Abstract
Background
Among patients with out-of-hospital cardiac arrest (OHCA) and ventricular fibrillation, more than half present with refractory ventricular fibrillation unresponsive to initial standard advanced cardiac life support (ACLS) treatment. We did the first randomised clinical trial in the USA of extracorporeal membrane oxygenation (ECMO)-facilitated resuscitation versus standard ACLS treatment in patients with OHCA and refractory ventricular fibrillation.
Methods
For this phase 2, single centre, open-label, adaptive, safety and efficacy randomised clinical trial, we included adults aged 18–75 years presenting to the University of Minnesota Medical Center (MN, USA) with OHCA and refractory ventricular fibrillation, no return of spontaneous circulation after three shocks, automated cardiopulmonary resuscitation with a Lund University Cardiac Arrest System, and estimated transfer time shorter than 30 min. Patients were randomly assigned to early ECMO-facilitated resuscitation or standard ACLS treatment on hospital arrival by use of a secure schedule generated with permuted blocks of randomly varying block sizes. Allocation concealment was achieved by use of a randomisation schedule that required scratching off an opaque layer to reveal assignment. The primary outcome was survival to hospital discharge. Secondary outcomes were safety, survival, and functional assessment at hospital discharge and at 3 months and 6 months after discharge. All analyses were done on an intention-to-treat basis. The study qualified for exception from informed consent (21 Code of Federal Regulations 50.24). The ARREST trial is registered with ClinicalTrials.gov, NCT03880565.
Findings
Between Aug 8, 2019, and June 14, 2020, 36 patients were assessed for inclusion. After exclusion of six patients, 30 were randomly assigned to standard ACLS treatment (n=15) or to early ECMO-facilitated resuscitation (n=15). One patient in the ECMO-facilitated resuscitation group withdrew from the study before discharge. The mean age was 59 years (range 36–73), and 25 (83%) of 30 patients were men. Survival to hospital discharge was observed in one (7%) of 15 patients (95% credible interval 1·6–30·2) in the standard ACLS treatment group versus six (43%) of 14 patients (21·3–67·7) in the early ECMO-facilitated resuscitation group (risk difference 36·2%, 3·7–59·2; posterior probability of ECMO superiority 0·9861). The study was terminated at the first preplanned interim analysis by the National Heart, Lung, and Blood Institute after unanimous recommendation from the Data Safety Monitoring Board after enrolling 30 patients because the posterior probability of ECMO superiority exceeded the prespecified monitoring boundary. Cumulative 6-month survival was significantly better in the early ECMO group than in the standard ACLS group. No unanticipated serious adverse events were observed.
Interpretation
Early ECMO-facilitated resuscitation for patients with OHCA and refractory ventricular fibrillation significantly improved survival to hospital discharge compared with standard ACLS treatment.
Introduction
Out-of-hospital cardiac arrest (OHCA) is responsible for more than 350 000 deaths each year in North America.1,2 A large proportion (60–80%) of patients surviving OHCA present with an initial shockable rhythm (ventricular fibrillation).1,2 However, even in this population that is most frequently resuscitated, half of patients with OHCA and ventricular fibrillation present with refractory ventricular fibrillation unresponsive to initial standard treatment, and thus have a poor prognosis. Among patients requiring more than 40 min of cardiopulmonary resuscitation almost all die.3–5 Most patients (70–85%) presenting with OHCA and refractory ventricular fibrillation (defined as failure of at least three shocks to establish return of spontaneous circulation [ROSC]) have coronary artery disease which, combined with poor perfusion during cardiopulmonary resuscitation, renders prolonged, standard, advanced cardiac life support (ACLS) ineffective.2,6,7
The Cardiovascular Division of The University of Minnesota (Minneapolis, MN, USA), in collaboration with three emergency medical services systems established an early veno-arterial extracorporeal membrane oxygenation (ECMO)-facilitated resuscitation protocol for OHCA and refractory ventricular fibrillation in the USA.3,6,8,9 Preliminary data suggested that survival could be improved by early transport from the field and expedited access to the cardiac catheterisation laboratory for ECMO-facilitated resuscitation. At the same time, programmes around the world have increased the use of ECMO-facilitated resuscitation without direct evidence that this expensive and resource-intensive therapeutic strategy increases survival. The purpose of the ARREST trial was to compare survival to hospital discharge between two standards of care in our community, after arrival at the hospital: emergency department-based standard ACLS resuscitation versus early ECMO-facilitated resuscitation.
Methods
Study design
The ARREST trial was a phase 2, single centre, open-label, safety and efficacy, pragmatic, randomised clinical trial supported by the National Heart, Lung, and Blood Institute (NHLBI). The trial used a hybrid design with Bayesian group-sequential monitoring and response adaptive randomisation calibrated with computer simulation to control frequentist type 1 and 2 error rates. The trial qualified for exception from informed consent under emergency circumstances (21 Code of Federal Regulations 50.24), with applicable requirements and oversight by the US Food & Drug Administration (FDA), an investigational device exemption, approval by the Institutional Review Board of the University of Minnesota, and monitoring by an independent NHLBI appointed Data and Safety Monitoring Board (DSMB). After admission to the hospital, patients who enrolled under exemption of informed consent had to provide written consent upon awakening. Until this was possible, the research team obtained consent to continue participation within 24 h from admission from the legally authorised representative. The representative and the patient had the freedom to withdraw from the study at any time. The study was done at the University of Minnesota Medical Center after receiving patients from three medical emergency systems with geographical proximity to the hospital. The systems transported the patients to the medical centre according to the established refractory ventricular fibrillation or tachycardia protocol of the Minnesota Resuscitation Consortium8 (MRC), based on criteria identical to this study’s inclusion criteria.3,8
We vouch for the completeness and accuracy of the data and all analyses and for the fidelity of the study to the trial protocol (appendix). The rationale, methods, and interventions of the ARREST trial were described previously.10
Patient population
We included all consecutive adults (presumed or known to be 18–75 years old) with an initial OHCA rhythm of ventricular fibrillation or pulseless ventricular tachycardia, no ROSC after three defibrillation shocks, body morphology able to accommodate a Lund University Cardiopulmonary Assist System, and an estimated transfer time to the emergency department shorter than 30 min. Exclusion criteria included valid do not resuscitate orders; blunt, penetrating, or burn-related injury; drowning; known overdose; known pregnancy; being a prisoner; being a nursing home resident; presence of an opt-out study bracelet; unavailability of the catheterisation laboratory; terminal cancer; absolute contraindications to emergent angiography; contrast allergies; and active gastrointestinal or internal bleeding. Sustainable ROSC within the first three shocks was an exclusionary criterion from the study, while ROSC achieved after the fourth shock did not exclude the patient because it was the main way that the standard ACLS group could achieve survival and it was a treatment goal of both groups.
Randomisation and masking
Included patients were randomly assigned to either standard ACLS resuscitation or early ECMO-facilitated resuscitation. On hospital arrival, at least one member of the research team (DY, JB, and EW) was available to verify inclusion or exclusion criteria and eligibility for the study, and they were responsible for enrolment and assignment of patients to the groups. After verification, randomisation to one of the two standards of care was immediately done by use of a secure schedule generated by the Statistical Data and Coordinating Center using permuted blocks with randomly varying block sizes.
The initial randomisation schedule was generated (by TAM, JC, and the Statistical Data and Coordinating Center) with use of a standard random number generator in R, with random permutations in blocks of two, four, and six to ensure approximate balance between the two groups and initially equal probability of assignment to either group. Allocation concealment was accomplished with a randomisation schedule with physical masking that required scratching off a completely opaque layer to determine assignment.
Emergency teams were masked to all aspects of the trial (pre-randomisation blinding). Treatment by ECMO-facilitated resuscitation or standard ACLS treatment was not masked. Investigators had no access to patients randomly assigned to emergency department-based standard ACLS treatment for the duration of the resuscitation and were not involved in any end-of-life decision making for patients. The critical care team was masked to group allocation, since both groups could present with or without the presence of an ECMO circuit.10 Functional assessment at hospital discharge and at 3 months and 6 months after hospital discharge were done by qualified evaluators masked to group allocation.
Procedures
The interventions of the ARREST trial have been described in detail previously.10 In the early ECMO-facilitated resuscitation group, patients gained immediate access to the cardiac catheterisation laboratory regardless of presence or absence of pulses on hospital arrival. In the catheterisation laboratory, patients undergoing cardiopulmonary resuscitation had arterial blood gas collected and, if resuscitation discontinuation criteria were met (two or more of the following: end-tidal CO2 <10 mm Hg, PaO2 <50 mm Hg or oxygen saturation <85%, and lactic acid >18 mmol/L), all further efforts were terminated and the patient was declared dead. If not, peripheral veno-arterial ECMO support was initiated and an angiogram immediately done with revascularisation as clinically indicated. If patients had a pulse and were stable upon arrival, they were treated with an angiogram or angioplasty and circulatory support as required.
In the standard ACLS resuscitation group, patients stayed in the emergency department under care of emergency physicians. In patients without pulses, the protocol dictated that the emergency department team continued treatment for at least 15 min after arrival to the department or for at least 60 min after the 911 call. Afterwards, if the patient did not achieve ROSC, continued resuscitation or declaration of death was at the emergency physician’s discretion. If the patient arrived with pulses or achieved ROSC at any point during resuscitation, the emergency physician transferred the patient for angiography, angioplasty, and circulatory support as needed per clinical protocol.
All patients who survived to hospital admission were treated in a dedicated cardiac intensive care unit (ICU) by a specialised cardiology critical care team. Post-resuscitation care was not protocolised but followed local standard of care for both groups. This standard of care includes 24 h of therapeutic hypothermia (target 34 °C for 24 h), minimisation of vasopressor support with optimisation of ECMO flow, no neuroprognostication for at least 72 h after cardiac arrest, head CT on admission and at day 3 for all patients, and continuous electroencephalogram monitoring until awakening. Using standard scales, masked certified research nurses obtained cerebral performance category and modified Rankin scores of patients during an interview at hospital discharge and 3 months and 6 months after discharge.
Outcomes
The primary outcome was survival to hospital discharge. Secondary endpoints were survival and functionally favourable status at hospital discharge and at 3 and 6 months after hospital discharge, defined as a modified Rankin score of 3 or lower (range from 0 [no symptoms] to 6 [death]) and a cerebral performance category scale of 2 or lower (range from 1 [good cerebral performance] to 5 [death]).11
The incidence of adverse events was recorded for all patients and presented by treatment group to the DSMB for review at regular meetings. All major adverse events and device-related adverse events were reported to the FDA according to federal regulations.
Statistical analysis
The primary endpoint was survival to hospital discharge with a null hypothesis of no difference in the probability of survival to hospital discharge between early ECMO-facilitated resuscitation and standard ACLS resuscitation. The primary null hypothesis was assessed by calculating the posterior probability that survival to hospital discharge was more probable with early ECMO-facilitated resuscitation than with standard ACLS treatment, on the basis of a β-binomial model with non-informative, independent uniform previous distributions. The primary analysis and safety analysis were based on the intention-to-treat principle.
The trial used a hybrid design with Bayesian group sequential monitoring and response adaptive randomisation calibrated with computer simulations to control frequentist type 1 error rates at 0·05 and type 2 at 0·10. The hybrid design dictated evaluation of the primary null hypothesis after each group of 30 participants were randomly assigned and followed up for the primary endpoint. The target effect hypothesised a probability of survival to hospital discharge of 0·37 with early ECMO-facilitated resuscitation versus 0·12 with standard ACLS treatment. To achieve 90% power with a 5% type 1 error rate for this target effect, computer simulation showed that up to five groups of 30 should be evaluated, or 150 total participants.
Additional comparative analyses included Barnard’s two-sample proportion test and a log-rank test and hazard ratio estimate from a Cox proportional hazards model for overall survival. Simulations were done with R, version 3.6.1.
If strong evidence was found of a difference in survival to hospital discharge rates between groups—a posterior probability in favour of either group being 0·986 or higher—the DSMB was obliged to provide a formal recommendation on whether to stop the trial. Otherwise, randomisation to the subsequent group of participants was to be weighted in proportion to the posterior probability of the superior treatment at the most recent analysis. Randomisation was restricted not to exceed 3:1 in either direction. For the first group of 30 patients, randomisation was 1:1 on the basis of permuted blocks of randomly varying sizes. Under the target effect, the expected number of participants was 77 (52 assigned to early ECMO and 25 to standard ACLS treatment.
Early stopping criteria and sequential monitoring used the posterior probability threshold of 0·986 using 10 000 computer simulations of the adaptive design to control type 1 error rate at 5% under the null scenario, with 12% response rates in both groups. The use of a constant boundary was analogous to a Pocock boundary that requires the same level of evidence to stop the trial at each preplanned analysis. The sequential multiple tests of the primary endpoint during the course of the trial were accounted for by setting the constant posterior probability boundary to be 0·986, which controlled the overall type 1 error rate at 5% under the analysis plan that assessed the primary endpoint after each group of 30 participants and allowed for prespecified adaptive modifications to the randomisation ratio. The statistical modelling and plan were approved by the FDA, DSMB statisticians, and the NHLBI leadership, before final approval of the protocol by the University of Minnesota Internal Review Board (00005086). The ARREST trial is registered with ClinicalTrials.gov, NCT03880565.
Role of the funding source
The funder had no role in study design, data collection, data analysis, data interpretation, or writing of the manuscript. The funder received a copy of the manuscript before submission for approval to submit for publication. DY, TPA, JC, TAM, and EW had access to all the data and analyses and made the final decision to submit the manuscript after input and comments from all other authors.
Results
The ARREST trial began on Aug 8, 2019, and was terminated early on June 14, 2020, by the NHLBI after unanimous recommendation from the DSMB members. The DSMB assessed the data from the first 30 randomly assigned patients, as dictated by the protocol, and recommended the termination of the study due to superiority of early ECMO-facilitated resuscitation versus standard ACLS treatment, because the posterior probability crossed the prespecified stopping boundary of 0·986. DSMB members determined, given that the primary endpoint was survival to hospital discharge, that there were ethical concerns to continue the trial in the presence of strong evidence for efficacy.
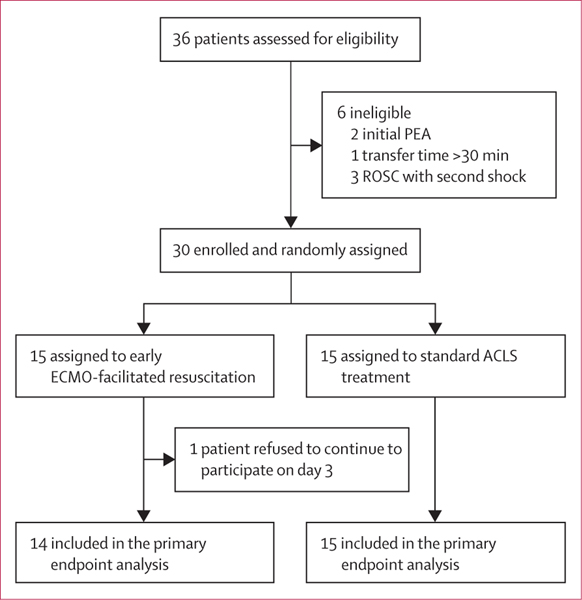
During this time, 36 patients were assessed. Six patients were excluded because of inaccurate MRC refractory ventricular fibrillation or tachycardia protocol selection by emergency teams: two had an initial cardiac arrest rhythm of pulseless electrical activity, one had a transport time of 48 min, and three patients had ROSC after the second shock at the scene before transport. Of 36 patients assessed, 30 met all inclusion criteria and no exclusion criteria (figure 1). Patients were randomly assigned to early ECMO (15 patients) or standard ACLS treatment (15 patients) on hospital arrival and were included in the intention-to-treat analysis. Overall, the mean age was 59 years (SD 10; range 36–73) and 25 (83%) of 30 patients were men. Demographics, past medical history, and current medications appeared balanced between groups (table 1). Apart from interventions associated with ECMO or standard ACLS treatment, characteristics, average times, and treatments in all phases of care appeared similar between groups (table 2).
Figure 1: ARREST trial profile.
ACLS=advanced cardiac life support. ECMO=extracorporeal membrane oxygenation. PEA=pulseless electrical activity. ROSC=return of spontaneous circulation.
Table 1:
Baseline characteristics of the intention-to-treat population
| ECMO-facilitated resuscitation (n=15) | Standard ACLS treatment (n=15) | Total (n=30) | |
|---|---|---|---|
| Demographics | |||
| Age, years | 59 (10) | 58 (11) | 59 (10) |
| Age range, years | 43–73 | 36–71 | 36–73 |
| Sex | |||
| Men | 14 (93%) | 11 (73%) | 25 (83%) |
| Women | 1 (7%) | 4 (27%) | 5 (17%) |
| Race | |||
| White | 5 (33%) | 2 (13%) | 7 (23%) |
| Black or African- American | 1 (7%) | 1 (7%) | 2 (7%) |
| Native American or Native Alaskan | 1 (7%) | 0 | 1 (3%) |
| Not specified by family | 9 (60%) | 11 (73%) | 20 (67%) |
| Medical history | |||
| Coronary artery disease | 2 (13%) | 4 (27%) | 6 (20%) |
| Previous myocardial infarction | 0 | 2 (13%) | 2 (7%) |
| CABG | 2 (13%) | 1 (7%) | 3 (10%) |
| PCI | 1 (7%) | 1 (7%) | 2 (7%) |
| Congestive heart failure | 1 (7%) | 0 | 1 (3%) |
| Previous cardiac arrest | 0 | 0 | 0 |
| General heart disease | 3 (20%) | 4 (27%) | 7 (23%) |
| Stroke | 0 | 1 (7%) | 1 (3%) |
| Hypertension | 2 (13%) | 5 (33%) | 7 (23%) |
| Hyperlipidaemia | 1 (7%) | 2 (13%) | 3 (10%) |
| Diabetes | 3 (20%) | 3 (20%) | 6 (20%) |
| Renal disease | 0 (0%) | 2 (13%) | 2 (7%) |
| Respiratory disease | 1 (7%) | 1 (7%) | 2 (7%) |
| Cancer | 1 (7%) | 1 (7%) | 2 (7%) |
| Smoking | 1 (7%) | 4 (27%) | 5 (17%) |
| Obesity | 0 | 1 (7%) | 1 (3%) |
| Alcoholism | 3 (20%) | 0 | 3 (10%) |
| Unknown | 8 (53%) | 5 (33%) | 13 (43%) |
| Current medications | |||
| ACE inhibitor | 0 | 3 (20%) | 3 (10%) |
| Aspirin | 0 | 2 (13%) | 2 (7%) |
| ß blocker | 1 (7%) | 2 (13%) | 3 (10%) |
| P2Y12 | 0 | 0 | 0 |
| Statin | 1 (7%) | 2 (13%) | 3 (10%) |
| Unknown | 12 (80%) | 11 (73%) | 23 (77%) |
Data are n (%) or mean (SD), unless otherwise specified. ACE=angiotensin-converting enzyme. ACLS=advanced cardiac life support. CABG=coronary artery bypass grafting. ECMO=extracorporeal membrane oxygenation. PCI=percutaneous coronary intervention. P2Y12=adenosine diphosphate receptor inhibitor.
Table 2:
Characteristics and treatments in all phases of care of the intention-to-treat population
| ECMO-facilitated resuscitation (n=15) |
Standard ACLS treatment (n=15) |
Risk difference or p value | |||
|---|---|---|---|---|---|
| Number of patients with data | Patients | Number of patients with data | Patients | ||
| Primary outcome (95% CrI) | |||||
| Survival to hospital discharge | 14 | 6 (43%, 21·3–67·7) | 15 | 1 (7%, 1·6–30·2) | 36% (3·7–59·2; posterior probability=0·9861) |
| Secondary outcomes (95% CI) | |||||
| Survival to 3 months | 14 | 6 (43%, 21·3–67·7) | 15 | 0 (0·0–20·4) | 0·0063 |
| Survival to 6 months | 14 | 6 (43%, 21·3–67·7) | 15 | 0 (0·0–20·4) | 0·0063 |
| CPC score at discharge | 6 | 2·5 (0·5) | 1 | 4 | NA |
| CPC score at 3 months | 6 | 1·16 (04) | 0 | NA | NA |
| CPC score at 6 months | 6 | 1·16 (04) | 0 | NA | NA |
| mRS score at discharge | 6 | 3·8 (0·7) | 1 | 5 | NA |
| mRS score at 3 months | 6 | 2 (1·2) | 0 | NA | NA |
| mRS score at 6 months | 6 | 1·3 (0·8) | 0 | NA | NA |
| Prehospital characteristics | |||||
| Primary VF cardiac arrest | 15 | 15 (100%) | 15 | 15 (100%) | .. |
| Public location of cardiac arrest | 15 | 8 (53%) | 15 | 8 (53%) | .. |
| Bystander witnessed | 15 | 11 (73·3%) | 15 | 13 (86·7%) | .. |
| Bystander CPR | 15 | 13 (86·7%) | 15 | 12 (80·0%) | .. |
| Time from 911 call to EMS arrival (min) | 15 | 6 (2·3) | 15 | 7 (2·5) | .. |
| Endotracheal intubation | 15 | 5 (33·3%) | 15 | 4 (26·6%) | .. |
| Epinephrine doses (1 mg) | 15 | 33 (23) | 15 | 4·4 (4·8) | .. |
| Amiodarone dose (mg) | 15 | 322 (165) | 15 | 375 (78) | .. |
| Number of shocks by EMS | 15 | 5 (2·5) | 15 | 6 (3) | .. |
| Time from cardiac arrest to first shock (min) | 15 | 8·5 (2) | 15 | 7 (2·5) | .. |
| Intermittent ROSC before ED arrival | 15 | 5 (33·3%) | 15 | 4 (26·6%) | .. |
| Arriving with ROSC at the ED | 15 | 0 | 15 | 0 | .. |
| Achieving ROSC in the ED | 15 | 0 | 15 | 2 (13·4%) | .. |
| EMS scene time (min) | 15 | 22·5 (6) | 15 | 23 (11) | .. |
| Transport time (min) | 15 | 19 (7) | 15 | 20 (10) | .. |
| Presenting arterial blood gases | |||||
| Initial lactate, mmol/L | 15 | 11·5 (4·5) | 15 | 10·7 (3·1) | .. |
| Initial pH | 15 | 6·9 (0·9) | 15 | 7·0 (0·11) | .. |
| Initial arterial oxygen, mm Hg | 15 | 86 (18) | 15 | 77 (26) | .. |
| Initial serum bicarbonate, mg/dL | 15 | 19·2 (6·5) | 15 | 20·8 (5·0) | .. |
| Initial end tidal CO2, mm Hg | 15 | 33 (15·2) | 15 | 28 (17·7) | .. |
| ED times for standard ACLS | |||||
| Time from 911 call to randomisation (min) | 15 | 48·5 (21) | 15 | 51·8 (13) | 0.61 |
| ACLS duration after ED arrival (min) | 15 | NA | 15 | 28·5 (17) | NA |
| Time of CPR duration from 911 call to death (min) | 15 | NA | 13 | 81 (20) | NA |
| Time of CPR duration from 911 call to ROSC (min) | 15 | NA | 2 | 83 (8·5) | NA |
| CCL treatment times | |||||
| Time from 911 call to VA-ECMO initiation, min | 12 | 59 (28) | 2 | NA | NA |
| Time from randomisation to VA-ECMO initiation, min | 12 | 12 (6) | 2 | NA | NA |
| Time from CCL arrival to VA-ECMO initiation, min | 12 | 7 (4) | 2 | NA | NA |
| CCL access and treatment | |||||
| Underwent angiography | 15 | 13 (87%) | 2 | 2 (100%) | 1·0 |
| Pronounced dead due to metabolic criteria | 15 | 2 (13%) | 2 | 0 | 1·0 |
| ECMO initiated | 15 | 12 (80%) | 2 | 0 | 0·07 |
| Intra-aortic balloon pump inserted | 15 | 6 (40%) | 2 | 1 (50%) | 1·0 |
| Culprit vessel | 13 | .. | 2 | .. | .. |
| Left anterior descending | 13 | 5 (38%) | 2 | 2 (100%) | 0·2 |
| Left circumflex | 13 | 0 | 2 | 0 | NA |
| Right coronary artery | 13 | 2 (15%) | 2 | 0 | 1·0 |
| Presence of chronic total occlusion | 13 | 2 (15%) | 2 | 2 (100%) | 0·06 |
| Total number of stents placed in all vessels | 13 | 2 (0·7) | 2 | 2 (1·4) | 0·7 |
| ICU interventions | |||||
| Tracheostomy | 12 | 1 (8%) | 2 | 1 (50%) | 0·035 |
| Bleeding requiring surgical intervention | 12 | 1 (8%) | 2 | ·· | 1·0 |
| Bleeding requiring transfusion of >3 units of PRBC | 12 | 5 (42%) | 2 | ·· | 0·5 |
| PEG tube | 12 | 0 | 2 | 1 (50%) | 0·14 |
| 24-h LVEF on echocardiogram in ICU, % | 13 | 12 (11) | 1 | 10 | NA |
| LVEF on hospital discharge, % | 6 | 42·5 (14) | 1 | 10 | NA |
| Time to decannulation, days; median (range) | |||||
| Survivors | 6 | 4(2·21) | 1 | NA | NA |
| Deceased | 6 | NA | 1 | NA | NA |
| Time to extubation, days; median (range) | |||||
| Survivors | 6 | 9·5 (4·21) | 1 | NA | NA |
| Deceased | 6 | NA | 1 | NA | NA |
| Length of ICU stay, days; median (range) | |||||
| Survivors | 6 | 21·5 (9·45) | 1 | 27 | NA |
| Deceased | 6 | 3·5 (1·22) | 1 | 1 | NA |
| Length of hospital stay, days; median (range) | |||||
| Survivors | 6 | 25·5 (11·48) | 1 | 46 | NA |
| Deceased | 6 | 3·5 (1·22) | 1 | 1 | NA |
Data are n (%) or mean (SD), unless otherwise specified. ACLS=advanced cardiac life support. CCL=cardiac catheterisation laboratory. CrI=credible interval. CPC=cerebral performance category. CPR=cardiopulmonary resuscitation. ECMO=extracorporeal membrane oxygenation. ED=emergency department. EMS=emergency medical services. ICU=intensive care unit. LVEF=left ventricular ejection fraction. mRS=modified Rankin score. NA=not applicable. PEG=percutaneous endoscopic gastrostomy. PRBC=packed red blood cells. ROSC=return of spontaneous circulation. VA=veno-arterial. VF=ventricular fibrillation.
Of the 30 patients enrolled, the primary outcome was obtained in 29 patients. One patient in the early ECMO group withdrew consent for continuation of participation on day 3 after randomisation. The primary endpoint of survival to hospital discharge was analysed with the same Bayesian model used for interim monitoring and was observed in six (43%, 95% credible interval 21·3–67·7) of 14 patients in the early ECMO group compared with one (7%, 1·6–30·2) of 15 in the standard ACLS treatment group (risk difference 36%, 3·7–59·2; 0·9861 posterior probability of ECMO superiority; table 2).
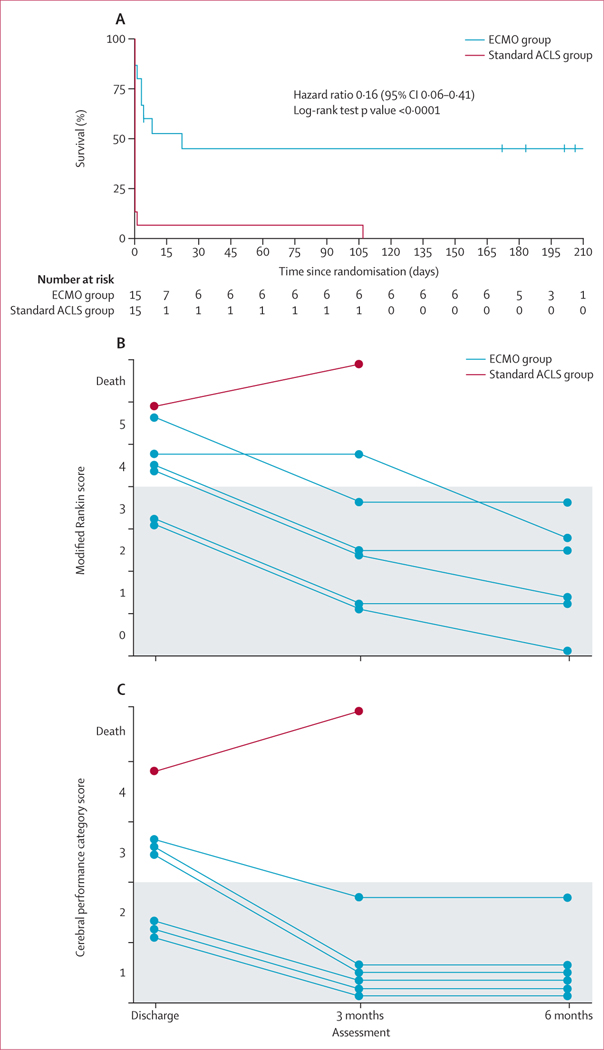
Secondary outcomes of cumulative survival, modified Rankin score, and cerebral performance category scores at hospital discharge, and at 3 and 6 months after hospital discharge were analysed with frequentist methods (figure 2). Cumulative survival was significantly better with early ECMO than with standard ACLS treatment (hazard ratio 0·16, 95% CI 0·06–0·41; log-rank test p<0·0001; figure 2A). Many patients who survived in the early ECMO group could not walk at the time of hospital discharge due to prolonged hospitalisation and physical deconditioning, reducing their functional assessment scores. Functional scores improved with time, physical rehabilitation, and reconditioning. All survivors had good functional assessment scores at 6 months (figure 2). The one patient in the standard ACLS group who survived to hospital discharge had a modified Rankin score of 5 and cerebral performance category of 4 at hospital discharge and died before the 3-month evaluation (figure 2). Because of the absence of survivors at 3 and 6 months in the standard ACLS group, statistical comparisons for neurological status between groups was not possible. Survival at 3 and 6 months was also improved in the early ECMO group (six of 14 patients at 3 months and 6 months) compared with that in the standard ACLS group (none of 15 at 3 and 6 months; p=0·0063; table 2).
Figure 2: Cumulative survival after randomisation (A) and functional scores in all survivors at hospital discharge and at 3 months and 6 months after discharge (B, C).
(A) Kaplan-Meier plot showing cumulative survival of patients from the index cardiac arrest to 6 months after discharge. Blinded modified Rankin scale (B) and cerebral performance category scale (C) scores in survivors at hospital discharge and 3 months and 6 months after hospital discharge. Neurological function was mainly preserved, and functional status scores were significantly improved after physical therapy and rehabilitation. Grey shading denotes the favourable range of neurological survival scores. ECMO=extracorporeal membrane oxygenation. ACLS=advanced cardiac life support.
As expected, serious multiorgan injury was frequent in the very critically ill population undergoing early ECMO, including cardiopulmonary resuscitation trauma, aspiration pneumonia, bleeding, cardiogenic shock, liver injury, and renal failure. No unanticipated serious adverse events related to the device were observed (table 3). We observed a single cracked tubing connector for the distal perfusion catheter that required replacement.
Table 3:
Adverse events in the intention-to-treat population
| ECMO-facilitated resuscitation (n=15) |
Standard ACLS treatment (n=15) |
|||
|---|---|---|---|---|
| Number of patients with data | Patients | Number of patients with data | Patients | |
| Number of adverse events | ||||
| Total number of adverse events | 15 | 166 | 15 | 47 |
| Patients with more than one event | 15 | 15 (100%) | 15 | 2 (13%) |
| Unsuccessful resuscitation from refractory cardiac arrest | ||||
| Death before admission | 15 | 2 (13%) | 15 | 13 (87%) |
| Circulatory events | ||||
| Cardiogenic shock | 13 | 12 (92%) | 2 | 2 (100%) |
| Inotropes or vasopressors in ICU | 13 | 11 (85%) | 2 | 2 (100%) |
| CNS events | ||||
| Cerebral oedema | 13 | 3 (23%) | 2 | 1 (50%) |
| CNS diffuse ischaemia | 13 | 6 (46%) | 2 | 1 (50%) |
| Seizure activity | 13 | 0 | 2 | 0 |
| Cardiopulmonary resuscitation trauma | ||||
| Retrosternal or intrathoracic bleeding | 13 | 4 (31%) | 2 | 1 (50%) |
| Rib fractures | 13 | 11 (85%) | 2 | 2 (100%) |
| Gastrointestinal events | ||||
| Acute liver failure or injury | 13 | 9 (69%) | 2 | 2 (100%) |
| Renal events | ||||
| Acute kidney injury requiring continuous renal replacement therapy or dialysis | 13 | 10 (77%) | 2 | 1 (50%) |
| Respiratory events | ||||
| Aspiration pneumonitis or pneumonia | 13 | 12 (92%) | 2 | 2 (100%) |
| Pulmonary oedema | 13 | 5 (38%) | 2 | 2 (100%) |
| Unanticipated device-related adverse events | ||||
| None | 13 | NA | 2 | NA |
| Procedure-related events | ||||
| Cracked tubing connector replaced | 13 | 1 (8%) | 2 | NA |
| Access-site bleeding requiring transfusion of >3 units of PRBC | 13 | 2 (15%) | 2 | NA |
| IVC trauma, retroperitoneal bleeding | 13 | 1 (8%) | 2 | NA |
Data are n (%), unless otherwise specified. ACLS=advanced cardiac life support. ECMO=extracorporeal membrane oxygenation. ICU=intensive care unit. NA=not applicable. PRBC=packed red blood cells. IVC=inferior vena cava.
In the standard ACLS group, 13 patients died due to unsuccessful resuscitation and inability to achieve ROSC despite prolonged resuscitation efforts in the emergency department, and never entered the catheterisation laboratory. Two patients achieved ROSC and were admitted to the hospital after catheterisation laboratory evaluation and treatment. One died from acute cerebral oedema and the other from severe anoxic brain injury after discharge. In the early ECMO group, two patients met resuscitation discontinuation criteria (both from meeting PaO2 and lactic acid criteria) and were declared dead before ECMO, and six patients died a median of 3·5 days [range 1–22] from admission due to severe anoxic brain injury and cerebral oedema. No other causes of death were identified.
Discussion
To our knowledge, the ARREST trial is the first to show that ECMO-facilitated resuscitation can improve survival compared with standard ACLS treatment in patients presenting with OHCA and refractory ventricular fibrillation or tachycardia. ECMO-facilitated resuscitation achieves three main goals when deployed in patients with refractory cardiac arrest: it normalises perfusion reliably, provides cardiopulmonary support to facilitate identification and treatment of the most common cause of refractory arrest (severe coronary artery disease with chronic and acute coronary occlusion)2,6,7 with consistent access to the catheterisation laboratory for angiography and angioplasty when needed, and becomes the bridge to recovery in ICU when the multiorgan injury sustained during long resuscitation can otherwise lead to accelerated deterioration and death. Therefore, it is important to note that early implementation of ECMO is the enabling and necessary condition that allows additional advanced targeted therapies to be delivered in these critical patients. In its absence, what follows is just not possible.
The ARREST trial confirmed that standard ACLS resuscitation alone for this patient population had a dismal outcome.3–5 Emergency teams exhausted every possibility for successful resuscitation before declaring death. The ARREST trial was stopped early because of the significant survival benefit observed with early ECMO. Contextual consistency of the survival rates observed in the ARREST trial with similar survival rates of extensively published cohorts of both ECMO-facilitated resuscitation and standard ACLS provided additional reinforcement of the validity of this trial’s results.3,4,6,8,12,13 Given that the average cardiopulmonary resuscitation duration for patients in the ARREST trial was close to 60 min, and survival rates with standard ACLS treatment lower than 5% reported in multiple international cohorts, DSMB members and the NHLBI deemed it unethical to continue to expose patients to that treatment in the presence of a mature ECMO-facilitated resuscitation programme.3,12,13
The ARREST trial outcomes reflect the importance of a highly orchestrated collaboration and coordinated implementation of the chain of survival throughout a community.14,15 ECMO-facilitated resuscitation is only the catalyst for the observed improvement in outcomes. Without the broader medical community coalition to coordinate each step of care and facilitate transfer to a high-volume ECMO resuscitation centre, these results would not have been possible.16
The ARREST trial used the cardiac catheterisation laboratory to start ECMO. We used this approach because interventional cardiologists are skilled subspecialists with extensive expertise in obtaining large bore percutaneous vascular access. Additionally, the immediate availability of fluoroscopy and ultrasound vascular access guidance in the catheterisation laboratory provides an additional level of safety and helps to keep vascular access complications to a minimum.17 This can serve as a model, but it is not the only potential successful approach. Vascular and bleeding adverse events were low and consistent with our previous published work in larger reported case series.9
The use of veno-arterial ECMO machines (Cardiohelp, Getinge, Sweden) did not result in any unexpected device-related adverse events and did as intended for the duration of the support. The extended use of ECMO machines beyond 6 h seems to be not only safe but also life-saving in this population.
Patients in the early ECMO group who arrived in the catheterisation laboratory with severe metabolic derangement and hypoxemia were not started on ECMO support. The criteria that we used in the ARREST trial have been consistent over the past 5 years, and patients presenting with two or more of those criteria have an extremely poor prognosis.3,8 Therefore, further resuscitation is considered futile. Patients who died early during ECMO support all had severe neurological injury and brain oedema and, eventually, care was withdrawn. We have previously shown that the time from 911 call to initiation of ECMO is the most important independent predictor of survival in this population.3 Strategies to further reduce average time to ECMO cannulation are warranted.
Survivors in the early ECMO group had a very long and complicated hospital stay, but they predictably and routinely overcame multiorgan injury with sustained ICU interventions and support.6 At the time of discharge, survivors mainly had extreme deconditioning and muscle weakness from the prolonged ICU and hospital stay. Neurological function was mainly preserved, as shown by the consistent recovery and improvement observed at the 3-months and 6-months follow-up visits, after physical therapy and rehabilitation was undertaken, which is consistent with our previous reports.18
The ARREST trial has some limitations. The outcomes observed at the University of Minnesota reflect local emergency health-care delivery characteristics and a highly experienced interventional critical care cardiology team providing continuity of care for all patients. Such expertise and resources might or might not be available in other places. The generalisation of this approach to the entire Minneapolis–St Paul community was published simultaneously with the ARREST trial and showed similar survival rates.19 The success of this programme, providing early ECMO-facilitated resuscitation to an entire metropolitan area, supports the contention that this approach is potentially generalisable to other locations and communities.
The need for a substantial systematic reorganisation of the emergency response infrastructure and centralisation of care, with highly trained and expert teams that respond within minutes to this time-sensitive emergency, cannot be understated. Organisational changes need to consider the geographical and health-care idiosyncrasies of the various metropolitan and rural areas. A cost analysis of this approach is very important and remains to be done.
In conclusion, early ECMO-facilitated resuscitation for patients with OHCA and refractory ventricular fibrillation significantly improved survival to hospital discharge and functional status compared with patients receiving standard ACLS resuscitation.
Supplementary Material
Research in context.
Evidence before this study
Survival from cardiac arrest has remained poor for decades. Refractory cardiac arrest is the most time-sensitive emergency and leads to death unless it can be reversed in a timely manner. Patients presenting with long resuscitation times, requiring cardiopulmonary resuscitation for longer than 30–40 min, essentially have no chance to survive with standard advanced cardiac life support (ACLS). This has been documented in multiple observational cohorts in the USA, Europe, and Japan. Over the past 5 years, several observational cohort studies have been published. Those studies assessed extracorporeal cardiopulmonary resuscitation, using peripheral extracorporeal membrane oxygenation (ECMO) devices, as a way to resuscitate and provide cardiopulmonary support in patients that did not have prompt return of spontaneous circulation. Most of those studies have shown promise and suggested an increase in survival for patients with refractory cardiac arrest. This was especially true for those patients presenting initially with a shockable rhythm. Other studies showed small or no effect on survival. No literature search was done because this subject has been extensively investigated and recently reported in a scientific statement from the American Heart Association about the role of the cardiac catheterisation laboratory in out-of-hospital cardiac arrest and in a statement by the Society of Cardiac Angiography and Interventions, in both of which DY had a contributing role.
Added value of this study
To our knowledge, the ARREST trial is the first randomised interventional trial to assess the effect of early ECMO-facilitated resuscitation compared with standard ACLS treatment for survival of patients with out-of-hospital refractory cardiac arrest. The results showed that, in a well organised and experienced system, survival for patients with refractory cardiac arrest can be significantly increased by the early implementation of ECMO. The results were materialised in a high-volume resuscitation centre that used interventional cardiologists as the lead resuscitators in the ECMO group, with technical expertise that is not widely available. The results also reflect a community based, systematic restructuring of the emergency medical service response for these patients that facilitated early transport and prompt activation and deployment of the ECMO team within 20 min of the prehospital 911 call.
Implications of all the available evidence
The ARREST trial, being a single centre trial, shows what it might be possible but does not definitively answer the question of whether this can be widely implemented. Reassuringly, the results of the ARREST trial accord with multiple cohorts. This suggests that the observed results might be replicated in other programmes. A definitive answer on this subject will require a multicentre phase 3 trial, but only after programmes have matured and restructured the systemic responses to these patients. A blue print of a community-wide programme expansion is provided in an accompanying paper published separately.
Acknowledgments
We would like to thank Sue Lowry for development and management of the ARREST trial database and nurses Gretchen Peichel, Barbara Bruhn-Ding, Deborah Wilder, and Julie Longman in the Lillehei Clinical Research Unit of the Division of Cardiology who contributed to the successful completion of this trial in obtaining consent, data collection, and analysis, as well as FDA related communications. We would also like to thank Brandy Harrington who facilitated all communications with the IRB.
Funding National Heart, Lung, and Blood Institute.
DY reports US National Institutes of Health (NIH) grants to study cardiopulmonary resuscitation (CPR) and cardiac arrest from NHLBI, and a grant from the Helmsley Charitable Trust for community implementation of a mobile ECMO programme in the St Paul Minneapolis metropolitan area. TPA reports NIH grants to study CPR and cardiac arrest from NHLBI.
Footnotes
Declaration of interests
All other authors declare no competing interests.
Data sharing
Data collected for the study, including individual participant data and a data dictionary defining each field in the set, will be made available 18 months after publication. We will provide deidentified participant data, data dictionary, study protocol, statistical analysis plan, and informed consent form. To gain access to datasets please send an email to ctsi@umn.edu or yanno001@umn.edu. Data will be shared only after approval of the proposal by the principal investigators of the ARREST trial and will be provided without investigator support and after a signed data agreement has been in place.
References
- 1.Link MS, Berkow LC, Kudenchuk PJ, et al. Part 7: adult advanced cardiovascular life support: 2015 American Heart Association Guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 2015; 132 (suppl 2): S444–64. [DOI] [PubMed] [Google Scholar]
- 2.Yannopoulos D, Bartos JA, Aufderheide TP, et al. The evolving role of the cardiac catheterization laboratory in the management of patients with out-of-hospital cardiac arrest: a scientific statement from the American Heart Association. Circulation 2019; 139: e530–52. [DOI] [PubMed] [Google Scholar]
- 3.Bartos JA, Grunau B, Carlson C, et al. Improved survival with extracorporeal cardiopulmonary resuscitation despite progressive metabolic derangement associated with prolonged resuscitation. Circulation 2020; 141: 877–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grunau B, Reynolds J, Scheuermeyer F, et al. Relationship between time-to-ROSC and survival in out-of-hospital cardiac arrest ECPR candidates: when is the best time to consider transport to hospital? Prehosp Emerg Care 2016; 20: 615–22. [DOI] [PubMed] [Google Scholar]
- 5.Grunau B, Reynolds JC, Scheuermeyer FX, et al. Comparing the prognosis of those with initial shockable and non-shockable rhythms with increasing durations of CPR: informing minimum durations of resuscitation. Resuscitation 2016; 101: 50–56. [DOI] [PubMed] [Google Scholar]
- 6.Yannopoulos D, Bartos JA, Raveendran G, et al. Coronary artery disease in patients with out-of-hospital refractory ventricular fibrillation cardiac arrest. J Am Coll Cardiol 2017; 70: 1109–17. [DOI] [PubMed] [Google Scholar]
- 7.Lamhaut L, Tea V, Raphalen JH, et al. Coronary lesions in refractory out of hospital cardiac arrest (OHCA) treated by extra corporeal pulmonary resuscitation (ECPR). Resuscitation 2017; 126: 154–59. [DOI] [PubMed] [Google Scholar]
- 8.Yannopoulos D, Bartos JA, Martin C, et al. Minnesota Resuscitation Consortium’s advanced perfusion and reperfusion cardiac life support strategy for out-of-hospital refractory ventricular fibrillation. J Am Heart Assoc 2016; 5: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bartos JA, Carlson K, Carlson C, et al. Surviving refractory out-of-hospital ventricular fibrillation cardiac arrest: critical care and extracorporeal membrane oxygenation management. Resuscitation 2018; 132: 47–55. [DOI] [PubMed] [Google Scholar]
- 10.Yannopoulos D, Kalra R, Kosmopoulos M, et al. Rationale and methods of the Advanced R2Eperfusion STrategies for refractory cardiac arrest (ARREST) trial. Am Heart J 2020; 229: 29–39. [DOI] [PubMed] [Google Scholar]
- 11.Geocadin RG, Callaway CW, Fink EL, et al. Standards for studies of neurological prognostication in comatose survivors of cardiac arrest: a scientific statement from the American Heart Association. Circulation 2019; 140: e517–42. [DOI] [PubMed] [Google Scholar]
- 12.Nagao K, Nonogi H, Yonemoto N, et al. Duration of prehospital resuscitation efforts after out-of-hospital cardiac arrest. Circulation 2016; 133: 1386–96. [DOI] [PubMed] [Google Scholar]
- 13.Grunau B, Reynolds JC, Scheuermeyer FX, et al. Comparing the prognosis of those with initial shockable and non-shockable rhythms with increasing durations of CPR: informing minimum durations of resuscitation. Resuscitation 2016; 101: 50–56. [DOI] [PubMed] [Google Scholar]
- 14.Panchal AR, Berg KM, Cabañas JG, et al. 2019 American Heart Association focused update on systems of care: dispatcher-assisted cardiopulmonary resuscitation and cardiac arrest centers: an update to the American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 2019; 140: e895–903. [DOI] [PubMed] [Google Scholar]
- 15.Panchal AR, Berg KM, Hirsch KG, et al. 2019 American Heart Association focused update on advanced cardiovascular life support: use of advanced airways, vasopressors, and extracorporeal cardiopulmonary resuscitation during cardiac arrest: an update to the American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 2019; 140: e881–94. [DOI] [PubMed] [Google Scholar]
- 16.Bougouin W, Dumas F, Lamhaut L, et al. Extracorporeal cardiopulmonary resuscitation in out-of-hospital cardiac arrest: a registry study. Eur Heart J 2020; 41: 1961–71. [DOI] [PubMed] [Google Scholar]
- 17.Danial P, Hajage D, Nguyen LS, et al. Percutaneous versus surgical femoro-femoral veno-arterial ECMO: a propensity score matched study. Intensive Care Med 2018; 44: 2153–61. [DOI] [PubMed] [Google Scholar]
- 18.Sideris G, Voicu S, Yannopoulos D, et al. Favourable 5-year postdischarge survival of comatose patients resuscitated from out-of-hospital cardiac arrest, managed with immediate coronary angiogram on admission. Eur Heart J Acute Cardiovasc Care 2014; 3: 183–91. [DOI] [PubMed] [Google Scholar]
- 19.Bartos JA, Frascone RJ, Conterato M, et al. The Minnesota Mobile Extracorporeal Cardiopulmonary Resuscitation Consortium for Treatment of Out-of-Hospital Refractory Ventricular Fibrillation: Program Description, Performance, and Outcomes. EClinicalMedicine 2020; published online November 13. 10.1016/j.eclinm.2020.100632. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.