Highlights
-
•
Cardiorespiratory fitness was associated with older adolescents’ cognitive control.
-
•
Muscular fitness was not associated with older adolescents’ cognitive control.
-
•
Cardiorespiratory fitness selectively influences cognition in late adolescence.
Keywords: Adolescence, Cognition, Physical activity, Physical fitness
Abstract
Background
Participation in physical activity supports greater cardiorespiratory fitness (CRF), a correlate of cognitive control. However, the relationship between muscular fitness (MF) and cognitive control is less clear. The present study investigated the differential relationship of CRF and MF with cognitive control in older adolescents.
Methods
This cross-sectional study involved students (15–17 years old, n = 541, 43% female) from 20 secondary schools who completed tests of inhibition (modified flanker task), working memory (n-back task), CRF (Progressive Aerobic Cardiovascular Endurance Run), and MF (standing long jump and push-up test). Multilevel analyses tested the association between CRF or MF and cognitive outcomes while accounting for the influence of the other fitness variable and relevant demographic factors.
Results
CRF predicted response accuracy during incongruent flanker trials, the condition requiring greater inhibition. For the working memory task, CRF predicted greater target accuracy and greater d' scores on the 1-back task, requiring lesser amounts of working memory. In the 2-back task, which requires greater amounts of working memory, CRF also predicted greater target and non-target accuracy and d' scores. Comparatively, MF did not predict any cognitive outcomes after adjustment for CRF.
Conclusion
CRF was selectively related to better performance during task conditions that require greater amounts of inhibition and working memory. This finding suggests that CRF, but not MF, may benefit cognitive control in older adolescents. This selective influence of CRF on older adolescents’ cognition highlights the value of aerobic physical activity.
Graphical Abstract
1. Introduction
The growing trend of physical inactivity is now considered a pandemic1 and is forecasted to rise even further in the coming decades.2 In 2013, scholars estimated that physical inactivity accounted for at least USD68.5 billion of the global economic burden due to expenses associated with healthcare costs and productivity loss.3 In adolescents,4 much of these costs are a consequence of increased prevalence of comorbid diseases, such as type 2 diabetes and the metabolic syndrome. Critically, adverse effects of physical inactivity further extend to cognitive health.5,6 Participation in physical activity, in part, may prevent the onset and severity of non-communicable diseases7,8 and can lead to gains in physical fitness,9 including both cardiorespiratory and muscular fitness (MF). Cardiorespiratory fitness (CRF) provides a number of health benefits in childhood and adolescence10 and predicts health status into adulthood.11 Concurrently, MF (i.e., muscle strength, endurance, and power) can benefit indices of health in both children and adolescents.10,12 The benefits of physical fitness have also been extended to cognitive and brain health. In particular, CRF promotes greater cognitive functioning across the lifespan13 and is related to enhanced academic achievement14, 15, 16 and brain development in children and adolescents.17,18
Childhood CRF has been associated with cognitive control,19,20 which is an umbrella term (also known as executive function, central executive, or executive control) that denotes a network of processes underlying adaptive, goal-directed behavior and is commonly categorized along the dimensions of inhibition, working memory, and mental flexibility.21 Children with higher CRF often outperform less fit children on tasks requiring greater amounts of cognitive control.22 While growing evidence has supported these benefits, less is known about whether the benefits are generalized across aspects of physical fitness or are specific to particular domains of fitness. Interestingly, research in children and adolescents has shown that greater CRF, but not MF, relates to higher academic achievement.15,23 However, Kao and colleagues24 found that greater MF, independent of CRF, predicted increased response accuracy on a working memory task, as well as on math performance, providing evidence that MF may be related to aspects of cognitive control and scholastic performance. Together, the link between CRF and cognitive control is well-established in the literature; however, the relationship between MF and cognitive control remains less clear.
Curiously, the literature concerning fitness and cognitive control in youth has involved preadolescent (∼8–10 years old) and younger adolescent (∼11–14 years old) populations, while late adolescent (∼15–18 years old) populations have largely been overlooked (see references25,26 for exceptions). The brain undergoes a multitude of changes from childhood to adolescence27,28 and cognitive control abilities consequently develop in their complexity as well.29 Thus, adolescence is vital to the development of brain mechanisms underlying cognitive control; however, it is also a volatile period of vulnerability to risk factors, including physical inactivity. Research indicates that <20% of adolescents are sufficiently active,30 which is concerning given that physical inactivity has been related to poor physical and mental health.31 Moreover, poor CRF and MF are linked to cardiovascular disease prevalence and metabolic risk in adolescents.32 Late adolescence also includes a period of schooling dominated by academic (e.g., standardized testing, rigorous curriculum, etc.) and social stressors, which can hinder physical activity opportunities in schools31. In Australia, for example, the outcome is reflected in the dismal 6% of older adolescents who meet daily physical activity recommendations.33 These issues warrant inquiry into late adolescents’ physical fitness in relation to cognitive control. Such an analysis may provide insight into factors that protect against harmful lifestyle factors.
The current study investigated the relationship between older adolescents’ physical fitness and cognitive control. Based on previous findings in studies of preadolescents,20,24,34 we hypothesized that CRF and MF would both be positively associated with cognitive outcomes and that the relationships would be greater for task conditions requiring greater amounts of cognitive control.
2. Methods
2.1. Participants
Participants were recruited for a large school-based project (Burn 2 Learn) conducted in New South Wales, Australia, between March and April in both 2018 and 2019.35 Ethics approval for the study was obtained from the University of Newcastle, Australia (H85 2016-0424), and from the New South Wales Department of Education (SERAP 2017116). School principals, teachers, parents, and study participants provided informed written consent prior to enrolment. The Burn 2 Learn trial was registered with the Australian and New Zealand Clinical Trials Registry (ACTRN12618000293268). Government schools with senior school students were recruited in 2 cohorts. In Cohort 1, ten schools located within a 90-min drive from the University of Newcastle were recruited. In Cohort 2, ten schools located within a 150-min drive from the University of Newcastle were recruited. The selected geographical regions (i.e., Hunter-Central Coast, Sydney, Northern Sydney, Western Sydney, and New England) are broadly representative of urban and regional secondary schools in New South Wales. Eligible participants were older adolescents (15–17 years old) in Grade 11 at consenting study schools. Participants had no health or medical conditions that would preclude their participation in vigorous physical activity. Prior to testing, all participants and their parents/guardians provided written informed assent and consent, respectively. The current investigation used baseline data that was collected prior to randomization to treatment conditions. A total of 669 students were recruited and performed baseline testing. Participants who provided complete data for all measures were included in the analyses (n = 541). Participants missing body mass index (n = 3) and socioeconomic status (n = 2) were imputed with the average within age, sex, and school.
2.2. Demographics
Participants completed a questionnaire in order to provide demographic information (Table 1), including age, sex, and cultural background. Height (cm) and weight (kg) were collected to calculate body mass index (kg/m2). Socioeconomic status was determined by population tertile using the Socio-Economic Indexes for Areas, Index of Relative Socioeconomic Disadvantage on the basis of residential postcode.36 For the analyses, sex (0 = female, 1 = male) and school (1–20) were dummy coded into variables.
Table 1.
Demographic information on participants.
| Female (n = 235) | Male (n = 306) | Total (n = 541) | |
|---|---|---|---|
| Characteristics (mean ± SD) | |||
| Age (year) | 16.5 ± 0.4 | 16.5 ± 0.4 | 16.5 ± 0.4 |
| SES (SEIFA percentile) | 51.5 ± 26.0 | 50.5 ± 24.8 | 51.0 ± 25.3 |
| BMI (kg/m2) | 23.1 ± 3.6 | 22.8 ± 4.4 | 22.9 ± 4.1 |
| PACER (lap) | 33.7 ± 18.8 | 61.5 ± 22.4 | 49.4 ± 25.0 |
| Push-ups (repetition) | 6.1 ± 6.4 | 16.1 ± 8.1 | 11.8 ± 8.9 |
| Standing long jump (cm) | 146.1 ± 22.9 | 199.7 ± 28.5 | 176.4 ± 37.3 |
| Cultural background (n (%)) | |||
| Australian | 175 (74.5) | 207 (67.6) | 382 (70.6) |
| European | 22 (9.4) | 27 (8.8) | 49 (9.1) |
| African | 2 (0.9) | 3 (1.0) | 5 (0.9) |
| Asian | 9 (3.8) | 26 (8.5) | 35 (6.5) |
| Middle Eastern | 2 (0.9) | 2 (0.7) | 4 (0.7) |
| Other | 25 (10.5) | 41 (13.4) | 66 (12.2) |
Note: SES was determined by population percentile using the socioeconomic indexes for areas of relative socioeconomic disadvantage on the basis of residential postcode; 2 participants did not provide their residential postcode.
Abbreviations: BMI = body mass index; PACER = progressive aerobic cardiovascular endurance run; SEIFA = socio-economic indexes for areas; SES = socio-economic status.
2.3. Cardiorespiratory assessment
The cardiorespiratory assessment was conducted by trained research assistants at the study schools during Term 1 of the school year (February–April). CRF was assessed via the 20-m Progressive Aerobic Cardiovascular Endurance Run (PACER) test using FitnessGram testing procedures (the Cooper Institute, Dallas, TX, USA).37 The PACER test is the most widely used field-based measure of CRF, demonstrating high reliability and validity in adolescent populations.38 Participants were instructed to run back and forth between 2 sets of lines 20 m apart in accordance with an accompanying audio file. Participants were familiarized with the testing parameters, and the test began at a speed of 8.5 km/h and progressively increased by 0.5 km/h with each minute. The test continued until participants failed to maintain the required pace for 2 consecutive laps, or upon volitional fatigue. Test administrators provided verbal encouragement to participants while completing the test in order to maximize motivation. The total number of PACER laps successfully completed was used as the measure of CRF.
2.4. MF assessment
MF was assessed using measures of upper body muscular endurance and lower body muscular power. Upper body muscular endurance was assessed using a modified version of the push-up test (push-ups completed),39 which has acceptable reliability in adolescents.40 Participants were instructed to perform as many push-ups as they could in accordance with a metronome set to a cadence of 40 beats per minute. The test concluded when the participants either failed to lower themselves to the required depth on 2 non-consecutive repetitions (warnings verbalized by assessor), failed to maintain movement coordinating with the metronome, or voluntarily ceased the exercise. The standing long jump test was used as a measure of lower body muscular power (maximum distance jumped), which has acceptable reliability and validity in adolescents.41 From a standing position behind the starting line marked at 0 cm, participants were instructed to take off and land with 2 feet. Upon landing, the distance was recorded from the heel of the rearmost foot. Participants performed a total of 2 jumps, with the longer of the 2 jumps providing a measure of lower body MF.42 Absolute scores of push-ups and standing long jump were standardized (i.e., (value – mean)/SD), accounting for body mass according to the weight-bearing nature of each test.43, 44, 45 A measure of total MF was calculated by adding each standardized test score together.46
2.5. Cognitive tasks
Cognitive tasks were completed on Dell Latitude E5470 laptops (Dell Computers, Round Rock, TX, USA) in the classroom. All cognitive tasks were completed prior to physical fitness tests. Participants were monitored by an experimenter to ensure their understanding of the task. All tasks used the buttons “Q” and “P”. The order of tasks was counterbalanced across participants. Cognitive tasks were presented focally on a black background of a 14-inch computer screen using PsychoPy software (Version 1.83.04; PsychoPy, Nottingham, UK).47 Behavioral performance data (response accuracy and reaction time (RT)) for the flanker task and the 1-back and 2-back tasks were processed and reduced using MATLAB 2017a (The Math Works Inc., Natick, MA, USA).
2.5.1 Flanker task
A modified flanker task48,49 was used to test inhibitory control. Participants responded to the directionality of a centrally presented target arrow amid either congruent (pointing in the same direction) or incongruent (pointing in the opposite direction) flanking arrows. Participants were instructed to respond by pressing the “Q” key with their left index finger if the middle arrow faced left or the “P” key with their right index finger if the middle arrow faced right. Participants were instructed to respond as quickly and accurately as possible. Congruency and directionality of the stimuli were equiprobable for each block of trials. Each participant completed 25 practice trials and an additional block if a 70% response accuracy or greater was not achieved. Subsequently, 1 block of 150 randomized trials were completed, where stimuli were presented for 100 ms with a variable inter-stimulus interval (900 ms, 1050 ms, and 1200 ms). Stimuli consisted of five 3-cm tall white arrows. Participants were presented with one of 2 counterbalanced sets of stimuli.
2.5.2. N-back task
A serial n-back task with 1-back and 2-back conditions was used to assess working memory. In the 1-back condition, participants were instructed to view a series of shapes. If the current shape matched the previous shape (i.e., target trial), participants responded with a “P” key press. If the current shape did not match the previous shape (i.e., non-target trial), they responded with a “Q” key press. In the 2-back condition, participants responded to whether the stimulus from 2 trials prior (i.e., n – 2) matched the current stimulus. In each condition, a match of the 1-back or 2-back stimulus was denoted as a target trial and a non-match was denoted as a non-target trial. Each condition started with a practice block consisting of 20 trials, and an additional practice block was completed if the response accuracy was ≥70%. Participants then completed 2 blocks of 72 trials (24 target, 48 non-target) each for the 1-back and 2-back conditions. Stimuli were each shown for 250 ms followed by an inter-stimulus interval of 2500 ms and consisted of 3 cm colored shapes (i.e., moon, circle, triangle, and star) on a black background. The order of the 1-back and 2-back tasks were counterbalanced across participants.
2.6. Statistical analyses
RT and response accuracy means were calculated for all cognitive measures. For the flanker task, interference effects were calculated as an index of differences in inhibitory control requirements between congruency conditions: congruent–incongruent response accuracy (i.e., response accuracy interference) and incongruent–congruent RT (i.e., RT interference). The d prime (d') was calculated as an additional measure of accuracy for the 1-back and 2-back tasks. The use of d' is advantageous because it factors in response accuracy along with false alarm rate (i.e., when a non-target is mistakenly given a target response). Specifically, a formula was used to subtract the z-score of false alarm rate from the z-score of target response accuracy.50 Maximum and minimum probability adjustments were made to correct for cases of 100% target accuracy (2–(1/n); n = number of trials) as well as cases with a false alarm rate of 0% (1 – (2–(1/n))). A high d' score demonstrates increased discriminatory ability between target and non-target stimuli, with a highest achievable score of 4.9.
Bivariate correlation analyses were conducted in SPSS (Version 23.0; IBM corp., Armonk, NY, USA) to explore associations among demographic, cognitive, MF, and CRF measures. Furthermore, multilevel modeling analyses were utilized to assess fitness associations with cognitive outcomes while accounting for the clustered nature of the data. R software (nlme package, Version 3.1-141; R Core Team, Vienna, Austria)51 was used for multilevel modeling, and the coefficients representing the strength of association between fitness and outcome variables were standardized to facilitate interpretation. As an a priori approach, all models included demographic factors known to influence physical fitness during adolescence (i.e., age, sex, and socioeconomic status)52,53 and were adjusted for school-level clustering. This strategy allowed for a robust assessment of the association of each fitness component to cognitive outcomes while accounting for individual differences and possible influences of the participants’ schools. MF and CRF were individually assessed in the first model (henceforth referred to as Model 1). If the fitness variable significantly predicted a cognitive outcome, a follow-up model was constructed to include the other fitness variable in order to adjust for its potential influence on the fitness variable being assessed (henceforth referred to as Model 2).
3. Results
A total of 541 participants were included in the analysis after excluding outliers (i.e., ±3 SD) in overall response accuracy and RT, as well as d' for the 1-back and 2-back tasks. Pearson product-moment correlation analyses were conducted for demographic variables and cognitive outcomes. CRF was positively correlated with congruent flanker response accuracy, non-target 1-back accuracy, and non-target and target 2-back accuracy and d', as well as negatively correlated with congruent and incongruent flanker RT, and with 1-back non-target RT. MF showed nearly the same pattern of correlations, with an additional significant negative association with 1-back target RT.
3.1. Muscular fitness
A summary of the multilevel modeling analyses can be found in Table 2.
Table 2.
Multilevel modeling summary for MF and cognitive outcomes.
| Model 1 |
Model 2 |
|||
|---|---|---|---|---|
| βMF (SE) | p | βMF (SE) CRF Adj. | p | |
| Flanker | ||||
| Congruent accuracy | 0.12 (0.05) | 0.010** | 0.07 (0.05) | 0.200 |
| Incongruent accuracy | 0.09 (0.05) | 0.050* | 0.03 (0.05) | 0.540 |
| Accuracy interference | –0.03 (0.05) | 0.480 | — | — |
| Congruent RT | –0.13 (0.05) | 0.005** | −0.08 (0.05) | 0.110 |
| Incongruent RT | –0.07 (0.05) | 0.130 | — | — |
| RT interference | 0.06 (0.05) | 0.230 | — | — |
| 1-back | ||||
| Non-target accuracy | 0.10 (0.05) | 0.020* | 0.06 (0.05) | 0.250 |
| Target accuracy | 0.05 (0.05) | 0.250 | — | — |
| d' | 0.09 (0.05) | 0.060 | — | — |
| Non-target RT | –0.11 (0.05) | 0.020* | –0.06 (0.05) | 0.260 |
| Target RT | –0.11 (0.05) | 0.020* | –0.08 (0.05) | 0.130 |
| 2-back | ||||
| Non-target accuracy | 0.10 (0.05) | 0.040* | 0.03 (0.05) | 0.560 |
| Target accuracy | 0.14 (0.05) | 0.002* | 0.09 (0.05) | 0.090 |
| d' | 0.14 (0.05) | 0.003* | 0.08 (0.05) | 0.120 |
| Non-target RT | –0.05 (0.05) | 0.340 | — | — |
| Target RT | –0.05 (0.05) | 0.250 | — | — |
*p ≤ 0.05, **p ≤ 0.01.
Abbreviations: CRF = cardiorespiratory fitness; MF = muscular fitness; CRF Adj. = adjusted for cardiorespiratory fitness; RT = reaction time; SE = standard error.
3.1.1. Model 1
MF significantly predicted congruent and incongruent flanker response accuracy as well as congruent flanker RT (all p ≤ 0.05). However, MF did not predict incongruent flanker RT, or interference outcomes for either response accuracy or RT (all p ≥ 0.13). For the 1-back task, MF significantly predicted non-target response accuracy as well as non-target and target RT (all p = 0.02). MF also showed a trend for d' (p = 0.06). However, MF did not predict target response accuracy (p = 0.25). For the 2-back task, MF predicted non-target and target response accuracy as well as d' (all p ≤ 0.04). However, MF did not predict non-target or target RT (all p ≥ 0.25).
3.1.2. Model 2
Follow-up analyses were conducted on outcomes significantly predicted by MF in Model 1. In these analyses, the association of MF with flanker and n-back outcomes failed to reach significance when CRF was accounted for in the model (all β (standard error (SE)) ≤ 0.09 (0.05), all p ≥ 0.09), suggesting that the variance accounted for by MF was not independent of CRF.
3.2. Cardiorespiratory fitness
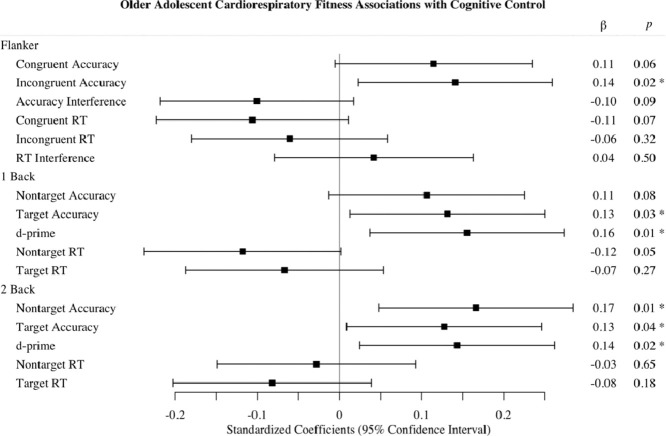
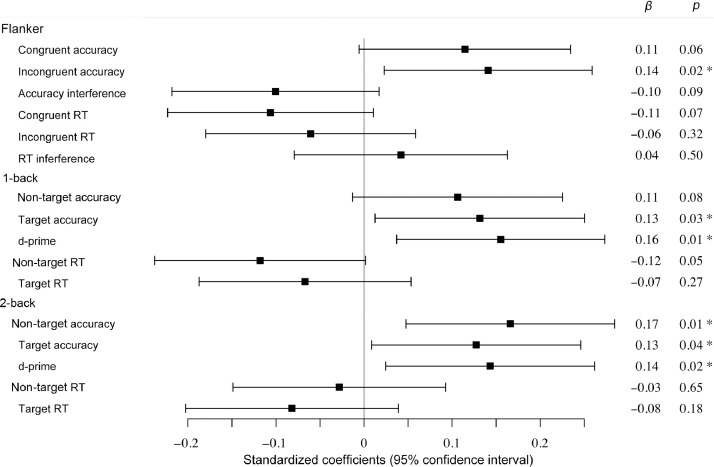
A summary of the full set of multilevel modeling analyses can be found in Table 3. Fig. 1 depicts the standardized linear coefficients with confidence intervals for the association of CRF with each cognitive outcome observed in Model 2.
Table 3.
Multilevel modeling summary for CRF and cognitive outcomes.
| Model 1 |
Model 2 |
|||
|---|---|---|---|---|
| βCRF (SE) | p | βCRF (SE) MF Adj. | p | |
| Flanker | ||||
| Congruent accuracy | 0.15 (0.05) | 0.004** | 0.11 (0.06) | 0.060 |
| Incongruent accuracy | 0.16 (0.05) | 0.002** | 0.14 (0.06) | 0.020* |
| Accuracy interference | 0.09 (0.05) | 0.070 | – | – |
| Congruent RT | –0.15 (0.05) | 0.003** | –0.11 (0.06) | 0.070 |
| Incongruent RT | –0.08 (0.05) | 0.100 | – | – |
| RT interference | 0.06 (0.05) | 0.250 | – | – |
| 1-back | ||||
| Non-target accuracy | 0.14 (0.05) | 0.009** | 0.11 (0.06) | 0.080 |
| Target accuracy | 0.13 (0.05) | 0.010* | 0.13 (0.06) | 0.030* |
| d' | 0.17 (0.05) | 0.001** | 0.16 (0.06) | 0.010** |
| Non-target RT | –0.15 (0.05) | 0.004** | –0.12 (0.06) | 0.053 |
| Target RT | –0.11 (0.05) | 0.030* | –0.07 (0.06) | 0.270 |
| 2-back | ||||
| Non-target accuracy | 0.18 (0.05) | <0.001** | 0.17 (0.06) | 0.007** |
| Target accuracy | 0.18 (0.05) | <0.001** | 0.13 (0.06) | 0.040* |
| d' | 0.19 (0.05) | <0.001** | 0.14 (0.06) | 0.020* |
| Non-target RT | –0.05 (0.05) | 0.400 | – | – |
| Target RT | –0.09 (0.05) | 0.090 | – | – |
* p ≤ 0.05, **p ≤ 0.01.
Abbreviations: CRF = cardiorespiratory fitness; MF = muscular fitness; MF Adj. = adjusted for muscular fitness; RT = reaction time; SE = standard error.
Fig 1.
Multilevel modeling CRF associations with cognitive outcomes. Standardized regression coefficients of CRF in Model 2 (adjusted for demographics and MF). *p < 0.05. Positive values for accuracy, interference, and d' = better performance; negative values for RT = better performance. CRF = cardiorespiratory fitness; RT = reaction time.
3.2.1. Model 1
CRF significantly predicted congruent and incongruent accuracy as well as congruent RT (all p ≤ 0.05) and showed a trend for accuracy interference (p = 0.07). CRF did not predict incongruent flanker RT or RT interference (all p ≥ 0.10). For the 1-back task, CRF significantly predicted all accuracy and RT outcomes across conditions (all p ≤ 0.03). For the 2-back task, CRF predicted non-target and target accuracy as well as d' (all p < 0.001), and showed a trend for target RT (p = 0.09). CRF did not predict non-target RT (p = 0.40).
3.2.2. Model 2
Follow-up analyses were conducted on outcomes that CRF significantly predicted in Model 1. CRF remained a significant predictor of incongruent response accuracy performance even when adjusting for MF (β (SE) = 0.16 (0.05), p = 0.002), suggesting that CRF was related to greater incongruent trial response accuracy. However, the association of CRF with congruent trial response accuracy (β (SE) = 0.11 (0.06), p = 0.06) and congruent trial RT (β (SE) = −0.11 (0.06), p = 0.08) were reduced to a trend when MF was included in Model 2. For the 1-back task, CRF remained a significant predictor of target response accuracy and d' even when adjusting for MF (β (SE) ≥ 0.13 (0.06), all p < 0.03), suggesting that greater CRF was related to better performance on this task condition. The association of CRF with non-target trial response accuracy (β (SE) = 0.10 (0.06), p = 0.09) and RT (β (SE) = –0.12 (0.06), p = 0.053) was reduced to trends when MF was included in Model 2. The association of CRF with target RT failed to reach significance when adjusting for MF. For the 2-back task, CRF remained a significant predictor of target and non-target response accuracy and d' even when adjusted for MF (all β (SE) ≥ 0.13 (0.06), all p < 0.04, suggesting that greater CRF was related to better performance across measures of response accuracy on the 2-back task.
4. Discussion
The current study investigated the relationship of older adolescents’ physical fitness with their performance on measures of cognitive control. Greater CRF was related to greater response accuracy across tasks that modulated inhibitory control and working memory demands. Specifically, CRF predicted response accuracy on the incongruent condition of the flanker task, even when MF was accounted for in the model, suggesting that higher levels of CRF are related to greater response accuracy during task conditions that require greater amounts of inhibitory control. Such a finding has been reported previously in preadolescent children.20 Greater CRF also predicted working memory performance in the 1-back and 2-back tasks. However, MF did not predict performance on any of the cognitive outcomes when CRF was included in the model. Accordingly, greater levels of CRF, independent of MF, were associated with task performance across tasks that manipulated different aspects of cognitive control. Together, these findings indicate that higher levels of CRF selectively support older adolescents’ cognitive operations during engagement in inhibitory control and working memory tasks.
Studies that have investigated the association of physical fitness with cognitive control have typically focused on CRF (see reference24 for an exception). While the current results support previous findings indicating a positive relationship between CRF and cognitive control,20,22,54,55 it was unexpected that MF would not demonstrate an independent relationship with any of the cognitive outcomes. This result contradicts a recent study by Kao and colleagues,24 which found a selective association between MF and working memory performance independent of CRF. However, there were notable differences between studies; for example, Kao et al.24 assessed preadolescent children and the current study assessed older adolescents. Furthermore, MF was assessed differently across studies, with Kao et al.24 using a full-body battery of assessments consisting of 7 different muscle endurance exercises, whereas the current study utilized push-up (muscular endurance) and standing long jump (maximal strength/power) tests. Finally, Kao et al.24 used a more valid and precise measure of CRF (i.e., VO2max test), whereas the current study used a field-based measure (i.e., PACER test) that was more suited for assessing large groups of participants. Despite this limitation, the PACER test remains a valid and reliable assessment of CRF.38 Therefore, the current findings may differ from prior reports because of the population sampled or the manner in which MF was assessed. Regardless, research investigating links between MF and cognitive control remains sparse. The hypothesis that MF would benefit cognition is logical given its link to a multitude of health benefits, including decreased central adiposity, reduction of metabolic risk factors, improved bone health, molecular growth factors, and psychological factors such as positive self-esteem.56 Such health benefits may support greater cognitive functioning;57,58 thus, the notion that MF could benefit cognitive control remains plausible. Indeed, MF may influence aspects of cognition (e.g., cognitive flexibility) that were not assessed in the current study protocol. Further research is necessary to fully unpack the findings, including the possibility of divergence in the association between MF and cognitive performance across adolescent development. Given that this has only been explored in cross-sectional studies, future research should explore causality with more robust study designs.
The literature has been more straightforward regarding the beneficial relationship between CRF and cognitive control. In preadolescent children, it has been previously established that greater CRF is associated with greater task performance (as measured via response accuracy) on tasks of cognitive control, including inhibition and working memory.20,34,59 Furthermore, increased CRF in preadolescent children has most often been reported relative to response accuracy compared to RT for tasks of inhibitory control20 and working memory.60,61 It is notable that such a pattern of results differs in older adulthood, where RT appears more sensitive during this period of the lifespan.62 The current study corroborated previous research in children, finding that greater CRF predicted increased response accuracy across cognitive tasks, particularly during conditions requiring greater amounts of inhibitory control and working memory. However, these findings differ from recent cross-sectional studies of CRF and cognitive control in younger adolescents.54,55 For instance, Huang and colleagues55 found that higher CRF was associated with shorter RT, rather than greater response accuracy, across congruency conditions in a modified flanker task, whereas Westfall et al.54 found that higher CRF was related to greater accuracy and shorter RT across congruency conditions on the same flanker task. Thus, while greater levels of CRF are specifically beneficial to response accuracy in preadolescent children, greater CRF in older adolescents may impart varying, more general benefits to response accuracy and RT. Regardless, the extant literature is consonant in reporting that CRF has a positive relationship with inhibitory control, which is consistent across studies sampling from populations at different stages of development. The current study also provided unique evidence of an association of greater CRF with response accuracy on tasks of working memory in older adolescents. Further research may help elucidate whether, similar to inhibitory control, the behavioral benefits of greater CRF on working memory in adolescents are variable or more specific to response accuracy, as observed during preadolescence. Nevertheless, the accumulating work in this area has further implications for adolescents, a population characterized by decreased physical activity behaviors. The current findings support the idea that physical activity is a vital component of the school day, especially given the heightened academic and social pressures that older adolescents often experience. Engaging in activities that support CRF may help to improve cognitive processes that underlie adolescents’ scholastic performance and psychological well-being.
As evidence mounts regarding the importance of fitness for cognition, there appears to be a clear advantage in maintaining activities that support CRF throughout childhood and adolescence. The current study supports this notion, albeit with cross-sectional evidence. The effects found in the current study were small yet significant in magnitude. However, even small effects are meaningful when considering that one's capacity for cognitive control is determined by a vast multitude of internal and external factors. The fact that small positive effects of fitness on cognition have been shown to be consistent and repeatable is inherently meaningful.63 Moreover, the current study parceled out other factors influential to cognitive performance (i.e., demographics) in its analyses. Thus, the current findings provide a robust platform for future research on fitness and cognition in older adolescents. A natural progression, therefore, would be to investigate the effect of physical activity interventions on cognitive and academic outcomes. Previous research on long-term, CRF training-centered interventions in preadolescent children has shown a positive effect on cognitive and brain function.34,59 Conducting research of this nature provides vital support for prioritizing physical activity in school curricula, especially for older adolescents, whose attendance in physical education classes remains below recommended levels.64 Such a trend is counterintuitive to the mounting findings of an association of physical fitness to academic achievement in children and adolescents.14,16,23 Moreover, physical activity interventions have also been found to be effective in improving time-on-task behavior in the classroom.65,66 Future research should continue to assess how the implementation of physical activity interventions (including physical education), especially those that promote CRF, may support scholastic performance and the underlying cognitive and brain processes.
One of the strengths of the current study is its sample size, albeit with the caveat that data collection occurred within classrooms across different schools. There was potential for selection bias because more of the classes that participated were senior physical education classes rather than other types of classes, which could have resulted in the inclusion of more physically active participants than would be expected in the broader population. There were also limitations imposed by environmental factors. Given the restricted quantity of equipment (i.e., laptops) and space constraints, cognitive assessments occurred while other participants were in the same room, which may have led to interference from a “busy” testing environment. Experimenters attempted to minimize distractions and supervise participants as much as possible to ensure that they understood and were engaged in the tasks; however, the participants’ performance could have been influenced by the presence of other people in the room. Collecting data in schools always poses these types of challenges, as well as requiring coordination with students’ course schedules. Testing conditions such as those utilized in the current study cannot replicate the control that a lab setting affords. Future research in school settings should endeavor to minimize participant distraction within the context of testing.
5. Conclusion
The current study investigated the associations of older adolescents’ physical fitness (i.e., CRF and MF) and cognitive control in a large sample of older adolescents. The main findings revealed significant associations of CRF on inhibitory control and working memory outcomes, even when MF was accounted for in the model. Greater CRF was associated with greater response accuracy across cognitive tasks, which is consistent with previous findings of a positive relationship between levels of CRF and behavior on tasks of cognitive control. On the other hand, MF did not predict performance on any of the cognitive measures, especially when CRF was accounted for in the model. These results provide support for emphasizing older adolescents’ engagement in activities that support CRF as a means for improving their cognitive function.
Acknowledgments
Support for this project was provided by the National Health and Medical Research Council (APP1120518). The study sponsors did not play a role in designing the study; collecting, analyzing, and interpreting the data; writing the report; or deciding to submit the manuscript for publication.
Authors’ contributions
All authors participated in the design of the study and contributed to the manuscript writing. AAL, JJS, NE, and DRL contributed to and/or oversaw data collection; TTS, AAL, JJS, and CHH contributed to data reduction/analysis. All authors have read and approved the final version of the manuscript, and agree with the order of presentation of the authors.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Peer review under responsibility of Shanghai University of Sport.
Supplementary materials
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.jshs.2020.05.004.
Appendix. Supplementary materials
References
- 1.Kohl HW, Craig CL, Lambert EV. The pandemic of physical inactivity: global action for public health. The Lancet. 2012;380:294–305. doi: 10.1016/S0140-6736(12)60898-8. [DOI] [PubMed] [Google Scholar]
- 2.Ng SW, Popkin BM. Time use and physical activity: a shift away from movement across the globe. Obes Rev. 2012;13:659–680. doi: 10.1111/j.1467-789X.2011.00982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ding D, Lawson KD, Kolbe-Alexander TL. The economic burden of physical inactivity: a global analysis of major non-communicable diseases. The Lancet. 2016;388:1311–1324. doi: 10.1016/S0140-6736(16)30383-X. [DOI] [PubMed] [Google Scholar]
- 4.Dabelea D, Mayer-Davis EJ, Saydah S. Prevalence of type 1 and type 2 diabetes among children and adolescents from 2001 to 2009. JAMA. 2014;311:1778–1786. doi: 10.1001/jama.2014.3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vaynman S, Gomez-Pinilla F. Revenge of the “sit”: how lifestyle impacts neuronal and cognitive health through molecular systems that interface energy metabolism with neuronal plasticity. J Neurosci Res. 2006;84:699–715. doi: 10.1002/jnr.20979. [DOI] [PubMed] [Google Scholar]
- 6.Lubans D, Richards J, Hillman C. Physical activity for cognitive and mental health in youth: a systematic review of mechanisms. Pediatrics. 2016;138 doi: 10.1542/peds.2016-1642. [DOI] [PubMed] [Google Scholar]
- 7.Janssen I, LeBlanc AG. Systematic review of the health benefits of physical activity and fitness in school-aged children and youth. Int J Behav Nutr Phys Act. 2010;7:40. doi: 10.1186/1479-5868-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee IM, Shiroma EJ, Lobelo F. Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. The Lancet. 2012;380:219–229. doi: 10.1016/S0140-6736(12)61031-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruiz JR, Rizzo NS, Hurtig-wennlöf A, Ortega FB, Wa J, Sjöström M. Relations of total physical activity and intensity to fitness and fatness in children: the European Youth Heart Study. Am J Clin Nutr. 2006;84:299–303. doi: 10.1093/ajcn/84.1.299. [DOI] [PubMed] [Google Scholar]
- 10.Ruiz JR, Castro-Piñero J, Artero EG. Predictive validity of health-related fitness in youth: a systematic review. Br J Sports Med. 2009;43:909–923. doi: 10.1136/bjsm.2008.056499. [DOI] [PubMed] [Google Scholar]
- 11.Lee D, Artero EG, Sui X, Blair SN. Review: mortality trends in the general population: the importance of cardiorespiratory fitness. J Psychopharmacol. 2010;24(Suppl. 4):S27–S35. doi: 10.1177/1359786810382057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.García-Hermoso A, Ramírez-Campillo R, Izquierdo M. Is muscular fitness associated with future health benefits in children and adolescents? A systematic review and meta-analysis of longitudinal studies. Sport Med. 2019;49:1079–1094. doi: 10.1007/s40279-019-01098-6. [DOI] [PubMed] [Google Scholar]
- 13.Hillman CH, Erickson KI, Kramer AF. Be smart, exercise your heart: exercise effects on brain and cognition. Nat Rev Neurosci. 2008;9:58–65. doi: 10.1038/nrn2298. [DOI] [PubMed] [Google Scholar]
- 14.Sardinha LB, Marques A, Minderico C. Longitudinal relationship between cardiorespiratory fitness and academic achievement. Med Sci Sports Exerc. 2016;48:839–844. doi: 10.1249/MSS.0000000000000830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Esteban-Cornejo I, Tejero-González CM, Martinez-Gomez D. Independent and combined influence of the components of physical fitness on academic performance in youth. J Pediatr. 2014;165:306–312. doi: 10.1016/j.jpeds.2014.04.044. [DOI] [PubMed] [Google Scholar]
- 16.Esteban-Cornejo I, Cadenas-Sanchez C, Contreras-Rodriguez O. A whole brain volumetric approach in overweight/obese children: examining the association with different physical fitness components and academic performance. The ActiveBrains project. Neuroimage. 2017;159:346–354. doi: 10.1016/j.neuroimage.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 17.Herting MM, Keenan MF, Nagel BJ. Aerobic fitness linked to cortical brain development in adolescent males: preliminary findings suggest a possible role of BDNF genotype. Front Hum Neurosci. 2016;10:327. doi: 10.3389/fnhum.2016.00327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Valkenborghs SR, Noetel M, Hillman C. The impact of physical activity on brain structure and function in youth: a systematic review. Pediatrics. 2019;144 doi: 10.1542/peds.2018-4032. [DOI] [PubMed] [Google Scholar]
- 19.Chaddock L, Erickson KI, Prakash RS. A functional MRI investigation of the association between childhood aerobic fitness and neurocognitive control. Biol Psychol. 2012;89:260–268. doi: 10.1016/j.biopsycho.2011.10.017. [DOI] [PubMed] [Google Scholar]
- 20.Raine LB, Kao SC, Pindus D. A large-scale reanalysis of childhood fitness and inhibitory control. J Cogn Enhanc. 2018;2:170–192. [Google Scholar]
- 21.Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: a latent variable analysis. Cogn Psychol. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- 22.Chaddock L, Hillman CH, Pontifex MB, Johnson CR, Raine LB, Kramer AF. Childhood aerobic fitness predicts cognitive performance one year later. J Sports Sci. 2012;30:421–430. doi: 10.1080/02640414.2011.647706. [DOI] [PubMed] [Google Scholar]
- 23.Kalantari HA, Esmaeilzadeh S. Association between academic achievement and physical status including physical activity, aerobic and muscular fitness tests in adolescent boys. Environ Health Prev Med. 2016;21:27–33. doi: 10.1007/s12199-015-0495-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kao SC, Westfall DR, Parks AC, Pontifex MB, Hillman CH. Muscular and aerobic fitness, working memory, and academic achievement in children. Med Sci Sports Exerc. 2017;49:500–508. doi: 10.1249/MSS.0000000000001132. [DOI] [PubMed] [Google Scholar]
- 25.Ruiz JR, Ortega FB, Castillo R. Physical activity, fitness, weight status, and cognitive performance in adolescents. J Pediatr. 2010;157:917–922. doi: 10.1016/j.jpeds.2010.06.026. [DOI] [PubMed] [Google Scholar]
- 26.Ross N, Yau PL, Convit A. Obesity, fitness, and brain integrity in adolescence. Appetite. 2015;93:44–50. doi: 10.1016/j.appet.2015.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giedd JN, Blumenthal J, Jeffries NO. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- 28.Sowell ER, Delis D, Stiles J, Jernigan TL. Improved memory functioning and frontal lobe maturation between childhood and adolescence: a structural MRI study. J Int Neuropsychol Soc. 2001;7:312–322. doi: 10.1017/s135561770173305x. [DOI] [PubMed] [Google Scholar]
- 29.Luna B. Developmental changes in cognitive control through adolescence. Adv Child Dev Behav. 2009;37:233–278. doi: 10.1016/s0065-2407(09)03706-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dumith SC, Gigante DP, Domingues MR, Kohl HW. Physical activity change during adolescence: a systematic review and a pooled analysis. Int J Epidemiol. 2011;40:685–698. doi: 10.1093/ije/dyq272. [DOI] [PubMed] [Google Scholar]
- 31.Hills AP, Dengel DR, Lubans DR. Supporting public health priorities: recommendations for physical education and physical activity promotion in schools. Prog Cardiovasc Dis. 2015;57:368–374. doi: 10.1016/j.pcad.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 32.Artero EG, Ruiz JR, Ortega FB. Muscular and cardiorespiratory fitness are independently associated with metabolic risk in adolescents: the HELENA study. Pediatr Diabetes. 2011;12:704–712. doi: 10.1111/j.1399-5448.2011.00769.x. [DOI] [PubMed] [Google Scholar]
- 33.Schranz NK, Olds T, Boyd R. Results from Australia's 2016 Report Card on Physical Activity for Children and Youth. J Phys Act Heal. 2016;13(Suppl. 2):S87–S94. doi: 10.1123/jpah.2016-0345. [DOI] [PubMed] [Google Scholar]
- 34.Hillman CH, Pontifex MB, Castelli DM. Effects of the FITKids Randomized controlled trial on executive control and brain function. Pediatrics. 2014;134:e1063–e1071. doi: 10.1542/peds.2013-3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leahy AA, Eather N, Smith JJ. School-based physical activity intervention for older adolescents: rationale and study protocol for the Burn 2 Learn cluster randomised controlled trial. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2018-026029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Australian Bureau of Statistics. Census ofPopulation and Housing: Socio-Economic Indexes for Areas (SEIFA). Canberra: Australian Bureau of Statistics; 2011.
- 37.Cureton KJ, Plowman SA, Mahar MT. Aerobic capacity assessments. In: Plowman SA, Meredith MD, editors. FITNESSGRAM/ACTIVITYGRAM reference guide. 4th ed. Dallas, TX: The Cooper Institute; 2013.
- 38.Lang JJ, Tomkinson GR, Janssen I. Making a case for cardiorespiratory fitness surveillance among children and youth. Exerc Sport Sci Rev. 2018;46:66–75. doi: 10.1249/JES.0000000000000138. [DOI] [PubMed] [Google Scholar]
- 39.Cooper Institute for Aerobics Research. Fitnessgram: Test administration manual. Champaign, IL: Human Kinetics, 1999.
- 40.Lubans DR, Morgan P, Callister R. Test–retest reliability of a battery of field-based health-related fitness measures for adolescents. J Sports Sci. 2011;29:685–693. doi: 10.1080/02640414.2010.551215. [DOI] [PubMed] [Google Scholar]
- 41.Ruiz JR, Castro-Piñero J, España-Romero V. Field-based fitness assessment in young people: the ALPHA health-related fitness test battery for children and adolescents. Br J Sports Med. 2011;45:518–524. doi: 10.1136/bjsm.2010.075341. [DOI] [PubMed] [Google Scholar]
- 42.Castro-Piñero J, Ortega FB, Artero EG. Assessing muscular strength in youth: usefulness of standing long jump as a general index of muscular fitness. J Strength Cond Res. 2010;24:1810–1817. doi: 10.1519/JSC.0b013e3181ddb03d. [DOI] [PubMed] [Google Scholar]
- 43.Artero EG, España-Romero V, Jiménez-Pavón D. Muscular fitness, fatness and inflammatory biomarkers in adolescents. Pediatr Obes. 2014;9:391–400. doi: 10.1111/j.2047-6310.2013.00186.x. [DOI] [PubMed] [Google Scholar]
- 44.Jaric S, Mirkov D, Markovic G. Normalizing physical performance tests for body size: a proposal for standardization. J Strength Cond Res. 2005;19:467–474. doi: 10.1519/R-15064.1. [DOI] [PubMed] [Google Scholar]
- 45.Smith JJ, Morgan PJ, Plotnikoff RC, Stodden DF, Lubans DR. Mediating effects of resistance training skill competency on health-related fitness and physical activity: the ATLAS cluster randomised controlled trial. J Sports Sci. 2016;34:772–779. doi: 10.1080/02640414.2015.1069383. [DOI] [PubMed] [Google Scholar]
- 46.Lubans DR, Cliff DP. Muscular fitness, body composition and physical self-perception in adolescents. J Sci Med Sport. 2011;14:216–221. doi: 10.1016/j.jsams.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 47.Peirce J, Gray JR, Simpson S. PsychoPy2: experiments in behavior made easy. Behav Res Methods. 2019;51:195–203. doi: 10.3758/s13428-018-01193-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eriksen BA, Eriksen CW. Effects of noise letters upon the identification of a target letter in a non-search task. Percept Psychophys. 1974;16:143–149. [Google Scholar]
- 49.Hillman CH, Motl RW, Pontifex MB. Physical activity and cognitive function in a cross-section of younger and older community-dwelling individuals. Heal Psychol. 2006;25:678–687. doi: 10.1037/0278-6133.25.6.678. [DOI] [PubMed] [Google Scholar]
- 50.Sorkin RD. Spreadsheet signal detection. Behav Res Methods Instrum Comput. 1999;31:46–54. doi: 10.3758/bf03207691. [DOI] [PubMed] [Google Scholar]
- 51.Pinheiro J, Bates D, DebRoy S, et al. Fit and compare Gaussian linear and nonlinear mixed-effects models. Available at: https://cran.r-project.org/package=nlme. [accessed 09.07.2019]
- 52.Armstrong N, Welsman JR. Aerobic fitness: what are we measuring? Med Sport Sci. 2007;50:5–25. doi: 10.1159/000101073. [DOI] [PubMed] [Google Scholar]
- 53.Coe DP, Peterson T, Blair C, Schutten MC, Peddie H. Physical fitness, academic achievement, and socioeconomic status in school-aged youth. J Sch Health. 2013;83:500–507. doi: 10.1111/josh.12058. [DOI] [PubMed] [Google Scholar]
- 54.Westfall DR, Gejl AK, Tarp J. Associations between aerobic fitness and cognitive control in adolescents. Front Psychol. 2018;9:1298. doi: 10.3389/fpsyg.2018.01298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huang T, Tarp J, Domazet SL. Associations of adiposity and aerobic fitness with executive function and math performance in Danish adolescents. J Pediatr. 2015;167:810–815. doi: 10.1016/j.jpeds.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 56.Smith JJ, Eather N, Morgan PJ, Plotnikoff RC, Faigenbaum AD, Lubans DR. The health benefits of muscular fitness for children and adolescents: a systematic review and meta-analysis. Sport Med. 2014;44:1209–1223. doi: 10.1007/s40279-014-0196-4. [DOI] [PubMed] [Google Scholar]
- 57.Kamijo K, Khan NA, Pontifex MB. The relation of adiposity to cognitive control and scholastic achievement in preadolescent children. Obesity (Silver Spring) 2012;20:2406–2411. doi: 10.1038/oby.2012.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Scudder MR, Khan NA, Lambourne K. Cognitive control in preadolescent children with risk factors for metabolic syndrome. Heal Psychol. 2015;34:243–252. doi: 10.1037/hea0000114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mora-Gonzalez J, Esteban-Cornejo I, Cadenas-Sanchez C. Fitness, physical activity, working memory, and neuroelectric activity in children with overweight/obesity. Scand J Med Sci Sports. 2019;29:1352–1363. doi: 10.1111/sms.13456. [DOI] [PubMed] [Google Scholar]
- 60.Drollette ES, Scudder MR, Raine LB. The sexual dimorphic association of cardiorespiratory fitness to working memory in children. Dev Sci. 2016;19:90–108. doi: 10.1111/desc.12291. [DOI] [PubMed] [Google Scholar]
- 61.Scudder MR, Lambourne K, Drollette ES. Aerobic capacity and cognitive control in elementary school-age children. Med Sci Sports Exerc. 2014;46:1025–1035. doi: 10.1249/MSS.0000000000000199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kramer AF, Hahn S, Cohen NJ. Ageing, fitness and neurocognitive function. Nature. 1999;400:418–419. doi: 10.1038/22682. [DOI] [PubMed] [Google Scholar]
- 63.Etnier JL, Salazar WW, Landers DM, Petruzzello SJ, Han M, Nowell P. The influence of physical fitness and exercise upon cognitive functioning: a meta-analysis. J Sport Exerc Psychol. 1997;19:249–277. [Google Scholar]
- 64.Clennin MN, Demissie Z, Michael SL. Secular changes in physical education attendance among U.S. high school students, 1991–2015. Res Q Exerc Sport. 2018;89:403–410. doi: 10.1080/02701367.2018.1502411. [DOI] [PubMed] [Google Scholar]
- 65.Mahar MT. Impact of short bouts of physical activity on attention-to-task in elementary school children. Prev Med (Baltim) 2011;52(Suppl. 1):S60–S64. doi: 10.1016/j.ypmed.2011.01.026. [DOI] [PubMed] [Google Scholar]
- 66.Mavilidi MF, Drew R, Morgan PJ, Lubans DR, Schmidt M, Riley N. Effects of different types of classroom physical activity breaks on children's on-task behaviour and academic achievement, and cognition. Acta Paediatr. 2020;109:158–165. doi: 10.1111/apa.14892. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.