Abstract
Introduction
Community-acquired bacterial meningitis may be complicated by cerebral venous thrombosis (CVT), but this has not systematically been studied.
Methods
We evaluated clinical characteristics and outcome of CVT in adults with community-acquired bacterial meningitis in a prospective nationwide cohort study of bacterial meningitis (2006–2018) in the Netherlands.
Results
CVT occurred in 26 of 2220 episodes with bacterial meningitis (1%). The diagnosis of CVT was made on the day of presentation in 15 patients (58%) and during hospital stay in 11 patients after a median of 6 days (IQR 2–7). Sinusitis or otitis was present in 16 of 24 patients (67%). Patients with CVT presented more often in a coma than those without CVT (53 vs. 18%; P = 0.001) and the clinical course was more often complicated by focal neurologic deficits (58 vs. 22%; P < 0.001). Twelve patients of 26 (46%) had parenchymal lesions on neuroimaging, of whom two (8%) were specific for CVT. The transverse sinus was most frequently thrombosed (18 of 26; 69%). Streptococcus pneumoniae was the most common causative pathogen, occurring in 17 of 26 patients (65%). Eleven patients (44%) received anticoagulant therapy with heparin and none of them developed intracerebral hemorrhage during admission. Unfavorable outcome, as defined as a score on the Glasgow Outcome Scale <5, occurred in 14 of 26 patients (54%) and 4 patients (15%) died.
Discussion and conclusion
CVT is a rare complication of bacterial meningitis and mainly occurs in patients with coma, ear, nose and throat infections, and focal neurologic deficits.
Keywords: Bacterial meningitis, cerebral venous thrombosis, thrombosis, cerebrovascular complication, stroke, Streptococcus pneumoniae
Introduction
Community-acquired bacterial meningitis is most commonly caused by Streptococcus pneumoniae and is associated with high mortality and morbidity rates.1–3 Neurological complications, e.g., hydrocephalus,4 seizures,5 and stroke 6 occur frequently and are important determinants of outcome.7,8 Stroke has been described to occur up to 30% of adults with bacterial meningitis,9 mainly consisting of cerebral infarctions. 10–12 An uncommonly reported cerebrovascular complication of bacterial meningitis is cerebral venous thrombosis (CVT). A retrospective study of 87 patients with pneumococcal meningitis, reported CVT in 9 of 87 (10%) of the patients.9 Other studies on CVT in bacterial meningitis patients are case reports and small case series mostly consisting of children.13–19 Vasculitis may be considered an underlying condition for CVT complicating bacterial meningitis, however, this is not systematically studied.9,20
Recognizing CVT complicating bacterial meningitis can be expected to be challenging because signs and symptoms of meningitis resemble CVT, such as headache, focal neurologic deficits, seizures, and lower level of consciousness.1,8,21–23 In CVT without bacterial meningitis, anticoagulant treatment is recommended and has been associated with a reduction in the risk of death.24 However, in bacterial meningitis, the use of anticoagulants has been associated with intracerebral hemorrhage.25 Patients with septic CVT were not included in international CVT trials that evaluated the efficacy and safety of anticoagulant therapy.26,27
Here, we describe clinical and radiological characteristics, treatment, and outcome in adults with bacterial meningitis complicated by CVT in a nationwide cohort study on community-acquired bacterial meningitis.
Materials and methods
The pseudonymized data is available on request by qualified researchers via www.MeninGene.eu. We conducted a nationwide prospective cohort study on community-acquired bacterial meningitis in the Netherlands between March 2006 - January 2018. We identified patients older than 16 years with bacterial meningitis defined by positive cerebrospinal fluid (CSF) culture or with CSF abnormalities predictive for bacterial meningitis (glucose level less than 1.9 mmol/L, CSF-blood glucose ratio less than 0.23, CSF protein level greater than 2.2 g/L, more than 2000 × 106/L leukocytes, or more than 1180 × 106/L polymorphonuclear leukocytes).28 The patients were either listed in the database of the Netherlands Reference Laboratory for Bacterial Meningitis (NRLBM) or reported to the investigators by their treating physician. The NRLBM receives CSF isolates from approximately 90% of all Dutch patients with bacterial meningitis. On a daily basis, updates were provided of the hospitals where patients with bacterial meningitis were admitted. Physicians were informed by telephone and asked to include their patients in the study. Physicians were also able to contact the investigators at all times to include patients, even without a reference laboratory report. The study was approved by the medical ethics committee of the Academic Medical Center in the Netherlands. Written informed consent was obtained from the patient or the legal representative. Patients with hospital-acquired meningitis, neurosurgical operation, or head trauma in the prior month, and patients with neurosurgical devices were excluded. We considered patients to be immunocompromised if there was a history of splenectomy, diabetes mellitus, current immunosuppressive drug use, HIV infection, or alcoholism. We used an online case record form (CRF) to collect data prospectively. The presence of CVT as a complication was scored in the CRF as a pre-specified complication. There were also separate sections where other complications and additional neuroimaging abnormalities were registered. This study is observational in nature, which means no standard neuroimaging was performed in all bacterial meningitis patients. All available data on neuroimaging during admission were archived.
We considered patients to have CVT when it was registered in the online CRF as a complication, as a radiological finding or when it was reported in the discharge letter. Subsequently, neuroimaging was re-evaluated to confirm the CVT diagnosis according to the American Heart Association guideline 26 by the investigators (SSD, MCB, JMC). Neuroimaging was assessed for the following findings: which cerebral sinus was involved, whether the thrombosis was adjacent to mastoiditis, and scored other abnormalities associated with CVT such as venous infarction and hemorrhage. All parenchymal lesions with edema (including vasogenic edema) have been scored as venous infarctions, according to the international CVT study criteria.23 We evaluated the discharge letters to assess the time of occurrence of the CVT, what clinical changes lead to the diagnosis of CVT in case it was not present on admission and whether anticoagulant therapy was used as a treatment of CVT.
Thrombosis in the following sinuses and veins was scored: cortical vein, superior sagittal sinus, deep cerebral veins, cavernous sinus, straight sinus, transverse sinus (L or R), sigmoid sinus (L or R) and internal jugular vein (L or R). The outcome at discharge was standardly graded in the online CRF according to the Glasgow Outcome Scale score (GOS), a functional outcome scale with scores varying from 1(death) to 5 (mild or no disability were the patient can return to work or school).29 A score of 5 is defined as a favorable outcome and a score of 1 to 4 is defined as an unfavorable outcome. The statistical analysis was performed with IBM SPSS Statistics, version 25. Descriptive analysis and non-parametric testing were conducted to describe the cohort baseline characteristics and continuous data. Chi-Square test and Fisher exact test were used to compare the categorical variables. We considered values of P < 0.05 to be statistically significant.
Results
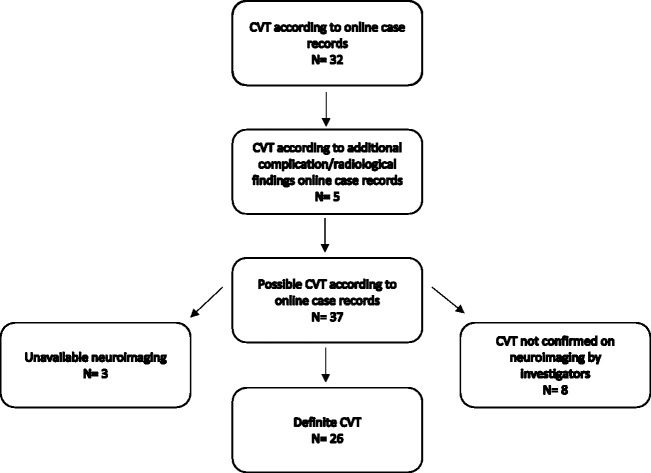
CVT was registered as a complication in 37 of 2220 bacterial meningitis episodes (1.6%). A total of 2001 of 2220 patients underwent neuroimaging (90%) of whom at least 360 patients had an MRI (16%). The neuroimaging (cerebral MRI, MR or CT venography) was available in 34 of 37 registered CVT cases. CVT was confirmed in 26 of 2220 episodes (1%; Figure 1). CVT was identified on the day of admission in 15 of 26 (58%) patients and during admission in the remaining 11 (42%) after a median of 6 days (IQR 2–7). Presenting clinical characteristics between patients with and without CVT were similar (Table 1).
Figure 1.
Flowchart of the study selection.
Table 1.
Characteristics of CVT and non-CVT patients.
| Patients characteristics | CVT group, n = 26 | Non-CVT group, n = 2220 |
|---|---|---|
| Age IQR | 57 (48–69) | 58 (47–70) |
| Female | 14/26 (53%) | 1025/2087 (49%) |
| Predisposing conditions | ||
| Otitis media | 12/24 (50%) | 542/1958 (28%)a |
| Sinusitis | 6/24 (25%) | 232/1936 (12%) |
| ENT infection | 16/24 (67%) | 723/1307(36%)a |
| Symptoms and signs on admission | ||
| Headache | 19/23 (83%) | 1482/1810 (82%) |
| Nausea | 12/21 (57%) | 1025/1705 (60%) |
| Neck stiffness | 17/23 (74%) | 1456/1596 (91%) |
| Temperature ≥ 38°C | 17/23 (74%) | 1541/1966 (78%) |
| Triad of neck stiffness, fever and altered mental status | 10/21 (48%) | 753/1915 (39%) |
| Seizures pre admission | 2/25 (8%) | 161/2074 (8%) |
| Altered mental status GCS < 14 | 22/26 (85%) | 1532/1942 (79%) |
| Coma on admission GCS ≤ 8 | 8/15 (53%) | 427/2177 (20%)a |
| Focal neurological deficit | 10/26 (38%) | 701/2050 (34%) |
| Mastoid opacification on neuroimaging | 12/26 (46%) | 253/1042 (24%)a |
| CSF values | ||
| Leukocyte count, 106/L | 5244 (126–15,517) | 2961 (715–8336) |
| Leukocyte count < 10,00,106/L | 7/26 (27%) | 604/2075 (29%) |
| Protein, g/L | 4,7 (1–12) | 3,7 (2–6) |
| CSF-blood-glucose-ratio | 0,1 (0–0.5) | 0,1 (0–0.3) |
| Laboratory results on admission | ||
| Leukocyte count, 109/L | 21 (17–28) | 16 (12–22) |
| C-reactive protein, nmol/L | 2209 (828–3047) | 183 (79–298) |
| Thrombocyte count, 109/L | 222 (178–314) | 199 (149–255) |
| Prothrombin time sec | 13 (11–18) | 15 (12–21) |
| Symptoms in patients with CVT on admission, day 0, n = 15 | ||
| Seizures | 1/15 (6%) | |
| Abnormal eye movements | 3/15 (20%) | |
| Altered mental status GCS < 14 | 13/15 (87%) | |
| Coma GCS < 8 | 8/15 (53%) | |
| Impaired vision | 1/15 (6%) | |
| Focal neurological signs | 3/15 (20%) | |
| Symptoms in patients with CVT during admission, day ≥ 1, n = 11 | ||
| Seizures | 3/11 (27%) | |
| Abnormal eye movements | 2/11 (18%) | |
| Altered mental status GCS < 14 | 5/11 (45%) | |
| Coma GCS < 8 | 4/11 (36%) | |
| Hemianopia | 2/11 (18%) | |
| Hemiparesis | 5/11 (45%) | |
| Aphasia | 3/11 (27%) | |
| Focal neurological signs | 8/11 (73%) | |
| Complications | ||
| Seizures during admission | 6/26 (23%) | 288/2077 (14%) |
| Focal neurological deficit | 15/26 (58%) | 438/2004 (22%)a |
| Transfer to ICU | 12/26 (46%) | 837/2088 (40%) |
| Neuroimaging findings | ||
| Hypodensity | 10/26 (38%) | 251/1078 (23%) |
| Intracerebral hemorrhage | 2/26 (8%) | 44/215 (20%) |
| Outcome | ||
| Time to discharge in days IQR | 18 (12–28) | 15 (11–22) |
| Poor outcome (GOS < 5) | 14/26 (54%) | 799/2165 (37%) |
aStatistically significant difference (P < 0.05).
Predisposing conditions for bacterial meningitis were present in 16 of 24 patients (67%; Table 1). An ear, nose and throat (ENT) infection focus was present in all 16 patients (67%): sinusitis (6 of 24; 25%), mastoiditis (9 of 25; 36%), and otitis media (12 of 24; 50%). Otitis media (50% vs 28%; P = 0.01) and mastoid opacification (46% vs 24%; P = 0.01) were more common in patients who presented or developed CVT as compared with those without CVT. Furthermore, sinusitis was seen in 6 of 24 (25%) in the CVT cohort and 232 of 1936 (12%) of the non-CVT patients (P = 0.05; Table 1).
All CVT patients underwent a lumbar puncture. Opening pressure was measured in 9 patients (median opening pressure 35 cm H2O; IQR 13–48). CSF analysis showed a median white cell count of 5244 × 106/L (126–15.517), protein levels 4.7 g/L (1–12), and CSF to blood glucose ratio 0.10 (0.00–0.50). In 7 of 26 patients (27%) the CSF leukocyte count was less than 1000 × 106/L. Causative organisms were Streptococcus pneumoniae 17 of 26 (65%), Streptococcus pyogenes in two patients (8%) and, Neisseria meningitidis and Streptococcus aureus both in one patient (4%). Cultures were negative in 5 of 26 patients (19%).
The triad of neck stiffness, fever, and altered mental status was present in 8 of 15 patients with CVT diagnosed on admission day (53%) and focal neurologic deficits were present in 3 of 15 (20%; Table 1). An altered mental status (defined by a score on the Glasgow Coma Scale (GCS) below 14) was present in 13 of 15 patients with CVT on admission day (87%); 8 of them were comatose (53%; GCS <8; Table 1). Coma on admission was more often observed in patients with CVT on admission day than those without CVT (8 of 15 [53%] vs. 427 of 2177 [20%]; P = 0.001; Table 1). Clinical deterioration prompted neuroimaging diagnosing CVT during admission in 11 patients: a decrease in the level of consciousness in 5 of these patients (45%), focal neurological symptoms in 8 (73%), seizures in 3 (27%), and abnormal eye movements in 2 (18%; Table 1).
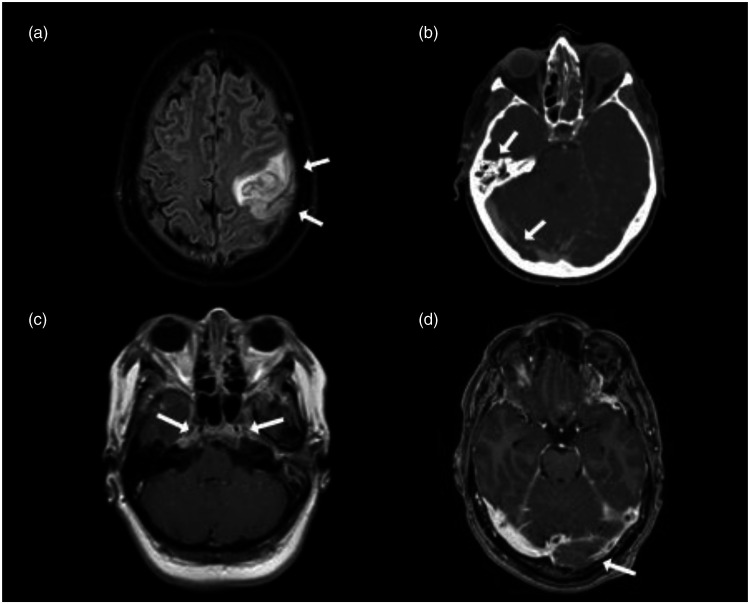
CVT was diagnosed using CT-venography in 14 of 26 (54%) and with MRV in 12 patients (46%). The most commonly thrombosed sinus was the transverse sinus 18 of 26 (69%) followed by the sigmoid sinus 12 of 26 (46%) and superior sagittal sinus 7 of 26 (27%). Internal jugular vein thrombosis was seen in 4 of 26 (15%), isolated cortical vein thrombosis in 3 of 26 (12%), and the cavernous sinus thrombosis in 2 of 26 (8%). In 12 of 26 (46%) of the cases, there was more than one cerebral venous channel involved. Mastoiditis in proximity to the venous thrombosis was seen in 8 of 26 (31%; Table 2). Additional findings on neuroimaging were parenchymal lesion(s) in 12 of 26 patients (46%) consisting of cerebral infarction in 10 of 26 (38%) and intracerebral hemorrhage in 2 of 26 (8%; Table 2). The two intracerebral hemorrhages (8%) were consistent with CVT as seen in patients without infection. One patient had a small, asymptomatic juxtacortical hemorrhage and the other patient had a hemorrhagic venous infarction (Figure 2).
Table 2.
Specific venous sinus details and additional neuroimaging findings.
| Cerebral venous sinus thrombosis details, n = 26 | |
| Cortical vein | 3/26 (12%) |
| Superior sagittal sinus | 7/26 (27%) |
| Deep cerebral veins | 0/26 (0%) |
| Cavernous sinus | 2/26 (8%) |
| Straight sinus | 0/26 (0%) |
| Transverse sinus L | 13/26 (50%) |
| Transverse sinus R | 5/26 (19%) |
| Sigmoid sinus L | 9/26 (34%) |
| Sigmoid sinus R | 3/26 (12%) |
| Internal jugular vein L | 4/26 (15%) |
| Internal jugular vein R | 0/26 (0%) |
| More than one cerebral sinus involved | 12/26 (46%) |
| Mastoiditis in proximity to CVT | 8/26 (31%) |
| Additional neuroimaging findings, n = 26 | |
| Generalized brain edema | 2/26 (8%) |
| Hydrocephalus | 1/26 (4%) |
| Signs of stroke | 12/26 (46%) |
| Signs of recent cerebral infarction | 10/26 (38%) |
| Intracerebral hemorrhage | 2/26 (8%) |
| Subdural effusion | 4/26 (15%) |
| Subdural empyema | 3/26 (12%) |
Figure 2.
Neuroimaging CVT. (a) MRI T2 Flair demonstrates venous infarction with secondary hemorrhage (arrow) of the left frontoparietal lobe in a patient with cortical vein thrombosis, (b) CT venography showing mastoid opacification (arrow) and right-sided transverse sinus thrombosis (arrow), (c) MRI T1 with enhancement shows bilateral pathological enhancement of the cavernous sinus (arrow) in a patient with cavernous sinus thrombosis, (d) MR venography demonstrates left-sided transverse sinus thrombosis (arrow).
Empirical antimicrobial treatment was administered in all CVT patients, mainly consisting of the combination of a third-generation cephalosporine and amoxicillin (20 of 26 (77%)). All CVT patients received adjunctive dexamethasone therapy, of whom 24 of 26 (92%) 10 mg every 6 hours for 4 days. Three of 26 patients (11%) underwent a mastoidectomy and another 3 patients (11%) had a tympanocentesis. One patient underwent a decompressive craniectomy because of cerebral empyema (4%), and one patient received an external ventricular drain to treat hydrocephalus (4%). Anticoagulant therapy (heparin) as a treatment for CVT was given in 11 of 25 (44%) and was withheld in the other 14. In one patient, data on anticoagulant treatment was unknown. None of the patients who started anticoagulant therapy developed intracerebral hemorrhage during admission.
The median time to discharge was 18 day (IQR 12–28) in CVT patients, and 15 days (IQR 11–22) in non-CVT patients. Four of the CVT patients (15%) died and 14 of 26 (54%) had an unfavorable outcome with a Glasgow Outcome Score of 1 – 4. The rate of unfavorable outcome of CVT patients was higher compared to 2165 patients without CVT but this did not reach statistical significance (54% vs. 37%; P = 0.07). Of the surviving CVT patients, 16 of 22 (73%) had neurological sequelae on discharge. Hearing impairment including deafness was seen in 9 patients (41%), cognitive impairment in 6 (27%), hemiparesis in 6 (27%), and aphasia in 4 (18%). Sensory deficit, blindness, and cranial nerve palsy other than VIII were present in one patient each (5%; Table 3).
Table 3.
Treatment, complications, clinical outcome and neurological sequelae.
| Treatment, n = 26 | |
| Anticoagulant therapy | 11/25 (44%) |
| No anticoagulant therapy | 14/25 (56%) |
| Decompressive craniectomy | 1/26 (4%) |
| Mastoidectomy | 3/26 (12%) |
| Tympanocentesis | 3/26 (12%) |
| Complications, n = 26 | |
| Transfer to intensive care unit (ICU) | 12/26 (46%) |
| Mechanical ventilation | 11/26 (42%) |
| Systemic complication | 12/26 (46%) |
| Pneumonia | 4/26 (15%) |
| Renal impairment | 2/26 (8%) |
| Vasculitis | 2/26 (8%) |
| Glasgow Outcome Scale Score n = 26 | |
| 1 death | 4/26 (15%) |
| 2 persistent vegetative state | 0/26 (0%) |
| 3 severe disability | 5/26 (19%) |
| 4 moderate disability | 5/26 (19%) |
| 5 low disability | 12/26 (46%) |
| Neurologic sequelae at discharge, n = 22 | |
| Neurologic sequelae | 16/22 (73%) |
| Hearing impairment including deafness | 9/22 (41%) |
| Cognitive impairment | 6/22 (27%) |
| Hemiparesis | 6/22 (27%) |
| Aphasia | 4/22 (18%) |
| Cranial nerve palsy other than VIII | 1/22 (5%) |
| Blindness | 1/22 (5%) |
| Sensory deficit | 1/22 (5%) |
Discussion
CVT is an uncommon complication of bacterial meningitis occurring in 1% of patients, mainly occurring in patients with coma, ENT infections, and focal neurologic deficits. In patients with unexplained coma or focal neurological signs, neuroimaging should be performed to determine the cause of deterioration and CVT as a complication should be considered.30 Our rate is considerably lower than a previous study (10%).9 This German study included patients with pneumococcal meningitis admitted to a neuro-ICU, a selected and more severely affected population. Furthermore, the German study was performed before the introduction of dexamethasone, which has been shown to reduce neurological complications.31,32
ENT infections were present in the majority of CVT patients. Mastoiditis in proximity to the CVT location (often transverse sinus or sigmoid sinus) was also often seen (31%), making a contiguous spread of the infection probable which is also seen in cases of subdural empyema or brain abscess.33,34 Whether the mastoiditis or the meningitis is the cause of CVT in these cases remains unclear. In our cohort, only 24% of the patients were surgically treated for mastoiditis. There is a lack of uniform guidelines on when and how surgical treatment of the ENT infection should take place in otogenic bacterial meningitis.35–38 Performing surgical treatment should be considered in patients with bacterial meningitis and co-existing mastoiditis complicated by CVT.38,39
Almost half of the patients with CVT in this cohort were started on anticoagulant therapy. In a cohort study, the use of anticoagulants on admission in patients with bacterial meningitis was associated with a higher incidence of intracranial hemorrhages (odds ratio 5.84, 95% confidence interval 2.17–15.76).25 A post hoc analysis of a large international study on CVT found that the risk of new intracranial hemorrhage after diagnosis of CVT was higher in patients with an infection, but there were insufficient data to determine if the use of anticoagulation influenced this risk.40 International guidelines for CVT do not provide a separate recommendation on the use of anticoagulation therapy in septic CVT cases.26,27 In our study none of the patients with CVT in whom anticoagulant therapy was started developed intracranial hemorrhages. Nevertheless, in the absence of proof of harm or benefit of anticoagulant treatment, anticoagulation therapy in patients with bacterial meningitis complicated by CVT remains highly controversial.
This study has several limitations. The first and major limitation is the lack of imaging review of the remaining patients, in which CVT was not specified. This could have led to an underestimation of the CVT cases.
Second, the additional clinical characteristics of CVT patients were not registered directly in the online CRF (time of onset, anticoagulant therapy, clinical changes before the CVT diagnosis) and were assessed retrospectively using the discharge letters. This could have led to a selection bias. Third, as the study was observational no standard neuroimaging was performed in all meningitis patients, which makes the available neuroimaging heterogeneous in nature (MRI, CT, or MR venography) and timing. Fourth, we were not able to retrieve three scans of possible CVT’s due to lack of available data and had to exclude them, which could have led to an underestimation of the incidence of CVT. Furthermore, outcome was scored on the GOS while the modified ranking scale is more common in CVT studies. Finally, we had no access to long term follow up data. Nevertheless, this is the largest collection of bacterial meningitis patients complicated by CVT in adults and therefore provides valuable insight about the incidence, clinical course, outcome, and the treatment of this group.
In conclusion, CVT is a rare complication in bacterial meningitis and is associated with coma, ENT infections, and focal neurologic deficits.
Acknowledgments
We are thankful to all the Dutch physicians and patients who participated in the MeninGene study.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study is supported by the Netherlands Organization for Health Research and Development (ZonMw; NWO-Vici-Grant [91819627] to D.v.d.B., NWO-Vidi-Grant [916.13.078] to M.C.B.), and the European Research Council (European Research Council Starting Grant to D.v.d.B.). The Netherlands Reference Laboratory is funded by the National Institute of Public Health and the Environment.
Informed consent: All subjects or their legal representatives gave written informed consent prior to their inclusion in this study.
Ethical approval: This study has been approved by the medical ethics committee of the Academic Medical Center in the Netherlands and has therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Guarantor: SSD.
Contributorship: SSD made substantial contributions to the conception of the work; analysis and interpretation of data; drafted the work, approved the version to be published, and agrees to be accountable for all aspects of the work. MB, JC, and DvdB made substantial contributions to the design of the work; interpretation of data; revised it critically for important intellectual content; approved the version to be published; and agree to be accountable for all aspects of the work.
ORCID iDs
Shahrzad S Deliran https://orcid.org/0000-0002-6402-6598
Jonathan M Coutinho https://orcid.org/0000-0002-8284-982X
References
- 1.van de Beek D, de Gans J, Spanjaard L, et al. Clinical features and prognostic factors in adults with bacterial meningitis. N Engl J Med 2004; 351: 1849–1859. [DOI] [PubMed] [Google Scholar]
- 2.Bijlsma MW, Brouwer MC, Kasanmoentalib ES, et al. Community-acquired bacterial meningitis in adults in The Netherlands, 2006-14: a prospective cohort study. Lancet Infect Dis 2016; 16: 339–347. [DOI] [PubMed] [Google Scholar]
- 3.van de Beek D, Brouwer M, Hasbun R, et al. Community-acquired bacterial meningitis. Nat Rev Dis Primers 2016; 2: 16074–16011. [DOI] [PubMed] [Google Scholar]
- 4.Kasanmoentalib ES, Brouwer MC, van der Ende A, et al. Hydrocephalus in adults with community-acquired bacterial meningitis. Neurology 2010; 75: 918–923. [DOI] [PubMed] [Google Scholar]
- 5.Zoons E, Weisfelt M, de Gans J, et al. Seizures in adults with bacterial meningitis. Neurology 2008; 70: 2109–2115. [DOI] [PubMed] [Google Scholar]
- 6.Schut ES, Lucas MJ, Brouwer MC, et al. Cerebral infarction in adults with bacterial meningitis. Neurocrit Care 2012; 16: 421–427. [DOI] [PubMed] [Google Scholar]
- 7.Lucas MJ, Brouwer MC, van de Beek D. Neurological sequelae of bacterial meningitis. J Infect 2016; 73: 18–27. [DOI] [PubMed] [Google Scholar]
- 8.van de Beek D, de Gans J, Tunkel AR, et al. Community-acquired bacterial meningitis in adults. N Engl J Med 2006; 354: 44–53. [DOI] [PubMed] [Google Scholar]
- 9.Kastenbauer S, Pfister HW. Pneumococcal meningitis in adults: spectrum of complications and prognostic factors in a series of 87 cases. Brain 2003; 126: 1015–1025. [DOI] [PubMed] [Google Scholar]
- 10.Weisfelt M, van de Beek D, Spanjaard L, et al. Clinical features, complications, and outcome in adults with pneumococcal meningitis: a prospective case series. Lancet Neurol 2006; 5: 123–129. [DOI] [PubMed] [Google Scholar]
- 11.Pfister HW, Borasio GD, Dirnagl U, et al. Cerebrovascular complications of bacterial meningitis in adults. Neurology 1992; 42: 1497–1504. [DOI] [PubMed] [Google Scholar]
- 12.Pfister HW, Feiden W, Einhaupl KM. Spectrum of complications during bacterial meningitis in adults. Results of a prospective clinical study. Arch Neurol 1993; 50: 575–581. [DOI] [PubMed] [Google Scholar]
- 13.Bozzola E, Bozzola M, Colafati GS, et al. Multiple cerebral sinus thromboses complicating meningococcal meningitis: a pediatric case report. BMC Pediatr 2014; 14: 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jorens PG, Parizel PM, Wojciechowski M, et al. Streptococcus pneumoniae meningoencephalitis with unusual and widespread white matter lesions. Eur J Paediatr Neurol 2008; 12: 127–132. [DOI] [PubMed] [Google Scholar]
- 15.Medhi N, Goswami P, Sarma P, et al. Klebsiella meningitis. A case report. Neuroradiol J 2008; 21: 323–326. [DOI] [PubMed] [Google Scholar]
- 16.Selvitop O, Poretti A, Huisman TA, et al. Cerebral sinovenous thrombosis in a child with Crohn's disease, otitis media, and meningitis. Neuroradiol J 2015; 28: 274–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharma KM, Ahn J. Cerebral venous sinus thrombophlebitis as a complication of acute otitis media. J Emerg Med 2015; 48: e9–e13. [DOI] [PubMed] [Google Scholar]
- 18.Wardle M, Mu A, Tong SYC. Streptococcus gallolyticus subsp. pasteurianus meningitis complicated by venous sinus thrombosis: a case report. Int J Infect Dis 2018; 71: 30–32. [DOI] [PubMed] [Google Scholar]
- 19.deVeber G, Andrew M, Adams C, et al.; Canadian Pediatric Ischemic Stroke Study Group. Cerebral sinovenous thrombosis in children. N Engl J Med 2001; 345: 417–423. [DOI] [PubMed] [Google Scholar]
- 20.Engelen-Lee JY, Brouwer MC, Aronica E, et al. Pneumococcal meningitis: clinical-pathological correlations (MeninGene-Path). Acta Neuropathol Commun 2016; 4: >26–03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bushnell C, Saposnik G. Evaluation and management of cerebral venous thrombosis. Continuum (Minneap Minn) 2014; 20: 335–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferro JM, Canhão P, Bousser M-G, et al. Cerebral vein and dural sinus thrombosis in elderly patients. Stroke 2005; 36: 1927–1932. [DOI] [PubMed] [Google Scholar]
- 23.Ferro JM, Canhao P, Stam J, et al.; ISCVT Investigators. Prognosis of cerebral vein and dural sinus thrombosis: results of the international study on cerebral vein and dural sinus thrombosis (ISCVT). Stroke 2004; 35: 664–670. [DOI] [PubMed] [Google Scholar]
- 24.Coutinho J, de Bruijn SF, Deveber G, et al. Anticoagulation for cerebral venous sinus thrombosis. Cochrane Database Syst Rev 2011; 8: CD002005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mook-Kanamori BB, Fritz D, Brouwer MC, et al. Intracerebral hemorrhages in adults with community associated bacterial meningitis in adults: should we reconsider anticoagulant therapy? PLoS One 2012; 7: e45271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saposnik G, Barinagarrementeria F, Brown RD, Jr, et al.; American Heart Association Stroke Council and the Council on Epidemiology and Prevention. Diagnosis and management of cerebral venous thrombosis: a statement for healthcare professionals from the American heart association/American stroke association. Stroke 2011; 42: 1158–1192. [DOI] [PubMed] [Google Scholar]
- 27.Einhaupl K, Stam J, Bousser MG, et al. EFNS guideline on the treatment of cerebral venous and sinus thrombosis in adult patients. Eur J Neurol 2010; 17: 1229–1235. [DOI] [PubMed] [Google Scholar]
- 28.Spanos A Harrell FE JrandDurack DT.. Differential diagnosis of acute meningitis. An analysis of the predictive value of initial observations. Jama 1989; 262: 2700–2707. [PubMed] [Google Scholar]
- 29.Jennett B, Teasdale G, Braakman R, et al. Predicting outcome in individual patients after severe head injury. Lancet 1976; 1: 1031–1034. [DOI] [PubMed] [Google Scholar]
- 30.van de Beek D, Cabellos C, Dzupova O, et al.; ESCMID Study Group for Infections of the Brain (ESGIB). ESCMID guideline: diagnosis and treatment of acute bacterial meningitis. Clin Microbiol Infect 2016; 22 Suppl 3: S37–S62. [DOI] [PubMed] [Google Scholar]
- 31.Brouwer MC, McIntyre P, Prasad K, et al. Corticosteroids for acute bacterial meningitis. Cochrane Database Syst Rev 2015; 9: CD004405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brouwer MC, Heckenberg SG, de Gans J, et al. Nationwide implementation of adjunctive dexamethasone therapy for pneumococcal meningitis. Neurology 2010; 75: 1533–1539. [DOI] [PubMed] [Google Scholar]
- 33.Osman Farah J, Kandasamy J, May P, et al. Subdural empyema secondary to sinus infection in children. Childs Nerv Syst 2009; 25: 199–205. [DOI] [PubMed] [Google Scholar]
- 34.Burton BN, Saliba J, Gabriel RA, et al. Risk factors associated with mortality in patients with otogenic brain abscess. Otol Neurotol 2019; 40: 471–477. [DOI] [PubMed] [Google Scholar]
- 35.Gower D, McGuirt WF. Intracranial complications of acute and chronic infectious ear disease: a problem still with us. Laryngoscope 1983; 93: 1028–1033. [DOI] [PubMed] [Google Scholar]
- 36.Khafif A, Halperin D, Hochman I, et al. Acute mastoiditis: a 10-year review. Am J Otolaryngol 1998; 19: 170–173. [DOI] [PubMed] [Google Scholar]
- 37.Hoppe JE, Koster S, Bootz F, et al. Acute mastoiditis–relevant once again. Infection 1994; 22: 178–182. [DOI] [PubMed] [Google Scholar]
- 38.Zanetti D, Nassif N. Indications for surgery in acute mastoiditis and their complications in children. Int J Pediatr Otorhinolaryngol 2006; 70: 1175–1182. [DOI] [PubMed] [Google Scholar]
- 39.Kaplan DM, Gluck O, Kraus M, et al. Acute bacterial meningitis caused by acute otitis media in adults: a series of 12 patients. Ear Nose Throat J 2017; 96: 20–28. [PubMed] [Google Scholar]
- 40.Zuurbier SM, Coutinho JM, Stam J, et al.; ISCVT Investigators. Clinical outcome of anticoagulant treatment in head or neck infection-associated cerebral venous thrombosis. Stroke 2016; 47: 1271–1277. [DOI] [PubMed] [Google Scholar]