Abstract
At least one third of HIV-1-afflicted individuals experience peripheral neuropathy. Although the underlying mechanisms are not known, they may involve neurotoxic HIV-1 proteins. We assessed the influence of the neurotoxic HIV-1 regulatory protein, Tat, on inflammatory and neuropathic nociceptive behaviors using transgenic male and female transgenic mice that conditionally expressed (or did not express) HIV-1 Tat1–86 in glial fibrillary acidic protein-expressing glia in the central and peripheral nervous systems. Tat induction significantly attenuated the time spent paw-licking following formalin injection (2.5%, i.pl.) in both male and female mice. However, significant sex differences were observed in the onset and magnitude of inflammation and sensory sensitivity following complete Freund’s adjuvant (CFA) injection (10%, i.pl.) after Tat activation. Unlike female mice, males showed a significant attenuation of paw swelling and an absence of mechanical/thermal hypersensitivity in response to CFA after Tat induction. Male Tat(+) mice also showed accelerated recovery from chronic constrictive nerve injury (CCI)-induced neuropathic mechanical and thermal hypersensitivity compared to female Tat(+) mice. Morphine (3.2 mg/kg) fully reversed CCI-induced mechanical hypersensitivity in female Tat(−) mice, but not in Tat(+) females. The ability of Tat to decrease edema, paw swelling, and limit allodynia suggest a sequela of events in which Tat-induced functional deficits precede the onset of mechanical hypersensitivity. Moreover, HIV-1 Tat attenuated responses to inflammatory and neuropathic insults in a sex-dependent manner. HIV-1 Tat appears to directly contribute to HIV sensory neuropathy and reveals sex differences in HIV responsiveness and/or the underlying peripheral neuroinflammatory and nociceptive mechanisms.
Keywords: Inflammatory Pain, NeuroHIV, Neuropathic Pain, Sex Differences, Trans-activator of Transcription
1. Introduction
Apart from regulatory actions to influence viremia and/or viral latency (Rice and Kimata, 2015), the HIV-1 trans-activator of transcription (Tat) may contribute to a number of co-morbidities experienced by HIV-infected individuals, including intractable pain. Tat can activate neuronal NMDA receptors (e.g., Shin and Thayer, 2013; Neri et al., 2007), disrupt the cell membrane directly (Chopard et al., 2018), dysregulate mitochondrial function (Ferri et al., 2000; Norman et al., 2007) and ATP production (Sorrell and Hauser, 2014), and dysregulate ion homeostasis (Fitting et al., 2014). These effects promote synaptodendritic injury and neuronal death (Kim et al., 2008). As such, it is perhaps not surprising that Tat can induce hyperexcitability and apoptosis of rat dorsal root ganglion (DRG) neurons (Chi et al., 2011). Notably, clade B HIV is associated with greater neuropathic pain than clade C HIV (Gandhi et al., 2009). Accordingly, we investigated the capacity of clade B Tat1–86 to modulate mechanical sensitivity and paw swelling using several, conventional inflammatory and neuropathic pain paradigms in mice.
HIV does not infect neurons but does infect microglia and to a lesser extent astroglia (important sources of inflammatory signaling; Brack-Werner, 1999; Churchill and Nath 2013; Churchill et al., 2009; Gorry et al., 2003; Kramer-Hammerle et al., 2005; Li et al., 2020; Tornatore et al., 1991). Accordingly, a frequent strategy to model neuroHIV is to express viral proteins in glia. Since microglial promoters are also found to be expressed by monocyte-derived macrophages (Bottcher et al., 2019; Goldmann et al., 2016; Kierdorf et al., 2019), previous attempts to restrict HIV protein expression to the nervous system have relied on the use of astrocytic promoters such as the glial fibrillary acidic protein (GFAP) promoter, which can also be expressed by satellite and Schwann cells, and enteric glia in the peripheral nervous system. The transgenic mouse model used herein conditionally expresses HIV-1 Tat1–86 in a nervous system-targeted manner via a GFAP-driven Tet-on (rtTA) promoter. In these mice, Tat transcripts are detected in the central (brain and spinal cord; Fitting et al., 2012; Wodarski et al., 2018) and peripheral nervous systems [dorsal root ganglia (DRG), enteric glia, and hindpaw skin presumably via expression by Schwann cells in peripheral nerves; Ngwainmbi et al., 2014; Wodarski et al., 2018] of Tat+, but not Tat−, transgenic mice following 1–6 weeks of DOX induction. Tat expression is presumed to originate from GFAP-expressing astrocytes in the dorsal spinal cord and peripheral glia including non-myelinating Schwann cells in the skin (Wodarski et al., 2018), and believed to contribute to hypersensitivity in the Tat transgenic model (Wodarski et al., 2018).
In the present report, male and female HIV-1 Tat transgenic mice were administered formalin, complete Freund’s adjuvant (CFA), or underwent chronic constriction injury (CCI) to assess the effects of Tat on mechanical nociception, thermal hyperalgesia, inflammatory swelling, and behavioral responding (i.e. paw licking). We anticipated that inducing HIV-1 Tat, would potentiate nociceptive responding among mice subject to these manipulations. Moreover, the influence of sex differences on these effects was sought prompted by clinical evidence suggesting an influence of sex on analgesic or nociceptive responding to HIV- and/or cART-related neuropathic pain (Aragonés-López et al., 2012; Katz et al., 2015; Mehta et al., 2011) and given the paucity of HIV-related studies that are stratified by sex (Sordo del Castillo et al., 2010).
2. Materials and Methods
2.1. Animals
Adult male and female transgenic mice conditionally expressing HIV-1 Tat1–86 were used. The HIV-Tat1–86 transgenic mouse model was developed on a C3H × C57BL/6J hybrid background as previously described (Bruce-Keller et al., 2008). Tat expression, which is under the control of a tetracycline responsive (Tet-on) rtTA, GFAP-selective promoter, was induced with a specially formulated chow containing 6 mg/g DOX (Harlan, product #TD.09282), fed to both the Tat controls and the inducible Tat mice. Mice were fed with DOX-containing chow for the duration of each experiment.
Mice were housed in a 21 °C humidity-controlled Association for Assessment and Accreditation of Laboratory Animal Care–accredited animal care facility. They were housed in groups of three as recommended by Virginia Commonwealth University and had free access to food and water. The rooms were on a 12-hour light/dark cycle (lights on at 7:00 AM). Mice were 8–12 weeks of age and weighed ~25–30 g at the start of all experiments. All experiments were performed during the light cycle, and the study was approved by the Institutional Animal Care and Use Committee of Virginia Commonwealth University. All studies were carried out in accordance with the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals.
2.2. Chemicals
Morphine and complete Freund’s adjuvant (CFA) were purchased from Sigma-Aldrich (St. Louis, MO). Formalin was obtained from Fisher Scientific (Pittsburgh, PA). CFA (10%) was diluted in mineral oil. Formalin (2.5%) was prepared in distilled water for intraplantar (i.pl.) injection. Morphine was dissolved in physiologic saline (0.9% sodium chloride) and injected subcutaneous (s.c.) at a total volume of 1 ml/100 g body weight. The dose of morphine is expressed as the free base.
2.3. Behavioral assessments
All behavioral experiments were performed during the light cycle by female well-trained observers (DB, MC). The observers were unaware of the genotype of the animals during conducting the behavioral experiments and doing the analysis of the data. A lab member made the observers blind to the groups by providing ear tags and coded cage labels, and randomized the animals into the groups. The genotype of the groups was revealed after the data analysis. However, the observers were able to differentiate female and male mice when both sexes were used.
2.3.1. Formalin test
The effects of HIV-1 Tat protein in the formalin test were measured in Tat(−) and Tat(+) mice after 3 weeks from DOX-diet induction (n=11/group). The formalin test was carried out as previously described (Bagdas et al., 2015). Animals were put in an open Plexiglas® cage (29 × 19 × 13 cm each), with a mirror placed at a 45-degree angle behind the cage to allow an unobstructed view of the paws. After mice acclimated for 15 min in the test cage, each animal was injected with 20 μl of (2.5%) formalin i.pl. to the right hindpaw. Each mouse was then immediately placed in the test cage and the time spent paw licking was evaluated from 0 to 5 min (phase 1) and from 20 to 45 min (phase 2) post-formalin injection. The duration of time spent licking the ipsilateral paw was recorded with a digital stopwatch during phases 1 and 2. Mice display minimal nociceptive behavior during the period between phase 1 and 2 (5 to 20 min post-formalin); their responses were not recorded during this time. Paw edema and paw diameter (see measurement of paw edema) were also measured before and at 1 h after formalin injection.
2.3.2. Complete Freund’s adjuvant (CFA)-induced inflammatory pain model
The effects of HIV-1 tat protein in the CFA model were measured in Tat(−) and Tat(+) mice. For this experiment, we first determined the mechanical thresholds (see von Frey test), and thermal latencies (see Hargreaves test) of naïve Tat(−) and Tat(+) mice before start of DOX-diet. Mice (male mice = 7/group; female mice n=9/group) were then exposed to DOX-diet and feeding with DOX-diet continued throughout the experiment. The thresholds and latencies were also tested 3 days after DOX-diet induction and mice were injected i.pl. 20 μl of CFA (10%) (day 0; Fig. 2A). To determine the mechanical hypersensitivity and its duration induced by CFA in Tat(−) and Tat(+) mice, animals were tested with Von Frey on days 1, 3, 7, 14, 21, and 28 after CFA injection. To decrease the stress on animals and not to sensitize the paw, Hargreaves thermal sensitivity test was performed 1 day after the von Frey test. In addition, paw diameter (see measurement of paw edema) was measured before and at 3, 7, and 14 days after CFA injection.
Fig. 2. 10% CFA-induced thermal and mechanical hypersensitivity and Inflammation in male Tat(−) and Tat(+) Mice.
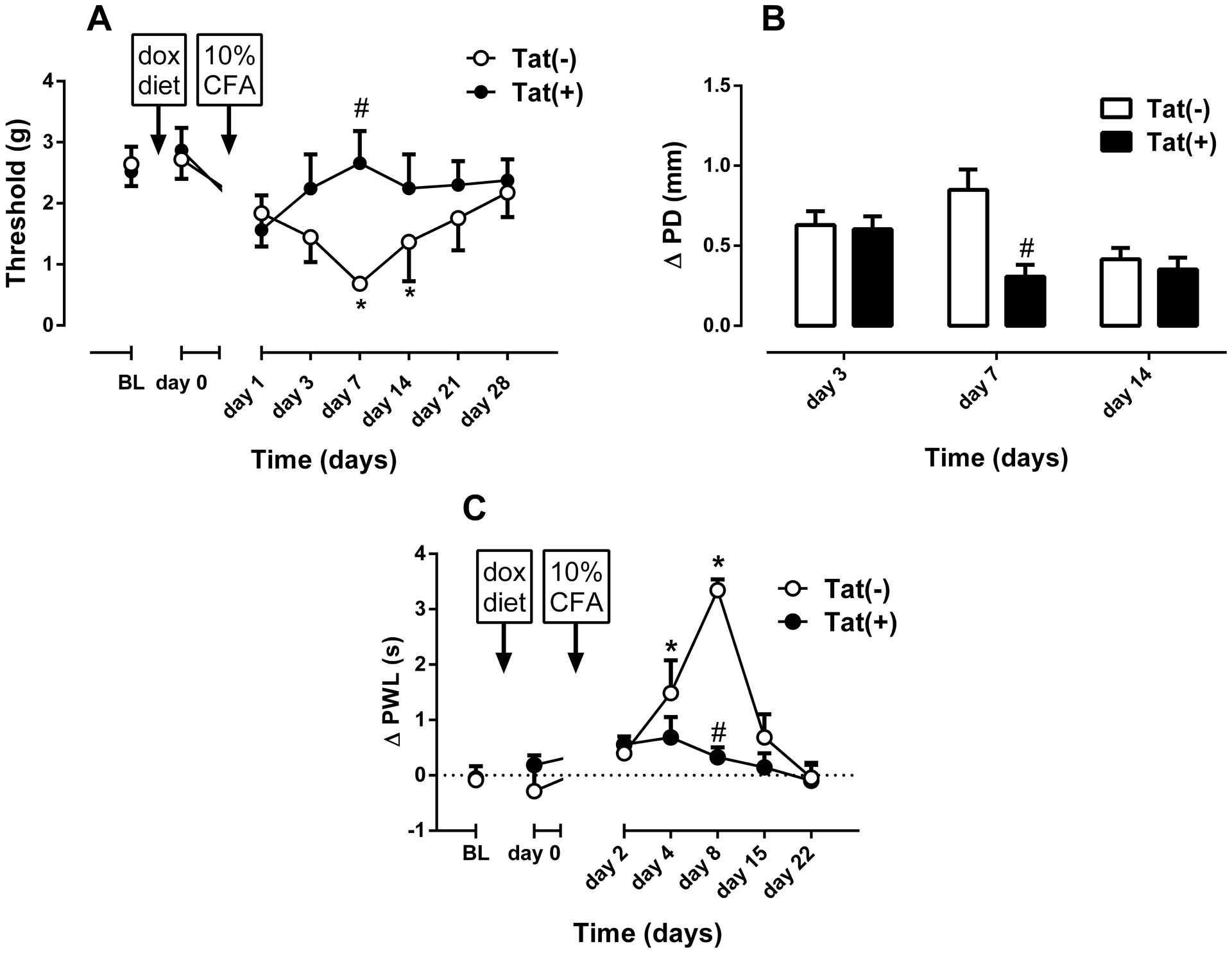
Mechanical paw withdrawal thresholds (A), degree of edema (ΔPD=difference in the ipsilateral paw diameter before and after injection of CFA) (B) and differences in paw withdrawal latencies (Δ PWL=contralateral–ipsilateral hindpaw latencies) (C) in Tat(−) and Tat(+) mice at different times after intraplantar injection of CFA (10% solution/20 μl, respectively). Data were expressed as the mean ± S.E.M. of 7 animals for each group. *p<0.05 significantly different from BL. #p<0.05 significantly different from Tat(−) group. BL: baseline
2.3.3. Chronic constrictive nerve injury (CCI)-induced neuropathic pain model
The effects of HIV-1 Tat protein in the CCI model were measured in Tat(−) and Tat(+) mice (male mice = 10/group; female mice n=9/group). We first determined baseline mechanical thresholds (see von Frey test) and thermal latencies (see Hargreaves test) of naïve Tat(−) and Tat(+) mice before starting the DOX diet. Mechanical thresholds and latencies were retested 3 days after the onset of DOX administration and then CCI surgery was performed on mice. Once DOX was started, mice were maintained on DOX-containing chow throughout the remainder of the experiment.
For CCI surgery, mice were anesthetized with pentobarbital (45 mg/kg, i.p.). An incision was made just below the hip bone, parallel to the sciatic nerve. The left common sciatic nerve was exposed at the level proximal to the sciatic trifurcation and a nerve segment 3–5 mm long was separated from the surrounding connective tissue. Two loose ligatures with 5–0 silk suture were made around the nerve with a 1.0–1.5 mm interval between each of them. Muscles were closed and the wound with suture. This procedure results in CCI of the ligated nerve (Bagdas et al., 2015). Mechanical thresholds were measured 1, 3, 7, 11, 14, and 21 days after surgery. Thermal paw withdrawal latencies were determined in the same mice 8, 15, and 22 days after surgery. In addition, on day 11 after CCI surgery, the effects of morphine (3.2 mg/kg, s.c.) on mechanical thresholds were tested in female Tat(−) and Tat(+) mice. The dose of morphine in the CCI model was chosen based on the previous report from our lab (Bagdas et al., 2018).
2.3.4. Hargreaves Test (Evaluation of Thermal sensitivity)
CFA administration and CCI surgery induce thermal hypersensitivity (Bagdas et al., 2015), which coincides with an increased sensitivity to painful stimuli (IASP, 1994). Therefore, we assessed the thermal withdrawal latencies in Tat(−) and Tat(+) mice following CFA administration or CCI surgery.
Thermal withdrawal latencies were measured via the Hargreaves test as described before (Bagdas et al., 2015). Mice were placed in clear plastic cylinders (9 × 11 cm) on an elevated surface and allowed to acclimatize to their environment before testing. The radiant heat source was directed to the plantar surface of each hind paw in the area immediately proximal to the toes. The paw withdrawal latency (PWL) was defined as the time from the onset of radiant heat to withdrawal of the mouse’s hind paw. The cut-off time was 20-s. An average of three measures of PWL were taken for each hind-paw. The results were expressed as withdrawal latency difference between paws [ΔPWL (s) = contralateral latency - ipsilateral latency].
2.3.5. Von Frey Test (Evaluation of Mechanical hypersensitivity)
Both CFA administration and CCI surgery induce mechanical hypersensitivity (Bagdas et al., 2015), which indicates a pain-like behavior resulting from a stimulus that does not normally provoke pain (IASP, 1994). Therefore, we assessed the mechanical withdrawal thresholds in Tat(−) and Tat(+) mice following CFA administration or CCI surgery.
Mechanical withdrawal thresholds were determined according to the method of Chaplan et al. (1994) with slight modifications (Bagdas et al., 2015). Mice were placed in a clear plastic cylinders (9 × 11 cm) with mesh metal flooring and allowed to acclimate for 20 min before testing. A series of calibrated von Frey filaments (Stoelting, Wood Dale, IL) with logarithmically incremental stiffness ranging numbers from 2.83 to 4.56 were applied to the paw using a modified up-down method (Dixon, 1965). Test was started by using 3.84 numbered filament for each mouse and continued up or down according to response. In the absence of a paw withdrawal response to the initially selected filament, a thicker filament corresponding to a stronger stimulus was presented. In the event of paw withdrawal, the next weaker stimulus was chosen. Each hair was presented perpendicularly against the paw, with enough force to cause slight bending, and held 2–3 s. The stimulation of the same intensity was applied three times to the hind paw at intervals of a few sec. Two responses out of three stimulations were coded as a positive response. Once a positive paw withdrawal response was detected, sequentially a weaker filament was used to assess the sensory threshold for each paw. The mechanical threshold was measured by the force applied in filament number and expressed in gram based on the filament number-force (g) conversion table provided by the manufacturer (see supplementary material), representing the force of the von Frey hair to which the mouse reacted (paw withdrawn). All behavioral testing was performed in a blinded manner.
2.3.6. Measurement of paw edema
The thickness of the formalin- or CFA-treated paws was measured both before and after injections at the times indicated above using a digital caliper (Traceable Calipers, Friendswood, TX). Data were recorded to the nearest ± 0.01 mm and expressed as change in paw thickness (ΔPD = the difference in the ipsilateral paw diameter before and after formalin or CFA injection).
2.4. Statistical Analysis
No data from animal studies was excluded. There was no prior statistical plan; after the study was performed, we planned a statistical plan and used this for the conducted experiments. Data were analyzed using GraphPad software, version 6.0 (GraphPad Software, Inc., La Jolla, CA) and were expressed as the mean ± S.E.M. A two-way analysis of variance (ANOVA), followed by Bonferroni’s post hoc testing, were used to determine group differences in the duration of paw licking following the formalin injection. An unpaired student’s t-test was used for simple two-group comparisons of formalin-induced paw edema. A repeated measures (RM), two-way ANOVA followed by the Bonferroni’s post hoc test were used in order to evaluate the effects of CFA administration and CCI surgery on mechanical thresholds, thermal latencies, and paw edema at different times. In addition, the effects of morphine in female mice after CCI surgery were tested by RM two-way ANOVA and Bonferroni’s post hoc test. Before ANOVA was performed, the data were first assessed for the normality of the residuals using the Shapiro-Wilk test and equal variance using the F test. The homogeneity of variance was evaluated by the Brown-Forsythe test. p values < 0.05 were considered significant.
3. Results
3.1. Paw licking and paw edema in Tat(−) and Tat(+) mice in the formalin test
The effect of HIV-1 Tat protein in the formalin test (2.5% concentration) was assessed in male (n = 11/group) and female (n = 8/group) Tat(−) and Tat(+) mice after 3 weeks of DOX-diet induction. Among male mice, two-way ANOVA revealed significant main effects of testing phase [Fphases(1,40) = 29.0; p < 0.001] and genotype [Fgenotype(1,40) = 13.34; p < 0.001] (Fig. 1A). Paw licking was significantly greater in phase II of the formalin test than in phase I; however, this responding was significantly attenuated among Tat(+) males compared to Tat(−) controls (Fig. 1A). Among female Tat(−) and Tat(+) mice, similar main effects were observed for paw licking to be greater in phase II of the formalin test [Fphases(1,28) = 6.62; p < 0.05], but attenuated among Tat(+) females compared to their Tat(−) counterparts [Fgenotype(1,28) = 12.56; p < 0.01] (Fig. 1C). Additionally, paw edema was measured in both groups 1 h after i.pl. injection of formalin; however, no significant differences were observed among male (Fig. 1B) or female (Fig. 1D) mice.
Fig. 1. Pain Behavior and Paw Edema in Tat(−) and Tat(+) Mice in The Formalin Test.
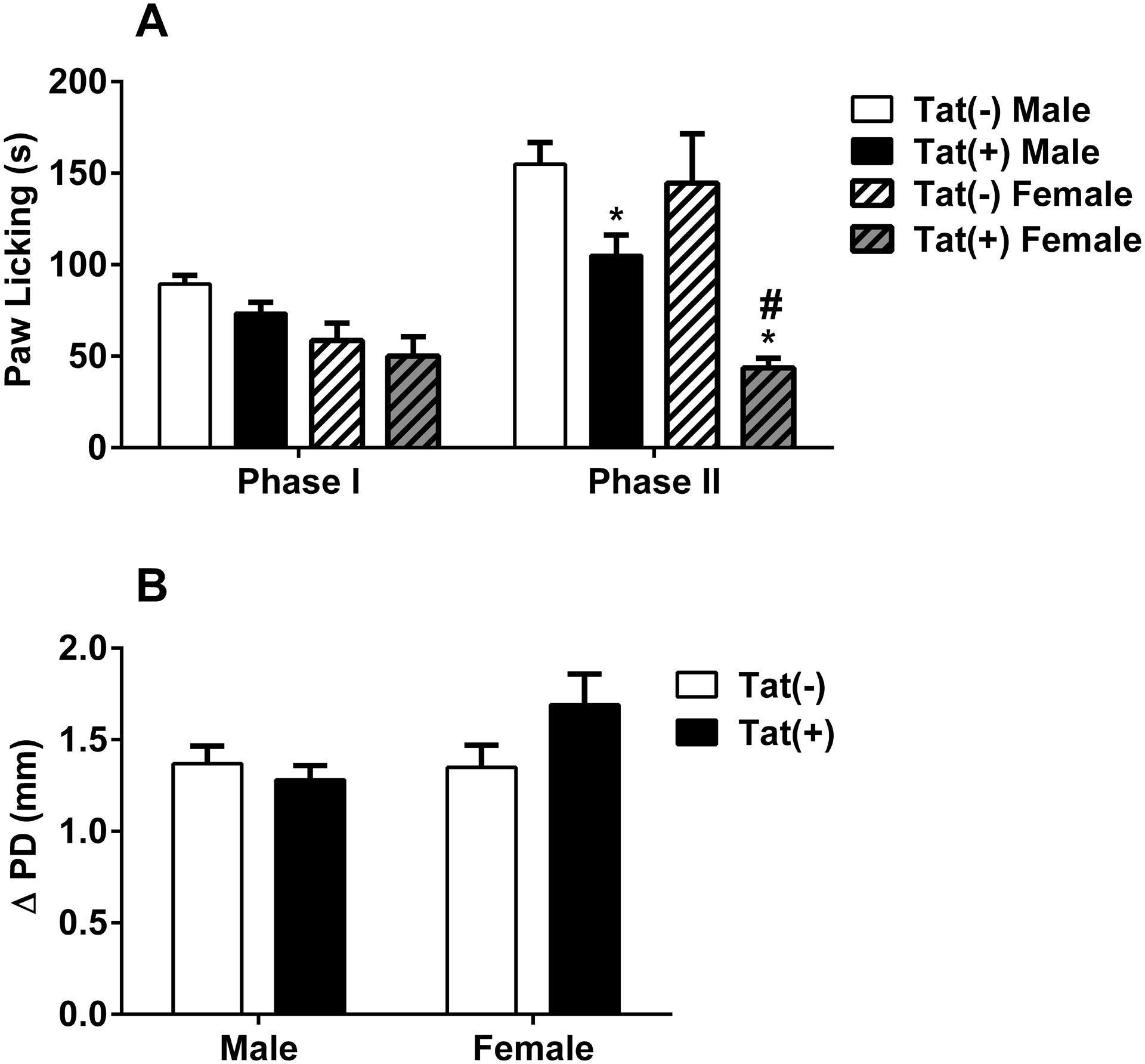
The paw licking response after intraplantar injection of (A) 2.5% formalin concentration into the right paw of both Tat(−) and Tat(+) mice. Changes in paw edema (B), as measured by the difference in the ipsilateral paw diameter before and after injection (ΔPD), in Tat(−) and Tat(+) mice 1 hour after intraplantar injection of formalin. Data were given as the mean ± S.E.M. of 11 animals for each group. *p<0.05 significantly different from Tat(−) group.
To test whether there was a difference between sexes, we compared male and female results of formalin tests. We found a significant interaction between genotype and sex [Fgenotype × sex(3,68) = 13.79; p < 0.001, Suppl. Fig. 1] and a significant main effect of phase [Fphase(1,68) = 27.53; p < 0.001, Suppl. Fig. 1]. The reduction of time spent in paw licking in Tat(+) mice was greater in female animals in phase II, compared to males within the same phase (p < 0.05, Suppl. Fig. 1).
3.2. Mechanical and thermal sensitivity of Tat(−) and Tat(+) mice in response to CFA-induced Inflammation
Mice were given an i.pl. injection of CFA (10% concentration) and assessed for mechanical threshold and edema 1, 3, 7, 14, 21, and 28 days later. In addition, thermal sensitivity was measured one day after mechanical assessments. Prior to CFA, neither Tat(−) nor Tat(+) mice differed in mechanical (Fig. 2A & 3A) or thermal (Fig. 2C & 3C) baselines before DOX-diet induction.
Fig. 3. 10% CFA-induced thermal and mechanical hypersensitivity and Inflammation in female Tat(−) and Tat(+) Mice.
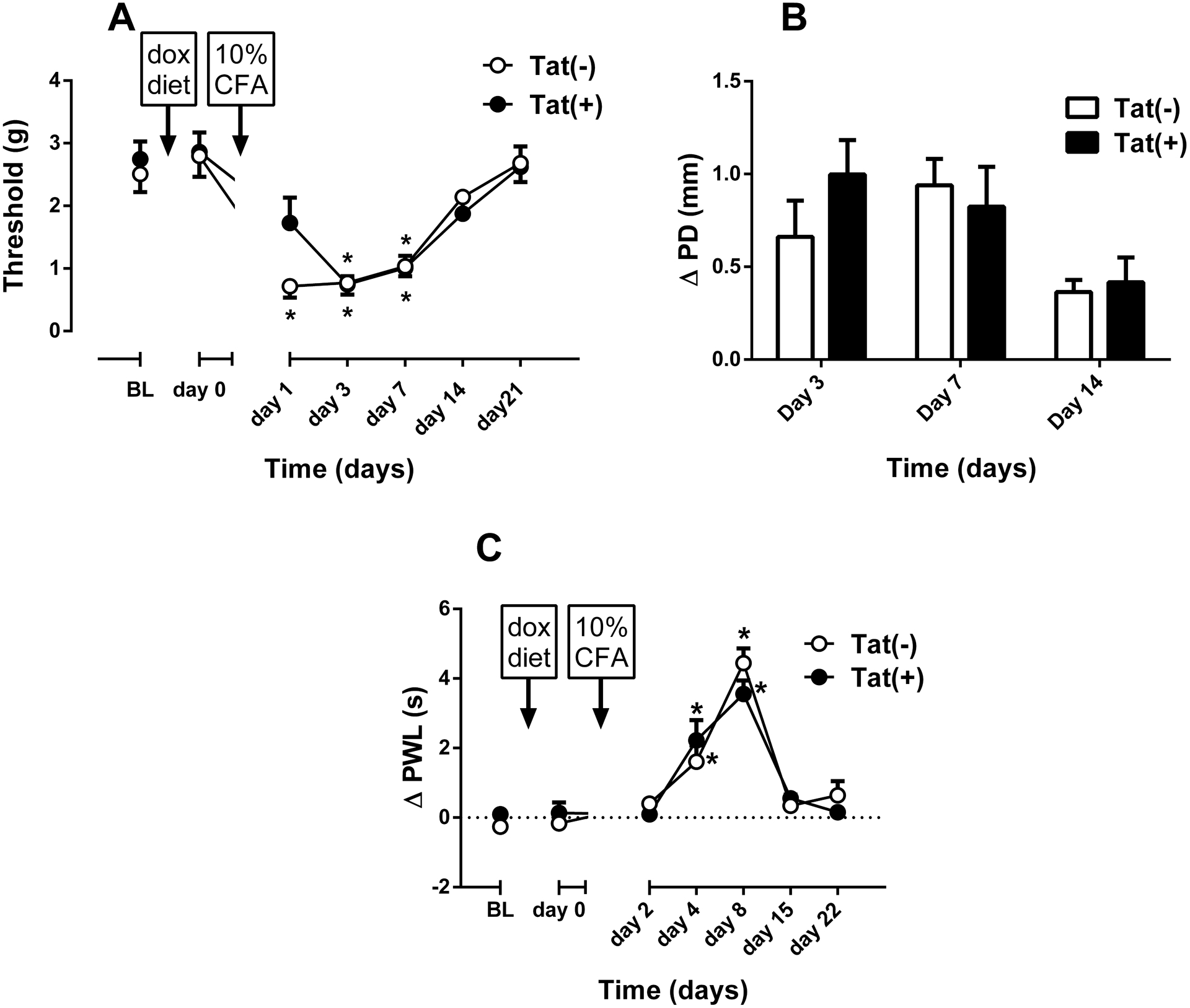
Mechanical paw withdrawal thresholds (A) and degree of edema (ΔPD=difference in the ipsilateral paw diameter before and after injection of CFA) in Tat(−) and Tat(+) mice at different times after intraplantar injection of CFA (10% solution/20 μl, respectively). Data were expressed as the mean ± S.E.M. of 9 animals for each group. *p<0.05 significantly different from BL. #p<0.05 significantly different from Tat(−) group. BL: baseline
Among males, there was a significant difference between Tat(−) (n = 7) and Tat(+) (n=7) mice in the development of CFA-induced inflammation as observed by changes in mechanical thresholds [Fgenotype(1,6) = 0.71, p = 0.429; Ftime(7,42) = 4.07, p < 0.01; Finteraction(7,42) = 2.36, p < 0.05; Fig. 2A] and paw edema [Fgenotype(1,6) = 17.26, p < 0.01; Ftime(2,12) = 4.34, p < 0.05; Finteraction(2,12) = 10.67, p < 0.01; Fig. 2B]. Bonferroni’s post hoc analysis revealed significant mechanical hypersensitivity among Tat(−) mice that was induced by CFA on days 7 and 14 (p < 0.05, Fig. 2A). However, Tat(+) mice did not show a significant change on mechanical thresholds at any time examined following day 0. Additionally, there was a significant attenuation of edema on day 7 in Tat(+) CFA-treated mice (p < 0.001) when compared to Tat(−) mice (Fig. 2B). In addition to the development of mechanical hypersensitivity, there was a marked difference in the development of thermal hypersensitivity between the groups [Fgenotype(1,6) = 33.65, p < 0.01; Ftime(6,36) = 9.894, p < 0.001; Finteraction(6,36) = 9.96, p < 0.001; Fig. 2C]. CFA-induced thermal hypersensitivity in Tat(−) mice (n = 7), which started 4 days post-treatment (p < 0.05) and lasted until day 15 after CFA injection (Fig. 2C). Conversely, there were no significant differences in the latency to respond to a thermal stimulus in Tat(+) mice following day 0, indicating an absence of thermal hypersensitivity (Fig. 2C). The attenuated response among Tat(+) mice significantly differed from that of Tat(−) controls on days 8 (p < 0.05, Fig. 2C).
Among females assessed for mechanical and thermal nociceptive responding, Tat(−) (n = 9) and Tat(+) (n = 9) mice did not initially differ on mechanical thresholds, nor did DOX-diet alter baseline values as observed on day 0 (Fig. 3A). I.pl. injection of CFA-induced mechanical hypersensitivity in both Tat(−) and Tat(+) mice without producing a significant difference on the development of hypersensitivity between the groups [Fgenotype(1,8) = 0.6116, p = 0.457; Ftime(6,48) = 27.7, p < 0.001; Finteraction(6,48) = 1.471, p=0.2083; Fig. 3A]. Bonferroni’s analysis revealed that while the hypersensitivity response against to CFA started 1 day after the CFA injection in Tat(−) mice, Tat(+) mice started to show an increased sensitivity on day 3 post-CFA (p<0.05; Fig. 3A). Hence, there was a reduction of mechanical hypersensitivity only on day 1 in Tat(+) mice. There was no significant difference in the paw edema ratio between the groups [Fgenotype(1,8) = 0.294, p = 0.602; Ftime(2,16) = 8.601, p < 0.01; Finteraction(2,16) = 2.506, p = 0.113; Fig. 3B]. Moreover, CFA-induced hypersensitivity was similar between Tat(−) and Tat(+) mice as seen in Fig. 3C [Fgenotype(1,8) = 0.028, p = 0.875; Ftime(6,48) = 57.13, p < 0.001; Finteraction(6,48) = 1.247, p = 0.299; Fig. 3C]. Significant thermal hypersensitivity was observed at day 4 and 8 in both genotypes (p < 0.05, Fig. 3C).
3.3. Mechanical and thermal sensitivity of Tat(−) and Tat(+) Mice in the CCI-induced Neuropathic Pain Model
The development of neuropathic pain was assessed in male mice that underwent CCI. Prior to any surgical procedure, Tat(−) (n = 10) and Tat(+) (n = 10) mice did not differ in mechanical and thermal baselines (Fig. 4A and B). Tat(+) mice displayed a significant attenuation of mechanical [Fgenotype(1,9) = 4.917, p = 0.0538; Ftime(6,54) = 28.43, p < 0.001; Finteraction(6,54) = 3.779, p < 0.01; Fig. 4A] and thermal [Fgenotype(1,9) = 5.032, p = 0.0516; Ftime(3,27) = 13.7, p < 0.001; Finteraction(3,27) = 7.064, p < 0.01; Fig. 4B] hypersensitivity compared with Tat(−) mice. CCI produced significant mechanical hypersensitivity in Tat(−) mice at all times examined. Surprisingly, Tat(+) mice demonstrated less mechanical hypersensitivity as evidenced by a significant elevation of mechanical thresholds observed on days 11 and 14, compared to Tat(−) mice (p < 0.05, Fig.4A). Moreover, mechanical hypersensitivity was absent by day 14 in Tat(+) mice. Similarly, a significant decrease in thermal hypersensitivity was observed at 1 and 2-weeks post-surgery (p < 0.05, Fig. 4B). Thermal hypersensitivity did not develop in Tat(+) mice.
Fig. 4. CCI-induced thermal and mechanical hypersensitivity in male Tat(−) and Tat(+) Mice.
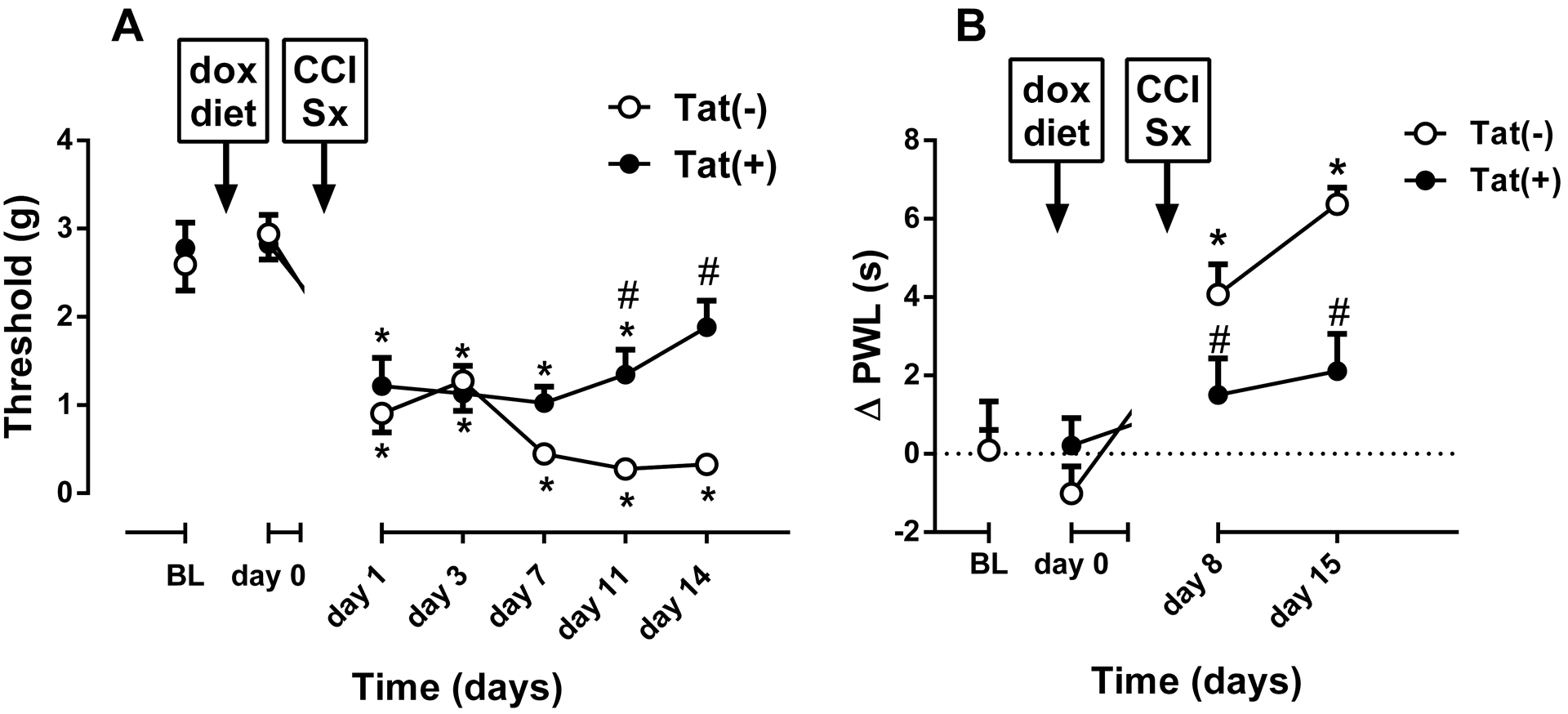
Mechanical paw withdrawal thresholds (A), and differences in paw withdrawal latencies (Δ PWL=contralateral–ipsilateral hindpaw latencies) (B) in Tat(−) and Tat(+) mice at different times after chronic constrictive nerve injury (CCI) operation. Data were expressed as the mean ± S.E.M. of 10 animals for each group. *p<0.05 significantly different from BL. #p<0.05 significantly different from Tat(−) group. BL: baseline
Among female Tat(−) (n = 9) and Tat(+) (n = 9) mice, CCI surgery induced mechanical hypersensitivity in both genotypes. On the other hand, there was no significant difference on development of hypersensitivity between the groups [Fgenotype(1,8) = 1.233, p = 0.299; Ftime(7,56) = 132.7, p < 0.001; Finteraction(7,56) = 1.122, p = 0.363; Fig. 5A]. There was only a slight but significant reduction in mechanical hypersensitivity in Tat(+) 1 day after CCI (p<0.05; Fig. 5A). Additionally, CCI surgery induced thermal hypersensitivity in both genotypes and the duration of hypersensitivity was significantly different between the groups [Fgenotype(1,8) = 13.87, p < 0.01; Ftime(4,32) = 21.02, p < 0.001; Finteraction(4,32) = 4.599, p < 0.01; Fig. 5B]. While Tat(+) females recovered from thermal hypersensitivity at post-CCI day 22, Tat(−) females still had significant hypersensitivity at the same time point (p < 0.05; Fig. 5B).
Fig. 5. CCI-induced thermal and mechanical hypersensitivity in female Tat(−) and Tat(+) Mice.
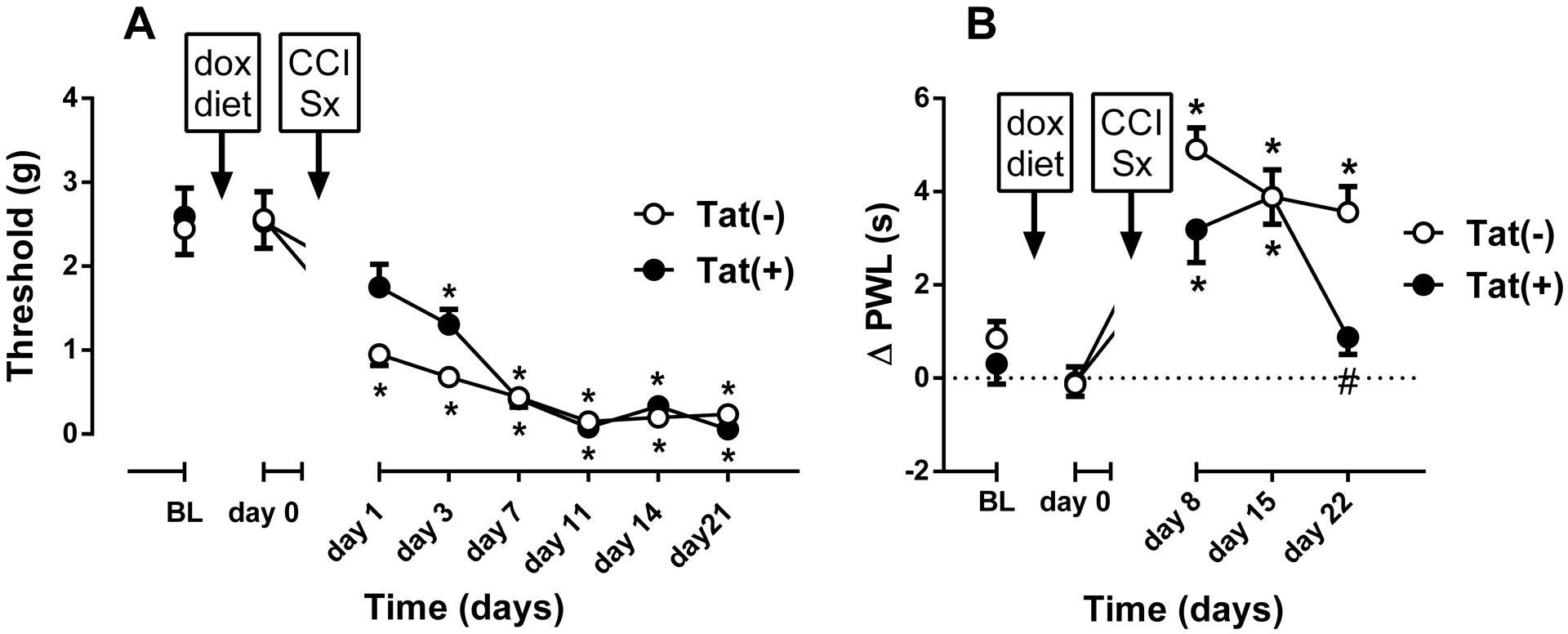
Mechanical paw withdrawal thresholds (A), and differences in paw withdrawal latencies (Δ PWL=contralateral–ipsilateral hindpaw latencies) (B) in Tat(−) and Tat(+) mice at different times after chronic constrictive nerve injury (CCI) operation. Data were expressed as the mean ± S.E.M. of 9 animals for each group. *p<0.05 significantly different from BL. #p<0.05 significantly different from Tat(−) group. BL: baseline
3.4. Impact of HIV-1 Tat protein on the antinociceptive effects of morphine in the CCI-induced neuropathic pain model
Since morphine was reported to be effective in the mouse CCI model of neuropathic pain (Bagdas et al., 2017), we tested its ability to reverse mechanical hypersensitivity produced by CCI in female Tat(−) and Tat(+) mice (n = 9/group). The effect of systemic morphine (3.2 mg/kg, s.c.) administration was tested on day 11 post-surgery. The effects of morphine were significantly different between genotypes [Fgenotype(1,8) = 40.59, p < 0.001; Ftime(5,40) = 58.73, p < 0.001; Finteraction(5,40) = 14.11, p < 0.001; Fig. 6]. Morphine significantly attenuated mechanical hypersensitivity in Tat(−) mice in a time-dependent manner, while morphine failed to limit mechanical hypersensitivity in Tat(+) mice at the times tested (Fig 6). Because mechanical hypersensitivity was not seen in male Tat(+) mice on day 11 following CCI (Fig. 4A), we did not test morphine in this group.
Fig. 6. Reversal of mechanical hypersensitivity by morphine in the CCI female Tat(−) and Tat(+) Mice.
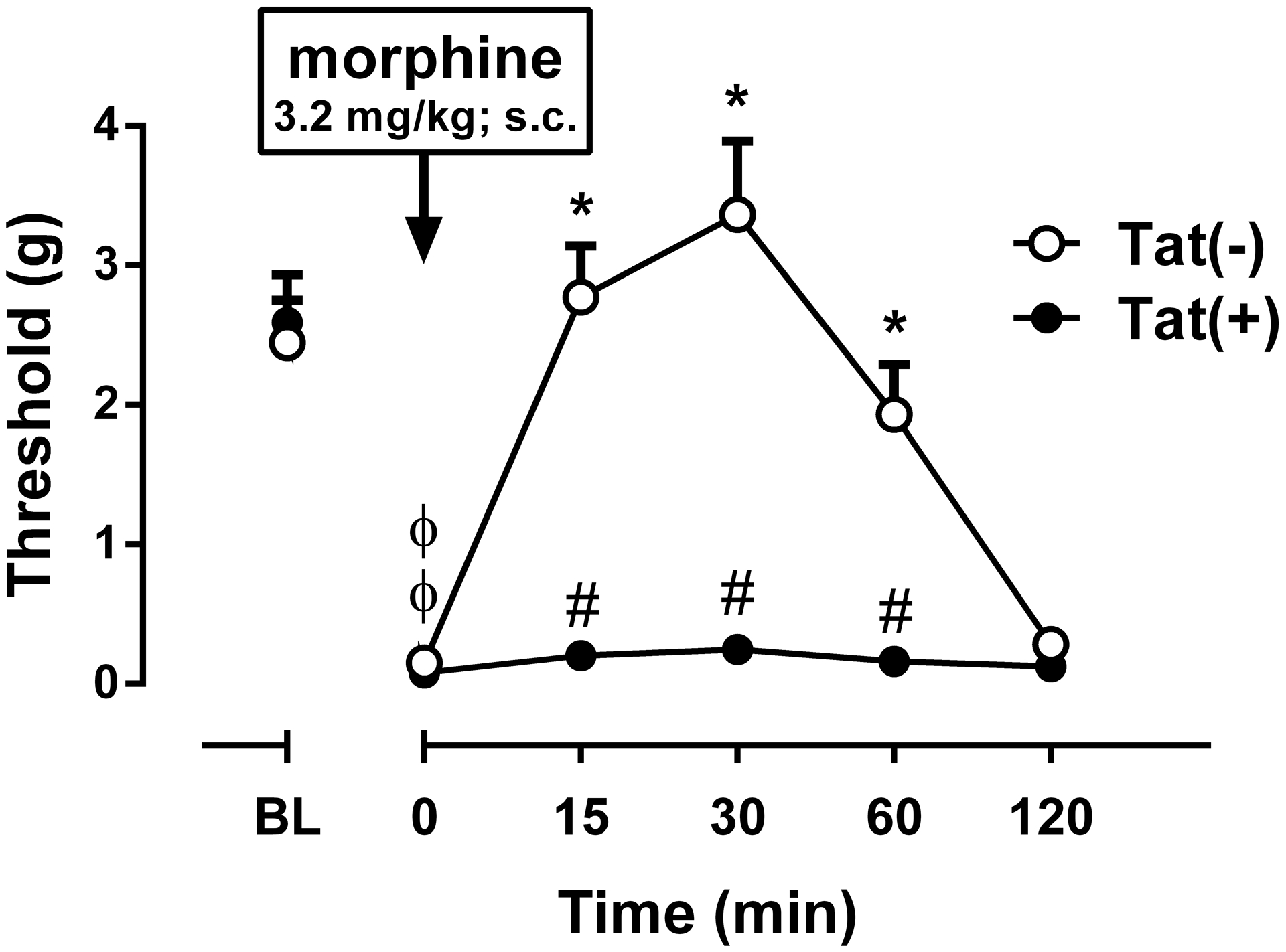
The mechanical paw withdrawal threshold values of ipsilateral paw for CCI are shown. Morphine (3.2 mg/kg, s.c.) reversed already-developed mechanical hypersensitivity produced by CCI (day 11 post surgery) in the Tat(−) mice but not in Tat(+) mice. Data were expressed as the mean ± S.E.M. of 9 animals for each group. ϕp<0.05 significantly different from BL. *p<0.05 significantly different from time 0 (zero). #p<0.05 significantly different from Tat(−) group. BL: baseline
4. Discussion
HIV-infected individuals experience greater distal polyneuropathies, headache, and additional chronic pain states (Keswani et al. 2002; Mirsattari et al, 1999) that are present in at least 38% of patients (Laycock et al., 2019). We examined the extent to which HIV-1 Tat expression modified formalin- and CFA-induced inflammatory and CCI-driven neuropathic nociception in male and female transgenic mice. We anticipated that Tat expression would exacerbate neuropathic and inflammatory pain-like responses in both male and female mice. Unexpectedly, Tat induction limited inflammatory paw swelling following CFA-injection in male mice and paw licking following formalin injection in both sexes, although females displayed a significantly greater reduction in paw licking than males. Intriguingly, males had attenuated nociceptive responses following Tat exposure that was not fully recapitulated in females. In male mice, Tat induction significantly attenuated phase II inflammatory (but not during phase I) paw licking in response to formalin and mechanical/thermal hypersensitivity to CFA and CCI, compared to Tat(−) controls. In contrast, Tat(+) female mice demonstrated a commensurate pain profile to that of their Tat(−) counterparts in the CFA-induced inflammatory pain or CCI-induced neuropathic models; yet, Tat(+) females were refractory to any reversal of mechanical hypersensitivity by morphine. These paradoxical findings demonstrate potentially fundamental sex differences in the effects of HIV-1 Tat on inflammation and neuropathic nerve injury and nociception.
These data are clinically-relevant given that Tat may influence, not only the response to pain, but also the response to the few medications available to treat it. In male mice, Tat exposure increases morphine tolerance, while decreasing the effects of physical withdrawal (Fitting et al., 2016) and morphine’s potency and efficacy in a thermal, tail flick (antinociceptive) assay (Fitting et al., 2012; Gonek et al., 2018). Moreover, Tat exposure also decreases MOR-mediated G-protein activation in an agonist, time, and regionally dependent manner in male mice (Hahn et al., 2016). These findings result, at least in part, from Tat-induced inflammatory chemokine and the activation of C-C chemokine type-5 receptors (CCR5) and heterologous desensitization of μ-opioid receptors (MOR) (Song et al., 2011; Steele et al., 2003). We find the CCR5 antagonist, maraviroc, can restore some of the sensitivity to morphine in vivo (Gonek et al., 2018) and prevent morphine from exacerbating the neurotoxic effects of Tat in vitro (Kim et al., 2018). Prompted by significant sex differences in neuropathic pain models (Sorge et al., 2014; Mapplebeck et al., 2018) and in HIV neuropathology (Hahn et al., 2015), we questioned whether sex differences on these measures might occur in the Tat transgenic mice.
By design, the present studies were performed during the first 3 weeks of Tat induction specifically to avoid the confounding effects of motor deficits. We do not see spontaneous motor deficits until ≥ 1 month of Tat exposure (Gonek et al., 2018; Hahn et al., 2016; Marks et al., 2016; Schier et al., 2017). The deficits that are observable on motor tests prior to 1 month of Tat exposure involve a cognitive component, such as the need to learn a rotarod paradigm (Fitting et al., 2012). By contrast, we do see anxiety-like behaviors (less time spent in the center of a well-lit open field or on the open arms of an elevated plus maze) after 1–4 weeks of Tat induction (Hahn et al., 2016; Paris et al., 2020; Paris et al., 2016); however, this does not significantly affect overall motor behavior in an open field (Gonek et al., 2018; Hahn et al., 2016; Schier et al., 2017) on a Barnes maze (Marks et al., 2016), or when analyzing gait (unpublished). Sex differences in anxiety-like behavior have been noted after 3 months of continuous Tat exposure, but effects on locomotor activity were more nuanced with males demonstrating a lesser capacity to learn a rotarod paradigm over 12 weeks (which may implicate cognitive processes over locomotor capacity; Hahn et al., 2015). Apart from the findings of the present study, sex differences with Tat exposure durations greater than 1 month have not been noted.
HIV-infected individuals with distal sensory neuropathies often first report a lack of sensation/numbness (hypoesthesia) followed by pain in the soles of the feet (Benito-León et al., 1998; Cornblath and McArthur, 1988; Mullin et al., 2011). In Tat transgenic mice, mechanical hypersensitivity is not evident after 4 weeks exposure and intradermal nerve fiber losses are evident at 6 weeks (Wodarski et al., 2018). Although attenuated responses to formalin, CFA, and CCI-induced nerve injury were seen following Tat induction, this is unlikely to reflect hypoesthesia, since they were not accompanied by increased baseline thresholds. Moreover, the lack of hypersensitivity is not due to a lack of Tat expression because significant increases in Tat mRNA expression occur in the DRG and skin at 8 d and significant astro- and microgliosis is detectable within the striatum 48 h following Tat induction (Bruce-Keller et al., 2008). Lastly, sublethal neuronal injury is well documented in vivo (Fitting et al., 2010) and is apparent within minutes of Tat exposure in vitro (Fitting et al., 2014; Ngwainmbi et al., 2014). Collectively, these findings suggest that during pathogenesis of Tat-induced neuropathy the attenuated responsiveness to formalin, CFA, and CCI-induced nerve injury precedes hypersensitivity, which may result from more prolonged nerve damage.
HIV-1 Tat levels are elevated in CSF (Henderson et al., 2019; Johnson et al., 2013) and trigger T cell type-17 immunological responsiveness in well cART-suppressed, aviremic persons infected with HIV (Johnson et al., 2013). In fact, cART may increase Tat expression by failing to prevent feedback inhibition and/or host suppression of proviral transcription (Johnson et al., 2013; Mbonye and Karn 2017; Henderson et al., 2019).
The sex differences observed in the present studies are intriguing. Unlike male mice, females demonstrated significant mechanical and thermal hypersensitivity following Tat induction. Although some clinical investigations find a greater incidence of HIV-related neuropathy in cART exposed (Chen at al., 2013) and cART-naïve women compared to men (Saylor et al., 2016), many studies do not reveal sex differences (Cherry et al., 2009; Ekenze et al., 2014; Forna et al., 2007; Hawkins et al., 2007; Hoffmann et al., 2008; Sacktor et al., 2009). Sex differences often result from cART-induced toxic neuropathy, the incidence of which is typically higher in women and more prevalent with older, more toxic cART drugs. For example, in a Kenyan cohort, HIV-peripheral neuropathy, resulting largely from treatment with stavudine (d4T), was nearly 10-times greater among women than men (Mehta et al., 2011). In a large Cuban cross-section of HIV-infected men and women on cART (n = 1,592), women reported greater levels of pain, more daily life interference by pain, and had an approximately 2.2-fold increase in the probability of experiencing pain, compared to men (Aragonés-López et al., 2012). Overall, women appear to be at greater risk for side effects associated with cART (Menezes et al., 2011; Osler et al., 2010; Pujades-Rodríguez et al., 2011; Wester et al., 2007) and discontinue treatment more frequently due to cART-induced toxic neuropathies (Currier et al., 2000; Kempf et al., 2009; Moore et al., 1996).
The sex differences in our findings may result from differences in the activation of microglia in male and T cells in female mice with inflammatory and neuropathic pain (Sorge et al., 2015; Mapplebeck et al., 2018). In males, nociception is mediated by a peroxisome proliferator activated receptor (PPAR) α-dependent activation of a microglia-neuron pathway involving P2X4 purinoreceptors, p38 MAPK and BDNF after spared nerve injury or plantar CFA injection; while in females, nociception is medicated via a PPARγ-dependent activation of T cells. At present, however, we cannot discern the extent to which Tat per se is triggering sexually dimorphic microglial versus T cell activation (Sorge et al., 2015) since the experimental procedures used (i.e., formalin, CFA, and CCI administration) themselves act through these different pathways in males and females.
Peripheral neuropathies in HIV-afflicted and unafflicted populations are often treated with anticonvulsant therapeutics (Finnerup et al., 2015); however, symptom relief is spurious and rarely fully attained (Cherry et al., 2016). As such, first-line medications have been pursued for symptom relief. In a sample of greater than 1,400 HIV-infected patients, chronic opioid regimens were prescribed for ~23% of individuals (Merlin et al., 2015). However, preclinical investigations demonstrate considerable interactions between opioids and HIV-1 Tat that may underlie some of the difficulties in treating HIV-related pain. Male Tat(+) mice demonstrate significantly increased tolerance to the anti-nociceptive effects of morphine in the tail-flick assay and reduced physical withdrawal symptoms compared to Tat(−) mice that are partially reversed by maraviroc (Gonek et al., 2018). These data suggest HIV-1 Tat disrupts morphine analgesic efficacy through excess CCL5, CCL3, and CCL4 chemokine production and CCR5 activation in the striatum. Whether sex differences in CCR5 signalling contribute to female Tat(+) mice being refractory to morphine analgesia in the present study is uncertain.
Identifying the potential mechanisms underlying HIV-related pain poses a challenge since multiple indirect and direct factors are thought to maintain chronic pain. Proinflammatory cytokines can promote and sustain neuropathic pain (DeLeo et al., 2004; Gao et al., 2010; Leung et al., 2010; Raghavendra et al., 2002; Watkins et al., 2001) and Tat increases their release from glia. Alternatively, HIV-1 Tat may exert direct excitatory effects on neurons that contribute to chronic pain. Application of high-concentration Tat causes rapid hyperexcitability in rat primary dorsal root ganglion neurons followed by apoptosis (Chi et al., 2011). Tat can directly modulate the NR1 subunit of the NMDA receptor (Song et al., 2003) and promotes phosphorylation of the NR2A and NR2B subunits (Haughey et al., 2001), thereby facilitating glutamate and NMDA receptor-mediated Ca2+ influx (Haughey et al., 2001; Krogh et al., 2014; Zhu et al., 2009). Tat interacts with the low-density lipoprotein receptor-related protein (LRP) to gain intracellular access, thereby indirectly activating the tyrosine kinase, Src, well-established for its requisite role in NMDA receptor activation (Eugenin et al., 2007; Krogh et al., 2014; Liu et al., 2000). In rats, selective Src-inhibition attenuates NMDA-mediated neuropathic pain sensitivity caused by intraplantar formalin or CFA (Liu et al., 2008) supporting the notion that Tat activation of Src may facilitate nociceptive signaling. In enteric neurons, Tat can increase the firing rate and reduce the threshold for triggering action potentials in part through actions on Nav1.7 and Nav1.8 sodium channels (Ngwainmbi et al., 2014). Nav1.7 appears to mediate key aspects of nociception in DRG neurons (Minett et al., 2014; Yang et al., 2018). Lastly, the role of mitochondrial dysfunction must be considered for as a target for Tat’s direct neurotoxic effects. Mitochondrial-derived reactive oxygen species are recently appreciated for their contribution to peripheral neuropathy (Flatters, 2015). Tat induces a pro-oxidative state that is driven in part via mitochondrial dysregulation (De Simone et al., 2016; Fitting et al., 2014; Hui et al., 2012; Norman et al., 2008). The extent to which these mechanisms may contribute to differences in Tat-related nociception males and females is important to consider.
Our work has some limitations, such as lack of comparison of morphine efficacy in male mice and lack of testing other HIV-1 neurotoxic proteins contribution to pain-like behavior in mice. Since different mechanical hypersensitivity was observed in Tat(−) and Tat(+) male mice by starting post-CCI surgery at day 7, we were not able to test the efficacy of morphine in male mice. A future study is needed to clarify the effects of morphine at an early stage of CCI in male mice, e.g. post-CCI day 3. In addition to Tat, other HIV-1 neurotoxic proteins contribute to hypersensitivity. In particular, the HIV-1 envelope glycoprotein, gp120, is directly toxic to sensory neurons (Keswani et al., 2003; Melli et al., 2006; Oh et al., 2001; Moss et al., 2015). Local paw injection of gp120 (Jolivalt et al., 2008) or delivery of gp120 to the sciatic nerve (Maratou et al., 2009) produce neuropathic mechanical hypersensitivity (without changes in thermal sensation; Wallace et al., 2007). Moreover, gp120 administered intrathecally or into the periaqueductal gray exacerbates formalin- (Hains et al., 2010) or cold-evoked mechanical hypersensitivity (Chen et al., 2011) suggesting there are also central sites of action. Tat may work in concert with soluble HIV proteins, including gp120, to promote sensory neuropathy. Parsing the distinct and/or possible interactive effects of individual pathogenic viral proteins in HIV-associated neuropathies may be warranted.
The present findings reveal intriguing sex differences in the effects of HIV-1 Tat on several modes of nociceptive responding and provides further evidence that Tat per se may contribute to HIV sensory neuropathy. Exposure to HIV-1 Tat caused impaired thermal and mechanical nociceptive responding in response to inflammatory, thermal, or neuropathic pain stimuli among males; females were significantly less affected, although female Tat(+) mice demonstrated a refractory response to morphine analgesia. HIV-1 Tat transgenic mouse may be a useful model to investigate the mechanisms that underlie sex differences in sensory neuropathy resulting from HIV and/or peripheral inflammation. The treatment of HIV-related neuropathy may be improved by considering sex as a clinical variable.
Supplementary Material
5. Acknowledgements
This work was supported by funds from the National Institutes of Health: R01 DA032246 (MID), R00 DA039791 (JJP), R01 DA024461 (PEK), R01 DA034231 (PEK and KFH), R01 DA045588 (KFH), K02 DA027374 (KFH), and a Pilot Project Award from the Massey Cancer Center at Virginia Commonwealth University (MID). Dr. Bagdas’ effort was supported in part by the National Institute on Drug Abuse of the National Institutes of Health under Award Number P50 DA036105 and the Center for Tobacco Products of the U.S. Food and Drug Administration. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Food and Drug Administration.
6. References
- Aragonés-López C, Pérez-Ávila J, Smith Fawzi MC, Castro A (2012). Quality of life of people with HIV/AIDS receiving antiretroviral therapy in Cuba: a cross-sectional study of the national population. Am J Public Health. 102, 884–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagdas D, AlSharari SD, Freitas K, Tracy M, Damaj MI (2015). The role of α5 nicotinic acetylcholine receptors in mouse models chronic inflammatory and neuropathic pain. Biochemical Pharmacology, 97(4), 590–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagdas D, Ergun D, Jackson A, Toma W, Schulte MK, Damaj MI. Allosteric modulation of α4β2* nicotinic acetylcholine receptors: desformylflustrabromine potentiates antiallodynic response of nicotine in a mouse model of neuropathic pain. 2018; Eur J Pain. 22:84–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benito-León J, Simón R, Miera C (1998). Numb chin syndrome as the initial manifestation of HIV infection. Neurology. 50(2), 511–512. [DOI] [PubMed] [Google Scholar]
- Bottcher C, Schlickeiser S, Sneeboer MAM, Kunkel D, Knop A, Paza E, Fidzinski P, Kraus L, Snijders GJL, Kahn RS, Schulz AR, Mei HE, Psy NBB, Hol EM, Siegmund B, Glauben R, Spruth EJ, de Witte LD, Priller J (2019). Human microglia regional heterogeneity and phenotypes determined by multiplexed single-cell mass cytometry. Nat Neurosci 22, 78–90. [DOI] [PubMed] [Google Scholar]
- Brack-Werner R (1999). Astrocytes: HIV cellular reservoirs and important participants in neuropathogenesis. AIDS, 13, 1–22. [DOI] [PubMed] [Google Scholar]
- Bruce-Keller AJ, Turchan-Cholewo J, Smart EJ, Geurin T, Chauhan A, Reid R, Xu R, Nath A, Knapp PE, Hauser KF (2008). Morphine causes rapid increases in glial activation and neuronal injury in the striatum of inducible HIV-1 Tat transgenic mice, Glia, 56, 1414–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL (1994). Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 53(1), 55–63. [DOI] [PubMed] [Google Scholar]
- Chen H, Clifford DB, Deng L, Wu K, Lee AJ, Bosch RJ, Riddler SA, Ellis RJ, Evans SR (2013). Peripheral neuropathy in ART-experienced patients: prevalence and risk factors. J Neurovirol, 19, 557–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Kirby LG, Palma J, Benamar K, Geller EB, Eisenstein TK, Adler MW (2011). The effect of gp120 on morphine’s antinociceptive and neurophysiological actions. Brain Behav Immun, 25, 1434–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry CL, Affandi JS, Imran D, Yunihastuti E, Smyth K, Vanar S, Kamarulzaman A, Price P (2009). Age and height predict neuropathy risk in patients with HIV prescribed stavudine. Neurology. 73, 315–20. [DOI] [PubMed] [Google Scholar]
- Chi X, Amet T, Byrd D, Chang KH, Shah K, Hu N, Grantham A, Hu S, Duan J, Tao F, Nicol G, Yu Q (2011). Direct effects of HIV-1 Tat on excitability and survival of primary dorsal root ganglion neurons: possible contribution to HIV-1-associated pain. PLoS One.6(9), e24412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopard C, Tong PBV, Toth P, Schatz M, Yezid H, Debaisieux S, Mettling C, Gross A, Pugniere M, Tu A, Strub JM, Mesnard JM, Vitale N, Beaumelle B (2018). Cyclophilin A enables specific HIV-1 Tat palmitoylation and accumulation in uninfected cells. Nature communications, 9: 2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill M, Nath A (2013). Where does HIV hide? A focus on the central nervous system. Curr Opin HIV AIDS, 8, 165–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill MJ, Wesselingh SL, Cowley D, Pardo CA, McArthur JC, Brew BJ, Gorry PR (2009). Extensive astrocyte infection is prominent in human immunodeficiency virus-associated dementia. Ann Neurol, 66, 253–258. [DOI] [PubMed] [Google Scholar]
- Cornblath DR, McArthur JC (1998). Predominantly sensory neuropathy in patients with AIDS and AIDS-related complex. Neurology. 38(5),794–796. [DOI] [PubMed] [Google Scholar]
- Currier JS, Spino C, Grimes J, Wofsy CB, Katzenstein DA, Hughes MD, Hammer SM, Cotton DJ (2000). Differences between women and men in adverse events and CD4+ responses to nucleoside analogue therapy for HIV infection. J Acquir Immune Defic Syndr, 24, 316–324. [DOI] [PubMed] [Google Scholar]
- DeLeo JA, Tanga FY, Tawfik VL (2004). Neuroimmune activation and neuroinflammation in chronic pain and opioid tolerance/hyperalgesia. Neuroscientist. 10(1), 40–52. [DOI] [PubMed] [Google Scholar]
- De Simone FI, Darbinian N, Amini S, Muniswamy M, White MK, Elrod JW, Datta PK, Langford D, Khalili K (2016). HIV-1 Tat and Cocaine Impair Survival of Cultured Primary Neuronal Cells via a Mitochondrial Pathway. J Neuroimmune Pharmacol, 11, 358–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon WJ (1965). The up-and-down method for small samples. J Am Stat Assoc, 60, 967–978. [Google Scholar]
- Ekenze OS, Nwosu CM, Ogunniyi A (2014). Frequency and risk factors for distal sensory polyneuropathy in HIV infection in a developing country. Int J STD AIDS., 25(3), 178–83. [DOI] [PubMed] [Google Scholar]
- Eugenin EA, King JE, Nath A, Calderon TM, Zukin RS, Bennett MV, Berman JW (2007). HIV-tat induces formation of an LRP-PSD-95- NMDAR-nNOS complex that promotes apoptosis in neurons and astrocytes. Proc Natl Acad Sci U S A. 104(9):3438–3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferri KF, Jacotot E, Blanco J, Esté JA, Kroemer G (2000). Mitochondrial control of cell death induced by HIV-1-encoded proteins. Ann N Y Acad Sci., 926, 149–164. [DOI] [PubMed] [Google Scholar]
- Finnerup NB, Attal N, Haroutounian S, McNicol E, Baron R, Dworkin RH, Gilron I, Haanpää M, Hansson P, Jensen TS, Kamerman PR, Lund K, Moore A, Raja SN, Rice AS, Rowbotham M, Sena E, Siddall P, Smith BH, Wallace M (2015). Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 14(2), 162–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitting S, Ngwainmbi J, Kang M, Khan FA, Stevens DL, Dewey WL, Knapp PE, Hauser KF, Akbarali HI (2015) Sensitization of enteric neurons to morphine by HIV-1 Tat protein. Neurogastroenterol Motil. 27(4), 468–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitting S, Scoggins KL, Xu R, Dever SM, Knapp PE, Dewey WL, Hauser KF (2012). Morphine efficacy is altered in conditional HIV-1 Tat transgenic mice. Eur J Pharmacol. 689(1–3), 96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitting S, Stevens DL, Khan FA, Scoggins KL, Enga RM, Beardsley PM, Knapp PE, Dewey WL, Hauser KF (2016). Morphine Tolerance and Physical Dependence Are Altered in Conditional HIV-1 Tat Transgenic Mice. J Pharmacol Exp Ther 356, 96–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitting S, Knapp PE, Zou S, Marks WD, Bowers MS, Akbarali HI, Hauser KF (2014). Interactive HIV-1 Tat and morphine-induced synaptodendritic injury is triggered through focal disruptions in Na+ influx, mitochondrial instability, and Ca2+ overload. J Neurosci 34, 12850–12864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flatters SJ (2015). The contribution of mitochondria to sensory processing and pain. Prog Mol Biol Transl Sci. 131:119–146. [DOI] [PubMed] [Google Scholar]
- Forna F, Liechty CA, Solberg P, et al. (2007). Clinical toxicity of highly active antiretroviral therapy in a home-based AIDS care program in rural Uganda. J Acquir Immune Defic Syndr. 44, 456–462. [DOI] [PubMed] [Google Scholar]
- Gandhi N, Saiyed Z, Thangavel S, Rodriguez J, Rao KV, Nair MP (2009). Differential effects of HIV type 1 clade B and clade C Tat protein on expression of proinflammatory and antiinflammatory cytokines by primary monocytes. AIDS Res Hum Retroviruses. 25(7), 691–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao YJ, Zhang L, Ji RR (2010). Spinal injection of TNF-alpha-activated astrocytes produces persistent pain symptom mechanical allodynia by releasing monocyte chemoattractant protein-1. Glia. 58(15),1871–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldmann T, Wieghofer P, Jordao MJ, Prutek F, Hagemeyer N, Frenzel K, Amann L, Staszewski O, Kierdorf K, Krueger M, Locatelli G, Hochgerner H, Zeiser R, Epelman S, Geissmann F, Priller J, Rossi FM, Bechman I, Kerschensteiner M, Linnarsson S, Jung S, Prinz M (2016). Origin, fate and dynamics of macrophages at central nervous system interfaces. Nature immunology. 17, 797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonek M, McLane VD, Stevens DL, Lippold K, Akbarali HI, Knapp PE, Dewey WL, Hauser KF, Paris JJ (2018). CCR5 mediates HIV-1 Tat-induced neuroinflammation and influences morphine tolerance, dependence, and reward. Brain Behav Immun. 69, 124–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorry PR, Ong C, Thorpe J, Bannwarth S, Thompson KA, Gatignol A, Vesselingh SL, Purcell DF (2003). Astrocyte infection by HIV-1: mechanisms of restricted virus replication, and role in the pathogenesis of HIV-1-associated dementia. Curr Hiv Res. 1, 463–473. [DOI] [PubMed] [Google Scholar]
- Hahn YK, Paris JJ, Lichtman AH, Hauser KF, Sim-Selley LJ, Selley DE, Knapp PE (2016). Central HIV-1 Tat exposure elevates anxiety and fear conditioned responses of male mice concurrent with altered mu-opioid receptor-mediated G-protein activation and beta-arrestin 2 activity in the forebrain. Neurobiol Dis. 92, 124–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn YK, Podhaizer EM, Farris SP, Miles MF, Hauser KF, Knapp PE (2015). Effects of chronic HIV-1 Tat exposure in the CNS: heightened vulnerability of males versus females to changes in cell numbers, synaptic integrity, and behavior. Brain structure & function. 220, 605–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hains LE, Loram LC, Weiseler JL, Frank MG, Bloss EB, Sholar P, Taylor FR, Harrison JA, Martin TJ, Eisenach JC, Maier SF, Watkins LR (2010). Pain intensity and duration can be enhanced by prior challenge: initial evidence suggestive of a role of microglial priming. J Pain.11(10),1004–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haughey NJ, Nath A, Mattson MP, Slevin JT, Geiger JD (2001). HIV-1 Tat through phosphorylation of NMDA receptors potentiates glutamate excitotoxicity. J Neurochem. 78(3):457–467. [DOI] [PubMed] [Google Scholar]
- Hawkins C, Achenbach C, Fryda W, Ngare D, Murphy R (2007). Antiretroviral durability and tolerability in HIV-infected adults living in urban Kenya. J Acquir Immune Defic Syndr. 45, 304–310. [DOI] [PubMed] [Google Scholar]
- Henderson LJ, Johnson TP, Smith BR, Reoma LB, Santamaria UA, Bachani M, Demarino C, Barclay RA, Snow J, Sacktor N, Mcarthur J, Letendre S, Steiner J, Kashanchi F, Nath A (2019). Presence of Tat and transactivation response element in spinal fluid despite antiretroviral therapy. AIDS. 33 Suppl 2:S145–S157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann CJ, Fielding KL, Charalambous S, et al. (2008). Antiretroviral therapy using zidovudine, lamivudine, and efavirenz in South Africa: tolerability and clinical events. AIDS. 22, 67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui L, Chen X, Bhatt D, Geiger NH, Rosenberger TA, Haughey NJ, Masino SA, Geiger JD (2012). Ketone bodies protection against HIV-1 Tat-induced neurotoxicity. J Neurochem. 122, 382–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IASP Task Force on Taxonomy (1994). Classification of Chronic Pain, “Part III: Pain Terms, A Current List with Definitions and Notes on Usage” (pp 209–214), Second Edition, edited by Merskey H and Bogduk N, IASP Press, Seattle. [Google Scholar]
- Johnson TP, Patel K, Johnson KR, Maric D, Calabresi PA, Hasbun R, Nath A (2013). Induction of IL-17 and nonclassical T-cell activation by HIV-Tat protein. Proc Natl Acad Sci U S A. 110(33):13588–13593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolivalt CG, Dacunha JM, Esch FS, Calcutt NA (2008) Central action of prosaptide TX14(A) against gp120-induced allodynia in rats. Eur J Pain.12(1), 76–81. [DOI] [PubMed] [Google Scholar]
- Kamerman PR, Wadley AL, Cherry CL (2012) HIV-associated sensory neuropathy: risk factors and genetics. Curr Pain Headache Rep. 16, 226–236. [DOI] [PubMed] [Google Scholar]
- Katz NP, Mou J, Paillard FC, Turnbull B, Trudeau J, Stoker M (2015). Predictors of Response in Patients With Postherpetic Neuralgia and HIV-Associated Neuropathy Treated With the 8% Capsaicin Patch (Qutenza). Clin J Pain. 31, 859–866. [DOI] [PubMed] [Google Scholar]
- Kempf MC, Pisu M, Dumcheva A, Westfall AO, Kilby JM, Saag MS (2009). Gender differences in discontinuation of antiretroviral treatment regimens. J Acquir Immune Defic Syndr. 52, 336–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keswani SC, Pardo CA, Cherry CL, Hoke A, McArthur JC (2002). HIV-associated sensory neuropathies. AIDS. 16(16), 2105–2117. [DOI] [PubMed] [Google Scholar]
- Keswani SC, Polley M, Pardo CA, Griffin JW, McArthur JC, Hoke A (2003). Schwann cell chemokine receptors mediate HIV-1 gp120 toxicity to sensory neurons. Ann. Neurol, 54(3), 287–296. [DOI] [PubMed] [Google Scholar]
- Kierdorf K, Masuda T, Jordao MJC, Prinz M (2019). Macrophages at CNS interfaces: ontogeny and function in health and disease. Nature reviews Neuroscience, 20, 547–562. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Martemyanov KA, Thayer SA (2008). Human immunodeficiency virus protein Tat induces synapse loss via a reversible process that is distinct from cell death. J Neurosci, 28(48), 12604–12613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Hahn YK, Podhaizer EM, McLane VD, Zou S, Hauser KF, Knapp PE (2018). A central role for glial CCR5 in directing the neuropathological interactions of HIV-1 Tat and opiates. J Neuroinflammation, 15, 285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer-Hammerle S, Rothenaigner I, Wolff H, Bell JE, Brack-Werner R (2005). Cells of the central nervous system as targets and reservoirs of the human immunodeficiency virus. Virus Res, 111, 194–213. [DOI] [PubMed] [Google Scholar]
- Krogh KA, Wydeven N, Wickman K, Thayer SA (2014). HIV-1 protein Tat produces biphasic changes in NMDA-evoked increases in intracellular Ca2+ concentration via activation of Src kinase and nitric oxide signaling pathways. J Neurochem. 130(5):642–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laycock H, Crawford V, Rice AS, Cox S (2019). Lessons learnt from establishing a high dose opioid review clinic for people living with HIV. Pain Manag. 9(1):37–44. [DOI] [PubMed] [Google Scholar]
- Leung L, Cahill CM (2010). TNF-alpha and neuropathic pain--a review. J. Neuroinflammation, 7:27 10.1186/1742-2094-7-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li GH, Maric D, Major EO, Nath A (2020). Productive HIV infection in astrocytes can be established via a non-classical mechanism. AIDS. 10.1097/QAD.0000000000002512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Wan Q, Pristupa ZB, Yu XM, Wang YT, Niznik HB (2000). Direct protein-protein coupling enables cross-talk between dopamine D5 and gamma-aminobutyric acid A receptors. Nature. 403: 274–280. [DOI] [PubMed] [Google Scholar]
- Liu XJ, Gingrich JR, Vargas-Caballero M, Dong YN, Sengar A, Beggs S, Wang SH, Ding HK, Frankland PW, Salter MW (2008). Treatment of inflammatory and neuropathic pain by uncoupling Src from the NMDA receptor complex. Nat Med. 14(12):1325–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mapplebeck JCS, Dalgarno R, Tu Y, Moriarty O, Beggs S, Kwok CHT, Halievski K, Assi S, Mogil JS, Trang T, Salter MW (2018). Microglial P2X4R-evoked pain hypersensitivity is sexually dimorphic in rats. Pain,159: 1752–1763. [DOI] [PubMed] [Google Scholar]
- Maratou K, Wallace VC, Hasnie FS, Okuse K, Hosseini R, Jina N, Blackbeard J, Pheby T, Orengo C, Dickenson AH, McMahon SB, Rice AS (2009). Comparison of dorsal root ganglion gene expression in rat models of traumatic and HIV-associated neuropathic pain. Eur J Pain, 13, 387–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks WD, Paris JJ, Schier CJ, Denton MD, Fitting S, McQuiston AR, Knapp PE, Hauser KF (2016). HIV-1 Tat causes cognitive deficits and selective loss of parvalbumin, somatostatin, and neuronal nitric oxide synthase expressing hippocampal CA1 interneuron subpopulations. J Neurovirol, 22, 747–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbonye U, Karn J (2017). The Molecular Basis for Human Immunodeficiency Virus Latency. Annu Rev Virol. 4(1):261–285. [DOI] [PubMed] [Google Scholar]
- Mehta S,A, Ahmed A, Laverty M, Holzman RS, Valentine F, Sivapalasingam S (2011). Sex differences in the incidence of peripheral neuropathy among Kenyans initiating antiretroviral therapy. Clin Infect Dis., 53, 490–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melli G, Keswani SC, Fischer A, Chen W, Höke A (2006). Spatially distinct and functionally independent mechanisms of axonal degeneration in a model of HIV-associated sensory neuropathy. Brain, 129, 1330–1338. [DOI] [PubMed] [Google Scholar]
- Menezes CN, Maskew M, Sanne I, Crowther NJ, Raal FJ (2011). A longitudinal study of stavudine-associated toxicities in a large cohort of South African HIV infected subjects. BMC Infect Dis.,11, 244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlin JS, Tamhane A, Starrels JL, Kertesz S, Saag M, Cropsey K (2016). Factors Associated with Prescription of Opioids and Co-prescription of Sedating Medications in Individuals with HIV. AIDS Behav, 20, 687–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minett MS, Falk S, Santana-Varela S, Bogdanov YD, Nassar MA, Heegaard AM, Wood JN (2014). Pain without nociceptors? Nav1.7-independent pain mechanisms. Cell Rep., 6, 301–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirsattari SM, Power C, Nath A (1999). Primary headaches with HIV infection. Headache, 39, 3–10. [DOI] [PubMed] [Google Scholar]
- Moore RD, Fortgang I, Keruly J, Chaisson RE (1996). Adverse events from drug therapy for human immunodeficiency virus disease. Am J Med., 101, 34–40. [DOI] [PubMed] [Google Scholar]
- Moss PJ, Huang W, Dawes J, Okuse K, McMahon SB, Rice AS (2015). Macrophage-sensory neuronal interaction in HIV-1 gp120-induced neurotoxicity. Br J Anaesth., 114, 499–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullin S, Temu A, Kalluvya S, Grant A, Manji H (2011) High prevalence of distal sensory polyneuropathy in antiretroviral-treated and untreated people with HIV in Tanzania. Trop Med Int Health, 16, 1291–1296. [DOI] [PubMed] [Google Scholar]
- Neri E, Musante V, Pittaluga A (2007). Effects of the HIV-1 viral protein TAT on central neurotransmission: role of group I metabotropic glutamate receptors. Int Rev Neurobiol., 82, 339–356. [DOI] [PubMed] [Google Scholar]
- Ngwainmbi J, De DD, Smith TH, El-Hage N, Fitting S, Kang M, Dewey WL, Hauser KF, Akbarali HI (2014). Effects of HIV-1 Tat on enteric neuropathogenesis. J Neurosci, 34, 14243–14251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman JP, Perry SW, Kasischke KA, Volsky DJ, Gelbard HA (2007). HIV-1 trans activator of transcription protein elicits mitochondrial hyperpolarization and respiratory deficit, with dysregulation of complex IV and nicotinamide adenine dinucleotide homeostasis in cortical neurons. J Immunol., 178, 869–876. [DOI] [PubMed] [Google Scholar]
- Norman JP, Perry SW, Reynolds HM, Kiebala M, De Mesy Bentley KL, Trejo M, Volsky DJ, Maggirwar SB, Dewhurst S, Masliah E, Gelbard HA (2008). HIV-1 Tat Activates Neuronal Ryanodine Receptors with Rapid Induction of the Unfolded Protein Response and Mitochondrial Hyperpolarization. PLoS ONE 3, e3731 10.1371/journal.pone.0003731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh SB, Tran PB, Gillard SE, Hurley RW, Hammond DL, Miller RJ (2001). Chemokines and glycoprotein120 produce pain hypersensitivity by directly exciting primary nociceptive neurons. J Neurosci., 21, 5027–5035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osler M, Stead D, Rebe K, Meintjes G, Boulle A (2010). Risk factors for and clinical characteristics of severe hyperlactataemia in patients receiving antiretroviral therapy: a case-control study. HIV Med., 11, 121–129. [DOI] [PubMed] [Google Scholar]
- Paris JJ, Liere P, Kim S, Mahdi F, Buchanan ME, Nass SR, Qrareya AN, Salahuddin MF, Pianos A, Fernandez N, Shariat-Madar Z, Knapp PE, Schumacher M, Hauser KF (2020). Pregnane steroidogenesis is altered by HIV-1 Tat and morphine: Physiological allopregnanolone is protective against neurotoxic and psychomotor effects. Neurobiology of Stress, 12, 100211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris JJ, Zou S, Hahn YK, Knapp PE, Hauser KF (2016). 5α-reduced progestogens ameliorate mood-related behavioral pathology, neurotoxicity, and microgliosis associated with exposure to HIV-1 Tat. Brain Behav Immun., 55, 202–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujades-Rodríguez M, Dantony E, Pinoges L, Ecochard R, Etard JF, Carrillo-Casas E, Szumilin E (2011). AIDS Working Group of Médecins Sans Frontières. Toxicity associated with stavudine dose reduction from 40 to 30 mg in first-line antiretroviral therapy. PLoS One, 6, e28112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavendra V, Rutkowski MD, DeLeo JA (2002). The role of spinal neuroimmune activation in morphine tolerance/hyperalgesia in neuropathic and sham-operated rats. J. Neurosci, 22, 9980–9989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice AP, Kimata JT (2015). Subversion of Cell Cycle Regulatory Mechanisms by HIV. Cell Host Microbe., 17, 736–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacktor N, Nakasujja N, Skolasky RL, Robertson K, Musisi S, Ronald A, Katabira E, Clifford DB (2009). Benefits and risks of stavudine therapy for HIV-associated neurologic complications in Uganda. Neurology, 72, 165–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saylor D, Dickens AM, Sacktor N, Haughey N, Slusher B, Pletnikov M, Mankowski JL, Brown A, Volsky DJ, McArthur JC (2016). HIV-associated neurocognitive disorder--pathogenesis and prospects for treatment. Nature reviews Neurology, 12, 234–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schier CJ, Marks WD, Paris JJ, Barbour AJ, McLane VD, Maragos WF, McQuiston AR, Knapp PE, Hauser KF (2017). Selective vulnerability of striatal D2 versus D1 dopamine receptor-expressing medium spiny neurons in HIV-1 Tat transgenic male mice. J Neurosci., 37, 5758–5769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin AH, Thayer SA (2013). Human immunodeficiency virus-1 protein Tat induces excitotoxic loss of presynaptic terminals in hippocampal cultures. Mol Cell Neurosci., 54, 22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song C, Rahim RT, Davey PC, Bednar F, Bardi G, Zhang L, Zhang N, Oppenheim JJ, Rogers TJ (2011). Protein kinase Czeta mediates micro-opioid receptor-induced cross-desensitization of chemokine receptor CCR5. J Biol Chem., 286, 20354–20365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sordo del Castillo L, Ruiz-Pérez I, Olry de Labry Lima A, (2010). Biological, psychosocial, therapeutic and quality of life inequalities between HIV-positive men and women - a review from a gender perspective. AIDS Rev.,12, 113–120. [PubMed] [Google Scholar]
- Sorge RE, Mapplebeck JC, Rosen S, Beggs S, Taves S, Alexander JK, Martin LJ, Austin JS, Sotocinal SG, Chen D, Yang M, Shi XQ, Huang H, Pillon NJ, Bilan PJ, Tu Y, Klip A, Ji RR, Zhang J, Salter MW, Mogil JS (2015). Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat Neurosci., 18, 1081–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrell ME, Hauser KF (2014). Ligand-gated purinergic receptors regulate HIV-1 Tat and morphine related neurotoxicity in primary mouse striatal neuron-glia co-cultures. J Neuroimmune Pharmacol. 9(2):233–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele AD, Henderson EE, Rogers TJ (2003). Mu-opioid modulation of HIV-1 coreceptor expression and HIV-1 replication. Virology, 309, 99–107. [DOI] [PubMed] [Google Scholar]
- Tornatore C, Nath A, Amemiya K, Major EO (1991). Persistent human immunodeficiency virus type 1 infection in human fetal glial cells reactivated by T-cell factor(s) or by the cytokines tumor necrosis factor alpha and interleukin-1 beta. J Virol, 65, 6094–6100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace VC, Blackbeard J, Pheby T, Segerdahl AR, Davies M, Hasnie F, Hall S, McMahon SB, Rice AS (2007). Pharmacological, behavioural and mechanistic analysis of HIV-1 gp120 induced painful neuropathy. Pain. 133(1–3):47–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins LR, Milligan ED, Maier SF (2001). Glial activation a driving force for pathological pain. Trends Neurosci., 24(8), 450–455. [DOI] [PubMed] [Google Scholar]
- Wester CW, Okezie OA, Thomas AM, Bussmann H, Moyo S, Muzenda T, Makhema J, van Widenfelt E, Musonda R, Novitsky V, Gaolathe T, Ndwapi N, Essex M, Kuritzkes DR, DeGruttola V, Marlink RG (2007). Higher-than-expected rates of lactic acidosis among highly active antiretroviral therapy-treated women in Botswana: preliminary results from a large randomized clinical trial. J Acquir Immune Defic Syndr., 46, 318–322. [DOI] [PubMed] [Google Scholar]
- Wodarski R, Bagdas D, Paris JJ, Pheby T, Toma W, Xu R, Damaj MI, Knapp PE, Rice ASC, Hauser KF (2018). Reduced intraepidermal nerve fibre density, glial activation, and sensory changes in HIV type-1 Tat-expressing female mice: involvement of Tat during early stages of HIV-associated painful sensory neuropathy. Pain reports, 3, e654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Mis MA, Estacion M, Dib-Hajj SD, Waxman SG (2018). NaV1.7 as a Pharmacogenomic Target for Pain: Moving Toward Precision Medicine. Trends Pharmacol Sci, 39, 258–275. [DOI] [PubMed] [Google Scholar]
- Zhu J, Mactutus CF, Wallace DR, Booze RM (2009). HIV-1 Tat protein-induced rapid and reversible decrease in [3H]dopamine uptake: dissociation of [3H]dopamine uptake and [3H]2beta-carbomethoxy-3-beta-(4-fluorophenyl)tropane (WIN 35,428) binding in rat striatal synaptosomes. J Pharmacol Exp Ther. 329(3):1071–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


