Abstract
Rumination, repetitively thinking about the causes, consequences, and one's negative affect, has been considered as an important factor of depression. The intrusion of ruminative thoughts is not easily controlled, and it may be useful to visualize one's neural activity related to rumination and to use that information to facilitate one's self‐control. Real‐time fMRI neurofeedback (rtfMRI‐nf) enables one to see and regulate the fMRI signal from their own brain. This proof‐of concept study utilized connectivity‐based rtfMRI‐nf (cnf) to normalize brain functional connectivity (FC) associated with rumination. Healthy participants were instructed to brake or decrease FC between the precuneus and the right temporoparietal junction (rTPJ), associated with high levels of rumination, while engaging in a self‐referential task. The cnf group (n = 14) showed a linear decrease in the precuneus‐rTPJ FC across neurofeedback training (trend [112] = −0.180, 95% confidence interval [CI] −0.330 to −0.031, while the sham group (n = 14) showed a linear increase in the target FC (trend [112] = 0.151, 95% CI 0.017 to 0.299). Although the cnf group showed a greater reduction in state‐rumination compared to the sham group after neurofeedback training (p < .05), decoupled precuneus‐rTPJ FC did not predict attenuated state‐rumination. We did not find any significant aversive effects of rtfMRI‐nf in all study participants. These results suggest that cnf has the capacity to influence FC among precuneus and rTPJ of a ruminative brain circuit. This approach can be applied to mood and anxiety patients to determine the clinical benefits of reduction in maladaptive rumination.
Keywords: fMRI neurofeedback, functional connectivity, precuneus, right temporoparietal junction, rumination
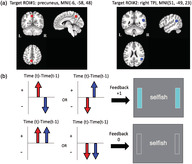
Regions of interest (ROI) for the connectivity‐based rtfMRI‐nf and the neurofeedback algorithm. (a) ROIs for the connectivity‐based rtfMRI‐nf. (b) Neurofeedback algorithm and display. Red circles and arrows indicate the precuneus ROI and BOLD activities, and blue circles and arrows indicate the right temporoparietal junction (rTPJ) ROI and BOLD activities. The sidebars on the screen were updated every 2‐s with positive feedback (+1: light blue color) or no feedback (0: blank color).
1. INTRODUCTION
Rumination is a thought pattern or a cognitive style that repetitively think about the causes, consequences, and one's negative affect (Nolen‐Hoeksema, Wisco, & Lyubomirsky, 2008; Smith & Alloy, 2009). Rumination can impair a person's ability to function because it prevents new insights or solutions on how to handle a situation and emotionally keeps a person in the cycle of repetitive negative thinking and intensifies one's negative feelings. Studies showed rumination as one of the important risk factors that predict the onset of depressive symptoms, and it has been consistently related to more depressive episodes, higher severity of depression, and longer depressive episodes (Mor & Winquist, 2002; Nolan, Roberts, & Gotlib, 1998; Roberts, Gilboa, & Gotlib, 1998; Spasojević & Alloy, 2001). Given the substantial overlap between depression and anxiety (Regier, 1990; Wittchen & Essau, 1993), rumination began receiving more attention as a factor potentially involved in the development of anxiety, or excessive worrying about future events (Ehring & Watkins, 2008). Further investigation has supported the role of rumination as a transdiagnostic risk factor; that is, rumination seems to increase the burden of treating mood and anxiety disorders (MAD) (Spinhoven et al., 2018).
Although effective treatments for MAD, such as pharmacotherapy, psychological intervention, and combination of both have been established, nearly two‐thirds of patients will not respond (Cain, 2007). Rumination also affects a slower response and poor outcome to both antidepressant medication and cognitive‐behavioral therapy (CBT; Jones, Siegle, & Thase, 2008; Schmaling, Dimidjian, Katon, & Sullivan, 2002). Unfortunately, it is unclear whether existing interventions can successfully improve rumination (Watkins, 2015). A recently developed intervention, Rumination‐Focused Cognitive Behavioral Therapy (RFCBT), showed its efficacy for depressive patients but requires twelve sessions to complete (Hvenegaard et al., 2020; Watkins et al., 2011). CBT requires patients to be conscious of their maladaptive ruminative thinking, so then they will be able to learn how to control it. Therefore, it will require multiple sessions until patients can feel progress in and improvement of their depressive symptoms. Importantly, the intrusion of ruminative thoughts is not easily controlled by one's own will, and it can be hard to get out of repetitive ruminative thinking, even if they want to stop it. Therefore, it may be useful to visualize one's internal state of mind outside of one's awareness and to use that information to facilitate one's self‐control. Also, these studies investigating the efficacy of RFCBT (Hvenegaard et al., 2020; Watkins et al., 2011) were not designed to determine what aspect of RFCBT contributes to reducing ruminations. Thus, it is necessary to identify and determine any underlying brain dysfunctions related to rumination.
Real‐time functional magnetic resonance imaging (rtfMRI) provides us blood‐oxygen‐level‐dependent (BOLD) signal processing and display simultaneously with image acquisition. It has enabled real‐time fMRI neurofeedback (rtfMRI‐nf), which allows a person to see and regulate the fMRI signal from their own brain (Cox, Jesmanowicz, & Hyde, 1995; deCharms, 2008). RtfMRI‐nf can be used for brain‐based therapies by providing individuals information about their brain activity and offering them an opportunity to learn how to control their brain activity by themselves (deCharms et al., 2004; Weiskopf et al., 2007; Young et al., 2017; Zotev et al., 2011, 2018). As long as the brain's regional representation associated with rumination symptoms and/or brain circuitry dysfunctions (e.g., abnormalities in functional connectivities related to rumination) are determined, this new method has the potential to be a promising way to target and influence rumination, which is difficult for pathological patients to be aware of. It also has another advantage in examining the relationship between changes in brain function and psychopathological symptoms, which contribute to further understandings of underlying features of psychiatric disorders in the aspects of brain functions. Therefore, rtfMRI‐nf targeting rumination‐related brain circuits will shed new light on revealing the mechanism of treating transdiagnostic symptoms and will give us a unique insight into the biophenotypes that a ruminative subgroup manifests. Defining which brain region to feedback and display is one of the most important elements of an rtfMRI‐nf study (Fede, Dean, Manuweera, & Momenan, 2020). Although targeting a single region of interest (ROI) is effective for rtfMRI‐nf (e.g., Young et al., 2014; Young et al., 2017; Zotev et al., 2011, 2018; Zotev, Phillips, Young, Drevets, & Bodurka, 2013), functional connectivity (FC) with other co‐recruited brain regions (e.g., posterior cingulate cortex, precuneus, hippocampus, insula, anterior cingulate gyrus, dorsomedial prefrontal cortex, etc.) also changes as a result of rtfMRI‐nf (Misaki et al., 2018; Young et al., 2018; Yuan et al., 2014). Correcting aberrant brain FC patterns holds promise because FC has been linked to behavioral, cognitive, and emotional symptoms. Recent studies show the potential of rt‐fMRI‐nf to train FC between brain regions (Kim, Yoo, Tegethoff, Meinlschmidt, & Lee, 2015; Koush et al., 2013, 2017; Liew et al., 2016; Megumi, Yamashita, Kawato, & Imamizu, 2015; Morgenroth et al., 2020; Ramot et al., 2017; Spetter et al., 2017; Yamada et al., 2017; Yamashita, Hayasaka, Kawato, & Imamizu, 2017; Zhao et al., 2019). There are several ways to calculate and estimate brain FCs. For example, the correlation‐based neurofeedback utilizes Pearson's correlation coefficients of BOLD signal time‐courses between two ROIs or even within multiple targeted connections (Kim et al., 2015; Liew et al., 2016; Megumi et al., 2015; Spetter et al., 2017; Yamada et al., 2017; Yamashita et al., 2017; Zhao et al., 2019). Another type of connectivity‐informed rtfMRI‐nf utilizes dynamic causal modeling (DCM; Koush et al., 2013; Koush et al., 2017). The DCM‐based neurofeedback is based on predefined models where the direction of information flow is hypothesized, and can strengthen unidirectional connectivity, while no model of causality in connectivity is presumed in the Pearson's correlation‐based neurofeedback. Moreover, recent technological evolution has enabled more complex calculations, and there are some studies applying regional brain pattern or whole‐brain dynamics as a target (Lorenzetti et al., 2018; Scheinost et al., 2020; Shibata, Watanabe, Sasaki, & Kawato, 2011; Taschereau‐Dumouchel et al., 2018). Those more advanced approaches have a significant advantage in decoding complex brain states with high sensitivity and accounting for individual variability, which enables us to identify personalized target locations or patterns. However, it is not always possible to fully utilize this approach for rtfMRI‐nf especially for the clinical populations, since not all diseases have an established brain biomarker representing abnormalities, and we do not know the ideal brain pattern. Similarly, those approaches use a task to create decoders or classifiers; however, there is no best‐established task to identify abnormalities (Misaki, Tsuchiyagaito, Al Zoubi, Paulus, & Bodurka, 2020). A consistent concern of those modeling methods is the translation of complex, regional brain patterns into clinically modifiable targets for intervention (Scheinost et al., 2019; Scheinost et al., 2020). The correlation‐based approach has taken advantage of the development of biomarkers of psychiatric disorders based on resting‐state functional connectivity (rsFC). Although studies suggest that rumination is associated with impairments in medial prefrontal cortex and post cingulate cortex/precuneus (Zhou et al., 2020), most studies use a priori‐defined seed region, which could misidentify the precise location of the FC associated with rumination. To identify the precise location of FC associated with rumination without a priori seed definition, we previously analyzed the first 500 participants (of 1,000) from an existing large naturalistic dataset (Tulsa 1000: T1000; Victor et al., 2018). After conducting a connectome‐wide association analysis (Misaki et al., 2020), we found that rsFC between the precuneus locus and the right temporoparietal junction (rTPJ) was positively correlated with the severity of rumination measured by the Ruminative Response Style Scale (RRS; Nolen‐Hoeksema & Morrow, 1991) in MAD patients (n = 223). Both the precuneus locus and the rTPJ are regions of the default mode network (DMN), which is responsible in part for intrinsic awareness and self‐referential processing (Andrews‐Hanna, Reidler, Sepulcre, Poulin, & Buckner, 2010; Davey, Pujol, & Harrison, 2016). The precuneus has been consistently considered a major hub of the DMN (Leech, Kamourieh, Beckmann, & Sharp, 2011), while the TPJ is related to emotional processing and theory of mind (Saxe & Kanwisher, 2003; Young, Camprodon, Hauser, Pascual‐Leone, & Saxe, 2010). The hyperconnectivity among them associated with RRS suggests that altered self‐referential processing might be an underlying feature of rumination. Therefore we posit that rebalancing (e.g., braking, decoupling, or decreased connectivity) this data‐driven identified ruminative brain circuit could be a promising way to improve a person's ruminative response and reduce ruminative thoughts. One of the difficulties in conducting connectivity‐based rtfMRI‐nf is to calculate online connectivity with adequate noise suppression. To give a person feedback signals in a timely manner, we need to optimize the number of time points to calculate FCs. In order to estimate FCs as feedback signals, we followed a framework introduced by Ramot and Gonzalez‐Castillo (2019) and optimized the real‐time neurofeedback signals (Misaki et al., 2020). Based on our previous study, we utilized a two‐point method to feedback FC in a timely manner (methodological details were described in Misaki et al., 2020 and Section 2.5).
To investigate the efficacy of rtfMRI‐nf, the study design has to consider the employment of the control group. Clinical trials investigating the efficacy of rt‐fMRI‐nf for MDD have been published primarily by two groups (Mehler et al., 2018; Young et al., 2017). However, there are mixed results in this field; for example, Young et al. (2017) found that rtfMRI‐nf LA training is effective for depressive populations compared to an active sham‐controlled group, while Mehler et al. (2018) could not find the superiority of rtfMRI‐nf based on upregulation of emotional brain areas to an active neurofeedback‐control group (i.e., neurofeedback based on upregulation of region activated by visual scenes), and both groups experienced clinical improvements together with increased self‐efficacy. Those mixed findings might be partially attributed to possible confounding factors, and one such factor could be a reward experience. We, and others, utilized an active sham‐control from alternative ROI (Young et al., 2014, 2017; Zotev et al., 2011, 2013, 2018); however, most of the studies did not control the reward experiences. Generally, target ROIs are selected based on the notion that those regions are supposed to be regulated by the explicit strategy, and alternative control ROIs are selected based on the notion that those regions are not related to both the explicit strategy and the BOLD signals from the targeted ROI. Therefore, participants in the active sham‐controlled group might not be able to receive the same reward experiences, which could be one of the confounding factors of neuronal and/or behavioral changes. On the other hand, in Mehler et al. (2018), an active control group received veridical feedback from a brain region which can be activated during mental imagery of scenes, and can be upregulated with rtfMRI‐nf. They aimed for an active neurofeedback‐control condition that would entail similar upregulation success and thus reward experience in both groups. As described above, both groups experienced clinical improvements together with increased self‐efficacy.
The underlying principle of neurofeedback protocols, in general, contains variables such as (i) mental strategies that trigger desired neurophysiological status, (ii) self‐monitoring, that is, meta‐cognition of what they are thinking and interoceptive observation of their internal state of mind, (iii) a learning process composed of operant learning of desired neurophysiological status and associative learning of feedback with their strategies (these two stages of learning were proposed in the dual‐process theory; Lacroix, 1986; Lacroix & Gowen, 1981), and (iv) reward experiences presented by positive feedback signals. To conclude that the neuronal changes and behavioral changes induced by rtfMRI‐nf are specific to the learning process of feedback from target brain regions, we have to exclude the general experience of self‐regulation, which could have a therapeutic effect. Therefore, we employed an active sham‐controlled feedback group, controlling reward experiences within a certain limit, that is, not exceeding reward experiences that an actual rtfMRI‐nf group would achieve. To exclude the general experience of self‐regulation such as (i), (ii), and (iv) as much as possible, we decided to compare the rtfMRI‐nf group receiving an actual feedback signal reflecting their own target FC with the sham group, which received artificially‐generated random feedback signals with controlled reward experiences. This design gave participants in both groups the opportunity to engage with the same kinds of mental strategies for viewing feedback signals with the same amount of reward experiences, regardless of the actual or artificially generated feedback signals.
Thus, this study targeted the precuneus‐rTPJ connectivity, which has been confirmed to be associated with rumination severity in MAD patients (Misaki et al., 2020), for a neurofeedback training to reduce this connectivity with the feedback signal of the two‐point method without a control ROI using an advanced real‐time fMRI data processing system with comprehensive real‐time noise reduction (Misaki et al., 2015). Since this work is the first proof‐of‐concept study to investigate the efficacy and tolerability of rtfMRI connectivity neurofeedback targeting ruminative brain circuits, we limited our participants to healthy individuals. Generally, rumination is common for depressive and/or anxious populations (Nolen‐Hoeksema, 2000); however, rumination can be separated into state and trait components: trait‐rumination refers to a person's tendency to ruminate, or a personality trait commonly observed among MAD patients, and state‐rumination refers to the act of ruminating induced by an acute stressor or a negative self‐referential task, which can be observed among healthy subjects (Key, Campbell, Bacon, & Gerin, 2008; LeMoult, Arditte, D'Avanzato, & Joormann, 2012). Therefore, we placed much value on state‐rumination as a behavioral outcome measure rather than on trait‐rumination for this proof‐of‐concept study. Our primary aim was to investigate whether healthy volunteers can learn how to decouple and reduce FC between the precuneus locus and the rTPJ in an identified ruminative brain circuit. Primary outcome was the precuneus‐rTPJ FC during the rtfMRI‐nf, which represents the rtfMRI‐nf regulation success, and we hypothesized that participants in the rtfMRI‐nf group would learn to decouple the target FC but not in the sham group. Secondary outcome was the level of state‐rumination, which represents behavioral changes, and we hypothesized that participants in the rtfMRI‐nf group would show significantly greater reduction of state‐rumination after the neurofeedback training, compared to those in the sham group. Other variables were all exploratory outcomes and we investigated changes in rsFC of the target regions before and after rtfMRI‐nf, and the association between rtfMRI‐nf regulation success and behavioral changes.
2. METHODS AND MATERIALS
2.1. Participants
We recruited 31 medically and psychiatrically healthy volunteers who are naive to rtfMRI‐nf experiments for two groups: the connectivity‐based rtfMRI‐nf group (cnf group, n = 16) and the sham control feedback group (sham group, n = 15). Participants were assigned to the cnf or sham group in alternating order, with stratification for age (younger vs. older than 18 years), and gender (male vs. female). The participants allocated to the cnf group received binary neurofeedback (0: no feedback or +1: the presence of positive feedback) derived from their own brain's neuronal activity and associated hemodynamic changes. The absence of feedback, equal to “0,” means coupling (increasing) in the rtfMRI‐nf targeted FC (e.g., precuneus‐rTPJ connectivity), and the presence of positive feedback, equal to “+1,” means decoupling (decreasing) of FC. The sham group participants received sham binary feedback signals, unrelated to subject performance and neurofeedback response (see details in Section 2.5). All participants provided consent for their participation in the experiments knowing that there would be a chance that they would be assigned to the sham group, and they were blinded to group allocation. Two subjects of the cnf group and one subject of the sham group had to be excluded (cnf group: MRI technical issues, noncompliance of instructions; sham group: noncompliance of instructions). In all, 14 subjects for both cnf and sham groups were included (cnf group: mean age/SD = 23/1 years, 10 females; sham group: mean age/SD = 22/1 years, 11 females). Although we did not conduct an a priori sample size calculations, post hoc power analyses were performed to evaluate the achieved power with G*power 3.1.
This study was conducted at the Laureate Institute for Brain Research with the research protocol (IRB# 20111188) approved by the Western Institutional Review Board (IRB). All participants provided written informed consent and received financial compensation for participation in the study. Prior to enrolling in the study, each subject underwent a screening evaluation, including medical and psychiatric history and neuromorphological MRI. Inclusion criteria included healthy volunteers (assessed by Mini International Neuropsychiatric Interview MINI; Sheehan et al., 1998), aged between 18 and 35 years and right‐handed according to the Edinburgh Inventory (Oldfield, 1971). Exclusion criteria included pregnancy, intake of any psychiatric medication, abnormal neuromorphological brain profile, and general contraindications against MRI examinations.
2.2. Imaging data collection and MRI parameters
Imaging was conducted on a 3 T MR750 Discovery (GE Healthcare). BOLD fMRI data were acquired using a T2*‐weighted gradient echo‐planar sequence with sensitivity encoding (ge‐EPI SENSE) with the following parameters: TR/TE = 2000/25 ms, acquisition matrix = 96 × 96, FOV/slice = 240/2.9 mm, flip angle = 90°, voxel size 2.5 × 2.5 × 2.9 mm; 40 axial slices, SENSE acceleration R = 2. For anatomical reference, T1‐weighted (T1w) MRI images were acquired with a magnetization‐prepared rapid gradient‐echo (MPRAGE) sequence with parameters of FOV = 240 × 192 mm, matrix = 256 × 256, 124 axial slices, slice thickness = 1.2 mm, 0.94 × 0.94 × 1.2 mm3 voxel volume, TR/TE = 5/2 ms, SENSE acceleration R = 2, flip angle = 8°, delay/inversion time TD/TI = 1400/725 ms, sampling bandwidth = 31.2 kHz, scan time = 4 min 59 s.
2.3. Real‐time fMRI processing
A Linux machine and an in‐house program written in Python were used for real‐time fMRI data transferring and processing. The real‐time fMRI processing included slice‐timing correction, motion correction, spatial smoothing with 6 mm‐FWHM Gaussian kernel within the brain mask, scaling to a percent change relative to the average for the first 28 TRs (in the initial rest period), and regressing out noise components (Misaki et al., 2015). The noise regressors were six motion parameters, eight RETROICOR (Glover, Li, & Ress, 2000) regressors (four cardiac and four respiration), white matter mean signal, ventricle mean signal, and Legendre polynomial models of slow signal fluctuation. This comprehensive noise reduction was performed in real‐time (less than 400 ms; Misaki et al., 2015).
The masks for the white matter and ventricles regions and the ROIs of precuneus and rTPJ were defined in the MNI template brain. The ROIs were defined as spheres of a 6 mm radius at the precuneus locus (MNI: −6, −58, 48) and the rTPJ (MNI: 51, −49, 23) in accordance with our previous research (Misaki et al., 2020). These masks and ROIs in the MNI space were warped to the individual subject brain for real‐time signal calculation. At first, a subject's anatomical image was aligned to a functional image of the first functional run (resting‐state scan). Then, the MNI template brain was warped to the aligned anatomical image using the Advanced Normalization Tools (ANTs; Avants, Epstein, Grossman, & Gee, 2008; http://stnava.github.io/ANTs/). At last, the warped masks and ROIs were resampled in functional image resolution to make masks and ROIs in the functional image space for real‐time calculation. The processing of the masks and ROIs was done during the resting‐state and baseline runs (Rest1, Think, View1 in Figure 1) before the neurofeedback runs. The functional image used as a reference for the alignment and resampling was also used as a reference for the real‐time motion correction.
FIGURE 1.
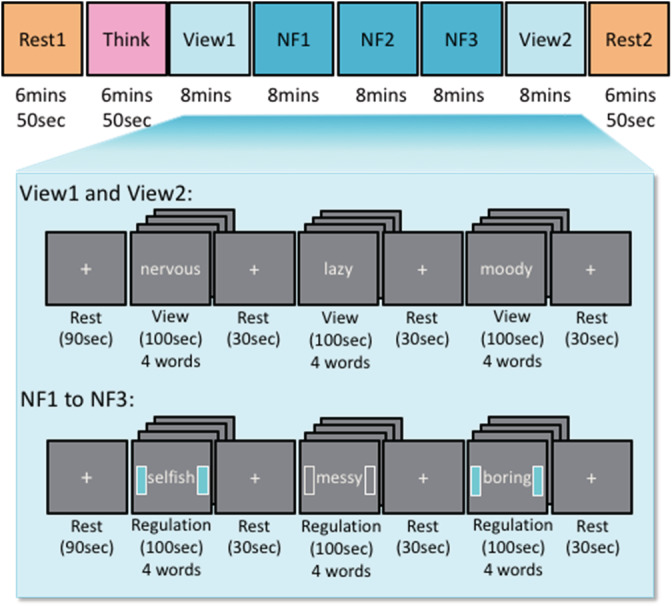
Experimental design. A neurofeedback session contains a first resting scan (Rest1), rumination‐inducing task scan (Think), is followed by five experimental runs: View1, Neurofeedback 1 to 3 (NF1, 2, 3) View2, and ends with the last resting scan (Rest2). Each experimental run started with a first “Rest” block (90‐s), followed by “View” or “Regulation” block (100‐s) during the presentation of four negative trait words (25‐s for each word) and “Rest” block (30‐s) alternatively. In the initial view scan (View1: Baseline), participants were instructed to naturally respond to those negative trait words during the “View” block, and in the last view scan (View2: Transfer), participants were instructed to use the mental strategy that worked best throughout three neurofeedback runs (NF1, 2, 3) during the “View” block. In the neurofeedback scan, participants were instructed to apply a mental strategy, such as cognitive reappraisal and acceptance (“it is OK” statement), while viewing negative trait words and instructed to regulate their brain activity represented by the sidebars during the “Regulation” block
2.4. Neurofeedback training paradigm
The session started with a 5‐min T1w MRI anatomical scan, a 6‐min, and 50‐s first fMRI resting‐state scan, a 6‐min, and 50‐s rumination‐inducing task fMRI scan (Think), followed by five experimental rtfMRI‐nf runs (View1 as a baseline, three NF trainings [NF1, 2, 3], and View2 as a transfer), each lasting 8‐min, and concluded with a 6‐min, and 50‐s resting‐state fMRI scan (Figure 1). The rumination‐inducing (Think) task was to induce participants' ruminative states in an experimental setting. During this task, participants were instructed to recall a memory where they were recently rejected by someone who meant a lot to them, and were instructed to think why they reacted the way they did while seeing the fixation sign. Data from the Think task were not analyzed in this study.
The experimental run (View1, 2, NF1, 2, 3 in Figure 1) started with a 90‐s resting block (for obtaining enough sample points for a real‐time noise reduction; Misaki et al., 2015), followed by three trial blocks, each composed of a 100‐s Regulation block during a presentation of four negative trait words (25‐s for each word) and a 30‐s rest. For the Rest block, participants were instructed to see the fixation cross displayed at the center of the presentation screen and to not think of anything in particular, in the same manner, as during the resting‐state scan. For the Regulation block, the participants were instructed to apply a mental strategy, such as cognitive reappraisal and acceptance (“it is OK” statement), while viewing negative personality trait words that were individually customized based on their own choice (see details in Table S1). Examples of cognitive reappraisal or acceptance were provided prior to the intervention: “Everyone has these traits (generalization), then it is OK if I have these negative traits.” “Sometimes, I behave like that, but not always (exception), then it is OK if I sometimes behave like that.” “Thinking about the good side of the negative trait. For example, being shy might mean that I am a humble and deep thinker rather than big‐headed. Then it is OK if I have these negative traits.” “Thinking about the solutions on how to overcome my negative traits or personality. Then it is OK, and I can overcome it.”
The first and last two experimental runs (View1, 2) were performed without neurofeedback signals, while the middle three experimental runs were performed with neurofeedback signals (NF1, 2, 3). In the initial view condition (View1: Baseline), participants were instructed to naturally respond to those negative trait words, and in the last view condition (View2: Transfer), they were instructed to use the mental strategy that works best through three neurofeedback runs (NF1, 2, 3). The View1 and View2 non‐neurofeedback runs were used to assess changes in the target FC between the precuneus and the rTPJ in the absence of neurofeedback signals.
2.5. Neurofeedback signal calculation utilizing two‐point method
Our previous study (Misaki et al., 2020) performed a simulation analysis to optimize an algorithm for online real‐time FC feedback signal to decouple the FC between the two target ROIs (i.e., the precuneus locus and the rTPJ). We evaluated two methods of online connectivity neurofeedback signal, that is, sliding‐window correlation (Gembris et al., 2000) and the two‐point method (Ramot et al., 2017). The sliding‐window correlation is a z‐transformed Pearson's correlation between two ROIs within a time window, and widths of a 3‐ to 10‐time points window were evaluated in the simulation. The two‐point method calculates a signal change direction in a consecutive two‐time points window and compares the change directions of the two target ROIs as a proxy measure of online connectivity. The feedback signal is calculated as a binary value. When the BOLD signal from the precuneus and the rTPJ moved in different directions (i.e., the change of BOLD signal from the precuneus increased and that from the rTPJ decreased, or that from the precuneus decreased and that from the rTPJ increased), participants saw blue bars displayed on both sides of the word stimulus as positive neurofeedback (coded as +1; Figure 2). When the changes of the BOLD signal from the precuneus and the rTPJ moved in the same direction, participants saw blank sidebars, which indicated the absence of feedback (coded as 0). The original introduction of the two‐point method utilized a control ROI to cancel a signal change unspecific to the target FC (Ramot et al., 2017). Both versions of the two‐point method, with and without the control ROI, were evaluated in the simulation. The simulation was performed on an advanced rtfMRI data processing system implementing comprehensive online noise reduction processes (Misaki et al., 2015; Misaki & Bodurka, 2019), which is the same system used for rtfMRI‐nf, with slice‐timing correction, motion correction, spatial smoothing, signal scaling and GLM with regressors of high‐pass filtering, six motion parameters, mean white matter signal, mean ventricle signal, and RETROICOR (Glover et al., 2000) for the estimation of online FC. The optimality of online feedback signals was evaluated with regard to three criteria, that is, correlation between online FC and offline FC, robustness to head motion, and timeliness of neurofeedback. The simulation results indicated a trade‐off between the correlation with offline FC and the risk of motion contamination. The higher correlation between online FC and offline FC was observed with more time points (i.e., sliding‐window correlations with 10 time points); however, the more time points were included, the more the feedback signal was correlated with head motions. Dependence of long signal history is also not favored regarding the timeliness of the neurofeedback. Including the control ROI in the two‐point method decreased the correlation between online FC and offline FC, which could be attributed to a reduced positive feedback frequency with restriction by the control ROI. Therefore, we decided to utilize the two‐point method without the control ROI for this study because it was robust to motion, less dependent on signal history, and more time sensitive for training to decouple target (see more details in Misaki et al. (2020)).
FIGURE 2.
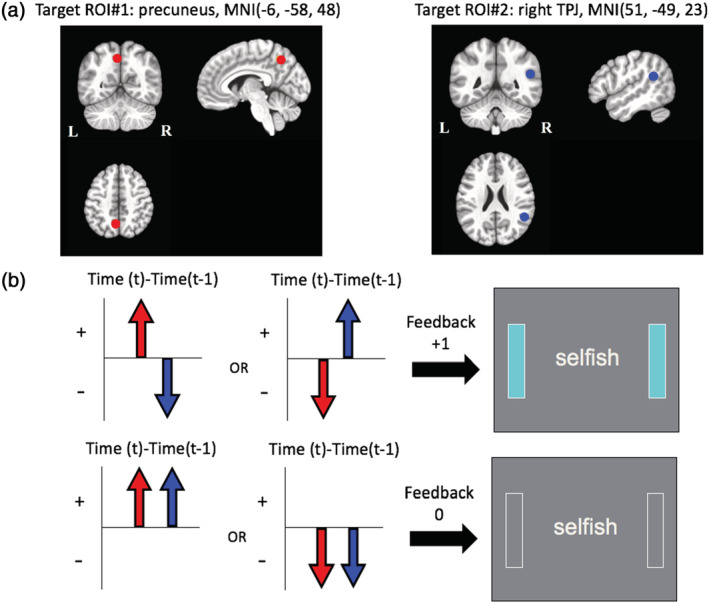
Regions of interest (ROI) for the connectivity‐based rtfMRI‐nf and the neurofeedback algorithm. (a). ROIs for the connectivity‐based rtfMRI‐nf. (b). Neurofeedback algorithm and display. Red circles and arrows indicate the precuneus ROI and BOLD activities, and blue circles and arrows indicate the right temporoparietal junction (rTPJ) ROI and BOLD activities. The sidebars on the screen were updated every 2‐s with positive feedback (+1: light blue color) or no feedback (0: blank color)
The neurofeedback presentation started 8 s after the onset of the Regulation block to wait for the hemodynamic response delay and to sample two points for connectivity calculation. The participants assigned to the sham group received the same feedback presentation except that the feedbacks were artificially generated unrelated to the target connectivity. With several technical and evaluation scans with healthy adults, we observed that the average success rate of decoupling the precuneus and the rTPJ during the Regulation block with true neurofeedback signals was around 40%. Therefore, participants assigned to the sham group were pre‐set to receive 40% of the positive feedback during the Regulation block, regardless of their actual brain connectivity patterns. The binary sham feedback signal was randomly generated from a Bernoulli distribution with a 0.4 probability of positive feedback at each TR. We also placed a safeguard process against an accidental correlation between the random sham and the real brain signals. We calculated the correlation between the sham signal and the real two‐point feedback signal (not presented to the participants in the sham group) in real‐time after the first block. A feedback signal that could reduce the correlation was presented only if the absolute correlation was larger than 0.3 instead of a random value; for example, if there was a high positive correlation, take the sham signal opposite to the real one, and vice versa. This ensured that the sham group received a feedback signal irrelevant to their brain activation.
The participants in both groups were instructed that the presence of blue sidebars indicates the subject's brain status is in the desired state (targeted FC related to rumination is decoupled), and were instructed to try and adjust their mental strategies based on the provided feedback throughout the experimental runs. Also, they were informed that there would be a 7‐s hemodynamic delay between brain changes and feedback signals and that their goal was to keep the sidebars blue as long as possible.
The Consensus on the Reporting and Experimental Design of clinical and cognitive‐behavioral Neurofeedback studies (CRED‐nf) best practices checklist 2020 (Ros et al., 2020) can be found in Table S2.
2.6. Experimental measures
Experimental measures comprised self‐rated scales and assessments along with fMRI connectivity analyses, as specified below.
2.6.1. Changes in neurofeedback‐related FC between the precuneus and the rTPJ
The change of FC between the precuneus and the rTPJ on the course of the experimental runs (View1, NF1‐3, and View2) was evaluated as a successful decoupling of the target FC.
2.6.2. Changes in state‐rumination after one rtfMRI‐nf session
The change of the subjective state‐rumination score during the self‐referential task between pre‐neurofeedback training (pre‐NF) and post‐neurofeedback training (post‐NF). The level of state‐rumination was assessed with the visual analog scale (VAS). Participants were asked to answer one question, “To what extent did you dwell on negative aspects of yourself?” using a button box rating from 1 (not at all) to 10 (extremely) at the end of View1 (pre‐NF) and View2 (post‐NF).
2.6.3. Changes in rsFC between the precuneus and the rTPJ after one rtfMRI‐nf session
The change of rsFC between the precuneus and the rTPJ before and after the neurofeedback training (Rest1 and Rest2) was evaluated to investigate whether the changes seen in rtfMRI‐nf could be observed in a more naturalistic fashion.
2.6.4. Exploratory behavioral variables related to state‐rumination
The following exploratory behavioral measures were used to examine whether there would be detectable behavioral changes after one session of rtfMRI‐nf. Also, mood changes were assessed with the State–Trait Anxiety Inventory‐State (STAI‐S; Spielberger, Gorsuch, Lushene, Vagg, & Jacobs, 1983) and the Profile of Mood States (POMS) Scale (McNair, Lorr, & Droppleman, 1971). POMS Total Mood Disturbance (TMD) subscale was checked before and after the neurofeedback intervention to ensure there are no harmful effects on participants in both groups. Prior to having their MRI scans, participants completed the following measures (pre‐NF); (i) Ruminative Response Style Scale (RRS; Nolen‐Hoeksema & Morrow, 1991); (ii) Toronto Alexithymia Scale (TAS; Bagby, Parker, & Taylor, 1994); (iii) Emotion Regulation Questionnaires (ERQ; Gross & John, 2003); (iv) State–Trait Anxiety Inventory‐Trait (STAI‐T; Spielberger et al., 1983); (v) State–Trait Anxiety Inventory‐State (STAI‐S; Spielberger et al., 1983); and (vi) Profile of Mood States (POMS) Scale (McNair et al., 1971).
After the neurofeedback session (post‐NF), they were asked to complete (v) STAI‐S and (vi) POMS again. In this study, the POMS TMD subscale was used to measure a negative mood. In addition, a follow‐up assessment was conducted 7–14 days after the neurofeedback session (Follow Up: FU), and participants were asked to complete (i) RRS, (ii) TAS, and (iii) ERQ. The changes in these scales between pre‐NF and post‐NF or FU was tested. All behavioral variables were answered and stored on the research electronic data capture (REDCap; Harris et al., 2009) by participants who were blinded to group allocation.
2.6.5. Post‐neurofeedback session questionnaire
We developed a post‐neurofeedback session questionnaire asking participants about their impressions of this training and mental strategies. Participants were asked to rate on a scale of 0–10 how pleasant/unpleasant the neurofeedback training was, how challenging the neurofeedback training was, how successful they were in stopping rumination during the scan, how often they found themselves dwelling on negative aspects of themselves, how successful they were in modulating their brain activity, how helpful they felt that this training was in preventing from rumination, how helpful they felt that this training would be in knowing how to stop their rumination in their daily life, and what strategies they felt were most useful to modulate their brain activity. See Table S3 for the data from these questionnaires.
2.7. Offline and statistical analysis
2.7.1. Changes in neurofeedback‐related FC between the precuneus and the rTPJ
The precuneus‐rTPJ FCs across the training runs were evaluated with a generalized psychophysiological interaction (gPPI) analysis (McLaren, Ries, Xu, & Johnson, 2012). We used offline‐processed fMRI images for the analysis. Analysis of Functional NeuroImages package (AFNI; http://afni.nimh.nih.gov/; Cox, 1996) was employed for the processing. The first five TRs were discarded from the analysis. The process included despike, RETROICOR (Glover et al., 2000) and respiration volume per time (RVT) correction (Birn, Smith, Jones, & Bandettini, 2008), slice‐timing and motion corrections, nonlinear warping to the MNI template brain with resampling to 2 mm3 voxels using the ANTs, spatial smoothing with 6 mm‐FWHM Gaussian kernel, and scaling signal to percent change relative to the mean in each voxel. Any time point with large motion (>0.20 mm frame‐wise displacement [FD]) was censored within the regression (Power, Schlaggar, & Petersen, 2015).
The design matrix of the general linear model (GLM) analysis for PPI included regressors of the task block modeled with a box‐car function convolved with hemodynamic response function (HRF), the three stimulus presentation (“word change,” “change to the positive feedback,” “change to no feedback”) modeled with a delta function convolved with HRF, and the PPI regressors of the precuneus ROI signal time‐series and the multiplication of the task‐block regressor and the ROI‐signal regressor as an interaction term. The design matrix also included noise regressors of three principal components of the ventricle signal, local white matter average signal (ANATICOR; Jo, Saad, Simmons, Milbury, & Cox, 2010), 12 motion parameters (three shift and three rotation parameters with their temporal derivatives), and low‐frequency fluctuation (third‐order Legendre polynomial model).
The ROI signal time‐series was extracted from the residual signal of the GLM analysis with the same design matrix except for the PPI regressors so that the signal changes associated with stimulus presentation and nuisance noises were excluded from the connectivity evaluation. The ROI‐signal regressor in the design matrix was orthogonalized with respect to the interaction regressor to avoid a collinearity problem. This regressor multiplication approach of gPPI is known as accurate and more robust to noise than the deconvolution approach for a block‐design experiment (Di & Biswal, 2017).
The PPI analysis from the precuneus ROI signal was performed in the rTPJ ROI for each task run (View1, 2, and NF1, 2, 3) independently. The t‐value of the beta coefficient for the interaction term was used as an estimate of the task‐related FC, and the average result within the rTPJ ROI was reported. The longitudinal change of the task‐related FC between the precuneus and rTPJ was tested by linear mixed effect model analysis (LME, lme4 package; Bates, Mächler, Bolker, & Walker, 2015) in R. The LME model included fixed effects of experimental runs (View1, NF1, 2, 3, and View2), group (cnf and sham), runs (time) by group interaction, age, gender, and a random effect of the subject on intercept. To evaluate the linear training effects across experimental runs, lstrends function with lsmeans R‐package (Lenth, 2016) was used with each experimental run as numbers (i.e., View1 = 0, NF1 = 1, NF2 = 2, NF3 = 3, and View2 = 4) to estimate and compare slopes of LME fitted lines across experimental runs between the groups. For all post hoc comparisons, we performed a False Discovery Rate (FDR) correction for multiple comparisons. These analyses were also conducted with rTPJ ROI as a seed instead of precuneus ROI.
2.7.2. Changes in state‐rumination after one rtfMRI‐nf session
For the secondary outcome variable, changes in the subjective state‐rumination score assessed by the VAS administered after View1 (pre‐NF) and View2 (post‐NF) were examined by LME model analysis. The LME model included fixed effects of time (pre‐NF and post‐NF), group (cnf and sham), time by group interaction, age, gender, and a random effect of the subject on intercept. For all post hoc comparisons, we performed an FDR‐correction for multiple comparisons.
2.7.3. Changes in rsFC between the precuneus and the rTPJ after one rtfMRI‐nf session
We examined the first resting‐state scan (Rest1) and the last resting‐state scan (Rest2), and evaluated the changes in rsFC between the precuneus and the rTPJ. The resting‐state fMRI data preprocessing was performed in the same manner shown in Section 2.7.1. For the noise reduction, three principal components of the ventricle signal, local white matter average signal (ANATICOR; Jo et al., 2010), and in addition, 12 motion parameters (three shift and three rotation parameters with their temporal derivatives), and low‐frequency fluctuation (third‐order Legendre polynomial model) were regressed out using AFNI 3dREMLfit. Any time point with large motion (>0.20 mm frame‐wise displacement [FD]) was censored within the regression (Power et al., 2015). For each ROI (precuneus and rTPJ, 6 mm radius), the average BOLD time‐series was calculated from the residual signal of the GLM analysis, and then, rsFC was calculated as Pearson's correlation between the BOLD time series of each ROI, followed by Fisher's r‐to‐z‐transformation. The resulting z‐scored rsFC was used for the statistical analysis. We predicted that the strength of rsFC between the precuneus and the rTPJ during Rest2 (post‐NF) would see a greater decrease (decouple) compared to Rest1 (pre‐NF) for the cnf group but not for the sham group. This hypothesis was examined by LME model analysis. The LME model included fixed effects of time (Rest1 and Rest2), group (cnf and sham), time by group interaction, age, gender, and a random effect of the subject on intercept.
2.7.4. Exploratory behavioral variables related to state‐rumination
Although our intention was to reduce the state‐rumination targeting ruminative brain network, we investigated the impact of this neurofeedback session on mood, trait‐rumination, emotional recognition, and emotional regulation. Changes in the moods assessed by POMS TMD and STAI‐S administered before and after the neurofeedback session (pre‐NF and post‐NF), and changes in the state‐rumination, alexithymia, and emotion regulation assessed by RRS, TAS, and ERQ administered before the neurofeedback session and 1–2 weeks later of the neurofeedback session (pre‐NF and FU) were statistically tested with the LME model to examine whether any behavioral changes could be detected after one rtfMRI‐nf session. The LME model included fixed effects of time (pre‐NF and post‐NF, or pre‐NF and FU), group (cnf and sham), time by group interaction, age, gender, and a random effect of the subject on intercept. For all post hoc comparisons, we performed an FDR correction for multiple comparisons.
2.7.5. Associations between neural changes and behavioral changes
We also explored the associations between neural changes and behavioral changes via rtfMRI‐nf, and used Spearman's rho (r) for the correlation analysis. The associations between changes in PPI estimates of the precuneus and rTPJ (i.e., changes of the precuneus and the rTPJ FC) at View2 from View1 and changes in each behavioral outcome at post‐NF or FU from pre‐NF (i.e., state‐rumination measured by VAS, POMS TMD, STAI‐S, RRS, TAS, and ERQ) were tested. Results with a p value <.05 were considered statistically significant and those with a p value <.1 were considered trending significant. We performed an FDR‐correction for multiple comparisons.
2.7.6. Changes in target ROIs during neurofeedback training
To examine whether correcting aberrant FC affected each target ROI, the GLM analysis was used to evaluate each ROI activity through neurofeedback sessions (View1, NF1, 2, 3, and View3). The design matrix included a modeled response to the Regulation block, 12 motion parameters (three shift and three rotation parameters with their temporal derivatives), three principal components of the ventricle signal, local white matter average signal (ANATICOR; Jo et al., 2010), and low‐frequency fluctuation (third‐order Legendre polynomial model). The beta‐coefficients of the Regulation block regressor were extracted for the estimation of percent BOLD signal changes for “Regulation” vs. “Rest” block contrast in the precuneus ROI and the rTPJ ROI, followed by LME model analysis. The LME model included fixed effects of experimental runs (View1, NF1, 2, 3, and View2), group (cnf and sham), runs (time) by group interaction, age, gender, and a random effect of the subject on intercept.
2.7.7. Seed‐to‐whole brain analysis investigating changes in FC during rtfMRI‐nf
We explored the effect of connectivity‐based rtfMRI‐nf on FCs from the precuneus to the whole brain using an AFNI program 3dLME. The PPI FC maps of interaction term between the precuneus and “Regulation” block was used as an estimate of the task‐related FC from the precuneus to whole brain. These maps indicate how correlated each voxel was with the precuneus ROI during the rtfMRI‐nf, and were used for 3dLME model analysis. The 3dLME model included fixed effects of experimental runs (NF1, NF2, and NF3), group (cnf and sham), runs (time) by group interaction, age, gender, and a random effect of the subject on intercept. The significance criterion was set with voxel‐wise p < .001 and cluster‐size correction at p < .05 (determined using AFNI 3dClustSim and the spatial autocorrelation function). This approach was used to identify the regions where FCs with the precuneus ROI differed from the sham group through NF1, NF2, and NF3 within the cnf group.
3. RESULTS
3.1. Demographic and behavioral measures
Table 1 shows age, gender, and changes of the behavioral outcome measures at the pre‐NF, the post‐NF, and the FU. The follow‐up data of five subjects in the cnf group and five subjects in the sham group were not available due to lack of time or motivation to participate. Two subjects in the sham group completed RRS but had missing data for the other measures due to time constraints or computer network issues. At the pre‐NF, there were no significant differences between the cnf and the sham groups in any variables.
TABLE 1.
Age, gender, and behavioral outcome measures at the pre‐, the post‐neurofeedback session, and the follow‐up
| Pre‐NF | Effect size | Post‐NF | Effect size | FU | Effect size | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cnf (n = 14) | Sham (n = 14) | Cnf (n = 14) | Sham (n = 14) | Cnf (n = 9) | Sham (n = 7 a ) | |||||
| Age in years | 23.43 (1.12) | 22.14 (0.97) | 0.33 | 23.43 (1.12) | 22.14 (0.97) | 0.33 | 23.89 (3.84) | 20.71 (2.71) | 0.93 | |
| Male:Female | 4:10 | 3:11 | 0.18 | 4:10 | 3:11 | 0.18 | 2:7 | 2:5 | 0.17 | |
| State‐rumination assessed by VAS | 6.71 (1.98) | 6.07 (2.40) | 0.29 | 2.21 (0.80) b , c | 3.93 (2.20) | −1.04 | – | – | – | |
| RRS | Total | 28.79 (7.95) | 28.43 (6.85) | 0.05 | – | – | – | 30.22 (10.43) | 30.11 (9.56) | 0.01 |
| Reflection | 7.00 (3.46) | 6.57 (2.87) | 0.13 | – | – | – | 8.67 (4.27) b | 7.33 (3.12) | 0.36 | |
| Brooding | 6.36 (1.78) | 6.57 (1.28) | −0.14 | – | – | – | 6.22 (1.48) | 6.44 (1.88) | −0.13 | |
| Depression | 15.43 (4.59) | 15.29 (3.41) | 0.04 | – | – | – | 15.33 (5.79) | 16.33 (6.02) | −0.17 | |
| TAS | Total | 32.50 (6.75) | 36.14 (6.35) | −0.56 | – | – | – | 30.22 (5.02) c | 38.57 (6.40) | −1.48 |
| Difficulty identify feeling | 8.79 (2.72) | 9.43 (2.59) | −0.24 | – | – | – | 7.78 (1.20) c | 11.14 (3.89) | −1.24 | |
| Difficulty describe feeling | 8.07 (3.45) | 9.71 (3.45) | −0.48 | – | – | – | 6.78 (2.28) c | 11.43 (4.31) | −1.41 | |
| Externally oriented thinking | 15.64 (2.31) | 17.00 (3.14) | −0.49 | – | – | – | 15.67 (3.28) | 16.00 (2.38) | −0.11 | |
| ERQ | Cognitive reappraisal | 35.29 (5.68) | 33.64 (4.89) | 0.31 | – | – | – | 37.11 (5.01) | 31.57 (11.63) | 0.65 |
| Cognitive suppression | 10.07 (3.71) | 11.07 (4.78) | −0.23 | – | – | – | 9.78 (4.09) | 14.00 (6.81) | −0.78 | |
| STAI | Trait | 28.00 (4.08) | 30.29 (4.23) | −0.55 | – | – | – | – | – | – |
| STAI | State | 24.21 (4.34) | 26.64 (4.55) | −0.55 | 25.21 (6.59) c | 30.71 (6.99) | −0.81 | – | – | – |
| POMS | Total mood disturbance | −4.64 (12.35) | −0.86 (9.37) | −0.35 | −9.64 (12.22) b , c | −0.57 (7.37) | −0.90 | – | – | – |
| Tension | 2.64 (2.50) | 4.43 (2.85) | −0.67 | 1.64 (1.69) c | 3.86 (2.35) | −1.08 | – | – | – | |
| Depression | 0.50 (0.76) | 1.00 (1.57) | −0.41 | 0.29 (0.61) | 0.86 (1.29) | −0.57 | – | – | – | |
| Anger | 0.86 (1.35) | 0.79 (1.37) | 0.05 | 0.14 (0.53) c | 0.43 (0.76) | −0.44 | – | – | – | |
| Fatigue | 3.64 (3.52) | 4.21 (2.91) | −0.18 | 3.07 (4.58) | 4.36 (2.84) | −0.34 | – | – | – | |
| Confusion | 3.43 (2.50) | 2.93 (1.33) | 0.25 | 2.29 (2.05) c | 3.5 (1.45) | −0.68 | – | – | – | |
| Vigor | 15.71 (6.09) | 14.21 (7.39) | 0.22 | 17.07 (6.82) | 13.57 (6.63) | 0.52 | – | – | – | |
Note: Means and standard deviations of behavioral outcome measures at the pre‐neurofeedback (Pre‐NF), the post‐neurofeedback (Post‐NF), and the follow up (FU).
Abbreviations: cnf group, connectivity‐based fMRI‐nf group; ERQ, Emotion Regulation Questionnaire; POMS, Profile of Mood States; RRS, Ruminative Response Scale; STAI, The State–Trait Anxiety Inventory; TAS, Toronto Alexithymia Scale; VAS, Visual Analogue Scale.
n = 9 for RRS.
A significant behavioral change between the Pre‐NF and the Post‐NF or between the Pre‐NF and the FU (p < .05, uncorrected for age and gender effects, uncorrected for multiple comparisons).
A significant group difference between cnf group and sham group (p < .05, uncorrected for age and gender effects, uncorrected for multiple comparisons).
3.2. Real‐time feedback presentation
To evaluate whether we had succeeded in controlling the positive feedback presentation in the sham groups compared to the cnf group, we computed the ratio of the positive feedback (the duration of presenting the blue bars) during the Regulation block in each subject in the cnf group. The average positive feedback ratio was 33.92% for NF1, 33.07% for NF2, and 38.15% for NF3 in the cnf group, while the positive feedback ratio was kept 40.00% in the sham group as described in Section 2.4. One‐sample t‐test with an FDR correction showed that the individuals in the sham group received significantly greater positive feedback presentations during NF1 and NF2, but not during NF3 (NF1: t [13] = −3.07, p = .03, FDR corrected), NF2: t [13] = −3.41, p = .01, FDR corrected, NF3: t [13] = −0.85, p = n.s., FDR corrected). Therefore, reward experiences for participants in the sham group were no less than those in the cnf group.
3.3. Post‐neurofeedback session questionnaires and mental strategies
The result of the Post‐Neurofeedback Session Questionnaires and mental strategies that subjects engaged in were listed in Table S3. Overall, there were no significant differences in the pleasantness/unpleasantness of this training (Question 1 and 2), task difficulties (Question 3), subjective evaluation of stopping rumination (Question 4 and 5), subjective evaluation of brain control (Question 6), and the evaluation of the scan time (Question 9) between both groups. Although there were no statistical differences, participants in the cnf group showed higher scores in Questions 7 and 8 (effect size d = 0.74 and 0.63, respectively), indicating that the neurofeedback training seemed to, in their opinions, be favorable in the aspect of learning how to prevent rumination.
3.4. Evaluation of training success: Changes in PPI estimates of the precuneus and rTPJ during neurofeedback training
There was no significant difference in the percentage censored due to large motion (>0.20 mm) during the neurofeedback training between groups (cnf group: mean percentage censored/SD = 10/8%, sham group: mean percentage censored/SD = 12/9%, t [26] = 0.65, p = n.s.). Figure 3 shows the main result of neuronal changes in the target rumination circuit (i.e., FC between the precuneus and the rTPJ) in both groups. As expected, LME analysis showed a significant time by group interaction effect on PPI estimates of the precuneus and the rTPJ FC, and there was a significant difference in experimental run slopes among the cnf and sham groups (F [1, 112] = 9.692, p = .002). For the cnf group, there was a negative linear relationship between the precuneus and the rTPJ FC across the experimental runs (trend [112] = −0.180, 95% confidence interval [CI] −0.330 to −0.031), while for the sham group, there was a trend for a linear positive relationship into the opposite direction (trend [112] = 0.151, 95% CI 0.017–0.299). Post hoc comparison within each group with 10 comparisons (View1 vs. NF1, View1 vs. NF2, View1 vs. NF3, View1 vs. View2, NF1 vs. NF2, NF1 vs. NF3, NF1 vs. View2, NF2 vs. MF3, NF2 vs. View2, NF3 vs. View2) revealed that the PPI estimates of the precuneus and the rTPJ FC were significantly lower at NF3 compared to NF1 for the cnf group (t [112] = 3.186, p = .019, FDR corrected, effect size d = 0.83), and this training effect tended to be maintained through View2 (t [112] = 2.600, p = .053, FDR corrected, effect size d = 0.68). Post hoc power analyses for the comparison between NF1 and NF3 in the cnf group (d = 0.83, n = 14, alpha = 0.05, one‐sided) revealed a power of 0.90, and those for the post hoc comparison between NF1 and View2 in the cnf group (d = 0.68, n = 14, alpha = 0.05, one‐sided) revealed a power of 0.78. When we compared the PPI estimates of the precuneus and the rTPJ FC between groups at each five‐time point (View1, NF1, NF2, NF3, and View2), those of the cnf group were lower than those of the sham group at NF3 (t [70] = −2.237, p = .029, uncorrected, effect size d = 0.81) and at View2: t [70] = −2.023, p = .047, uncorrected, effect size d = 0.74), although after FDR corrections, those effects did not remain statistically significant. The results indicated that BOLD activity in the rTPJ was negatively coupled to BOLD activity in the precuneus through experimental runs in the cnf group, while the opposite trend was observed in the sham group. Moreover, the same analysis was conducted based on the PPI estimates map of the rTPJ and the precuneus FC and showed the same trends, indicating that BOLD activity in the precuneus was also negatively coupled to BOLD activity in the rTPJ (Figure S1).
FIGURE 3.
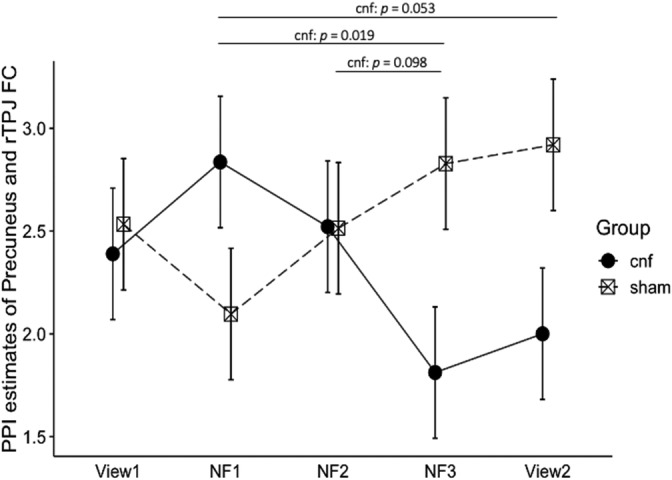
Changes in PPI estimates (t‐value) of the precuneus and the right temporoparietal junction (rTPJ) functional connectivity (FC) between the connectivity‐based rtfMRI‐nf group (cnf group) and the sham group through the neurofeedback session (View1 and View2: no‐neurofeedback run with self‐referential task, NF1, 2, 3: neurofeedback run with self‐referential task). The error bars represent the standard error of the mean
3.5. Changes in state‐rumination after one rtfMRI‐nf session
Figure 4 shows the changes in state‐rumination measured by VAS before and after the neurofeedback training in both groups. The LME analysis showed a significant main effect of time and the interaction between time and group for the primary behavioral outcome (time: F [1, 28] = 73.788, p < .001, interaction: F [1, 28] = 9.291, p = .005). Both groups showed a significant reduction in the state‐rumination score; however, when we performed post hoc analysis comparing groups at each two‐time point, the cnf group showed a significantly lower score of state‐rumination at post‐NF compared to the sham group (t [50] = −2.325, p = .048, FDR corrected, effect size d = 0.84). The result supported the evidence that rtfMRI‐nf targeting ruminative circuit is more effective than sham feedback to reduce state‐rumination.
FIGURE 4.
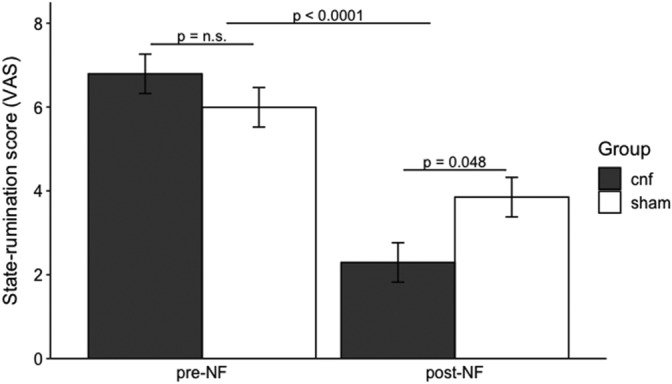
Changes in state‐rumination measured by the visual analogue scale (VAS) between the connectivity‐based rtfMRI‐nf group (cnf group) and the sham group, comparing pre‐neurofeedback (Pre‐NF) and post‐neurofeedback (Post‐NF)
3.6. Changes in rsFC between the precuneus and the rTPJ after one rtfMRI‐nf session
There was no significant main effect nor an interaction between time and group effect. The result indicated that no sustained effects of this neurofeedback training were observed during the resting‐state.
3.7. Exploratory analysis of behavioral changes related to state‐rumination
Measures of mood, trait‐rumination, emotion recognition, and emotion regulation were evaluated, and group comparisons on assessment measures are presented in Table 1. We did not find a significant effect of rtfMRI‐nf in the aversive direction (e.g., average scores of POMS TMD and STAI‐S were not significantly increased after the rtfMRI‐nf in both groups). Although we found differences in several behavioral measurements between groups at post‐NF or at FU, any of those did not remain significant after correcting multiple comparison. Of all behavioral measures, the LME analysis showed a trend‐wise significant interaction between time and group (interaction: F [1, 19] = 3.183, p = .090) for the TAS difficulty identify feeling (DIF) subscale. A post hoc analysis comparing groups at each two‐time point revealed that the cnf group showed a trend wisely lower score of the TAS DIF subscale at FU compared to the sham group (t [44] = −2.314, p = .0051, FDR corrected, effect size d = 1.18). Nevertheless, the cnf group was not found to be superior over the sham group on any of the other behavioral measures with the LME analysis, including trait‐rumination. The result indicated that this neurofeedback session was effective for improving emotion recognition, such as the ability to identify feelings. As for the trait‐rumination assessed with RRS, a paired t‐test revealed that average scores of the RRS Reflection subscale were significantly increased in the cnf group (p uncorrected <.05), while the RRS Brooding subscale and RRS Depressive rumination subscale were slightly decreased in this group (Table 1); however, any changes of these scores did not reach statistical significance after adjusting multiple comparisons.
3.8. Associations between neural changes and behavioral changes
In all participants, the magnitude of change in FC between the precuneus and the rTPJ was nominally correlated with the magnitude of change in the RRS Brooding subscale (rho = 0.48, p = .04, uncorrected), which was driven by the association in the sham group (rho = 0.63, p = .07, uncorrected). Also, the magnitude of change in FC between the precuneus and the rTPJ was nominally and trend‐wisely correlated with the magnitude of change in the TAS DIF subscale (rho = 0.43, p = .09, uncorrected), which was driven by the association in the cnf group (rho = 0.64, p = .06, uncorrected). When considering only the cnf group, the magnitude of change in FC between the precuneus and the rTPJ was inversely correlated with the magnitude of change in the ERQ cognitive‐reappraisal subscale in the cnf group (rho = −0.70, p = .04, uncorrected). Also, the magnitude of change in FC between the precuneus and the rTPJ was trend‐wisely but inversely correlated with the magnitude of change in the state‐rumination score in the cnf group (rho = −0.51, p = .06, uncorrected). Although there were several associations between neural changes and behavioral changes, these results were no longer significant after controlling for multiple comparisons (Table S4).
3.9. Changes in target ROIs during neurofeedback training
The LME analysis found no significant main effect nor interaction between the time and group effect in neither the precuneus ROI nor the rTPJ ROI (Figure S2A,B). The result indicated that the current connectivity‐based rtfMRI‐nf did not affect the percent BOLD signal changes from each ROI through the experimental runs both in the cnf group and the sham group.
3.10. Seed‐to‐whole brain analysis investigating changes in FC during rtfMRI‐nf
From NF1 to NF3, reduced FC with the precuneus ROI was found in the right superior parietal gyrus in the cnf group with compared to the sham group, and from NF2 to NF3, reduced FCs with the precuneus ROI were found in the bilateral inferior parietal gyrus and left superior parietal gyrus in the cnf group with compared to the sham group (Table S5). No significant regions were found from NF1 to NF2.
4. DISCUSSION
This single‐blind, sham‐controlled proof‐of‐concept study investigated the feasibility of a novel rtfMRI‐nf protocol targeting a rumination‐related brain circuit in healthy participants. It was proven that participants assigned to the cnf group would learn to brake or decrease FC between the precuneus and the rTPJ while engaging with self‐referential processes compared to the sham group. Furthermore, it was proposed that participants undergoing rtfMRI‐nf showed a decreased state‐rumination at post‐NF compared to the participants receiving sham feedback; however, we could not find a significant association between those neural changes and the reduction of state‐rumination. Contrary to our expectations, we could not find a significant change in rsFC among the target circuit before and after rtfMRI‐nf training. Importantly, our exploratory analysis showed no harmful effects because mood changes assessed by STAI‐S and POMS TMD did not worsen after this intervention in both groups. Therefore, this newly developed FC‐based rtfMRI‐nf method was safe and can be used for future clinical trials targeting MAD.
4.1. Primary neural changes supported training success
As part of our fMRI‐nf system, we employed an advanced real‐time processing capacity and the two‐point method to compute on‐the‐fly estimates of FC as neurofeedback signal (Misaki et al., 2020). Our findings preliminarily showed that individuals can modulate the specific FC between the precuneus and the rTPJ, and also recruit relevant brain networks in real‐time with binary presented feedback signals. The two‐point method allowed us time‐sensitive neurofeedback compared with other methods (such as calculating mean average correlations). Of the note and to our knowledge, this is the first study that shows that the two‐point method can be effective in the direction of negative FC coupling (e.g., brake or decreasing FC). This two‐point method estimates the FC using only two‐time points, and it can be argued that this method is less stable than other methods; however, we valued more time‐sensitive feedback presentations suppressing potential motion artifacts. There are several ways to estimate FCs (as we introduced in Section 1), and there are pros and cons for each method. Although more time points would provide us more stable estimation of FCs, those FCs are under risks of motion artifacts. We previously simulated the most convenient way to calculate the target FC for the purpose of feedback, and we adopted two‐point method for this study. Importantly, we could not find any time trends in each target region (Figure S2A,B), which indicates that this dynamic modulation of the target circuit via connectivity‐based rtfMRI‐nf is distinctly different from existing neurofeedback training targeting single ROI activities.
It could be argued that the changes in the target circuit were not a result of the feedback, but rather of the repeated effects of mental strategies during the self‐referential processing. In this study, we used the sham feedback group as a control, giving them equivalent or more positive feedbacks statistically, and participants in the sham group went through the exact same procedure as participants in the cnf group unless there was a difference in the source of the feedback. Therefore, there were no differences in experiences with exposure to the mental strategies and rather, the participants in the sham group received more rewards than those in the cnf group, which provides strong evidence that the neural changes in the cnf group occurred because of the modulation of the target circuit rather than mental strategies or reward experiences. Although our intention was not to discuss what kind of learning theory underpinned this neurofeedback training, the cnf group showed a linear trend of decoupling the target circuit, which indicates that a desired neural pattern triggered by mental strategies was reinforced with trial‐and‐error learning in an operant conditioning manner. We aimed that mental strategies, such as reappraisal and acceptance, would trigger desired neural patterns because those mental strategies were often used in CBT for MAD patients to stop rumination (Watkins, 2018) and we showed that the triggered neural patterns were reinforced by the neurofeedback signal in a timely manner within the cnf group, but not within the sham group. However, we have to note that, by giving frequent reward signals, participants in the sham group might be induced to think that they are effectively controlling (decoupling) their target FC, while their target FC actually was coupled (increased). This might explain why participants in the sham group showed an increased trend of coupling FC through NF1, NF2, and NF3, and therefore could make it statistically easier to detect the differences in target FC between groups, especially at NF3. This potential effect of sham feedback should be considered when we interpret the result and should be carefully considered in future clinical trials.
4.2. Reduction in state‐rumination after one rtfMRI‐nf session
Although the purpose of this study was to determine the feasibility of influencing a rumination‐related brain circuit using the two‐point method rather than to test any potential clinical efficacy, our findings preliminarily give us the notion that connectivity‐based rtfMRI‐nf has the potential to reduce state‐rumination. Since we found a significant reduction in both groups, we could not deny the possibility that repetitively conducting mental strategies, habituation to the self‐referential task, and/or some kinds of placebo expectations had effects on the reduction of state‐rumination. However, participants in the cnf group showed a lower score of state‐rumination at post‐NF compared to the sham group, suggesting that the connectivity‐based rtfMRI‐nf exceeded those possible nuisance effects. These results need a careful interpretation because the lower state‐rumination score for the cnf group at post‐NF showed a marginal significant difference compared to the sham group although the effect size was large (p = .048, FDR corrected, effect size d = 0.84), and we did not find any evidence of relationships in those neural changes together with behavioral changes. We, therefore, need to conduct more larger clinical trials to investigate the clinical efficacy for MDD. Importantly, our results suggest that applying this protocol should not induce harmful effects on the clinical population. Although, and likely, a more careful methodology could be considered, connectivity‐based rtfMRI‐nf targeting a ruminative circuit may be most effective in individuals with aberrant brain circuit regions and a high tendency in ruminative thinking and less effective in individuals who do not suffer from ruminative thoughts. Future research enabled by this work will be needed to examine who can benefit more from connectivity‐based rtfMRI‐nf targeting a specific symptom.
4.3. Changes in rsFC between the precuneus and the rTPJ after one rtfMRI‐nf session
Changes during neurofeedback training will not ensure the generalization of the learning effect. Therefore, we investigated the rsFC change in the target circuit out of the task‐related context. In more detail, we evaluated the neurofeedback success while participants were instructed and motivated to regulate their target circuit in the context of the self‐referential processing, that is, context‐dependent (discussed in Section 4.1), and moving forward; we investigated the rsFC changes in this target circuit without any explicit instruction during the resting‐state scan, that is, context‐free. We could not find statistically significant changes in the precuneus and the rTPJ rsFC between Rest1 and Rest2 in the cnf group, and this result indicated that rtfMRI‐nf did not change the subject baseline brain connectivity with more naturalistic settings compared to task‐related brain connectivity. The changes in rsFC among target circuits were somewhat more modest than the changes in FC among the target circuit seen during neurofeedback training, and therefore, could not reach the statistically significant difference. These modest changes of rsFC were consistent with other neurofeedback studies (Ramot et al., 2017). This may be explained based on the learning theory. In general, operant conditioning occurs in a context‐dependent manner, and showing the instruction on the screen and instructing participants to engage mental strategies during the self‐referential processing served as a context in this study. However, during the resting‐state scan, participants were context‐independent and instructed not to think about anything in particular, which reflects real‐world settings. Therefore, to detect the rsFC changes in the target circuit, the learning effects established during the rtfMRI‐nf should be beyond the context and be generalized into a real‐world setting, and this may not be realized in 1 day. Generalization of what was learned from rtfMRI‐nf to everyday life is another aspect of the evaluation of successful training. Emerging research suggests that improvement in behavior or symptoms can continue for weeks to months after the final neurofeedback session (Mehler et al., 2018; Paret et al., 2019; Rance et al., 2018). As Rance et al. (2018) suggested, the neural changes acquired in the rtfMRI‐nf session may be reinforced over time in everyday life, because correcting an aberrant brain circuit will improve bias to emotional stimuli, and daily experiences will be more normalized with this newly acquired brain status. At this point, updated daily experiences become a social reinforcement and neural learning may continue. The critical question is how long it will take to establish the generalization of this rtfMRI‐nf, and it should be tested in future clinical trials. Future clinical trials should be designed to reveal the underlying mechanism of the time‐course of effectiveness in rtfMRI‐nf. To claim that rtfMRI‐nf follows the aforementioned hypothesized improvement processes, we have to evaluate and track changes in brain activity during the resting‐state scan, participant cognitive bias and their symptoms. Identifying individual differences in the use of different regulation strategies is also important, if we aim to enhance the effectiveness of these generalization processes. For example, a record of post‐training feedback from participants would be helpful in identifying any trends in the establishment and usage of those mental strategies. To facilitate these generalizations of neural leaning, clarifying participant experiences during a successful neurofeedback run and the writing down experiences and strategies established through the neurofeedback training will be needed. We can encourage participants to remember those experiences and use those strategies in everyday life, which will contribute to the augmentation of neural intervention and psychotherapies.
4.4. Functional networks which have been involved during the rtfMRI‐nf
Both target ROIs (i.e., precuneus and rTPJ) belong to the DMN, and we explored comodulated other FCs during rtfMRI‐nf, conducting seed‐to‐whole brain FC analysis. We found decreased (decoupled) FCs from the precuneus seed to bilateral superior parietal gyri and bilateral inferior parietal gyri from NF1 to NF3 and from NF2 to NF3 in the cnf group compared to the sham group. The superior parietal gyrus has close links to the precuneus region, and is involved in mental imagery and recalling personal experiences as well as in attention and visuospatial perception, while the inferior parietal gyrus lies close to the TPJ, or the junction of the visual, auditory and somatosensory cortices, and consists of the supramarginal and the angular gyrus, contributing to language processing (Johns, 2014). Both areas are considered a part of the DMN and are involved in the monitoring of body and emotional state and in self‐referenced cognition (Davey et al., 2016). These results indicated that decoupling specific FC between the precuneus and rTPJ via rtfMRI‐nf influenced other FCs within the DMN.
Depression has been consistently associated with increased connectivity within the DMN as well as increased connectivity between the salience network (SN) and DMN and decreased connectivity between the DMN and the executive control network (ECN; Mulders, van Eijndhoven, Schene, Beckmann, & Tendolkar, 2015), which indicates that failure to suppress DMN during effortful cognitive processing while the ECN and SN appropriately modulate DMN, may result in interference from internal mentation or emotional processing (Sheline et al., 2009). In our study, we could not find any evidence showing that ECN and SN comodulate DMN, and rather we found decreases within the DMN subnetwork in posterior to parietal DMN. Generally, excessive activations in posterior DMN subnetworks as well as midline core within the DMN were reported to relate to depression and rumination (Li et al., 2013). Reduction in the subnetwork between posterior and parietal DMN during effortful cognitive processing may reflect the reduced brain's self‐referential activity. Nevertheless, the lack of evidence that DMN subnetworks comodulate ECN and SN might be attributable to the fact that we recruited healthy participants and their ruminative symptoms were not comparable to clinical levels.
It has been reported that the change of within‐DMN FCs extends to other regions in the default mode modules, and is also associated with FCs in the frontoparietal module (Di & Biswal, 2015). Therefore, changing within DMN subnetwork, especially within posterior to inferior and superior parietal subnetworks, may have the potential to influence other major networks, including anterior DMN, CEN, and SN, which may contribute to recovery from depression. The precise interplay between these networks is still unclear, as many FCs and networks confound these mutual influences. To the best of our knowledge, there is not yet an answer to the question of if abnormality of FCs is a cause of depression or depression itself changes FC, same as ruminative symptoms. There is a limitation that we cannot really know if the correlational relationship could be causal, and much more work is needed to reveal how modulating subnetworks within the DMN will change its connectivity to other main networks or vice versa.
There are several limitations to this study. First, the sample size of this study was relatively small, and therefore, the result of changes in behavioral measures (especially at the follow up) might have been biased, and need to be considered carefully. Second, the study design needs to be improved, especially for View1 (Baseline) and View2 (Transfer) runs. In the initial view condition (View1), participants naturally responded to negative trait words without any volitional control of their brain activities, while in the last view condition (View2), they applied the mental strategy that works best for them with volitional control of their brain activities. Many rtfMRI‐nf studies compared first neurofeedback run (NF1 in this study) and the last transfer run (View2 in this study) without any baseline (View1 in this study), and we proved a reduction in the target FC from NF1 to View2 (trend‐wise significant after FDR correction) in the cnf group; however direct comparison between View1 and View2 could be problematic. Although both groups were instructed in the same way and it was less problematic to interpret the group different than the direct comparison between View1 and View2 within each group, we need to improve the study design for future clinical trials, that is, the same instruction between View1 and View2. Third, we need to improve the sham‐controlled condition for the future clinical trials. The average ratio of the positive feedback (the duration of presenting the blue bars) during the Regulation block in each subject in the cnf group was 33.92% for NF1, 33.07% for NF2, and 38.15% for NF3 (see results presented in Section 3.2). We applied an active sham‐controlled group and gave them 40.00% reward experiences at all neurofeedback runs. Although this controlled reward experiences approach was conservative since the sham group received more positive feedback than the cnf group, the cnf group experienced linear learning trends. Those growing learning experiences should also be controlled in future clinical trials. For example, we can simulate the feedback sequence with the same conditional probabilities (i.e., the probability of the positive feedback sequence: the probability of positive feedback after the positive feedback and after the negative feedback, respectively) as the real cnf group which reflects the growing learning experiences. There are several approaches of selecting the control group (Sorger, Scharnowski, Linden, Hampson, & Young, 2018), and the advantage of using a synthesized signal rather than other approaches is that we can control the specific aspect of the feedback signal (e.g., the probability of the reinforcement). Forth, there are a few technical limitations related to the two‐point method. Since this new approach utilized the two‐point method, feedback signals are designed in a binary manner (+1: the presence of positive feedback or 0: no presence of feedback). Therefore, if the subject's target FC are strongly coupled, one might be discouraged or frustrated by no presence of feedback because of the difficulty in decoupling the target FC, especially the beginning of rtfMRI‐nf. When we compare rtfMRI‐nf with EEG neurofeedback, one of the big differences is a “shaping” method that EEG neurofeedback usually adopts to reinforce one's learning. The process of the shaping method refers to gradually molding or training a person to perform a specific response by reinforcing any responses that are similar to the desired response (Skinner, 1938). Adjusting the task difficulties or developing the feedback display system to consider a shaping method to give them an adequate experience of positive feedback will allow participants to have more opportunities to be reinforced. Since MAD patients can be expected to have stronger FC in the target circuit, based on our previous study (Misaki et al., 2020) and the suggestion that they might have less brain plasticity, MAD patients may have fewer opportunities to be reinforced in decoupling the target circuit, especially in the first and second neurofeedback runs (NF1 and 2). To conduct a clinical trial with this clinical population, we need to consider giving them enough time to practice their mental strategies which trigger desired neural changes with the presence of neurofeedback signals, or we may need to consider adjusting task difficulties and feedback presentation to motivate them, especially in the earlier stages of the neurofeedback training. Fifth, we would like to emphasize that online and offline analyses were preprocessed and calculated in a different way. To evaluate the change in target FC, the two‐point method was used for online feedback calculations in a timely manner and the gPPI was used for an offline analysis so that the signal changes associated with stimulus presentation and nuisance noises (e.g., physiological changes, motions, etc.) were excluded from the FC evaluation. For example, for the online preprocessing, we are not able to adopt censoring approaches because we have no choice but to remove the TRs with large motion, and removing the TR will result in the absence of feedback signal at that time point (which means coupling or increasing in the target FC and can mislead participants’ neurofeedback learning). Although we previously conducted simulation analyses (described in Section 2.5) and selected the two‐point method as the most appropriate approach to calculate FC, online and offline calculation of FC are not exactly the same. To design and apply rtfMRI‐nf, online calculations in a timely manner are favorable; however, it always requires a trade‐off between the time‐sensitiveness and the accuracy of the feedback. Finally, we applied state‐rumination as a secondary outcome; however, participants in this study were healthy volunteers, and their levels of state‐rumination were not compatible to pathological rumination observed in clinical populations. To evaluate clinical efficacy of rtfMRI‐nf in clinical populations, we are better to utilize a task fMRI to measure FCs during their ruminative state, as well as measuring changes in trait‐rumination in a real‐life setting.
5. CONCLUSION
The present study examined the feasibility of FC based rtfMRI‐nf training targeting a rumination‐related circuit between the precuneus and the rTPJ. This study provided the proof‐of‐concept that healthy participants can break or decouple the target FC positively associated with RSS, volitionally with a binary neurofeedback signal. Also, the cnf group showed a greater reduction in state‐rumination scores compared to the sham group after neurofeedback training. Results indicate and suggest that this new approach is feasible for healthy participants and also applicable to individuals with mood and anxiety disorders. More data will be needed to ensure the clinical benefit of this approach for the reduction of maladaptive rumination. Future clinical trials should be designed to answer the following research questions: (a) can MAD patients with high ruminative thinking also learn to modulate the target circuit, (b) can this rtfMRI‐nf improve trait‐rumination and will those behavioral changes be predicted by neural changes, and (c) can time‐courses and generalized leaning effects of the effectiveness of rtfMRI‐nf be assessed with changes of rsFC, cognitive biases, and other rumination‐related symptoms.
CONFLICT OF INTEREST
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
AUTHOR CONTRIBUTIONS
Aki Tsuchiyagaito, Masaya Misaki, Obada Al Zoubi, and Jerzy Bodurka conceived and developed the study design; Masaya Misaki and Jerzy Bodurka developed rtfMRI‐nf system; Masaya Misaki and Jerzy Bodurka developed data acquisition and analysis infrastructure; Masaya Misaki and Jerzy Bodurka developed analysis framework; Martin Paulus, Jerzy Bodurka and Tulsa 1000 (T1000) investigators designed T1000 study and collected data; Aki Tsuchiyagaito performed experiments; Aki Tsuchiyagaito and Masaya Misaki analyzed the data; Aki Tsuchiyagaito and Jerzy Bodurka wrote the original draft paper; Masaya Misaki, Obada Al Zoubi, Jerzy Bodurka, Martin Paulus provided guidance on analyses and critical review of the paper, all authors provided comments on the manuscript.
Supporting information
Appendix S1: Supporting information
ACKNOWLEDGMENTS
We are grateful to Julie Arterbury, Bill Alden, Julie DiCarlo, and Greg Hammond for helping with MRI and EEG‐fMRI scanning. The study was supported by Laureate Institute for Brain Research, the William K. Warren Foundation, and in part by the National Institute of General Medical Sciences Center (Grant Award Number 1P20GM121312).
Tsuchiyagaito A, Misaki M, Zoubi OA, Tulsa 1000 Investigators, Paulus M, Bodurka J. Prevent breaking bad: A proof of concept study of rebalancing the brain's rumination circuit with real‐time fMRI functional connectivity neurofeedback. Hum Brain Mapp. 2021;42:922–940. 10.1002/hbm.25268
DATA AVAILABILITY STATEMENT
The data that support the findings and the data analysis scripts used in this study are available from the corresponding author upon reasonable request.
REFERENCES
- Andrews‐Hanna, J. R. , Reidler, J. S. , Sepulcre, J. , Poulin, R. , & Buckner, R. L. (2010). Functional‐anatomic fractionation of the Brain's default network. Neuron, 65, 550–562. 10.1016/j.neuron.2010.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants, B. B. , Epstein, C. L. , Grossman, M. , & Gee, J. C. (2008). Symmetric diffeomorphic image registration with cross‐correlation: Evaluating automated labeling of elderly and neurodegenerative brain. Medical Image Analysis, 12, 26–41. 10.1016/j.media.2007.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagby, R. M. , Parker, J. D. A. , & Taylor, G. J. (1994). The twenty‐item Toronto alexithymia scale—I. item selection and cross‐validation of the factor structure. Journal of Psychosomatic Research, 38, 23–32. 10.1016/0022-3999(94)90005-1 [DOI] [PubMed] [Google Scholar]
- Bates, D. , Mächler, M. , Bolker, B. , & Walker, S. (2015). Fitting linear mixed‐effects models using lme4. Journal of Statistical Software, 67, 1–48. 10.18637/jss.v067.i01 [DOI] [Google Scholar]
- Birn, R. M. , Smith, M. A. , Jones, T. B. , & Bandettini, P. A. (2008). The respiration response function: The temporal dynamics of fMRI signal fluctuations related to changes in respiration. NeuroImage, 40, 644–654. 10.1016/j.neuroimage.2007.11.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain, R. A. (2007). Navigating the sequenced treatment alternatives to relieve depression (STAR*D) study: Practical outcomes and implications for depression treatment in primary care. Primary Care, 34, 505–519. 10.1016/j.pop.2007.05.006 [DOI] [PubMed] [Google Scholar]
- Cox, R. W. (1996). AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research, 29, 162–173. 10.1006/cbmr.1996.0014 [DOI] [PubMed] [Google Scholar]
- Cox, R. W. , Jesmanowicz, A. , & Hyde, J. S. (1995). Real‐time functional magnetic resonance imaging. Magnetic Resonance in Medicine, 33, 230–236. 10.1002/mrm.1910330213 [DOI] [PubMed] [Google Scholar]
- Davey, C. G. , Pujol, J. , & Harrison, B. J. (2016). Mapping the self in the brain's default mode network. NeuroImage, 132, 390–397. 10.1016/j.neuroimage.2016.02.022 [DOI] [PubMed] [Google Scholar]
- deCharms, R. C. (2008). Applications of real‐time fMRI. Nature Reviews Neuroscience, 9, 720–729. 10.1038/nrn2414 [DOI] [PubMed] [Google Scholar]
- deCharms, R. C. , Christoff, K. , Glover, G. H. , Pauly, J. M. , Whitfield, S. , & Gabrieli, J. D. E. (2004). Learned regulation of spatially localized brain activation using real‐time fMRI. NeuroImage, 21, 436–443. 10.1016/j.neuroimage.2003.08.041 [DOI] [PubMed] [Google Scholar]
- Di, X. , & Biswal, B. B. (2015). Dynamic brain functional connectivity modulated by resting‐state networks. Brain Structure & Function, 220, 37–46. 10.1007/s00429-013-0634-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di, X. , & Biswal, B. B. (2017). Psychophysiological interactions in a visual checkerboard task: Reproducibility, reliability, and the effects of Deconvolution. Frontiers in Neuroscience, 11, 573 10.3389/fnins.2017.00573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehring, T. , & Watkins, E. R. (2008). Repetitive negative thinking as a Transdiagnostic process. International Journal of Cognitive Therapy, 1, 192–205. 10.1521/ijct.2008.1.3.192 [DOI] [Google Scholar]
- Fede, S. J. , Dean, S. F. , Manuweera, T. , & Momenan, R. (2020). A guide to literature informed decisions in the design of real time fMRI neurofeedback studies: A systematic review. Frontiers in Human Neuroscience, 14, 60 10.3389/fnhum.2020.00060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gembris, D. , Taylor, J. G. , Schor, S. , Frings, W. , Suter, D. , & Posse, S. (2000). Functional magnetic resonance imaging in real time (FIRE): Sliding‐window correlation analysis and reference‐vector optimization. Magnetic Resonance in Medicine, 43, 259–268. [DOI] [PubMed] [Google Scholar]
- Glover, G. H. , Li, T. , & Ress, D. (2000). Image‐based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magnetic Resonance in Medicine, 44, 162–167. [DOI] [PubMed] [Google Scholar]
- Gross, J. J. , & John, O. P. (2003). Individual differences in two emotion regulation processes: Implications for affect, relationships, and well‐being. Journal of Personality and Social Psychology, 85, 348–362. 10.1037/0022-3514.85.2.348 [DOI] [PubMed] [Google Scholar]
- Harris, P. A. , Taylor, R. , Thielke, R. , Payne, J. , Gonzalez, N. , & Conde, J. G. (2009). Research electronic data capture (REDCap)—A metadata‐driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics, 42, 377–381. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hvenegaard, M. , Moeller, S. B. , Poulsen, S. , Gondan, M. , Grafton, B. , Austin, S. F. , … Watkins, E. R. (2020). Group rumination‐focused cognitive‐behavioural therapy (CBT) v. group CBT for depression: Phase II trial. Psychological Medicine, 50, 11–19. 10.1017/S0033291718003835 [DOI] [PubMed] [Google Scholar]
- Jo, H. J. , Saad, Z. S. , Simmons, W. K. , Milbury, L. A. , & Cox, R. W. (2010). Mapping sources of correlation in resting state FMRI, with artifact detection and removal. NeuroImage, 52, 571–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns, P. (2014). Chapter 3—Functional neuroanatomy In Johns P. (Ed.), Clinical Neuroscience (pp. 27–47). London, UK: Churchill Livingstone; 10.1016/B978-0-443-10321-6.00003-5 [DOI] [Google Scholar]
- Jones, N. P. , Siegle, G. J. , & Thase, M. E. (2008). Effects of rumination and initial severity on remission to cognitive therapy for depression. Cognitive Therapy and Research, 32, 591 10.1007/s10608-008-9191-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Key, B. L. , Campbell, T. S. , Bacon, S. L. , & Gerin, W. (2008). The influence of trait and state rumination on cardiovascular recovery from a negative emotional stressor. Journal of Behavioral Medicine, 31, 237–248. 10.1007/s10865-008-9152-9 [DOI] [PubMed] [Google Scholar]
- Kim, D.‐Y. , Yoo, S.‐S. , Tegethoff, M. , Meinlschmidt, G. , & Lee, J.‐H. (2015). The inclusion of functional connectivity information into fMRI‐based Neurofeedback improves its efficacy in the reduction of cigarette cravings. Journal of Cognitive Neuroscience, 27, 1552–1572. 10.1162/jocn_a_00802 [DOI] [PubMed] [Google Scholar]
- Koush, Y. , Meskaldji, D.‐E. , Pichon, S. , Rey, G. , Rieger, S. W. , Linden, D. E. J. , … Scharnowski, F. (2017). Learning control over emotion networks through connectivity‐based neurofeedback. Cerebral Cortex, 27, 1193–1202. 10.1093/cercor/bhv311 [DOI] [PubMed] [Google Scholar]
- Koush, Y. , Rosa, M. J. , Robineau, F. , Heinen, K. , Rieger, S. W. , Weiskopf, N. , … Scharnowski, F. (2013). Connectivity‐based neurofeedback: Dynamic causal modeling for real‐time fMRI. NeuroImage, 81, 422–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacroix, J. M. (1986). Mechanisms of biofeedback control In Davidson R. J., Schwartz G. E., & Shapiro D. (Eds.), Consciousness and self‐regulation: Advances in research and theory (Vol. 4, pp. 137–162). Boston, MA: Springer US; 10.1007/978-1-4757-0629-1_6 [DOI] [Google Scholar]
- Lacroix, J. M. , & Gowen, A. H. (1981). The Acquisition of Autonomic Control through Biofeedback: Some tests of discrimination theory. Psychophysiology, 18, 559–572. 10.1111/j.1469-8986.1981.tb01826.x [DOI] [PubMed] [Google Scholar]
- Leech, R. , Kamourieh, S. , Beckmann, C. F. , & Sharp, D. J. (2011). Fractionating the default mode network: Distinct contributions of the ventral and dorsal posterior cingulate cortex to cognitive control. The Journal of Neuroscience, 31, 3217–3224. 10.1523/jneurosci.5626-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeMoult, J. , Arditte, K. A. , D'Avanzato, C. , & Joormann, J. (2012). State rumination: Associations with emotional stress reactivity and attention biases. Journal of Experimental Psychopathology, 4, 471–484. 10.5127/jep.029112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenth, R. V. (2016). Least‐squares means: The R package lsmeans. Journal of Statistical Software, 69, 1–33. 10.18637/jss.v069.i01 [DOI] [Google Scholar]
- Li, B. , Liu, L. , Friston, K. J. , Shen, H. , Wang, L. , Zeng, L.‐L. , & Hu, D. (2013). A treatment‐resistant default mode subnetwork in major depression. Biological Psychiatry, Sources of Treatment Resistance in Depression: Inflammation and Functional Connectivity, 74, 48–54. 10.1016/j.biopsych.2012.11.007 [DOI] [PubMed] [Google Scholar]
- Liew, S.‐L. , Rana, M. , Cornelsen, S. , Fortunato de Barros Filho, M. , Birbaumer, N. , Sitaram, R. , … Soekadar, S. R. (2016). Improving motor Corticothalamic communication after stroke using real‐time fMRI connectivity‐based Neurofeedback. Neurorehabilitation and Neural Repair, 30, 671–675. 10.1177/1545968315619699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzetti, V. , Melo, B. , Basílio, R. , Suo, C. , Yücel, M. , Tierra‐Criollo, C. J. , & Moll, J. (2018). Emotion regulation using virtual environments and real‐time fMRI Neurofeedback. Frontiers in Neurology, 9, 390 10.3389/fneur.2018.00390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaren, D. G. , Ries, M. L. , Xu, G. , & Johnson, S. C. (2012). A generalized form of context‐dependent psychophysiological interactions (gPPI): A comparison to standard approaches. NeuroImage, 61, 1277–1286. 10.1016/j.neuroimage.2012.03.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNair, D. , Lorr, M. , & Droppleman, L. (1971). Manual for the profile of mood states (POMS). San Diego: Educational and Industrial Testing Service. [Google Scholar]
- Megumi, F. , Yamashita, A. , Kawato, M. , & Imamizu, H. (2015). Functional MRI neurofeedback training on connectivity between two regions induces long‐lasting changes in intrinsic functional network. Frontiers in Human Neuroscience, 9, 160 10.3389/fnhum.2015.00160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehler, D. M. A. , Sokunbi, M. O. , Habes, I. , Barawi, K. , Subramanian, L. , Range, M. , … Linden, D. E. J. (2018). Targeting the affective brain—A randomized controlled trial of real‐time fMRI neurofeedback in patients with depression. Neuropsychopharmacology, 43, 1–8. 10.1038/s41386-018-0126-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misaki, M. , Barzigar, N. , Zotev, V. , Phillips, R. , Cheng, S. , & Bodurka, J. (2015). Real‐time fMRI processing with physiological noise correction—Comparison with off‐line analysis. Journal of Neuroscience Methods, 256, 117–121. 10.1016/j.jneumeth.2015.08.033 [DOI] [PubMed] [Google Scholar]
- Misaki, M. , & Bodurka, J. (2019). Comprehensive fMRI real‐time processing improves online functional connectivity evaluation. Rome Italy: Annual Meeting Organization for Human Brain Mapping. [Google Scholar]
- Misaki, M. , Phillips, R. , Zotev, V. , Wong, C.‐K. , Wurfel, B. E. , Krueger, F. , … Bodurka, J. (2018). Real‐time fMRI amygdala neurofeedback positive emotional training normalized resting‐state functional connectivity in combat veterans with and without PTSD: A connectome‐wide investigation. NeuroImage. Clinical, 20, 543–555. 10.1016/j.nicl.2018.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misaki, M. , Tsuchiyagaito, A. , Al Zoubi, O. , Paulus, M. , & Bodurka, J. (2020). Connectome‐wide search for functional connectivity locus associated with pathological rumination as a target for real‐time fMRI neurofeedback intervention. NeuroImage: Clinical, 26, 102244 10.1016/j.nicl.2020.102244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mor, N. , & Winquist, J. (2002). Self‐focused attention and negative affect: A meta‐analysis. Psychological Bulletin, 128, 638–662. 10.1037/0033-2909.128.4.638 [DOI] [PubMed] [Google Scholar]
- Morgenroth, E. , Saviola, F. , Gilleen, J. , Allen, B. , Lührs, M. , Eysenck, M. W. , & Allen, P. (2020). Using connectivity‐based real‐time fMRI neurofeedback to modulate attentional and resting state networks in people with high trait anxiety. NeuroImage: Clinical, 25, 102191 10.1016/j.nicl.2020.102191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulders, P. C. , van Eijndhoven, P. F. , Schene, A. H. , Beckmann, C. F. , & Tendolkar, I. (2015). Resting‐state functional connectivity in major depressive disorder: A review. Neuroscience & Biobehavioral Reviews, 56, 330–344. 10.1016/j.neubiorev.2015.07.014 [DOI] [PubMed] [Google Scholar]
- Nolan, S. A. , Roberts, J. E. , & Gotlib, I. H. (1998). Neuroticism and ruminative response style as predictors of change in depressive symptomatology. Cognitive Therapy and Research, 22, 445–455. 10.1023/a:1018769531641 [DOI] [Google Scholar]
- Nolen‐Hoeksema, S. (2000). The role of rumination in depressive disorders and mixed anxiety/depressive symptoms. Journal of Abnormal Psychology, 109, 504–511. 10.1037/0021-843x.109.3.504 [DOI] [PubMed] [Google Scholar]
- Nolen‐Hoeksema, S. , & Morrow, J. (1991). A prospective study of depression and posttraumatic stress symptoms after a natural disaster: The 1989 Loma Prieta earthquake. Journal of Personality and Social Psychology, 61, 115–121. 10.1037/0022-3514.61.1.115 [DOI] [PubMed] [Google Scholar]
- Nolen‐Hoeksema, S. , Wisco, B. E. , & Lyubomirsky, S. (2008). Rethinking rumination. Perspectives on Psychological Science, 3, 400–424. 10.1111/j.1745-6924.2008.00088.x [DOI] [PubMed] [Google Scholar]
- Oldfield, R. C. (1971). The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia, 9, 97–113. 10.1016/0028-3932(71)90067-4 [DOI] [PubMed] [Google Scholar]
- Paret, C. , Goldway, N. , Zich, C. , Keynan, J. N. , Hendler, T. , Linden, D. , & Kadosh, K. C. (2019). Current progress in real‐time functional magnetic resonance‐based neurofeedback: Methodological challenges and achievements. NeuroImage, 202, 116107 10.1016/j.neuroimage.2019.116107 [DOI] [PubMed] [Google Scholar]
- Power, J. D. , Schlaggar, B. L. , & Petersen, S. E. (2015). Recent progress and outstanding issues in motion correction in resting state fMRI. NeuroImage, 105, 536–551. 10.1016/j.neuroimage.2014.10.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramot, M. , & Gonzalez‐Castillo, J. (2019). A framework for offline evaluation and optimization of real‐time algorithms for use in neurofeedback, demonstrated on an instantaneous proxy for correlations. NeuroImage, 188, 322–334. 10.1016/j.neuroimage.2018.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramot, M. , Kimmich, S. , Gonzalez‐Castillo, J. , Roopchansingh, V. , Popal, H. , White, E. , … Martin, A. (2017). Direct modulation of aberrant brain network connectivity through real‐time neurofeedback. eLife, 6, e28974 10.7554/elife.28974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rance, M. , Walsh, C. , Sukhodolsky, D. G. , Pittman, B. , Qiu, M. , Kichuk, S. A. , … Hampson, M. (2018). Time course of clinical change following neurofeedback. NeuroImage, 181, 807–813. 10.1016/j.neuroimage.2018.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regier, D. A. (1990). Comorbidity of mental disorders with alcohol and other drug abuse. Results from the epidemiologic catchment area (ECA) study. JAMA: The Journal of the American Medical Association, 264, 2511–2518. 10.1001/jama.264.19.2511 [DOI] [PubMed] [Google Scholar]
- Roberts, J. E. , Gilboa, E. , & Gotlib, I. H. (1998). Ruminative response style and vulnerability to episodes of Dysphoria: Gender, neuroticism, and episode duration. Cognitive Therapy and Research, 22, 401–423. 10.1023/a:1018713313894 [DOI] [Google Scholar]
- Ros, T. , Enriquez‐Geppert, S. , Zotev, V. , Young, K.D. , Wood, G. , Whitfield‐Gabrieli, S. , … Thibault, R.T. (2020). Consensus on the reporting and experimental design of clinical and cognitive‐behavioural neurofeedback studies (CRED‐nf checklist). Brain, 143, 1674–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe, R. , & Kanwisher, N. (2003). People thinking about thinking people: The role of the temporo‐parietal junction in “theory of mind.”. NeuroImage, 19, 1835–1842. 10.1016/S1053-8119(03)00230-1 [DOI] [PubMed] [Google Scholar]
- Scheinost, D. , Hsu, T. W. , Avery, E. W. , Hampson, M. , Constable, R. T. , Chun, M. M. , & Rosenberg, M. D. (2020). Connectome‐based neurofeedback: A pilot study to improve sustained attention. NeuroImage, 212, 116684 10.1016/j.neuroimage.2020.116684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheinost, D. , Noble, S. , Horien, C. , Greene, A. S. , Lake, E. M. R. , Salehi, M. , … Constable, R. T. (2019). Ten simple rules for predictive modeling of individual differences in neuroimaging. NeuroImage, 193, 35–45. 10.1016/j.neuroimage.2019.02.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmaling, K. B. , Dimidjian, S. , Katon, W. , & Sullivan, M. (2002). Response styles among patients with minor depression and dysthymia in primary care. Journal of Abnormal Psychology, 111, 350–356. 10.1037//0021-843x.111.2.350 [DOI] [PubMed] [Google Scholar]
- Sheehan, D. V. , Lecrubier, Y. , Sheehan, K. H. , Amorim, P. , Janavs, J. , Weiller, E. , … Dunbar, G. C. (1998). The Mini‐international neuropsychiatric interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM‐IV and ICD‐10. The Journal of Clinical Psychiatry, 59(Suppl 20), 22–33 quiz 34–57. [PubMed] [Google Scholar]
- Sheline, Y. I. , Barch, D. M. , Price, J. L. , Rundle, M. M. , Vaishnavi, S. N. , Snyder, A. Z. , … Raichle, M. E. (2009). The default mode network and self‐referential processes in depression. Proceedings of the National Academy of Sciences of the United States of America, 106, 1942–1947. 10.1073/pnas.0812686106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata, K. , Watanabe, T. , Sasaki, Y. , & Kawato, M. (2011). Perceptual learning incepted by decoded fMRI neurofeedback without stimulus presentation. Science, 334, 1413–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner, B. F. (1938). The behavior of organisms: An experimental analysis. New York: Appleton‐Century‐Crofts. [Google Scholar]
- Smith, J. M. , & Alloy, L. B. (2009). A roadmap to rumination: A review of the definition, assessment, and conceptualization of this multifaceted construct. Clinical Psychology Review, 29, 116–128. 10.1016/j.cpr.2008.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorger, B. , Scharnowski, F. , Linden, D. E. J. , Hampson, M. , & Young, K. D. (2018). Control freaks: Towards optimal selection of control conditions for fMRI neurofeedback studies. NeuroImage, 186, 256–265. 10.1016/j.neuroimage.2018.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spasojević, J. , & Alloy, L. B. (2001). Rumination as a common mechanism relating depressive risk factors to depression. Emotion, 1, 25–37. 10.1037/1528-3542.1.1.25 [DOI] [PubMed] [Google Scholar]
- Spetter, M. S. , Malekshahi, R. , Birbaumer, N. , Lührs, M. , van der Veer, A. H. , Scheffler, K. , … Hallschmid, M. (2017). Volitional regulation of brain responses to food stimuli in overweight and obese subjects: A real‐time fMRI feedback study. Appetite, 112, 188–195. 10.1016/j.appet.2017.01.032 [DOI] [PubMed] [Google Scholar]
- Spielberger, C. D. , Gorsuch, R. L. , Lushene, R. , Vagg, P. R. , & Jacobs, G. A. (1983). Manual for the state‐trait anxiety inventory. Palo Alto, CA: Consulting Psychologists Press. [Google Scholar]
- Spinhoven, P. , Klein, N. , Kennis, M. , Cramer, A. O. J. , Siegle, G. , Cuijpers, P. , … Bockting, C. L. (2018). The effects of cognitive‐behavior therapy for depression on repetitive negative thinking: A meta‐analysis. Behaviour Research and Therapy, 106, 71–85. 10.1016/j.brat.2018.04.002 [DOI] [PubMed] [Google Scholar]
- Taschereau‐Dumouchel, V. , Cortese, A. , Chiba, T. , Knotts, J. D. , Kawato, M. , & Lau, H. (2018). Towards an unconscious neural reinforcement intervention for common fears. Proceedings of the National Academy of Sciences of the United States of America, 115, 3470–3475. 10.1073/pnas.1721572115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victor, T. A. , Khalsa, S. S. , Simmons, W. K. , Feinstein, J. S. , Savitz, J. , Aupperle, R. L. , … Paulus, M. P. (2018). Tulsa 1000: A naturalistic study protocol for multilevel assessment and outcome prediction in a large psychiatric sample. BMJ Open, 8, e016620 10.1136/bmjopen-2017-016620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins, E. (2015). Psychological treatment of depressive rumination. Current Opinion in Psychology, Depression, 4, 32–36. 10.1016/j.copsyc.2015.01.020 [DOI] [Google Scholar]
- Watkins, E. R. (2018). Rumination‐focused cognitive‐behavioral therapy for depression. New York, NY: Guilford Publications. [Google Scholar]
- Watkins, E. R. , Mullan, E. , Wingrove, J. , Rimes, K. , Steiner, H. , Bathurst, N. , … Scott, J. (2011). Rumination‐focused cognitive‐behavioural therapy for residual depression: Phase II randomised controlled trial. The British Journal of Psychiatry, 199, 317–322. 10.1192/bjp.bp.110.090282 [DOI] [PubMed] [Google Scholar]
- Weiskopf, N. , Sitaram, R. , Josephs, O. , Veit, R. , Scharnowski, F. , Goebel, R. , … Mathiak, K. (2007). Real‐time functional magnetic resonance imaging: Methods and applications. Magnetic Resonance Imaging, 25, 989–1003. 10.1016/j.mri.2007.02.007 [DOI] [PubMed] [Google Scholar]
- Wittchen, H. U. , & Essau, C. A. (1993). Comorbidity and mixed anxiety‐depressive disorders: Is there epidemiologic evidence? The Journal of Clinical Psychiatry, 54, 9–15. [PubMed] [Google Scholar]
- Yamada, T. , Hashimoto, R. , Yahata, N. , Ichikawa, N. , Yoshihara, Y. , Okamoto, Y. , … Kawato, M. (2017). Resting‐state functional connectivity‐based biomarkers and functional MRI‐based neurofeedback for psychiatric disorders: A challenge for developing theranostic biomarkers. International Journal of Neuropsychopharmacology, 20, pyx059 10.1093/ijnp/pyx059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita, A. , Hayasaka, S. , Kawato, M. , & Imamizu, H. (2017). Connectivity Neurofeedback training can differentially change functional connectivity and cognitive performance. Cerebral Cortex, 27, 4960–4970. 10.1093/cercor/bhx177 [DOI] [PubMed] [Google Scholar]
- Young, K. D. , Siegle, G. J. , Zotev, V. , Phillips, R. , Misaki, M. , Yuan, H. , … Bodurka, J. (2017). Randomized clinical trial of real‐time fMRI amygdala Neurofeedback for major depressive disorder: Effects on symptoms and autobiographical memory recall. AJP, 174, 748–755. 10.1176/appi.ajp.2017.16060637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young, K. D. , Zotev, V. , Phillips, R. , Misaki, M. , Drevets, W. C. , & Bodurka, J. (2018). Amygdala real‐time functional magnetic resonance imaging neurofeedback for major depressive disorder: A review. Psychiatry and Clinical Neurosciences, 72, 466–481. 10.1111/pcn.12665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young, K. D. , Zotev, V. , Phillips, R. , Misaki, M. , Yuan, H. , Drevets, W. C. , & Bodurka, J. (2014). Real‐time fMRI Neurofeedback training of amygdala activity in patients with major depressive disorder. PLoS One, 9, e88785 10.1371/journal.pone.0088785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young, L. , Camprodon, J. A. , Hauser, M. , Pascual‐Leone, A. , & Saxe, R. (2010). Disruption of the right temporoparietal junction with transcranial magnetic stimulation reduces the role of beliefs in moral judgments. Proceedings of the National Academy of Sciences of the United States of America, 107, 6753–6758. 10.1073/pnas.0914826107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan, H. , Young, K. D. , Phillips, R. , Zotev, V. , Misaki, M. , & Bodurka, J. (2014). Resting‐state functional connectivity modulation and sustained changes after real‐time functional magnetic resonance imaging Neurofeedback training in depression. Brain Connectivity, 4, 690–701. 10.1089/brain.2014.0262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, Z. , Yao, S. , Li, K. , Sindermann, C. , Zhou, F. , Zhao, W. , … Becker, B. (2019). Real‐time functional connectivity‐informed Neurofeedback of amygdala‐frontal pathways reduces anxiety. Psychotherapy and Psychosomatics, 88, 5–15. 10.1159/000496057 [DOI] [PubMed] [Google Scholar]
- Zhou, H.‐X. , Chen, X. , Shen, Y.‐Q. , Li, L. , Chen, N.‐X. , Zhu, Z.‐C. , … Yan, C.‐G. (2020). Rumination and the default mode network: Meta‐analysis of brain imaging studies and implications for depression. NeuroImage, 206, 116287 10.1016/j.neuroimage.2019.116287 [DOI] [PubMed] [Google Scholar]
- Zotev, V. , Krueger, F. , Phillips, R. , Alvarez, R. P. , Simmons, W. K. , Bellgowan, P. , … Bodurka, J. (2011). Self‐regulation of amygdala activation using real‐time fMRI Neurofeedback. PLoS One, 6, e24522 10.1371/journal.pone.0024522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zotev, V. , Phillips, R. , Misaki, M. , Wong, C. K. , Wurfel, B. E. , Krueger, F. , … Bodurka, J. (2018). Real‐time fMRI neurofeedback training of the amygdala activity with simultaneous EEG in veterans with combat‐related PTSD. NeuroImage: Clinical, 19, 106–121. 10.1016/j.nicl.2018.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zotev, V. , Phillips, R. , Young, K. D. , Drevets, W. C. , & Bodurka, J. (2013). Prefrontal control of the amygdala during real‐time fMRI Neurofeedback training of emotion regulation. PLoS One, 8, e79184 10.1371/journal.pone.0079184 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting information
Data Availability Statement
The data that support the findings and the data analysis scripts used in this study are available from the corresponding author upon reasonable request.


