Highlights
-
•
Physical exercise efficiently prevents the progression of Alzheimer's disease and mitigates the risk of developing the disease.
-
•
Physical exercise acts against the development and progression of Alzheimer's disease via promoting mitochondrial fitness.
-
•
Physical exercise may exert its therapeutic influence on Alzheimer's disease through the reversal of mitochondrial dysfunction via SIRT1-FOXO1/3-PINK1-Parkin–mediated mitophagy.
Alzheimer's disease (AD) is the most common form of age-related neurodegenerative disease. Importantly, the prevalence of AD is expected to increase as the population ages, and it will place a huge burden on health care in the future. AD is clinically characterized by confusion, disorientation, and progressive memory and language decline. Diagnosis is confirmed histologically by the accumulation of extracellular β-amyloid (Aβ) plaques, intracellular tangles of abnormally phosphorylated tau protein, and progressive neuronal loss in the brain.1 Current treatment focuses on symptom alleviation since there is not yet a cure for AD, despite the wealth of research on the mechanisms underlying the disease and its pathophysiology.2
Interestingly, numerous investigations clearly suggest a beneficial role of physical exercise in AD.3 In fact, it is thought that physical exercise may not only prevent the progression of AD, but also mitigate the risk of developing the disease. Various mechanisms have been proposed for the beneficial properties of exercise in AD, including a reduction in abnormal protein aggregates and neuroinflammation and a boost in neurogenesis.3 However, the underlying mechanisms accounting for the benefits of exercise are not well-understood. Identifying these mechanisms could help promote the development of novel pharmaceutical agents in the treatment of AD.
Recent studies have highlighted the contribution of mitochondrial dysfunction and bioenergetic deficit to the Aβ and phosphorylated tau depositions in the brain of AD patients and animals, suggesting that mitochondrial dysfunction is an early event in AD pathology.4 In addition to reduced activity of mitochondrial respiratory enzymes, ultrastructural deficits, including the loss of cristae, the development of vacuolar intramedullary lesions, and increased permeability, have been demonstrated in brain tissue in AD patients.5 Consequently, much evidence has been reported showing that excessive reactive oxygen species and reduced adenosine triphosphate (ATP) levels are present in neurons, thereby exacerbating mitochondrial dysfunction and triggering apoptotic neuronal cell death in AD.6 In fact, multiple lines of evidence have shown that exercise can play a therapeutic role in the development and progression of AD via promoting mitochondrial fitness (see the review by Bernardo et al.7). In addition to decreased reactive oxygen species production, other exercise-induced effects, such as changes in glucose uptake, activity of mitochondrial respiratory enzymes, ATP levels, and mitochondrial biogenesis, have all been described as benefits of exercise in combatting mitochondrial dysfunction in AD.8, 9, 10 However, the underlying mechanisms accounting for the benefits of exercise on mitochondrial fitness are still elusive.
In this opinion piece, we propose a novel idea: physical exercise may exert its therapeutic influence on AD through the reversal of mitochondrial dysfunction via a Sirtuin1 (SIRT1)-mediated mitophagy. Under physiological conditions, damaged mitochondria are surrounded by autophagosomal vesicles, before fusion with lysosomes. The resulting autolysosomes are crucial in the removal of the damaged mitochondria through the action of proteases and other catabolic enzymes. Interestingly, postmortem brain tissue examination in AD patients exhibits abnormal accumulation of mitochondrial constituents within autophagosomal vacuoles.11,12 Abnormal changes in the mitophagy-linked pathway PARK6 (phosphatase and tensin homologue (PTEN) induced putative kinase 1 (PINK1))–Parkin have been found to contribute to AD.13 Notably, recent publications14,15 in Nature and Nature Neuroscience suggest that impaired mitophagy activity plays a critical role in AD onset and progression. The authors of these 2 studies discovered that nicotinamide adenine dinucleotide (NAD+) precursors, such as nicotinamide mononucleotide and nicotinamide riboside, could inhibit Aβ and phosphorylated tau aggregation, improve mitochondrial dysfunction, and reverse cognitive deficits in GMC101 worms and APPswe/PS1De9 (APP/PS1) mouse models of AD via enhancing PINK1–Parkin-dependent mitophagy activity.14,15 Although NAD+ replenishment may serve as a promising candidate for combatting AD in animals, how to translate it to humans remains a challenge.
It is common knowledge that NAD+ is an essential cofactor for SIRT1. A seminal paper from 2012 suggested that a high NAD+/nicotinamide adenine dinucleotide (reduced) (NADH) ratio can mediate mitophagy through an increase in SIRT1 activation.16 Previous studies have revealed that the NAD+-dependent SIRT1 upregulates mitophagy by activating the forkhead box O1/3(FOXO1/3)–PINK1–Parkin pathway.17,18 In this regard, extensive research has confirmed that physical exercise significantly alters the NAD+/NADH ratio and results in enhanced expression of SIRT1 in the brain.19 More important, exercise has been shown to attenuate cognitive decline, improve synaptic dysfunction and enhance survival factors, and decrease the Aβ burden via the activation of the SIRT1 signaling pathway in mouse models of AD.20,21
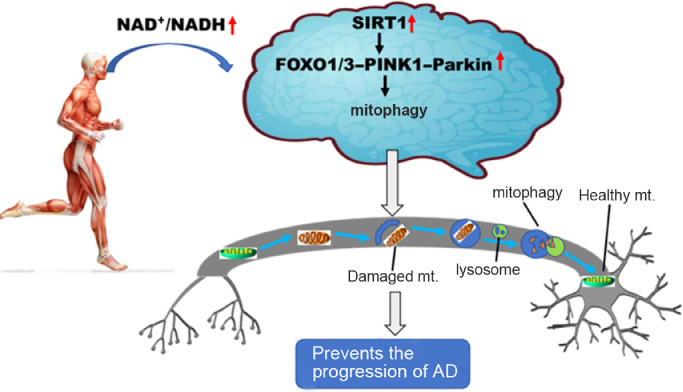
Moreover, it has recently been demonstrated that treadmill exercise activates autolysosomes function in wild-type mice brains through the SIRT1 pathway, and it has also been found that inhibiting the deacetylation effect of SIRT1 abolishes the transcription of lysosomal genes that are regulated by exercise.22 Recent studies have demonstrated that PINK1–Parkin-dependent mitophagy is enhanced after treadmill exercise, resulting not only in increased resistance to age-related and doxorubicin-induced mitochondrial alterations but also in attenuated Aβ-induced cognitive decline and mitochondrial dysfunction in AD animal models.23, 24, 25 It is worth mentioning that long-term exercise is associated with the improvement of mitochondrial quality control in the hippocampus of aged Sprague Dawley rats and that the autophagy pathway is essential for this process.26 Therefore, we suggest that exercise is likely to enhance mitophagy activity by activating the SIRT1–FOXO1/3–PINK1–Parkin pathway through a high NAD+/NADH ratio, thus contributing to the mitigation of the mitochondrial dysfunction associated with AD neurodegeneration. Alternatively, activating the SIRT1 pathway by physical exercise balances both mitophagy and mitochondrial biogenesis, allowing for the maintenance of the functional integrity of mitochondria in the brains of AD (Fig. 1).
Fig. 1.
Physical exercise may exert its potential curative effects in Alzheimer's disease (AD) via SIRT1–FOXO1/3–PINK1–Parkin-mediated mitophagy. NAD+/NADH ↑ means that the NAD+/NADH ratio is increased; SIRT1 ↑ means that SIRT1's activity is increased; FOXO1/3–PINK1–Parkin ↑ means that FOXO1/3–PINK1–Parkin signaling pathway is up-regulated. FOXO = forkhead box O; mt. = mitochondria; NAD+ = nicotinamide adenine dinucleotide; NADH = nicotinamide adenine dinucleotide (reduced); PINK1 = phosphatase and tensin homologue (PTEN) induced putative kinase 1; SIRT1 = Sirtuin1.
It is worth mentioning that we cannot exclude the possibility that SIRT1 may exert its therapeutic influence on AD through pathways other than the mitophagy pathway. For example, findings suggest that lactate mediates the effects of exercise on learning and memory through SIRT1-dependent activation of hippocampal brain-derived neurotrophic factor (BDNF).27 We believe that an exercise-induced increase in BDNF levels is closely related to the activation of SIRT1. A great deal of evidence has confirmed that SIRT1 is an important upstream molecule that activates BDNF.28,29 Perhaps, the benefits of exercise lie in the diversity of “pills of exercise”, and these “pills” perform dual functions by regulating multiple factors, including SIRT1 and BDNF.
We believe that the benefit of exercise on mitophagy is of broad interest to the neuroscientific community and that the results of this interest will inspire further studies that investigate the role of SIRT1-mediated mitophagy in AD prevention and treatment.
Acknowledgments
Acknowledgment
This study was supported by the Fundamental Research Funds for the Central Universities (40500-20104-222290).
Authors’ contributions
NZ was responsible for the overall concept and content of this opinion article and writing the first draft of the manuscript; JX and BX critically reviewed the manuscript. All authors have read and approved the final version of the manuscript, and agree with the order of presentation of the authors.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Peer review under responsibility of Shanghai University of Sport.
References
- 1.Tanzi RE, Bertram L. Twenty years of the Alzheimer's disease amyloid hypothesis: a genetic perspective. Cell. 2005;120:545–555. doi: 10.1016/j.cell.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 2.Long JM, Holtzman DM. Alzheimer disease: an update on pathobiology and treatment strategies. Cell. 2019;179:312–339. doi: 10.1016/j.cell.2019.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McGurran H, Glenn JM, Madero EN, Bott NT. Prevention and treatment of Alzheimer's disease: biological mechanisms of exercise. J Alzheimers Dis. 2019;69:311–338. doi: 10.3233/JAD-180958. [DOI] [PubMed] [Google Scholar]
- 4.Völgyi K, Háden K, Kis V. Mitochondrial proteome changes correlating with beta-Amyloid accumulation. Mol Neurobiol. 2017;54:2060–2078. doi: 10.1007/s12035-015-9682-4. [DOI] [PubMed] [Google Scholar]
- 5.Manczak M, Calkins MJ, Reddy PH. Impaired mitochondrial dynamics and abnormal interaction of amyloid beta with mitochondrial protein Drp1 in neurons from patients with Alzheimer's disease: implications for neuronal damage. Hum Mol Genet. 2011;20:2495–2509. doi: 10.1093/hmg/ddr139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Swerdlow RH. Mitochondria and mitochondrial cascades in Alzheimer's disease. J Alzheimers Dis. 2018;62:1403–1416. doi: 10.3233/JAD-170585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bernardo TC, Marques-Aleixo I, Beleza J, Oliveira PJ, Ascensao A, Magalhaes J. Physical exercise and brain mitochondrial fitness: the possible role against Alzheimer's disease. Brain Pathol. 2016;26:648–663. doi: 10.1111/bpa.12403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bo H, Kang W, Jiang N, Wang X, Zhang Y, Ji LL. Exercise-induced neuroprotection of hippocampus in APP/PS1 transgenic mice via upregulation of mitochondrial 8-oxoguanine DNA glycosylase. Oxid Med Cell Longev. 2014;2014 doi: 10.1155/2014/834502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yan QW, Zhao N, Xia J, Li BX, Yin LY. Effects of treadmill exercise on mitochondrial fusion and fission in the hippocampus of APP/PS1 mice. Neurosci Lett. 2019;701:84–91. doi: 10.1016/j.neulet.2019.02.030. [DOI] [PubMed] [Google Scholar]
- 10.Li B, Liang F, Ding X. Interval and continuous exercise overcomememory deficits related to beta-amyloid accumulation through modulating mitochondrial dynamics. Behav Brain Res. 2019;376 doi: 10.1016/j.bbr.2019.112171. [DOI] [PubMed] [Google Scholar]
- 11.Nixon RA. The role of autophagy in neurodegenerative disease. Nature Med. 2013;19:983–997. doi: 10.1038/nm.3232. [DOI] [PubMed] [Google Scholar]
- 12.Nixon RA, Yang DS. Autophagy failure in Alzheimer's disease–locating the primary defect. Neurobiol Dis. 2011;43:38–45. doi: 10.1016/j.nbd.2011.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reddy PH, Oliver DM. Amyloid beta and phosphorylated tau-induced defective autophagy and mitophagy in Alzheimer's disease. Cells. 2019;8:488. doi: 10.3390/cells8050488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sorrentino V, Romani M, Mouchiroud L. Enhancing mitochondrial proteostasis reduces amyloid-beta proteotoxicity. Nature. 2017;552:187–193. doi: 10.1038/nature25143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fang EF, Hou Y, Palikaras K. Mitophagy inhibits amyloid-beta and tau pathology and reverses cognitive deficits in models of Alzheimer's disease. Nat Neurosci. 2019;22:401–412. doi: 10.1038/s41593-018-0332-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jang SY, Kang HT, Hwang ES. Nicotinamide-induced mitophagy: event mediated by high NAD+/NADH ratio and SIRT1 protein activation. J Biol Chem. 2012;287:19304–19314. doi: 10.1074/jbc.M112.363747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Das S, Mitrovsky G, Vasanthi HR, Das DK. Antiaging properties of a grape-derived antioxidant are regulated by mitochondrial balance of fusion and fission leading to mitophagy triggered by a signaling network of Sirt1-Sirt3-Foxo3-PINK1-PARKIN. Oxid Med Cell Longev. 2014;2014 doi: 10.1155/2014/345105. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Hariharan N, Maejima Y, Nakae J, Paik J, Depinho RA, Sadoshima J. Deacetylation of FoxO by Sirt1 plays an essential role in mediating starvation-induced autophagy in cardiac myocytes. Circ Res. 2010;107:1470–1482. doi: 10.1161/CIRCRESAHA.110.227371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Radak Z, Suzuki K, Posa A, Petrovszky Z, Koltai E, Boldogh I. The systemic role of SIRT1 in exercise mediated adaptation. Redox Biol. 2020;35 doi: 10.1016/j.redox.2020.101467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Revilla S, Sunol C, Garcia-Mesa Y, Gimenez-Llort L, Sanfeliu C, Cristofol R. Physical exercise improves synaptic dysfunction and recovers the loss of survival factors in 3xTg-AD mouse brain. Neuropharmacology. 2014;81:55–63. doi: 10.1016/j.neuropharm.2014.01.037. [DOI] [PubMed] [Google Scholar]
- 21.Koo JH, Kang EB, Oh YS, Yang DS, Cho JY. Treadmill exercise decreases amyloid-beta burden possibly via activation of SIRT-1 signaling in a mouse model of Alzheimer's disease. Exp Neurol. 2017;288:142–152. doi: 10.1016/j.expneurol.2016.11.014. [DOI] [PubMed] [Google Scholar]
- 22.Huang J, Wang X, Zhu Y. Exercise activates lysosomal function in the brain through AMPK-SIRT1-TFEB pathway. CNS Neurosci Ther. 2019;25:796–807. doi: 10.1111/cns.13114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marques-Aleixo I, Santos-Alves E, Balca MM. Physical exercise mitigates doxorubicin-induced brain cortex and cerebellum mitochondrial alterations and cellular quality control signaling. Mitochondrion. 2016;26:43–57. doi: 10.1016/j.mito.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 24.Marques-Aleixo I, Santos-Alves E, Balca MM. Physical exercise improves brain cortex and cerebellum mitochondrial bioenergetics and alters apoptotic, dynamic and auto(mito)phagy markers. Neuroscience. 2015;301:480–495. doi: 10.1016/j.neuroscience.2015.06.027. [DOI] [PubMed] [Google Scholar]
- 25.Zhao N, Yan QW, Xia J. Treadmill exercise attenuates Abeta-induced mitochondrial dysfunction and enhances mitophagy activity in APP/PS1 transgenic mice. Neurochem Res. 2020;45:1202–1214. doi: 10.1007/s11064-020-03003-4. [DOI] [PubMed] [Google Scholar]
- 26.Luo L, Dai JR, Guo SS. Lysosomal proteolysis is associated with exercise-induced improvement of mitochondrial quality control in aged hippocampus. J Gerontol A Biol Sci Med Sci. 2017;72:1342–1351. doi: 10.1093/gerona/glw242. [DOI] [PubMed] [Google Scholar]
- 27.EI Hayek L, Khalifeh M, Zibara V. Lactate mediates the effects of exercise on learning and memory through SIRT1-dependent activation of hippocampal brain-derived neurotrophic factor (BDNF) J Neurosci. 2019;39:2369–2382. doi: 10.1523/JNEUROSCI.1661-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zocchi L, Sassone-Corsi P. SIRT1-mediated deacetylation of MeCP2 contributes to BDNF expression. Epigenetics. 2012;7:695–700. doi: 10.4161/epi.20733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang X, Zhao Y, Zhou Z. Resveratrol mitigates sevoflurane-induced neurotoxicity by the SIRT1-dependent regulation of BDNF expression in developing mice. Oxid Med Cell Longev. 2020;2020 doi: 10.1155/2020/9018624. [DOI] [PMC free article] [PubMed] [Google Scholar]