost to follow-up.
Participant inclusion was considered complete once the goal of 1500 patients fulfilling the inclusion criteria was reached with at least 686 inclusions per group. The power calculation was conducted with the R-Package samplesize.17 Because there was scarce information on the anticipated effect size, we conducted an interim analysis for a blinded sample size reestimation after a primary end point assessment of 300 patients. The results of the interim analysis did not lead to any change of the sample size calculation.
To compare group characteristics, we calculated unadjusted odds ratios (ORs) for dichotomous variables, mean differences of z standardized values for normally distributed continuous variables and mean differences of ranked and z standardized values of ordinal or nonnormally distributed variables. For all those measures, we computed 95% CIs (Table 1). Because this was not a randomized study, we planned and reported estimates from ordinal regression models (ordinal, logistic or linear, depending on the scale of the outcome variable) adjusted for potential sources of confounding.
Table 1. Baseline Parameters, Clinical Information, and Process Parameters in Patients of the Full Analysis Seta.
| Patients, No. (%) | Mean difference or odds ratio (95% CI) | ||
|---|---|---|---|
| With mobile stroke unit dispatch (n = 749) | With conventional ambulance (n = 794) | ||
| Demographics | |||
| Age, mean (SD), y | 73 (13) | 74 (13) | −0.11 (−0.21 to −0.01)b |
| Median (IQR) | 75 (65 to 82) | 77 (67 to 83) | |
| Sex | |||
| Women | 346 (46.2) | 377 (47.5) | 0.95 (0.78 to 1.16)c |
| Men | 403 (53.8) | 417 (52.5) | 1.05 (0.86 to 1.29)c |
| Comorbidities | |||
| Arterial hypertension | 589 (78.6) | 649 (81.7) | 0.82 (0.64 to 1.06)c |
| Atrial fibrillation | 216 (28.8) | 209 (26.3) | 1.13 (0.91 to 1.42)c |
| Diabetes | 191 (25.5) | 201 (25.3) | 1.01 (0.80 to 1.27)c |
| Functional status before stroke | |||
| Living at home without assistance | 601 (80.2) | 624 (78.6) | 1.11 (0.86 to 1.42)c |
| Living in nursing care facility | 84 (11.2) | 96 (12.1) | 0.92 (0.67 to 1.25)c |
| Clinical information | |||
| EMS documentation of neurological deficits | 668 (89.2) | 638 (80.4) | 2.02 (1.51 to 2.69)c |
| NIHSS score, median (IQR) | |||
| First assessedd,e | 4 (2-9) | 4 (2-9) | 0.03 (−0.07 to 0.13)b |
| No. | 746 | 789 | |
| At hospital arrivald | 3 (1-7) | 4 (2-9) | −0.10 (−0.20 to −0.003)b |
| No. | 736 | 789 | |
| Transient ischemic attackf | 124 (16.6) | 131 (16.5) | 1.00 (0.77 to 1.31)c |
| Ischemic stroke | 625 (83.4) | 663 (83.5) | 1.00 (0.76 to 1.30)c |
| Large vessel occlusion documented in acute vessel imaging | 163 (21.8) | 177 (22.3) | 0.97 (0.76 to 1.23)c |
| Process parameter times, median (IQR), min | |||
| Onset or last seen well to dispatch | 36 (13 to 95) | 39 (16 to 87) | −0.05 (−0.15 to 0.06)b |
| No. | 736 | 751 | |
| Dispatch to first ambulance arrival | 8 (6 to 10) | 8 (6 to 10) | −0.02 (−0.12 to 0.09)b |
| No. | 748 | 794 | |
| Dispatch to MSU arrival, 197 times not arrived | 15 (12 to 19) | NA | |
| No. | 550 | NA | |
| Dispatch to hospital arrival | 67 (46 to 82) | 37 (31 to 44) | 1.17 (1.09 to 1.25)b |
| Only patients with tPA | |||
| No. of patients receiving thrombolysis | 451 | 382 | |
| First assessed NIHSS score, median (IQR)d,e | 6 (3 to 11) | 7 (4 to 11) | −0.12 (−0.23 to −0.01) |
| No. | 450 | 380 | |
| tPA treatment start within 60 min | 96 (12.8) | 32 (4.0) | 2.96 (1.93 to 4.53)c |
| Hospital door-to-needle time, median (IQR), min | NA | 30 (22 to 40) | |
| No. | NA | 380 | |
| Only patients with endovascular treatment times, median (IQR), min | |||
| No. of patients receiving endovascular treatment | 103 | 113 | |
| Dispatch to start of procedure | 137 (117 to 166) | 125 (110 to 154) | 0.22 (−0.06 to 0.49)b |
| No. | 100 | 103 | |
| Onset or last-seen-well to start of procedureg | 170 (137 to 216) | 157 (126 to 228) | 0.12 (−0.16 to 0.40)b |
| No. | 100 | 100 | |
Abbreviations: EMS, emergency medical services; IQR, interquartile range; MSU, mobile stroke unit; NA, not applicable; NIHSS, National Institutes of Health Stroke Scale; tPA, tissue plasminogen activator.
For process parameters predefined as secondary outcomes, see eTable 2 in Supplement 2.
Mean difference of z standardized values (95% CI).
Unadjusted odds ratio (95% CI).
The NIHSS is a score ranging from 0 to 42, with higher scores indicating greater neurological deficits.
Assessment of patients cared for by MSU or patients transported by conventional ambulance until treated at the emergency department.
Transient ischemic attack was defined as a transient neurological dysfunction caused by loss of blood flow in the brain (according to International Statistical Classification of Diseases and Related Health Problems, Tenth Revision: g45.x except g45.4).
Last seen well was assessed or documented by medical personnel who asked the patient (or a relative) at what time the event occurred.
The primary analysis of the primary outcome (mRS) was performed on the full analysis set, which used estimated values in case of missing values from multiple imputations, with results expressed as common ORs and corresponding 95% CIs. For the primary and all other outcome analyses, the regression models were adjusted for the following potential confounders: age, sex, arterial hypertension, diabetes mellitus, atrial fibrillation, neurological deficits at the time of conventional ambulance or MSU arrival, and living situation before stroke (independent at home or living in a nursing care facility). All analyses were additionally adjusted for center heterogeneity by including a random intercept for each center.
The proportional odds assumption was tested using the Brant test.18 In case of violation of this assumption in the ordinal regression analyses, we used partial proportional odds models.19
When appropriate, the primary analysis also included the analysis of the coprimary outcome. This 3-level ordinal outcome was analyzed with an ordinal logistic regression model with adjustment for the same covariates as for the primary outcome, with results expressed as common ORs and corresponding 95% CIs.
As proposed by Rahlfs et al,20 we calculated stratified analyses and pooled measures using the Cochran-Mantel-Haenszel analyses and multiple ordinal regressions. Stratification of analysis results was performed for the onset-to-dispatch time (0-60, 61-120, 121-180, ≥181 minutes). Additionally, we performed a Mann-Whitney test to compare outcomes between groups among those patients without missing values as a sensitivity analysis.
The primary analysis included estimates for missing values after multiple imputation using data of all patients who fulfilled the inclusion criteria, comparing patients for whom the MSU was available and dispatched to patients for whom MSU was unavailable (hence not sent). Multiple imputation of missing values (10 imputed data sets, predictive mean matching, multiple imputation for chained equations) for primary and coprimary outcome analyses was conducted using the following variables: age, sex, hypertension, diabetes mellitus, atrial fibrillation, living at home without assistance or living in nursing care facility, neurological deficits documented at EMS arrival, first assessed National Institutes of Health Stroke Scale (NIHSS, range, 0-42, with higher scores indicating greater neurological deficits) score, time from onset or the time last-seen-well to dispatch, mRS assessed at discharge, EQ-5D total score and visual analog scale score, and MSU dispatch. As documentation of neurological symptoms at first EMS arrival was not available for all cases and might be unbalanced between the 2 groups, another sensitivity analysis restricted to only those patients with such documentation was conducted.
Additional predefined sensitivity analyses were performed. The first analysis involved patients for whom an MSU and a conventional ambulance were dispatched and compared patients who were evaluated by an MSU with those who were not evaluated by an MSU because the MSU unavailable. The second compared patients who were evaluated by the MSU team with all patients without an MSU evaluation independent of whether an MSU was dispatched, which includes those for whom the MSU was initially dispatched but did not arrive or those for whom no MSU was dispatched.
In a post hoc analysis, we quantified the relationship between time to treatment and the dichotomous mRS outcome (defining an excellent outcome as having an mRS score of 0 or 1) among patients receiving tPA treatment. The onset-to-treatment time was calculated as the interval between the time of onset or time last seen well (if within 30 minutes of the emergency call) and the start of thrombolysis. We analyzed the associations of onset to treatment time in the following increments: 0 to 60 minutes, 61 to 120 minutes, 121 to 180 minutes and 181 to 270 minutes (with the latest category as the reference).
Additionally, the sensitivity analyses (1) comparing patients for whom MSU was dispatched but canceled before arrival vs those for whom no MSU was dispatched and (2) comparing patients evaluated by MSU personnel vs those not evaluated by MSU personnel included additional adjustment for first measured NIHSS score.
Primary and coprimary outcomes were compared between patients with and patients without MSU dispatch. To account for multiple testing, we implemented the Benjamini-Hochberg21 procedure with an overall false-discovery rate of .05 (see the statistical analysis plan in Supplement 1). Accordingly, P values for both primary and coprimary outcomes were ordered from smaller to higher P value. Two-sided significance levels for each outcome corresponding to an overall false-discovery rate of .05 were defined as .025 for the smaller P value and .05 for the larger P value (because of 2 outcome tests in total).
Statistical testing of secondary hypotheses and prespecified subgroup analyses was done within an exploratory framework without adjustment for multiple testing, using 2-sided tests. Interpretation of results for secondary outcomes was therefore based on effect estimates and 95% CIs. For each of the 3 subgroup analyses, the subgroup × treatment interaction was tested and subgroup-specific associations and 95% CIs for the primary and coprimary outcomes were reported. The prespecified subgroups were (1) patients with time from onset or last seen well to dispatch of 60 minutes or less vs more than 60 minutes, (2) first measured NIHSS score of 6 or less vs more than 6, and (3) first 750 included patients vs included patients thereafter. Statistical analyses were performed with SPSS statistics for Windows, version 25.0; Stata statistical software version 15 (StataCorp LLC), and R, package tableone (https://www.R-project.org/; https://CRAN.R-project.org/package=tableone).
Results
Patients
Between February 1, 2017, and May 8, 2019, 1637 patients fulfilled the inclusion criteria and 1543 patients (94%) did not opt-out and participated in the follow-up assessments. Of these, MSUs were dispatched to 749 patients (49%) and conventional ambulances to 794 (51%) (Figure 1). Of the 749 patients, the MSU dispatch was canceled for 197 patients prior to its arrival. Dispatch times of day and distribution over the week and weekend days were similar for both groups (eFigure 2 in Supplement 2). There was no relevant association of the distance of hospitals to the city center and the proportion of patients treated on an MSU (eFigure 3 in Supplement 2). The mean (SD) age of patients with an MSU dispatched was 73 years (13 years) and 74 years (13 years) for those without an MSU dispatched (mean difference, −0.11; 95% CI, −0.21 to −0.01). The median NIHSS score was 4 (interquartile range [IQR], 2-9) at the first assessment in both groups (mean difference, −0.03; 95% CI, −0.07 to 0.13). The median NIHSS score was lower among patients for whom the MSU was dispatched but later canceled (2; IQR, 1-4) than it was among patients with MSU evaluation (5; IQR, 2-10). Transient ischemic attacks were more frequent among patients with MSU cancellations (26.4%) than among those evaluated by the MSU team (13.0%). For an overview of all baseline parameters, see Table 1.
Outcomes
The mRS, the primary outcome, was assessed in 1337 patients (87%) and the coprimary outcome, either the mRS score or 3-tiered disability scale, in 1506 (98%). Modes of outcome ascertainment are detailed in Figure 1. Based on the full analysis set (including imputed data), the 749 patients for whom an MSU was dispatched had significantly less global disability as indicated by a favorable shift in the distribution of the mRS score at 3 months than did the 794 patients for whom MSU was not dispatched (median [IQR] mRS score, 1 [0-3] vs 2 [0-3]; adjusted common OR for worse outcome, 0.71, 95% CI, 0.58-0.86; P < .001) (Table 2). Patients in the MSU-dispatched group had a better outcome throughout the range of the mRS scores in unadjusted analysis than did those in the control group (eTable 1 in Supplement 2; Figure 3, P = .004 in Mann-Whitney U test; OR, 0.77; 95% CI, 0.64-0.92; P = .004). The adjusted common OR for the coprimary outcome at 3 months also indicated a significant association between MSU dispatch and a favorable shift in the distribution of outcomes (common OR for worse functional outcome, 0.73; 95% CI, 0.54-0.99; P = .04). Complete results are displayed in Table 2. Baseline parameters, process indicators, and short-term outcomes of patients without a 3-month mRS assessment were similar to those of the total cohort (eTable 2 in Supplement 2; Table 1).
Table 2. Primary and Secondary Outcomes.
| Outcomes and unadjusted associations, No. (%) of patients | Absolute difference (95% CI) | MSU dispatch vs conventional ambulancea | |||
|---|---|---|---|---|---|
| MSU dispatch (n = 749) | Conventional ambulance (n = 794) | Adjusted common OR (95% CI) | P value | ||
| Primary outcomes at 3 months | |||||
| No. | 654 | 683 | |||
| mRS score, median (IQR) | 1 (0-3) | 2.0 (0-3) | −1 (−3 to −0.5)b | 0.71 (0.58 to 0.86)c | <.001 |
| Coprimary efficacy outcomes at 3 months,d | |||||
| No. | 730 | 776 | |||
| mRS 0-3 or living at home | 586 (80.3) | 605 (78.0) | 2.3 (−1.8 to 6.4) | 0.73 (0.54 to 0.99)c | .04 |
| mRS 4-5 or living in nursing care institution | 92 (12.6) | 103 (13.3) | −0.7 (−4.1 to 2.7) | ||
| Death | 52 (7.1) | 68 (8.8) | −1.7 (−4.4 to 1.1) | ||
| Secondary outcomes | |||||
| No. | 748 | 794 | |||
| tPA | 451 (60.2) | 382 (48.1) | 12.1 (7.2 to 17.0) | 1.62 (1.32 to 2.00) | <.001 |
| Endovascular treatments | 103 (13.8) | 103 (13.0) | 0.8 (−2.6 to 4.2) | 0.80 (0.58 to 1.10) | .17 |
| No. | 716 | 787 | |||
| Dispatch to imaging, median (IQR), min | 45 (32 to 75) | 60 (49 to 77) | −15 (−18 to −12) | Mean difference, −20 (−26 to −15)e | <.001 |
| Selected short-term outcomes | |||||
| No. | 749 | 794 | |||
| Discharge | |||||
| Home | 453 (60.5) | 445 (56.0) | 4.5 (−0.5 to 9.4) | 1.42 (1.12 to 1.81) | .004 |
| Rehabilitation | 157 (21.0) | 151 (19.0) | 2.0 (−2.0 to 5.9) | 0.91 (0.69 to 1.19) | .49 |
| Nursing care home | 49 (6.5) | 60 (7.6) | −1.1 (−3.6 to 1.5) | 1.05 (0.57 to 1.93) | .87 |
| Inhospital death | 19 (2.5) | 30 (3.8) | −1.3 (−3.0 to 0.5) | 0.61 (0.33 to 1.12) | .11 |
| Symptomatic secondary intracranial hemorrhage | 24 (3.2) | 22 (2.8) | 0.4 (−1.3 to 2.1) | 1.20 (0.66 to 2.19) | .56 |
| 7-Day mortality | 13 (1.7) | 24 (3.0) | −1.3 (−2.8 to 0.2) | 0.54 (0.26 to 1.12) | .10 |
| mRS scoref | |||||
| No. | 654 | 683 | |||
| 0 | 199 (30.4) | 171 (25.0) | 5.4 (0.6 to 10.2) | 0.71 (0.58 to 0.86)a | <.001 |
| 1 | 134 (20.5) | 118 (17.3) | 3.2 (−1.0 to 7.4) | ||
| 2 | 79 (12.1) | 99 (14.5) | −2.4 (−6.0 to 1.2) | ||
| 3 | 114 (17.4) | 133 (19.5) | −2.1 (−6.2 to 2.1) | ||
| 4 | 76 (11.6) | 94 (13.8) | −2.2 (−5.7 to 1.4) | ||
| ≥5 | 52 (8.0) | 68 (10.0) | −2.0 (−5.1 to 1.1) | ||
| 3-Month outcomes | |||||
| No. | 635 | 669 | |||
| Unfavorableg | 295 (46.5) | 359 (53.7) | −7.2 (−12.6 to −1.8) | 0.70 (0.56 to 0.87) | .002 |
| Quality of life, mean (SD) | |||||
| No. | 605 | 631 | |||
| EQ-5D visual analog scaleh | 60 (29) | 55 (29) | 5 (2 to 8) | Mean difference, 4.3 (1.5 to 7.2) | .003 |
| No. | 618 | 649 | |||
| EQ-5D total scorei | 63 (34) | 59 (35) | 4 (0.3 to 8) | Mean difference, 4.4 (1.2 to 7.7) | .007 |
| Sensitivity analysis of patients with documentation of neurological deficits (MSU dispatch, n = 668; conventional ambulance, n = 638) | |||||
| No. | 584 | 550 | |||
| mRS score, median (IQR)j | 2 (0 to 3) | 2 (1 to 4) | 0 (−0.3 to 0.3) | 0.73 (0.59 to 0.91)b | .005 |
| Times to tPA, median (IQR), min | |||||
| No. | 421 | 371 | |||
| Imaging to tPA | 12 (7 to 22) | 15 (10 to 23) | −3(−5 to −1) | Mean difference, −0.1 (−0.2 to 0.03)e | .17 |
| No. | 448 | 377 | |||
| Onset or last-seen-well to tPA | 95 (60 to 149) | 110 (80 to 165) | −15 (−27 to −3) | Mean difference, −13 (−21 to −4) | .003 |
| No. | 448 | 377 | |||
| Mean (SD) | 112 (62) | 126 (61) | |||
| No. | 449 | 380 | |||
| Dispatch to tPA | 50 (43 to 64) | 70 (59 to 86) | −20 (−23 to −17) | Mean difference, −26 (−31 to −22)e | <.001 |
Abbreviations: EQ-5D, European Quality of Life 5 Dimensions; IQR, interquartile range; mRS, modified Rankin Scale; MSU, mobile stroke unit; OR, odds ratio; tPA, tissue plasminogen activator.
Adjusted for age, sex, hypertension, diabetes, living situation, documentation of deficits at MSU arrival, atrial fibrillation, and center heterogeneity (by including random intercepts). Multiple imputation was performed with 10 imputed data sets.
Derived from quantile regression.
Common ORs for ordinal outcomes are based on partial proportional odds models using the proportional odds model only for variables for which the assumptions for the model were met and multinomial model parameters otherwise. Odds ratios for binary outcomes are based on binary logistic regression models; adjusted mean differences, on linear regression models. All models were adjusted for center heterogeneity by including random intercepts for the centers. Common ORs less than 1.00 indicate a favorable shift in the outcome distribution, reducing odds of higher levels of disability.
See the Methods section for coprimary outcome definitions.
Log-transformed values. Mean differences (95% CIs) are presented as percentages.
mRS scores were analyzed across 6 levels of 0, 1, 2, 3, with 4 and 5 collapsed, and 6.
Defined as mRS 2 to 6 in patients 80 years or younger and mRS of 3 to 6 in patients older than 80 years or living at home with help or living in a nursing facility before stroke.
A measure of generic health status, ranging from 0 (the worst health you can imagine) to 100 (the best health you can imagine).
EQ-5D score, ranges from 0 to 100, is a descriptive measure of mobility, self-care, usual activities, pain or discomfort and anxiety or depression with each of the 3 levels: no problems, some problems, and extreme problems the most appropriate statement in each of the five dimensions results in a 1-digit number.
Analysis restricted to patients with documented neurological deficits at EMS arrival.
Figure 3. Modified Rankin Scale Score Distribution at 3 Months, Unadjusted Only for Patients With Respective Follow-up.
The modified Rankin Scale (mRS) score is a measure of disability ranging from 0, no neurological deficits, to 6, death.
aThree-tier disability scale (none to moderate disability; severe disability; dead) with tier assignment were based on mRS scores if available and place of residence if the mRS scores were not available. None to moderate disability was considered present if the mRS score was 0 to 3 or when mRS score were not available, if the patient was residing at home. Severe disability was considered present if the mRS score was 4 to 5 or, when mRS score was not available, if the patient was residing in a nursing care facility.
Secondary Outcomes
A total of 451 patients (60.2%) with an MSU dispatch and 382 (48.1%) without an MSU dispatch received thrombolysis (adjusted OR, 1.62; 95% CI,1.32-2.00); the median times from dispatch to initiating thrombolysis for patients with an MSU dispatch were 50 minutes (IQR, 43-64 minutes) and without an MSU dispatch, 70 minutes (IQR, 59-86 minutes) (mean difference in percentage based on log-transformed values, −27%; 95% CI, −31% to −22%). The proportional distribution of times to thrombolysis is shown in eFigure 4 in Supplement 2.
Technical failures (not a prespecified outcome) with subsequent inhospital cerebral imaging and possible treatment delays occurred in 7 patients during MSU care with 6 cases of CT dysfunction and 1 case of stretcher-CT alignment dysfunction. Thirteen deaths (1.7%) occurred within 7 days among patients with an MSU dispatch and 24 deaths (3.0%) among patients without an MSU dispatch (adjusted OR, 0.54; 95% CI, 0.26-1.12; Table 2).
Symptomatic secondary intracranial hemorrhage occurred in 24 patients (3.2%) with an MSU dispatch and 22 patients (2.8%) without an MSU dispatch (adjusted OR, 1.20; 95% CI, 0.66-2.19). For additional secondary outcomes see Table 2.
The sensitivity analysis restricted to patients with available documentation for neurological symptoms when EMS first arrived resulted in a common OR of 0.73 (95% CI, 0.59-0.91) for the primary outcome (Table 2).
eTables 3, 4, 5, and 6 and eFigure 1 in Supplement 2 provide baseline parameters, process indicators, and outcomes analyzed according to the sensitivity analyses.
In the post hoc analysis probing the associations between onset-to-treatment time and outcome, shorter times were found to be associated with excellent outcomes (mRS 0-1) among 687 patients receiving tPA treatment. Compared with patients with times ranging from 181 to 270 minutes, the adjusted OR for favorable outcome was highest among those with times of 60 minutes or less (common OR, 3.25; 95% CI, 1.72-6.13), followed by times ranging from 61 to 120 minutes (common OR, 2.54; 95% CI, 1.44-4.49), and by times ranging from 121 to 180 minutes (common OR, 2.11; 95% CI, 1.12-3.94).
Discussion
Among patients prompting an MSU dispatch code and without contraindications to thrombolysis and thrombectomy, MSU dispatch compared with dispatch of conventional ambulance alone was significantly associated with better mRS scores at 3 months. The analysis of the coprimary outcome also showed a significant association between patients for whom an MSU was dispatched and a favorable shift in the distribution of outcomes at 3 months.
The rate of thrombolysis was higher and the median dispatch-to-thrombolysis time was lower among patients with an MSU dispatch than among patients without an MSU dispatch. The combined effect of these parameters may explain the better outcomes in the MSU group. The absolute differences of 8.6% for survival without any disability (mRS, 0-1) and 4% for survival without moderate to severe disability (mRS, 0-3) are clinically relevant (Table 2).
In the prespecified sensitivity analysis patients evaluated by the MSU team had better primary outcomes than did patients for whom an MSU was not dispatched. However, that association was weakened when comparing patients evaluated by MSU personnel vs those not evaluated by MSU personnel for the primary outcome and in both sensitivity analyses of the coprimary outcome with fewer granular data, ie, without differentiation of no, mild, and moderate disability (eFigure 1 in Supplement 2). The fairly balanced distribution of baseline characteristics between groups suggests that MSU availability was a decent proxy for randomization. Therefore, the comparison between patients with and without an MSU dispatch may have been more robust than the other analyses, which left more room for bias in either direction. For instance, median stroke severity was lower in patients for whom the MSU was dispatched but canceled, and this may have contributed to weakened associations observed in some of the sensitivity analyses. Overall, compared with many randomized clinical trials of thrombolysis, the median NIHSS score was low, reflecting that this study captured data from patients in prehospital clinical settings rather than a highly selected trial population.
Rates of serious adverse events were similar in both groups. The 7-day death rate and the rate of symptomatic intracranial hemorrhage were low in both groups despite the use of a broad definition for the latter.
In this study, MSU dispatch was not significantly associated with shorter time from dispatch to thrombectomy, which contrasts with recent reports from other MSU sites.22,23 Although MSU staff did notify the hospital teams en route if the CT angiographic scan taken aboard the MSU demonstrated the need for endovascular treatment, patients underwent another scan in most emergency departments. The main reason was that interventionalists requested imaging of the aortic arch and the proximal carotid arteries that were not covered by the small MSU CT scanner. Further streamlining of the angiography suite may be necessary in the Berlin setting. In comparison with the present study, prior MSU studies showed higher rates for both prehospital thrombolysis and the so-called “golden hour” thrombolysis, demonstrating additional room for improvement.5,6,7 Although the present study was designed to evaluate the whole MSU approach rather than the effects of prehospital thrombolysis, effects of ultra early thrombolysis might have contributed to the results.24,25 Substantially more patients received tPA treatment within 70 minutes of MSU dispatch (eFigure 4 in Supplement 2). The post hoc analysis provided additional evidence of an association between shorter treatment times and better outcomes, which is in line with similar previous reports from the Get With The Guidelines-Stroke program.26
Future research including cost-effectiveness analysis27,28 and ongoing evaluations of MSU implementations should also focus on optimizing triage systems. Improvement of dispatch algorithms should aim for higher numbers of MSU treatment candidates, ie, increasing sensitivity and specificity. Dispatches to patients with nonstroke diseases tie up MSUs, making them unavailable for treatment candidates. Sensitivity and specificity at dispatch, time to treatment with and without MSU attendance, hospital distribution, and performance in the service area, as well as characteristics of the stroke population may all affect treatment effectiveness.29 Future implementations of MSU services in other locations should carefully consider the idiosyncrasies that come with each new MSU project.12
Limitations
This study has several limitations. First, the intervention in this study was not randomized; instead, patients were allocated to the MSU or conventional care groups based on availability of MSUs. This approach was chosen because active randomization was not feasible after MSUs had become part of provisional regular care in Berlin. Second, while assessments of the recordings used for outcome assessment were blinded, the interviewer on the phone was not. This left room for bias via suggestive questions. However, phone calls were recorded and conducted in a standardized way to minimize this risk. This preferred mode of primary outcome assessment was only available for fewer than 60% of patients (Figure 2). Sources of information for the majority of the remaining cases, however, included death and written questionnaires, both not susceptible to biased rating by study personnel either. Third, documentation of the presence or absence of neurological deficits at EMS arrival was unbalanced between groups, with a 9% higher documentation rate for this information in the MSU group (Table 1). However, restricting the analysis to patients with documented deficits in a sensitivity analysis did not meaningfully change the observed associations between outcome and MSU dispatch. Fourth, this study did not assess the outcome of patients with final diagnoses other than acute ischemic stroke or TIA, thereby excluding intracerebral bleeds and stroke mimics. As for the stroke mimics, the previous Prehospital Acute Neurological Treatment and Optimization of Medical care in Stroke Study (PHANTOM-S)6 showed similar thrombolysis rates and no thrombolysis-related complications in stroke mimics between groups.
Conclusions
In this prospective, nonrandomized, controlled intervention study of patients with acute ischemic stroke in Berlin, Germany, the dispatch of mobile stroke units, compared with conventional ambulances alone, was significantly associated with lower global disability at 3 months. Clinical trials in other regions are warranted.
Trial Protocol and Statistical Analysis Plan
Post hoc analyses
eFigure 1. Outcomes in different datasets and pre-specified subgroups
eFigure 2. Distribution of weekdays
eFigure 3. Association of percentage of patients actually treated by MSU to distance of hospital to city center
eFigure 4. Comparison of times from onset to needle in patients with and without MSU dispatch
eTable 1. Unadjusted outcomes in the full analysis set (intention-to-treat analysis)
eTable 2. Patients included in primary analysis without mRS assessment at 3 months
eTable 3. Analysis of patients for whom an MSU was dispatched and arrived at scene compared to patients without MSU dispatch; baseline parameters
eTable 4. Analysis of patients for whom an MSU was dispatched and arrived at scene compared to patients without MSU dispatch; adjusted primary and secondary outcomes
eTable 5. Analysis comparing patients with evaluation aboard an MSU to patients without MSU evaluation independent of dispatch; baseline parameters
eTable 6. Analysis comparing patients with evaluation aboard an MSU to patients without MSU evaluation independent of dispatch; adjusted primary and secondary outcomes
Study Investigators
References
- 1.Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic Stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344-e418. doi: 10.1161/STR.0000000000000211 [DOI] [PubMed] [Google Scholar]
- 2.Fassbender K, Walter S, Liu Y, et al. “Mobile stroke unit” for hyperacute stroke treatment. Stroke. 2003;34(6):e44. doi: 10.1161/01.STR.0000075573.22885.3B [DOI] [PubMed] [Google Scholar]
- 3.Audebert H, Fassbender K, Hussain MS, et al. ; PRESTO Group . The PRE-hospital Stroke Treatment Organization. Int J Stroke. 2017;12(9):932-940. doi: 10.1177/1747493017729268 [DOI] [PubMed] [Google Scholar]
- 4.Fassbender K, Grotta JC, Walter S, Grunwald IQ, Ragoschke-Schumm A, Saver JL. Mobile stroke units for prehospital thrombolysis, triage, and beyond: benefits and challenges. Lancet Neurol. 2017;16(3):227-237. doi: 10.1016/S1474-4422(17)30008-X [DOI] [PubMed] [Google Scholar]
- 5.Walter S, Kostopoulos P, Haass A, et al. Diagnosis and treatment of patients with stroke in a mobile stroke unit versus in hospital: a randomised controlled trial. Lancet Neurol. 2012;11(5):397-404. doi: 10.1016/S1474-4422(12)70057-1 [DOI] [PubMed] [Google Scholar]
- 6.Ebinger M, Winter B, Wendt M, et al. ; STEMO Consortium . Effect of the use of ambulance-based thrombolysis on time to thrombolysis in acute ischemic stroke: a randomized clinical trial. JAMA. 2014;311(16):1622-1631. doi: 10.1001/jama.2014.2850 [DOI] [PubMed] [Google Scholar]
- 7.Ebinger M, Kunz A, Wendt M, et al. Effects of golden hour thrombolysis: a Prehospital Acute Neurological Treatment and Optimization of Medical Care in Stroke (PHANTOM-S) substudy. JAMA Neurol. 2015;72(1):25-30. doi: 10.1001/jamaneurol.2014.3188 [DOI] [PubMed] [Google Scholar]
- 8.Wendt M, Ebinger M, Kunz A, et al. ; STEMO Consortium . Improved prehospital triage of patients with stroke in a specialized stroke ambulance: results of the pre-hospital acute neurological therapy and optimization of medical care in stroke study. Stroke. 2015;46(3):740-745. doi: 10.1161/STROKEAHA.114.008159 [DOI] [PubMed] [Google Scholar]
- 9.Kunz A, Ebinger M, Geisler F, et al. Functional outcomes of pre-hospital thrombolysis in a mobile stroke treatment unit compared with conventional care: an observational registry study. Lancet Neurol. 2016;15(10):1035-1043. doi: 10.1016/S1474-4422(16)30129-6 [DOI] [PubMed] [Google Scholar]
- 10.Nolte CH, Ebinger M, Scheitz JF, et al. Effects of prehospital thrombolysis in stroke patients with prestroke dependency. Stroke. 2018;49(3):646-651. doi: 10.1161/STROKEAHA.117.019060 [DOI] [PubMed] [Google Scholar]
- 11.Kobayashi A, Czlonkowska A, Ford GA, et al. European Academy of Neurology and European Stroke Organization consensus statement and practical guidance for pre-hospital management of stroke. Eur J Neurol. 2018;25(3):425-433. doi: 10.1111/ene.13539 [DOI] [PubMed] [Google Scholar]
- 12.Yamal JM, Rajan SS, Parker SA, et al. Benefits of stroke treatment delivered using a mobile stroke unit trial. Int J Stroke. 2018;13(3):321-327. doi: 10.1177/1747493017711950 [DOI] [PubMed] [Google Scholar]
- 13.Ebinger M, Harmel P, Nolte CH, Grittner U, Siegerink B, Audebert HJ. Berlin prehospital or usual delivery of acute stroke care—study protocol. Int J Stroke. 2017;12(6):653-658. doi: 10.1177/1747493017700152 [DOI] [PubMed] [Google Scholar]
- 14.Wilson JT, Hareendran A, Hendry A, Potter J, Bone I, Muir KW. Reliability of the modified Rankin Scale across multiple raters: benefits of a structured interview. Stroke. 2005;36(4):777-781. doi: 10.1161/01.STR.0000157596.13234.95 [DOI] [PubMed] [Google Scholar]
- 15.Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med. 2001;33(5):337-343. doi: 10.3109/07853890109002087 [DOI] [PubMed] [Google Scholar]
- 16.Kaplan RM The minimally clinically important difference in generic utility-based measures. COPD. 2005;2(1):91-97. doi: 10.1081/COPD-200052090 [DOI] [PubMed] [Google Scholar]
- 17.Zhao YD, Rahardja D, Qu Y. Sample size calculation for the Wilcoxon-Mann-Whitney test adjusting for ties. Stat Med. 2008;27(3):462-468. doi: 10.1002/sim.2912 [DOI] [PubMed] [Google Scholar]
- 18.Brant R Assessing proportionality in the proportional odds model for ordinal logistic regression. Biometrics. 1990;46(4):1171-1178. doi: 10.2307/2532457 [DOI] [PubMed] [Google Scholar]
- 19.Williams R. Generalized ordered logit/partial proportional odds models for ordinal dependent variables. Stata J. 2006;6(1):55-82. doi: 10.1177/1536867X0600600104 [DOI] [Google Scholar]
- 20.Rahlfs VW, Zimmermann H, Lees KR. Effect size measures and their relationships in stroke studies. Stroke. 2014;45(2):627-633. doi: 10.1161/STROKEAHA.113.003151 [DOI] [PubMed] [Google Scholar]
- 21.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Stat Society: Series B (Method). 1995;57(1): 289-300 doi: 10.1111/j.2517-6161.1995.tb02031.x [DOI] [Google Scholar]
- 22.Zhao H, Coote S, Easton D, et al. Melbourne mobile stroke unit and reperfusion therapy: greater clinical impact of thrombectomy than thrombolysis. Stroke. 2020;51(3):922-930. doi: 10.1161/STROKEAHA.119.027843 [DOI] [PubMed] [Google Scholar]
- 23.Helwig SA, Ragoschke-Schumm A, Schwindling L, et al. Prehospital stroke management optimized by use of clinical scoring vs mobile stroke unit for triage of patients with stroke: a randomized clinical trial. JAMA Neurol. 2019. doi: 10.1001/jamaneurol.2019.2829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kunz A, Nolte CH, Erdur H, et al. Effects of ultraearly intravenous thrombolysis on outcomes in ischemic stroke: the STEMO (Stroke Emergency Mobile) group. Circulation. 2017;135(18):1765-1767. doi: 10.1161/CIRCULATIONAHA.117.027693 [DOI] [PubMed] [Google Scholar]
- 25.Kim JT, Fonarow GC, Smith EE, et al. Treatment with tPA in the “Golden Hour” and the shape of the 4.5 hour time-benefit curve in the national us get with the guidelines-stroke population. Circulation. 2017;135(2):128-139. doi: 10.1161/CIRCULATIONAHA.116.023336 [DOI] [PubMed] [Google Scholar]
- 26.Saver JL, Fonarow GC, Smith EE, et al. Time to treatment with intravenous tissue plasminogen activator and outcome from acute ischemic stroke. JAMA. 2013;309(23):2480-2488. doi: 10.1001/jama.2013.6959 [DOI] [PubMed] [Google Scholar]
- 27.Gyrd-Hansen D, Olsen KR, Bollweg K, Kronborg C, Ebinger M, Audebert HJ. Cost-effectiveness estimate of prehospital thrombolysis: results of the PHANTOM-S study. Neurology. 2015;84(11):1090-1097. doi: 10.1212/WNL.0000000000001366 [DOI] [PubMed] [Google Scholar]
- 28.Dietrich M, Walter S, Ragoschke-Schumm A, et al. Is prehospital treatment of acute stroke too expensive? an economic evaluation based on the first trial. Cerebrovasc Dis. 2014;38(6):457-463. doi: 10.1159/000371427 [DOI] [PubMed] [Google Scholar]
- 29.Rajan SS, Baraniuk S, Parker S, Wu TC, Bowry R, Grotta JC. Implementing a mobile stroke unit program in the United States: why, how, and how much? JAMA Neurol. 2015;72(2):229-234. doi: 10.1001/jamaneurol.2014.3618 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol and Statistical Analysis Plan
Post hoc analyses
eFigure 1. Outcomes in different datasets and pre-specified subgroups
eFigure 2. Distribution of weekdays
eFigure 3. Association of percentage of patients actually treated by MSU to distance of hospital to city center
eFigure 4. Comparison of times from onset to needle in patients with and without MSU dispatch
eTable 1. Unadjusted outcomes in the full analysis set (intention-to-treat analysis)
eTable 2. Patients included in primary analysis without mRS assessment at 3 months
eTable 3. Analysis of patients for whom an MSU was dispatched and arrived at scene compared to patients without MSU dispatch; baseline parameters
eTable 4. Analysis of patients for whom an MSU was dispatched and arrived at scene compared to patients without MSU dispatch; adjusted primary and secondary outcomes
eTable 5. Analysis comparing patients with evaluation aboard an MSU to patients without MSU evaluation independent of dispatch; baseline parameters
eTable 6. Analysis comparing patients with evaluation aboard an MSU to patients without MSU evaluation independent of dispatch; adjusted primary and secondary outcomes
Study Investigators