Abstract
Colorectal cancer (CRC) is one of the most aggressive malignancies worldwide. Increasing evidence has indicated that microRNA (miR)-599 is involved in the occurrence and development of different types of tumors, such as breast cancer and glioma. However, the role of miR-599 in CRC remains unclear. Thus, the present study aimed to identify the regulatory mechanism of miR-599 in CRC progression. Reverse transcription-quantitative PCR was used to analyze the expression levels of MCM3AP-AS1, miR-599 and ARPP19, and Cell Counting Kit-8 and Transwell assays were used to determine the cell proliferation and migration of CRC cells. In addition, a Dual-luciferase reporter assay was used to analyze the direct interaction between miR-599 and MCM3AP-AS1 or ARPP19. Reverse transcription-quantitative PCR analysis demonstrated that miR-599 expression decreased in patients with CRC and in CRC cell lines, while miR-599 overexpression inhibited cell proliferation and migration abilities in vitro. MCM3AP-AS1 was identified as a molecular sponge of miR-599, and further investigation indicated that MCM3AP-AS1 silencing inhibited cell proliferation and migration of the CRC cell lines. In addition, ARPP19 was identified as a target gene of miR-599, and MCM3AP-AS1-knockdown decreased ARPP19 mRNA expression and increased miR-599 expression. Furthermore, silencing ARPP19 inhibited the proliferation and migration of the CRC cell lines. The results also demonstrated that MCM3AP-AS1 promoted CRC cell progression by regulating the miR-599/ARPP19 axis. Taken together, the results of the present study suggest that MCM3AP-AS1 may be a novel therapeutic target for patients with CRC.
Keywords: MCM3AP-AS1, microRNA-599, colorectal cancer, ARPP19
Introduction
Colorectal cancer (CRC) is one of the most common malignancies with 1.4 million new cases and >690,000 associated mortalities in 2012 (1). Recent advancements in prognosis and therapeutic strategies in CRC have been made (2); however, due to metastasis and the fast-growing nature, the 5-year survival rate of patients with CRC remains poor, at only ~14% (3–5). Thus, it remains critical to develop novel reliable molecular markers for CRC treatment.
MicroRNAs (miRNAs/miRs) are small RNA molecules, 21–25 nucleotides in length, which have the ability to induce mRNA degradation or repress translation by binding to the 3′-untranslated region of RNA transcripts (6). It has been reported that dysregulated miRNAs are frequently involved in different types of human cancer, as both oncogenes and tumor suppressors. For example, overexpression of miR-187 inhibits cell proliferation and metastasis of glioma by downregulating SMAD1 expression (7). miR-599 has been reported to act as a tumor suppressor in several types of cancer. For example, miR-599 suppresses glioma progression by targeting RAB27B (8). However, the regulatory mechanism of miR-599 in CRC remains unclear.
Long non-coding (lnc)RNAs are RNA molecules, >200 nucleotides in length, which are involved in different types of cellular processes, such as proliferation, migration and differentiation (9–13). Previous studies have demonstrated that lncRNAs are important regulatory factors that participate in cell proliferation, invasion and metastasis (14–16). For example, lncRNA RBM5-AS1 promotes the aggressive behaviors of oral squamous cell carcinoma by regulating the miR-1285-3p/YAP1 axis (17). A previous study also demonstrated that lncRNAs can regulate the progression of different types of cancer by sponging miRNAs. For example, lncRNA RBMS3-AS3 acts as a miR-4534 sponge to inhibit prostate cancer progression by upregulating VASH1 expression (18). lncRNA SNHG17 acts as a ceRNA of miR-324-3p to contribute the progression of osteosarcoma (19). MCM3AP-AS1 has been reported to be involved in several types of cancer. For example, Yang et al (20) demonstrated that the MCM3AP-AS1/miR-211/KLF5/AGGF1 axis regulates glioblastoma angiogenesis, while Li et al (21) reported that overexpression of lncRNA MCM3AP-AS1 promotes lung cancer progression via the miR-340-5p/KPNA4 axis. In addition, Yang et al (22) revealed that MCM3AP-AS1 sponges miR-138 by modulating FOXK1 expression to promote pancreatic cancer cell proliferation and migration. However, the molecular mechanism of MCM3AP-AS1 in CRC remains largely unknown.
The present study investigated the effects and mechanisms of lncRNA MCM3AP-AS1 on the proliferation and migration of CRC cells.
Materials and methods
Patients and tissue samples
CRC tissues and adjacent normal tissues were collected from 30 patients (18 males and 12 females) with a median age of 56 years (range, 38–67 years) between January 2018 and January 2019 at The Bishan Hospital of Chongqing (Chongqing, China). Adjacent normal tissues of the patients were at least 1-cm away from the tumor tissues. The specimens were immediately preserved at −80°C following surgical resection. The present study was approved by the Ethics Committee of The Bishan Hospital of Chongqing and written informed consent was provided by all patients prior to the study start.
CRC cell lines
Human CRC cell lines (HCT-116, SW620, SW480 and LoVo), the normal colorectal cell line (NCM460), and 293T cells were purchased from the American Type Culture Collection. All cell lines were maintained in Dulbecco's modified Eagle's medium (DMEM; Invitrogen; Thermo Fisher Scientific, Inc.) supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.), at 37°C in a humidified atmosphere with 5% CO2.
Cell transfection
Short hairpin (sh)RNAs targeting MCM3AP-AS1 (sh-MCM3AP-AS1; 10 nM; 5′-GCGCCUUCCCUCUAACCUUAA-3′) and non-targeting scrambled negative control (sh-NC; 10 nM; 5′-GGCAAGAUGAACGUCUGAAAU-3′), and miR-599 mimics (10 nM; 5′-AGCAGCAUUGUACAGGGCUAUCA-3′) and their NC (NC mimics; 10 nM; 5′-UGUAACGUACGUUCGUACCGUGA-3′) were all purchased from Shanghai GenePharma Co., Ltd. For MCM3AP-AS1 and ARPP19 overexpression, the full-length MCM3AP-AS1 and ARPP19 gene sequences were amplified and subcloned into the pcDNA3.1 vector (Invitrogen; Thermo Fisher Scientific, Inc.) to synthesize pcDNA3.1/MCM3AP-AS1 and pcDNA3.1/ARPP19, respectively. Lipofectamine® 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) was used for all transfections, according to the manufacturer's instructions, and incubated at 37°C for 48 h. All functional experiments were performed 48 h post-transfection.
Reverse transcription-quantitative (RT-q)PCR
Total RNA was extracted from CRC tissues, adjacent normal tissues, HCT-116, SW620, SW480, LoVo and NCM460 cells using TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc.), and reverse transcribed into cDNA using the RT kit (cat. no. RR047A; Takara Biotechnology Co., Ltd.), according to the manufacturer's protocol. qPCR was subsequently performed using the SYBR-Green PCR Master Mix kit (cat. no. DRR041A; Takara Biotechnology Co., Ltd.) according to the manufacturer's protocol. The following thermocycling conditions were used for qPCR: Pre-denaturation at 95°C for 15 sec, denaturation at 94°C for 30 sec, annealing at 60°C for 20 sec and extension at 72°C for 40 sec for 40 cycles. The primers were designed as follows: MCM3AP-AS1 forward, 5′-CGATTTGGCGTTAACTTATTGA-3′; and reverse, 5′-ATCATTACAGAACATAATCAACAGGTA-3′; ARPP19 forward, 5′-ACTCATGCAGCGGAGCTCTAG-3′; and reverse, 5′-GTTCAGAGATGAAGGTCCGAACA-3′; miR-599 forward, 5′-TCGGCAGGUUCAAGCCAGGGG-3′; and reverse, 5′-CACTCAACTGGTGTCGTGGA-3′; GAPDH forward, 5′-CGGAGTCAACGGATTTGGTCGTAT-3′; and reverse, 5′-AGCCTTCTCCATGGTGGTGAAGAC-3′; U6 forward, 5′-GCTTCGGCAGCACATATACTAAAAT-3′; and reverse, 5′-CGCTTCACGAATTTGCGTGTCAT-3′. GAPDH was used as an internal control for MCM3AP-AS1 and ARPP19, while U6 was used as an internal control for miR-599. Relative mRNA expression levels were quantified using the 2−ΔΔCq method (23).
Cell Counting Kit-8 (CCK-8) assay
Cell proliferation was assessed via the CCK-8 assay (Dojindo Molecular Technologies, Inc.). Briefly, HCT-116 and SW480 cells were seeded into 96-well plates at a density of 2×104 cells/well. Following transfection for 48 h, CCK-8 reagent was added to the wells at 37°C for 2 h, according to the manufacturer's instructions and cell proliferation was subsequently analyzed at a wavelength of 450 nm, using a microplate reader (Thermo Fisher Scientific, Inc.).
Bioinformatic prediction and Dual-luciferase reporter assay
StarBase 2.0 (http://starbase.sysu.edu.cn) was used to predict the potential downstream targets of MCM3AP-AS1 and miR-599. To assess the binding ability between different RNAs, wild type or mutant fragments (MCM3AP-AS1 and ARPP19) were amplified and cloned into the pGL3 luciferase vector (Promega Corporation). The wild-type or mutant fragments were subsequently transfected into 293T cells (American Type Culture Collection), which had been co-transfected with miR-599 mimics or NC mimics. Transfection was performed using Lipofectamine® 2000 (Invitrogen; Thermo Fisher Scientific, Inc.). Following incubation for 48 h at 37°C, a Dual-luciferase reporter system (Promega Corporation) was used to measure the luciferase gene activity. Firefly luciferase activity was normalized to that of Renilla luciferase.
Migration assay
The migration efficiency of the CRC cell lines was assessed via the Transwell assay. The transfected HCT-116 and SW480 cells were plated in the upper chamber at the density of 2×105 cells per well in serum-free DMEM. DMEM supplemented with 10% FBS was plated in the lower chambers. Following incubation for 48 h at 37°C, the migratory cells were stained with 0.1% crystal violet for 20 min at room temperature.and counted under a light microscope (magnification, ×200; Olympus Corporation).
Statistical analysis
Statistical analysis was performed using SPSS v20.0 software (IBM Corp.). All experiments were performed in triplicate and data are presented as the mean ± standard deviation. Comparisons among multiple groups were performed using one-way ANOVA, followed by Tukey's post hoc test. Comparisons between CRC tissues and adjacent normal tissues were performed using a paired Student's t-test, while comparisons between the experimental and control groups was performed using an unpaired Student's t-test. P<0.05 was considered to indicate a statistically significant difference.
Results
Decreased miR-599 expression affects the migratory ability of CRC cell lines
The results demonstrated that miR-599 expression was significantly decreased in the CRC tissues and cell lines (HCT-116, SW620, SW480 and LoVo) compared with that in adjacent normal tissues and the NCM460 cell line (Fig. 1A and B). Especially, miR-599 expression was significantly higher in HCT-116 and SW480 cells, compared with NCM460 cells. Thus, HCT-116 and SW480 cells were selected to perform subsequent experiments. RT-qPCR analysis demonstrated that transfection with miR-599 mimics significantly enhanced miR-599 expression in HCT-116 and SW480 cells (Fig. 1C). Furthermore, the results from the CCK-8 assay indicated that overexpression of miR-599 significantly inhibited cell proliferation (Fig. 1D), while the results from the Transwell assay demonstrated that miR-599 overexpression significantly decreased the migratory ability of the CRC cell lines (Fig. 1E). Collectively, these results suggest that miR-599 has an antitumor effect on CRC cell lines.
Figure 1.
Decreased miR-599 expression affects the migratory ability of CRC cell lines. (A and B) Reverse transcription-quantitative PCR was used to assess miR-599 expression in the CRC tissues and cell lines (HCT-116, SW620, SW480 and LoVo) compared with that in adjacent normal tissues and NCM460 cell line. (C) Transfection with miR-599 mimics upregulated miR-599 expression in HCT-116 and SW480 cells. (D) Cell Counting Kit-8 assay was performed to assess cell proliferation following overexpression of miR-599. (E) Transwell assay (magnification, ×200) was performed to assess the migratory ability of the CRC cell lines following overexpression of miR-599. *P<0.05 vs. NC mimics. miR, microRNA; CRC, colorectal cancer; NC, negative control; OD, optical density.
MCM3AP-AS1 sponges miR-599 to accelerate CRC progression
lncRNAs exert their functions by interacting with downstream target miRNAs (24). In the present study, a potential target site between miR-599 and MCM3AP-AS1 was predicted using StarBase software (Fig. 2A). A Dual-luciferase reporter assay was performed for validation, and the results demonstrated that transfection with miR-599 mimics significantly suppressed the luciferase activity of wild-type MCM3AP-AS1; however, no effect was observed on mutant MCM3AP-AS1 (Fig. 2B). In addition, MCM3AP-AS1 mRNA expression was significantly elevated in the CRC cell lines compared with that in the NCM460 cell line (Fig. 2C).
Figure 2.
MCM3AP-AS1 sponges miR-599 to accelerate CRC progression. (A) Binding sequences between MCM3AP-AS1 and miR-599 were predicted using StarBase software. (B) Dual-luciferase reporter assay was used to verify the binding ability between MCM3AP-AS1 and miR-599 in 293T cells. (C) RT-qPCR analysis was performed to detect MCM3AP-AS1 expression in the CRC cell lines and normal NCM460 cell line. (D) RT-qPCR analysis demonstrated that MCM3AP-AS1 expression decreased following transfection with sh-MCM3AP-AS1 in HCT-116 and SW480 cells. (E) RT-qPCR analysis was performed to detect miR-599 expression following transfection with sh-MCM3AP-AS1 in HCT-116 and SW480 cells. (F) Cell Counting Kit-8 and (G) Transwell assays (magnification, ×200) were performed to detect cell proliferation and migration following MCM3AP-AS1-knockdown, respectively. *P<0.05 vs. shNC. miR, microRNA; CRC, colorectal cancer; RT-qPCR, reverse transcription-quantitative PCR; sh, short hairpin; NC, negative control; WT, wild-type; MUT, mutant; OD, optical density.
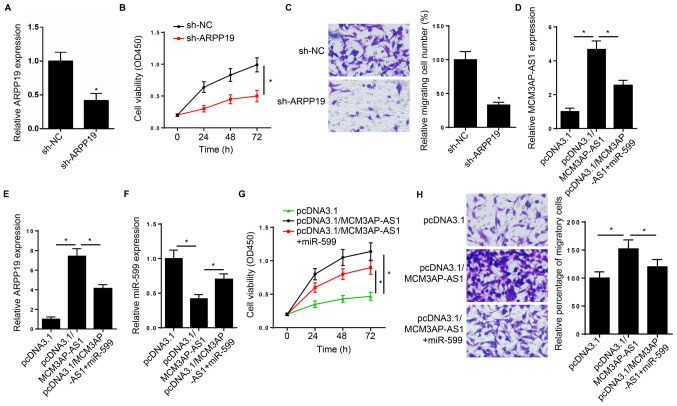
To further investigate the biological function of MCM3AP-AS1 in CRC, HCT-116 and SW480 cells were transfected with sh-MCM3AP-AS1 or sh-NC. RT-qPCR analysis confirmed that MCM3AP-AS1 mRNA expression significantly decreased with sh-MCM3AP-AS1 transfection (Fig. 2D). In addition, miR-599 expression significantly increased in HCT-116 and SW480 cells following MCM3AP-AS1-knockdown (Fig. 2E). The results from the CCK-8 and Transwell assays demonstrated that MCM3AP-AS1-knockdown significantly attenuated cell proliferation and migration, respectively (Fig. 2F and G). Taken together, these results suggest that MCM3AP-AS1 acts as a sponge of miR-599, and is involved in CRC.
MCM3AP-AS1 interacts with miR-599 to regulate ARPP19 in CRC
Previous studies have demonstrated that lncRNAs exert their functions by sponging miRNAs to regulate mRNAs (25–27). The StarBase software predicted that ARPP19 was a direct target of miR-599 (Fig. 3A), and RT-qPCR analysis demonstrated that ARPP19 mRNA expression increased in SW480 cells compared with that in normal NCM460 cells (Fig. 3B). In addition, transfection with sh-MCM3AP-AS1 or miR-599 mimics significantly decreased ARPP19 mRNA expression in SW480 cells (Fig. 3C). Notably, MCM3AP-AS1 expression was significantly enhanced in SW480 cells following transfection with pcDNA3.1/MCM3AP-AS1 (Fig. 3D). Furthermore, inhibition of miR-599 mimics based on luciferase activity in wild-type ARPP19 was decreased following overexpression of MCM3AP-AS1, while there was no change in luciferase activity in mutant ARPP19 (Fig. 3E). Collectively, these results suggest that MCM3AP-AS1 regulates ARPP19 by modulating miR-599 in CRC.
Figure 3.
MCM3AP-AS1 interacts with miR-599 to regulate ARPP19 in colorectal cancer. (A) The binding sites between ARPP19 and miR-599. (B) RT-qPCR analysis demonstrated that ARPP19 expression was significantly increased in SW480 cells compared with that in normal NCM460 cells. (C) RT-qPCR analysis was performed to detect ARPP19 expression in SW480 cells following overexpression of miR-599 and MCM3AP-AS1-knockdown, respectively. (D) RT-qPCR analysis was performed to detect MCM3AP-AS1 expression in SW480 cells following transfection with pcDNA3.1/MCM3AP-AS1. (E) Dual-luciferase reporter assay validated the interaction between ARPP19 and miR-599, following overexpression of MCM3AP-AS1. *P<0.05 vs. NC mimics. miR, microRNA; RT-qPCR, reverse transcription-quantitative PCR; sh, short hairpin; NC, negative control; WT, wild-type; MUT, mutant.
MCM3AP-AS1 accelerates CRC progression via the miR-599/ARPP19 axis
To determine whether MCM3AP-AS1 promoted CRC progression via the miR-599/ARPP19 axis, a series of rescue experiments were performed. The results demonstrated that ARPP19 mRNA expression significantly decreased following transfection with sh-ARPP19 in SW480 cells (Fig. 4A). In addition, SW480 cell proliferation and migration were attenuated following ARPP19-knockdown, respectively (Fig. 4B and C). SW480 cells were transfected with pcDNA3.1, pcDNA3.1/MCM3AP-AS1 and pcDNA.1/MCM3AP-AS1+miR-599, and the results demonstrated that MCM3AP-AS1 and ARPP19 expression levels increased following overexpression of MCM3AP-AS1, the effects of which were partially reversed following transfection with the miR-599 mimic (Fig. 4D and E). In addition, miR-599 expression decreased following overexpression of MCM3AP-AS1, the effect of which was partially reversed following transfection with the miR-599 mimic (Fig. 4F). The results from the CCK-8 and Transwell assays demonstrated that overexpression of MCM3AP-AS1 promoted SW480 cell proliferation and migration, respectively, the effects of which were partially reversed following overexpression of miR-599 (Fig. 4G and H). Taken together, these results suggest that MCM3AP-AS1 sponges miR-599 and increases ARPP19 mRNA expression to accelerate CRC progression.
Figure 4.
MCM3AP-AS1 accelerates colorectal cancer progression via the miR-599/ARPP19 axis. (A) RT-qPCR analysis was performed to detect ARPP19 expression in SW480 cells following ARPP19-knockdown. (B) CCK-8 and (C) Transwell assays (magnification, ×200) were performed to assess the proliferation and migration of SW480 cells following ARPP19-knockdown, respectively. RT-qPCR analysis was performed to detect (D) MCM3AP-AS1, (E) ARPP19 and (F) miR-599 expression levels in SW480 cells transfected with pcDNA3.1, pcDNA3.1/MCM3AP-AS1 and pcDNA3.1/MCM3AP-AS1+miR-599. (G) CCK-8 and (H) Transwell assays (magnification, ×200) were performed to assess the proliferation and migration of SW480 cells transfected with pcDNA3.1, pcDNA3.1/MCM3AP-AS1 and pcDNA3.1/MCM3AP-AS1+miR-599, respectively. *P<0.05 vs. pcDNA3.1. miR, microRNA; RT-qPCR, reverse transcription-quantitative PCR; CCK-8, Cell Counting Kit-8; sh, short hairpin; NC, negative control; OD, optical density.
Discussion
Increasing evidence has indicated that miRNAs may have an antitumor role in different types of tumor. For example, miR-192 has been demonstrated to inhibit the progression of lung cancer bone metastasis by regulating TRIM44 (28). miR-506-3p inhibits papillary thyroid cancer cells tumorigenesis via targeting YAP1 (29). miR-296-5p suppresses the tumorigenesis of esophageal squamous cell carcinoma cells via inhibiting STAT3 signaling (30). The present study demonstrated that miR-599 was involved in CRC, whereby its expression was decreased in the examined CRC cell lines. In addition, overexpression of miR-599 inhibited cell proliferation and migration in CRC.
lncRNAs have been reported to act as competing endogenous (ce)RNAs that sponge miRNAs. For example, lncRNA FAL1 acts as a ceRNA of miR-1236 to promote cell proliferation and migration in hepatocellular carcinoma (HCC) (31), while lncRNA-UCA1 acts as a ceRNA of Sox4 to promote cell proliferation in esophageal cancer (32). In the present study, MCM3AP-AS1 was demonstrated to negatively regulate miR-599 via direct interaction. Notably, MCM3AP-AS1 has been reported to participate in the progression of different types of cancer. For example, Liang et al (33) demonstrated that MCM3AP-AS1 promotes proliferation and invasion in papillary thyroid cancer by regulating the miR-211-5p/SPARC axis. In addition, Wang et al (34) reported that MCM3AP-AS1 promotes HCC progression by regulating the miR-194-5p/FOXA1 axis. However, the molecular mechanism of MCM3AP-AS1 in CRC remains unclear. The results from the present study demonstrated that MCM3AP-AS1 sponged miR-599 to accelerate CRC progression. In addition, MCM3AP-AS1 mRNA expression was increased in the CRC cell lines. In vitro functional assays were performed and the results demonstrated that MCM3AP-AS1-knockdown significantly decreased the proliferation and migration of CRC cells. Collectively, the results of the present study demonstrated that MCM3AP-AS1 accelerated CRC progression by binding to miR-599.
Previous studies have reported that miRNAs are involved in tumor progression by targeting mRNAs. For example, miR-26a inhibits thyroid cancer cell proliferation by targeting ARPP19 (35). In addition, miR-9-5p targets GOT1 to inhibit pancreatic cancer cell proliferation, invasion and metabolism (36). In the present study, bioinformatics analysis and the Dual-luciferase reporter assay confirmed that miR-599 directly binds to ARPP19. Furthermore, MCM3AP-AS1-knockdown decreased ARPP19 mRNA expression by promoting miR-599 expression. The results also demonstrated that ARPP19-knockdown attenuated the proliferation and migration of CRC cells. In addition, overexpression of MCM3AP-AS1 increased ARPP19 mRNA expression, the effect of which was reversed following transfection with miR-599 mimics. Notably, MCM3AP-AS1 promoted CRC progression via the miR-599/ARPP19 axis.
In conclusion, the results of the present study suggest that lncRNA MCM3AP-AS1 exerts its biological functions by modulating the miR-599/ARPP19 axis, thus MCM3AP-AS1 may be an effective therapeutic target for CRC. However, the current study is limited by the small sample size. Besides, in vivo interactions among MCM3AP-AS1, miR-599 and ARPP19 have not been explored. Future research is required to include in vivo experiments and enroll more patients to further verify these conclusions.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
YY designed the study. YY, SL and XP performed the research and analyzed the data. XP and YY wrote the manuscript. All authors read and approved the final version of the manuscript.
Ethics approval and consent to participate
The protocol of this research has been approved by the Ethics Committee of The Bishan Hospital of Chongqing (Chongqing, China; approval no. 2017A110502) and written informed consent was provided by all patients prior to the start of the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Chen X, Zeng K, Xu M, Hu X, Liu X, Xu T, He B, Pan Y, Sun H, Wang S. SP1-induced lncRNA-ZFAS1 contributes to colorectal cancer progression via the miR-150-5p/VEGFA axis. Cell death & disease. 2018;9:1–18. doi: 10.1038/s41419-018-0962-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmed S, Johnson K, Ahmed O, Iqbal N. Advances in the management of colorectal cancer: From biology to treatment. Int J Colorectal Dis. 2014;29:1031–1042. doi: 10.1007/s00384-014-1928-5. [DOI] [PubMed] [Google Scholar]
- 4.Mele V, Sokol L, Kölzer VH, Pfaff D, Muraro MG, Keller I, Stefan Z, Centeno I, Terracciano LM, Dawson H, et al. The hyaluronan-mediated motility receptor RHAMM promotes growth, invasiveness and dissemination of colorectal cancer. Oncotarget. 2017;8:70617–70629. doi: 10.18632/oncotarget.19904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang KM, Park IJ, Lee JL, Kim CW, Yoon YS, Lim SB, Yu CS, Kim JC. Benefits of repeated resections for liver and lung metastases from colorectal cancer. Asian J Surg. 2020;43:102–109. doi: 10.1016/j.asjsur.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 6.Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 7.Gulinaer AJ, Ju AN, Gao M, Luo Y, Bo YL. Over-expression of miR-187 inhibited cell proliferation and metastasis of glioma via down-regulating SMAD1. Eur Rev Med Pharmacol Sci. 2019;23:10908–10917. doi: 10.26355/eurrev_201912_19794. [DOI] [PubMed] [Google Scholar]
- 8.Jiang Y, Wang X, Zhang J, Lai R. MicroRNA-599 suppresses glioma progression by targeting RAB27B. Oncol Lett. 2018;16:1243–1252. doi: 10.3892/ol.2018.8727. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9.Sheng XY, Wang CH, Wang CF, Xu HY. Long-chain non-coding SOX21-AS1 promotes proliferation and migration of breast cancer cells through the PI3K/AKT signaling pathway. Cancer Manag Res. 2020;12:11005–11014. doi: 10.2147/CMAR.S270464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang G, Luo L, Zhang J, Zhai D, Huang D, Yin J, Zhou Q, Zhang Q, Zheng G. lncRNA LINC01057 promotes mesenchymal differentiation by activating NF-KB signaling in glioblastoma. Cancer Lett. 2020 Oct 31; doi: 10.1016/j.canlet.2020.10.047. (Online ahead of print) [DOI] [PubMed] [Google Scholar]
- 11.Liu Y, Yang Y, Li L, Liu Y, Geng P, Li G, Song H. LncRNA SNHG1 enhances cell proliferation, migration, and invasion in cervical cancer. Biochem Cell Biol. 2018;96:38–43. doi: 10.1139/bcb-2017-0188. [DOI] [PubMed] [Google Scholar]
- 12.Batista PJ, Chang HY. Long noncoding RNAs: Cellular address codes in development and disease. Cell. 2013;152:1298–1307. doi: 10.1016/j.cell.2013.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang L, Froberg JE, Lee JT. Long noncoding RNAs: FResh perspectives into the RNA world. Trends Biochem Sci. 2014;39:35–43. doi: 10.1016/j.tibs.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang H, Bai M, Zeng A, Si L, Yu N, Wang X. LncRNA HOXD-AS1 promotes melanoma cell proliferation and invasion by suppressing RUNX3 expression. Am J Cancer Res. 2017;7:2526–2535. [PMC free article] [PubMed] [Google Scholar]
- 15.Lian Y, Cai Z, Gong H, Xue S, Wu D, Wang K. HOTTIP: A critical oncogenic long non-coding RNA in human cancers. Mol Biosyst. 2016;12:3247–3253. doi: 10.1039/C6MB00475J. [DOI] [PubMed] [Google Scholar]
- 16.Lv M, Zhong Z, Huang M, Tian Q, Jiang R, Chen J. lncRNA H19 regulates epithelial-mesenchymal transition and metastasis of bladder cancer by miR-29b-3p as competing endogenous RNA. Biochim Biophys Acta Mol Cell Res. 2017;1864:1887–1899. doi: 10.1016/j.bbamcr.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 17.Li C, Ye J, Zhang Z, Gong Z, Lin Z, Ding M. Long non-coding RNA RBM5-AS1 promotes the aggressive behaviors of oral squamous cell carcinoma by regulation of miR-1285-3p/YAP1 axis. Biomed Pharmacother. 2019;123:109723. doi: 10.1016/j.biopha.2019.109723. [DOI] [PubMed] [Google Scholar]
- 18.Jiang Z, Zhang Y, Chen X, Wu P, Chen D. Long noncoding RNA RBMS3-AS3 acts as a microRNA-4534 sponge to inhibit the progression of prostate cancer by upregulating VASH1. Gene Ther. 2020;27:143–156. doi: 10.1038/s41434-019-0108-1. [DOI] [PubMed] [Google Scholar]
- 19.Zhang YT, Yang GY. LncRNA SNHG17 acts as a ceRNA of miR-324-3p to contribute the progression of osteosarcoma. J Biol Regul Homeost Agents. 2020;34:1529–1533. doi: 10.23812/20-302-L. [DOI] [PubMed] [Google Scholar]
- 20.Yang C, Zheng J, Xue Y, Yu H, Liu X, Ma J, Liu L, Wang P, Li Z, Cai H, Liu Y. The effect of MCM3AP-AS1/miR-211/KLF5/AGGF1 axis regulating glioblastoma angiogenesis. Front Mol Neurosci. 2018;10:437. doi: 10.3389/fnmol.2017.00437. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Li X, Yu M, Yang C. YY1-mediated overexpression of long noncoding RNA MCM3AP-AS1 accelerates angiogenesis and progression in lung cancer by targeting miR-340-5p/KPNA4 axis. J Cell Biochem. 2020;121:2258–2267. doi: 10.1002/jcb.29448. [DOI] [PubMed] [Google Scholar]
- 22.Yang M, Sun S, Guo Y, Qin J, Liu G. Long non-coding RNA MCM3AP-AS1 promotes growth and migration through modulating FOXK1 by sponging miR-138-5p in pancreatic cancer. Mol Med. 2019;25:55. doi: 10.1186/s10020-019-0121-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 24.Paraskevopoulou MD, Hatzigeorgiou AG. Analyzing MiRNA-LncRNA interactions. Methods Mol Biol. 2016;1402:271–286. doi: 10.1007/978-1-4939-3378-5_21. [DOI] [PubMed] [Google Scholar]
- 25.Liu Z, Li Z, Xu B, Yao H, Qi S, Tai J. Long noncoding RNA PRR34-AS1 aggravates the progression of hepatocellular carcinoma by adsorbing microRNA-498 and thereby upregulating FOXO3. Cancer Manag Res. 2020;12:10749–10762. doi: 10.2147/CMAR.S263619. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Wang S, Wang T, Liu D, Kong H. LncRNA MALAT1 aggravates the progression of non-small cell lung cancer by stimulating the expression of COMMD8 via targeting miR-613. Cancer Manag Res. 2020;12:10735–10747. doi: 10.2147/CMAR.S263538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou Y, Shi H, Du Y, Zhao G, Wang X, Li Q, Liu J, Ye L, Shen Z, Guo Y, Huang Y. lncRNA DLEU2 modulates cell proliferation and invasion of non-small cell lung cancer by regulating miR-30c-5p/SOX9 axis. Aging (Albany NY) 2019;11:7386–7401. doi: 10.18632/aging.102226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zou P, Zhu M, Lian C, Wang J, Chen Z, Zhang X, Yang Y, Chen X, Cui X, Liu J, et al. miR-192-5p suppresses the progression of lung cancer bone metastasis by targeting TRIM44. Sci Rep. 2019;9:19619. doi: 10.1038/s41598-019-56018-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen L, Wang X, Ji C, Hu J, Fang L. MiR-506-3p suppresses papillary thyroid cancer cells tumorigenesis by targeting YAP1. Pathol Res Pract. 2020;216:153231. doi: 10.1016/j.prp.2020.153231. [DOI] [PubMed] [Google Scholar]
- 30.Wang ZZ, Luo YR, Du J, Yu Y, Yang XZ, Cui YJ, Jin XF. MiR-296-5p inhibits cell invasion and migration of esophageal squamous cell carcinoma by downregulating STAT3 signaling. Eur Rev Med Pharmacol Sci. 2019;23:5206–5214. doi: 10.26355/eurrev_201906_18185. [DOI] [PubMed] [Google Scholar]
- 31.Li B, Mao R, Liu C, Zhang W, Tang Y, Guo Z. LncRNA FAL1 promotes cell proliferation and migration by acting as a CeRNA of miR-1236 in hepatocellular carcinoma cells. Life Sci. 2018;197:122–129. doi: 10.1016/j.lfs.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 32.Jiao C, Song Z, Chen J, Zhong J, Cai W, Tian S, Chen S, Yi Y, Xiao Y. lncRNA-UCA1 enhances cell proliferation through functioning as a ceRNA of Sox4 in esophageal cancer. Oncol Rep. 2016;36:2960–2966. doi: 10.3892/or.2016.5121. [DOI] [PubMed] [Google Scholar]
- 33.Liang M, Jia J, Chen L, Wei B, Guan Q, Ding Z, Yu J, Pang R, He G. LncRNA MCM3AP-AS1 promotes proliferation and invasion through regulating miR-211-5p/SPARC axis in papillary thyroid cancer. Endocrine. 2019;65:318–326. doi: 10.1007/s12020-019-01939-4. [DOI] [PubMed] [Google Scholar]
- 34.Wang Y, Yang L, Chen T, Liu X, Guo Y, Zhu Q, Tong X, Yang W, Xu Q, Huang D, Tu K. A novel lncRNA MCM3AP-AS1 promotes the growth of hepatocellular carcinoma by targeting miR-194-5p/FOXA1 axis. Mol Cancer. 2019;18:28. doi: 10.1186/s12943-019-0957-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gong Y, Wu W, Zou X, Liu F, Wei T, Zhu J. MiR-26a inhibits thyroid cancer cell proliferation by targeting ARPP19. Am J Cancer Res. 2018;8:1030–1039. [PMC free article] [PubMed] [Google Scholar]
- 36.Wang J, Wang B, Ren H, Chen W. miR-9-5p inhibits pancreatic cancer cell proliferation, invasion and glutamine metabolism by targeting GOT1. Biochem Biophys Res Commun. 2019;509:241–248. doi: 10.1016/j.bbrc.2018.12.114. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.