Abstract
Previous studies have reported that GATA3 is downregulated in multiple types of tumours, including gastric cancer and osteosarcoma. The aim of this study was to explore whether GATA3 serves as a tumour suppressor to inhibit hepatocellular carcinoma (HCC) development. Tumour tissue specimens and adjacent normal tissue specimens were obtained from 162 patients diagnosed with HCC in the Affiliated Hospital of Shaoxing University from July 2000 to May 2018. The result of the present study demonstrated that GATA3 was downregulated in HCC tumour tissues compared with that of adjacent normal tissues. The expression of GATA3 was also negatively associated with tumour size, TNM stage and lymph node metastasis. Additionally, analysis of the follow-up data revealed that low GATA3 expression was closely correlated with poor survival. Gain and loss of function analyses revealed that overexpression of GATA3 decreased the ability of proliferation, migration and invasion in HCC cell lines, whereas inhibition of GATA3 promoted the ability of proliferation, migration and invasion. In addition, GATA3 suppressed EMT through the regulation of slug expression. Additionally, slug overexpression attenuated the inhibitory effects of GATA3 overexpression on cancer cell proliferation, migration and invasion. Thus, GATA3 is downregulated in HCC, and suppresses cell proliferation, migration and invasion. Moreover, GATA3 transcriptionally inhibits slug expression, thereby suppressing EMT in HCC.
Keywords: GATA3, proliferation, migration, invasion, epithelial-mesenchymal transition
Introduction
Liver cancer has become one of the most malignant cancers worldwide (1), with hepatocellular carcinoma (HCC) accounting for ~80% cases of all cases (2). Due to the high rate of recurrence or intrahepatic metastasis after curative resection, the overall prognosis of patients with HCC remains poor despite marked improvements in surgical techniques and perioperative management (2–4). The overall 5-year survival rate of HCC is still <20% (5); thus, an improved understanding of the molecular mechanisms underlying HCC metastasis will help prevent HCC recurrence and metastasis.
Epithelial-mesenchymal transition (EMT) is a crucial event in tumour metastasis, where epithelial cell layers lose polarity and cell-cell contact, which results in dramatic remodelling of the cytoskeleton (6). The main characteristic of EMT is loss of E-cadherin expression, which is associated with tumour invasiveness, metastasis and poor prognosis (7–9). The activation of various ligands, such as vascular endothelial growth factor (VEGF), epidermal growth factor (EGF) and transforming growth factor-β (TGF-β) can induce the expression of several EMT-associated transcription factors, including zinc finger e-box binding homeobox (ZEB) 1, snail, slug and Twist (10). A previous study has demonstrated positive correlations between EMT-associated transcription factors and poor clinical outcomes in cancer, such as lung cancer, breast cancer, melanoma and HCC (10).
GATA3, a member of the GATA family (11,12), serves a crucial role in T-cell proliferation and differentiation (13). In addition, numerous studies have demonstrated that GATA3 serves different roles in different cancers, for example, GATA3 serves as a tumour activator in soft tissue sarcomas, endometrial carcinomas and neuroblastomas (14–16). In addition, GATA3 suppresses cell proliferation, migration and invasion in osteosarcoma (17). Furthermore, a recent study demonstrated that zinc finger protein 503 (ZNF503) accelerates HCC cell aggressiveness by downregulating GATA3 expression via microRNA-495, suggesting that GATA3 may serve as a tumour suppressor in HCC (18). However, the detailed function of GATA3 in HCC remains unclear.
The aim of present study was to explore whether GATA3 serves as a tumour suppressor to inhibit HCC development and further investigate whether GATA3 may be a molecular therapy target in HCC.
Materials and methods
Tumour samples
A total of 162 HCC tissues and adjacent non-tumour tissues (resected 1–2 cm from the malignant tumor) were obtained from the Affiliated Hospital of Shaoxing University from July 2000 to May 2018. These patients with gastric cancer included 92 males and 70 females aged between 23–78 years, with a mean age of 42.3 years. Tumour tissue (TT) and adjacent non-tumour tissue (ANT) were resected by surgical excision. No prior treatments (including chemotherapy or radiotherapy) were conducted before liver resection surgery. Pathological staging was determined according to the seventh edition of the tumour node metastasis (TNM) classification of the International Union Against Cancer (19). All tissue samples were confirmed using histopathological evaluation and stored at −80°C until further use. The tissue samples were used in accordance with the policies of the institutional review board at the Affiliated Hospital of Shaoxing University, China. The study was approved the by the review board of the Affiliated Hospital of Shaoxing University and written informed consent was obtained from all patients.
Cell culture
HCC cell lines, including MHCC97-H and Hep3B were obtained from the Institute of Biochemistry and Cell Biology (Chinese Academy of Sciences). All cells were cultured in DMEM medium (Thermo Fisher Scientific, Inc.) supplemented with 100 U/ml penicillin-streptomycin mixture (Beyotime Institute of Biotechnology) and 10% FBS (Sigma-Aldrich: Merck KGaA) at 37°C with 5% CO2.
Cell transfection
Transfections were performed using Lipofectamine® 2000 or Lipofectamine® RNAiMAX Reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's instructions. FLAG-GATA3 (pcDNA3.1) and FLAG-slug (pcDNA3.1) plasmids were obtained from Vigene Bioscience Inc. The sequences of small interfering (si)RNAs were as follows: Scramble siRNA (SCR): 5′-UUCUCCGAACGUGUCACGU-3′; siGATA3#1: 5′-AAACUAGGUCUGAUAUUCAUU-3′; siGATA3#2: 5′-CUUUAUUGCAUCUGGGUAGUU-3′. For overexpression of GATA3 or/and slug, cells were transfected with 2.5 µg FLAG-GATA3 and/or 2.5 µg FLAG-slug using Lipofectamine® 2000. For inhibition of GATA3, cells were transfected with 40 nM siRNAs using Lipofectamine® RNAiMAX Reagent. After transfection for 48 h, cells were collected.
RNA extraction and quantitative reverse transcription quantitative (RT-q)PCR
Total RNA was extracted from tissue samples or cells using TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's instructions. A total of 2 µg RNA was used to synthesize cDNA using a PrimeScript Reverse Transcriptase kit (Takara Biotechnology Co., Ltd.). The reverse transcription protocol was as follows: initial denaturation at 37°C for 15 min and then 85°C for 5 sec. The samples were stored at 4°C. Subsequently, RT-qPCR was performed by SYBRGreen (Takara Bio, Inc.) in the Applied Biosystems 7500 Sequence Detection system (Thermo Fisher Scientific Inc.). The PCR cycles were performed by initial denaturation at 95°C for 15 min, followed by 40 cycles at 95°C for 20 sec, 57°C for 35 sec and elongation at 72°C for 2 min. Relative mRNA levels were normalized against that of GAPDH, and the levels of the transcripts were quantified using the 2−ΔΔCq method (20). The sequences of primers as follows: GATA3 forward, 5′-GTTGTGCTCGGAGGGTTTCT-3′, reverse, 5′-GCACGCTGGTAGCTCATACA-3′; Slug forward, 5′-ATCACTGTGTGGACTACCGC-3′, reverse, 5′-TCACTCGCCCCAAAGATGAG-3′; Snail forward, 5′-GTTTACCTTCCAGCAGCCCT-3′, reverse, 5′-TCCCAGATGAGCATTGGCAG-3′; ZEB1 forward, 5′-GATGACCTGCCAACAGACCA-3′, reverse: 5′-CTGTGTCATCCTCCCAGCAG-3′; and GAPDH forward, 5′-GAAAGCCTGCCGGTGACTAA-3′, reverse, 5′-AGGAAAAGCATCACCCGGAG-3′. Each experiment was performed at least three times.
Western blotting
Cell lysates were prepared using RIPA lysis buffer (Pierce; Thermo Fisher Scientific, Inc.) with a protease inhibitor cocktail (Roche Applied Science) and protein concentration was measured using a bicinchoninic acid kit (Beyotime Institute of Biotechnology) according to manufacturer's protocol. Subsequently, 40 µg protein was separated on 12% SDS-PAGE and then transferred onto a PVDF membrane (EMD Millipore). After blocking with 5% non-fat milk in TBST for 1 h at room temperature, membranes were incubated with the indicated primary antibodies at 4°C overnight. The primary antibodies used were as follows: GATA3 (1:1,000; cat. no. ab199428; Abcam); E-cadherin (1:1,000; cat. no. 14472; CST Biological Reagents Co., Ltd.); N-cadherin (1:1,000; cat. no. 13116; CST Biological Reagents Co. Ltd.); and Slug (1:1,000; cat. no. ab51772; Abcam). The membranes were then washed with TBST three times and incubated with horse radish peroxide-conjugated goat anti-rabbit IgG (1:2,000; cat. no. ab6721; Abcam) or goat anti-mouse IgG (1:4,000; cat. no. ab6789; Abcam) secondary antibodies for 1 h at room temperature. The specific bands were visualized using the enhanced chemiluminescence (ECL; Merck KGaA) according to manufacturer's protocol. β-actin (1:5,000; cat. no. ab8226; Abcam) was used as the internal control. Each experiment was performed at least three times. Western blotting densitometry was analysed using Image J software (v1.48; National Institutes of Health).
Cell Counting Kit-8 (CCK-8) assay
CCK-8 (Dojindo Molecular Technologies, Inc.) was utilized to determine the effect of GATA3 on cell proliferation according to the manufacturer's instructions. Briefly, Hep3B and MHCC97-H cells were transfected with FLAG-GATA3 or GATA3 siRNA. After transfection for 48 h, ~2×103 cells were seeded in 96-well plates with 200 µl DMEM at 37°C with 5% CO2. After 24 and 48 h of incubation, 20 µl CCK-8 reagent was added to each well and cultured for 2 h. Finally, the absorbance at 450 nm measured using a microplate reader (Bio-Rad laboratories, Inc.). Each experiment was performed at least three times.
Colony formation assay
To evaluate the effect of GATA3 on cell proliferation in HCC, colony formation assay was performed. In brief, 5×103 transfected Hep3B and MHCC97-H cells were plated into 6-well plate. After incubation at 37°C with 5% CO2 for 2 weeks, cells were fixed in 4% paraformaldehyde at room temperature for 10 min and stained with 0.5% crystal violet at room temperature for 10 min. Finally, the colonies were counted. Each experiment was performed at least three times.
Wound healing assay
GATA3 was overexpressed or knocked down in Hep3B and MHCC97-H cells. After transfection for 48 h, 5×106 cells were grown in 6-well plates. After cell density reached 100% confluency, the monolayer was scratched with a 20 µl sterile plastic pipette tip. After washing with PBS three times, cells were cultured in serum-free DMEM at 37°C with 5% CO2 for 48 h. Then, wound closure was imaged under a light microscope (magnification, ×40) and the rate of wound closure was measured using Image J software version 1.2 (National Institutes of Health). Relative distance of cell migration = [wound closure (0 h) - wound closure (48 h)]/wound closure (0 h) ×100. Each experiment was performed at least three times.
Transwell assay
A transwell invasion assay was performed to determine the effect of GATA3 on the capacity of cell invasion. The upper chamber was precoated with BioCoat Matrigel (BD Biosciences) and PBS (1:8) at 37°C with 5% CO2 for 30 min. GATA3 was overexpressed or knocked down in MHCC97-H cells. After transfection for 48 h, 2×105 cells were placed into upper chamber containing 400 μl serum-free DMEM and 500 µl DMEM medium containing 10% FBS was added into the lower chamber. After incubation at 37°C with 5% CO2 for 24 h, cells were stained with 0.5% crystal violet for 5 min at room temperature and non-invading cells on the upper chamber surface were removed using a cotton swab. Finally, invading cells were imaged under a light microscope (magnification, ×40). Each experiment was performed at least three times.
Enzyme-linked immunosorbent assay (ELISA)
MHCC97-H cells were collected and plated into 6-well culture plates at a density of 4×105 cells/well. Cell supernatants in serum-free medium were homogenized and harvested 72 h later and centrifuged at 1,000 × g for 30 min. The levels of MMP2 and MMP9 were subsequently determined using commercially available ELISA kits (cat. nos. E0100Hu and E0553Hu; Wuhan USCN Business Co., Ltd.) according to the manufacturer's instructions.
Chromatin immunoprecipitation (ChIP) and quantitative (q) ChIP assay
ChIP and qChIP analyses were performed using an EZ-ChIP kit (EMD Millipore) according to the manufacturer's instructions. Briefly, Hep3B and MHCC97-H cells were cultured to 80–100% confluence and the chromatin was cross-linked by 1% formaldehyde at 37°C for 10 min. Subsequently, cross-linked chromatin was sonicated (20 kHz; amplitude, 40%; 30 cycles, 1 sec on and 1 sec off) at 4°C to generate 200–1,000 bp fragments. Next, 4 µg of anti-GATA3 (cat. no. ab199428; Abcam) or anti-IgG antibody (cat. no. ab171870; Abcam) were used to immunoprecipitate chromatin fragments at 4°C overnight. IgG antibody was used as the control. The protein-DNA complexes were incubated with protein A Sepharose beads (Thermo Fisher Scientific, Inc.), and eluted in 1% SDS/0.1 M NaHCO3. The protein-DNA cross-link was reversed by heating at 65°C for 6 h. The DNA was purified using QIAEX II Gel Extraction kit (Qiagen GmbH) according to manufacturer's instructions. After purifying the antibody-interact DNA, RT-qPCR was conducted to analyze the precipitated chromatin DNA, as aforementioned. The primer sequences were as follows: Slug forward, 5′-TCCGGTGGTTCCAAATGACA-3′; and reverse, 5′-TCCGGTGGTTCCAAATGACA-3′. The qPCR conditions were as follows: 5 min at 98°C, denaturation at 98°C for 30 sec, annealing at 56°C for 30 sec and extension at 72°C for 20 sec, performed for 32 cycles.
Luciferase reporter assay
The sequence of the slug promoter region was amplified from human genomic DNA. The sequence was then cloned into pGL3-basic luciferase reporter vector (Promega Corporation). A total of 2 µg pGL3-slug, Renilla and GATA3 or 50 nM GATA3 siRNA were co-transfected into Hep3B and MHCC97-H cells using Lipofectamine® 2000 (Invitrogen; Thermo Fisher Scientific, Inc.). After transfection for 24 h, the relative luciferase activity was measured using Dual-Luciferase Reporter Assay System (Promega Corporation) according to the manufacturer's instructions. Renilla activity was used as the internal control. The relative luciferase activity was normalized with Renilla luciferase activity. Each experiment was performed at least three times.
Statistical analysis
All data were represented as the mean ± SD. The association between GATA3 expression and clinicopathological features of HCC was analysed by χ2 test. Student's t-test was used to compare the statistical differences between two groups and one-way ANOVA followed by Tukey's post-hoc test was used to compare the statistical differences between multiple groups. The overall survival of patients with HCC with high or low level of GATA3 was estimated using the Kaplan-Meier method. All data were analysed by SPSS 18.0 software (SPSS, Inc.). P<0.05 was considered to indicate a statistically significant difference.
Results
GATA3 is downregulated in HCC
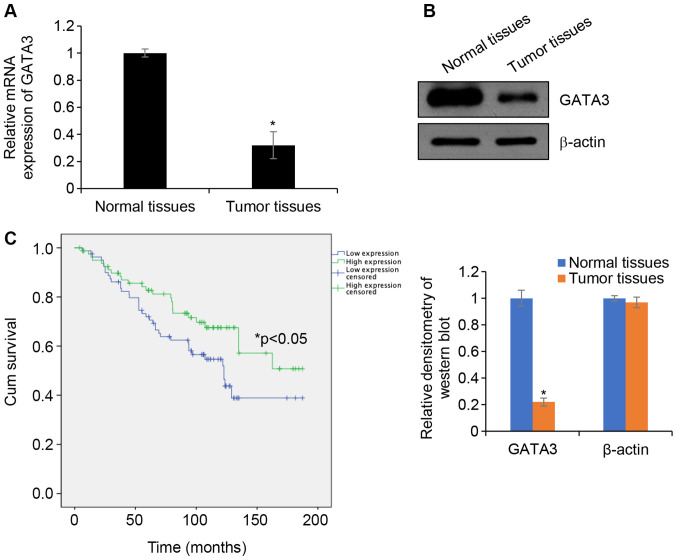
To investigate whether GATA3 serves as a tumour suppressor in HCC, the expression of GATA3 in HCC tissues was determined. HCC tissue samples and adjacent normal tissues samples were collected and RT-qPCR was performed to determine GTAT3 mRNA levels. The results demonstrated that the expression of GATA3 mRNA in HCC tissues was significantly downregulated in TT compared with that in ANT (Fig. 1A). In addition, western blotting analyses demonstrated that the GATA3 protein expression in HCC tissues was lower in TT compared with that in ANT (Fig. 1B). To further determine the roles of GATA3 in HCC, the association of GATA3 and the clinicopathological features of HCC were analysed. GATA3 expression was negatively associated with tumour size, pathological grade and lymph node metastasis, but no significant association was observed between GATA3 and other factors, such as age and sex (Table I). Additionally, the survival curve demonstrated that patients with high expression of GATA3 had an improved prognosis compared with patients exhibiting low GATA3 expression (Fig. 1C). Collectively, the results indicated that GATA3 serves a key function in HCC.
Figure 1.
GATA3 is downregulated in HCC cell lines and tissues. (A) GATA3 mRNA levels in tumour and adjacent normal tissues were established using reverse transcription-quantitative PCR analysis. *P<0.05. (B) GATA3 protein levels in tumour and adjacent normal tissues was established using western blotting analysis. The relative densitometry of the western blots was analysed using Image J software. *P<0.05. (C) Kaplan-Meier analysis was used to identify the effect of GATA3 expression on the prognosis of patients with HCC. *P<0.05 high vs. low expression. HCC, hepatocellular carcinoma.
Table I.
Clinicopathological variables in 162 patients with hepatocellular carcinoma
| GATA3 expression | ||||
|---|---|---|---|---|
| Variables | No. (n=162) | Low (n=111) | High (n=51) | P-value |
| Age, years | 0.527 | |||
| ≥40 | 83 | 55 | 28 | |
| <40 | 79 | 56 | 23 | |
| Sex | 0.503 | |||
| Male | 92 | 65 | 27 | |
| Female | 70 | 46 | 24 | |
| Tumour size, cm | 0.009a | |||
| Large (≥2) | 94 | 72 | 22 | |
| Small (<2) | 68 | 39 | 29 | |
| Pathological grade | 0.047a | |||
| I–II | 83 | 51 | 32 | |
| III–IV | 79 | 60 | 19 | |
| Lymph node metastasis | 0.049a | |||
| Yes | 82 | 62 | 20 | |
| No | 80 | 49 | 31 | |
| Slug expression | <0.001a | |||
| High | 77 | 63 | 14 | |
| Low | 85 | 48 | 37 | |
P<0.05.
GATA3 suppresses HCC cell proliferation
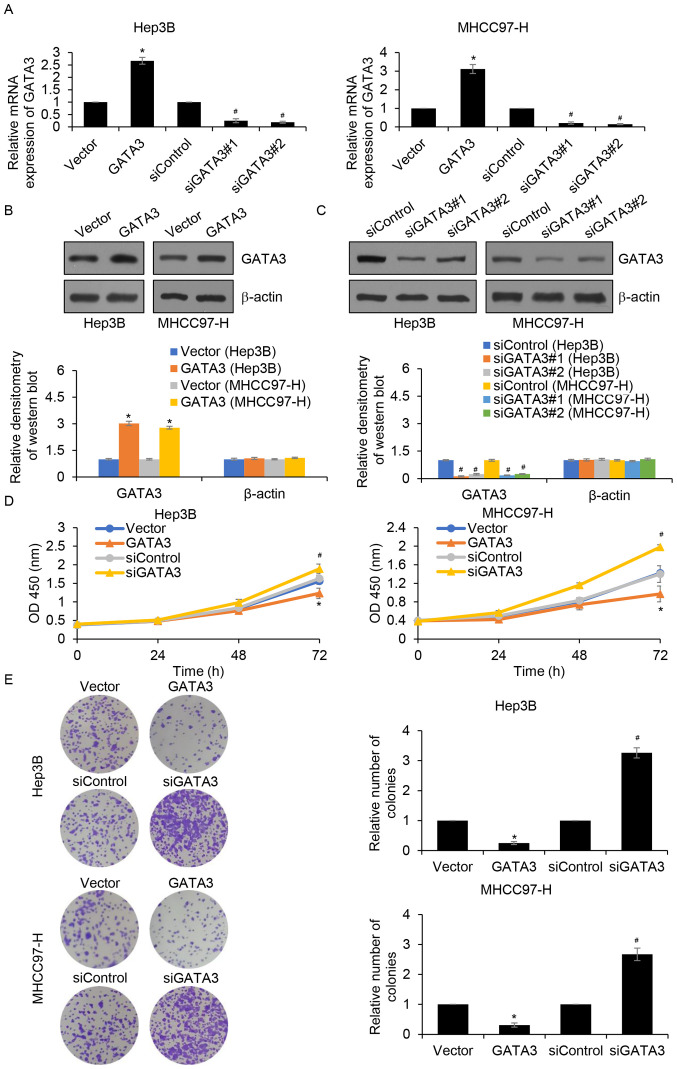
Since GATA3 expression was associated with tumour size, it was hypothesized that GATA3 may suppress cell proliferation in HCC. To verify this hypothesis, GATA3 was overexpressed or knocked down in Hep3B and MHCC97-H cells and the expression of GATA3 was established by RT-qPCR (Fig. 2A) and western blotting (Fig. 2B and C). The results demonstrated that GATA3 mRNA and protein levels were significantly increased in Hep3B and MHCC97-H cells transfected with FLAG-GATA3 compared with those of the vector group. In addition, GATA3 mRNA and protein levels were significantly decreased following siGATA3 transfection compared with those of the siControl group. CCK-8 and colony formation assays were subsequently performed to detect the effects of GATA3 on cell proliferation. The results of the CCK-8 assay demonstrated that, when compared with the control groups, GATA3 overexpression significantly suppressed cell proliferation, whereas inhibition of GATA3 significantly increased cell proliferation (Fig. 2D). Additionally, the colony formation assay demonstrated that GATA3 overexpression significantly reduced the number of colonies compared with the control groups, whereas inhibition of GATA3 significantly increased the number of colonies, suggesting that GATA3 may suppress cell proliferation in HCC (Fig. 2E).
Figure 2.
GATA3 suppresses HCC cell proliferation. (A) GATA3 was overexpressed or knocked down in Hep3B and MHCC97-H cells using FLAG-GATA3 plasmid or GATA3 siRNAs, respectively. After transfection for 48 h, the expression of GATA3 was determined using reverse transcription-quantitative PCR. GATA3 was (B) overexpressed or (C) knocked down in Hep3B and MHCC97-H cells using FLAG-GATA3 plasmid or GATA3 siRNAs, respectively. The expression of GATA3 was determined using western blotting. The relative densitometry of the western blots was analysed using Image J software. (D) GATA3 was overexpressed or knocked down in Hep3B and MHCC97-H cells, after which cell proliferation rate was detected using a Cell Counting Kit-8 assay. (E) After GATA3 was overexpressed or knocked down in Hep3B and MHCC97-H cells, colony formation assay was assessed to determine the effect of GATA3 on cell proliferation. *P<0.05 vs. vector; #P<0.05 siGATA3 vs. siControl. HCC, hepatocellular carcinoma; si, small interfering.
GATA3 inhibits cell migration and invasion in HCC in vitro
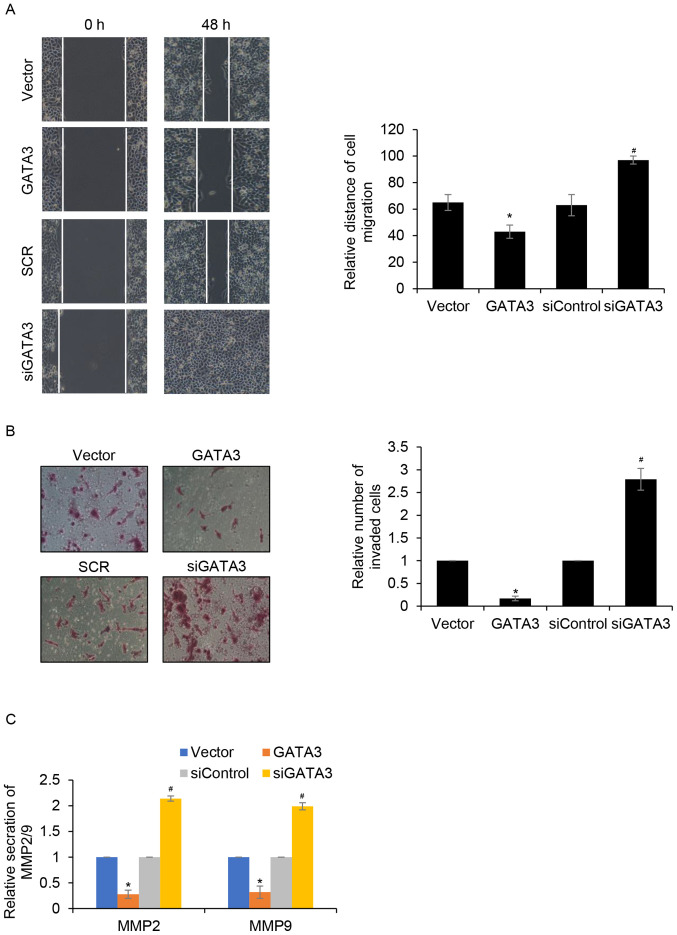
The effects of GATA3 on cell migration and invasion in MHCC97-H cells was investigated. The results of wound healing analysis demonstrated that MHCC97-H cell migration was significantly reduced when GATA3 was overexpressed compared with the control group. Furthermore, MHCC97-H cell migration was significantly increased when GATA3 was knocked down compared with the siControl group (Fig. 3A). Transwell invasion analysis demonstrated that GATA3 overexpression significantly suppressed the invasion of MHCC97-H cells compared with the control group. Additionally, inhibition of GATA3 promoted MHCC97-H cell invasion compared with that of the siControl group (Fig. 3B). Metalloproteinase (MMP)2 and MMP9 have been reported to be strongly associated with tumour aggressiveness and poor prognosis in multiple types of cancer, such as lung adenocarcinoma and tongue carcinoma (21,22). Thus, whether GATA3 promotes HCC cell migration and invasion through regulation of MMP2 or MMP9 was explored. The effect of GATA3 on MMP2 and MMP9 secretion was determined using ELISA assay. The results demonstrated that GATA3 inhibition significantly increased MMP2 and MMP9 secretion compared with the siControl group (Fig. 3C). By contrast, GATA3 overexpression significantly suppressed MMP2 and MMP9 secretion compared with the same group. These data suggested that GATA3 may suppress HCC cell migration and invasion by inhibiting MMP2 or MMP9 secretion.
Figure 3.
GATA3 inhibits cell migration and invasion in HCC in vitro. (A) Wound healing assay was performed to detect the effect of GATA3 on cell migration. (B) Transwell invasion assay was performed to detect the effect of GATA3 on cell invasion. (C) The secretion of MMP2 and MMP9 was measured using MMP2 and MMP9 Elisa kits. *P<0.05 GATA3 vs. vector; #P<0.05 siGATA3 vs. siControl. HCC, hepatocellular carcinoma; si, small interfering; MMP, metalloproteinase.
GATA3 inhibits EMT in HCC by regulating Slug
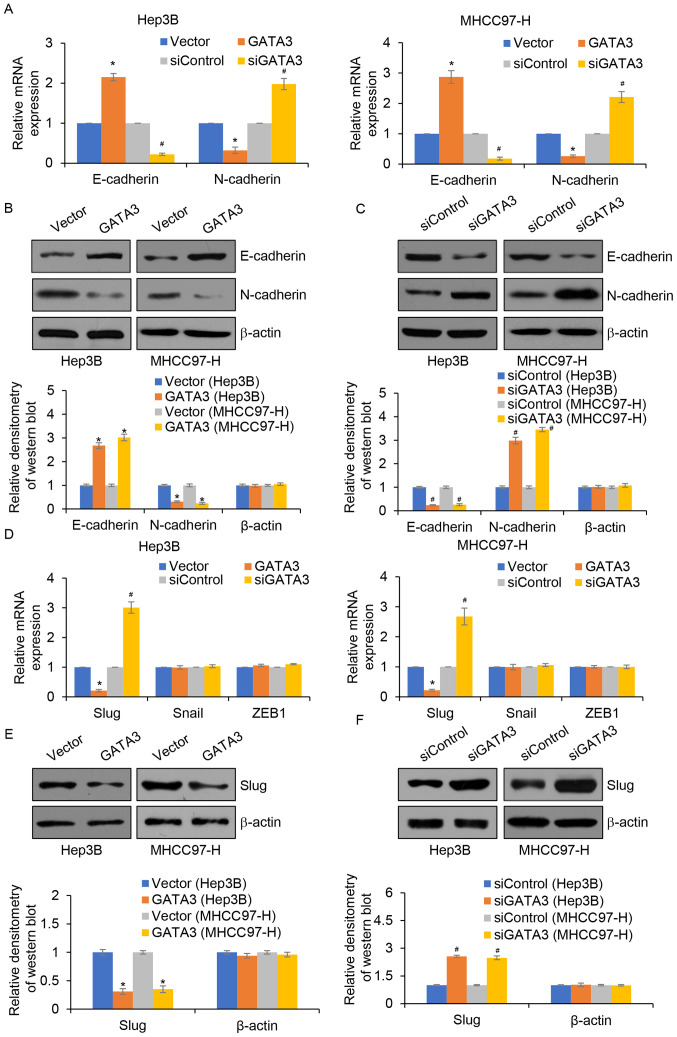
EMT is a complex process associated with metastasis (23); thus, whether GATA3 suppresses migration and invasion through the regulation of EMT in HCC was explored. The results demonstrated that GATA3 overexpression significantly increased the mRNA and protein expression of E-cadherin, an epithetical marker. However, the mRNA and protein expression of N-cadherin, a mesenchymal marker was significantly reduced compared with the control group (Fig. 4A and B). By contrast, inhibition of GATA3 significantly reduced the mRNA and protein expression levels of E-cadherin, but significantly increased the mRNA and protein expression levels of N-cadherin and vimentin compared with those of the control group (Fig. 4A and C). Notably, a previous study demonstrated that GATA3 regulates the expression of slug in osteosarcoma (17). Thus, whether GATA3 regulated slug expression in HCC was determined. The results demonstrated that compared with the controls, GATA3 overexpression significantly decreased the expression of slug, whereas inhibition of GATA3 significantly increased slug levels (Fig. 4D, E and F).
Figure 4.
GATA3 inhibits epithelial-mesenchymal transition in hepatocellular carcinoma through the regulation of Slug. (A) The mRNA levels of E-cadherin and N-cadherin in GATA3-overexpression or GATA3-depletion MHCC97-H cells were determined by RT-qPCR. The protein levels of E-cadherin and N-cadherin in (B) GATA3-overexpressed or (C) knocked down MHCC97-H cells were determined by western blotting. The relative densitometry of the western blots was analysed using Image J software. (D) The mRNA levels of slug, snail and ZEB1 in GATA3-overexpressed or knocked down MHCC97-H cells were determined by RT-qPCR assay. GATA3 vs. vector, siGATA3 vs. siControl. (E and F) The protein level of slug in GATA3-overexpression or GATA3-depletion MHCC97-H cells were determined by RT-qPCR assay. The relative densitometry of the western blots was analysed using Image J software. *P<0.05 GATA3 vs. vector; #P<0.05 siGATA3 vs. siControl. si, small interfering; ZEB1, zinc finger e-box binding homeobox 1; RT-qPCR, reverse transcription-quantitative PCR.
Slug is a direct target of GATA3 in HCC cells
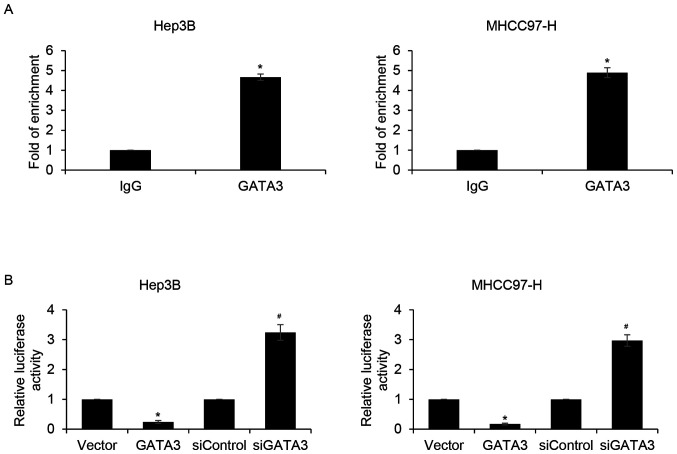
To further determine whether GATA3 inhibits EMT in HCC through the regulation of slug expression, slug levels were detected in HCC specimens. The results demonstrated that slug expression was negatively associated with GATA3 (Table I). GATA3 is a transcription factor; thus, to further determine whether GATA3 directly binds to the promoter region of slug, qChIP and dual luciferase reporter assays were performed. The results demonstrated that the relative enrichment of GATA3 in the promoter region of slug was significantly increased compared with that of the IgG group (Fig. 5A). In addition, the relative luciferase activity of Hep3B and MHCC97-H cells were significantly reduced in GATA3-overexpression cells compared with that of the vector group (Fig. 5B). By contrast, inhibition of GATA3 significantly increased relative luciferase activity compared the siControl group (Fig. 5B). The results suggested that GATA3 may transcriptionally inhibit slug expression in HCC cells.
Figure 5.
Slug is a direct target of GATA3 in hepatocellular carcinoma cells. (A) qChIP assay was performed using in Hep3B and MHCC97-H cells. IgG was used as internal control. GATA3 vs. IgG, *P<0.05. (B) Hep3B and MHCC97-H cells were transfected with FLAG-GATA3 plasmid or GATA3 siRNA. After transfection for 24 h, dual luciferase assay was performed. *P<0.05 GATA3 vs. IgG; GATA3 vs. vector; #P<0.05 siGATA3 vs. siControl. si, small interfering.
Overexpression of slug partially attenuates GATA3 inhibited migration, invasion and proliferation
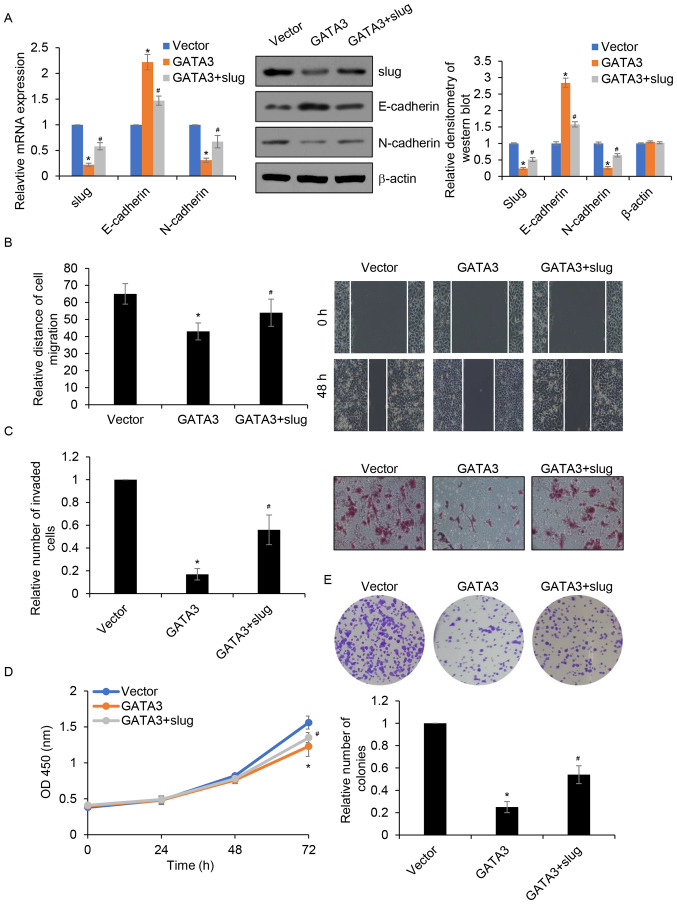
Slug serves as a downstream target of GATA3; thus, whether the roles of GATA3 in HCC occurred through the transcriptional regulation of slug was investigated. GATA3 and slug was simultaneously overexpressed in MHCC97-H cells and the expression of slug was determined by RT-qPCR and western blotting. The results demonstrated that compared with those the control group, the mRNA and protein levels of slug were significantly reduced in the GATA3 group and that this was partially restored in the GATA3+ slug (Fig. 6A). To further confirm whether GATA3 regulated cell migration and invasion by transcriptionally inhibiting slug expression, wound healing and Transwell invasion assays were performed. The results demonstrated that overexpression of slug partially rescued the GATA3 overexpression-induced suppression of MHCC97-H cell migration and invasion (Fig. 6B and C). Furthermore, CCK-8 and colony formation assays demonstrated that overexpression of slug in GATA3-overexpressed MHCC97-H cells partially reversed the dampening effect of GATA3 overexpression on cell proliferation (Fig. 6D and E). Together, the results suggested that slug acts as a downstream effector of GATA3 in the regulation of migration, invasion and proliferation of HCC cells.
Figure 6.
Overexpression of Slug partially attenuates GATA3 inhibited migration, invasion and proliferation of MHCC97-H cells. (A) MHCC97-H cells were transfected with FLAG-GATA3 and/or slug plasmid. After transfection for 48 h, the mRNA and protein levels of slug were determined using reverse transcription-quantitative PCR and western blotting. The relative densitometry of the western blots was analysed using Image J software. (B) MHCC97-H cells were transfected with FLAG-GATA3 or/and slug plasmid. Wound healing assay was used to determine the effect of slug on GATA3 inhibited cell migration. (C) MHCC97-H cells were transfected with FLAG-GATA3 or/and slug plasmid. Transwell invasion assay was used to effect of slug on GATA3 inhibited cell invasion. (D) MHCC97-H cells were transfected with FLAG-GATA3 or/and slug plasmid, the cell proliferation rate was detected by Cell Counting Kit-8 assay. (E) MHCC97-H cells were transfected with FLAG-GATA3 and/or slug plasmid, colony formation assay was used to determine the effect of slug on cell proliferation. *P<0.05 GATA3 vs. vector; #P<0.05 GATA3+ slug vs. GATA3.
Discussion
The function of GATA3 varies in different cancers. For example, previous studies have demonstrated that GATA3 is a poor prognostic marker in soft tissue sarcomas, endometrial carcinomas and neuroblastomas (14–16,24). However, GATA3 has also been demonstrated to suppress cell proliferation, migration and invasion in osteosarcoma (17). In breast cancer cells, GATA3 interacts with the G9A/NuRD (MTA3) complex to suppress tumour growth factor (TGF) β1, ZEB2 and other EMT-related genes (25). By contrast, GATA3 facilitates the cell cycle by activating the transcription of cyclin D1 in luminal-type breast cancer cells (26). However, the detailed mechanisms underlying the distinct roles of GATA3 in HCC remains unclear.
The present study suggested that GATA3 may act as a tumour suppressor in HCC. The results of the present study demonstrated that GATA3 expression is downregulated in HCC tissue species and cell lines and that the expression of GATA3 is associated with tumour size, lymph node metastasis, TNM stage and prognosis. Additionally, the present study demonstrated that GATA3 overexpression inhibited cell proliferation, migration and invasion in HCC. In addition, GATA3 overexpression suppressed EMT by transcriptionally regulating slug expression.
Several gain of function and loss of function analyses were performed to identify the functions of GATA3 in HCC. Colony formation and CCK-8 assays revealed that overexpression of GATA3 suppressed cell proliferation in HCC. In addition, wound healing assay and Transwell invasion assays demonstrated that overexpression of GATA3 suppressed cell migration and invasion in HCC. EMT serves key roles in cancer metastasis (27). In corroboration with previous studies (17,28), the present study demonstrated that GATA3 suppressed EMT in HCC cells by increasing the expression of E-cadherin and decreasing the expression of N-cadherin and vimentin. In addition, qChIP and dual luciferase reporter assays demonstrated that GATA3 transcriptionally regulated slug, thereby inhibiting EMT in HCC.
A previous study revealed that GATA3 may serve as a tumour suppressor in HCC (18). However, Guan et al (29) identified that hepatitis B virus-regulated GATA3 helps HCC cells escape from natural killer cell surveillance by regulating major histocompatibility complex class I polypeptide-related sequence B, ligands of the NKG2D receptor. These contradictory findings imply that the roles of GATA3 in HCC is complex and that the detailed mechanism of GATA3 in HCC needs to be further investigated.
In summary, the current study suggested that GATA3 may act as a tumour suppressor in HCC. Mechanistically, GATA3 directly regulated slug expression to suppress the EMT process, thereby inhibiting HCC cell migration and invasion. In addition, GATA3 also reduced cell proliferation. Thus, the current study provides a strong rationale for GATA3 as a therapeutic target in HCC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analysed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
ZZ and XF conceived and designed the study. ZZ, XF, JZ and GX performed the experiments. XF, GX and JZ wrote the manuscript. ZZ, XF, GX and JZ reviewed and edited the manuscript. All authors read and approved the manuscript and agree to be accountable for all aspects of the research in ensuring that the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ethics approval and consent to participate
The present study was approved by the Ethics Committee of the Affiliated Hospital of Shaoxing University (approval no. LK1999006). All patients provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Bruix J, Gores GJ, Mazzaferro V. Hepatocellular carcinoma: Clinical frontiers and perspectives. Gut. 2014;63:844–855. doi: 10.1136/gutjnl-2013-306627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Torres HA, Vauthey JN, Economides MP, Mahale P, Kaseb A. Hepatocellular carcinoma recurrence after treatment with direct-acting antivirals: First, do no harm by withdrawing treatment. J Hepatol. 2016;65:862–864. doi: 10.1016/j.jhep.2016.05.034. [DOI] [PubMed] [Google Scholar]
- 4.Tang ZY, Ye SL, Liu YK, Qin LX, Sun HC, Ye QH, Wang L, Zhou J, Qiu SJ, Li Y, et al. A decade's studies on metastasis of hepatocellular carcinoma. J Cancer Res Clin Oncol. 2004;130:187–196. doi: 10.1007/s00432-003-0511-1. [DOI] [PubMed] [Google Scholar]
- 5.Chapman BC, Paniccia A, Hosokawa PW, Henderson WG, Overbey DM, Messersmith W, McCarter MD, Gleisner A, Edil BH, Schulick RD, et al. Impact of Facility Type and Surgical Volume on 10-Year Survival in Patients Undergoing Hepatic Resection for Hepatocellular Carcinoma. J Am Coll Surg. 2017;224:362–372. doi: 10.1016/j.jamcollsurg.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 6.Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A, Weinberg RA. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927–939. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 7.Hirohashi S, Kanai Y. Cell adhesion system and human cancer morphogenesis. Cancer Sci. 2003;94:575–581. doi: 10.1111/j.1349-7006.2003.tb01485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sugimachi K, Taguchi K, Aishima S, Tanaka S, Shimada M, Kajiyama K, Sugimachi K, Tsuneyoshi M. Altered expression of beta-catenin without genetic mutation in intrahepatic cholangiocarcinoma. Mod Pathol. 2001;14:900–905. doi: 10.1038/modpathol.3880409. [DOI] [PubMed] [Google Scholar]
- 9.Rhim AD, Mirek ET, Aiello NM, Maitra A, Bailey JM, McAllister F, Reichert M, Beatty GL, Rustgi AK, Vonderheide RH, et al. EMT and dissemination precede pancreatic tumor formation. Cell. 2012;148:349–361. doi: 10.1016/j.cell.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: An alliance against the epithelial phenotype? Nat Rev Cancer. 2007;7:415–428. doi: 10.1038/nrc2131. [DOI] [PubMed] [Google Scholar]
- 11.Ho IC, Pai SY. GATA-3 - not just for Th2 cells anymore. Cell Mol Immunol. 2007;4:15–29. [PubMed] [Google Scholar]
- 12.Simon MC. Gotta have GATA. Nat Genet. 1995;11:9–11. doi: 10.1038/ng0995-9. [DOI] [PubMed] [Google Scholar]
- 13.Hosoya T, Maillard I, Engel JD. From the cradle to the grave: Activities of GATA-3 throughout T-cell development and differentiation. Immunol Rev. 2010;238:110–125. doi: 10.1111/j.1600-065X.2010.00954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haraguchi T, Miyoshi H, Hiraoka K, Yokoyama S, Ishibashi Y, Hashiguchi T, Matsuda K, Hamada T, Okawa T, Shiba N, et al. GATA3 Expression Is a Poor Prognostic Factor in Soft Tissue Sarcomas. PLoS One. 2016;11:e0156524. doi: 10.1371/journal.pone.0156524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Engelsen IB, Stefansson IM, Akslen LA, Salvesen HB. GATA3 expression in estrogen receptor alpha-negative endometrial carcinomas identifies aggressive tumours with high proliferation and poor patient survival. Am J Obstet Gynecol. 2008;199:543.e1–7. doi: 10.1016/j.ajog.2008.04.043. [DOI] [PubMed] [Google Scholar]
- 16.Peng H, Ke XX, Hu R, Yang L, Cui H, Wei Y. Essential role of GATA3 in regulation of differentiation and cell proliferation in SK-N-SH neuroblastoma cells. Mol Med Rep. 2015;11:881–886. doi: 10.3892/mmr.2014.2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma L, Xue W, Ma X. GATA3 is downregulated in osteosarcoma and facilitates EMT as well as migration through regulation of slug. OncoTargets Ther. 2018;11:7579–7589. doi: 10.2147/OTT.S176534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yin G, Liu Z, Wang Y, Sun L, Wang L, Yao B, Liu R, Chen T, Niu Y, Liu Q. ZNF503 accelerates aggressiveness of hepatocellular carcinoma cells by down-regulation of GATA3 expression and regulated by microRNA-495. Am J Transl Res. 2019;11:3426–3437. [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng Y, Wang DD, Wang W, Pan K, Huang CY, Li YF, Wang QJ, Yuan SQ, Jiang SS, Qiu HB, et al. Reduced expression of uroplakin 1A is associated with the poor prognosis of gastric adenocarcinoma patients. PLoS One. 2014;9:e93073. doi: 10.1371/journal.pone.0093073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 21.Cai X, Zhu H, Li Y. PKCζ, MMP-2 and MMP-9 expression in lung adenocarcinoma and association with a metastatic phenotype. Mol Med Rep. 2017;16:8301–8306. doi: 10.3892/mmr.2017.7634. [DOI] [PubMed] [Google Scholar]
- 22.Zhao Y, Zhou FL, Li WP, Wang J, Wang LJ. Slit2 Robo1 signaling promotes the adhesion, invasion and migration of tongue carcinoma cells via upregulating matrix metalloproteinases 2 and 9, and downregulating E cadherin. Mol Med Rep. 2016;14:1901–1906. doi: 10.3892/mmr.2016.5518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liao TT, Yang MH. Revisiting epithelial-mesenchymal transition in cancer metastasis: The connection between epithelial plasticity and stemness. Mol Oncol. 2017;11:792–804. doi: 10.1002/1878-0261.12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mehra R, Varambally S, Ding L, Shen R, Sabel MS, Ghosh D, Chinnaiyan AM, Kleer CG. Identification of GATA3 as a breast cancer prognostic marker by global gene expression meta-analysis. Cancer Res. 2005;65:11259–11264. doi: 10.1158/0008-5472.CAN-05-2495. [DOI] [PubMed] [Google Scholar]
- 25.Si W, Huang W, Zheng Y, Yang Y, Liu X, Shan L, Zhou X, Wang Y, Su D, Gao J, et al. Dysfunction of the reciprocal feedback loop between GATA3- and ZEB2-nucleated repression programs contributes to breast cancer metastasis. Cancer Cell. 2015;27:822–836. doi: 10.1016/j.ccell.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 26.Shan L, Li X, Liu L, Ding X, Wang Q, Zheng Y, Duan Y, Xuan C, Wang Y, Yang F, et al. GATA3 cooperates with PARP1 to regulate CCND1 transcription through modulating histone H1 incorporation. Oncogene. 2014;33:3205–3216. doi: 10.1038/onc.2013.270. [DOI] [PubMed] [Google Scholar]
- 27.Takai M, Terai Y, Kawaguchi H, Ashihara K, Fujiwara S, Tanaka T, Tsunetoh S, Tanaka Y, Sasaki H, Kanemura M, et al. The EMT (epithelial-mesenchymal-transition)-related protein expression indicates the metastatic status and prognosis in patients with ovarian cancer. J Ovarian Res. 2014;7:76. doi: 10.1186/1757-2215-7-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wei S, Zhong L, Wang X, Zhang W. Low expression of GATA3 promotes cell proliferation and metastasis in gastric cancer. Cancer Manag Res. 2017;9:769–780. doi: 10.2147/CMAR.S147973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guan Y, Li W, Hou Z, Han Q, Lan P, Zhang J, Tian Z, Zhang C. HBV suppresses expression of MICA/B on hepatoma cells through up-regulation of transcription factors GATA2 and GATA3 to escape from NK cell surveillance. Oncotarget. 2016;7:56107–56119. doi: 10.18632/oncotarget.11271. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the present study are available from the corresponding author on reasonable request.