Abstract
A variety of G-protein coupled receptors (GPCRs) have been implicated in the pathogenesis of pulmonary fibrosis, largely through their promotion of profibrotic fibroblast activation. In contrast, recent work has highlighted the beneficial effects Gαs-coupled GPCRs exert on reducing fibroblast activation and fibrosis. This review highlights how fibrosis promoting and inhibiting GPCR signaling converges on downstream signaling and transcriptional effectors, and how the diversity and dynamics of GPCR expression challenge efforts to identify effective therapies for IPF. Next generation strategies to overcome these challenges, focusing on target selection, polypharmacology and personalized medicine approaches, are discussed as a path toward more effective GPCR-targeted therapies for pulmonary fibrosis.
Keywords: G–protein coupled receptor, ROCK, YAP, TAZ, MRTF, fibrosis
GPCRs as targets for pulmonary fibrosis
G-protein Coupled Receptors (GPCRs)(see Glossary) are a class of over 800 receptors, making up one of the largest and most diverse families of proteins in the genome[1]. The basic function of GPCRs is to communicate extracellular cues into intracellular signals. The transduction of extracellular stimuli is mediated by the interaction of each receptor with one or more of four major unique G-protein families: Gαi/o, Gαq/11, Gα12/13, and Gαs [2, 3] (Fig. 1). Physiological appropriate responses are ensured by coordinated cell-specific expression of receptors [4, 5] and presentation of their endogenous ligands. Their diverse expression, along with their well-defined binding pockets and cell membrane expression, have made them frequent targets for therapeutic development [6], accounting for ~35% of all FDA approved drugs [7]. Multiple prior and ongoing drug discovery campaigns and clinical trial efforts have targeted GPCRs for treatment of idiopathic pulmonary fibrosis (IPF) (Table 1).
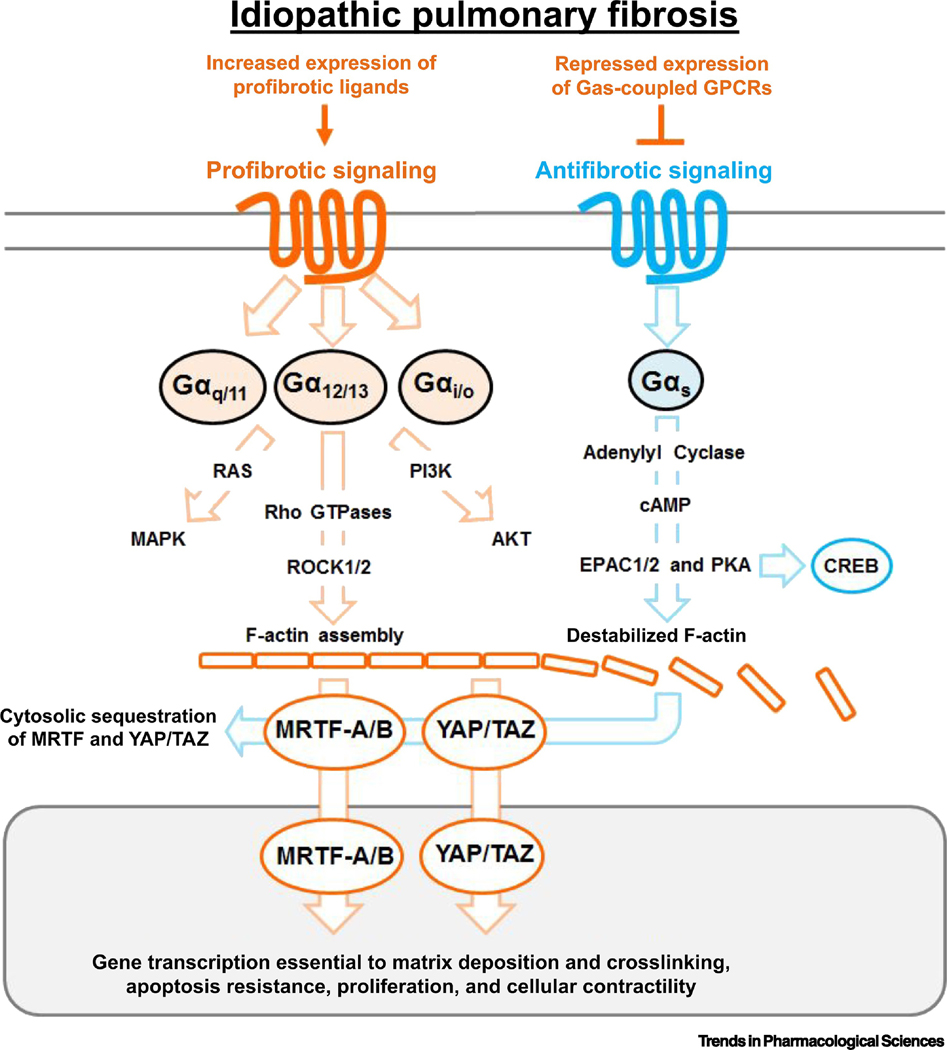
Fig. 1. GPCR Signaling Promotes profibrotic and antifibrotic signaling.
In IPF there is increased abundance of profibrotic ligands, notably: LPA, endothelin, and serotonin that activate receptors coupled to Gαi/o, Gαq/11, Gα12/13 promoting multiple pathways including Rho and ROCK which regulates the actin cytoskeleton. MRTF-A/B and YAP/TAZ are cytoskeletal sensitive profibrotic transcription co-factors essential to fibroblast activation. Known transcript targets for MRTF and YAP/TAZ are profibrotic genes: COL1A1, COL1A2, CTGF,and ACTA2. GPCRs which couple to Gαs are antifibrotic and negatively regulate MRTF-A/B and YAP/TAZ, but are often repressed in IPF patient fibroblasts. Profibrotic signaling is shown in orange and antifibrotic signaling is show in blue.
Table 1.
Summary of GPCR Targeting Clinical Trials for IPF
| Receptor activity | Drug | Outcomes | Status | Trial Number Citation |
|---|---|---|---|---|
| ETA and ETB antagonist | Bosentan | No significant Improvements. | Phase 2/3 (completed) | NCT00071461 [106] |
| ETA and ETB antagonist | Bosentan | No significant Improvements. | Phase 3 (completed) | NCT00391443 [107] |
| ETA antagonist | Ambrisentan | More patients experienced disease progression and death in treatment group compared to placebo. | Phase 3 (terminated) | NCT00768300 [108] |
| ETA and ETB antagonist | Macitentan | No significant Improvements. | Phase 2 (completed) | NCT00903331 [109] |
| LPA1 antagonist | BMS-986020 | Improvement in forced vital capacity for the 600 mg/bid group compared to placebo. | Phase 2 (completed) | NCT01766817 [110] |
| GPR40 agonist and GPR84 antagonist | PBI-4050 | PBI-4050 alone or in combination was well tolerated. | Phase 2 (completed) | NCT02538536 [111] |
| AT1 antagonist | Losartan | Improvement in forced vital capacity. | Pilot Study | NCT00879879 [112] |
| β2 Adrenergic agonist | Formoterol | Treatment significantly improved forced expiratory volume and flow. | Pilot Study | EudraCT: 2013-004404-19 [113] |
| Leukotriene antagonist | Tipelukast | - | Phase 2 (recruiting) | NCT02503657 |
| Prostanoid antagonist | Treprostinil | - | Phase 2 (terminated) | NCT00703339 |
| Prostanoid antagonist | Treprostinil | - | Phase 2 (completed) | NCT00705133 |
| GPR84 antagonist | GLPG1205 | - | Phase 2 (recruiting) | NCT03725852 |
| Smoothened antagonist | Vismodegib | - | Phase 1 (completed) Phase 2 (terminated) | NCT02648048 NCT02168530 |
| Serotonergic and Dopaminergic ligand | RP5063 | - | Phase 2 (planning) | - |
IPF is a progressive chronic lung disorder characterized by uncontrolled deposition of fibrous connective tissue, notably collagen I, predominantly affecting the alveolar interstitium where it results in a replacement of healthy gas exchange tissue with fibrotic scar, leading to respiratory failure and eventual death [8]. IPF affects ~3 million people worldwide with a median life expectancy after diagnosis of 2–4 years and an increasing annual incidence of 3 to 18 cases per 100,000 people in the United States and Europe [8, 9]. Nintedanib and Pirfenidone were approved by the FDA in 2014 as the first pharmaceutical treatments for IPF based on their ability to reduce the decline of pulmonary function [10, 11] however both drugs show only a modest improvement in mortality [11]. The prevailing hypothesis for the initiation and propagation of pulmonary fibrosis is that repeated epithelial insult and impaired repair leads to recruitment or activation of extracellular matrix (ECM) depositing fibroblasts [12–14], potentially supported by additional disease-associated cells such as macrophages [15–17]. This can be modeled experimentally by introducing an epithelial injury in mice by a onetime intratracheal administration of bleomycin which promotes inflammation, injury, and lung fibrosis [18]. Although fibroblasts are transiently activated following tissue injury, in pulmonary fibrosis they maintain an active state, driven by profibrotic soluble factors, mechanosignaling, altered metabolism, and cellular aging [14, 19]. This review focuses on the roles of GPCR signaling in progression and resolution of pulmonary fibrosis and as targets for therapeutic intervention. While not a focus of this review, the parallels to GPCR signaling in asthma, another chronic lung disease, are striking, and include similar challenges with receptor redundancy and desensitization [20, 21]. Consideration of the approaches used there, including multi-drug and personalized strategies, may provide a useful path for GPCR targeting in IPF.
Gαi/o, Gαq/11, Gα12/13 are pro-fibrotic fibroblast regulators
Multiple GPCR ligand/receptor pairs have been recognized as potential drivers of fibrogenic fibroblast activation in vitro, and fibrosis progression in vivo. The specific ligands implicated in pulmonary fibroblast activation and fibrosis include: endothelin (ET-1) [22–24], lysophosphatidic acid (LPA) [25–29], serotonin (5-HT) [30], sphingosine-1-phosphate (S1P) [31] and angiotensin [32]. Although each ligand can interact with multiple cognate receptor subtypes, specific receptors including LPA receptor 1, endothelin receptor A, serotonin receptors 2A and 2B, and sphingosine-1-phosphate receptor 1 have been identified as primary drivers of ligand-mediated fibrogenic activation of human lung fibroblasts. These receptors are capable of activating Gαi/o, Gαq/11, and Gα12/13 subclasses of G-proteins [33]. The remarkable diversity of these upstream GPCRs all capable of engaging very similar patterns of fibroblast activation strongly suggests the presence of convergent downstream mechanisms common to all three receptor families. While a diverse array of signaling and transcriptional programs is activated by these receptors [34], recent work has identified a common intersecting effect on activation of Rho GTPases and actin cytoskeletal assembly [35] (Fig. 1). Together the activation of these pathways promotes nuclear translocation of myocardin-related transcription factors (MRTF-A/B) and the Hippo pathway effectors yes-associated protein (YAP) and transcriptional coactivator with PDZ-binging motif (TAZ) [36, 37], both of which are essential to the activation of fibroblasts to the contractile and matrix synthetic states that drive fibrosis [38–40]. Interestingly, mechanosensitive signaling through integrin and focal adhesion mediated pathways also promote fibroblast activation through the same convergent pathways [41]. Genetic and pharmacological approaches have confirmed the central roles for MRTFA/B and YAP/TAZ in experimental pulmonary fibrosis [38–40, 42], highlighting the potential for identifying and targeting specific upstream GPCRs driving these effects. Additionally, MRTFA/B and YAP/TAZ cooperate and crosstalk with SMAD3 downstream of TGFβ [43, 44], further highlighting their attractiveness as targets for pulmonary fibrosis. Multiple clinical trials have already been conducted or are ongoing to test antagonists of specific GPCRs (Table 1). Below we detail some of the challenges that this approach faces, and later we propose some opportunities for identifying more effective GPCR targeted therapies for IPF.
Gαs is an anti-fibrotic fibroblast regulator
Receptors that couple to Gαs activate adenylyl cyclase, elevating intracellular levels of cyclic adenosine monophosphate (cAMP). Through the use of the cAMP enhancing pharmaco-tool forskolin, elevated cAMP was identified as a means to block transforming growth factor beta (TGFβ) induced fibroblast activation [45]. Later it was recognized that elevation of cAMP through Gαs coupled GPCRs inhibits fibroblast proliferation, expression of ECM proteins, and cellular contractility [46, 47]. Downstream, cAMP interacts with two main effector proteins, protein kinase A (PKA) and exchange factor directly activated by cAMP 1/2 (EPAC1/2) each sharing unique responsibilities for antifibrotic effects [45]. PKA activation causes phosphorylation and activation of the transcription factor cAMP response element-binding protein (CREB) which is itself an antifibrotic mediator [48] (Fig. 1). In harmony with these findings phosphodiesterase inhibitors, which enhance intracellular cAMP, block fibroblast activation and reduce lung fibrosis in vivo [49]. In contrast to all other classes of G-proteins discussed above, Gαs/cAMP signaling promotes phosphorylation and inhibition of YAP/TAZ nuclear translocation [37, 50], as well as reduced MRTF nuclear localization [51]. Ligands and agonists that stimulate Gαs coupled GPCRs promote matrix degradation in multiple tissues [52, 53], and exert protective effects against lung fibroblast activation and fibrosis [46, 47]. Thus treatments that can selectively enhance Gαs signaling in fibroblasts should have promise for anti-fibrotic therapies, and we discuss this opportunity in detail below.
Challenges
Redundancy
As already outlined above, GPCRs are a large class of receptors, several of which are capable of activating similar profibrotic features in pulmonary fibroblasts. Confirmed expression of multiple profibrotic GPCR ligands in human disease and mouse models of pulmonary fibrosis further supports the potential for widespread redundancy in fibroblast activating mechanisms in vivo. For example, LPA has been shown to be increased in the bronchial alveolar lavage fluid (BALF) [29], and exhaled breath condensate (EBC) [25] from patients with idiopathic pulmonary fibrosis, and in rodent models of pulmonary fibrosis [26–28]. Similar reports have been observed for ET-1 [22–24], serotonin [30] and S1P [31]. While mouse models have reported beneficial effects of therapies targeting individual GPCRs in these studies, the complex environment in human IPF may not be as amenable to such an approach. Beyond GPCR ligands, additional biochemical and biomechanical signals contribute to fibroblast activation. These results strongly suggest pulmonary fibrosis is promoted by a profibrotic ligand and extracellular matrix milieu of diverse molecules with overlapping and convergent effects on fibroblast activation, challenging the concept that efficacy in this disease be achieved by blockade of an individual GPCR.
Loss of Gαs coupled GPCRs
An alternative strategy to antagonizing profibrotic signaling would be to agonize antifibrotic signaling, as already highlighted above through Gαs -coupled elevation of cAMP. Strikingly however, widespread repression of Gs coupled GPCRs has been documented in pulmonary fibrosis, posing a challenge to this approach. The most well characterized example is the prostaglandin family of receptors. In cultured human lung fibroblasts prostaglandin E2 stimulates antifibrotic responses [47]. However, in fibroblasts derived from patients with IPF the anti-fibrotic efficacy of prostaglandin E2 is muted by reduced expression of the prostaglandin receptor PTGER2 [54–56]. This phenomenon is also observed in experimental lung fibrosis [57]. Similarly, the relaxin receptor RXFP1 has also been reported to be repressed in IPF patient samples [58]. Data from an RNAseq analysis of TGFβ1-stimulated IPF fibroblasts (GSE136534) also documents a pervasive repression of Gαs coupled GPCRs [59]. Together these findings suggest a coordinated transcriptional repression of Gαs/cAMP signaling is a central feature of pathogenic fibroblast activation. While genomic datasets support this mechanism, detailed functional studies, as already conducted for prostaglandin E2, are still necessary to broadly validate receptor downregulation and its roles in IPF.
Ubiquitous Receptor Expression
Another potential challenge in the development of GPCR regulators for the treatment of pulmonary fibrosis is the widespread expression of these receptors. In many cell types and multiple organs, receptors for endothelin, LPA, serotonin, angiotensin, S1P, and prostaglandins are some of the most highly expressed GPCRs [4, 60, 61]. At a systemic level targeting these receptors could cause dose limiting deleterious effects that preclude beneficial effects in the lung. Even within the lung there are likely to be confounding roles for some of these receptors in specific cell types important for the treatment of fibrosis. A prominent example coming into focus is the alveolar epithelium. Alveolar injury is a hallmark of IPF and repair of the epithelium is likely to be essential for successful resolution [13]. Following alveolar injury, alveolar type II (AEII) cells proliferate and differentiate into alveolar type I (AEI) cells, a process essential to lung repair [13]. The same pathway identified above as a desirable target in fibroblasts (YAP/TAZ) is essential to epithelial repair [62]. Specifically TAZ is required for epithelial differentiation following injury and genetic deletion of TAZ in AEII cells worsens fibrosis in the lungs [63]. In another example, Gαq/11 genetic deletion in AEII cells causes pulmonary inflammation and alveolar enlargement consistent with emphysema [64]. The abundant overlap in GPCR expression between alveolar epithelium and fibroblasts [65], thus poses a substantial challenge for GPCR therapeutics in IPF. Similarly, overlap between the GPCRs expressed in fibroblasts and additional disease relevant cell types such as endothelium and macrophages adds further complexity that is only now beginning to be appreciated.
Next Generation Strategies
Solutions to Redundancy: Target downstream, polypharmacology, personalized medicine
As discussed above a multitude of receptors may be activated in IPF that all have the capacity to promote fibroblast activation. However, these receptors appear to rely on common intracellular pathways for this effect (Fig. 1). Thus, one strategy would be to target the common downstream pathways. Gαq is one of the major profibrotic heterotrimeric G-proteins activated by LPA, ET-1, ATII, and 5-HT signaling. Synthetic and naturally derived Gαq inhibitors have been investigated for efficacy in experimental models of asthma but have yet to be tested in pulmonary fibrosis [66]. Rho-associated coiled-coil containing kinases (ROCK1/2) are downstream effectors to Rho GTPases, and ROCK1/2 inhibitors effectively block fibroblast activation and pulmonary fibrosis in rodent models [67]. However the pleotropic roles of ROCK kinases and the well-known side effects of their inhibition, has raised concerns regarding their potential as therapeutic targets [68]. A recent investigation has found ROCK1- or ROCK2-haploinsufficient mice are equally resistant to pulmonary fibrosis, and targeting ROCK1 may have a beneficial effect in preventing alveolar epithelial apoptosis [69], opening the door to developing ROCK1/2 selective inhibitors that could offer greater safety while still effectively targeting lung fibrosis. Continuing downstream, another opportunity could be in targeting transcriptional regulators. Multiple small-molecule inhibitors of the MRTFA/B pathway have been developed and tested in models of tissue fibrosis [39, 70–72]. Notably, CCG-203971 reduced collagen lung content and enhanced apoptosis of activated fibroblasts in two rodent models of pulmonary fibrosis [42]. Similar approaches have been pursued in developing inhibitors of YAP/TAZ transcriptional activity. The first focused effort in developing a YAP/TAZ inhibitor resulted in the identification of porphyrin family rings, specifically verteporfin, as a feasible mechanism to therapeutically inhibit YAP/TAZ function [73]. Later studies showed effective antifibrotic effects of verteporfin in a silicosis model of pulmonary fibrosis [74]. Additionally, dihydrotanshinone I, a natural compound was found to reduce collagen expression through disruption of YAP/TAZ nuclear localization and transcriptional activity, shows efficacy in rodent models of liver [75] and lung [74] fibrosis. Finally, substantial effort has focused on targeting YAP and TAZ through modulating RhoGTPase membrane association via mevalonate metabolism, one effect of inhibiting HMG-CoA reductase using statin drugs [76]. Although this is a means of indirectly targeting YAP/TAZ, statins have already shown beneficial effect in rodent models of pulmonary fibrosis [77, 78] and in clinical data emerging from retrospective analysis [79, 80]. Together these data support the potential of targeting downstream of GPCRs to solve the problem of activating receptor redundancy. However, the potential challenge inherent in the widespread expression and function of these pathways, addressed below, remains.
Polypharmacology is the design and development of pharmaceutical agents with the ability to simultaneously interact with multiple targets and signaling pathways. Recently, polypharmacology has gained interest for its potential to generate higher efficacy agents with more predictable pharmacokinetic profile and reduced drug resistance [81]. Aided by advancements in GPCR structural and molecular biology, compounds can be rationally designed to target multiple receptors as agonist and antagonist [82]. Intriguingly, one of the two drugs approved for the treatment of IPF is a clear example of a polypharmacology. Nintedanib is a tyrosine kinase inhibitor initially designed to target proangiogenic pathways. Nintedanib acts as an ATP-competitive inhibitor of fibroblast growth factor receptor (FGFR)-1, vascular endothelial growth factor receptor (VEGFR)-2, platelet-derived growth factor receptors (PDGFRs), Flt-3 and members of the Src-family, such as Src, Lyn and Lck [83],[84]. Two new GPCR ligands in clinical trials for IPF may also elicit their effects through polypharmacology (Table 1). RP5063 is a modulator of dopamine and serotonin receptors developed primarily for the treatment of schizophrenia and neuropsychiatric disorders. It has a partial agonism and potent binding affinity with dopamine D2, D3, D4, and serotonin 5-HT1A and 5-HT2A receptors, and antagonist activity at the serotonin 5-HT2B, 5-HT6 and 5-HT7 receptors. Moreover, it exhibits moderate binding affinity for 5-HT2C, α2-adrenergic, and muscarinic acetylcholine receptors [85]. PBI-4050 is a synthetic analog of a medium-length chain fatty acid recognized as an agonist of GPR40 and an antagonist of GPR84, both Free Fatty Acid Receptors (FFAR). As it exerts anti-inflammatory and anti-fibrotic properties, it is being studied for the treatment of Alström Syndrome, Liver and Lung fibrosis, and Chronic Kidney Diseases [86]. PBI-4050 diminishes bleomycin-induced pulmonary fibrosis in mice and reduces TGF-β induced fibroblast activation in vitro [87]. The recent enthusiasm for polypharmacology is a potentially powerful solution to the complexity of GPCR actions in IPF. However, an inherent challenge to this strategy is how much “polypharmacology” is enough, especially when considering the diversity of ligands present in IPF. For example it is unlikely that one molecule could antagonize serotonin receptors (small-molecule) and endothelin receptors (peptide). Polypharmacology will likely benefit from an individualized patient strategy.
Personalized medicine involves a customized approach to disease, identifying the specific characteristics of a patient and tailoring their therapy accordingly. To date, clinical trials testing GPCR ligands for the treatment of IPF have taken a non-personalized approach, one compound for all enrolled patients (Table 1). More broadly, trials in IPF patients have thus far not considered “endotypes”, or subtypes of a disease with distinct pathophysiological or molecular mechanisms [88], a strategy embraced in other lung diseases including asthma and COPD [89, 90]. Several methods are available to ascertain levels of molecules in patients with IPF, including exhaled breath condensate, and serum sampling [91, 92]. These techniques could be used to identify the relative expression of potential GPCR ligands and tailor therapies based on molecular profiles. Going one step further, patient derived cells can be studied to rapidly identify suitable therapeutic strategies. For example, patient derived multicellular “pulmospheres” have shown responses that were predictive of therapeutic response to approved IPF drugs pirfenidone and nintedanib [93]. Future clinical trials may divide patient populations based on their respective endotypes, and analysis of ongoing and past trials of GPCR targeted therapeutics may provide critical insight into molecular classifiers that identify patients most likely to benefit from specific therapeutic approaches. Given the diversity of GPCRs implicated in IPF, such an approach seems warranted.
Solutions to Loss of Gαs Receptors: Rescue downregulated receptors, or target those that remain
Agonists of Gαs receptors display impressive anti-fibrotic effects [46, 47, 52, 53]. However, decreased expression of receptors of this class in diseased tissue or in TGFβ-stimulated fibroblasts provides a challenge to an agonist based therapeutic. Significant efforts have been devoted to understanding the reduced responsiveness of IPF fibroblasts to prostaglandin E2 and have identified epigenetic repression of the PTGER2 receptor through promoter hypermethylation as critical [54]. Inhibitors of PI3K and Akt reduce methylation of the PTGER2 promoter and enhance its transcription, suggesting a mechanism to restore receptor expression and function [54]. In a recent study, TGFβ induced repression of GPCR encoding genes including prostanoid receptors (PTGER2, PTGER4, and PTGIR), adenosine receptors (ADORA2A and ADORA2B), as well as the beta-2 adrenergic receptor (ADRB2). This repression is dependent on histone deacetylase (HDAC) activity, and treatment with an investigational HDAC inhibitor pracinostat (SB939) enhanced expression of these Gαs coupled receptors [59]. MicroRNAs have also been identified to function in TGFβ induced repression of Gαs coupled receptors. Expression of miR-144–3p is enhanced in lungs of patients with IPF and stimulated by TGFβ in cultured lung fibroblasts. Expression of miR-144–3p causes reduced expression of the Gαs coupled receptor, RFXP1, whereas blocking miR-144–3p with a targeted antagomir enhances expression of the receptor and reduces fibroblast activation [94]. These studies identify potential mechanisms to rescue Gαs coupled receptors in patients with IPF by targeting the signaling pathways, epigenetic regulators, or microRNAs that repress their presentation.
A simpler approach is to target Gαs coupled receptors not repressed in IPF. The calcitonin-receptor-like receptor (CRLR), which is activated by adrenomedullin and couples to Gαs to promote cAMP, is actually increased in expression by TGFβ in fibroblasts and in the mouse lung following bleomycin induced fibrosis [95]. Likewise, the dopamine receptor D1 (DRD1) couples to Gαs, and is not decreased in IPF patient fibroblasts or in freshly sorted fibroblasts from bleomycin injured mice [65]. More broadly, RNA-seq analysis of TGFβ stimulated fibroblasts identified three receptors that exclusively couple to Gαs that were not repressed by TGFβ; dopamine receptor D1, G-protein bile acid receptor 1, and an orphan GPCR, GPR3. Further identification of GPCRs preserved in disease settings, such as through emerging single cell RNA-seq analyses [17], will help to define the Gαs coupled receptors available for targeting in IPF patients.
Solutions to Ubiquitous Receptor Expression: Allosteric modulators, target selectively expressed receptors
The final and perhaps most challenging aspect of GPCR targeting is their widespread expression and function. One potential solution to this challenge is the use of allosteric modulators, molecules that bind to a unique site on a receptor to influence the activity of the ligand that binds to the orthosteric, or principle binding site [96]. This approach takes advantage of the potentially unique tissue and compartment-specific expression of endogenous ligands, such that it modulates GPCR function only where these ligands are present. This limits some of the problems that may occur with direct GPCR agonists and antagonists that will function throughout the organism [96]. Importantly, allosteric molecules can enhance (positive allosteric modulators) or repress (negative allosteric modulators) the activity of ligand-receptor signaling. Two allosteric modulators have been FDA approved and there are several more in clinical trials [97]. Although not yet investigated for utility in pulmonary fibrosis, multiple allosteric modulators have been identified and developed for receptors of interest in IPF including serotonin, adenosine, adrenergic, dopamine, endothelin, free fatty acid and prostaglandin receptors [98–100]. The toolbox of allosteric modulators may thus provide unique opportunities to target widely expressed GPCRs safely and effectively for IPF.
A simpler solution, when available, is to identify and target receptors expressed uniquely in the cell type or compartment of interest. For example, the “GPCRome” of fibroblasts and alveolar epithelial cells displays considerable overlap, but there are multiple receptors uniquely expressed in each cell type [65]. The D1 dopamine receptor is the most preferentially expressed receptor in fibroblasts compared to alveolar epithelial cells and lung endothelial cells, and it can be selectively activated in vitro and in vivo without apparent effects on either lung epithelial or endothelial cells. D1 agonism in the mouse lung reduces lung collagen abundance after bleomycin injury, and in vitro stimulation of the D1 receptor prompts fibroblasts to take on a pro-resolution phenotype by enhancing gene expression of matrix degrading enzymes, reducing expression of matrix crosslinking genes, and promoting fibroblasts to produce a less stiff ECM. This approach to selectively target Gαs in lung fibroblasts shows early promise as a targeted therapy for IPF. Expansion of this approach to include analysis of systematic datasets, including single cell RNA-seq profiles, may further refine our understanding of GPCR and ligand expression patterns across cell populations in health and disease, allowing for identification of additional selective targets for therapeutic intervention. The number of drugs which target GPCRs is very large; however the number of distinct GPCRs targeted by those drugs is actually only a small percentage of the more than 800 unique GPCRs [101]. Until recently, investigations into GPCR signaling in disease was focused on non-chemosensory, class A receptors (almost all of the receptors mentioned in this review). However enthusiasm has shifted towards lesser known adhesion receptors, orphan receptors, and chemosensory receptors [102], paving the way for new discoveries in pulmonary fibrosis.
Concluding Remarks
GPCR targeting approaches have thus far yielded disappointing results as IPF therapies (Table 1). Despite this, several promising therapies continue to enter and progress through clinical trials, raising hopes for future success. Several challenges have emerged as outlined above, and major questions remain to be addressed (see Outstanding Questions) as we progress toward next generation therapeutic targets and candidates. The widespread expression and complex cell-specific effects of GPCR modulators will clearly need to be considered in refining our approaches to target identification and early testing. Single cell RNA-seq datasets will provide unique perspectives on receptor distribution [17, 103], while more complex human cellular model systems such as organoids and organ on chip models may provide useful early testing strategies for identifying promising directions [104, 105]. Personalized approaches that target molecular endotypes may split the patient population into smaller segments better served by specific candidate therapies. Candidate molecules that employ polypharmacology or target common downstream mechanisms may overcome the challenge of redundant fibrosis-promoting signals. Finally, Gαs targeting strategies, particularly those focused on receptors uniquely expressed on fibrosis-promoting activated fibroblasts may provide an effective new approach to IPF treatment. Collectively, these advances in understanding offer a fresh perspective on the challenges and opportunities in targeting GPCRs in IPF, and will underpin efforts to identify new strategies that offer therapeutic benefit.
Outstanding Questions.
Can targeting a single GPCR effectively treat most patients with IPF?
When identifying GPCR-based targets for antifibrotic therapy, when and how should receptor expression and function in diverse cell types be considered?
Is the field too focused on molecular target identification and not enough on understanding the complex multicellular interactions and integrated effects of GPCR-based therapeutic candidates?
Highlights.
Multiple GPCR ligand receptor pairs are implicated in IPF, and clinical trials are currently underway targeting GPCR pathways for the treatment of IPF.
Individual GPCRs can promote profibrotic or antifibrotic phenotypes in lung fibroblasts, depending on the receptor class and downstream signaling pathways.
The convergence of downstream pathways on common signaling and transcriptional mechanisms integrates diverse GPCR effects and may provide a path to overcome redundancy.
Signaling programs downstream of GPCR signaling are also essential to alveolar epithelial regeneration and repair, highlighting the need to identify strategies that account for this complexity.
Acknowledgements
We thank the past and present members of the Tschumperlin Lab and our collaborators and colleagues who have contributed to our understanding of GPCR pathways and downstream effectors in fibrosis. We acknowledge funding from the NIH (HL133320 and HL092961) and US Department of Defense (PR181132), the Boehringer Ingelheim Discovery Award in Interstitial Lung Disease, the American Lung Association Catalyst Award, and the Pulmonary Fibrosis Foundation Scholars Award.
Glossary
- Allosteric Modulators
Molecules that affect protein activity by binding to a secondary, allosteric, site rather than a primary, orthosteric, site
- Alveolar and Alveolus
Refers to an alveolus (plural: alveoli), a small, balloon-shaped air cavity arranged in clusters at the end of the respiratory tree in the lung parenchyma
- Bronchial Alveolar Lavage Fluid (BALF)
Fluid collected following the insertion of a device that can infuse solutions into specific parts of the lung
- Cyclic Adenosine Monophosphate (cAMP)
Second messenger molecule which is elevated following Gαs activation
- Endotypes
A subtype of a condition defined by a distinct pathophysiological mechanism
- Exhaled Breath Condensate (EBC)
Breath is condensed following exhalation and allows the determination of biomolecules present in respiratory compartments
- Extracellular Matrix (ECM)
Non-cellular component that provides structural support to cells and organs
- Fibroblasts
Spindle-shape cells in the connective tissue responsible for the synthesis and degradation of extracellular matrix
- Fluorescence-Activated Cell Sorting (FACS)
The process of sorting cells for a desired population through the use of metrics such as size, protein fused fluorescent proteins, and cell surface markers
- G-protein Coupled Receptors (GPCRs)
Largest family of cell membrane receptors that mediate physiological responses through the transduction of extracellular
- Histone Heacetylase (HDAC)
A class of enzymes which removes acetyl groups from histones. This removal causes DNA to bind more tightly, and an overall decrease in gene expression
- Idiopathic Pulmonary Fibrosis (IPF)
A progressive lung disease characterized by the uncontrolled scarring of the lung connective tissue by activated fibroblasts
- Myocardin-Related Transcription Factor (MRTFA/B)
Transcriptional coactivators which are regulated by cytoskeletal dynamics, regulate expression of profibrotic genes
- Nintedanib
An approved small molecule, therapeutic for the treatment of IPF. It has been shown to elicit antifibrotic activity through the inhibition of a wide range of tyrosine kinase receptors
- Pirfenidone
An approved small molecule, therapeutic for the treatment of IPF. It has proven anti-fibrotic activity, but an unknown mechanism of action
- Polypharmacology
Design and development of pharmaceutical agents that simultaneously interact with multiple targets
- Profibrotic and Antifibrotic
A property that promotes or reduces fibrosis - the pathological accumulation of fibrous proteins in an organ
- Rho-associated coiled-coil containing kinases (ROCK1/2)
Are a pair of serine/threonine kinases that are important for a wide range of processes in IPF
- Transforming Growth Factor-Beta (TGFβ)
A profibrotic cytokine involved in fibroblast proliferation and recruitment, cell differentiation, and matrix regulation
- Yes-Associated Protein (YAP)/Transcriptional Coactivator with PDZ-binding Motif (TAZ)
Transcriptional coactivators which are regulated by G-protein and mechanosignaling, regulate expression of profibrotic genes
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kroeze WK, Sheffler DJ, and Roth BL, G-protein-coupled receptors at a glance. Journal of Cell Science, 2003. 116(24): p. 4867–4869. [DOI] [PubMed] [Google Scholar]
- 2.Vass M, et al. , Chemical Diversity in the G Protein-Coupled Receptor Superfamily. Trends in Pharmacological Sciences, 2018. 39(5): p. 494–512. [DOI] [PubMed] [Google Scholar]
- 3.Milligan G. and Kostenis E, Heterotrimeric G-proteins: a short history. British Journal of Pharmacology, 2006. 147: p. S46–S55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Insel PA, et al. , GPCR expression in tissues and cells: Are the optimal receptors being used as drug targets? British Journal of Pharmacology, 2012. 165(6): p. 1613–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Insel PA, et al. , GPCRomics: An Approach to Discover GPCR Drug Targets. Trends in Pharmacological Sciences, 2019. 40(6): p. 378–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hauser AS, et al. , Pharmacogenomics of GPCR Drug Targets. Cell, 2018. 172(1–2): p. 41-+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sriram K. and Insel PA, G Protein-Coupled Receptors as Targets for Approved Drugs: How Many Targets and How Many Drugs? Molecular Pharmacology, 2018. 93(4): p. 251–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martinez FJ, et al. , Idiopathic pulmonary fibrosis. Nature Reviews Disease Primers, 2017. 3. [DOI] [PubMed] [Google Scholar]
- 9.Richeldi L, Collard HR, and Jones MG, Idiopathic pulmonary fibrosis. Lancet, 2017. 389(10082): p. 1941–1952. [DOI] [PubMed] [Google Scholar]
- 10.Canestaro WJ, et al. , Drug Treatment of Idiopathic Pulmonary Fibrosis: Systematic Review and Network Meta-Analysis. Chest, 2016. 149(3): p. 756–66. [DOI] [PubMed] [Google Scholar]
- 11.Maher TM and Strek ME, Antifibrotic therapy for idiopathic pulmonary fibrosis: time to treat. Respir Res, 2019. 20(1): p. 205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Winters NI, et al. , Epithelial Injury and Dysfunction in the Pathogenesis of Idiopathic PulmonaryFibrosis. Am J Med Sci, 2019. 357(5): p. 374–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kulkarni T, et al. , Alveolar epithelial disintegrity in pulmonary fibrosis. American Journal of Physiology-Lung Cellular and Molecular Physiology, 2016. 311(2): p. L185–L191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.King TE Jr., Pardo A, and Selman M, Idiopathic pulmonary fibrosis. Lancet, 2011. 378(9807): p. 1949–61. [DOI] [PubMed] [Google Scholar]
- 15.Misharin AV, et al. , Monocyte-derived alveolar macrophages drive lung fibrosis and persist in the lung over the life span. J Exp Med, 2017. 214(8): p. 2387–2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morse C, et al. , Proliferating SPP1/MERTK-expressing macrophages in idiopathic pulmonary fibrosis. European Respiratory Journal, 2019. 54(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reyfman PA, et al. , Single-Cell Transcriptomic Analysis of Human Lung Provides Insights into the Pathobiology of Pulmonary Fibrosis. American Journal of Respiratory and Critical Care Medicine, 2019. 199(12): p. 1517–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moore BB and Hogaboam CM, Murine models of pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol, 2008. 294(2): p. L152–60. [DOI] [PubMed] [Google Scholar]
- 19.Pardo A. and Selman M, Lung Fibroblasts, Aging, and Idiopathic Pulmonary Fibrosis. Ann Am Thorac Soc, 2016. 13 Suppl 5: p. S417–S421. [DOI] [PubMed] [Google Scholar]
- 20.Deshpande DA and Penn RB, Targeting G protein-coupled receptor signaling in asthma. Cell Signal, 2006. 18(12): p. 2105–20. [DOI] [PubMed] [Google Scholar]
- 21.Wendell SG, Fan H, and Zhang C, G Protein-Coupled Receptors in Asthma Therapy: Pharmacology and Drug Action. Pharmacol Rev, 2020. 72(1): p. 1–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uguccioni M, et al. , Endothelin-1 in Idiopathic Pulmonary Fibrosis. Journal of Clinical Pathology, 1995. 48(4): p. 330–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mutsaers SE, et al. , Increased endothelin-1 and its localization during the development of bleomycin-induced pulmonary fibrosis in rats. Am J Respir Cell Mol Biol, 1998. 18(5): p. 611–9. [DOI] [PubMed] [Google Scholar]
- 24.Saleh D, et al. , Elevated expression of endothelin-1 and endothelin-converting enzyme-1 in idiopathic pulmonary fibrosis: possible involvement of proinflammatory cytokines. Am J Respir Cell Mol Biol, 1997. 16(2): p. 187–93. [DOI] [PubMed] [Google Scholar]
- 25.Montesi SB, et al. , Docosatetraenoyl LPA is elevated in exhaled breath condensate in idiopathic pulmonary fibrosis. Bmc Pulmonary Medicine, 2014. 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cong CC, et al. , Regulation of silicosis formation by lysophosphatidic acid and its receptors. Experimental Lung Research, 2014. 40(7): p. 317–326. [DOI] [PubMed] [Google Scholar]
- 27.Black KE, et al. , Autotaxin activity increases locally following lung injury, but is not required for pulmonary lysophosphatidic acid production or fibrosis. Faseb Journal, 2016. 30(6): p. 2435–2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tager A, et al. , The lysophosphatidic acid receptor LPA1 links pulmonary fibrosis to lung injury by mediating fibroblast recruitment and vascular leak. Inflammation Research, 2007. 56: p. S347–S347. [DOI] [PubMed] [Google Scholar]
- 29.Tager AM, et al. , The lysophosphatidic acid receptor LPA1 links pulmonary fibrosis to lung injury by mediating fibroblast recruitment and vascular leak. Nat Med, 2008. 14(1): p. 45–54. [DOI] [PubMed] [Google Scholar]
- 30.Fabre A, et al. , Modulation of bleomycin-induced lung fibrosis by serotonin receptor antagonists in mice. Eur Respir J, 2008. 32(2): p. 426–36. [DOI] [PubMed] [Google Scholar]
- 31.Huang LS and Natarajan V, Sphingolipids in pulmonary fibrosis. Adv Biol Regul, 2015. 57: p. 55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tan WSD, et al. , Targeting the renin-angiotensin system as novel therapeutic strategy for pulmonary diseases. Current Opinion in Pharmacology, 2018. 40: p. 9–17. [DOI] [PubMed] [Google Scholar]
- 33.Flock T, et al. , Selectivity determinants of GPCR-G-protein binding. Nature, 2017. 545(7654): p. 317-+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Radeff-Huang J, et al. , G protein mediated signaling pathways in lysophospholipid induced cell proliferation and survival. J Cell Biochem, 2004. 92(5): p. 949–66. [DOI] [PubMed] [Google Scholar]
- 35.Maekawa M, et al. , Signaling from rho to the actin cytoskeleton through protein kinases ROCK and LIM-kinase. Science, 1999. 285(5429): p. 895–898. [DOI] [PubMed] [Google Scholar]
- 36.Yu OM, Miyamoto S, and Brown JH, Myocardin-Related Transcription Factor A and Yes-Associated Protein Exert Dual Control in G Protein-Coupled Receptor- and RhoA-Mediated Transcriptional Regulation and Cell Proliferation. Mol Cell Biol, 2016. 36(1): p. 39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu FX, et al. , Regulation of the Hippo-YAP Pathway by G-Protein-Coupled Receptor Signaling. Cell, 2012. 150(4): p. 780–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu F, et al. , Mechanosignaling through YAP and TAZ drives fibroblast activation and fibrosis. American Journal of Physiology-Lung Cellular and Molecular Physiology, 2015. 308(4): p. L344–L357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsou PS, et al. , Cellular mechanisms of tissue fibrosis. 8. Current and future drug targets in fibrosis: focus on Rho GTPase-regulated gene transcription. Am J Physiol Cell Physiol, 2014. 307(1): p. C2–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bernau K, et al. , Megakaryoblastic leukemia-1 is required for the development of bleomycin-induced pulmonary fibrosis. Respiratory Research, 2015. 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tschumperlin DJ, et al. , Mechanosensing and fibrosis. J Clin Invest, 2018. 128(1): p. 74–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sisson TH, et al. , Inhibition of myocardin-related transcription factor/serum response factor signaling decreases lung fibrosis and promotes mesenchymal cell apoptosis. Am J Pathol, 2015. 185(4): p. 969–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Speight P, et al. , Context-dependent switch in chemo/mechanotransduction via multilevel crosstalk among cytoskeleton-regulated MRTF and TAZ and TGFbeta-regulated Smad3. Nat Commun, 2016. 7: p. 11642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miranda MZ, et al. , TGF-beta1 regulates the expression and transcriptional activity of TAZ protein via a Smad3-independent, myocardin-related transcription factor-mediated mechanism. J Biol Chem, 2017. 292(36): p. 14902–14920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Insel PA, et al. , cAMP and Epac in the regulation of tissue fibrosis. Br J Pharmacol, 2012. 166(2): p. 447–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Della Latta V, et al. , The role of the adenosinergic system in lung fibrosis. Pharmacological Research, 2013. 76: p. 182–189. [DOI] [PubMed] [Google Scholar]
- 47.Bozyk PD and Moore BB, Prostaglandin E2 and the pathogenesis of pulmonary fibrosis. Am J Respir Cell Mol Biol, 2011. 45(3): p. 445–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu Y, et al. , Dibutyryl-cAMP attenuates pulmonary fibrosis by blocking myofibroblast differentiation via PKA/CREB/CBP signaling in rats with silicosis. Respiratory Research, 2017. 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sisson TH, et al. , Phosphodiesterase 4 inhibition reduces lung fibrosis following targeted type II alveolar epithelial cell injury. Physiological Reports, 2018. 6(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zmajkovicova K, et al. , The Antifibrotic Activity of Prostacyclin Receptor Agonism Is Mediated through Inhibition of YAP/TAZ. American Journal of Respiratory Cell and Molecular Biology, 2019. 60(5): p. 578–591. [DOI] [PubMed] [Google Scholar]
- 51.Penke LR, et al. , Prostaglandin E2 inhibits alpha-smooth muscle actin transcription during myofibroblast differentiation via distinct mechanisms of modulation of serum response factor and myocardin-related transcription factor-A. J Biol Chem, 2014. 289(24): p. 17151–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Neumann E, Khawaja K, and Muller-Ladner U, G protein-coupled receptors in rheumatology. Nature Reviews Rheumatology, 2014. 10(7): p. 429–436. [DOI] [PubMed] [Google Scholar]
- 53.Samuel CS, et al. , Anti-fibrotic actions of relaxin. British Journal of Pharmacology, 2017. 174(10): p. 962–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huang SK, et al. , Hypermethylation of PTGER2 confers prostaglandin E2 resistance in fibrotic fibroblasts from humans and mice. Am J Pathol, 2010. 177(5): p. 2245–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huang SK, et al. , Variable prostaglandin E2 resistance in fibroblasts from patients with usual interstitial pneumonia. Am J Respir Crit Care Med, 2008. 177(1): p. 66–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mukherjee S, et al. , Prostaglandin E2 inhibits profibrotic function of human pulmonary fibroblasts by disrupting Ca(2+) signaling. Am J Physiol Lung Cell Mol Physiol, 2019. 316(5): p. L810–L821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moore BB, et al. , Bleomycin-induced E prostanoid receptor changes alter fibroblast responses to prostaglandin E2. J Immunol, 2005. 174(9): p. 5644–9. [DOI] [PubMed] [Google Scholar]
- 58.Tan JN, et al. , Expression of RXFP1 Is Decreased in Idiopathic Pulmonary Fibrosis Implications for Relaxin-based Therapies. American Journal of Respiratory and Critical Care Medicine, 2016. 194(11): p. 1392–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jones DL, et al. , TGFbeta-induced fibroblast activation requires persistent and targeted HDAC-mediated gene repression. J Cell Sci, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Insel PA, et al. , GPCRomics: An Approach to Discover GPCR Drug Targets. Trends Pharmacol Sci, 2019. 40(6): p. 378–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Snead AN and Insel PA, Defining the cellular repertoire of GPCRs identifies a profibrotic role for the most highly expressed receptor, protease-activated receptor 1, in cardiac fibroblasts. Faseb Journal, 2012. 26(11): p. 4540–4547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.LaCanna R, et al. , Yap/Taz regulate alveolar regeneration and resolution of lung inflammation. Journal of Clinical Investigation, 2019. 129(5): p. 2107–2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sun TH, et al. , TAZ is required for lung alveolar epithelial cell differentiation after injury. Jci Insight, 2019. 4(14). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.John AE, et al. , Loss of epithelial Gq and G11 signaling inhibits TGFbeta production but promotes IL-33-mediated macrophage polarization and emphysema. Sci Signal, 2016. 9(451): p. ra104. [DOI] [PubMed] [Google Scholar]
- 65.Haak AJ, et al. , Selective YAP/TAZ inhibition in fibroblasts via dopamine receptor D1 agonism reverses fibrosis. Sci Transl Med, 2019. 11(516). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Carr R 3rd, et al. , Interdicting Gq Activation in Airway Disease by Receptor-Dependent and Receptor-Independent Mechanisms. Mol Pharmacol, 2016. 89(1): p. 94–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Knipe RS, Tager AM, and Liao JK, The Rho kinases: critical mediators of multiple profibrotic processes and rational targets for new therapies for pulmonary fibrosis. Pharmacol Rev, 2015. 67(1): p. 103–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Feng YB, et al. , Rho Kinase (ROCK) Inhibitors and Their Therapeutic Potential. Journal of Medicinal Chemistry, 2016. 59(6): p. 2269–2300. [DOI] [PubMed] [Google Scholar]
- 69.Knipe RS, et al. , The Rho Kinase Isoforms ROCK1 and ROCK2 Each Contribute to the Development of Experimental Pulmonary Fibrosis. American Journal of Respiratory Cell and Molecular Biology, 2018. 58(4): p. 471–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Johnson LA, et al. , Novel Rho/MRTF/SRF inhibitors block matrix-stiffness and TGF-beta-induced fibrogenesis in human colonic myofibroblasts. Inflamm Bowel Dis, 2014. 20(1): p. 154–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Haak AJ, et al. , Targeting the Myofibroblast Genetic Switch: Inhibitors of Myocardin-Related Transcription Factor/Serum Response Factor-Regulated Gene Transcription Prevent Fibrosis in a Murine Model of Skin Injury. Journal of Pharmacology and Experimental Therapeutics, 2014. 349(3): p. 480–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kahl DJ, et al. , 5-Aryl-1,3,4-oxadiazol-2-ylthioalkanoic Acids: A Highly Potent New Class of Inhibitors of Rho/Myocardin-Related Transcription Factor (MRTF)/Serum Response Factor (SRF)-Mediated Gene Transcription as Potential Antifibrotic Agents for Scleroderma. J Med Chem, 2019. 62(9): p. 4350–4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu-Chittenden Y, et al. , Genetic and pharmacological disruption of the TEAD-YAP complex suppresses the oncogenic activity of YAP. Genes & Development, 2012. 26(12): p. 1300–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li SY, et al. , Targeting Mechanics-Induced Fibroblast Activation through CD44-RhoA-YAP Pathway Ameliorates Crystalline Silica-Induced Silicosis. Theranostics, 2019. 9(17): p. 4993–5008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ge M, et al. , The anti-hepatic fibrosis effects of dihydrotanshinone I are mediated by disrupting the yes-associated protein and transcriptional enhancer factor D2 complex and stimulating autophagy. Br J Pharmacol, 2017. 174(10): p. 1147–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sorrentino G, et al. , Metabolic control of YAP and TAZ by the mevalonate pathway. Nature Cell Biology, 2014. 16(4): p. 357-+. [DOI] [PubMed] [Google Scholar]
- 77.Tulek B, et al. , Effects of simvastatin on bleomycin-induced pulmonary fibrosis in female rats. Biological Research, 2012. 45(4): p. 345–350. [DOI] [PubMed] [Google Scholar]
- 78.Zhu B, et al. , Atorvastatin attenuates bleomycin-induced pulmonary fibrosis via suppressing iNOS expression and the CTGF (CCN2)/ERK signaling pathway. Int J Mol Sci, 2013. 14(12): p. 24476–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kreuter M, et al. , Effect of statins on disease-related outcomes in patients with idiopathic pulmonary fibrosis. Thorax, 2017. 72(2): p. 148–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kreuter M, et al. , Statin Therapy and Outcomes in Trials of Nintedanib in Idiopathic Pulmonary Fibrosis. Respiration, 2018. 95(5): p. 317–326. [DOI] [PubMed] [Google Scholar]
- 81.Anighoro A, Bajorath J, and Rastelli G, Polypharmacology: Challenges and Opportunities in Drug Discovery. Journal of Medicinal Chemistry, 2014. 57(19): p. 7874–7887. [DOI] [PubMed] [Google Scholar]
- 82.Jacobson KA, New paradigms in GPCR drug discovery. Biochem Pharmacol, 2015. 98(4): p. 541–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Roth GJ, et al. , Nintedanib: from discovery to the clinic. J Med Chem, 2015. 58(3): p. 1053–63. [DOI] [PubMed] [Google Scholar]
- 84.Wollin L, et al. , Mode of action of nintedanib in the treatment of idiopathic pulmonary fibrosis. Eur Respir J, 2015. 45(5): p. 1434–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rajagopal L, et al. , RP5063, an atypical antipsychotic drug with a unique pharmacologic profile, improves declarative memory and psychosis in mouse models of schizophrenia. Behavioural Brain Research, 2017. 332: p. 180–199. [DOI] [PubMed] [Google Scholar]
- 86.Li Y, et al. , Fatty acid receptor modulator PBI-4050 inhibits kidney fibrosis and improves glycemic control. Jci Insight, 2018. 3(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nguyen QT, et al. , PBI-4050 Reduces Pulmonary Hypertension, Lung fibrosis and Right Ventricular Dysfunction in Heart Failure. Cardiovasc Res, 2019. [DOI] [PubMed] [Google Scholar]
- 88.Goodwin AT and Jenkins G, Molecular Endotyping of Pulmonary Fibrosis. Chest, 2016. 149(1): p. 228–237. [DOI] [PubMed] [Google Scholar]
- 89.Svenningsen S. and Nair P, Asthma Endotypes and an Overview of Targeted Therapy for Asthma. Front Med (Lausanne), 2017. 4: p. 158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Garudadri S. and Woodruff PG, Targeting Chronic Obstructive Pulmonary Disease Phenotypes, Endotypes, and Biomarkers. Ann Am Thorac Soc, 2018. 15(Suppl 4): p. S234–S238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hayton C, et al. , Breath biomarkers in idiopathic pulmonary fibrosis: a systematic review. Respir Res, 2019. 20(1): p. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Spagnolo P, Tzouvelekis A, and Maher TM, Personalized medicine in idiopathic pulmonary fibrosis: facts and promises. Current Opinion in Pulmonary Medicine, 2015. 21(5): p. 470–478. [DOI] [PubMed] [Google Scholar]
- 93.Surolia R, et al. , 3D pulmospheres serve as a personalized and predictive multicellular model for assessment of antifibrotic drugs. Jci Insight, 2017. 2(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bahudhanapati H, et al. , MicroRNA-144–3p targets relaxin/insulin-like family peptide receptor 1 (RXFP1) expression in lung fibroblasts from patients with idiopathic pulmonary fibrosis. J Biol Chem, 2019. 294(13): p. 5008–5022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kach J, et al. , Regulation of myofibroblast differentiation and bleomycin-induced pulmonary fibrosis by adrenomedullin. Am J Physiol Lung Cell Mol Physiol, 2013. 304(11): p. L757–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Christopoulos A, Advances in G protein-coupled receptor allostery: from function to structure. Mol Pharmacol, 2014. 86(5): p. 463–78. [DOI] [PubMed] [Google Scholar]
- 97.Hauser AS, et al. , Trends in GPCR drug discovery: new agents, targets and indications. Nat Rev Drug Discov, 2017. 16(12): p. 829–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gentry PR, Sexton PM, and Christopoulos A, Novel Allosteric Modulators of G Protein-coupled Receptors. J Biol Chem, 2015. 290(32): p. 19478–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jiang JX, et al. , Discovery of 2-Piperidinyl Phenyl Benzamides and Trisubstituted Pyrimidines as Positive Allosteric Modulators of the Prostaglandin Receptor EP2. Acs Chemical Neuroscience, 2018. 9(4): p. 699–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Luderman KD, et al. , Identification of Positive Allosteric Modulators of the D-1 Dopamine Receptor That Act at Diverse Binding Sites. Molecular Pharmacology, 2018. 94(4): p. 1197–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wacker D, Stevens RC, and Roth BL, How Ligands Illuminate GPCR Molecular Pharmacology. Cell, 2017. 170(3): p. 414–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hauser AS, et al. , Trends in GPCR drug discovery: new agents, targets and indications. Nature Reviews Drug Discovery, 2017. 16(12): p. 829–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Xie T, et al. , Single-Cell Deconvolution of Fibroblast Heterogeneity in Mouse Pulmonary Fibrosis. Cell Rep, 2018. 22(13): p. 3625–3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gkatzis K, et al. , Use of three-dimensional organoids and lung-on-a-chip methods to study lung development, regeneration and disease. European Respiratory Journal, 2018. 52(5). [DOI] [PubMed] [Google Scholar]
- 105.Clevers H, Modeling Development and Disease with Organoids. Cell, 2016. 165(7): p. 1586–1597. [DOI] [PubMed] [Google Scholar]
- 106.King TE Jr., et al. , BUILD-1: a randomized placebo-controlled trial of bosentan in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med, 2008. 177(1): p. 75–81. [DOI] [PubMed] [Google Scholar]
- 107.King TE Jr., et al. , BUILD-3: a randomized, controlled trial of bosentan in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med, 2011. 184(1): p. 92–9. [DOI] [PubMed] [Google Scholar]
- 108.Raghu G, et al. , Treatment of idiopathic pulmonary fibrosis with ambrisentan: a parallel, randomized trial. Ann Intern Med, 2013. 158(9): p. 641–9. [DOI] [PubMed] [Google Scholar]
- 109.Raghu G, et al. , Macitentan for the treatment of idiopathic pulmonary fibrosis: the randomised controlled MUSIC trial. Eur Respir J, 2013. 42(6): p. 1622–32. [DOI] [PubMed] [Google Scholar]
- 110.Palmer SM, et al. , Randomized, Double-Blind, Placebo-Controlled, Phase 2 Trial of BMS-986020, a Lysophosphatidic Acid Receptor Antagonist for the Treatment of Idiopathic Pulmonary Fibrosis. Chest, 2018. 154(5): p. 1061–1069. [DOI] [PubMed] [Google Scholar]
- 111.Khalil N, et al. , Phase 2 clinical trial of PBI-4050 in patients with idiopathic pulmonary fibrosis. European Respiratory Journal, 2019. 53(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Couluris M, et al. , Treatment of idiopathic pulmonary fibrosis with losartan: a pilot project. Lung, 2012. 190(5): p. 523–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wright CE, et al. , Inhaled beclomethasone/formoterol in idiopathic pulmonary fibrosis: a randomised controlled exploratory study. ERJ Open Res, 2017. 3(4). [DOI] [PMC free article] [PubMed] [Google Scholar]