Abstract
Introduction
Non-invasive brain stimulation (NIBS) with speech therapy might improve recovery from post-stroke aphasia. This three-armed sham-controlled blinded prospective proof-of-concept study tested 1 Hz subthreshold repetitive transcranial magnetic stimulation (rTMS) and 2-mA cathodal transcranial direct current stimulation (ctDCS) on the right pars triangularis in subacute post-stroke aphasia.
Patients and methods
Sixty-three patients with left middle cerebral artery infarcts were recruited in five hospitals (Canada/United States/Germany, 01–2014/03–2018) and randomized to receive rTMS (N = 20), ctDCS (N = 24) or sham stimulation (N = 19) with ST for 10 days. Primary outcome variables were Z-score changes in naming, semantic fluency and comprehension tests and adverse event frequency. Secondary outcome variable was the percent change in the Unified Aphasia Score. Intention-to-treat analyses tested between-group effects at days 1 and 30 post-treatment with a pre-planned subgroup analysis for lesion location (affecting Broca’s area or not).
Results
Naming was significantly improved by rTMS (median = 1.91/interquartile range = 0.77/p = .01) at 30 days versus ctDCS (median = 1.11/interquartile range = 1.51) and sham stimulation (median = 1.02/interquartile range = 1.71). All other primary results were non-significant. The rTMS effect was driven by the patient subgroup with intact Broca’s area where NIBS tended to improve UnAS (median = 33.2%/interquartile range = 46.7%/p = .062) versus sham stimulation (median = 12.5%/interquartile range = 7.9%) at day 30. Conversely, in patients with infarcted Broca’s area, UnAS tended to improve more with sham stimulation (median = 75.0%/interquartile range = 86.9%/p = .053) versus NIBS (median = 12.7%/interquartile range = 31.7).
Conclusion: We found a delayed positive effect of low-frequency rTMS targeting the right pars triangularis on the recovery of naming performance in subacute post-stroke aphasia. This intervention may be beneficial only in patients with morphologically intact Broca’s area.
Keywords: Aphasia, stroke, transcranial magnetic stimulation, transcranial direct current stimulation, speech therapy, language therapy, randomized control trial
Introduction
Aphasia affects 15–40% of patients with acute stroke and independently predicts prolonged hospitalization and poor outcome.1 Speech therapy (ST) is currently the only effective aphasia treatment and therefore constitutes the recommended therapeutic approach to aphasia rehabilitation.2 However, the intensity of ST provided in usual care settings is likely insufficient to achieve significant treatment effects on the language deficit beyond spontaneous recovery.1
Non-invasive brain stimulation (NIBS) can modulate the excitability and activity of targeted cortical regions by inducing cortical currents of short duration through rapidly changing magnetic fields, such as repetitive transcranial magnetic stimulation (rTMS), or by applying long-lasting but weak direct currents to the skull, as with transcranial direct current stimulation (tDCS).3 Combined imaging studies with 1-Hz rTMS or cathodal tDCS (ctDCS) suggest an inhibitory effect on cortical recruitment.4,5 These modalities have been used to down-regulate the activity of the non-affected hemisphere during ST to promote restitution of language networks in the affected, usually left hemisphere.6 This intrahemispheric compensation has been associated with better language recovery than recruitment of compensatory networks of the right hemisphere.7,8 A meta-analysis of small randomized controlled trials (RCT) has concluded that low-frequency rTMS, targeting the pars triangularis of the right inferior frontal gyrus (the right homologue to Broca’s area), may have a positive effect on language recovery, including naming, repetition, writing and comprehension.9 Evidence for equivalent ctDCS protocols is weaker. A recent Cochrane review suggests some effect of tDCS on object naming irrespective of the tDCS modality (anodal, cathodal or bihemispheric).10 A comparative meta-analysis found a small-to-medium effect size of tDCS on naming performance, whereas the effect size of rTMS was medium to large.11 Direct comparison of both techniques has not yet been performed but would be clinically relevant, because tDCS is much easier to apply and associated costs are lower.
Based on this evidence, NORTHSTAR (NOn-invasive Repeated THerapeutic STimulation for Aphasia Recovery) was designed12 as the first multilingual blinded and sham-controlled proof-of-concept study to directly compare the effect of 1-Hz rTMS, ctDCS and sham stimulation as add-on therapy for rehabilitation of subacute post-stroke aphasia.
Here, we report the results of the intention-to-treat analysis on primary and secondary outcomes and of a planned subgroup analysis for lesion location. It was hypothesized that 10 treatment sessions of NIBS in combination with ST would improve naming performance, verbal fluency and comprehension more than sham stimulation on days 1 and 30 after last treatment, with no difference in adverse events (AE) between groups (primary hypotheses). It was further hypothesized that a similar NIBS effect would be observed in global language function. The treatment effect on verbal fluency and naming would be larger in subjects with stroke in the anterior MCA territory (affecting Broca’s area), whereas the effect on recovery in language comprehension would be larger in subjects with posterior MCA territory infarct in whom Broca’s area is spared (secondary hypotheses).12
Methods
Trial design
In this three-armed sham-controlled blinded prospective proof-of-concept study, patients were randomized to sham, rTMS or ctDCS treatment (allocation ratio 1:1:1). rTMS-allocated patients received sham ctDCS; ctDCS patients received sham rTMS; patients in the sham group received both sham stimulations. For 10 sessions over two weeks, patients received 45 min of individualized ST by a certified therapist according to best-practice guidelines.2 ST started immediately following real/sham rTMS and during real/sham ctDCS. Outcome measures were assessed 1 and 30 days following the last therapy session.
Study settings
The study took place from January 2014 to March 2018 in Canada at the Jewish General Hospital (JGH, Montreal), CHUM-Hôpital Notre-Dame (Montreal) and Toronto Rehabilitation Hospital (Toronto); in the United States at Burke Rehabilitation Hospital (White Plains) and in Germany at the Rehabilitation Hospital RehaNova (Cologne). The trial protocol was approved by the Research Ethics Boards both at the central coordinating center (JGH) and each participating site.
Patient population
Eligible participants were right-handed adults aged 18–90 years, with English, French or German as language of daily use. Patients were recruited 5–45 days after left middle cerebral artery ischemic stroke. Exclusion criteria, such as prior stroke and contraindication for electrical or magnetic stimulation, are listed in Thiel et al.12 Recruitment age-range and time-window were extended from the initial protocol (age 50–85 and 5–30 days post-stroke) 14 months after study start to accelerate recruitment.
Intervention
rTMS was applied over the non-affected right hemisphere (pars triangularis of the right inferior frontal gyrus) using a figure-of-eight coil at 1 Hz for 900 pulses (15 min) at 90% resting motor threshold (RMT). RMT was determined prior to each treatment session over the right primary motor area.13 ST sessions were given immediately following the rTMS procedure to ensure treatment within the period of maximum rTMS after-effect (about 45 min).14 For sham-stimulation, the coil was placed over the inter-hemispheric fissure at the vertex, and stimulation was applied with 10% RMT.
ctDCS was applied with 5-cm2 sponge electrodes placed over the target area (cathode) and the forehead over the right eye (anode). Stimulation with a 2-mA current started immediately before and lasted throughout the ST session. For sham stimulation, the current was turned on for 30 s to elicit a typical skin sensation and then turned off (see Supplementary material for details).
The stimulation target (right pars triangularis) was localized using the patient’s T1-weighted MRI or CT scan and transferred to the patient’s head using the TMS device neuronavigation system or a modification of the surface distance measurements method.15
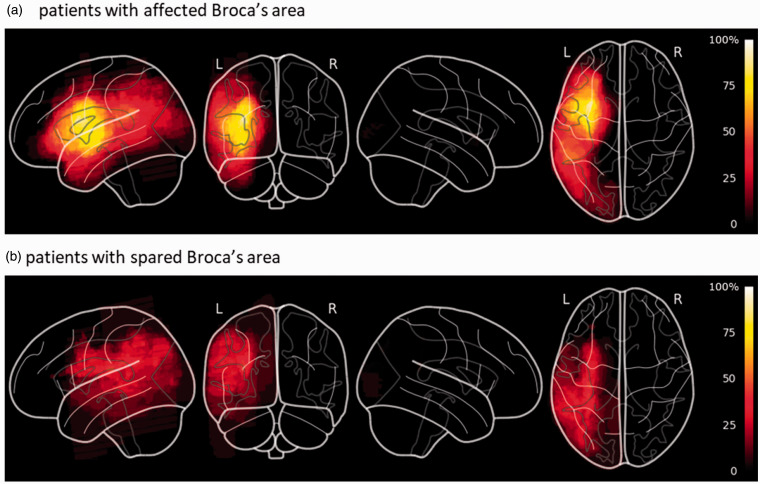
Two raters (AT and AZ) independently stratified patient’s scans by infarct location prior to analysis and unblinding (Figure 3). “Extended” Broca’s area included the inferior or middle frontal gyri, anterior insula, premotor cortex and underlying white matter, and basal ganglia.16 For divergent classification, consensus was reached by joint review.
Figure 3.
Lesion overlap maps spatially normalized to MNI-stereotaxic space for subgroups of patients with affected (Panel A) or non-affected (Panel B) Broca’s area. Color scale represents the percentage of patients who had infarcted tissue at that voxel.
Randomization and blinding
Computer-generated, non-restricted randomization by site was performed through an online system located at the Department of Clinical Epidemiology at the JGH (Montreal). Data and eligibility were entered by each study site coordinator into a web-based data capturing system. Only the technician performing the stimulation had access to the randomization information when logging into the study platform, on the first day of treatment. Patients, therapist, principal investigators and research personnel assessing clinical outcomes were blinded to the treatment assignment. Therapists did not attend rTMS sessions and had no access to the tDCS device settings.
Primary outcome measures and variables
Primary outcome measures were core language functions measured by tests commonly used in English, French and German and are comparable across languages. These were lexical access (Boston Naming Test, BNT17 and SF1min, a widely used semantic verbal fluency task18–20) and simple to complex sentence comprehension (36-item Token Test, TT21). Treatment effect was the difference in standardized Z-scores at days 1 and 30 post-treatment, relative to baseline for each primary outcome.
Secondary outcome measures and variable
Secondary outcome measures were integral measures of aphasic impairment. In the absence of a single test for aphasic impairment normalized in all three languages, we used approved language specific batteries: Aachener Aphasie Test in German,22 the Protocole Montréal-Toulouse-86 in French23 and the Western Aphasia Battery in English.24 We derived a standardized T-score, the Unified Aphasia Score (UnAS) based on the normative data available for each battery (Supplementary material).
The secondary outcome variable was the percent difference of UnAS at days 1 and 30 post-treatment, relative to baseline.
AE and serious adverse events (SAE) outcomes and variables
AE during or ≤1 h after session (headache, scalp dysesthesia/paresthesia at stimulation site, muscle pain of temporal or neck muscles) and SAE during or following session (seizures) were documented after each therapy session. Cumulative AE and SAE during 10 days of therapy are reported.
Sample size and statistical analysis
We planned to recruit 33 patients in each group (rTMS, tDCS and Sham), but the study was terminated after enrolment of 63 subjects because lower than expected recruitment rates would have resulted in further study extension. Detail of the sample size determination is available in Thiel et al.12
In this intention-to-treat analysis, missing data were replaced by the mean of each corresponding randomization group. Data were checked for outliers. Only data entry errors were corrected.
Baseline characteristics were normally distributed and were thus compared across groups with ANOVAs. Due to non-normality and multiple extreme outliers in the primary and secondary outcome variables, the conservative Mood’s median test for independent samples (two-tailed) was used, and medians (Mdn) and interquartile ranges (IQRs) as well as effect sizes (ϕ) are reported. The significance threshold was p < 0.05, and the Bonferroni correction was applied for post-hoc pairwise median tests. Analyses were performed on SPSS-24.0 (IBM Corp.).
Results
Participant flow
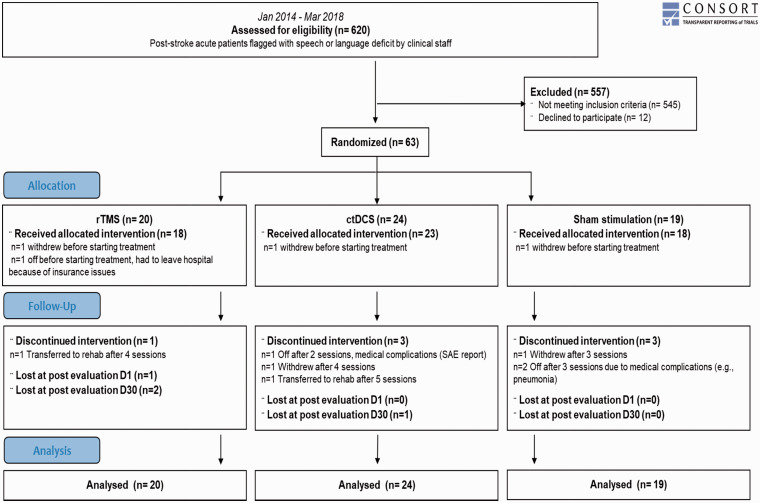
A total of 620 stroke patients presenting with speech or language problems were screened. Out of 75 eligible patients, 12 declined to participate. The remaining 63 patients were randomized in the three study arms. Figure 1 displays the participant flow.
Figure 1.
Participant flow.
Baseline characteristics
Baseline characteristics were similar across intervention groups (Table 1). In the subgroup of patients with affected Broca’s area, baseline UnAS was significantly higher in rTMS-allocated (vs. sham) patients (Supplemental Table 1).
Table 1.
Demographic and baseline data per group.
| rTMS | ctDCS | Sham | Between-group ANOVAs p-value | |
|---|---|---|---|---|
| N | 20 | 24 | 19 | |
| Male; female | 10; 10 | 14; 10 | 12; 7 | |
| English; French; German language | 6; 6; 8 | 12; 3; 9 | 7; 5; 7 | |
| Lesion in Broca’s complex, N (% participants) | 8 (40%) | 11 (46%) | 9 (47%) | |
| Age, mean (SD) | 66.7 (9.8) | 65.3 (13.2) | 67.4 (11.7) | .844 |
| Days post-stroke at recruitment, mean (SD) | 21.2 (13.2) | 20.4 (14.7) | 15.9 (11.2) | .405 |
| Naming Z-score, mean (SD) | –6.00 (3.38) | –7.01 (5.16) | –7.36 (4.97) | .634 |
| Semantic fluency Z-score, mean (SD) | –2.73 (1.04) | –2.98 (1.01) | –2.65 (1.13) | .560 |
| Comprehension Z-score, mean (SD) | –7.11 (5.20) | –9.55 (4.76) | –9.48 (5.25) | .218 |
| Unified Aphasia Score, mean (SD) | 46.9 (22.4) | 45.2 (22.6) | 38.5 (26.7) | .514 |
Primary outcomes
Any real versus sham stimulation
Comparing sham to any real stimulation across all patients showed no significant difference in language recovery at day 1 or day 30 post-intervention (Supplemental Table 2).
rTMS versus ctDCS versus Sham
There was no significant difference in language recovery at day 1 post-treatment between the intervention groups. At day 30, however, there was a significant between-group difference in naming (p = .01) and comprehension recovery (p = .03) (Table 2). Post-hoc tests revealed significantly greater change in BNT Z-score for rTMS compared to sham stimulation (χ2(1) = 5.867; p = .046; ϕ = 0.39, medium-large effect), and ctDCS (χ2(1) = 9.167; p = .007; ϕ = 0.46, medium-large effect). The ctDCS group improved significantly more than the rTMS group on the TT (χ2(1) = 5.867; p = .046; ϕ = 0.37, medium-large effect). There was also a medium-to-large effect of ctDCS over sham for TT improvement, but this was not significant after Bonferroni correction (p = .054).
Table 2.
rTMS versus ctDCS versus sham – change in primary outcomes relative to baseline at post-treatment (day 1) and at one-month follow-up (day 30).
| Z-score change | rTMS | ctDCS | Sham | p-Value | Post-hoc tests |
|---|---|---|---|---|---|
| Naming (BNT) | |||||
| Day 1 | 0.90 (1.19) | 1.02 (1.18) | 0.73 (0.94) | .131 | |
| Day 30 | 1.91 (0.77) | 1.11 (1.51) | 1.02 (1.71) | .010 | rTMS > ctDCS (p = .007)rTMS > sham (p = .046) |
| Verbal fluency (SF) | |||||
| Day 1 | 0.00 (0.46) | 0.05 (0.29) | 0.00 (0.20) | .366 | |
| Day 30 | 0.48 (0.75) | 0.38 (0.75) | 0.73 (1.14) | .107 | |
| Comprehension (TT) | |||||
| Day 1 | 0.87 (1.99) | 1.05 (1.58) | 1.12 (1.87) | .580 | |
| Day 30 | 1.54 (2.22) | 2.58 (2.86) | 2.07 (2.57) | .033 | ctDCS > rTMS (p = .046) |
Medians (and interquartile ranges) are displayed for each intervention condition as well as p-values of median tests. Significance level =.05. Post-hoc tests p-values are adjusted with the Bonferroni correction.
BNT: Boston naming test; SF: semantic fluency, TT: token test.
Planned subgroup analysis by lesion location
We found a significant intervention effect on naming 30 days post-treatment only in patients with intact Broca’s area (rTMS: N = 8; Mdn = +1.95, IQR = 0.33; ctDCS: N = 12, Mdn = +1.33, IQR = 1.42; sham: N = 10, Mdn = +0.86, IQR = 1.95; p = .01; Supplemental Figures 1 and 2). Pairwise post-hoc median tests were not significant.
Effect of covariates
There was no significant correlation between change in naming or comprehension and baseline performance (Supplemental Table 3), but there was a significant correlation between recruitment time post-stroke and comprehension change. Considering recruitment time in a non-parametric analysis of covariance (Quade’s test), the ctDCS effect on comprehension became a trend (p = .086). The rTMS effect was unchanged.
Secondary outcome
Any real versus sham stimulation
The percent change on the UnAS was similar across sham and real stimulation groups at day 1 and day 30 post-intervention.
rTMS versus ctDCS versus sham
There was no significant difference between the three study groups in the UnAS percent change at day 1 or 30 post-intervention.
Planned subgroup analysis by lesion location
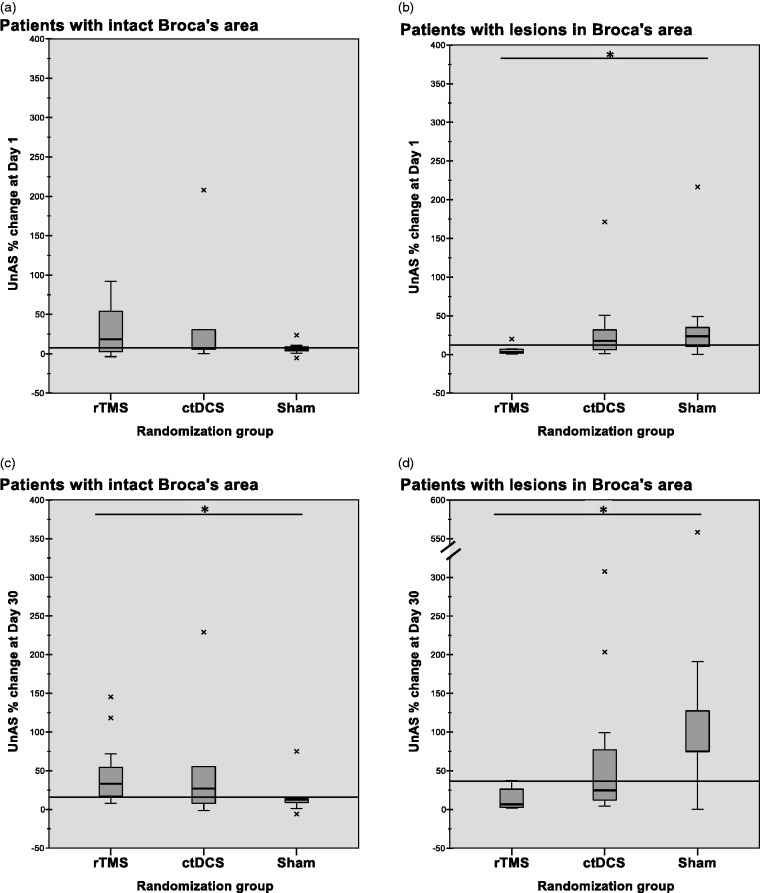
There was a significant and large effect on the percent change in the UnAS at day 30 in favour of any real stimulation for patients with spared Broca’s complex (real: N = 25, Mdn = +33.2%, IQR = 46.7%; sham: N = 10, Mdn = +12.5%, IQR = 7.9%; χ2(1) = 8.338, p = .01, ϕ = 0.488). Conversely, a significant and large effect in favour of sham stimulation was found in patients where Broca’s complex was affected (real: N = 19, Mdn = +12.7%, IQR = 31.7; sham: N = 9, Mdn = +75.0%, IQR = 86.9%; χ2(1)=8.023, p = .01, ϕ = 0.535).
Comparing the three study groups at day 30, there was a significant and very large superiority effect of rTMS over sham stimulation in patients with spared Broca’s complex and the reverse pattern in patients with affected Broca’s complex. The latter negative effect was also significant at day 1 post-treatment (Figure 2).
Figure 2.
Unified Aphasia Score (UnAS) percent change: Panel A: rTMS (Mdn = 18.3, IQR = 53.5), ctDCS (Mdn = 7.4, IQR = 25.8), sham (Mdn = 5.6, IQR = 6.5), p = .789; Panel B: rTMS (Mdn = 3.0, IQR = 5.9), ctDCS (Mdn = 17.5, IQR = 27.4), sham (Mdn = 23.6, IQR = 36.7), p = .042; Post-hoc tests show sham > rTMS (χ2(1) = 7.244; p = .021; ϕ = 0.65, large effect); Panel C: rTMS (Mdn = 33.2, IQR = 46.8), ctDCS (Mdn = 26.8, IQR = 47.5), sham (Mdn = 12.5, IQR = 7.9), p = .009; post-hoc tests show rTMS > sham (χ2(1) = 11.733; p = .002; ϕ = 0.73, very large effect); Panel D: rTMS (Mdn = 6.7, IQR = 27.5), ctDCS (Mdn = 24.7, IQR = 87.1), sham (Mdn = 75.0, IQR = 86.9), p = .007; post-hoc tests show sham > rTMS (χ2(1) = 13.442; p = .001; ϕ = 0.89, very large effect).
Effect of covariates
There was a significant correlation between the change in UnAS and baseline score as well as recruitment time (Supplementary Table 3). Subgroup results with both covariates (Quade’s test) became trends (p = .062 in Broca’s-spared groups and p = .053 in Broca’s-affected groups).
Safety
AEs were rare and their cumulative numbers did not differ between groups. We reported one SAE for one ctDCS patient, which was not thought to have been related to the study intervention. (For individual AE and SAE, see Supplementary material.)
Discussion
This multicentre, multilingual RCT was designed to test the additional effect of low-frequency rTMS or ctDCS targeting the right pars triangularis when combined with ST in patients with subacute post-stroke aphasia. There are three key results: (1) the differential efficacy of rTMS and ctDCS for naming recovery, a core language function, (2) a delayed treatment effect in the absence of an immediate effect and (3) a possible impact of lesion location on treatment response.
Stimulation modality-specific effects
The superiority of low-frequency rTMS on naming recovery versus sham stimulation confirms previous results of several smaller studies.9 We did not find such an effect with ctDCS. A recent meta-analysis reported greater effect size with rTMS than tDCS on naming abilities in chronic aphasia.11 Our direct comparison also suggests that low-frequency rTMS is superior to ctDCS over the right pars triangularis for naming improvement in sub-acute aphasia. Other tDCS setups, however, might be more efficient for improving naming nouns.10
Although our data showed a trend towards a beneficial effect of ctDCS compared to rTMS on a comprehension test (TT), we did not find a significant difference between ctDCS and sham. In one RCT, post-stroke comprehension in the subacute stage was improved with ctDCS versus sham over the right Wernicke’s area.25 With electrode placement over the right pars triangularis, it is possible – given the relatively large stimulation area with tDCS – that the superior temporal gyrus may have been stimulated in some, but not all patients resulting in improved comprehension in a subset of participants. The more focal stimulation with rTMS may not have reached those regions.
There was no significant effect on semantic verbal fluency. The change in Z-score was surprisingly low (0.00–0.73) compared to the other primary outcomes (BNT: 0.73–1.91; TT: 0.87–2.58). Administration of the SF1min test is easy, and this is one reason why it has been widely used in the literature. However, there is a growing uncertainty about the construct validity of this language test.26 Future studies for post-stroke language recovery may need to include other measures of verbal fluency which are less affected by non-verbal aspects of the task.
Delayed treatment effect
Significant results emerged 30 days after the intervention. This delay might result from the dynamics underlying treatment-related neuroplasticity. NIBS may initially cause a small facilitating effect which, however, may trigger the development of an alternative pathway. With subsequent preferential use of these new pathways, the performance on the behavioural level also improves. The idea of a recovery trigger is consistent with tendencies in meta-analyses. In a recent review,10 tDCS had a small-to-medium effect on naming nouns just after the intervention (11 studies; SMD = 0.42; 95% CI = 0.19–0.66) but the effect doubled in size six months later (two studies; SMD = 0.87; 95% CI = 0.25–1.48). The effect size computed from eight rTMS studies with follow-up measures also tends to increase from post-treatment (SMD = 0.66; 95% CI = 0.37, 0.95) to follow-up (SMD = 0.71; 95% CI = 0.43–1.00).11 Delayed effects may be even more important than immediate effects in clinical settings, meaning that a limited course of NIBS in combination with ST could still improve the effectiveness of subsequent ST sessions. Future NIBS studies should thus be designed to specifically investigate longer follow-up periods (6–12 months) and sequential outcome assessments to better characterize this effect.
Lesion-dependent treatment effect
One important finding in our study is a possible lesion location-specific effect on language recovery. Compared to sham, inhibitory NIBS, especially low-frequency rTMS, over the right homologue of Broca’s area significantly improved language abilities in the subgroup of patients with intact Broca’s area, but not in patients with damage in this region. In fact, these patients improved more on the UnAS if they did not receive real stimulation.
Following the hierarchical model of brain post-stroke compensation strategies,27,28 efficient recovery is usually only achieved if a sufficient amount of left-hemispheric language networks is left intact. The rationale for using inhibitory NIBS is to facilitate the recruitment of such spared networks by suppressing right-hemispheric activity.29 It has recently been shown with functional imaging that right-sided low-frequency rTMS does indeed restitute left hemisphere networks.5 In this trial, no functional imaging was performed, but the exclusion criteria were chosen in a way that patients with very severe aphasia (who most likely exclusively depend on the right hemisphere) were excluded from the study. In none of the patients, Broca’s complex was completely destroyed, and all patients were able to perform the language tasks at baseline. This finding points towards the necessity of personalized NIBS protocols for aphasia in subacute stroke. Further studies are needed to identify which imaging criteria or which combination of behavioral and imaging criteria should be used to cost-efficiently identify candidates for NIBS.
The possible negative effect of rTMS on patients with affected Broca’s complex was only observed for global language impairment as measured with the UnAS but not for specific core language functions as primary outcome measures. The effect may thus have also been caused by non-language effects (e.g. attention, executive function, mood) of the intervention. While the beneficial effect of rTMS in the Broca’s-spared groups emerged only after 30 days, there was a significant, negative effect in the Broca’s-affected group already one day post-treatment. Given the influence of treatment onset time on the observed change in UnAS, with the Broca’s-affected patients in the sham group having been recruited eight days earlier, a larger spontaneous recovery in these patients may have contributed to this seemingly deleterious effect (Supplementary Tables 1 and 3).
Clinical relevance
A crucial concern for the applicability of new therapeutic strategies is the magnitude of the effect of the new treatment compared to placebo. Our results show a medium-to-large effect of rTMS, which almost doubles naming recovery compared to ST alone. This effect size is in line with the conclusions of recent meta-analyses and supports the potential clinical relevance of rTMS.
Even more important for clinical practice are the individual treatment effects. We found very large but opposite effects of rTMS on aphasia recovery (UnAS) depending on the lesion location. It suggests that (1) rTMS can make a clinically significant difference and (2) it may be necessary to tailor stimulation protocols to avoid counterproductive results. In contrast, the clinical relevance of ctDCS is not yet clear and needs further research.
Challenges of multilingual aphasia trials, safety and consequences for future trial design
This proof-of-concept trial has some limitations. First, we did not achieve our recruitment goal of 99 patients with five recruiting centers within the study funding period. This highlights the need for future trials to include more study sites to achieve larger numbers for possible phase-III RCTs. While the inclusion of patients with different languages may have introduced additional variability, it is important for future trials to demonstrate effects independent of the patient’s language. In view of larger international trials or even national trials in countries with multiple official languages, our trial offers strategies for how to address specific problems of multilingual aphasia trials but also highlights the need for better assessment tools that are standardized and validated across different languages. Last, this study again confirms that NIBS is a safe procedure if performed in accordance with present guidelines. Absolute numbers of AE/SAE were very few, and no difference in the frequency of AE/SAE was found between treatment groups.
Conclusion
Contralesional NIBS is a safe add-on therapy for post-stroke aphasia. Low-frequency rTMS improved naming recovery one month after a 10-day treatment course. ctDCS effect was not significantly different from sham stimulation. Our results raise the possibility that inhibitory NIBS over the right pars triangularis may have negative effects in patients where Broca’s Area is affected, supporting the view that NIBS presently should not be applied outside clinical trials. Future trials should specifically investigate individual factors for patient stratification (e.g. lesion location) and include longer-term follow-up outcome measures (>6 months).
NORTHSTAR study group
Alexander Thiel, Anna Zumbansen, Sharon Shapiro, Latifa Lazzouni, Stephanie Houston, Mica Vincent, Dominique Gillis, Mélissa Bouchard and Caroline Paquette (Jewish General Hospital, Montreal)
Alexander Hartmann, Ilona Rubi-Fessen, Heike Kneifel and Thomas Rommel (RehaNova, Cologne)
Wolf-Dieter Heiss (Max Planck Institute für Stoffwechsel Forschung – MPI for Metabolism Research & Universität zu Köln, Cologne)
Josef Kessler (Universität zu Köln, Cologne)
Dylan Edwards, Jennie Valles, and Susan Wortman-Jutt (Burke Neurological Institute, White Plains)
Sylvain Lanthier, Marlène Lapierre, Diana Mina, Liliana Jastrzabek, Paul Lespérance, and Walid El-Abyad (CHUM, Montreal)
Elizabeth Rochon, Laura Laird, Fiona Höbler, Lisa McQueen, Ruth Tannenbaum, Joanna Wong, Amy Lewis and Alyssa Bobkin (Toronto Rehabilitation Institute, Toronto)
Sandra Black, George Mochizuki, Joyce Chen, Valerie Closson, Andrew Centen and Tyler Saumur (Sunnybrook Hospital, Toronto)
Supplemental Material
Supplemental material, sj-pdf-1-eso-10.1177_2396987320934935 for Non-invasive brain stimulation as add-on therapy for subacute post-stroke aphasia: a randomized trial (NORTHSTAR) by Anna Zumbansen, Sandra E Black, Joyce L Chen, Dylan J Edwards, Alexander Hartmann, Wolf-Dieter Heiss, Sylvain Lanthier, Paul Lesperance, George Mochizuki, Caroline Paquette, Elizabeth A Rochon, Ilona Rubi-Fessen, Jennie Valles, Heike Kneifel, Susan Wortman-Jutt, Alexander Thiel and on behalf of the NORTHSTAR-study group in European Stroke Journal
Appendix 1. CONSORT checklist
| Section/Topic | Item No | Checklist item | Reported on page No |
|---|---|---|---|
| Title and abstract | |||
| 1a | Identification as a randomised trial in the title | Title | |
| 1b | Structured summary of trial design, methods, results, and conclusions | 1 | |
| Introduction | |||
| Background and objectives | 2a | Scientific background and explanation of rationale | 2 |
| 2b | Specific objectives or hypotheses | 2 | |
| Methods | |||
| Trial design | 3a | Description of trial design (such as parallel, factorial) including allocation ratio | 2 |
| 3b | Important changes to methods after trial commencement (such as eligibility criteria), with reasons | 3 & 4 | |
| Participants | 4a | Eligibility criteria for participants | 3 |
| 4b | Settings and locations where the data were collected | 2--3 | |
| Interventions | 5 | The interventions for each group with sufficient details to allow replication, including how and when they were actually administered | 3 |
| Outcomes | 6a | Completely defined pre-specified primary and secondary outcome measures, including how and when they were assessed | 3 |
| 6b | Any changes to trial outcomes after the trial commenced, with reasons | None | |
| Sample size | 7a | How sample size was determined | 4 |
| 7b | When applicable, explanation of any interim analyses and stopping guidelines | Not applicable | |
| Randomisation: | |||
| Sequence generation | 8a | Method used to generate the random allocation sequence | 3 |
| 8b | Type of randomisation; details of any restriction (such as blocking and block size) | 3 | |
| Allocation concealment mechanism | 9 | Mechanism used to implement the random allocation sequence (such as sequentially numbered containers), describing any steps taken to conceal the sequence until interventions were assigned | 3 |
| Implementation | 10 | Who generated the random allocation sequence, who enrolled participants, and who assigned participants to interventions | 3 |
| Blinding | 11a | If done, who was blinded after assignment to interventions (for example, participants, care providers, those assessing outcomes) and how | 3 |
| 11b | If relevant, description of the similarity of interventions | 3 | |
| Statistical methods | 12a | Statistical methods used to compare groups for primary and secondary outcomes | 4 |
| 12b | Methods for additional analyses, such as subgroup analyses and adjusted analyses | 3 & 4 | |
| Results | |||
| Participant flow (a diagram is strongly recommended) | 13a | For each group, the numbers of participants who were randomly assigned, received intended treatment, and were analysed for the primary outcome | 4 & Figure 1 (flow diagram) |
| 13b | For each group, losses and exclusions after randomisation, together with reasons | Figure 1 (flow diagram) | |
| Recruitment | 14a | Dates defining the periods of recruitment and follow-up | Figure 1 (flow diagram) |
| 14b | Why the trial ended or was stopped | 4 & 9 | |
| Baseline data | 15 | A table showing baseline demographic and clinical characteristics for each group | Table 1 |
| Numbers analysed | 16 | For each group, number of participants (denominator) included in each analysis and whether the analysis was by original assigned groups | 4 & Figure 1 (flow diagram) |
| Outcomes and estimation | 17a | For each primary and secondary outcome, results for each group, and the estimated effect size and its precision (such as 95% confidence interval) | 4–6 |
| 17b | For binary outcomes, presentation of both absolute and relative effect sizes is recommended | Not applicable | |
| Ancillary analyses | 18 | Results of any other analyses performed, including subgroup analyses and adjusted analyses, distinguishing pre-specified from exploratory | 4 & 6 |
| Harms | 19 | All important harms or unintended effects in each group | 6 |
| Discussion | |||
| Limitations | 20 | Trial limitations, addressing sources of potential bias, imprecision, and, if relevant, multiplicity of analyses | 9 |
| Generalisability | 21 | Generalisability (external validity, applicability) of the trial findings | 9 |
| Interpretation | 22 | Interpretation consistent with results, balancing benefits and harms, and considering other relevant evidence | 6 & 8--9 |
| Other information | |||
| Registration | 23 | Registration number and name of trial registry | 9 |
| Protocol | 24 | Where the full trial protocol can be accessed, if available | 4 & 9 |
| Funding | 25 | Sources of funding and other support (such as supply of drugs), role of funders | 9 |
Footnotes
Trial registration: The NORTHSTAR trial was preregistered at the US National Institutes of Health (ClinicalTrials.gov) #NCT02020421.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This trial was supported by research grants from the Canadian Institutes for Health Research (CIHR, MOP#125954), W.-D. Heiss Foundation, and the Lady Davis Institute at the JGH (CLIPP#2014). AZ was funded by a CIHR postdoctoral fellowship.
Ethical approval: The trial protocol was approved by the Research Ethics Boards both at the central coordinating center (JGH) and at each participating site. Institutional Review Board of the Faculty of Medicine, McGill University (study approval ID A05-M47-13A).
Informed consent: Patients and their relatives received written and conversational information about the study. Supplementary pictographic material was used if needed. Procedures were in accordance with institutional guidelines. Written informed consent was obtained from all subjects before the study.
Guarantor: AT
Contributorship: AZ, SB, JC, AH, WDH, SL, GM, CP, ER, and AT conceived the study. All authors were involved in the design of the study, the recruitment of participants and the data acquisition. AZ and AT analyzed the data and drafted the manuscript. All authors made critical revision of the manuscript.
Supplemental Material: Supplemental material for this article is available online.
References
- 1.Code C, Petheram B. Delivering for aphasia. Int J Speech Lang Pathol. 2011; 13: 3–10. [DOI] [PubMed] [Google Scholar]
- 2.Hebert D, Lindsay MP, McIntyre A, et al. Canadian stroke best practice recommendations: stroke rehabilitation practice guidelines, update 2015. Int J Stroke 2016; 11: 459–484. [DOI] [PubMed] [Google Scholar]
- 3.Gandiga PC, Hummel FC, Cohen LG. Transcranial dc stimulation (TDCS): a tool for double-blind sham-controlled clinical studies in brain stimulation. Clin Neurophysiol 2006; 117: 845–850. [DOI] [PubMed] [Google Scholar]
- 4.Paquette C, Sidel M, Radinska BA, et al. Bilateral transcranial direct current stimulation modulates activation-induced regional blood flow changes during voluntary movement. J Cereb Blood Flow Metab 2011; 31: 2086–2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thiel A, Hartmann A, Rubi-Fessen I, et al. Effects of noninvasive brain stimulation on language networks and recovery in early poststroke aphasia. Stroke 2013; 44: 2240–2246. [DOI] [PubMed] [Google Scholar]
- 6.Zumbansen A, Thiel A. Recent advances in the treatment of post-stroke aphasia. Neural Regen Res 2014; 9: 703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heiss WD, Kessler J, Thiel A, et al. Differential capacity of left and right hemispheric areas for compensation of poststroke aphasia. Ann Neurol 1999; 45: 430–438. [DOI] [PubMed] [Google Scholar]
- 8.Rosen H, Petersen S, Linenweber M, et al. Neural correlates of recovery from aphasia after damage to left inferior frontal cortex. Neurology 2000; 55: 1883–1894. [DOI] [PubMed] [Google Scholar]
- 9.Ren C-L, Zhang G-F, Xia N, et al. Effect of low-frequency RTMS on aphasia in stroke patients: a meta-analysis of randomized controlled trials. PloS One 2014; 9: e102557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elsner B, Kugler J, Pohl M, et al. Transcranial direct current stimulation (TDCS) for improving aphasia in adults with aphasia after stroke. Cochrane Datab Syst Rev 5(5): CD009760. doi: 10.1002/14651858.CD009760.pub4. [DOI] [PMC free article] [PubMed]
- 11.Bucur M, Papagno C. Are transcranial brain stimulation effects long-lasting in post-stroke aphasia? A comparative systematic review and meta-analysis on naming performance. Neurosci Biobehav Rev 2019; 102: 264–289. [DOI] [PubMed] [Google Scholar]
- 12.Thiel A, Black SE, Rochon EA, et al. Non-invasive repeated therapeutic stimulation for aphasia recovery: a multilingual, multicenter aphasia trial. J Stroke Cerebrovasc Dis 2015; 24: 751–758. [DOI] [PubMed] [Google Scholar]
- 13.Mishory A, Molnar C, Koola J, et al. The maximum-likelihood strategy for determining transcranial magnetic stimulation motor threshold, using parameter estimation by sequential testing is faster than conventional methods with similar precision. J Ect 2004; 20: 160–165. [DOI] [PubMed] [Google Scholar]
- 14.Avanzino L, Bove M, Trompetto C, et al. 1‐Hz repetitive TMS over ipsilateral motor cortex influences the performance of sequential finger movements of different complexity. Eur J Neurosci 2008; 27: 1285–1291. [DOI] [PubMed] [Google Scholar]
- 15.Weiduschat N, Habedank B, Lampe B, et al. Localizing Broca’s area for transcranial magnetic stimulation: comparison of surface distance measurements and stereotaxic positioning. Brain Stimul 2009; 2: 93–102. [DOI] [PubMed] [Google Scholar]
- 16.Aboitiz F, Garcıa R. The evolutionary origin of the language areas in the human brain. A neuroanatomical perspective. Brain Res Rev 1997; 25: 381–396. [DOI] [PubMed] [Google Scholar]
- 17.Tombaugh TN, Hubiey AM. The 60-item Boston naming test: norms for cognitively intact adults aged 25 to 88 years. J Clin Exp Neuropsychol 1997; 19: 922–932. [DOI] [PubMed] [Google Scholar]
- 18.Tombaugh TN, Kozak J, Rees L. Normative data stratified by age and education for two measures of verbal fluency: F.A.S. and animal naming. Arch Clin Neuropsychol 1999; 14: 167–177. [PubMed] [Google Scholar]
- 19.Cardebat D, Doyon B, Puel M, et al. Performance and dynamics of production as a function of sex, age and educational level. Acta Neurol Belg 1989; 90: 207–217. [PubMed] [Google Scholar]
- 20.Aschenbrenner S, Tucha O, Lange KW. Regensburger wortflüssigkeits-test. Hogrefe: Verlag für Psychologie, 2000.
- 21.De Renzi E, Faglioni P. Normative data and screening power of a shortened version of the token test. Cortex 1978; 14: 41–49. [DOI] [PubMed] [Google Scholar]
- 22.Huber W, Poeck K, Weniger D, et al. Der aachner aphasie test. Göttingen, Germany: Hogrefe, 1983.
- 23.Nespoulous J, Lecours A, Lafond D, et al. Protocole montréal-toulouse d’examen linguistique de l’aphasie [Montreal-Toulouse protocol of aphasia linguistic examination]. Isbergues, France: L’Ortho-Edition, 1992.
- 24.Kertesz A. Western aphasia battery – revised. Austin, TX: Pro-Ed, 2006. [Google Scholar]
- 25.You DS, Kim D-Y, Chun MH, et al. Cathodal transcranial direct current stimulation of the right Wernicke’s area improves comprehension in subacute stroke patients. Brain Lang 2011; 119: 1–5. [DOI] [PubMed] [Google Scholar]
- 26.Shao Z, Janse E, Visser K, et al. What do verbal fluency tasks measure? Predictors of verbal fluency performance in older adults. Front Psychol 2014; 5: 772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heiss W-D, Thiel A. A proposed regional hierarchy in recovery of post-stroke aphasia. Brain Lang 2006; 98: 118–123. [DOI] [PubMed] [Google Scholar]
- 28.Anglade C, Thiel A, Ansaldo AI. The complementary role of the cerebral hemispheres in recovery from aphasia after stroke: a critical review of literature. Brain Inj 2014; 28: 138–145. [DOI] [PubMed] [Google Scholar]
- 29.Thiel A, Zumbansen A. The pathophysiology of post-stroke aphasia: a network approach. Restor Neurol Neurosci 2016; 34: 507–518. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-eso-10.1177_2396987320934935 for Non-invasive brain stimulation as add-on therapy for subacute post-stroke aphasia: a randomized trial (NORTHSTAR) by Anna Zumbansen, Sandra E Black, Joyce L Chen, Dylan J Edwards, Alexander Hartmann, Wolf-Dieter Heiss, Sylvain Lanthier, Paul Lesperance, George Mochizuki, Caroline Paquette, Elizabeth A Rochon, Ilona Rubi-Fessen, Jennie Valles, Heike Kneifel, Susan Wortman-Jutt, Alexander Thiel and on behalf of the NORTHSTAR-study group in European Stroke Journal