Abstract
Carry‐over effects on brain states have been reported following emotional and cognitive events, persisting even during subsequent rest. Here, we investigated such effects by identifying recurring co‐activation patterns (CAPs) in neural networks at rest with functional magnetic resonance imaging (fMRI). We compared carry‐over effects on brain‐wide CAPs at rest and their modulation after both affective and cognitive challenges. Healthy participants underwent fMRI scanning during emotional induction with negative valence and performed cognitive control tasks, each followed by resting periods. Several CAPs, overlapping with the default‐mode (DMN), salience, dorsal attention, and social cognition networks were impacted by both the preceding events (movie or task) and the emotional valence of the experimental contexts (neutral or negative), with differential dynamic fluctuations over time. Temporal metrics of DMN‐related CAPs were altered after exposure to negative emotional content (compared to neutral) and predicted changes in subjective affect on self‐reported scores. In parallel, duration rates of another attention‐related CAP increased with greater task difficulty during the preceding cognitive control condition, specifically in the negative context. These findings provide new insights on the anatomical organization and temporal inertia of functional brain networks, whose expression is differentially shaped by emotional states, presumably mediating adaptive homeostatic processes subsequent to behaviorally challenging events.
Keywords: brain networks, co‐activation patterns, cognitive control, dynamic functional connectivity (dFC), emotions, negative affect
Dynamic intrinsic functional connectivity brain networks were altered after exposure to negative emotional content and after emotionally influenced cognitive control. Both brain networks fluctuations were associated with changes in self‐reported affect state and cognitive control performance.
1. INTRODUCTION
Transient emotions can lead to carry over effects in brain activity that can be traced after the eliciting episodes (Borchardt et al., 2017; Eryilmaz, van de Ville, Schwartz, & Vuilleumier, 2011; Eryilmaz, van de Ville, Schwartz, & Vuilleumier, 2014; Harrison et al., 2008), including changes in functional connectivity (FC) of the default‐mode network (DMN) normally active at rest (Buckner & DiNicola, 2019). Thus, following exposure to fearful or joyful events (e.g., in brief movie excerpts), several core regions of the DMN such as precuneus, PCC, and VMPFC show enhanced coupling with limbic brain areas including insula, anterior cingulate cortex (ACC), amygdala, and/or striatum as compared to standard neutral rest conditions (Eryilmaz et al., 2011; Eryilmaz et al., 2014). These lingering effects of emotional events on brain states may promote adaptive changes in response to challenging situations, but also provide a neural substrate for maladaptive emotional inertia and ruminative processing characterized by altered emotion regulation (Kuppens, Allen, & Sheeber, 2010). Moreover, changes in emotional states (e.g., following emotional induction or depending on affective traits) are associated with behavioral modifications in various emotional, social, and cognitive tasks performance (Qiao‐Tasserit et al., 2017; Qiao‐Tasserit, Corradi‐Dell'Acqua, & Vuilleumier, 2018).
Emotion carry‐over effects on intrinsic brain activity and connectivity might constitute an important biomarker of spontaneous homeostatic regulation processes which allow the brain to return to “normal” neutral states following transient situational challenges and acute stress responses (Hermans, Henckens, Joëls, & Fernández, 2014). A better understanding of neural systems mediating faster or more complete recovery following exposure to negative emotional episodes would therefore be valuable to better assess or predict maladaptive stress responses and emotional resilience. However, previous studies on emotion carry‐over effects (Eryilmaz et al., 2011; Eryilmaz et al., 2014; Harrison et al., 2008) have focused on highly arousing (e.g., fearful) emotions, and used paradigms with relatively brief emotional stimuli immediately followed by short resting periods (<2 min). It remains to be determined whether similar carry‐over effects also occur after low arousing emotional episodes (e.g., sadness) and for longer periods of time after intervening stimuli or tasks.
Besides emotions, cognitive challenges with high attentional demands can also influence subsequent brain activity at rest (Barnes, Bullmore, & Suckling, 2009; Wexler, Duyck, & Mamassian, 2015), possibly through a remodeling of specific neural pathways engaged by the preceding tasks or stimuli (Kanai & Verstraten, 2005). Behaviorally, prior high demands on executive control abilities can alter subsequent performance on both cognitive and affective tasks (van Steenbergen, 2015; van Steenbergen, Band, & Hommel, 2010), suggesting an impact on partly shared brain systems for coping with stressful situations and self‐regulation such as ACC and insula (Critchley & Garfinkel, 2017). Interestingly, negative emotions may hamper cognitive resource restoration (Dolcos et al., 2020; Gendolla, Tops, & Koole, 2015), while reduced attentional capacities tend to increase rumination in individuals with low mood (Apazoglou et al., 2019). However, it remains unresolved whether the impact of cognitive challenges on subsequent rest involves similar networks as those modulated by emotional events, and whether such impact depends on prior affective state.
In the present study, we aimed to provide new insights on those issues by addressing two related questions: (a) can negative affect (NA) produce lasting changes in the functional dynamics of brain networks at rest? and (b) how those NA‐related effects interact with (i.e., amplify or attenuate) those elicited by prior engagement of effortful cognitive control mechanisms? To address these questions, we implemented a novel paradigm (Figure 1a) in which we probed for the impact of NA on spontaneous brain activity during longer resting periods (5 min), directly subsequent to movie clips with negative (sad) emotional valence (research aim 1). NA is reliably induced by such movies (Borchardt et al., 2017; Raz et al., 2012; Raz et al., 2016; Shiota & Levenson, 2009; Sonkusare, Breakspear, & Guo, 2019), and commonly associated with emotional inertia and ruminative thinking (Kuppens et al., 2010). In addition, we examined whether changes in neural dFC observed at rest after high demands on cognitive control (Schmidt, Notebaert, & van den Bussche, 2015) are also influenced by negative emotions, compared to neutral conditions (research aim 2). For this purpose, we used Stroop and Flanker tasks for eliciting cognitive control, as both tasks engage executive control to respond to goal‐relevant targets in the presence of distractors (Botvinick, Braver, Barch, Carter, & Cohen, 2001; Egner, 2007), and both have been shown to be sensitive to emotion and stressful situations (Egner, Etkin, Gale, & Hirsch, 2008; Hommel, Proctor, & Vu, 2004; Mayr, Awh, & Laurey, 2003; Mayr & Awh, 2009; Schuch & Koch, 2014).
FIGURE 1.
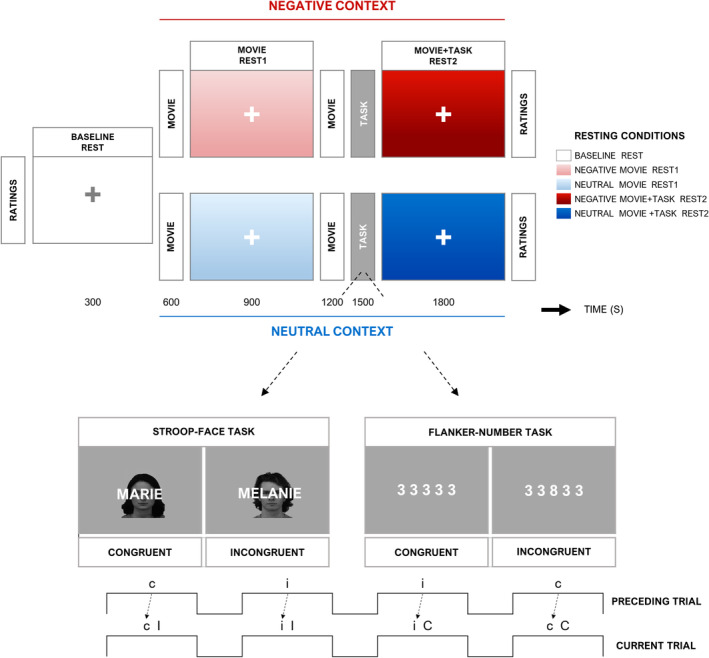
Paradigm design. (a) Initially, a baseline block (baseline rest) was recorded, preceded by an affective screening. Subsequently two experimental contexts (neutral and negative) were presented in the same functional magnetic resonance imaging (fMRI) scanning session. Each context consisted of: 1) a movie clip (5 min) followed by a rest period (movie rest1). 2) A second movie clip (5 min) and a cognitive control task (~5 min) preceding a second rest period (movie + task rest2). The contexts were differentiated by the affective valence of the clips (e.g., two negative films in the negative context). Then, 18 min (approx.) elapsed between the first and second experimental context, in order to implement a second affective screening and the clips subjective assessment. The order of the affective context was counterbalanced across participants. The Stroop and Flanker tasks were equally counterbalanced across participants and affective contexts. (b) Top: Illustration of stimuli in the Stroop task (left) and Flanker task (right). Bottom: Trial types of each cognitive task, including congruent (C) and incongruent (I) trials that could be preceded by either the same or opposite condition (“c” or “i” trials), in a semi‐random but balanced order. This resulted in four trial types, allowing subsequent behavioral analysis according to both current congruence (indicated by upper‐case letters “C” and “I”) and congruence of the preceding trial (indicated by lower‐case letters “c” and “i”)
To investigate spontaneous brain network connectivity, we employed a dynamic FC (dFC) approach (Hutchison, Womelsdorf, et al., 2013; Preti, Bolton, & van de Ville, 2017) based on functional magnetic resonance imaging (fMRI)fMRI with CAPs analysis (Chen, Chang, Greicius, & Glover, 2015; Liu, Zhang, Chang, & Duyn, 2018), in which transient brain configurations are captured at single time points (each time point corresponding to one TR) (Tagliazucchi, Balenzuela, Fraiman, & Chialvo, 2012). We predicted that emotional episodes would produce distinctive changes among functionally interactive brain‐wide networks at rest, with both temporally and anatomically specific modulations as a function of the preceding events. In particular, we hypothesized that (a) exposure to negative emotional events could produce lasting changes in subsequent connectivity and dynamics of spontaneous resting state networks, such as the DMN, salience (SN), or dorsal attention (DAN) networks underlying emotion regulation processes; (b) negative events could also alter the connectivity patterns of posttask resting activity subsequent to high cognitive control demands; and (c) emotional and cognitive carry‐over effects on intrinsic brain network activity might be associated with concomitant differences in subjective affect and behavioral task performance, respectively.
2. METHODS
2.1. Participants
Nineteen French‐speaking female volunteers (mean age = 23.2 ± 4.3) provided written informed consent according to the regional research ethics committee (CCER), University of Geneva. Only female participants were recruited because pilot testing suggested stronger emotional induction in women, compared to males, particularly with the negative movie clips used here. Inclusion criteria were: no history of neurological and psychiatric disease; known menstrual phase and no contraceptive method to rule out hormonal effects on emotional processing and FC (Petersen, Kilpatrick, Goharzad, & Cahill, 2014; Pletzer, Crone, Kronbichler, & Kerschbaum, 2016; Protopopescu et al., 2005); no major head movement during the scanning sessions (head motion >1.5 mm translation or >1.5° rotation in any of the axes). Scanning was scheduled for each participant in the early stage of the menstrual follicular phase, when the levels of estradiol and progesterone hormones are moderate (Andreano, Touroutoglou, Dickerson, & Feldman, 2018; Endicott, 1993). Nicotine and caffeine consumption were prohibited 10 hr before scanning.
2.2. Experimental design
Upon arrival, participants started by briefly practicing the two cognitive control tasks used in the subsequent fMRI session (25 trials from the Flanker and 25 from the Stroop). Details on these tasks and their standardization are described in Supp. Info. (“Cognitive control tasks” section). Next, to assess their subjective affective state, participants filled in the French version of the positive and NA schedule (PANAS). Subsequently, they underwent the fMRI scanning session for approximately 60 min (see experimental sequence illustrated in Figure 1a). A first resting period (baseline rest [5 min]) was recorded to provide a general control for non‐specific effects of time/repetition. Then, two experimental contexts (neutral and negative) were sequentially presented. Each context comprised a movie clip (5 min) followed by an experimental resting period (“movie rest1” condition [5 min]), which allowed us to probe for carry‐over effects as a function of the affective valence of the previous movie. Subsequently, a second movie (5 min) followed by a cognitive control task (~5 min) preceded a second experimental resting period (“movie + task rest2” condition (5 min]). This allowed us to examine how affective carry‐over effects elicited by the second movie modulated any other differential effect produced by the intervening cognitive demands.
The cognitive tasks were based on the Stroop and Flanker paradigms (Figure 1b). Both tasks recruit similar executive components (Frühholz, Godde, Finke, & Herrmann, 2011; Verbruggen, Liefooghe, Notebaert, & Vandierendonck, 2005; Westerhausen et al., 2010). Each participant underwent the cognitive control load twice, once in the “neutral” context, and once in the “negative” context. These tasks (Flanker or Stroop) were always given after the second movie in each experimental context (see Figure 1a), since exerting control demands during the cognitive task prior to the first movie could have modified emotion regulation and emotion experience during this movie (Baumeister, 2003). Note we did not aim at comparing “purely” cognitive and “purely” emotional rest conditions in a fully factorial design but focused on how emotion carry‐over effects were modulated by an intervening task engaging cognitive control processes.
The affective valence of the movies differentiated the two experimental contexts of interest (i.e., two negative clips were presented in the negative context, and two neutral clips were presented in the neutral context, respectively). See experimental sequence in Figure 1. Detailed information on the movies, affective ratings (PANAS), and behavioral task is found in Supp. Info. (“Supplementary Methods” section). Self‐assessments on the movie clips, as well as another affective screening (PANAS) were carried out at the end of each experimental context.
2.3. MRI data acquisition
MRI data were acquired using a 3 T scanner (Siemens TIM Trio) at the Brain & Behavior Laboratory, University of Geneva. A multiband sequence was implemented, with a voxel size of 2 mm isometric and a TR of 1.3 s. See Supp. Info. (“fMRI data acquisition” section), for details on data acquisition. Data preprocessing is described in Supp. Info. (“Preprocessing for CAP analysis on resting periods” section).
2.4. dFC analysis
Our study focused on dFC of brain activity during resting periods only (methods and results on the brain response to movies and cognitive task blocks is reported in supplementary material). Our analysis followed the CAPs methodology described elsewhere (Chen et al., 2015; Liu, Zhang, et al., 2018), and comprised three subsequent steps as described below.
2.5. Seed selection
A preliminary step of the CAP method is the selection of a brain seed region whose connectivity can then be compared between conditions. In keeping with our main goals, we selected a region of interest (ROI) in the precuneus because: (a) it constitutes a major hub of brain networks active at rest, particularly the DMN (Raichle et al., 2001); (b) previous studies showed a reliable modulation during resting state after exposure to negative and positive emotional events (Eryilmaz et al., 2011; Eryilmaz et al., 2014); (c) this region is also frequently recruited during affective and social tasks, particularly in relation to self‐reflective processing and introspection (Cavanna & Trimble, 2006; Engen, Kanske, & Singer, 2017; Fox et al., 2018; Saarimäki et al., 2018); and (d) an independent GLM analysis of the movie blocks (not used in subsequent CAP analysis) revealed significant increases in negative compared to neutral movies (MNI peak 4,‐52, 54; K = 486 voxels; z = 6.50. p < .05 FWE corrected; see Figure 2a, Table S1). This ROI allowed us to define a relevant “anchor” for referencing our CAP analysis of dynamic brain connectivity during the subsequent rest periods, in accordance with the “task–rest interaction” framework of other studies investigating modulations of intrinsic brain networks by preceding tasks or emotions (Eryilmaz et al., 2011; Eryilmaz et al., 2014; Northoff, Qin, & Nakao, 2010).
FIGURE 2.
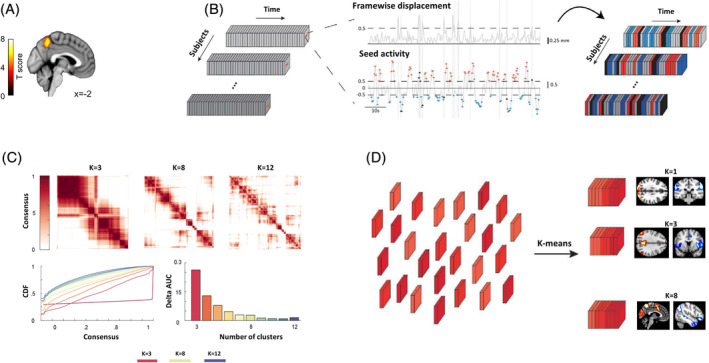
Co‐activation pattern (CAP) analysis pipeline. (a) Seed region of interest (ROI) in precuneus used for the CAP analysis and identified by a preliminary GLM analysis, showing increased activity during negative versus neutral movies. Full GLM results on the movie clips are found in Table S1. (b) The activity time‐course from the precuneus seed is computed for each subject across all resting state blocks, and the frames for which it exceeds a threshold T seed = 0.5 either positively (red) or negatively (blue) are tagged. Frames corrupted by a framewise displacement are also tagged (black) and removed from analysis. (c) A consensus resampling‐based clustering algorithm was implemented to obtain the number and membership (consensus) of reliable CAPs within our dataset. Parameters used to calculate the “consensus rate” between all pairs of samples included 80% of item resampling, a maximum k of 12, and 50 resamplings, with Euclidean distance indices as the distance measurement. Up: Heatmaps of consensus matrices for k = 3, k = 8, k = 12, where values range from 0 (samples are never clustered together across consensus folds) to 1 (always clustered together), marked by white to dark red colors. Bottom left: consensus cumulative distribution function (CDF) of the consensus matrices from k = 2 to k = 12 (k = 3, k = 8, and k = 12; indicated by red, laguna yellow, and purple, respectively), describing how consensus entries distribute for each case. Bottom right: Delta area under the curve plot, indicating the relative change in area under the CDF curve (a larger area change implies a larger increase in the quality of clustering at the assessed k). These results provide qualitative (top matrices) and quantitative (bottom plots) information suggesting that k = 8 is the optimal number of clusters for classifying our functional magnetic resonance imaging (fMRI) rest dataset. (d) Retained frames across subjects (depicted by different shades of red) undergo k‐means clustering to be separated into K different CAPs, each defined as the arithmetic mean between the subset of frames denoting one particular network of regions (voxelwise), with which the seed was strongly co‐(de)active at the same time points. Adapted from reference Bolton et al. (2020)
2.6. Spatiotemporal CAPs generation
Three different metrics were computed to assess temporal fluctuations in the expression of each brain CAP across conditions. See Figure 2, and (Bolton et al., 2020) for a complete description of the CAPs pipeline. “Occurrences” corresponded to the sum of frames assigned to each CAP among all the retained frames, across the entire fMRI scanning runs. “Entries” referred to the number of times that the brain entered a particular state of co‐activation or co‐deactivation corresponding to this CAP. Both metrics were normalized by the total number of each index (i.e., occurrences or entries) relative to other CAPs, for a given condition. Therefore, they were expressed in terms of proportions (%). In addition, “duration” (in seconds) was computed as the average time during which a CAP was sustained when it emerged (i.e., the average of the number of frames, divided by the entries and multiplied by the TR). Finally, the average of each cluster of volumes across all blocks yielded the anatomical CAP maps, representing the spatial configuration of these networks over all resting conditions.
2.7. Statistical analysis of CAPs across conditions
To compare the presence and temporal dynamics of each CAP across conditions, we computed linear mixed models (LMMs) with the “lmerTest” package (Kuznetsova, Brockhoff, & Christensen, 2017), applied to the three quantitative metrics of CAPs (occurrence, entry, duration) from each experimental condition (i.e., baseline rest, negative movie rest1, neutral movie rest1, negative movie + task rest2, neutral movie + task rest2; see Figure 1). The first step of our analysis allowed us to identify the most relevant CAPs (i.e., those responding to specific conditions), by conducting an omnibus analysis with a set of eight LMMs (one for each CAP identified by CAP analysis. See Figure S3c). Occurrences represent the most basic ‐and therefore the main quantitative parameter reflecting the relative importance of a given CAP in a given condition, whereas entries and durations are derivative metrics of CAPs occurrences. To account for nonindependence of repeated data, individual “participants” were modeled as random factors, while the resting “conditions” were introduced as a fixed factor with five levels (i.e., baseline rest, negative movie rest1, neutral movie rest1, negative movie + task rest2, neu‐movie + task rest2). The dependent variable was the “occurrences” computed for each resting condition. The R‐based formula syntax was:
Only those CAPs showing significant variance in occurrences across these different rest conditions were considered for all further analyses (see Section 3 and Figure S3c). To compare the dynamics of the relevant CAPs, a second set of LMMs was carried out on the two other metrics (entries and durations) for those CAPs across the same five resting conditions.
Finally, another set of LMMs was performed in which the “rest baseline” condition was excluded to perform a 2 (emotion context) × 2 (task condition) analysis, using only the four critical resting blocks defined by our experimental manipulation. This analysis comprised the fixed factor “context” with two levels determined by the movies affective valence (negative or neutral) and another fixed factor “event” type with two levels determined by the just preceding condition (movie or cognitive task). Their respective R‐based formula syntax was:
This 2 × 2 analysis allowed for evaluating separately the main effects of preceding stimulus exposure (movie vs. cognitive control) and emotional valence (negative vs. neutral), as well as their interactions, for each temporal metric of each of the selected CAPs. In all analyses, the resulting p values were corrected for multiple comparisons using false discovery rate (FDR) for multiple testing under dependency (Benjamini & Hochberg, 1995; Yekutieli & Benjamini, 2001).
Finally, we tested whether the temporal metrics of relevant brain CAPs (during rest) predicted differences in our measures of subjective affective state (PANAS) and behavioral indices of performance in the cognitive control tasks (interference cost). To probe for partial associations between the different CAP parameters and behavioral measures, we used generalized estimated equations (GEEs) (Ghisletta & Spini, 2004). Here, the CAPs metrics were introduced as multiple outcomes, and the behavioral indices (affective PANAS and RT of the tasks) were the predictor variables. As in the LMMs, GEEs p values were adjusted by the FDR method for multiple testing under dependency.
3. RESULTS
3.1. Behavioral and neural indices of effective emotional induction by movies
We first verified that movies with negative content induced distinctive patterns in both behavior and brain measures, compared to neutral movies. Affective ratings of movie clips confirmed a reliable difference in emotional experience with more negative valence and higher arousal elicited by the negative compared to neutral movies (Supp. Info. [“Supplementary results” section] and Figure S1a). PANAS scores of subjective affect also differed between the prescanning and postscanning measures according to the emotional context, with significant increases in the NA scores following negatively valenced movies (Figure S1b). Further behavioral results concerning the movies are described in Supp. Info. (“Psychological indices of affective stimulation” section).
As expected, fMRI data showed higher brain activity for the contrast “negative > neutral” movies (FWE p < .05) across several regions including anterior insula, putamen, orbitofrontal cortex, middle frontal gyrus, as well as the lingual, inferior occipital, and precuneus (see Table S1, for detailed GLM‐based fMRI results). These increases are consistent with greater engagement of perceptual processes, social cognition (SC), emotional appraisal, and memory systems in response to sad scenarios in these movies (Chen, Gilmore, Nelson, & McDermott, 2017; Elman, Cohn‐Sheehy, & Shimamura, 2013; Göttlich, Ye, Rodriguez‐Fornells, Münte, & Krämer, 2017).
3.2. Behavioral performance in cognitive control tasks
Preliminary assessment of cognitive performance comparing the Flanker and Stroop tasks confirmed an effective standardization of both tasks, as established during our pilot testing. Importantly, there was a reliable interference cost (I > C trials) on response times (RTs) during both tasks (Flanker RT: t(18) = 1.73, p < .001; Stroop RT: t(18) = 1.73, p < .001), with a similar magnitude (mean = 58 vs. 56 ms, [F(1,18) = 1.3, p = .5]; Figure S2a). This interference was found after both the negative clips (t(18) = 3.24, p < .001) and the neutral clips (t(18) = 2.12, p < .05). In addition, interferences costs were larger in the negative than neutral context (mean = 62 vs. 41 ms, t(18) = 2.60. p < .01) (see Figure S2c, and Table S2), consistent with a modulation of cognitive control performance by negative emotional information as reported in previous studies (Song et al., 2017; van Steenbergen, 2015).
We also verified the presence of a congruence sequence effect (CSE; Egner, 2007) (Supp. Info. “Behavioral indices of cognitive control across tasks” section). As expected, it was found in both tasks, with longer RTs on the “cI” trials where attention conflict is exacerbated by weaker task‐set preparation from the preceding trial (Botvinick et al., 2001), compared to the “iI” trials where incongruent stimuli are repeated (Stroop 627 vs. 642, Flanker vs. 649 ms). Interestingly, we also observed a selective increase of RTs on these more difficult trials in the negative compared to neutral context (F(1,126) = 4.9, p = .03); Figure S2d, Table S2), while there was no effect of emotion on the (easiest) “cC” trials (repetition of congruent trials, (F(1,126) = 4.9, p = .99; see Figure S2d, Table S2). Importantly, there was no difference between the Stroop and Flanker tasks, neither in the “negative” context (F(1,9) = 0..31, p = .2) nor in the “neutral” context (F(1,9) = 0..31, p = .4). Taken together, these data confirm not only that higher attentional demand was successfully produced (RT cost due to incongruence between target and distractors) and monitored by participants (CSEs across trials) during both cognitive control tasks, but also indicate that this effect was amplified by a negative affective context, in line with previous research.
3.3. Affective modulation of brain CAPs at rest
We next turned to the main goal of our study, namely, to identify networks active at rest and differentially modulated by the preceding emotional and cognitive control demands. To do so, we applied the CAP methodology on fMRI data from resting blocks to (a) define configurations of brain areas dynamically co‐activated with the precuneus over time, and (b) determine how the expression of these networks varied after exposure to emotional events in movies (”negative movie rest1” and “neutral movie rest1”), and after exposure to both emotional movies and cognitive control tasks (“negative movie + task rest2” and “neutral movie + task rest2”).
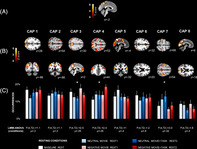
A set of reliable resting state networks was identified by our data‐driven CAP analysis with the precuneus seed ROI (see Figure 2a, Methods and Table S1). Using a clustering with consensus selection procedure (Figure 2c), we found K = 8 as the optimal number of distinct brain maps in terms of replicability across all resting blocks. These eight clusters constitute the most dominant configurations of co‐activated areas with the precuneus over the whole brain during rest blocks in our dataset (see graphical summary in Figure S3). Several of these networks resemble well‐established resting state networks found in previous studies (Sridharan, Levitin, & Menon, 2008), including DMN, DAN, and visual networks among others.
To assess how activity in these networks fluctuated according to experimental conditions, we computed the occurrence rates for each CAP across all resting blocks (baseline, negative movie rest1, neutral movie rest1, negative movie + task rest2, neutral movie + task rest2) (Figure S3). We conducted a first omnibus LMM‐analysis of variance (ANOVA) with five rest conditions (see Section 2.7) to compare these values and identify any effect of condition on the expression of different networks. This analysis showed significant differences in occurrence rates for three of the eight networks: CAP3, CAP4, and CAP7. All differences were predominantly due to emotional context, with no effect driven only by the preceding cognitive task performance. These three CAPs were therefore selected for all further analyses.
CAP3 exhibited co‐active patterns in areas overlapping with the DMN, together with co‐deactive patterns in areas associated with the SN (Figure 3b, Table S4). The omnibus LMM‐ ANOVA of this CAP revealed higher occurrence in the “negative movie rest1” condition (Figure 3a), relative to the “neutral movie rest1” and “neutral movie2 + task rest” conditions (Table 1 for full statistics). The same analysis applied to other temporal parameters showed similar differences for entry rates, consistent with more frequent engagement of this network during rest after negative movies, but no difference in durations.
FIGURE 3.
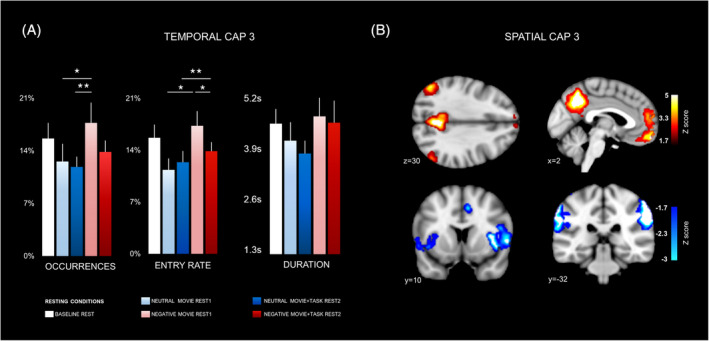
Spatial and temporal characteristics of CAP3. (a) Occurrence rate (left), entry rate (middle), and duration (right), are shown for each experimental condition. Stars indicate significant differences among conditions in a mixed model‐based analysis of variance (ANOVA) (see Section 3 and Table 1). Error bars indicate SEM. (b) Spatial configuration of CAP3. Hot colors (above) represent areas with transient positive co‐activation with the precuneus seed, while cold colors (below) represent areas with transient negative co‐deactivation with precuneus (see Table S4 for details). Slice coordinates are provided in MNI space. Brain regions were observed at Z = <−1.7 (p < .05)
TABLE 1.
Results of LMM‐ANOVA analysis on temporal parameters of relevant CAPs across all rest conditions. Only three CAPs showed significant modulations by experimental conditions. The expression of each of these CAPs was computed in terms of occurrences, entry rates, and durations, and compared between the five resting state blocks (one‐way analysis with five levels)
| Estimates β (SE) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CAP | ANOVA | B | N | NT | A | AT | CONTRAST | CIlow | CIhigh | p |
| CAP3 OCCURRENCES | F(4,72) = 2.4. p = .05 | 15.6 (2.0) | 12.5 (2.0) | 11.8 (2.0) | 18.5 (2.0) | 14.0 (2.0) | A > B | −07.3 | 01.9 | .20 |
| A > N | −10.8 | −01.13 | .01 | |||||||
| A > NT | −11.6 | −01.8 | .00 | |||||||
| A > AT | −0.30 | 09.46 | .11 | |||||||
| CAP3 ENTRIES | F(4,72) = 2.8. p = .03 | 15.6 (1.3) | 11.3 (1.3) | 13.8 (1.3) | 17.3 (1.3) | 13.8 (1.3) | A > B | −0.05 | 0.02 | .43 |
| A > N | −0.10 | −0.01 | .00 | |||||||
| A > NT | −0.01 | 0.09 | .02 | |||||||
| A > A | −0.01 | 0.07 | .04 | |||||||
| CAP4 OCCURRENCES | F(4,72) = 2.5. p = .05 | 11.1 (2.0) | 14.1 (2.0) | 13.8 (2.0) | 13.8 (2.0) | 18.6 (2.0) | AT > B | −12.3 | −2.65 | .00 |
| AT > N | −9.29 | 0.36 | .07 | |||||||
| AT > NT | −9.63 | 0.01 | .04 | |||||||
| AT > A | −9.61 | 0.04 | .05 | |||||||
| CAP4 DURATION | F(4,72) = 2.4. p = .05 | 3.0 (0.3) | 3.1 (0.3) | 2.6 (0.3) | 3.5 (0.3) | 4.1 (0.3) | AT > B | −2.14 | −0.11 | .03 |
| AT > N | −1.94 | 0.08 | .07 | |||||||
| AT > NT | −2.47 | −0.44 | .00 | |||||||
| AT > A | −1.55 | 0.47 | .29 | |||||||
| CAP7 OCCURRENCES | F(4,72) = 2.4. p = .02 | 7.5 (2.6) | 11.2 (2.6) | 13.0 (2.6) | 5.5 (2.6) | 7.7 (2.6) | NT > B | −10.4 | −0.55 | .03 |
| N > A | −10.64 | −0.78 | .02 | |||||||
| NT > A | −12.4 | −2.54 | .00 | |||||||
| NT > AT | 0.35 | 10.21 | .03 | |||||||
| CAP7 ENTRIES | F(4,72) = 3.8. p = .01 | 8.0 (2.2) | 12.1 (2.2) | 12.9 (2.2) | 5.6 (2.2) | 9.2 (2.2) | NT > B | −0.09 | −0.00 | .03 |
| N > A | −0.10 | −0.02 | .00 | |||||||
| NT > N | −0.05 | 0.03 | .73 | |||||||
| NT > A | −0.11 | −0.02 | .00 | |||||||
Note: B, baseline rest; N, neutral movie rest1; NT, neutral movie + task rest2; A, negative movie rest1; AT, negative movie + task rest2. p > .1; * p > .05; ** p > .01; ***p > .001.). p‐Adjustment by the FDR method for multiple testing under dependency.
Abbreviations: ANOVA, analysis of variance; CAP, co‐activation patterns; FDR, false discovery rate; LMM, linear mixed model.
The temporal parameters of CAPs were also assessed using a LMM‐based ANOVA with a 2 × 2 factorial design, probing separately for the effects of “emotional context” (negative vs. neutral) and preceding “event” type (movie vs. movie + task), without the baseline condition (presented only once). For CAP3, this analysis yielded a main effect of the “context” factor, due to higher occurrences and higher entry rates at rest after negative movies compared to neutral movies overall (Figure 3a. See full statistics in Table S5). There was no effect or interaction involving the preceding “event” type factor. Differences in the duration of CAP3 were not significant.
The second relevant network, CAP4, encompassed several brain areas typically associated with the DAN network (Fox, Corbetta, Snyder, Vincent, & Raichle, 2006), including bilateral frontoparietal regions and dorsal ACC (dACC). Conversely, co‐deactive areas included parts of the SC network (Pillemer, Holtzer, & Blumen, 2017; Puhan et al., ), such as the bilateral superior temporal sulcus (STS) and temporo‐parietal junction (TPJ), the left orbital inferior frontal gyrus, as well as lateral occipital visual regions (Figure 4b, Table S4). The omnibus analysis of temporal metrics of this CAP across all five resting conditions indicated predominant differences in both the occurrence rates and durations during the “negative movie + task rest2” block, compared to the “rest baseline,” “neutral movie1rest,” and “neutral movie + task rest2” (Figure 4a, Table 1). The subsequent 2 × 2 factorial analysis (without baseline) further showed a significant main effect of “emotional context” (negative vs. neutral) for the duration metric, reflecting more prolonged dwell times of CAP4 both after negative movies alone (negative movie rest1) and after the cognitive control task following such negative movies (negative movie + task rest2) (Table S5).
FIGURE 4.
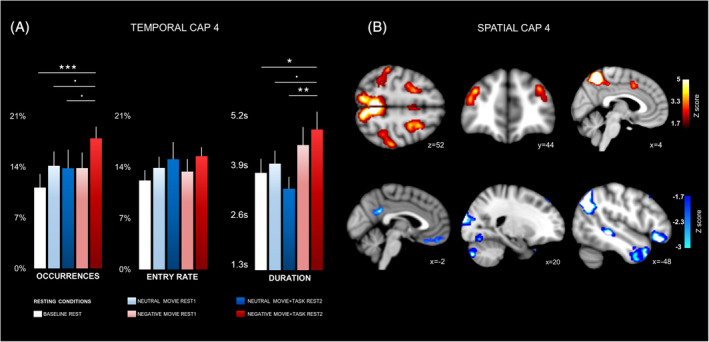
Spatial and temporal characteristics of CAP4. (a) Occurrence rate (left), entry rate (middle), and duration (right) across all participants are shown for all conditions. Stars indicate significant differences between conditions (see Section 3 and Table 1). Error bars indicate SEM. (b) Spatial configuration of CAP4. Hot colors (above) represent areas with transient positive co‐activation with the precuneus seed, while cold colors (below) represent areas with transient negative co‐deactivation with precuneus (see Table S4 for details). Slice coordinates are provided in MNI space. Brain regions were observed at Z = < −1.7 (p < .05)
The third CAP7 comprised CAPs in both lateral brain regions (postcentral and precentral gyri) and more limited medial frontal regions (ACC and supplementary motor cortex), overlapping with sensorimotor networks. Co‐deactivation patterns selectively involved the thalamus (in medial dorsal and ventral anterior portions; Figure 5b. Table S4). The omnibus analysis of temporal parameters across all five rest conditions showed significant differences in occurrence and entry rates during the “neutral movie rest1” and “neutral movie + task rest2” conditions relative to all other conditions, that is, “baseline rest,” “negative movie rest1”, and “negative movie + task rest2” blocks (see Figure 5a, Table 1). Likewise, a significant main effect of “emotional context” was also observed in the 2 × 2 factorial analysis for the same metrics (occurrences and entry rates), indicating that this specific CAP was generally less present after exposure to negative than neutral movie content (Table S5).
FIGURE 5.
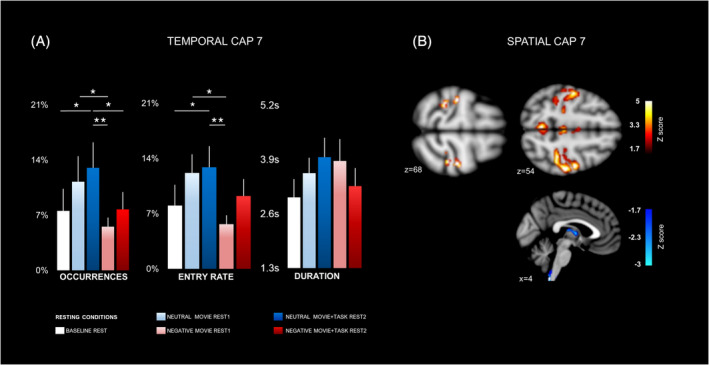
Spatial and temporal characteristics of CAP7. (a) Occurrence rate (left), entry rate (middle), and duration (right) across all participants are shown for all conditions. Stars indicate significant differences between conditions (see Section 3 and Table 1). Error bars indicate SEM. (b) Spatial configuration of CAP7. Hot colors (above) represent areas with transient positive co‐activation with the precuneus seed, while cold colors (below) represent areas with transient negative co‐deactivation with precuneus (see Table S4 for a details). Slice coordinates are provided in MNI space. Brain regions were observed at Z = <−1.7 (p < .05)
3.4. Linking brain CAPs during rest to affective and behavioral measures
Finally, we examined whether the differential expression of resting brain networks across conditions could be related to differences in affective states and behavioral measures. To this aim, we performed partial association analyses of CAP parameters with our indices of subjective affect and cognitive control efforts, respectively, using two sets of GEE‐based models.
A first analysis examined the subjective affective impact of negatively valenced events alone by comparing the first “movie rest1” blocks across the two emotional contexts. The temporal metrics of the three relevant CAPs (occurrences, entries, and durations) from these conditions were introduced in the model as multiple outcomes, while the subjective affective measures (prescanning and postscanning scores on PANAS) were used as predictor variable. A second analysis examined the affective aftermath of negative events followed by the cognitive control task, using the same CAP metrics but now from the “movie + task rest2” blocks as multiple predictors, and with behavioral indices of cognitive control (mean RT in each task condition and interference cost [i.e., RT difference on I > C trials]) as outcome variables.
Results from these analyses are shown in Figure 6. Concerning subjective affect measures, we found a significant and highly selective relationship of the entry rates of CAP3 during the “negative movie rest1” condition, with the NA scores assessed after exposure to the negative context (Figure 6a; β = .48, 95% CI (0.13–0.83), p = .03). This partial association was specific for this CAP (Figure 6a). In other words, the more frequent the expression of CAP3 during “negative movie rest1” blocks, the more negative the affective ratings of participants were after exposure to the negative movies. In contrast, the “positive affect” (PA) score on PANAS (postcontexts) was associated with none of the CAPs in the same (negative or neutral) contexts. For completeness, further inspection of the data from other indicated that CAP3 was also positively associated with NA scores only at the end of the negative context blocks conditions (Figure S4a), while it was negatively associated with PA scores prior to the movies in the neutral context (Figure S4a, β = −1.69, 95% CI (−2.40; −0.99), p < .01). Finally, concerning behavioral indices of cognitive control, we found that entry rates of CAP3 in the “negative movie + task rest2” condition were positively associated with the interference cost on RTs (I > C) during the preceding cognitive task (β = 10.49, 95% CI [4.73; 16.2], p < .01). This was again specific to this CAP and to the NA context (Figure 6b).
FIGURE 6.
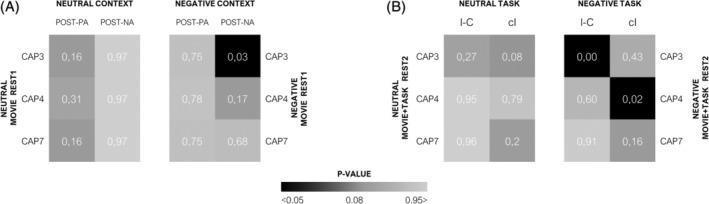
Functional relationship of co‐activation patterns (CAPs) expression with behavioral indices. (a) Probability (p) values of partial associations between affective scores (PANAS) and the three CAPs of interest. PANAS ratings measured after each experimental context (post movie exposure) were modeled as predictors, whereas entry rates of CAPs from the “movie rest1” conditions were introduced as multiple predicted variables in the Generalized estimated equations (GEEs) models. (b) Probability (p) values of partial associations between cognitive control load and the relevant CAPs. Cognitive load was measured by the interference effect (I–C trials) and response times (RTs) on “cI” trials (most difficult task condition) and used as predictors, whereas the CAPs (entries and durations) from the “movie + task rest2” conditions were outcome variables. The p value significance was adjusted by the false discovery rate (FDR) method for multiple testing under dependency
In contrast, CAP4 showed a clearly distinct pattern of associations, with longer durations during the “negative movie + task rest2” condition linked to longer RTs on conflict trials in the preceding cognitive task (Figure 6b; β = 18.50. 95% CI [6.29; 30.7], p < .02). Remarkably, this partial association was selective for incongruent trials when these were preceded by congruent ones (“cI” condition), that is, the most attention‐demanding condition (see also behavioral results above). This association between CAP4 and RTs in the negative context was not found for other trial types (Figure S4d), and absent in the neutral context (Figure S4c).
No significant association was identified between CAP7 and our cognitive control measures of interest. Altogether, these results underscore a differential significance of relevant CAPs and their modulation by negative emotion in both the spontaneous and posttask rest conditions.
4. DISCUSSION
4.1. Lingering effects of emotion on subsequent resting brain activity
NA elicited by movies produced lasting influences on spontaneous brain network activity during subsequent rest, not only immediately after the movie but also after the performance of an intervening cognitive control task engaging attention to other nonemotional stimuli. Such aftermath of NA influenced specific networks overlapping with the DMN and SN (CAP3), the DAN (CAP4), and sensorimotor areas (CAP7), with the first two (CAP3 and CAP4) showing enhanced expression in negative context‐related resting conditions, and the latter (CAP7) showing reduced expression. Remarkably, modulations of CAP3 and CAP4 were linked to specific behavioral indices of subjective affect and cognitive performance after negative movies, further supporting their functional relevance. Taken together, our findings demonstrate that negative affective episodes can alter brain networks over prolonged periods of time following emotional episodes themselves, presumably reflecting regulatory mechanisms acting to adapt behavior and restore normal homeostatic conditions in response to environmental challenges (Critchley & Garfinkel, 2017; Eryilmaz et al., 2014; Hermans et al., 2014). Below we discuss the main CAPs associated with these changes and their possible functional significance.
4.2. Emotional aftermath reflected in CAP3
CAP3 partly overlapped with the DMN and showed greater expression (higher occurrence and entry rates) at rest after exposure to negative movies. Apart from a key role in self‐referential mental activity and introspection (Engen et al., 2017; Kober et al., 2008; Liemburg et al., 2012), DMN activity has consistently been related to emotion processing and mood disorders (Hyett et al., 2015; Lanius, Frewen, Tursich, Jetly, & McKinnon, 2015; Liemburg et al., 2012; Sheline et al., 2009; Song, Zhang, & Huang, 2016), and was for instance reported as a biomarker for depressive rumination (Belleau, Taubitz, & Larson, 2015; Hamilton, Farmer, Fogelman, & Gotlib, 2015; Sambataro, Wolf, Pennuto, Vasic, & Wolf, 2014; Whitfield‐Gabrieli & Ford, 2012). Accordingly, in our participants, more frequent occurrences of CAP3 after negative movies predicted more negative subjective affect in postcontext ratings. This observation suggests that the increased dFC of DMN‐like CAP3 at rest in this condition (negative movie rest1) may be critically involved in emotion regulation and self‐referential processes engaged by negatively valenced information. Furthermore, we also observed a negative relationship between CAP3 and PA scores prior to scanning (baseline ratings), confirming a functional link of this specific brain activity pattern at rest with affective state. Thus, the higher an individual experienced low PA or high NA, the more this DMN‐like network appeared and fluctuated at rest.
In addition, there was a significant relationship between the entry rates of CAP3 and the interference magnitude on RTs during cognitive control (I > C trials) after exposure to the negative context. These findings demonstrate that the aftermath of negative events may produce sustained changes in brain network dynamics that do not only modify spontaneous resting state activity and subjective affect, but also impact on cognitive performance during behavioral challenges. This is consistent with an interwoven functional relationship between DMN and attentional networks and their modulation by negative mood (Piguet et al., 2016), and with previous reports of altered cognitive performance after exposure to negative emotions (Dolcos et al., 2020; Gendolla et al., 2015). Accordingly, our behavioral results during the cognitive control tasks showed higher RT costs (larger interference effect and larger CSEs) in the negative compared to the neutral context, in agreement with other studies on emotion–cognition interaction (Schuch & Koch, 2014; van Steenbergen et al., 2010). Altogether, these data converge to suggest that negative emotional events may trigger lingering self‐regulatory processes partly mediated by the DMN, which reflect changes in subjective affect and reduce the efficacy of selective attention and strategic cognitive control in attention‐demanding conditions.
Simultaneously, the positive CAP in DMN associated with CAP3 was accompanied by co‐deactivation in a saliency‐related network (SN). Such anticorrelated DMN‐SN configuration, in the aftermath of negative emotional events, accords with studies reporting that the generation of affective states may involve a coordinated recruitment of DMN and SN regions, each mediating dissociable emotion component processes (Engen et al., 2017). In this scheme, dynamic interactions between these two networks might subserve shifts from externally oriented attention to internally oriented or self‐related cognition (Menon & Uddin, 2010). The co‐deactive SN could thus amplify interoceptive signals and subjective emotion feelings (Chang, Yarkoni, Khaw, & Sanfey, 2013) through their interaction with self‐reference representations elaborated in DMN areas, implying a distinctive antagonistic dynamics of connectivity of each network with the precuneus, and ultimately contributing to subjective emotional experiences. More generally, such DMN‐SN push‐pull balance is consistent with other effects of negative emotions and stress on selective attention and thought content. This is typically observed for ruminations, characterized by repetitive retrieval of aversive and threat information about the self and past events, which is also exacerbated by negative contextual cues (Piguet et al., 2014; Trapnell & Campbell, 1999).
We conclude that both the temporal and spatial features of CAP3 reveal a functional inertia in the dynamics of large‐scale brain networks that follows negative emotional events, overlapping with components of both the DMN and SN. This inertia is intimately connected to changes in subjective emotional experience and self‐monitoring, presumably reflecting adaptive or homeostatic (possibly automatic and implicit) emotion regulation mechanisms.
4.3. Aftermaths of emotion and cognitive control associated with CAP4
A more dorsal network pattern overlapping with the DAN was found in CAP4, which showed distinctive increases in occurrence as well as duration after negative movies. This was particularly the case when the rest period followed the intervening cognitive control task (negative movie + task rest2) relative to other conditions, that is, after cognitive resources were mobilized in a negative emotional context (Figure 4). CAP4 comprised several regions responsible for selective attention (Fox et al., 2006), including not only frontoparietal areas involved in executive control (Ptak, 2012; Shulman et al., 2010) but also dorsal ACC, associated with the maintenance of alertness (Sadaghiani & D'Esposito, 2015) as well as response conflict monitoring (Botvinick et al., 2001). Given its modulation by the just preceding cognitive task, the higher occurrence rates of CAP4 might constitute a functional fingerprint of high attentional load. Accordingly, this functional pattern was selectively amplified by the negative context, while we found no similar effect when the cognitive control task was performed after neutral movies. Further evidence to support a link of such emotionally amplified cognitive load and resource depletion with heightened dFC of CAP4 in subsequent rest comes from the significant association between CAP4 duration rates and RT measures on incongruent (cI) trials during the cognitive control task, observed only in the negative affective context (see Figure 6b).
The current data therefore add to previous research indicating that negative emotions are associated with reduced attention, decreased problem‐solving abilities, and impaired execution of instrumental behaviors (Bonanno, Goorin, & Coifman, 2008; Nolen‐Hoeksema, 1991; Nolen‐Hoeksema, Wisco, & Lyubomirsky, 2008). Here, we show that such effects may imply a durable reconfiguration and a dynamic modulation of large‐scale network connectivity that is apparent even during resting state. Such changes may impact on the subsequent mobilization of efficient neural resources during cognitive task performance as well as during subsequent recovery periods allowing resource restoration.
In addition, a co‐deactive network was also observed in CAP4 implicating STS, TPJ, temporal poles, as well as PCC and vmPFC/OFC (Pillemer et al., 2017). This network overlaps with areas commonly involved in SC and value‐based decision‐making (Fox et al., 2018; Ochsner, Silvers, & Buhle, 2012; Sinha, Lacadie, Constable, & Seo, 2016). Such effects may reflect a relative disengagement of social information processing and empathic responding normally evoked after negative movies, but partly suppressed after performance of a difficult cognitive task. Noteworthy, some of these areas were co‐active with CAP3 when rest directly followed the negatively valenced movies. However, further analyses investigating the exact functional relationships between spontaneous mental processes engaged at rest in different affective contexts and concomitant brain activation patterns will be necessary to support our interpretations.
4.4. Preferential expression of CAP7 after neutral rather than negative affective conditions
Unlike CAP3 and CAP4, CAP7 network was functionally enhanced during rest periods in the neutral context, comprising a thalamocortical network centered on sensorimotor areas (Llinás, Ribary, Contreras, & Pedroarena, 1998; Ribary, 2005; Ribary et al., 1991), unrelated to higher‐level cognitive or affective functions associated with other CAPs. Thalamocortical circuits similar to CAP7 have been associated with regulation of sensory gating (McCormick & Bal, 1994) as well as arousal or wakefulness (Liu et al., 2018). Moreover, CAPs in thalamus and postcentral cortex have been associated with cognitive load during working memory and visual attention tasks (Tomasi, Ernst, Caparelli, & Chang, 2006). The modulation of CAP7 may therefore reflect more externally oriented attention to sensory and “cold” cognitive information during rest after exposure to neutral movies (as opposed to more introspective and “warm” socio‐affective processing after negatively affective movies). While this interpretation remains speculative, the distinctive functional profile of CAP7 further highlights the selectivity of changes in connectivity dynamics induced by affective and cognitive challenges.
4.5. Limitations and future research
Our study included only female participants. This gender selection was based on pilot results, in which women reported higher emotional ratings. It is possible that, in our paradigm, women assumed more easily an empathetic perspective in response to movie clips, where female characters were often depicted facing difficult emotional situations (e.g., with a child or a partner) that might occur in real life situations. Accordingly, emotional induction with social material was also found to be more reliable in females and motivated similar selection procedure in other studies on emotion regulation (Vrtička, Bondolfi, Sander, & Vuilleumier, 2012). Our results therefore need to be replicated in males, even though there is no reason why the overall pattern of postaffect and posttask changes in resting state would differ between genders (while they may differ in magnitude to some extent).
Another possible limitation is that the movie and task blocks were presented in the same order in both contexts, even though the emotional valence (negative vs. neutral) and nature of tasks (Stroop vs. Flanker) were fully counterbalanced across participants. This was chosen by design for the sake of experimental efficiency and to minimize any impact of attentional depletion after cognitive control on subsequent emotional responses to movies. Thus, any order effect between the first and second rest periods would not undermine our main results, since our focus concerned the rest conditions and their comparison between the two emotion contexts. A direct comparison of posttask versus postemotion carry‐over effects on brain activity at rest was beyond the scope of the present study and will require further research.
Finally, we used rest blocks with relatively short time windows (5 min). Many resting state fMRI studies employ longer durations (8–10 min), but our paradigm design required briefer sessions to allow measuring several resting conditions in the same participants, while avoiding discomfort and boredom, which could have altered our results. Moreover, other postemotion rest studies (Eryilmaz et al., 2011; Eryilmaz et al., 2014) found reliable changes with even shorter rest periods (<2 min). Also, this issue was controlled in the present study by two related technical aspects. First, fMRI scanning was performed with a multiband sequence with a fast repetition time (TR = 1.3), which enabled us to acquire a greater number of fMRI time points in order to increase our statistical power. Second, and more important, the optimal fMRI data necessary for a reliable analysis of brain dFC with the CAP approach is dependent on the number of timepoints, that is, image volumes, rather than the length of the scanning (Liu, Zhang, et al., 2018).
5. CONCLUSION
Our work extends previous studies revealing a lasting impact of NA on brain states, beyond the transient events eliciting particular emotions (Eryilmaz et al., 2011, 2014), and highlights their selective impact on the FC dynamics of networks associated with self‐regulation, salience processing, and attention. In addition, we show that such affective aftermaths modulate brain activity at rest not only immediately following emotional events but also after engaging attention to a different task requiring high cognitive control, beyond the direct influence of affect on task performance itself. Future work probing functional CAPs and their temporal dynamics in patients with mood disorders and affective regulation deficits might provide new insights on potential neuromarkers of emotion inertia (Kuppens & Verduyn, 2017) associated with clinical symptoms, risk, or prognosis of mental diseases.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICS STATEMENT
This work was approved by the regional research ethics committee (CCER), University of Geneva.
PARTICIPANT CONSENT
Participants of this study provided written informed consent according to the guidelines of the regional research ethics committee (CCER), university of Geneva.
Supporting information
APPENDIX S1: Supporting information
FIGURE S1 Supporting information
ACKNOWLEDGMENTS
This work was supported by the Swiss Excellence Scholarship Program, the Schmidheiny Foundation, and the Colombian Science Ministry (J. G.), as well as by a Sinergia Grant No. 180319 from the Swiss National Science Foundation (SNF), by the Swiss Center of Affective Sciences financed by UNIGE and SNF (Grant No. 51NF40_104897), and by the Société Académique de Genève (SACAD). This study was conducted on the imaging platform at the Brain and Behavior Lab (BBL) and benefited from support of the BBL technical staff. Imaging analysis was carried out at University of Geneva on the high‐performance computing (HPC) BAOBAB server. The authors thank Ben Meuleman, Alejandro Salazar Couso, and Paolo Ghisletta for their advices regarding the statistics implemented on CAPs data. Also, special thanks to Dr Sevada Hovsepyan for his support during data analysis.
Gaviria J, Rey G, Bolton T, Delgado J, Van De Ville D, Vuilleumier P. Brain functional connectivity dynamics at rest in the aftermath of affective and cognitive challenges. Hum Brain Mapp. 2021;42:1054–1069. 10.1002/hbm.25277
Funding information Ministerio de Ciencia Tecnología e Innovación. Colombia; Schmidheiny Foundation; Société Académique de Genève; Swiss Center of Affective Sciences, Grant/Award Number: 51NF40_104897; Swiss National Science Foundation, Grant/Award Number: 180319; Sinergia; Colombian Science Ministry; Swiss Excellence Scholarship Program
DATA AVAILABILITY STATEMENT
Derived data supporting the findings of this study are available from the corresponding author (J. G.) on request.
REFERENCES
- Andreano, J. M. , Touroutoglou, A. , Dickerson, B. , & Feldman, B. L. (2018). Hormonal cycles, brain network connectivity, and windows of vulnerability to affective disorder. Trends in Neurosciences, 41, 660–676. 10.1016/j.tins.2018.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apazoglou, K. , Küng, A.‐L. , Cordera, P. , Aubry, J.‐M. , Dayer, A. , Vuilleumier, P. , & Piguet, C. (2019). Rumination related activity in brain networks mediating attentional switching in euthymic bipolar patients. International Journal of Bipolar Disorders, 7, 3 10.1186/s40345-018-0137-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes, A. , Bullmore, E. T. , & Suckling, J. (2009). Endogenous human brain dynamics recover slowly following cognitive effort. PLoS One, 4, e6626 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumeister, R. F. (2003). Ego depletion and self‐regulation failure: A resource model of self‐control. Alcoholism, Clinical and Experimental Research, 27, 281–284. [DOI] [PubMed] [Google Scholar]
- Belleau, E. L. , Taubitz, L. E. , & Larson, C. L. (2015). Imbalance of default mode and regulatory networks during externally focused processing in depression. Social Cognitive and Affective Neuroscience, 10, 744–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini, Y. , & Hochberg, Y. (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society: Series B, 57, 289–300. http://doi.wiley.com/10.1111/j.2517-6161.1995.tb02031.x. [Google Scholar]
- Bolton, T. A. W. , Tuleasca, C. , Wotruba, D. , Rey, G. , Dhanis, H. , Gauthier, B. , … van de Ville, D. (2020). TbCAPs: A toolbox for co‐activation pattern analysis. NeuroImage, 211, 116621 http://arxiv.org/abs/1910.06113. [DOI] [PubMed] [Google Scholar]
- Bonanno, G. A. , Goorin, L. , & Coifman, K. G. (2008). Sadness and grief In The handbook of emotion, 3rd ed. New York, NY: The Guilford Press. pp 797–810. [Google Scholar]
- Borchardt, V. , Fan, Y. , Dietz, M. , Melendez, A. L. H. , Bajbouj, M. , Gärtner, M. , Li M, Walter M, Grimm S. (2017). Echoes of affective stimulation in brain connectivity networks. Cerebral Cortex, 28, 4365–4378. Retrieved from http://academic.oup.com/cercor/advance-article/doi/10.1093/cercor/bhx290/4637595. [DOI] [PubMed] [Google Scholar]
- Botvinick, M. M. , Braver, T. S. , Barch, D. M. , Carter, C. S. , & Cohen, J. D. (2001). Conflict monitoring and cognitive control Matthew. Psychological Review, 108, 624–652. [DOI] [PubMed] [Google Scholar]
- Buckner, R. L. , & DiNicola, L. M. (2019). The brain's default network: Updated anatomy, physiology and evolving insights. Nature Reviews. Neuroscience, Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/31492945, 20, 593–608. [DOI] [PubMed] [Google Scholar]
- Cavanna, A. E. , & Trimble, M. R. (2006). The precuneus: A review of its functional anatomy and behavioural correlates. Brain, 129, 564–583. [DOI] [PubMed] [Google Scholar]
- Chang, L. J. , Yarkoni, T. , Khaw, M. W. , & Sanfey, A. G. (2013). Decoding the role of the insula in human cognition: Functional parcellation and large‐scale reverse inference. Cerebral Cortex, 23, 739–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, H.‐Y. , Gilmore, A. W. , Nelson, S. M. , & McDermott, K. B. (2017). Are there multiple kinds of episodic memory? An fMRI investigation comparing autobiographical and recognition memory tasks. The Journal of Neuroscience, 37, 2764–2775 Retrieved from http://www.jneurosci.org/lookup/doi/10.1523/JNEUROSCI.1534-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, J. E. , Chang, C. , Greicius, M. D. , & Glover, G. H. (2015). Introducing co‐activation pattern metrics to quantify spontaneous brain network dynamics. NeuroImage, 111, 476–488. 10.1016/j.neuroimage.2015.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley, H. D. , & Garfinkel, S. N. (2017). Interoception and emotion. Current Opinion in Psychology, 17, 7–14. 10.1016/j.copsyc.2017.04.020. [DOI] [PubMed] [Google Scholar]
- Dolcos, F. , Katsumi, Y. , Moore, M. , Berggren, N. , de Gelder, B. , Derakshan, N. , … Dolcos, S. (2020). Neural correlates of emotion‐attention interactions: From perception, learning, and memory to social cognition, individual differences, and training interventions. Neuroscience and Biobehavioral Reviews, 108, 559–601. 10.1016/j.neubiorev.2019.08.017. [DOI] [PubMed] [Google Scholar]
- Egner, T. (2007). Congruency sequence effects and cognitive control. Cognitive, Affective, & Behavioral Neuroscience, 7, 380–390 Retrieved from http://link.springer.com/article/10.3758%2FCABN.7.4.380#/page-1. [DOI] [PubMed] [Google Scholar]
- Egner, T. , Etkin, A. , Gale, S. , & Hirsch, J. (2008). Dissociable neural systems resolve conflict from emotional versus nonemotional distracters. Cerebral Cortex, 18, 1475–1484. Retrieved from https://academic.oup.com/cercor/article-lookup/doi/10.1093/cercor/bhm179. [DOI] [PubMed] [Google Scholar]
- Elman, J. A. , Cohn‐Sheehy, B. I. , & Shimamura, A. P. (2013). Dissociable parietal regions facilitate successful retrieval of recently learned and personally familiar information. Neuropsychologia, 51, 573–583. 10.1016/j.neuropsychologia.2012.12.013. [DOI] [PubMed] [Google Scholar]
- Endicott, J. (1993). The menstrual cycle and mood disorders. Journal of Affective Disorders, 29, 193–200. [DOI] [PubMed] [Google Scholar]
- Engen, H. G. , Kanske, P. , & Singer, T. (2017). The neural component‐process architecture of endogenously generated emotion. Social Cognitive and Affective Neuroscience, 12, 197–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eryilmaz, H. , van de Ville, D. , Schwartz, S. , & Vuilleumier, P. (2011). Impact of transient emotions on functional connectivity during subsequent resting state: A wavelet correlation approach. NeuroImage, 54, 2481–2491. 10.1016/j.neuroimage.2010.10.021. [DOI] [PubMed] [Google Scholar]
- Eryilmaz, H. , van de Ville, D. , Schwartz, S. , & Vuilleumier, P. (2014). Lasting impact of regret and gratification on resting brain activity and its relation to depressive traits. The Journal of Neuroscience, 34, 7825–7835. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/24899706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox, K. C. R. , Andrews‐Hanna, J. R. , Mills, C. , Dixon, M. L. , Markovic, J. , Thompson, E. , & Christoff, K. (2018). Affective neuroscience of self‐generated thought. Annals of the New York Academy of Sciences, 1426, 25–51. [DOI] [PubMed] [Google Scholar]
- Fox, M. D. , Corbetta, M. , Snyder, A. Z. , Vincent, J. L. , & Raichle, M. E. (2006). Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proceedings of the National Academy of Sciences of the United States of America, 103, 9381–9386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frühholz, S. , Godde, B. , Finke, M. , & Herrmann, M. (2011). Spatio‐temporal brain dynamics in a combined stimulus‐stimulus and stimulus‐response conflict task. NeuroImage, 54, 622–634. 10.1016/j.neuroimage.2010.07.071. [DOI] [PubMed] [Google Scholar]
- Gendolla, G. H. E. , Tops, M. , & Koole, S. L. (2015). Handbook of biobehavioral approaches to self‐regulation (Vol. 101). New York, NY: Springer New York; Retrieved from http://link.springer.com/10.1007/978-1-4939-1236-0. [Google Scholar]
- Ghisletta, P. , & Spini, D. (2004). An introduction to generalized estimating equations and an application to assess selectivity effects in a longitudinal study on very old individuals. Journal of Educational and Behavioral Statistics, 29, 421–437. [Google Scholar]
- Göttlich, M. , Ye, Z. , Rodriguez‐Fornells, A. , Münte, T. F. , & Krämer, U. M. (2017). Viewing socio‐affective stimuli increases connectivity within an extended default mode network. NeuroImage, 148, 8–19. 10.1016/j.neuroimage.2016.12.044. [DOI] [PubMed] [Google Scholar]
- Hamilton, J. P. , Farmer, M. , Fogelman, P. , & Gotlib, I. H. (2015). Depressive rumination, the default‐mode network, and the dark matter of clinical neuroscience. Biological Psychiatry, 78, 224–230. 10.1016/j.biopsych.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison, B. J. , Pujol, J. , Ortiz, H. , Fornito, A. , Pantelis, C. , & Yücel, M. (2008). Modulation of brain resting‐state networks by sad mood induction. PLoS One, 3, e1794 Retrieved from https://dx.plos.org/10.1371/journal.pone.0001794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans, E. J. , Henckens, M. J. A. G. , Joëls, M. , & Fernández, G. (2014). Dynamic adaptation of large‐scale brain networks in response to acute stressors. Trends in Neurosciences, 37, 304–314. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/24766931. [DOI] [PubMed] [Google Scholar]
- Hommel, B. , Proctor, R. W. , & Vu, K. P. L. (2004). A feature‐integration account of sequential effects in the Simon task. Psychological Research, 68, 1–17. [DOI] [PubMed] [Google Scholar]
- Hutchison, R. M. , Womelsdorf, T. , Allen, E. A. , Bandettini, P. A. , Calhoun, V. D. , Corbetta, M. , … Chang, C. (2013). Dynamic functional connectivity: Promise, issues, and interpretations. NeuroImage, 80, 360–378. 10.1016/j.neuroimage.2013.05.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyett, M. P. , Parker, G. B. , Guo, C. C. , Zalesky, A. , Nguyen, V. T. , Yuen, T. , & Breakspear, M. (2015). Scene unseen: Disrupted neuronal adaptation in melancholia during emotional film viewing. NeuroImage: Clinical, 9, 660–667. 10.1016/j.nicl.2015.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai, R. , & Verstraten, F. A. J. (2005). Perceptual manifestations of fast neural plasticity: Motion priming, rapid motion aftereffect and perceptual sensitization. Vision Research, 45, 3109–3116. [DOI] [PubMed] [Google Scholar]
- Kober, H. , Feldman, B. L. , Joseph, J. , Bliss‐Moreau, E. , Lindquist, K. A. , & Wager, T. D. (2008). Functional grouping and cortical‐subcortical interactions in emotion: A meta‐analysis of neuroimaging studies. NeuroImage, 42, 998–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuppens, P. , Allen, N. B. , & Sheeber, L. B. (2010). Emotional inertia and psychological maladjustment. Psychological Science, 21, 984–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuppens, P. , & Verduyn, P. (2017). Emotion dynamics. Current Opinion in Psychology, 17, 22–26. 10.1016/j.copsyc.2017.06.004. [DOI] [PubMed] [Google Scholar]
- Kuznetsova, A. , Brockhoff, P. B. , & Christensen, R. H. B. (2017). lmerTest package: Tests in linear mixed effects models. Journal of Statistical Software, 82, 1–26. http://www.jstatsoft.org/v82/i13/. [Google Scholar]
- Lanius, R. A. , Frewen, P. A. , Tursich, M. , Jetly, R. , & McKinnon, M. C. (2015). Restoring large‐scale brain networks in ptsd and related disorders: A proposal for neuroscientifically‐informed treatment interventions. European Journal of Psychotraumatology, 6, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liemburg, E. J. , Swart, M. , Bruggeman, R. , Kortekaas, R. , Knegtering, H. , Ćurčić‐Blake, B. , & Aleman, A. (2012). Altered resting state connectivity of the default mode network in alexithymia. Social Cognitive and Affective Neuroscience, 7, 660–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, X. , de Zwart, J. A. , Schölvinck, M. L. , Chang, C. , Ye, F. Q. , Leopold, D. A. , & Duyn, J. H. (2018). Subcortical evidence for a contribution of arousal to fMRI studies of brain activity. Nature Communications, 9, 1–10. 10.1038/s41467-017-02815-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, X. , Zhang, N. , Chang, C. , & Duyn, J. H. (2018). Co‐activation patterns in resting‐state fMRI signals. NeuroImage, 180, 1–10. 10.1016/j.neuroimage.2018.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás, R. , Ribary, U. , Contreras, D. , & Pedroarena, G. (1998). The neuronal basis for consciousness. Philosophical Transactions of the Royal Society B: Biological Sciences, 353, 1841–1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayr, U. , & Awh, E. (2009). The elusive link between conflict and conflict adaptation. Psychological Research, 73, 794–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayr, U. , Awh, E. , & Laurey, P. (2003). Conflict adaptation effects in the absence of executive control. Nature Neuroscience, 6, 450–452. [DOI] [PubMed] [Google Scholar]
- McCormick, D. A. , & Bal, T. (1994). Sensory gating mechanisms of the thalamus. Current Opinion in Neurobiology, 4, 550–556. [DOI] [PubMed] [Google Scholar]
- Menon, V. , & Uddin, L. Q. (2010). Saliency, switching, attention and control: A network model of insula function. Brain Structure & Function, 214, 655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolen‐Hoeksema, S. (1991). Responses to depression and their effects on the duration of depressive episodes. Journal of Abnormal Psychology, 100, 569–582. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/1757671. [DOI] [PubMed] [Google Scholar]
- Nolen‐Hoeksema, S. , Wisco, B. E. , & Lyubomirsky, S. (2008). Rethinking rumination. Perspectives on Psychological Science, 3, 400–424. Retrieved from http://journals.sagepub.com/doi/10.1111/j.1745-6924.2008.00088.x. [DOI] [PubMed] [Google Scholar]
- Northoff, G. , Qin, P. , & Nakao, T. (2010). Rest‐stimulus interaction in the brain: A review. Trends in Neurosciences, 33, 277–284. Retrieved from http://linkinghub.elsevier.com/retrieve/pii/S0166223610000317. [DOI] [PubMed] [Google Scholar]
- Ochsner, K. N. , Silvers, J. A. , & Buhle, J. T. (2012). Functional imaging studies of emotion regulation: A synthetic review and evolving model of the cognitive control of emotion. Annals of the New York Academy of Sciences, 1251, E1–E24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen, N. , Kilpatrick, L. A. , Goharzad, A. , & Cahill, L. (2014). Oral contraceptive pill use and menstrual cycle phase are associated with altered resting state functional connectivity. NeuroImage, 90, 24–32. 10.1016/j.neuroimage.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piguet, C. , Cojan, Y. , Sterpenich, V. , Desseilles, M. , Bertschy, G. , & Vuilleumier, P. (2016). Alterations in neural systems mediating cognitive flexibility and inhibition in mood disorders. Human Brain Mapping, 37, 1335–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piguet, C. , Desseilles, M. , Sterpenich, V. , Cojan, Y. , Bertschy, G. , & Vuilleumier, P. (2014). Neural substrates of rumination tendency in non‐depressed individuals. Biological Psychology, 103, 195–202. 10.1016/j.biopsycho.2014.09.005. [DOI] [PubMed] [Google Scholar]
- Pillemer, S. , Holtzer, R. , & Blumen, H. M. (2017). Functional connectivity associated with social networks in older adults: A resting‐state fMRI study. Social Neuroscience, 12, 242–252. 10.1080/17470919.2016.1176599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pletzer, B. , Crone, J. S. , Kronbichler, M. , & Kerschbaum, H. (2016). Menstrual cycle and hormonal contraceptive‐dependent changes in intrinsic connectivity of resting‐state brain networks correspond to behavioral changes due to hormonal status. Brain Connectivity, 6, 572–585. Retrieved from http://online.liebertpub.com/doi/10.1089/brain.2015.0407. [DOI] [PubMed] [Google Scholar]
- Preti, M. G. , Bolton, T. A. W. , & van de Ville, D. (2017). The dynamic functional connectome: State‐of‐the‐art and perspectives. NeuroImage, 160, 41–54. [DOI] [PubMed] [Google Scholar]
- Protopopescu, X. , Pan, H. , Altemus, M. , Tuescher, O. , Polanecsky, M. , McEwen, B. , … Stern, E. (2005). Orbitofrontal cortex activity related to emotional processing changes across the menstrual cycle. Proceedings of the National Academy of Sciences of the United States of America, 102, 16060–16065. 10.1073/pnas.0502818102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptak, R. (2012). The frontoparietal attention network of the human brain: Action, saliency, and a priority map of the environment. The Neuroscientist, 18, 502–515. [DOI] [PubMed] [Google Scholar]
- Qiao‐Tasserit, E. , Corradi‐Dell'Acqua, C. , & Vuilleumier, P. (2018). The good, the bad, and the suffering. Transient emotional episodes modulate the neural circuits of pain and empathy. Neuropsychologia, 116, 99–116. 10.1016/j.neuropsychologia.2017.12.027. [DOI] [PubMed] [Google Scholar]
- Qiao‐Tasserit, E. , Quesada, M. G. , Antico, L. , Bavelier, D. , Vuilleumier, P. , & Pichon, S. (2017). Transient emotional events and individual affective traits affect emotion recognition in a perceptual decision‐making task. PLoS One, 12, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle, M. E. , MacLeod, A. M. , Snyder, A. Z. , Powers, W. J. , Gusnard, D. A. , & Shulman, G. L. (2001). A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America, 98, 676–682. Retrieved from http://www.pnas.org/cgi/doi/10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz, G. , Touroutoglou, A. , Wilson‐Mendenhall, C. , Gilam, G. , Lin, T. , Gonen, T. , … Barrett, L. F. (2016). Functional connectivity dynamics during film viewing reveal common networks for different emotional experiences. Cognitive, Affective, & Behavioral Neuroscience, 16, 709–723. Retrieved from http://link.springer.com/10.3758/s13415-016-0425-4. [DOI] [PubMed] [Google Scholar]
- Raz, G. , Winetraub, Y. , Jacob, Y. , Kinreich, S. , Maron‐Katz, A. , Shaham, G. , … Hendler, T. (2012). Portraying emotions at their unfolding: A multilayered approach for probing dynamics of neural networks. NeuroImage, 60, 1448–1461. Retrieved from http://linkinghub.elsevier.com/retrieve/pii/S1053811912000250. [DOI] [PubMed] [Google Scholar]
- Ribary, U. (2005). Dynamics of thalamo‐cortical network oscillations and human perception. Progress in Brain Research, 150, 127–142. [DOI] [PubMed] [Google Scholar]
- Ribary, U. , Ioannides, A. A. , Singh, K. D. , Hasson, R. , Bolton, J. P. , Lado, F. , … Llinas, R. (1991). Magnetic field tomography of coherent thalamocortical 40‐Hz oscillations in humans. Proceedings of the National Academy of Sciences of the United States of America, 88, 11037–11041. Retrieved from http://www.pnas.org/cgi/doi/10.1073/pnas.88.24.11037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saarimäki, H. , Ejtehadian, L. F. , Glerean, E. , Jääskeläinen, I. P. , Vuilleumier, P. , Sams, M. , & Nummenmaa, L. (2018). Distributed affective space represents multiple emotion categories across the human brain. Social Cognitive and Affective Neuroscience, 13, 471–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadaghiani, S. , & D'Esposito, M. (2015). Functional characterization of the cingulo‐opercular network in the maintenance of tonic alertness. Cerebral Cortex, 25, 2763–2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambataro, F. , Wolf, N. D. , Pennuto, M. , Vasic, N. , & Wolf, R. C. (2014). Revisiting default mode network function in major depression: Evidence for disrupted subsystem connectivity. Psychological Medicine, 44, 2041–2051. [DOI] [PubMed] [Google Scholar]
- Schmidt, J. R. , Notebaert, W. , & van den Bussche, E. (2015). Is conflict adaptation an illusion?[Editorial]. Frontiers Research Topics, 6, 10.3389/fpsyg.2015.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuch, S. , & Koch, I. (2015). Mood states influence cognitive control: The case of conflict adaptation. Psychological Research, 79, 759–772. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/25100233. [DOI] [PubMed] [Google Scholar]
- Sheline, Y. I. , Barch, D. M. , Price, J. L. , Rundle, M. M. , Vaishnavi, S. N. , Snyder, A. Z. , … Raichle, M. E. (2009). The default mode network and self‐referential processes in depression. Proceedings of the National Academy of Sciences of the United States of America, 106, 1942–1947. Retrieved from http://www.pnas.org/lookup/doi/10.1073/pnas.0812686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiota, M. N. , & Levenson, R. W. (2009). Effects of aging on experimentally instructed detached reappraisal, positive reappraisal, and emotional behavior suppression. Psychology and Aging, 24, 890–900. Retrieved from http://doi.apa.org/getdoi.cfm?doi=10.1037/a0017896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman, G. L. , Pope, D. L. W. , Astafiev, S. V. , McAvoy, M. P. , Snyder, A. Z. , & Corbetta, M. (2010). Right hemisphere dominance during spatial selective attention and target detection occurs outside the dorsal frontoparietal network. The Journal of Neuroscience, 30, 3640–3651 Retrieved from http://www.jneurosci.org/cgi/doi/10.1523/JNEUROSCI.4085-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha, R. , Lacadie, C. M. , Constable, R. T. , & Seo, D. (2016). Dynamic neural activity during stress signals resilient coping. Proceedings of the National Academy of Sciences of the United States of America, 113, 8837–8842. Retrieved from http://www.pnas.org/lookup/doi/10.1073/pnas.1600965113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, S. , Zilverstand, A. , Song, H. , Uquillas, O. , d'Oleire Uquillas, F. , Wang, Y. , … Zou, Z. (2017). The influence of emotional interference on cognitive control: A meta‐analysis of neuroimaging studies using the emotional Stroop task. Scientific Reports, 7, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, Z. , Zhang, M. , & Huang, P. (2016). Aberrant emotion networks in early major depressive disorder patients: An eigenvector centrality mapping study. Translational Psychiatry, 6, e819 10.1038/tp.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonkusare, S. , Breakspear, M. , & Guo, C. (2019). Naturalistic stimuli in neuroscience: Critically acclaimed. Trends in Cognitive Sciences, 23, 699–714. 10.1016/j.tics.2019.05.004. [DOI] [PubMed] [Google Scholar]
- Sridharan, D. , Levitin, D. J. , & Menon, V. (2008). A critical role for the right fronto‐insular cortex in switching between central‐executive and default‐mode networks. Proceedings of the National Academy of Sciences of the United States of America, 105, 12569–12574. Retrieved from http://www.pnas.org/cgi/doi/10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagliazucchi, E. , Balenzuela, P. , Fraiman, D. , & Chialvo, D. R. (2012). Criticality in large‐scale brain fMRI dynamics unveiled by a novel point process analysis. Frontiers in Physiology, 3, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi, D. , Ernst, T. , Caparelli, E. C. , & Chang, L. (2006). Common deactivation patterns during working memory and visual attention tasks: An intra‐subject fMRI study at 4 tesla. Human Brain Mapping, 27, 694–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell, P. D. , & Campbell, J. D. (1999). Private self‐consciousness and the five‐factor model of personality: Distinguishing rumination from reflection. Journal of Personality and Social Psychology, 76, 284–304. [DOI] [PubMed] [Google Scholar]
- van Steenbergen, H. (2015). Affective modulation of cognitive control: A biobehavioral perspective Henk In: Gendolla, GHE, Tops, M & Koole, SL, (Eds.) Handbook of biobehavioral approaches to self‐regulation (pp.89‐107). New York, NY: Springer. 10.1007/978-1-4939-1236-0_7. [DOI] [Google Scholar]
- van Steenbergen, H. , Band, G. P. H. , & Hommel, B. (2010). In the mood for adaptation: How affect regulates conflict‐driven control. Psychological Science, 21, 1629–1634. Retrieved from http://pss.sagepub.com/lookup/doi/10.1177/0956797610385951. [DOI] [PubMed] [Google Scholar]
- Verbruggen, F. , Liefooghe, B. , Notebaert, W. , & Vandierendonck, A. (2005). Effects of stimulus‐stimulus compatibility and stimulus‐response compatibility on response inhibition. Acta Psychologica, 120, 307–326. https://linkinghub.elsevier.com/retrieve/pii/S0001691805000624. [DOI] [PubMed] [Google Scholar]
- Vrtička, P. , Bondolfi, G. , Sander, D. , & Vuilleumier, P. (2012). The neural substrates of social emotion perception and regulation are modulated by adult attachment style. Social Neuroscience, 7, 473–493. [DOI] [PubMed] [Google Scholar]
- Westerhausen, R. , Moosmann, M. , Alho, K. , Belsby, S. O. , Hämäläinen, H. , Medvedev, S. , … Hugdahl, K. (2010). Identification of attention and cognitive control networks in a parametric auditory fMRI study. Neuropsychologia, 48, 2075–2081. [DOI] [PubMed] [Google Scholar]
- Wexler, M. , Duyck, M. , & Mamassian, P. (2015). Persistent states in vision break universality and time invariance. Proceedings of the National Academy of Sciences of the United States of America, 112, 14990–14995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield‐Gabrieli, S. , & Ford, J. M. (2012). Default mode network activity and connectivity in psychopathology. Annual Review of Clinical Psychology, 8, 49–76. [DOI] [PubMed] [Google Scholar]
- Yekutieli, D. , & Benjamini, Y. (2001). The control of the false discovery rate in multiple testing under dependency. The Annals of Statistics, 29, 1165–1188. Retrieved from http://projecteuclid.org/euclid.aos/1013699998. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
APPENDIX S1: Supporting information
FIGURE S1 Supporting information
Data Availability Statement
Derived data supporting the findings of this study are available from the corresponding author (J. G.) on request.


